Abstract
Background
Revascularization strategies for chronic mesenteric ischemia (CMI) include open (OR) and endovascular (ER) modalities. The primary objective of this study was to analyze the safety and effectiveness of OR and ER and the impact of clinical and morphological variables on early and midterm outcomes in a consecutive series of CMI patients in a tertiary referral center.
Patients and methods
From 2004 to 2017, all CMI patients treated with OR and ER were retrospectively identified. Patient records, preoperative imaging, as well as peri- and postoperative outcomes were analyzed. Univariable and multivariable analysis was performed to identify clinical or morphological variables affecting reintervention rates within 2 years.
Results
In total, 63 patients (33% male; mean age 71, range 60–76 years) were treated by ER (41 patients) or OR (22 patients) for CMI. Mean follow-up was 26 (10–71) months. 30-day mortality was 0.0% after ER and 4.5% after OR (p = 0.069); 30-day morbidity was 9.8% vs. 31.8%, respectively (p = 0.030). Length of stay was significantly longer after OR (14 vs. 4 days; p < 0.001). Freedom from reintervention rate after 2 years was 82% after OR and 73% after ER (p = 0.14). Overall survival did not differ after 2 years (OR 85% vs. ER 86%; p = 0.35). Multivariable analysis revealed that smoking was associated with higher risk of reintervention (hazard ratio, HR: 4.14; 95% confidence interval, CI 1.11–15.53; p = 0.03). Additionally, a nonsignificant trend of lower reintervention rates after OR was detected (HR 0.23 95% CI 0.05–1.08; p = 0.06).
Conclusion
Due to a lower invasiveness, despite the higher reintervention rate, an “endovascular first” strategy is justified and recommended.
Introduction
Although the prevalence of severe atherosclerosis of the mesenteric arteries supposedly ranges between 30 and 50% in the elderly population (> 65 years), the exact incidence of chronic mesenteric ischemia (CMI) is unknown. Clinically, CMI is a rarely diagnosed cause of abdominal pain [1, 2]. Scott J. Boley, a pioneer in the field of mesenteric ischemia, has been studying mesenteric circulation and vascular disorders of the intestines since the early 1960s. A particular concern was to focus physicians’ attention on the methods for, and potential success of, early diagnosis and aggressive treatment of these conditions [3, 4].
In approximately 90% of patients, it is caused by an atherosclerotic stenosis or occlusion of the superior mesenteric artery (SMA) and/or the celiac trunk (CTr) [5, 6]. Stenoses or occlusions of the inferior mesenteric artery (IMA) are usually clinically silent.
The most characteristic symptoms are postprandial abdominal pain, “food fear,” and subsequent unintended weight loss. However, patients can also present with nonspecific symptoms such as malaise, appetite loss, nausea, diarrhea, and weight loss of unclear origin [7]. The correct diagnosis is often made only after several months and, consequently, the majority of cases are recognized in late stages [8]. Persistent and refractory complaints are an indicator of advanced stage with chronic mesenteric malperfusion. Due to a vast collateral network of mesenteric vessels, symptoms usually only occur if more than one artery is affected. This is in contrast to acute mesenteric ischemia (AMI), where embolic occlusion of the SMA usually constitutes a life-threatening situation [9].
Revascularization has been proven to be beneficial in cases of symptomatic CMI, regardless of the number of affected vessels [10]. Additionally, invasive treatment can be indicated in selected cases of non-symptomatic atherosclerotic mesenteric arteries, to prevent AMI in the further course [11].
While computed tomography angiography (CTA) is the diagnostic method of choice, the optimal revascularization strategy for individual patients sometimes remains unclear [12].
The first successful open revascularization (OR) for CMI was reported in 1958 by RS Shaw from the Massachusetts General Hospital when performing endarterectomy of the SMA [13]. Due to the rapid development of endovascular techniques and the assumed lower procedural morbidity and mortality compared to OR [14–16], the therapeutic approach has progressively moved from open repair (OR) to a primary endovascular approach. ER is now applied initially in 70–80% cases [15, 17].
Since randomized controlled trials (RCTs) comparing OR and ER are lacking, the primary objective of this study was to examine early and midterm outcomes in a consecutive series of CMI patients treated by OR and ER at a tertiary referral center. In particular, clinical and morphological variables and their potential impact on reintervention rates and long-term survival were analyzed.
Patients and methods
This study is a retrospective analysis of a consecutively treated cohort of CMI patients at a single university hospital center between January 2004 and December 2017. Identification of the study patients was based on the hospital information system that includes all medical records. Patients were retrieved using the German modification of the International Classification of Diseases (ICD)-10 code for CMI (K55.1). Inclusion criteria were surgical or endovascular treatment of CMI. Patients who required additional surgical treatment (e.g., for concurrent aortic aneurysm repair or renal revascularization) and patients with acute mesenteric ischemia (AMI) were excluded.
Diagnostic and surgical strategies
All patients underwent CTA to assess individual anatomy and determine the feasibility of an endovascular approach. All CTAs were evaluated by an interdisciplinary vascular board (vascular surgery, radiology, and cardiology) for different therapeutic strategies. The choice of therapy was affected by anatomy, comorbidities, the urgency of repair, and the patient’s preference. ER was performed by percutaneous transluminal angioplasty (PTA) and/or stenting of one or more visceral vessels under local anesthesia whenever possible. OR was performed by transposition of the SMA (or IMA in one patient) into the aorta, bypass grafting, or endarterectomy.
Data acquisition
Baseline clinical data included sex, age, clinical presentation (postprandial abdominal pain, weight loss, diarrhea, and duration of abdominal complaints), surgical and medical history, and comorbidities. Specific data included the number of affected mesenteric arteries, surgical strategy, primary technical success, length of stay (LoS), complications (cardiac, respiratory, renal, gastrointestinal, wound healing, and puncture site complications), and reintervention rates. Morphological data included type, length, location of lesion, previous interventions, and grade of calcification as described by Zacharias et al. [18]. For calcification scoring, two experienced readers separately evaluated the CTA data. Disagreements were solved by consensus. The circumference of the affected vessel was divided into thirds and classified into low-, middle-, and high-grade calcification, similar to methods reliably used in the coronary or carotid arteries [19]. Technical success was defined as residual stenosis of less than 30% by angiography.
Data acquisition was in accordance with the Declaration of Helsinki and written informed consent was obtained from all patients.
Follow-up was carried out in accordance with a standardized protocol and included a clinical examination and duplex ultrasound of the visceral arteries 6 weeks after discharge and annually thereafter in an outpatient setting. Patients with recurrent symptoms were additionally evaluated with CTA or diagnostic angiography. If not seen within the standard surveillance protocol, patients or their family doctors were contacted by phone and an appointment was made for the patient for a follow-up evaluation at the time of data collection in March 2018.
Endpoints
Primary endpoints were 30-day mortality (safety endpoint) and freedom from reintervention (efficacy endpoint). Secondary endpoints were perioperative (30-day) morbidity, LoS, and overall survival (OS).
Technical success in the case of ER was defined as residual stenosis of less than 30%, confirmed at the end of the endovascular procedure. In OR cases, technical success was characterized by patent reconstruction of the visceral arteries. Clinical success was defined as resolution of clinical symptoms within 3 days after OR or ER. Medical complications within the first 30 days postoperatively defined 30-day morbidity, whereby we differentiated between seven groups of complications.
Statistics
Categorical data were compared using Fisher’s exact and Chi-square tests, and continuous data were assessed by the Mann–Whitney U test. Primary patency and survival rates were estimated using Kaplan–Meier analysis and compared with a log-rank test. Univariable and multivariable analysis on demographic, clinical, and morphological characteristics was performed to assess their association with the risk of reintervention. For the primary safety and efficacy endpoints, a baseline Cox proportional hazards model was fitted using demographic variables (age and sex) only. Subsequently, another clinical or morphological variable was added to the baseline model, evaluated, and finally removed again. Statistical analyses were performed using Med-Calc© version 9.6.4.0 (MedCalc Software, Mariakerke, Belgium), with p ≤ 0.05 considered statistically significant.
Results
Patient characteristics (Table 1)
Table 1.
Patient demographics, comorbidities, and morphological criteria
| Total (n = 63) | ER (n = 41) | OR (n = 22)a | p value | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) median (range) | 71 (60–76) | 71 (64–78) | 70 (53–73) | 0.145 |
| Sex (male%) | 21 (33.3) | 11 (26.8) | 10 (45.5) | 0.137 |
| Symptoms | ||||
| Weight loss | 38 (60.3) | 22 (53.7) | 16 (72.7) | 0.145 |
| Postprandial pain | 53 (84.1) | 36 (87.8) | 17(77.3) | 0.281 |
| Nausea and vomiting | 12 (19.0) | 7 (17.1) | 5 (22.7) | 0.593 |
| Diarrhea | 15 (23.8) | 12 (29.3) | 3 (13.6) | 0.167 |
| Symptom onset (months), median (range) | 3 (0.5–11) | 5 (0.5–14) | 2 (0.6–6) | 0.165 |
| Comorbidities | ||||
| Hypertension | 40 (63.5) | 27 (65.9) | 13 (59.1) | 0.596 |
| Hyperlipidemia | 25 (39.7) | 17(41.5) | 8(36.4) | 0.696 |
| CHD | 25 (39.7) | 18 (43.9) | 7 (31.8) | 0.353 |
| Ever smoker | 31 (49.2) | 21 (51.2) | 10 (45.5) | 0.669 |
| Diabetes | 20 (31.7) | 13 (31.7) | 7 (31.8) | 0.994 |
| ESRD | 7 (11.1) | 4 (9.8) | 3 (13.6) | 0.650 |
| Morphological parameter | ||||
| High-grade calcification | 36 (57.1) | 23 (56.1) | 13 (59.1) | 0.820 |
| Middle-grade calcification | 8 (12.7) | 5 (12.2) | 3 (13.6) | 0.875 |
| Low-grade calcification | 18 (28.6) | 13 (31.7) | 5 (22.7) | 0.455 |
| Location of vessel occlusion > 2 cm from origin | 15 (23.8) | 9 (22.0) | 6 (27.3) | 0.641 |
| Length of stenosis > 2 cm | 9 (14.3) | 4 (9.8) | 5 (22.7) | 0.167 |
| Occlusion | 11 (17.5) | 4 (9.8) | 7 (31.8) | 0.030 |
| Previous interventions | 8 (12.7) | 6 (14.6) | 2 (9.1) | 0.535 |
When not stated otherwise, results are given as numbers (%)
ER endovascular repair, OR open repair, CHD coronary heart disease, ESRD end-stage renal disease
a2 patients were converted after failed ER within 7 days and further considered as OR
p values were calculated by Mann–Whitney U or Chi-square tests, significant difference is highlighted in bold
A total of 63 patients (33% male; mean age 71, range 60–76 years) fulfilled the inclusion criteria. Over the study period, an increasing number of patients (2002 n = 2, 2017 n = 8) were treated with ER.
Main clinical symptoms were abdominal angina and weight loss, found in 84 and 60%, of patients, respectively. Atherosclerotic risk factors, comorbidities, and patient demographics were quite evenly distributed between the OR and ER groups. Most patients (87%) had no prior surgical or endovascular intervention of the mesenteric arteries. However, the majority (80%) had already been treated for atherosclerotic diseases in another vascular region.
Morphological variables (Table 1)
Of all patients, 60% suffered from multivessel disease. In 89% of patients, the SMA was affected. Whereas the calcification grade did not differ between OR and ER, significantly more occlusions were treated in the OR group (32 vs. 10%, p = 0.030).
Surgical treatment (Table 2)
Table 2.
Technical details and comparison of 30-day peri- and postoperative outcomes for open (OR) and endovascular repair (ER) of chronic mesenteric ischemia
| Total (n = 63) | ER (n = 41) | OR (n = 22) | p value | |
|---|---|---|---|---|
| Technical procedures | ||||
| Number of artery (n) | 69 | 45 | 24 | N/A |
| Angioplasty | – | 17 (18) | – | N/A |
| Stenting | – | 24 (27) | – | N/A |
| Failure of endovascular therapy | – | 2 (2) | – | N/A |
| Endarterectomy | – | – | 5 (7) | N/A |
| Mesenteric bypass or transposition | – | – | 14 (14) | N/A |
| Thrombectomy | – | – | 1 (1) | N/A |
| Resection of arcuate ligament | – | – | 2 (2) | N/A |
| Early outcome | ||||
| 30-day morbidity | 11 (17.5) | 4 (9.8) | 7 (31.8) | 0.030 |
| 30-day freedom from reintervention | 96.7% (92.4–100) | 97.4% (92.6–100) | 95.5% (87.1–100) | 0.069 |
| 30-day mortality | 1.6% (0 – 4.8) | 0% (0–0) | 4.5% (0–2.9) | 0.768 |
| Length of stay (days) | 7 (3–12.5) | 4 (3–7) | 14 (10–17) | < 0.001 |
Numbers represent median and range (Q1–Q3); technical details are given in number of patients n (number of arteries n); p values were calculated by Mann–Whitney U or Chi-square test, significant differences are highlighted in bold
ER was used in 41 patients (45 arteries) and OR in 22 patients (24 arteries) and was performed by angioplasty alone in 17/41 patients (17 arteries). An additional stent was used in 24/41 patients (24 arteries). The most common procedure in the OR group was transposition of the SMA in the aorta or aortomesenteric bypass surgery (14/22 patients). In both groups, usually only one visceral artery was treated (OR 80% vs. ER 86%). The technical success rate was 88% in the ER group and 100% in the OR group. Two patients had to be converted to OR after failed ER within 7 days. Further, they were considered to OR group.
Early outcomes (Table 2)
Mortality (30-day and in-hospital) was 1.6% (0% for ER; 4.5% for OR; p = 0.069), with one fatality on day 12 due to necrotizing pancreatitis. A total of 11 patients (17.5%) suffered from perioperative complications (30-day morbidity), with significantly higher numbers in the OR group as compared to ER (31.8% vs. 9.8%; p = 0.030).
There were mild complications in the ER group in three cases (angina pectoris (n = 1), ischemic rectitis (n = 1) and one false aneurysm in the femoral puncture site (n = 1)) and severe complications in one case (pneumonia and urosepsis). In the OR group, five of seven patients had severe complications (myocardial infarction (n = 2), pneumonia (n = 1), acute renal failure (n = 1), pancreatic fistula (n = 1), and pancreatitis (n = 1)) and two mild complications (postoperative delirium, deep vein thrombosis).
LoS was longer after OR compared to ER (14, Q1–Q3: 10–17 vs. 4:Q1–Q33–7 days, p < 0.001).
The freedom from reintervention rates at 30 days was 96% vs. 97% after OR and ER, respectively (p = 0.069).
Midterm and late outcomes (Tables 2, 3)
Table 3.
Univariable analysis of clinical, morphological, and operative variables and their impact on the 2-year rates of reintervention after endovascular or open revascularization for chronic mesenteric ischemia
| HR(95% CI) | p value | |
|---|---|---|
| Baseline characteristics | ||
| Age ≥65 versus <65 years | 0.64 (0.21–1.90) | 0.42 |
| Female/male | 1.50 (0.49–4.60) | 0.48 |
| Clinical parameter | ||
| Hypertension (yes/no) | 1.67 (0.51–5.44) | 0.39 |
| Hyperlipidemia (yes/no) | 1.32 (0.44–3.93) | 0.62 |
| CHD (yes/no) | 1.87 (0.63–5.57) | 0.26 |
| Ever smoker (yes/no) | 3.91 (1.08–14.23) | 0.04 |
| Diabetes (yes/no) | 2.46 (0.82–7.35) | 0.11 |
| ESRD (yes/no) | 1.52 (0.34–6.88) | 0.58 |
| Morphological parameter | ||
| High-grade versus low-/mid-grade calcification | 1.15 (0.39–3.45) | 0.80 |
| Stenosis/occlusion >2 cm versus <2 cm from origin | 1.07 (0.29–3.90) | 0.92 |
| Lesion length >2 cm/<2 cm | 1.67 (0.46–6.10) | 0.44 |
| Occlusion versus stenosis | 1.16 (0.32–4.23) | 0.82 |
| Previous interventions (yes/no) | 1.84 (0.51–6.68) | 0.36 |
| Mode of revascularization | ||
| OR versus ER | 0.27 (0.06–1.23) | 0.09 |
HR hazard ratio, CI confidence interval, OR open revascularization, ER endovascular revascularization, CHD coronary heart disease, ESRD end-stage renal disease
P values were calculated by Mann–Whitney U or Chi-square tests, significant differences are highlighted
Clinical success was achieved in 95% in both groups. The remaining 5% had persistent complaints despite technical success; initial misdiagnosis thus had to be considered, and patients were transferred to the gastroenterology department.
Mean follow-up was 26 (Q1–Q3: 10–71) months. Longer follow-up was available in 18 patients (28.5%). In Kaplan–Meier analysis freedom from reintervention rates within 2 years were 82% for OR and 73% for ER (p = 0.14). Recurrent stenosis with or without symptoms indicated reintervention.
There was also no significant difference between OR and ER in Kaplan–Meier analysis for OS, with 30-day and 2-year survival rates of 95 and 85% for OR, and 100 and 86% for ER, respectively (p = 0.35).
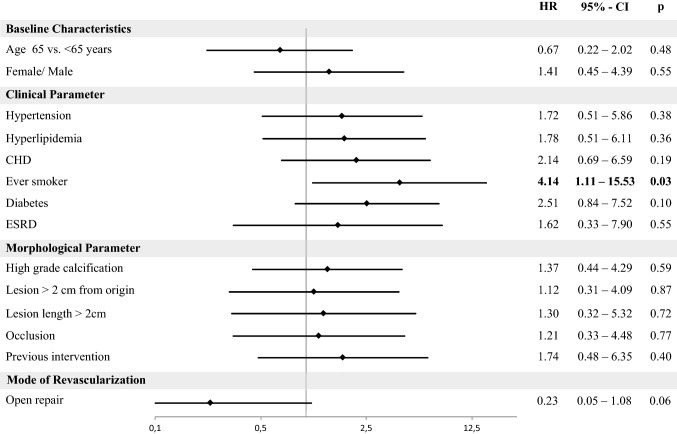
In the uni- and multivariable analysis (Table 3, Fig. 1) adjusted for clinical and morphological parameters, a positive smoking history or current smoking was associated with a significantly higher risk of reintervention (hazard ratio, HR: 4.14; 95% confidence interval, CI 1.11–15.53; p = 0.03; Fig. 1), while OR tends toward a lower reintervention risk (HR 0.23, 95% CI 0.05–1.08; p = 0.06).
Fig. 1.
Clinical, morphological parameters and mode of revascularization were adjusted for age and sex. HR, hazard ratio; CI, confidence interval; OR, open revascularization; ER, endovascular revascularization, CHD, coronary heart disease, ESRD, end-stage renal disease
Discussion
In this consecutive cohort of patients suffering from chronic mesenteric ischemia (CMI), there were no significant differences in terms of reintervention rate, 30-day mortality, or overall survival (OS) between endovascular (ER) or open vascular surgical revascularization (OR) during a mean follow-up of 26 months. As with many other studies in the field, most likely due to the small sample number, we only observed trends for a lower reintervention rate in OR and better OS in ER. OR was associated with a higher rate of 30-day morbidity and longer LoS. In multivariable analysis, smoking history was associated with higher rates of reintervention.
As in other studies, a significant higher perioperative morbidity for OR in our cohort was observed, while there was no significant difference between ER and OR in terms of 30-day mortality (Table 2) [20]. Overall, 30-day mortality in the OR group was 4.5% and thus almost identical to the 5.5% of a recently published meta-analysis [21]. However, perioperative mortality due to cardiovascular events can be as high as 15% after OR [22]. In our department, a complete preoperative cardiologic workup including possible noninvasive imaging in all elective patients precedes treatment.
However, due to the lower invasiveness of ER, there are not only differences in perioperative morbidity, but also in LoS. These results have been confirmed in various other studies [20, 22, 23]. In addition, lower costs could be calculated for ER compared to OR [24, 25].
Attention should be paid to the fact that not all lesions in our cohort are suitable for ER, because of long-distance occlusions. In our study, seven patients who were primarily treated with OR would not have been eligible for an endovascular procedure, which may well have led to a bias in favor of ER.
One of the main problems of endovascular treatment in general is its durability, mostly in terms of target vessel patency [26]. Some of the newer methods (e.g., drug eluting balloons or stents) are not yet used in clinical routine for treating mesenteric vessels. However, although freedom from reintervention showed no significant difference between ER and OR in our study, 30-day and 2-year freedom from reintervention showed a tendency toward favoring OR (albeit without statistical correction for various endovascular means applied). This is likely due to the low case number, and those patients lost to follow-up. Another reason for the high reintervention rate could be the treatment without stenting. In our study, nearly half of the ER group (17 of 41 patients) was treated with angioplasty alone. Prospective trials to compare angioplasty with primary stenting are missing. But experts agree, and the 2017 Guidelines from the European Society for Vascular Surgery (ESVS) recommend that primary mesenteric stenting is indicated [9, 27–30].
Nevertheless, the reintervention rate of 27% by ER after 2 years is in accordance with the published results [20, 31]. Therefore, reintervention rates and worse primary patency rates need to be considered when opting for an “endo-first” strategy [22, 32].
Moreover, the multivariable analysis of the current study confirmed a tendency of lower reintervention rates for OR. Thus, OR remains a viable option in patients who are fit for surgery and are morphologically no good candidates for ER. Particularly, an early change of regimen after failed ER should be considered as supported by the ESVS guidelines [9].
Decision-making should also support clinical or morphological variables which might be associated with lower patency rates [33]. Even though the numbers of CMI patients were relatively low in this study, our multivariable analysis revealed that patients with a history of smoking or current smoking have a fourfold higher risk of reintervention. This seems plausible, since although hit has not been described for mesenteric arteries, other investigations of different areas and techniques have reported similar results regarding smoking as a risk factor for restenosis [34–38]. Regarding the complexity of lesions, Oderich et al. investigated endovascular procedures. In this study, female sex as well as long (< 20 mm) and calcified lesions significantly increased the risk of in-stent restenosis [31, 39]. These factors were also analyzed in our cohort, but did not show the significant results due to the small sample size (Table 3).
Not only the high rate of reintervention plays an important role in arteriosclerotic disease, but also the poor overall survival has (OS) [40]. Patients with severe atherosclerotic burden have a poor survival prognosis; for example, patients with chronic limb threatening ischemia have a 1-year survival probability of only about 75% if affected by CMI [41]. However, even patients with CMI alone have a poor OS [42]. Therefore, it is satisfying that OS in this study was 98.4% at 30 days and 86.1% at 2 years. We were not able to detect any difference between the two treatment options, and these results thus correspond to the results of the 2018 published meta-analysis, where also no significant difference in the 3-year OS of 78% was found.
Another treatment option that should be mentioned but was not used in our cohort is a hybrid procedure with retrograde stenting of SMA. This option may be selected when transaortic stenting and open reconstruction are impossible, e.g., in case of extensive aortoiliac disease and no good source of inflow, and it is mainly recommended in those with acute mesenteric ischemia. The use of a hybrid approach provides one of the most expeditious methods of revascularization in patients with difficult SMA occlusions [9, 43–45].
Several shortcomings of this study need to be addressed. First, selection bias is possible as it is a single-center analysis. Second, the cardiovascular and morphological risk factors used in the multivariate analysis represent only a small number of the factors influencing OS and freedom from reintervention. Finally, this study had the inherent limitations of a retrospective analysis.
Conclusion
CMI is a rare disease that can be effectively treated by ER and OR. ER tends to have higher rates of reintervention, while 30-day morbidity is higher and hospital LoS is longer in patients treated with OR. Due to the less invasive nature of ER, the results support the “endovascular-first” strategy that has been established in recent years.
Acknowledgements
Open Access funding provided by Projekt DEAL.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.White CJ. Chronic mesenteric ischemia: diagnosis and management. Prog Cardiovasc Dis. 2011;54:36–40. doi: 10.1016/j.pcad.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Kolkman JJ, Bargeman M, Huisman AB, et al. Diagnosis and management of splanchnic ischemia. World J Gastroenterol. 2008;14:7309–7320. doi: 10.3748/wjg.14.7309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boley SJ, Brandt LJ, Veith FJ. Ischemic disorders of the intestines. Curr Probl Surg. 1978;15:1–85. doi: 10.1016/s0011-3840(78)80018-5. [DOI] [PubMed] [Google Scholar]
- 4.Boley SJ, Brandt LJ, Sammartano RJ. History of mesenteric ischemia. The evolution of a diagnosis and management. Surg Clin N Am. 1997;77:275–288. doi: 10.1016/s0039-6109(05)70548-x. [DOI] [PubMed] [Google Scholar]
- 5.Clair DG, Beach JM. Mesenteric ischemia. N Engl J Med. 2016;374:959–968. doi: 10.1056/NEJMra1503884. [DOI] [PubMed] [Google Scholar]
- 6.Derrick JR, Pollard HS, Moore RM. The pattern of arteriosclerotic narrowing of the celiac and superior mesenteric arteries. Ann Surg. 1959;149:684–689. doi: 10.1097/00000658-195905000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biolato M, Miele L, Gasbarrini G, et al. Abdominal angina. Am J Med Sci. 2009;338:389–395. doi: 10.1097/MAJ.0b013e3181a85c3b. [DOI] [PubMed] [Google Scholar]
- 8.Pecoraro F, Rancic Z, Lachat M, et al. Chronic mesenteric ischemia: critical review and guidelines for management. Ann Vasc Surg. 2013;27:113–122. doi: 10.1016/j.avsg.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 9.Writing C, Bjorck M, Koelemay M, et al. Editor’s choice—management of the diseases of mesenteric arteries and veins: clinical practice guidelines of the european society of vascular surgery (ESVS) Eur J Vasc Endovasc Surg. 2017;53:460–510. doi: 10.1016/j.ejvs.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Mensink PB, van Petersen AS, Geelkerken RH, et al. Clinical significance of splanchnic artery stenosis. Br J Surg. 2006;93:1377–1382. doi: 10.1002/bjs.5481. [DOI] [PubMed] [Google Scholar]
- 11.Acosta S. Epidemiology of mesenteric vascular disease: clinical implications. Semin Vasc Surg. 2010;23:4–8. doi: 10.1053/j.semvascsurg.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Oliva IB, Davarpanah AH, Rybicki FJ, et al. ACR appropriateness criteria (R) imaging of mesenteric ischemia. Abdom Imaging. 2013;38:714–719. doi: 10.1007/s00261-012-9975-2. [DOI] [PubMed] [Google Scholar]
- 13.Shaw RS, Maynard EP., 3rd Acute and chronic thrombosis of the mesenteric arteries associated with malabsorption; a report of two cases successfully treated by thromboendarterectomy. N Engl J Med. 1958;258:874–878. doi: 10.1056/NEJM195805012581803. [DOI] [PubMed] [Google Scholar]
- 14.Foley TR, Rogers RK. Endovascular therapy for chronic mesenteric ischemia. Curr Treat Options Cardiovasc Med. 2016;18:39. doi: 10.1007/s11936-016-0463-9. [DOI] [PubMed] [Google Scholar]
- 15.Lima FV, Kolte D, Kennedy KF, et al. Endovascular versus surgical revascularization for chronic mesenteric ischemia: insights from the national inpatient sample database. JACC Cardiovasc Interv. 2017;10:2440–2447. doi: 10.1016/j.jcin.2017.09.033. [DOI] [PubMed] [Google Scholar]
- 16.Resch T, Lindh M, Dias N, et al. Endovascular recanalisation in occlusive mesenteric ischemia–feasibility and early results. Eur J Vasc Endovasc Surg. 2005;29:199–203. doi: 10.1016/j.ejvs.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Zettervall SL, Lo RC, Soden PA, et al. Trends in treatment and mortality for mesenteric ischemia in the United States from 2000 to 2012. Ann Vasc Surg. 2017;42:111–119. doi: 10.1016/j.avsg.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zacharias N, Eghbalieh SD, Chang BB, et al. Chronic mesenteric ischemia outcome analysis and predictors of endovascular failure. J Vasc Surg. 2016;63:1582–1587. doi: 10.1016/j.jvs.2016.02.051. [DOI] [PubMed] [Google Scholar]
- 19.Shemesh J, Henschke CI, Shaham D, et al. Ordinal scoring of coronary artery calcifications on low-dose CT scans of the chest is predictive of death from cardiovascular disease. Radiology. 2010;257:541–548. doi: 10.1148/radiol.10100383. [DOI] [PubMed] [Google Scholar]
- 20.Oderich GS, Bower TC, Sullivan TM, et al. Open versus endovascular revascularization for chronic mesenteric ischemia: risk-stratified outcomes. J Vasc Surg. 2009;49(1472–1479):e1473. doi: 10.1016/j.jvs.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Alahdab F, Arwani R, Pasha AK, et al. A systematic review and meta-analysis of endovascular versus open surgical revascularization for chronic mesenteric ischemia. J Vasc Surg. 2018;67:1598–1605. doi: 10.1016/j.jvs.2017.12.046. [DOI] [PubMed] [Google Scholar]
- 22.Sivamurthy N, Rhodes JM, Lee D, et al. Endovascular versus open mesenteric revascularization: immediate benefits do not equate with short-term functional outcomes. J Am Coll Surg. 2006;202:859–867. doi: 10.1016/j.jamcollsurg.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 23.Atkins MD, Kwolek CJ, LaMuraglia GM, et al. Surgical revascularization versus endovascular therapy for chronic mesenteric ischemia: a comparative experience. J Vasc Surg. 2007;45:1162–1171. doi: 10.1016/j.jvs.2007.01.067. [DOI] [PubMed] [Google Scholar]
- 24.Erben Y, Protack CD, Jean RA, et al. Endovascular interventions decrease length of hospitalization and are cost-effective in acute mesenteric ischemia. J Vasc Surg. 2018;68:459–469. doi: 10.1016/j.jvs.2017.11.078. [DOI] [PubMed] [Google Scholar]
- 25.Erben Y, Jean RA, Protack CD, et al. Improved mortality in treatment of patients with endovascular interventions for chronic mesenteric ischemia. J Vasc Surg. 2018;67:1805–1812. doi: 10.1016/j.jvs.2017.10.071. [DOI] [PubMed] [Google Scholar]
- 26.Kayssi A, Al-Atassi T, Oreopoulos G et al (2016) Drug-eluting balloon angioplasty versus uncoated balloon angioplasty for peripheral arterial disease of the lower limbs. Cochrane Database Syst Rev CD011319 [DOI] [PMC free article] [PubMed]
- 27.Sarac TP, Altinel O, Kashyap V, et al. Endovascular treatment of stenotic and occluded visceral arteries for chronic mesenteric ischemia. J Vasc Surg. 2008;47:485–491. doi: 10.1016/j.jvs.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 28.Aksu C, Demirpolat G, Oran I, et al. Stent implantation in chronic mesenteric ischemia. Acta Radiol. 2009;50:610–616. doi: 10.1080/02841850902953873. [DOI] [PubMed] [Google Scholar]
- 29.Fioole B, van de Rest HJ, Meijer JR, et al. Percutaneous transluminal angioplasty and stenting as first-choice treatment in patients with chronic mesenteric ischemia. J Vasc Surg. 2010;51:386–391. doi: 10.1016/j.jvs.2009.08.055. [DOI] [PubMed] [Google Scholar]
- 30.Kougias P, Huynh TT, Lin PH. Clinical outcomes of mesenteric artery stenting versus surgical revascularization in chronic mesenteric ischemia. Int Angiol. 2009;28:132–137. [PubMed] [Google Scholar]
- 31.Tallarita T, Oderich GS, Macedo TA, et al. Reinterventions for stent restenosis in patients treated for atherosclerotic mesenteric artery disease. J Vasc Surg. 2011;54(1422–1429):e1421. doi: 10.1016/j.jvs.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Arya S, Kingman S, Knepper JP, et al. Open mesenteric interventions are equally safe as endovascular interventions and offer better midterm patency for chronic mesenteric ischemia. Ann Vasc Surg. 2016;30:219–226. doi: 10.1016/j.avsg.2015.07.038. [DOI] [PubMed] [Google Scholar]
- 33.Bisdas T, Stavroulakis K. Endovascular-first approach for chronic mesenteric ischemia: the critical need for reporting standards and high-grade evidence. JACC Cardiovasc Interv. 2017;10:2448–2450. doi: 10.1016/j.jcin.2017.10.029. [DOI] [PubMed] [Google Scholar]
- 34.Chan RC, Chan YC, Cheung GC, et al. Predictors of restenosis after carotid endarterectomy: 17-year experience in a tertiary referral vascular center. Vasc Endovasc Surg. 2014;48:201–206. doi: 10.1177/1538574413518117. [DOI] [PubMed] [Google Scholar]
- 35.Beck AW, Murphy EH, Hocking JA, et al. Aortic reconstruction with femoral-popliteal vein: graft stenosis incidence, risk and reintervention. J Vasc Surg. 2008;47:36–43. doi: 10.1016/j.jvs.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 36.Pedrini L, Pisano E, Donato Di Paola M, et al. Late occlusion of aortofemoral bypass graft: surgical treatment. Cardiovasc Surg. 1994;2:763–766. [PubMed] [Google Scholar]
- 37.Davies MG, El-Sayed HF. Objective performance goals after endovascular intervention for critical limb ischemia. J Vasc Surg. 2015;62:1555–1563. doi: 10.1016/j.jvs.2015.06.228. [DOI] [PubMed] [Google Scholar]
- 38.Bonati LH, Ederle J, McCabe DJ, et al. Long-term risk of carotid restenosis in patients randomly assigned to endovascular treatment or endarterectomy in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): long-term follow-up of a randomised trial. Lancet Neurol. 2009;8:908–917. doi: 10.1016/S1474-4422(09)70227-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gustavo S, Oderich TAM, Malgor R, Ricotta JJ, Vrtiska T, Duncan AA, Kalra M, Gloviczki P. RR26 Natural history of mesenteric artery stent restenoses and clinical and anatomic predictors for re-intervention in patients with chronic mesenteric ischemia. J Vasc Sur. 2009;49:54. [Google Scholar]
- 40.Rothwell PM, Coull AJ, Silver LE, et al. Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study) Lancet. 2005;366:1773–1783. doi: 10.1016/S0140-6736(05)67702-1. [DOI] [PubMed] [Google Scholar]
- 41.Norgren L, Hiatt WR, Dormandy JA, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II) Eur J Vasc Endovasc Surg. 2007;33(Suppl 1):S1–75. doi: 10.1016/j.ejvs.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 42.Cai W, Li X, Shu C, et al. Comparison of clinical outcomes of endovascular versus open revascularization for chronic mesenteric ischemia: a meta-analysis. Ann Vasc Surg. 2015;29:934–940. doi: 10.1016/j.avsg.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 43.Wyers MC, Powell RJ, Nolan BW, et al. Retrograde mesenteric stenting during laparotomy for acute occlusive mesenteric ischemia. J Vasc Surg. 2007;45:269–275. doi: 10.1016/j.jvs.2006.10.047. [DOI] [PubMed] [Google Scholar]
- 44.Oderich GS, Gloviczki P, Bower TC. Open surgical treatment for chronic mesenteric ischemia in the endovascular era: when it is necessary and what is the preferred technique? Semin Vasc Surg. 2010;23:36–46. doi: 10.1053/j.semvascsurg.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 45.Pisimisis GT, Oderich GS. Technique of hybrid retrograde superior mesenteric artery stent placement for acute-on-chronic mesenteric ischemia. Ann Vasc Surg. 2011;25(132):e111–e137. doi: 10.1016/j.avsg.2010.04.004. [DOI] [PubMed] [Google Scholar]