Abstract
Role of rhizobacteria and zinc (Zn) was investigated in the management of charcoal rot disease in mungbean [Vigna radiata (L.) Wilczek] caused by Macrophomina phaseolina (Tassi) Goid. In vitro, screening tests with eight rhizobacteria [Bacillus subtilis (FCBP-0324), B. subtilis (FCBP-0189), Rhizobacter daucus (FCBP-0450), Azospirillum brasilense (FCBP-0025), Azospirillum lipoferum (FCBP-0022), Pseudomonas malophilia (FCBP-0099), Pseudomonas florescense (FCBP-0083) and Ochrobactrum ciceri (FCBP-0727)] were conducted against M. phaseolina and FCBP-0727 were found as the most effective biocontrol agent. Molecular analyses of 16S rDNA combined with cultural and biochemical analyses confirmed FCBP-0727 identification (GeneBank Accession No. LC415039). Cell-free culture filtrate (CFCF) and cell culture of O. ciceri were separated and antifungal trials of both substrates indicated inhibition in mycelial growth and suppression in sclerotia formation, although the CFCF appeared to be more destructive against the pathogen. Ethyl-acetate and chloroform extracts of bacterial secondary metabolites completely halted the growth of M. phaseolina. The GC–MS analysis of CFCF of chloroform extract proved to be rich sources of bioactive fungicide like phthalates, adipic acid, propanoic acid, and linoleic acid. Likewise, CFCF of ethyl acetate also exhibited important organic compounds like phthalates, diisopropylglycol and octasiloxan. Pot experiment revealed that soil inoculation with O. ciceri in combination with Zn (2.5 mg/kg) protected mungbean plants against M. phaseolina through improving photosynthetic pigment, total protein content and activities of antioxidant enzymes (catalase, peroxidase and polyphenol oxidase). The present study will open new vistas for biological management of charcoal rot disease of mungbean using a combination of rhizobacteria and Zn.
Electronic supplementary material
The online version of this article (10.1007/s12298-020-00817-y) contains supplementary material, which is available to authorized users.
Keywords: Antifungal biological control agents, Antioxidants, Charcoal rot, Ochrobactrum cicero, Volatile organic compounds, Zinc
Introduction
Mungbean (Vigna radiata L.) is a summer-growing, short duration (75–90 days) legume crop, which is cultivated widely in tropics and subtropic regions. It grows best at the temperature range of 28–35 °C and exhibits wider adaptability to adverse climatic conditions (Khan et al. 2018). The presence of the high amount of starch, proteins, amino acids, oligosaccharides and polyphenols in seed and sprout of mungbean are known for antimicrobial, anti-inflammatory, antidiabetic, anticancer, anti-obesity and antitumor activities (Ganesan and Xu 2018). In Pakistan, mungbean ranks second after chickpea and is cultivated mostly in rainfed regions as these areas provide a favorable environment for the cultivation of this crop. However, during the last few years, the total cultivated area for mungbean has been reduced from 237,000 ha (2002–2003) to 118,000 ha (2017–2018) (Aziz-ur-Rehman et al. 2019).
Macrophomina phaseolina is one of the most devastatic biotic constraints causing charcoal rot disease leading to yield loss up to 60% (Atiq et al. 2014) and in severe cases, 100% yield loss may occur. It is a soil and seed-borne pathogenic, ascomycete fungus of the family Botryosphaeriaceae, which can attack over 500 plant species including legume crops (Iqbal and Mukhtar 2014). It is a heat and drought favored fungus, which produces large quantities of black colored and cushion-shaped microsclerotia. Germination of these microsclerotia occurs throughout the growing season within a temperature range of 28–35 °C under low water potential. Microsclerotia overwinter in soil both as free sclerotia and over plant/crop residues and acts as a initial source of inoculum in the soil. Sclerotia can survive in soil up to one decade or more and abide a temperature range of 60–65 °C (Iqbal and Mukhtar 2014). The pathogen can grow intercellularly as well as intracellularly and produce disease symptoms in the plant at any growth stage (Sharma and Singh 2000). Disease incidence increases with high temperature and moisture stress to the plant. The pathogen infection results in wilting followed by rapid leaves dryness and eventually yield loss. Chlorosis, as well as red to brown discoloration on the stem that turns into brown to black was observed on infected plants. Infected plants may also give greyish-black appearance but these symptoms do not appear until mid-end of growing season (Khan et al. 2018).
Biocontrol of fungal diseases by using bacterial strains are getting immense attention because these are less expensive, eco-friendly as compared to chemical fungicides (Awan and Shoaib 2019). Bacterial pesticides make up the most of biopesticides comprising about 74% of the total worldwide production of biopesticides (Thakore 2006). Different mechanisms were reported regarding antagonistic activities of rhizobacteria which help in making disease suppressive soil through secretion of various antifungal metabolites (hydrogen cyanide, siderophore, hydrolytic enzymes, and antibiotics) and by increasing the production of indole 3-acetic acid, plant defensive enzymes, phosphate solubilization, and nitrogen fixation (Expósito et al. 2017). Many bacterial strains like Rhizobium, Mesorhizobium, Ensifer (Sinorhizobium), Pseudomonas, Devosia, Ochrobacturm, Phyllobacterium, Herbspirillum, Shinella, Alcaligenes, Azospirillum, Azotobacter, Bacillus, Clostridium, Enterobacter and Klebsiella have been successfully used worldwide to increase growth and suppress diseases in plants (Mazzola et al. 1992; Korejo et al. 2017). Unfortunately, the durability of these bacterial biopesticides and bio-fertilizers is compromised in field conditions due to the diversity of environmental conditions. Understanding the difference between chemical and physical properties of environmental factors that produce a major impact on the activity of plant growth-promoting bacteria found in the rhizosphere is a major step in increasing the performance and reliability of these bacteria (Expósito et al. 2017).
Activities of the rhizobacteria can also influence the availability of the nutrients such as zinc (Zn), by the production of organic acids, proton and humic substances (Azarmi et al. 2016). Zn shows a vital character in the synthesis of several phyto-enzymes that keep cellular membranes integrity, metabolism of nitrogen and carbohydrate, energy transfer, redox reactions, regulation of auxin synthesis, activation of defense mechanism and pollen formation (Hafeez et al. 2013; Khan et al. 2018). Zn defends the plant from amplified reactive oxygen species (ROS) production through reduced production of membrane-bounded NADPH oxidase. Zn deficiency causes significant damage to the ultrastructure of the chloroplasts because of its interaction with the phospholipids and sulfhydryl groups of the membrane protein (Zarmehri et al. 2013). Soil and foliar applications of Zn fertilizer are recommended for correcting deficiencies in plants (Hafeez et al. 2013). Zn based fungicides have been applied effectively to manage important diseases caused by many phytopathogenic fungi (Alternaria solani, Fusarium oxysporum, Phytophthora capsici, Monilia frucigena and Botrytis cinerea) (Nikolov and Ganchev 2010). Zn amended soil promotes growth, yield and protected mungbean plant from charcoal rot disease (Khan et al. 2018).
In Pakistan, the soil is calcareous by nature in the major mungbean cultivated areas where it displays low availability of micronutrients especially Zn and have a low amount of organic matter. Interactive effect of Zn and bacterial strain might provide a better possibility to manage the charcoal rot disease by inducing resistance in host plant. The present study is aimed to investigate the role of rhizobacteria and Zn in the mungbean plant to manage a charcoal rot disease.
Materials and methods
In vitro bioassays
Screening of bacterial strains against M. phaseolina
In vitro, antifungal activity of eight rhizobacteria [Bacillus subtilis (FCBP-0324), B. subtilis (FCBP-0189), Rhizobacter daucus (FCBP-0450), Azospirillum brasilense (FCBP-0025), Azospirillum lipoferum (FCBP-0022), Pseudomonas malophilia (FCBP-0099), Pseudomonas florescense (FCBP-0083) and Ochrobactrum ciceri (FCBP-0727)] was checked against M. phaseolina (FCBP-0721) in 2% malt extract agar (MEA) by dual culture technique. The dual culture method of bacterial strains screening was performed following the method of Shoaib et al. (2019). A fungal disc (5 mm) was cut from an actively grown pure culture (7-days old), placed at one side of the Petri plates aseptically and a loopful of actively growing bacterial strains was streaked 3 cm apart from the fungal disc on the same plate and incubated at 30 °C for 5 days. After the incubation period, growth inhibition (%) in M. phaseolina by different bacterial antagonists was calculated with respect to control treatment (inoculated with M. phaseolina only).
Characterization and identification and of O. ciceri
Based on the screening trial, FCBP-0727 was found the most effective in inhibiting the growth of M. phaseolina. Therefore, identification and characterization of FCBP-0727 were first verified through standard phenotypic, biochemical and 16S rRNA methods. Phenotypic characterization was carried out by using Gram staining, catalase test, indole test, and hydrogen sulfide test. The rest of biochemical tests were assessed by using a biochemical kit (Microgen biochemical identification kit). For 16S rRNA gene amplification, the chromosomal DNA was isolated using a bacterial DNA extraction kit (Genomic DNA mini kit-Thermo Fisher Scientific) following the manufacturer’s instructions. Amplification by PCR was performed using universal primers 27f (5′AGAGTTTGATCCTGGCTCAG-3′) and 1492r (5′-TACGGTTACCTTGTTACGACT-3′).
Antifungal assays with cell-free supernatant and the cell pellet
A pure inoculum of O. ciceri was inoculated in 400 mL of LB broth medium (1% Trypton, 1% NaCl and 0.5% Yeast extract) in 1000 mL of Erlenmeyer flask for 72 h at 30 °C in an orbital shaker (120 rpm). Cell-free supernatant (CFS) and bacterial cell pellet (CP) were separated by centrifugation at 12,000 rpm and 4 °C for 30 min from the exponential growth phase. The cell pellet was washed with autoclaved distilled water to remove excess culture and resuspended in sterilized water to be later used, while cell-free supernatant was filtered through a millipore membrane filter (Pore size: 0.22 µm). For antifungal assays, MEA (2%) medium was homogeneously mixed with bacterial cell pellet (106 CFU) and cell-free supernatant (100%), separately. After media pouring in glass Petri plates (9 cm), solidified medium was inoculated with a 5 mm fungal disc. Medium plates inoculated with a fungal pathogen only served as control. The plates were incubated at 30 °C for 5 days. After incubation, macroscopic (colony morphology) and microscopic (hyphae and sclerotia) characteristics of the fungal pathogen were noticed under a compound microscope.
Characterization and antifungal assays with cell-free supernatant
Cell-free supernatant was diluted with sterilized water to make six concentrations (0, 20, 40, 60, 80 and 100%). The experiment was set in 10 mL test tubes containing 1 mL of 2% ME broth inoculated with 10 µL of M. phaseolina cultural suspension (2.0 × 104) along with six different concentrations of bacterial metabolites. ME broth inoculated with fungal suspension only served as control and test tubes were incubated at 30 °C for 5 days. After incubation, fungal biomass was filtered; dried and weighed (mg) and percentage inhibition in biomass was calculated.
Extraction and purification of antifungal cell-free supernatant
For extraction, centrifuged cell-free supernatant (500 mL) was concentrated at 40 °C for three days. The concentrate containing crude metabolites (72 mL) was filtered again and used for successive extractions with double volume of chloroform and ethyl acetate and shaken for 30 min in an orbital shaker. The individual organic layer was carefully collected and evaporated to dryness at 40 °C in a 1000 mL of round bottle flask on a rotary evaporator to obtain the slimy mass of crude metabolites.
Fractions of cell-free supernatant were checked for their antimycotic activity. Initially, the stock solution of each organic fraction was prepared for the experiment by adding 100 mg of dried fraction in 1 mL of the respective organic solvent (chloroform and ethyl acetate). Stock solution (100 mg mL−1) was diluted with respective organic solvents to make final concentrations of 20, 40, 60 and 80 mg mL−1. Antifungal assays were conducted in 5 mL glass test tubes each containing 1 mL of medium (2% ME), 10 µL fraction concentration and 10 µL of cultural suspension of M. phaseolina (2.0 × 104) aseptically. For control (0 mg mL−1), the test tubes were added with 1 mL 2% ME, 10 µL of pure organic solvent of n chloroform and ethyl acetate separately, and 10 µL of conidial suspension. Test tubes were incubated at 30 °C for 3 days. After incubation, fungal biomass was filtered, dried and weighed (mg). Percentage inhibition in fungal biomass was calculated.
The GC–MS analysis of sub-fractions
The GC–MS analysis was performed with Perkin Elmer Turbo Mass Spectrophotometer (Norwalk, CTO6859, and USA). Perkin Elmer Autosampler XLGC with Perkin Elmer Elite-5 capillary columns (measuring 30 m × 25 mm with a film thickness of 0.25 mm) was composed of 95% dimethylpolysiloxane. Operating conditions used helium as the carrier gas (flow rate of 1 mL min−1). The column was set at 75 °C for 3 min, followed by an increase from 75 to 175 °C at the rate of 3 °C per minute, which was finally maintained at 220 °C for 7 min. Source pressure was 7 Pa, filament voltage was 70 eV and a scan rate was employed of 1.9 scan s−1. The m/z peaks (representing mass-to-charge ratios) characteristic for the antimicrobial fraction, were compared with those in the mass spectrum library (National Institute for Standards and Technology). Turbo-Mass-OCPTVS-Demo SPL software was used to measure peak areas and data processing. GCMS Postrun analysis software was used to measure peak areas and data processing. The constituents of peaks were finally recognized after comparing with available data in the NIST-14 mass spectrum library (National Institute of Standards and Technology, USA) (Syed-Ab-Rahman et al. 2018).
In planta assays
The soil was sterilized with 2% formalin solution filled (0.612 kg pot−1) in pots (10′′ length × 8′′ width) and inoculated with cultural suspension (15 mL pot−1) of the M. phaseolina. After 3 days of pathogen inoculation, surface-sterilized seeds of susceptible variety of mungbean (MUNAYAT-7) were sown in potting soil (4 seeds pot−1) (Khan et al. 2018). The pathogen inoculated soil was then inoculated with bacterial suspension measuring 108 cells mL−1 (OD595nm = 0.5) right after seed sowing. After 3 days of seed germination, basal doses of zinc (2.5 mg/kg and 5 mg/kg) were applied in the form of zinc sulfate (ZnSO4·H2O). The pots were kept under natural environmental conditions in a completely randomized design. The experiment consisted of 12 treatments, T1: −ve control (without any treatment/inoculation); T2: + ve control [M. phaseolina (MP)]; T3: Ochrobactrum ciceri (OC); T4: MP + OC; T5: Zn (1X: 2.5 mg/kg); T6: MP + Zn (1X); T7: OC + Zn (1X); T8: MP + OC + Zn (1X); T9: Zn (2X: 5 mg/kg); T10: MP + Zn (2X); T11: OC + Zn (2X); T12: MP + OC + Zn (2X). The experiment was regularly monitored and the data for physicochemical and disease attributes was assessed.
Physicochemical variations [total chlorophyll content, carotenoids and total protein content, catalase (CAT), peroxidase (POX) and polyphenol oxidase (PPO)] in mungbean leaf were assessed after 20 days of seed by randomly taking leaf samples from each treatment in triplicate following the protocols described by Khan et al. (2018).
Disease incidence (% DI) was calculated after 25 days of seed sowing and plant mortality (% PM) was also calculated in each treatment after 30 days of seed sowing (Khan et al. 2018).
Statistical analysis
For each in vitro and in planta experiments, each treatment was replicated thrice and the experiments were conducted in a completely randomized design. Data regarding the effect of different treatments in the laboratory as well as in pots assays were analyzed statistically and the data were subjected to analysis of variance (ANOVA). The treatment means were delineated by LSD test at p ≤ 0.05 level of significance using computer software Statistix 8.1.
Results
In vitro bioassays
Screening of bacterial strains against M. phaseolina
Among eight antagonists, O. ciceri (FCBP-0727) found to be a strong antagonist, it significantly inhibited the radial growth of M. phaseolina by 77%. Three bacterial strains (FCBP-0083, FCBP-0097 and FCBP-0450) also exhibited significantly higher antifungal activity i.e. 70–73%, thus characterized as good antagonists. A. brasilense and P. malophilia (moderate antagonists) significantly decreased fungal growth by 65–66%. A. lipoferum and B. subtilis exhibited the lowest antifungal activity and were categorized as weak antagonists. Based on a screening test, O. ciceri was selected for further experiments (Table 1).
Table 1.
Antagonistic effect of bacterial strains on the growth of Macrophomina phaseolina
| Sr. no | Antagonist type | Strains | FCBP-Accession | Growth (cm) | Growth inhibition (%) over control |
|---|---|---|---|---|---|
| Control | Macrophomina phaseolina (Pathogen) | 0721 | 8.9a | – | |
| 1 | Strong | Ochrobactrum ciceri | 0727 | 2.1e | 77a |
| 2 | Good | Pseudomonas fluorescens | 0083 | 2.4de | 73b |
| 3 | Pseudomonas malophilia | 0097 | 2.7cd | 70bc | |
| 4 | Rhizobacter daucus | 0450 | 2.7cd | 70bc | |
| 5 | Moderate | Azospirillum brasilense | 0025 | 3.0bc | 66cd |
| 6 | Pseudomonas malophilia | 0099 | 3.1c | 65d | |
| 7 | Weak | Azospirillum lipoferum | 0022 | 6.6b | 26e |
| 8 | Bacillus subtilis | 0324 | 6.9b | 23e |
Each treatment in the experiment has three independent biological replicates (n = 3). Values with different letters in superscript show a significant difference (p ≤ 0.05) as determined by LSD-test
Characterization and identification and of O. ciceri
Cells of O. ciceri were Gram-negative, aerobic (catalase and oxidase positive), non-spore former appeared as short rods commonly observed as single cells under the light microscope. Cells showed motility at the exponential phase but no motility at the stationary phase. White to cream colonies on LB agar were generally circular, low-convex, shiny, large (3 to 4 mm) mucoid, translucent with smooth margins. Acid production was observed from inositol, arabinose, and rhamnose but not from sorbitol, sucrose, lactose, adnonitol, and raffinose. It showed a positive reaction for hydrogen sulfide and gelatin hydrolysis. Besides, it was positive for the assimilation of arginine dihydrolase but negative for salicin and malonate. The selected antifungal bacterium was confirmed to belong to the genus Ochrobactrum (Table 2). The obtained sequence of O. ciceri (FCPB-0727; GeneBank, Accession No. LC415039) was compared with the 16S rDNA sequences gathered in the NCBI databases. Phylogenetic analysis based on 16S rDNA sequence of FCBP-0727 and the other 10 reference strains of O. ciceri revealed the presence of two separate clusters. The first cluster grouped 10 strains, while FCBP-0727 occupied in the second cluster as an outlier. However, sequences of FCBP-0727 exhibited 97% identity with all 10 reference strains including Y17 (JX646639.1), SN336 (MG890329.1), CP2 (MH507567.1), NT11 (MH507563.1), HRJ1 (KP140839.1), L22 (JX646649.1), Y27 (JX646640.1), Ca-34 (NR_115819.1), SW1 (KX185944.1) and W3 (KX185946.1). Strains Y17, SN336, CPT2, NT11, HRJ1, L22 and Y27 have 99% homology among them, and 94% similarity with strain Ca-34. However, strains SW1 and W3 exhibited 99% homology with strains Y17, SN336, CPT2, NT11, HRJ1, L22, Y27 and Ca-34 (Fig. 1).
Table 2.
Differential morphological and biochemical characteristics of the Ochrobactrum ciceri
| Cultural characters | |
| Colony | White to creamy, shiny, circular, low convex with smooth margins |
| Growth at | 25–30 °C |
| Cell morphology | |
| Shape | Rod |
| Gram type | − |
| Motile | + |
| Endospore | 4.1 |
| Reactions | |
| Catalase | + |
| Oxidase | + |
| Indole acetic acid | + |
| Hydrogen sulfides | + |
| Gelatin hydrolysis | + |
| Malonate | − |
| Inositol | + |
| Sorbitol | − |
| Rhamnose | + |
| Sucrose | − |
| Lactose | − |
| Arabinose | + |
| Adnonitol | − |
| Raffinose | − |
| Salicin | − |
| Arginine dihydrolase | + |
Biochemical tests were performed from each of three independent biological replicates (n = 3) and data is presented as + and − signs for positive or negative reaction, respectively
Fig. 1.
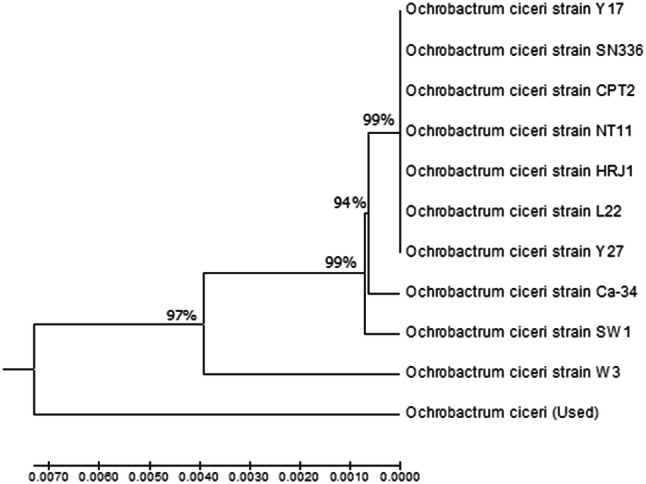
A phylogenetic tree based on 16S rDNA of Ochrobactrum ciceri (FCBP-0727; LC415039) made with the neighbour-joining method using MEGA7 software
Antifungal assays with cell-free supernatant (CFS) and culture (CP)
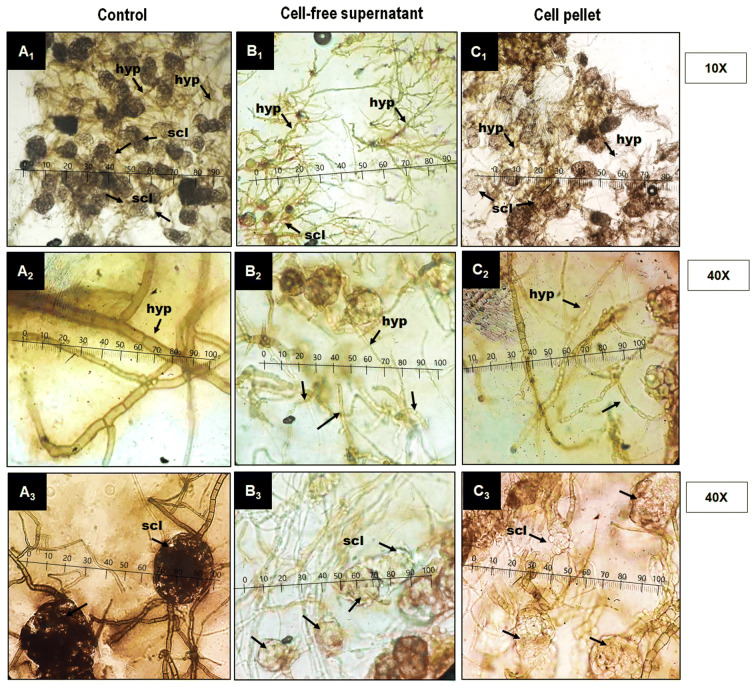
The changes in fungal mycelial and sclerotial characteristics due to fungicidal action of CFS and CP of O. ciceri were observed under a microscope at 10 × and 40 × (Fig. 2). In control, hyphae of the fungus were cylindrical and branched. Sclerotia were globose, dark brown to black and arranged compactly. CFS and CP caused substantial changes in hyphal and sclerotial morphology. Generally, hyphae became thin, lost normal branching and distorted. Sclerotia fragmented, contracted, lysed and pigmentation was lost. However, M. phaseolina was found to be more suscpeible to CFS as compared to CP, therefore further in vitro assays were carried with CFS.
Fig. 2.
a–c Effect of cell free supernatant (CFS) and cell pellet (CP) on fungal hyphae (hyp) and scletotium (scl) under compound microscope A1–A3 (A1 regular size hyphae and sclerotia; A2 cylindrical, septate and branched hyphae; A3 globose compactly arranged sclerotia; B1–B3, C1–C3 (B1, C1 irregular hyphae and sclerotia; B2, C2 deformations of the hypha; B3, C3 fragmentation, lysis, contraction and loss of pigmentation in sclerotia)
Characterization and antifungal assays with organic fractions of CFS
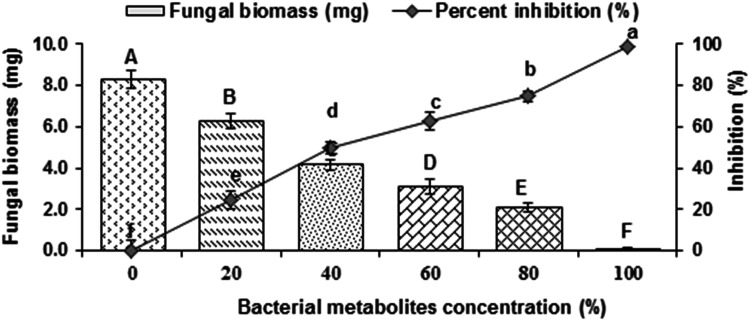
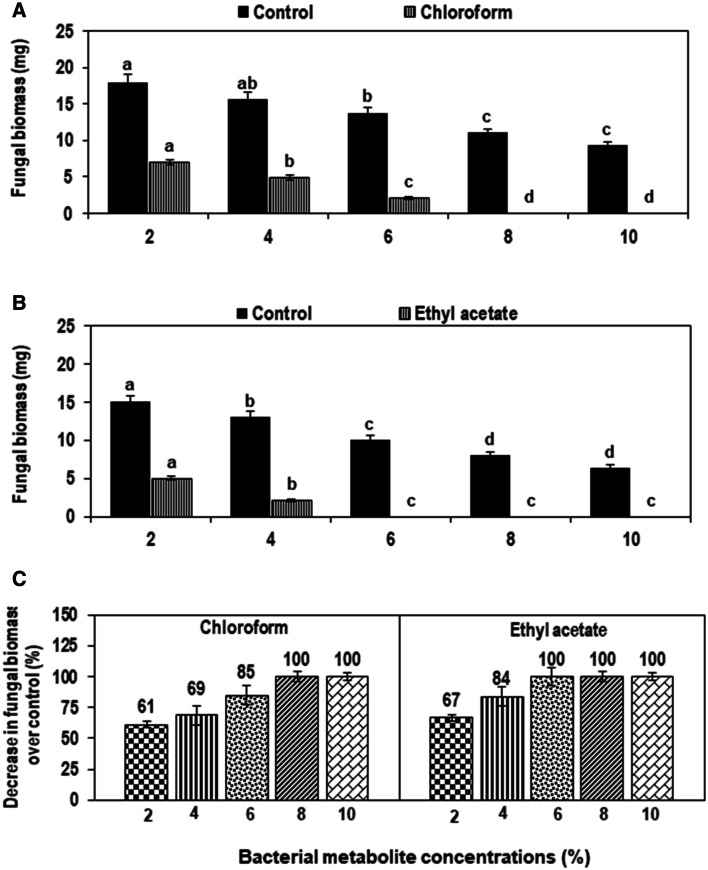
Results revealed that increasing concentration (20–100%) of CFS significantly inhibited the fungal biomass by 24–99% as relavent to control (Fig. 3). Therefore, both ethyl acetate and chloroform extracts of metabolites were analyzed through GC–MS. Ethyl acetate and chloroform extracts of CFS also demonstrated considerable fungicidal effect against the pathogen. Various concentrations of chloroform extract of metabolites considerably suppressed biomass of M. phaseolina by 61–100% compared with respective control (Fig. 4a). Likewise, different concentrations (2–10%) of ethyl acetate extract of metabolite reduced fungal biomass significantly by 67–100% over corresponding control treatment (Fig. 4b).
Fig. 3.
Effect of different concentrations of cell-free supernatant (CFS) of Ochrobactrum ciceri on the growth of Macrophomina phaseolina after incubation of 5 days at 30 °C. Vertical bars show standard errors of means of three independent biological replicates (n = 3). Values with different letters at their top show a significant difference (p ≤ 0.05) as determined by LSD Test
Fig. 4.
a–c Effect of different concentrations of an organic solvent extract of cell-free supernatant (CFS) of Ochrobactrum ciceri on the growth of Macrophomina phaseolina after incubation of 3 days at 30 °C. a Effect of chloroform extract on fungal biomass; b effect of ethyl acetate extract on fungal biomass. c Percentage decrease in fungal biomass over control. Vertical bars show standard errors of means of three independent biological replicates (n = 3). Values with different letters at their top show a significant difference (p ≤ 0.05) as determined by the LSD test
GC–MS analysis of organic fractions of CFS
The compounds identified from chloroform fraction of CFS were bis(2-ethylhexyl) phthalate; 1,3-benzenedicarboxylic acid, bis(2-ethylhexyl) ester; distearyl thiodipropionate, 1-methylene-2B-hydroxymethyl,-3,3-dimethyl-4B-(3-methylbut-2-enyl)-CY, and 1-monolinoleoylglycerol trimethylsilyl ether (Table 3). Likewise, GC–MS chromatogram of ethyl acetate extract of CFS revealed the presence of important organic compounds including bis(2-ethylhexyl) phthalate; meso-2,5-dimethyl-,3,3-hexanediol; hexadecamethyloctasiloxane; didodecyl phthalate and octasiloxane, hexadecamethyl- (Table 4).
Table 3.
Compounds identified from chloroform extract of Ochrobactrum ciceri of cell-free culture filtrate through GC–MS analysis
| # | Names of compounds | Molecular formula | Molecular weight | Retention time (min) | Peak area (%) |
|---|---|---|---|---|---|
| 1 | Bis(2-ethylhexyl) phthalate | C24H38O4 | 390 | 27.54 | 6575 |
| 2 | 1,3-Benzenedicarboxylic acid, bis(2-ethylhexyl) ester | C24H38O4 | 390 | 31.22 | 7453 |
| 3 | Distearyl thiodipropionate | C42H82O4S | 683 | 31.76 | 7583 |
| 4 | 1-Methylene-2B-hydroxymethyl,-3,3-dimethyl-4B-(3-methylbut-2-enyl)-CY | C15H26O | 222 | 32.51 | 7762 |
| 5 | 1-Monolinoleoylglycerol trimethylsilyl ether | C27H54O4Si2 | 498 | 33.65 | 8033 |
Table 4.
Compounds identified from ethyl acetate of Ochrobactrum ciceri of cell-free culture filtrate through GC–MS analysis
| # | Names of compounds | Molecular formula | Molecular weight | Retention time (min) | Peak area (%) |
|---|---|---|---|---|---|
| 1 | Bis(2-ethylhexyl) phthalate | C24H38O4 | 390 | 27.359 | 6533 |
| 2 | Meso-2,5-dimethyl-,3,3-hexanediol | C8H18O | 146 | 29.55 | 7054 |
| 3 | OctasiloxaneHexadecamethyl-, 1,1,3,3,5,5,7,7,9,9,11,11,13,13,15,15- | C16H50O7Si8 | 578 | 30.38 | 7253 |
| 4 | Didodecyl phthalate | C35H54O4 | 502 | 31.22 | 7454 |
| 5 | Octasiloxane, 1,1,3,3,5,5,7,7,9,9,11,11,13,13,15,15-hexadecamethyl- | C16H50O7Si8 | 578 | 31.59 | 7542 |
In planta assays
Effect of O. ciceri and Zn on disease and plant mortality
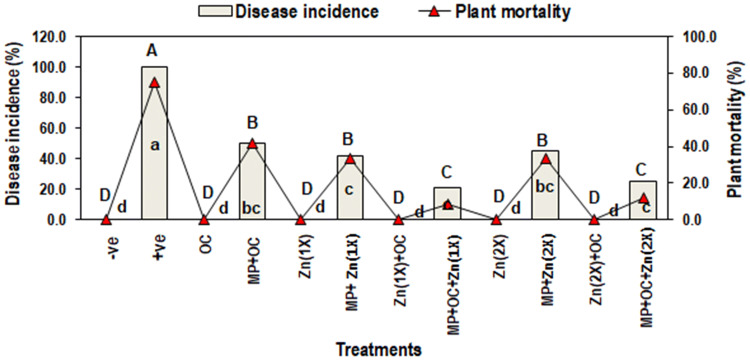
Plants in positive control (T2) treatment displayed the 100% disease incidence (DI) and 75% plant mortality (PM) with respect to the negative control (T1). Application of O. ciceri and Zn either combined or alone significantly managed charcoal rot disease in all five treatments inoculated with MP (T4, T6, T8 T10, and T12) as compared to a positive control (T2). However, combined effect of biocontrol bacteria and Zn (2.5 mg/kg) in T8, showed the greatest suppression in disease incidence (21%) and plant mortality (8.3%). Higher dose of Zn (5 mg/kg) along with O. ciceri (T12) also showed significantly less disease incidence (25%) and plant mortality (12%). Rest of the treatments viz., T4 (MP + O. ciceri), T6 and T10 [(MP + Zn (IX and 2X)] less effectively manage disease, hence the DI and PM were ranged between 42–50% and 33–42%, respectively (Fig. 5).
Fig. 5.
Effect of Macrophomina phaseolina (MP), Ochrobactrum ciceri (OC) and zinc (Zn) on disease incidence (%) and plant mortality (%) of mungbean. Vertical bars show standard errors of means of three independent biological replicates (n = 3). Values with different letters show a significant difference (p ≤ 0.05) as determined by LSD-test. T1: −ve control (without any treatment/inoculation); T2: + ve control [M. phaseolina (MP)]; T3: Ochrobactrum ciceri (OC); T4: MP + OC; T5: Zn (1X: 2.5 mg/kg); T6: MP + Zn (1X); T7: OC + Zn (1X); T8: MP + OC + Zn (1X); T9: Zn (2X: 5 mg/kg); T10: MP + Zn (2X); T11: OC + Zn (2X); T12: MP + OC + Zn (2X)
Effect of O. ciceri and Zn on physicochemical attributes
Photosynthetic pigment
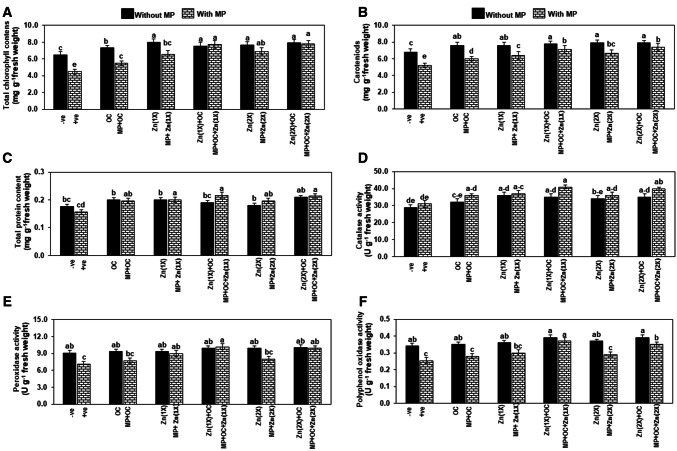
M. phaseolina inoculation significantly reduced total chlorophyll content (TCC) and carotenoids (CC) by 31 and 23%, respectively in the positive control (T2) as compared to the negative control (T1). O. ciceri and Zn either alone or in combination significantly enhanced the TCC and CC by 12–23% in the treatments without MP (T3, T5, T7, T9 and T11) over negative control. However, in all MP inoculated treatments (T4, T6, T8 T10, and T12), the application of Zn alone or in combined with bacteria also significantly improved the TCC by 22–73% and CC by 20–40%, maximum being was observed in T8 and T12,when compared with positive control (T2) (Fig. 6a, b).
Fig. 6.
A–F Effect of Macrophomina phaseolina (MP), Ochrobactrum ciceri (OC) and zinc (Zn) on physiology and biochemistry of mungbean leaf. Vertical bars show standard errors of means of three independent biological replicates (n = 3). Values with different letters show a significant difference (p ≤ 0.05) as determined by LSD-test. T1: −ve control (without any treatment/inoculation); T2: + ve control [M. phaseolina (MP)]; T3: Ochrobactrum ciceri (OC); T4: MP + OC; T5: Zn (1X: 2.5 mg/kg); T6: MP + Zn (1X); T7: OC + Zn (1X); T8: MP + OC + Zn (1X); T9: Zn (2X: 5 mg/kg); T10: MP + Zn (2X); T11: OC + Zn (2X); T12: MP + OC + Zn (2X)
Total protein content and antioxidant enzymes
Total protein conent (TPC) and catalase activity were insignificantly affected in inoculated plants (T2) under pathogen stress as compared to a healthy plants (T1). These attributes were insignificantly increased in all treatments without MP (T3, T5, T7, T9 and T11) over negative control, while significantly improved by 20–40% in treatments with MP (T4, T6, T8, T10 and T12) over positive control (Fig. 6c, d).
Peroxidase (POX) activity declined considerably by 21% in T2 as compared to T1. Interactive effect of Zn or bacteria insignificantly affected POX activity in treatments without MP but significantly improved in MP inoculated plants. The highest increase of 43% in POX activity was noticed in T8 followed by 39% in T12 and 27% in T6. In the rest of MP inoculated treatments, the POX activity was significantly increased as compared to T2 (Fig. 6e).
In MP inoculated treatment (T2), the polyphenol oxidase (PPO) activty decreased significantly (25%) as compared to the uninoculated plants (T1). OC or Zn did not enzyme activity significantly in all five treatments where MP was not inoculated. However, it was insignificantly increased in T4, T6 and T10, while significantly amplified by 46 and 38% in T8 and T12 with respect to T2 (Fig. 6f).
Discussion
In vitro bioassays
Among eight rhizobacterial strains (P. flourescene, P. malophilia 1, P. malophilia 2, R. daucus, A. brasilense, A. lipoferum, B. subtilis and O. ciceri), the highest antifungal activity was recorded by O. ciceri. The difference in the antifungal potential of these rhizobacteria could be due to the difference in the production of antimycotic compounds (hydrogen cyanide, siderophores, phenazines, pyrrolnitrin, 2,-diacetylphloroglucinol, pyoluteorin, viscosinamide, and tensin) (Reetha et al. 2014). The strong antimycotic potential of O. ciceri might be associated with the inherent adaptability potential of genus Ochrobactrum to extreme habitats with phosphate solubilization, siderophore and hydrogen sulfide production potential (Imran et al. 2015). Hydrogen sulfide is known to act as a fungicide and it can induce oxidative damage to molecules crucial for mycelial growth and spore germination (Fu et al. 2014).
Morphological and biochemical characterization of O. ciceri confirmed as gram-negative rods and non-spore former (Patel and Minocheherhomji 2018). The positive reaction to indole acetic acid, hydrogen sulfide and hydrolysis of gelatin along with assimilation of arginine dihydrolase has also been reported earlier by Imran et al. (2010). The obtained sequence of FCBP-0727 (GeneBank, accession no. LC415039) was compared with the 16S rDNA sequences gathered in the NCBI databases. The reference strains used for the phylogenetic tree belong to different localities. Like Y17 (JX646639), HRJ1 (KP140839), L22 (JX646649), Y27 (JX646640), and SN336 (MG890329) were isolated from Xinjiang China. However, CPT2 (MH507567) and NT11 (Mthe H507563) were isolated from the soil of the agricultural field of West Bengal, India. The strain Ca-34 (NR_115819.1), were isolated from plant nodule, while SW1(KX185944.1) and W3 (KX185946.1) were isolated from industrial effluents. The homology of all these strains with the subjected O. ciceri strain (FCBP-0727) is 97% as shown on neighbor-joining tree, FCBP-0727 is in cluster II suggesting this strain might be genetically more diverse due to variation in source (chickpea rhizosphere) and regions.
Observations under the compound microscope revealed, that CFS and CP inhibited mycelial growth and suppressed the formation of sclerotia although the CFS appeared to be more destructive to the pathogen than the CP. Cell wall lysis of M. phaesolina may occur due to the coordinated action of hydrolytic enzymes and antifungal lipopeptides (e.g. subtilosin, subtilisin, protease and β-1,3-glucanase) of the O. ciceri, which may lead to swelling and distortion, deformation in hyphae and sclerotia. Similar observations have been observed by CFS of B. subtilis against many drastic phytopathogenic fungi (Macrophomina sp., Colletotrichum gloeosporioides, Botrytis cinerea, and Sclerotium rolfsii) (Abdelmoteleb et al. 2017). Likewise, Zhang et al. (2017) reported the inhibition in the growth of Gaeumannomyces graminis var. tritici due to the secretion of diverse compounds in the culture filtrate of B. subtilis Z-14. Furthermore, to date, there have been no reports regarding the effect of O. ciceri (cell-free supernatant and cell pellet) against M. phaseolina.
Cell-free supernatant (CFS) of O. ciceri found to be more inhibitory for M. phaesolina, and further assessment using different concentrations of CFS as well as and its fractions (chloroform and ethyl acetate fractions) also exhibited significant suppression to complete inhibition in growth of the fungus. The previous investigation also confirmed that chloroform and ethyl acetate fractions of CFS of Bacillus strains hold high antimycotic activity owing to chemical multiplicity (Balouiri et al. 2016). The GC–MS analysis of CFS of chloroform and ethyl acetate extracts proved to be rich sources of volatile organic compounds. In CFS of chloroform, bis(2-ethylhexyl) phthalate (DEPH), 1,3-benzenedicarboxylic acid (m-Phthalic acid), bis(2-ethylhexyl) ester (adipic acid), distearyl thiodipropionate (antioxidant STDP), 1-methylene-2B-hydroxymethyl,-3,3-dimethyl-4B-(3-methylbut-2-Enyl)-CY and 1-monolinoleoylglycerol trimethylsilyl ether were present. Five organic compounds [bis(2-ethylhexyl) phthalate, 2,5-dimethyl-3,4-hexanediol (diisopropylglycol); 5H-hexadecamethyloctasiloxane, didodecyl phthalate] were present in ethyl acetate extract contained. DEHP is an antimicrobial as well as pro-inflammatory agent; it is toxic to cells of living things and also suppresses their carbohydrate metabolism (Wang et al. 2018). Jasmine et al. (2013) and Parthipan et al. (2015) reported the antimicrobial, anti-inflammatory and antihyperlipidemic properties of 1-methylene-2B-hydroxymethyl,-3,3-dimethyl-4B-(3-methylbut-2-enyl)-CY, 1,3-benzenedicarboxylic acid, bis(2-ethylhexyl) ester, 1-monolinoleoylglycerol trimethylsilyl ether, while distearyl thiodipropionate is an antioxidant agent (Diamante et al. 2010). Venkatesh and Kalaivani (2014) described antimicrobial activity of octasiloxane,1,1,3,3,5,5,7,7,9,9,11,11,13,13,15,15-hexadecamethyl. Didodecyl phthalate belong to the class of phthalates. Phthalates are used as gelling agents, film formers, stabilizers, dispersants, lubricants, binders, emulsifying agents and agricultural adjuvants (Raorane et al. 2012). Therefore, the antimycotic activity of O. ciceri against M. phaseolina might be ascribed to the occurrence of many important and active organic compounds.
In planta assays
Pot experiment revealed that as compared to healthy plants in negative control (T1), the infection of M. phaseolina in the positive control (T2) casued metabolic hijacking in the mungbean plant, which resulted in disturbance in physicochemical attributes of the host plant along with the highest disease pressure (Awan et al. 2019). O. ciceri and Zn either alone or in combination reduced 50–80% disease pressure by improving potential mediators of the resistance (physicochemical attributes). However, the synergism of O. ciceri and Zn [(T8: IX & T12: 2X)] showed the greastest reduction of 79 and 75% in disease pressure, respectively along with maximum improvement in the physicochemical attributes. Priyanka and Sevugapperuma (2018) have documneted 77% suppression in Botrytis leaf blight with an increase in the expression profile of defense-related genes (phenylalanine ammonia-lyase and ascorbate peroxidase) in Lilium due to foliar application of O. ciceri (MM17). Hassan et al. (2014) result revealed 44–52% management of dry rot in sugar cane due to the application of Ochrobactrum intermedium strain (NH-5). Role of O. ciceri in managing disease has been ascribed to elicit induce systemic resistance aside its potential in mineral nutrient utilization, production of siderophores and antibiotic in the host plant (Imran et al. 2015; Priyanka and Sevugapperuma 2018). So far, O. ciceri along with Zn seems to develop efficient signal transduction in host cells through Ca2+ influx and biphasic accumulation of reactive oxygen species (ROS), which has been indicated by improvement in the activities of ROS scavenging enzymes i.e. POX and CAT. Stimulation in the activities of POX and PPO could also be ascribed to enhanced phenols oxidation, lignin cotent and suberization of host plant cells. Current findings are in accordance with many earlier reports, where management of mungbean charcoal rot and tomato early blight was correlated with improvement in mediators of the resistance after application of Zn or Zn + biocontrol (Khan et al. 2018; Awan et al. 2018, 2019). Improved resistance in mungbean with Zn doses may be achieved by joint additive or synergic effects between Zn and other soil nutrients. Over and above, disease-resistant has been associated with improvement of cellular integrity through application of Zn (Machado et al. 2018). Zn also acts as co-enzyme in many biochemical functions related to photosynthesis, respiration and growth stimulation (Awan et al. 2019), while Zn might also be a factor to induce the production of antimicrobial compounds in O. ciceri (Siddiqui et al. 2002). Besdies, application of biocontrol and Zn also improved physicochemical attitbutes in those treatments (T3, T5, T7, T9 and T11), which were not inoculated with pathogen, though only photosynthetic pigment and PPO activity were improved significantly as compared to T1. Higher total chlorophyll conent, carotenoids, and PPO might be reason for benefical role of CAT and POX in improving general defense status of mungbean plants due to better supply of nutrients by O. cicieri and Zn.
It is proposed that Zn was indirectly responsible for improving plant health leading to better plant-bacteria interactions resulting in better efficacy of O. ciceri to manage Macrophomina root rot in mungbean. It was concluded that combined effect of O. ciceri and Zn (2.5 mg/kg) could be effectively used to manage disease by modulating plant physiological efficiency.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Authors are thankful to the University of Punjab to provide funding to accomplish this research work. Authors have no conflict of interest regarding the publication of this paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdelmoteleb A, Troncoso-Rojas R, Gonzalez-Soto T, González-Mendoza D. Antifungical activity of autochthonous Bacillus subtilis isolated from Prosopis juliflora against phytopathogenic fungi. Mycobiology. 2017;45:385–391. doi: 10.5941/MYCO.2017.45.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atiq M, Asad S, Rafique M, Khan NA, Rehman AU, Younis M, Shafiq M, Ahmad K, Bashir N, Khan WA. Identification of source of resistance in mungbean germplasm against charcoal rot disease. Pak J Phytopathol. 2014;26:133–136. [Google Scholar]
- Awan ZA, Shoaib A. Combating early blight infection by employing Bacillus subtilis in combination with plant fertilizers. Curr Plant Biol. 2019;20:100125. doi: 10.1016/j.cpb.2019.100125. [DOI] [Google Scholar]
- Awan ZA, Shoaib A, Khan KA. Variations in total phenolics and antioxidant enzymes cause phenotypic variability and differential resistant response in tomato genotypes against early blight disease. Sci Hortic. 2018;239:216–223. doi: 10.1016/j.scienta.2018.05.044. [DOI] [Google Scholar]
- Awan ZA, Shoaib A, Khan KA. Crosstalk of Zn in combination with other fertilizers underpins interactive effects and induces resistance in tomato plants against early blight disease. J Plant Pathol. 2019;35:330–340. doi: 10.5423/PPJ.OA.01.2019.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azarmi F, Mozafari V, Dahaji PA, Hamidpour M. Biochemical, physiological and antioxidant enzymatic activity responses of pistachio seedlings treated with plant growth-promoting rhizobacteria and Zn to salinity stress. Acta Physiol Plant. 2016;38:21. doi: 10.1007/s11738-015-2032-3. [DOI] [Google Scholar]
- Aziz-ur-Rehman KME, Kaukab S, Saeed S, Aqeel M, Riasat G, Rafiq CM. Prospects of Mungbean as an additional crop in rice wheat system of Punjab Pakistan. Univ J Agric Res. 2019;7:136–141. [Google Scholar]
- Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal. 2016;6:71–79. doi: 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamante C, Fiume MZ, Bergfeld WF, Belsito DV, Hill RA, Klaassen CD, Liebler DC, Marks JG, Shank RC, Slaga TJ, Snyder PW, Alan Andersen F. Final safety assessment of thiodipropionic acid and its dialkyl esters as used in cosmetics. Int J Toxicol. 2010;29:137–150. doi: 10.1177/1091581810373150. [DOI] [PubMed] [Google Scholar]
- Expósito RG, Bruijn ID, Postma J, Raaijmakers JM. Current insights into the role of rhizosphere bacteria in disease suppressive soils. Front Microbiol. 2017;8:1–12. doi: 10.3389/fmicb.2017.02529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu LH, Hu KD, Hu LY, Li YH, Hu LB, Yan H, Liu YS, Zhang H. An antifungal role of hydrogen sulfide on the postharvest pathogens Aspergillus niger and Penicillium italicum. PLoS ONE. 2014;9:e104206. doi: 10.1371/journal.pone.0104206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan K, Xu B. A critical review on phytochemical profile and health-promoting effects of mungbean (Vigna radiata) Food Sci Hum Wellness. 2018;7:11–33. doi: 10.1016/j.fshw.2017.11.002. [DOI] [Google Scholar]
- Hafeez B, Khanif YM, Saleem M. Role of zinc in plant nutrition—a review. Am J Exp Agric. 2013;3:374–391. [Google Scholar]
- Hassan MN, Afghan S, Hassan Z, Hafeez FY. Biopesticide activity of sugarcane associated rhizobacteria: Ochrobactrum intermedium strain NH-5 and Stenotrophomonas maltophilia strain NH-300 against red rot under field conditions. Phytopathol Mediterr. 2014;5:27–37. [Google Scholar]
- Imran A, Hafeez FY, Fruhling A, Schumann P, Malik KA, Stackebrandt E. Ochrobactrumciceri sp. nov. Isolated from nodules of Cicer arietinum. Int J Syst Evol Microbiol. 2010;60:1548–1553. doi: 10.1099/ijs.0.013987-0. [DOI] [PubMed] [Google Scholar]
- Imran A, Mirza MS, Shah TM, Malik KA, Hafeez FY. Differential response of kabuli and desi chickpea genotypes toward inoculation with PGPR in different soils. Front Microbiol. 2015;6:859. doi: 10.3389/fmicb.2015.00859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal U, Mukhtar T (2014) Morphological and pathogenic variability among Macrophomina phaseolina isolates associated with mungbean (Vigna radiata L.) Wilczek from Pakistan. Sci World J. Article ID 950175 [DOI] [PMC free article] [PubMed]
- Jasmine JM, Latha K, Vanaja R. Determination of bioactive components of decholestrate, a polyherbal formulation by GC–MS analysis. TIC. 2013;1(e8):12–39. [Google Scholar]
- Khan KA, Shoaib A, Arshad Awan Z, Basit A, Hussain M. Macrophomina phaseolina alters the biochemical pathway in Vigna radiata chastened by Zn2+ and FYM to improve plant growth. J Plant Interact. 2018;13:131–140. doi: 10.1080/17429145.2018.1441451. [DOI] [Google Scholar]
- Korejo F, Noreen R, Ali SA, Humayun F, Rahman A, Sultana V, Ehteshamul-haque S. Evaluation of antibacterial and antifungal potential of endophytic fluorescent pseudomonas associated with Salvadora persica L. and Salvadorao oleoides Decne. Pak J Bot. 2017;49:1995–2004. [Google Scholar]
- Machado PP, Steiner F, Zuffo AM, Machado RA. Could the supply of boron and zinc improve the resistance of potato to early blight? Potato Res. 2018;61:169–182. doi: 10.1007/s11540-018-9365-4. [DOI] [Google Scholar]
- Mazzola M, Cook RJ, Thomashow LS, Weller D, Pierson LS. Contribution of phenazine antibiotic biosynthesis to the ecological competence of Pseudomonads fluorescent in soil habitats. App Environ Microb. 1992;58:2616–2624. doi: 10.1128/AEM.58.8.2616-2624.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolov A, Ganchev D. Effect of zinc undecylenates on plant pathogenic fungi. Bulg J Agric Sci. 2010;16:220–226. [Google Scholar]
- Parthipan B, Suky MGT, Mohan VR. GC–MS analysis of phytocomponents in Pleiospermium alatum (Wall. ex Wight & Arn.) Swingle, (Rutaceae) J Pharma Phytochem. 2015;4:216–222. [Google Scholar]
- Patel TS, Minocheherhomji FP. Review: plant growth promoting rhizobacteria: blessing to agriculture. Int J Pure Appl Biosci. 2018;6:481–492. doi: 10.18782/2320-7051.6383. [DOI] [Google Scholar]
- Priyanka R, Sevugapperuma N. Ochrobactrum ciceri mediated induction of defence genes and antifungal metabolites enhance the biocontrol efficacy for the management of Botrytis leaf blight of Lilium under protected conditions. J Plant Pathol. 2018;101:323–337. doi: 10.1007/s42161-018-00212-3. [DOI] [Google Scholar]
- Raorane CJ, Gavimath CC, Kukreja GP, Kalsekar DP, Kulkarni SM, Ravishankar BE, Hooli RS. Isolation, identification and phylogenetic analysis of phthalic acid degrading bacteria. Int J Biotechnol Res. 2012;3:804–809. [Google Scholar]
- Reetha AK, Pavani SL, Mohan S. Hydrogen cyanide production ability by bacterial antagonist and their antibiotics inhibition potential on Macrophomina phaseolina (Tassi.) Goid. Int J Curr Microbiol Appl Sci. 2014;3:172–178. [Google Scholar]
- Sharma RM, Singh RR. Harvesting, postharvest handling and physiology of fruits and vegetables. Postharvest Technol Fruits Veg. 2000;1:94–147. [Google Scholar]
- Shoaib A, Awan ZA, Khan KA. Intervention of antagonistic bacteria as potential inducer of disease resistance in tomato to mitigate early blight. Sci Hortic. 2019;252:20–28. doi: 10.1016/j.scienta.2019.02.073. [DOI] [Google Scholar]
- Siddiqui IA, Shaukat SS, Hamid M. Role of zinc in rhizobacteria-mediated suppression of root-infecting fungi and root-knot nematode. J Phytopathol. 2002;150:569–575. doi: 10.1046/j.1439-0434.2002.00805.x. [DOI] [Google Scholar]
- Syed-Ab-Rahman SF, Carvalhais LC, Chua E, Xiao Y, Wass TJ, Schenk PM. Identification of soil bacterial isolates suppressing different Phytophthora spp. and promoting plant growth. Front Plant Sci. 2018;9:1–18. doi: 10.3389/fpls.2018.01502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakore Y. The biopesticide market for global agricultural use. Ind Biotechnol. 2006;2:194–198. doi: 10.1089/ind.2006.2.194. [DOI] [Google Scholar]
- Venkatesh VR, Kalaivani K. Gas chromatography and mass spectrometry analysis of Solanum villosum (Mill) (solanaceae) R. Int J Pharm Sci Res. 2014;5:5283–5287. [Google Scholar]
- Wang Y, Ren W, Li Y, Xu Y, Teng Y, Christie P, Luo Y. Non-targeted metabolomics analysis to unravel the impact of di (2-ethylhexyl) phthalate stress on root exudates of alfalfa (Medicago sativa) Sci Total Environ. 2018;646:212–219. doi: 10.1016/j.scitotenv.2018.07.247. [DOI] [PubMed] [Google Scholar]
- Zarmehri SG, Moosavi SG, Zabihi HR, Seghateslami MJ. The effect of plant growth promoting rhizobacteria (PGPR) and zinc fertilizer on forage yield of maize under water deficit stress conditions. Technol J Eng Appl Sci. 2013;3:3281–3290. [Google Scholar]
- Zhang D, Gao T, Li H, Lei B, Zhu B. Identification of antifungal substances secreted by Bacillus subtilis Z-14 that suppress Gaeumannomyces graminis var. tritici. Biocontrol Sci Technol. 2017;27:237–251. doi: 10.1080/09583157.2016.1275522. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.