Abstract
Hepatocellular carcinoma (HCC) is one of the foremost causes of cancer related morbidity worldwide. An increasing number of studies have confirmed that microRNAs play an important role in the development, progression and metastasis of HCC. From those important miRNAs are miR-98 and miR-214. This study were conducted to explore the effect of these two miRNAs on some apoptotic and angiogenic genes namely, BCL-2, survivin, CCND1, CDC2, P53 and P21, VEGF, Hif-1α, MMP-2, MMP-9, Ang-1, Ang-2, and FGF-1. miRNAs mimics and inhibitors transfection was used to investigate the role of both studied molecules in apoptosis and angiogenesis in HepG2 cells. QRT-PCR was used for Quantitative gene and miRNA expression analyses. The study revealed that miR-98 could serve as a pro-apoptotic factor through the upregulation of P53 gene expression levels. Besides, the anti-angiogenic effect of this miRNA was evident through the down regulation of Ang-1 and FGF-1 genes. Meanwhile, miR-214 showed a pro-apoptotic role and anti-angiogenic effects. These effects were verified through the significant down regulation of BCL-2, CDC2, VEGF, Ang-1 and MMP-2. These results introduced a possible positive role played by both miR-98 and miR-214 on some pro-apoptotic and anti-angiogenic genes.
Keywords: Hepatocellular carcinoma, Apoptosis, Angiogenesis, miR-98, miR-214
Introduction
Hepatocellular carcinoma (HCC) is ranked as the third leading cause of cancer morbidity worldwide [1]. Therapeutic options include surgery, radiotherapy, and chemotherapy. In chemotherapy, anti-neoplastic drug (s) that interfere with cancer cell proliferation and tumor growth are administrated. However, drug resistance occasionally are developed in chemotherapy leading to the failure of treatment and mortality [2]. This poor outcome is due to intrahepatic metastasis, a prominent hallmark of HCC. In HCC, tumor cells invade the major branches of portal vein, resulting in intrahepatic metastasis. Surgical resection/liver transplantation are used as the standard treatment strategies to improve survival benefit for patients with HCC. Therefore, there is an urgent need to elucidate the molecular mechanisms of apoptosis and agiogenesis and to introduce novel therapeutic targets for HCC.
MicroRNAs (miRNAs) are considered as a post-transcription factor consisting of small non-coding oligonucletides that downregulate target genes and initiate the breakdown of mRNA or counteract with its translation pathway [3]. A single miR- can control the transcription of many genes, consequently, researchers were motivated to do extensive researches on miRNAs as a transcription factors. Modulation of miRNA could be a useful tool to attack heterogeneous populations of cancer cells in tumor mass, introducing a promising therapeutic value in cancer treatment [4]. Besides, recent experimental evidences have emphasized the important roles of miRNAs in apoptosis and angiogenesis [5].
Several miRNAs were found to be overexpressed in HCC while few were down regulated and associated with HCC development and progression. miR-224, miR-106b and miR-21 were found to be upregulated [6, 7], meanwhile, miR-98 and miR-214 were down regulated in HCC [8, 9]. MiR-98, one of the let-7/miR-98 family members was found to act as a tumor suppressor gene in some human cancers through downregulation of its different target genes [10, 11]. For example, miR-98 was found to inhibit the proliferation and metastasis of oral squamous cell carcinoma by targeting of IGF1R expression [12]. Besides, miR-98 inhibits proliferation, migration, and invasion of non-small-cell lung cancer by downregulating ITGB3 [13]. Li et al. [14] found that miR-98 supressed melanoma metastasis via a negative feedback loop with its target gene IL-6. Nevertheless, very little is known about the role of miR98 in HCC in humans. Recently, the tumor suppressor function of miR-214 has gained a lot of research interests [15, 16]. It was found that miR-214 ectopic over expression has a positive role in suppressing cell proliferation by inducing G1-S checkpoint arrest. While, miR-214 downregulation significantly promoted cell cycle advancement and growth of HCC cells. Up regulation of miR-214-3p greatly enhanced the sensitivity of esophageal cancer cells to cis-platin through targeting both survivin and CUG-BP1. Moreover, E2F2, CDK3 and CDK6 were all targets of miR-214 [15].
Materials and Methods
Cell Culture
Hepatocellular carcinoma HepG2 cells were purchased from ATCC (American Type Culture Collection) and maintained in the proper conditions. The cells were cultured in Dulbecco’s modified Eagle’s Medium (DMEM) (Lonza, Beligium) supplemented by 10% fetal bovine serum (FBS), 4 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin sulfate at 37 °C in a humidified incubator with 5% CO2. The cells harvested after trypsinization (0.025% trypsin and 0.02% EDTA) and washed twice with Dulbecco’s phosphate-buffered saline (DPBS). When the cell density reached approximately 80%, cells were split for further culture. The experiments were made up when the cells were in the logarithmic growth phase.
Transfection of HepG2/Dox Cells with miR-98 and miR-214
HepG2/Dox cells were transfected with miR-98-3p mimic, miR-98-3p inhibitor, miR-214-3p mimic, miR-214-3p inhibitor and miRNA negative control (Qiagen, USA) using Hiperfect transfection reagent, Qiagen, according to the following protocol: Before transfection, 1.5x105 cells were seeded per well of two 24-well plate in 1.5 ml of DMEM culture medium containing 10% FBS serum and antibiotics. The second day, cells were transfected with 100 nM miR-mimics or inhibitors in a serum-free media using 1 µl of the Hiperfect transfection reagent. MiScript Inhibitor negative control which has no homology to any known mammalian gene was used as negative control. The third day, the media were changed with fresh complete media and the cells were incubated at the proper conditions for another 48 h. The third day, the cells in the 1st 24 well plate were harvested using Qiazole reagent and were subjected to miRNAs expression level estimation. At the end of the experimental period, the other plate were used for further gene expressions.
miR-98 and miR-214 Quantitation Using RT-qPCR
miR-98 and miR-214 were determined using the Qiagen miscript system (Qiagen, USA) according to manufacturer instructions. miRNeasy kit was used for Purification of RNA containing miRNA, after that cDNA generation from RNA containing miRNA was performed using miscript RT-kit. Real-Time PCR was used for detection of mature miRNA using miRNA specific primers to hsa-miR-98 and hsa-miR-214 in separate reactions. The cycling conditions were as follow: denaturation for 15 s at 94 °C, annealing for 30 s at 55 °C and extension for 30 s at 70 °C. Fluorescence data were collected using MiniOpticon Bio-Rad Real Time Thermal Cycler.
Quantitative Real Time Gene Expression Analysis
This was done using SYBR® Green one-step RT-PCR (Qiagen, USA). Briefly, total RNA was extracted from HepG2/Dox cells (1.5 × 105 cells/well) treated with miR-98 mimic, miR-98 inhibitor, miR-214 mimic, miR-214 inhibitor and negative control using Qiazole lysis reagent (Qiagen, USA) according to the manufacturer instructions. Primer sequence for β-actin, BCL-2, survivin, CCND1, CDC2, P53 and P21, VEGF, Hif-1α, MMP-2, MMP-9, Ang-1, Ang-2, and FGF-1 are listed in Table 1. The copy numbers were normalized to 100,000 copies of the housekeeping beta-actin gene. The RT and subsequent PCR cycling conditions were as follows: 50 °C for 10 min, 95 °C for 5 min, 60 °C for 30 s and then 95 °C for 15 s; the number of cycles were 40 cycles. BioRad Miniopticon™ real time PCR cycler was used for quantitative estimation.
Table 1.
Primers used for apoptotic and angiogenic pathway analyses
| Gene | Primer forward (5′–3′) | Primer reverse (5′–3′) |
|---|---|---|
| β–β-actin | CCTTCCTGGGCATGGAGTCCT | GGAGCAATGATCTTGATCTTC |
| BCL-2 | CCTGGTGGACAACATCGCC | AATCAAACAGAGGCCG CATGC |
| CCND1 | GAGGAAGAGGAGGAGGAGGA | GAGATGGAAGGGGGAAAGAG |
| CDC2 | CAAATATAGTCAGTCTTCAGGATG | CCTGTAGGATTTGGTATAAATAAC- |
| P53 | AGA GTC TAT AGG CCC ACC CC | GCT CGA CGC TAG GAT CTG AC |
| Vascular endothelial growth factor (VEGF) | TACCTCCACCATGCCAAGTG | ATGATTCTGCCCTCCTCCTTC |
| MMP-2 | ATGCTTCCAAACTTCACGCTCT- | CAGGGTTTCCCATCAGCATT |
| Angiopoietin-1 (Ang-1) | AAAGGTCACACTGGGACAGC | TTCTGACATTGCGCTTTCAA |
| Angiopoietin-2 (Ang-2) | TCCAAGCAAAATTCCATCATTG | GCCTCCTCCAGCTTCCATGT |
| Fibroblast growth factor 1 (FGF-1) | AAGCCCGTCGGTGTCCATGG | GATGGCACAGTGGATGGGAC |
Results and Discussion
This study was conducted to investigated the role of both miR-98 and miR-214 in apoptotic and angiogenic pathways in HepG2 cells through screening the effect of mimics and inhibitors of miR-98 and miR-214 on various apoptoitic and angiogenic genes including BCL-2, survivin, CCND1, CDC2, P53, VEGF, MMP-2, Ang-1, Ang-2, and FGF-1. HepG2 cells were transfected with miR-98 mimic, miR-98 inhibitor, miR-214 mimic and miR-214 inhibitor and the expression levels of miR-98 and miR214 in HepG2 cells have been determined by qRT-PCR (Fig. 1).
Fig. 1.
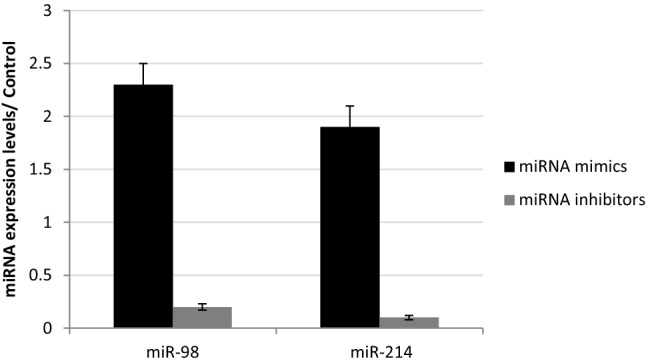
Expression levels of miR-98 and miR214 in HepG2 cells transfected with miR-98 and miR-214 mimics and inhibitors
Building on many previous observations it is accepted to expect that there are numerous miRNAs having both tumor-promoting and tumor-suppressing roles. The exact role of miR-mediated tumor suppression in the different complex pathways and different cancers is not fully yet elucidated. Zhang et al. [17] suggested that miR-98 functioned as a tumor suppressor by targeting Wnt/β-catenin signal pathway. As it directly targets and inhibits EZH2 gene expression. In the same context, Zou et al. [18] found that miR-98 plays an inhibition role in the proliferation, migration, invasion and epithelial mesenchymal transition of HCC cells, partly at least, via directly inhibition of SALL4.
In the current study, miR-98 mimics significantly upregulated the expression level of P53 gene as compared to negative control treated HepG2 cells (Fig. 3). However, the inhibition of miR-98 significantly downregulated BCL-2, CDC2, and CCND1 gene expression levels as compared to negative control treated cells (Figs. 2, 3). Besides, miR-98 mimics significantly upregulated the CCND1 gene expression levels (Fig. 3). All the above mentioned findings could suggest that the miR-98 could serve as a pro-apoptotic factor through the upregulation of P53 gene expression levels. It was reported that the downregulation of miR-98 resulted in increased expression of cellular MYC and subsequent elevation of cell proliferation as well as MYC-induced apoptosis [19]. Much evidences showed that c-Myc plays a critical role in the control of cell proliferation, regulation of cell cycle, and serves as a link between proliferation and cell death by inducing p53-dependent apoptosis. c-Myc has been documented to be both a positive and a negative signal for induction of apoptosis. It was established that overexpression of c-Myc enhances normal cell apoptosis. On contrary, down-regulation of c-Myc expression may be a must for induction of apoptosis in many cancer cells, such as leukemia cells, prostate cancer cells, lung cancer cells, and liver cancer cells [20]. It was found that miR-98 inhibits the proliferation, migration, invasion and apoptosis through the down-regulation of PAK1 expression in non-small cells carcinoma lung cancer cells [21]. Our finding is in accordance with Pathak et al. [21] who reported that after radiation and SN38 (a topoisomerase I inhibitor) treatment, miR-98 expression significantly was up-regulated and this upregulation was dependent on p53 status in colon cancer cells.
Fig. 3.

The effect of transfection of HepG2 cells with miR-98 and miR-214 mimics and inhibitors on expression levels of CCND1 and P53, NC = miRNA negative control; miR-98 M = miR-98 mimic; miR-98I = miR-98 inhibitor; miR-214 M = miR-214 mimic; miR-214I = miR-214 inhibitor. Data are represented as Means ± SEM, the data were reproducible, * P < 0.05
Fig. 2.
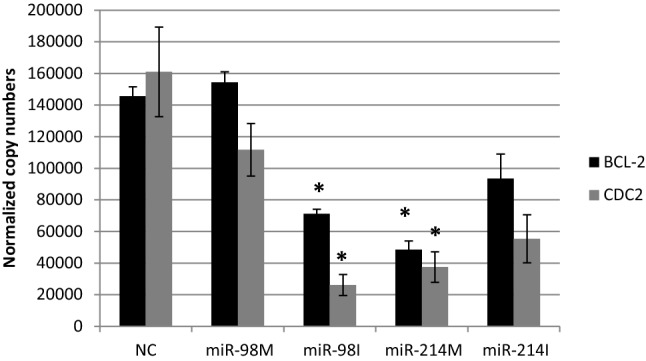
The effect of transfection of HepG2 cells with miR-98 and miR-214 mimics and inhibitors on expression levels of Bcl-2 and CDC2 NC = miRNA negative control; miR-98 M = miR-98 mimic; miR-98I = miR-98 inhibitor; miR-214 M = miR-214 mimic; miR-214I = miR-214 inhibitor. Data are represented as Means ± SEM, the data were reproducible, *P < 0.05
miR-214 is upregulated in several human tumors, such as ovarian cancer, gastric cancer, Sezary syndrome, and melanoma [22–25]. However, miR-214 expression was found to be reduced in cervical cancer [26], pancreatic cancer [27], hepatocellular carcinoma [28] and breast cancer [29], suggesting a tumor suppressor function. In the present investigations, transfecting HepG2 cells with miR-214 mimic resulted in significant down regulation in BCL-2 and CDC2 gene expression levels as compared to negative control treated cells (Fig. 2). These finding could suggest a pro-apoptotic effect of miR-214 as both BCL-2 and CDC2 are considered as anti-apoptotic factors. BCL-2 is considered as anti-apoptotic gene, it blocks apoptosis through its cooperation with c-myc during cell transformation [30]. Growing body of evidences refers to the Tyr15 residue of Cdc2 as the main target of the G2/M. Inhibition of CDC2–cyclin B1 complex results in G2/M arrest [31]. However miR-214 significantly upregulated CCND1 gene expression levels and its inhibition down regulated CCND1 gene expression levels (Fig. 3). Besides, miR-214 significantly down regulated P53 expression level (Fig. 3). This finding could be similar to that of Wang et al. [32] who demonstrated that the upregulation of miR-214 down regulated the expression of p53, leading to the enhancement of breast cancer cell invasion ability.
VEGF, MMP-2, MMP-9, Ang-1, Ang-2, and FGF-1 genes are considered as pro-angiogenic factors [33]. Our findings indicated that miR-98 inhibition significantly upregulated Ang-1 and FGF-1 and downregulated Ang-2 expression levels in HepG2 cells as compared to negative control treated cells (Figs. 5, 6). However, the over expression of miR-98 significantly upregulated VEGF expression levels, meanwhile the inhibition of miR-98 significantly down regulated this gene (Fig. 4). Moreover, the over-expression of miR-98 significantly upregulated MMP2 expression level (Fig. 7).
Fig. 5.
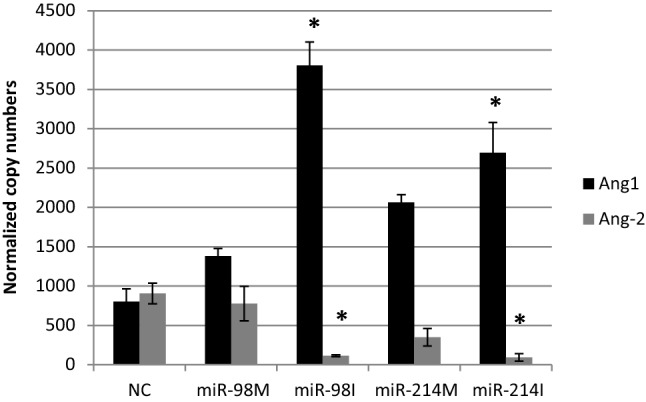
The effect of transfection of HepG2 cells with miR-98 and miR-214 mimics and inhibitors on expression levels of Ang-1 and Ang-2, NC = miRNA negative control; miR-98 M = miR-98 mimic; miR-98I = miR-98 inhibitor; miR-214 M = miR-214 mimic; miR-214I = miR-214 inhibitor. Data are represented as Means ± SEM, the data were reproducible, * P < 0.05
Fig. 6.

The effect of transfection of HepG2 cells with miR-98 and miR-214 mimics and inhibitor on expression levels of FGF-1, NC = miRNA negative control; miR-98 M = miR-98 mimic; miR-98I = miR-98 inhibitor; miR-214 M = miR-214 mimic; miR-214I = miR-214 inhibitor. Data are represented as Means ± SEM, the data were reproducible, * P < 0.05
Fig. 4.
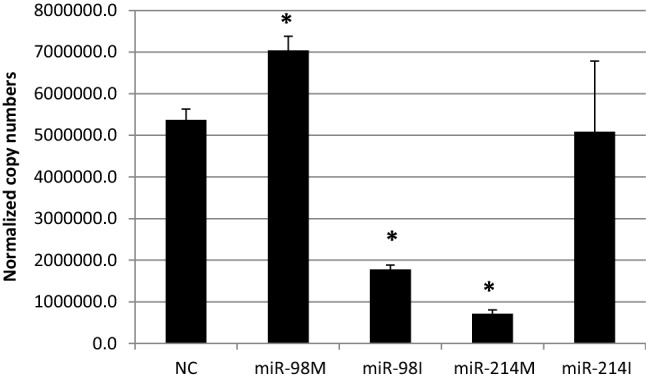
The effect of transfection of HepG2 cells with miR-98 and miR-214 mimics and inhibitors on expression levels of VEGF, NC = miRNA negative control; miR-98 M = miR-98 mimic; miR-98I = miR-98 inhibitor; miR-214 M = miR-214 mimic; miR-214I = miR-214 inhibitor. Data are represented as Means ± SEM, the data were reproducible, * P < 0.05
Fig. 7.
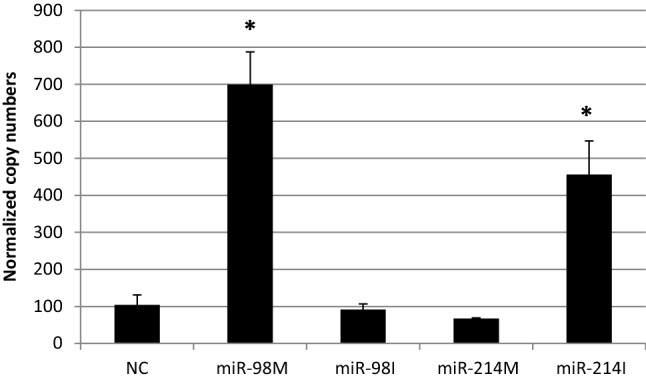
The effect of transfection of HepG2 cells with miR-98 and miR-214 mimics and inhibitor on expression levels of MMP-2, NC = miRNA negative control; miR-98 M = miR-98 mimic; miR-98I = miR-98 inhibitor; miR-214 M = miR-214 mimic; miR-214I = miR-214 inhibitor. Data are represented as Means ± SEM, the data were reproducible, * P < 0.05
Angiopoietin-1 (Ang-1) was reported to enhance angiogenesis through enhancing vascular branching, pericyte recruitment and endothelial survival [34]. Fibroblast growth factor receptor 1 (FGFR1) is overexpressed in HCC. FGFR1 overexpression increased cancer cell growth and infiltration and was involved in angiogenesis, which promoted the progression of HCC [35]. Consequently, we can suggest that the antiangiogenic effect of miR-98 could be mediated through the down regulation of Ang-1 and FGF-1 genes.
On the other hand, the transfection of miR-214 mimics into HepG2 cells resulted in down regulation of VEGF (Fig. 4), however, its inhibition down regulated Ang-2 and upregulated Ang-1 and MMP2 (Figs. 5, 7). These findings is Suggesting that the anti-angiogenic effect of miR-214 could be played through their inhibitory effects on VEGF, Ang-1 and MMP-2.
Conclusion
This study concluded that both miR-98 and miR-214 have a pro-apoptotic and anti-angiogenic effect on some apoptotic and angiogenic genes, however, this effect was not established for all apoptotic and angiogenic genes. miR-98 could serve as a pro-apoptotic factor through the upregulation of P53 gene expression levels. Besides, miR-214 could serve as a pro-apoptotic agent through the downregulation of both BCL-2 and CDC2. On the other hand, the anti-angiogenic effect of miR–98 could be mediated through the down regulation of Ang-1 and FGF-1 genes. Moreover, the anti-angiogenic effect of miR-214 could be played through their inhibitory effects on VEGF, Ang-1 and MMP-2.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Huang EC, Lai WY, Lau WP, Zhou F, Shen ZY, Pan SY, et al. Posthepatectomy HBV reactivation in hepatitis B-related hepatocellular carcinoma influences postoperative survival in patients with preoperative low HBV-DNA levels Ann. Surg. 2013;257:490–505. doi: 10.1097/SLA.0b013e318262b218. [DOI] [PubMed] [Google Scholar]
- 2.Alfarouk KO, Stock CM, Taylor S, Walsh M, Muddathir AK, Verduzco D. Resistance to cancer chemotherapy: failure in drug response from ADME to P-gp. Cancer Cell Int. 2015;15:71. doi: 10.1186/s12935-015-0221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.To KK. MicroRNA: a prognostic biomarker and a possible druggable target for circumventing multidrug resistance in cancer chemotherapy. J Biomed Sci. 2013;20:99. doi: 10.1186/1423-0127-20-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gong Z, Dong Z, Yang L, Yang J, Li J. The role of MicroRNA in lung cancer drug resistance and targeted therapy. In: Sarkar FH, editor. MicroRNA targeted cancer therapy. New York: Springer; 2014. pp. 51–82. [Google Scholar]
- 5.Peng Y, Croce CM. The role of MicroRNAs in human cancer. Signal Transduct Target Ther. 2012;1:15004. doi: 10.1038/sigtrans.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scisciani C, Vossio S, Guerrieri F, Schinzari V, De Iaco R, D’Onorio de Meo P. Transcriptional regulation of miR-224 upregulated in human HCCs by NFkappaB inflammatory pathways. J Hepatol. 2012;56(4):855–861. doi: 10.1016/j.jhep.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 7.Zhou L, Yang ZX, Song W, Li QJ, Yang F, Wang DS. MicroRNA-21 regulates the migration and invasion of a stem-like population in hepatocellular carcinoma. Int J Oncol. 2013;43(2):661–669. doi: 10.3892/ijo.2013.1965. [DOI] [PubMed] [Google Scholar]
- 8.Siragam V, Rutnam J, Yang W, Fang L, Luo L, Yang X. MicroRNA miR-98 inhibits tumor angiogenesis and invasion by targeting activin receptor-like kinase-4 and matrix metalloproteinase-11. Oncotarget. 2012;11:1370–1385. doi: 10.18632/oncotarget.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Lee AT, Ma JZ, Wang J, Ren J, Yang Y. Profiling microRNA expression in hepatocellular carcinoma reveals microRNA-224 up-regulation and apoptosis inhibitor-5 as a microRNA-224-specific target. J Biol Chem. 2008;283(19):13205–13215. doi: 10.1074/jbc.M707629200. [DOI] [PubMed] [Google Scholar]
- 10.Rizos E, Siafakas N, Katsantoni E, Skourti E, Salpeas V, Rizos I, et al. mir-98 and mir-183 as biomarkers for cancer and schizophrenia. PLoS ONE. 2015;10:e0123522. doi: 10.1371/journal.pone.0123522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wendler A, Keller D, Albrecht C, Luso JJ, Wehling M. Involvement of let-7/miR-98 microRNAs in the regulation of progesterone receptor membrane component 1 expression in ovarian cancer cells. Oncol Rep. 2011;25:273–279. [PubMed] [Google Scholar]
- 12.Du Y, Li Y, Lv H, Zhou S, Sun Z, Wang M. miR-98 suppresses tumor cell growth and metastasis by targeting IGF1R in oral squamous cell carcinoma. Int J Clin Exp Pathol. 2015;8:12252–12259. [PMC free article] [PubMed] [Google Scholar]
- 13.Ni R, Huang Y, Wang J. miR-98 targets ITGB3 to inhibit proliferation, migration, and invasion of non-small-cell lung cancer. Onco Targets Ther. 2015;8:2689–2697. doi: 10.2147/OTT.S90998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li F, Li XJ, Qiao L, Shi F, Liu W, Li Y, et al. miR-98 suppresses melanoma metastasis through a negative feedback loop with its target gene IL-6. Exp Mol Med. 2014;46:e116. doi: 10.1038/emm.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang P, Chen S, Fang H, Wu X, Chen D, Peng L. miR-214/199a/199a* cluster levels predict poor survival in hepatocellular carcinoma through interference with cell-cycle regulators. Oncotarget. 2015;7(1):929–945. doi: 10.18632/oncotarget.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phatak P, Byrnes KA, Mansour D, Liu L, Cao S, Li R. Overexpression of miR-214-3p in esophageal squamous cancer cells enhances sensitivity to cisplatin by targeting survivin directly and indirectly through CUG-BP1. Oncogene. 2016;35(16):2087–2097. doi: 10.1038/onc.2015.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang JJ, Chen JT, Hua L, Yao KH, Wang CY. miR-98 inhibits hepatocellular carcinoma cell proliferation via targeting EZH2 and suppressing Wnt/β-catenin signaling pathway. Biomed Pharmacother. 2017;85:472–478. doi: 10.1016/j.biopha.2016.11.053. [DOI] [PubMed] [Google Scholar]
- 18.Zhou W, Zou B, Liu L, Cui K, Gao J, Yuan S, et al. MicroRNA-98 acts as a tumor suppressor in hepatocellular carcinoma via targeting SALL4. Oncotarget. 2016;7(45):74059–74073. doi: 10.18632/oncotarget.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Secombe JS, Pierce SB, Eisenman RN. Myc: a weapon of mass destruction. Cell. 2004;117:153–156. doi: 10.1016/S0092-8674(04)00336-8. [DOI] [PubMed] [Google Scholar]
- 20.Peng Z, Wang Y, Fan J, Lin X, Liu C, Ji WYX, Yan C, et al. Costunolide and dehydrocostuslactone combination treatment inhibit breast cancer by inducing cell cycle arrest and apoptosis through c-Myc/p53 and AKT/14-3-3 pathway. Sci Rep. 2017;7:41254. doi: 10.1038/srep41254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang G, Zhang X, Shi J. MiR-98 inhibits cell proliferation and invasion of non-small cell carcinoma lung cancer by targeting PAK1. Int J Clin Exp Med. 2015;8(11):20135–20145. [PMC free article] [PubMed] [Google Scholar]
- 22.Pathak S, Meng W, Nandy SK, Ping J, Bisgin A, Helmfors AP, et al. Radiation and SN38 treatments modulate the expression of microRNAs, cytokines and chemokines in colon cancer cells in a p53-directed manner. Oncotarget. 2015;6(42):44758–44780. doi: 10.18632/oncotarget.5815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yin G, Chen R, Alvero AB, Fu HH, Holmberg J, et al. TWISTing stemness, inflammation and proliferation of epithelial ovarian cancer cells through MIR199A2/214. Oncogene. 2010;29:3545–3553. doi: 10.1038/onc.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ueda T, Volinia S, Okumura H, Shimizu M, Taccioli C. Relation between microRNA expression and progression and prognosis of gastric cancer: a microRNA expression analysis. Lancet Oncol. 2010;11:136–146. doi: 10.1016/S1470-2045(09)70343-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Narducci MG, Arcelli D, Picchio MC, Lazzeri C, Pagani E. MicroRNA profiling reveals that miR-21, miR486 and miR-214 are upregulated and involved in cell survival in Sezary syndrome. Cell Death Dis. 2011;2:e151. doi: 10.1038/cddis.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Penna E, Orso F, Cimino D, Tenaglia E, Lembo A. MicroRNA-214 contributes to melanoma tumour progression through suppression of TFAP2C. EMBO J. 2011;30:1990–2007. doi: 10.1038/emboj.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qiang R, Wang F, Shi YL, Liu M, Chen S. Plexin-B1 is a target of miR-214 in cervical cancer and promotes the growth and invasion of HeLa cells. Int J Biochem Cell Biol. 2011;43:632–641. doi: 10.1016/j.biocel.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Zhang XJ, Ye H, Zeng CW, He B, Zhang H. Dysregulation of miR-15a and miR-214 in human pancreatic cancer. J Hematol Oncol. 2010;3:46. doi: 10.1186/1756-8722-3-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duan Q, Wang X, Gong W, Ni L, Chen C. ER stress negatively modulates the expression of the miR-199a/214 cluster to regulates tumor survival and progression in human hepatocellular cancer. PLoS ONE. 2012;7:e31518. doi: 10.1371/journal.pone.0031518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Derfoul A, Juan AH, Difilippantonio MJ, Palanisamy N, Ried T. Decreased microRNA-214 levels in breast cancer cells coincides with increased cell proliferation, invasion and accumulation of the Polycomb Ezh2 methyltransferase. Carcinogenesis. 2011;32:1607–1614. doi: 10.1093/carcin/bgr184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDonnell TJ, Korsmeyer SJ. Progression from lymphoid hyperplasia to high-grade malignant lymphoma in mice transgenic for the t (14; 18) Nature. 1991;349:254–256. doi: 10.1038/349254a0. [DOI] [PubMed] [Google Scholar]
- 32.Taylor WR, Stark GR. Regulation of the G2/M transition by p53. Oncogene. 2001;20:1803–1815. doi: 10.1038/sj.onc.1204252. [DOI] [PubMed] [Google Scholar]
- 33.Krock BL, Skuli N, Simon MC. Hypoxia-induced angiogenesis: good and evil. Genes Cancer. 2011;2(12):1117–1133. doi: 10.1177/1947601911423654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayes AJ, Huang WQ, Yu J, Maisonpierre PC, Liu A, Kern FG, et al. Expression and function of angiopoietin-1 in breast Cancer. Br J Cancer. 2000;83(9):1154–1160. doi: 10.1054/bjoc.2000.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J, Li J, Wang X, Zheng C, Maa W. Downregulation of microRNA-214 and overexpression of FGFR-1 contribute to hepatocellular carcinoma metastasis. Biochem Biophys Res Commun. 2013;439:47–53. doi: 10.1016/j.bbrc.2013.08.032. [DOI] [PubMed] [Google Scholar]


