Abstract
Risk-stratification is an essential management tool in defining prognosis of haematological neoplasms, both from patient and physician perspective. We define a new prognostic term “Dynamic Response Assessment Method(s) (DRAM)” as “method(s) used for re-stratifying disease prognosis at fixed intervals during the treatment course”. The risk stratification is done after a fixed duration of treatment or chemotherapy cycles using sensitive techniques. The information obtained then can be used for further therapeutic decisions and prognostication. Currently, there is enough evidence that response to treatment improves the prognostic value of baseline disease variables in the management of Chronic Myeloid Leukemia, Hodgkin lymphoma, Diffuse Large B cell Lymphoma, and Multiple Myeloma. Through this review, we discuss the current evidence based application of “DRAM” to guide therapeutic decisions in these malignancies. We also discuss how the results of “DRAM” can be incorporated for redefining prognosis and counselling the patients with these selected hematologic malignancies.
Keywords: Prognosis, Risk-stratification, Chronic Myeloid Leukemia, Hodgkin lymphoma, Diffuse large B-cell lymphoma, Multiple Myeloma
Introduction
Risk-stratification and prognostication of patients with various haematological malignancies is conventionally performed at the time of initial diagnosis. However, with increasing experience in treating these disorders, it has been realized that response to treatment is one of the strongest prognostic factors, and can potentially re-stratify these patients into different risk groups. We define “method(s) used for re-stratifying disease prognosis at fixed intervals during the treatment course” as Dynamic Response Assessment Method(s).The strategy uses sensitive techniques to estimate the depth of response and this information is used for making therapeutic decisions and prognostication. Data has shown that the results of DRAM overpower the prognostic impact of baseline prognostic markers in certain hematologic malignancies. A clear understanding of the prognosis by the patients or their caregivers is of utmost importance in making informed decisions. Up to 77.6% patients with hematologic malignancies have a discordant understanding of their prognosis as compared to their physician even in the pre-transplant setting [1]. The results of DRAM can be used for re-defining prognosis of patients on follow-up, and therefore contribute towards patient-centric approach for decision making. Although response criteria have been defined for most hematologic malignancies, uniform use of DRAM is limited by considerable heterogeneity in the timing and techniques used. DRAM-based treatment algorithms have been successfully incorporated in the prognostication and management of Chronic Myeloid Leukemia (CML) and Hodgkin Lymphoma (HL), while their role in Diffuse Large B-cell lymphoma (DLBCL), and Multiple Myeloma (MM) continues to evolve. We review the current evidence, and basis of using DRAM for the management of CML, HL, DLBCL, and MM.
Chronic Myeloid Leukemia
CML is one disease that best elucidates the role of DRAM based on robust long-term data. Sokal score for baseline prognostication in CML was derived from patient cohorts treated with chemotherapy and Hasford score was derived from patient cohorts treated with Interferon [2, 3]. As Sokal and Hasford metrics failed to predict treatment success and failures with adequate sensitivity and specificity in patients receiving imatinib, EUTOS score was derived in 2011 [4]. However, Jabbour et al. and Marin et al. showed no difference in rates of Major Molecular Remission (MMR), Treatment Free Survival (TFS),Event Free Survival (EFS), or Overall Survival (OS) between EUTOS-defined low-risk and high-risk patients [5, 6]. Moreover these scores have limited discriminatory capacity for predicting outcomes in patients treated with highly effective second-generation tyrosine kinase inhibitors (2nd Gen-TKIs) as these drugs improve outcomes across all baseline risk groups [7].Therefore in chronic malignancies like CML with prolonged survival, baseline prognostic scores have limited significance after 3–6 months of therapy whereas dynamic response assessment variables are strong surrogates of survival and transformation. DRAM for CML include hematological, cytogenetic and molecular tests done at specified time-points to assess the rate of decline or disappearance of BCR-ABL transcript and/or emergence of resistance to TKI [8]. World Health Organization (WHO) 2016 classification of myeloid neoplasms introduced ‘provisional’ definition of accelerated phase CML (CML-AP) based on treatment-response and emergence of kinase-domain (KD) mutations while on TKI therapy [9]. Survival of CML patients now parallels the general population, therefore the goals of therapy in the current era are not only restricted to the attainment of 12-month complete cytogenetic remission (CCyR), but also include treatment-free remission (TFR), avoidance of long-term toxicities associated with prolonged TKI use and reduction of the financial burden [10]. DRAM in CML could help identify early treatment failures/suboptimal responders, and allow early therapy modification.
DRAM Based on Specific Time Points
In CML, both European Leukemia Network (ELN) and National Comprehensive Cancer Network (NCCN) treatment milestones at specific time points (3, 6 and 12 months) exemplify the use of DRAM in clinical practise [11, 12]. Early molecular response (EMR) defined as BCR-ABL1 < 10% by RQ-PCR on international scale at 3 months is the best dynamic prognosticator in CML, both in the front-line and second-line TKI setting [13]. EMR failure after frontline imatinib seen in 24–28% cases is associated with poor progression-free survival (PFS) and OS, although it is not considered treatment failure by either ELN or NCCN guidelines [14]. The concept of halving time (i.e. time taken for 50% reduction of BCR-ABL1 from the baseline) may allow better prognostication of patients with CML within the initial 3-month time period. Patients with EMR failure but short having time have a better PFS and OS as compared to those with a longer halving time (> 76 days) [15]. We have previously reported “Complete Hematologic Response (CHR) velocity” as a prognostic indicator that independently predicts EMR at 3 months in chronic phase CML (CML-CP) patients receiving Imatinib [16]. Change of therapy based on EMR failure is debatable, due to a lack of proven OS benefit although it is known to improve CCyR and MMR rates [17]. Achievement of MMR at 12 months is considered ‘optimal’ response and correlates with improved PFS and OS across most studies [12]. Failure to attain CCyR is seen in ~ 30% of patients at 12-months and is considered TKI failure [17]. Change of therapy to 2nd-generation TKI for patients in CCyR but not in MMR fails to provide PFS and OS benefit, although it leads to deepening of the molecular responses, thereby increasing the eligibility rates for TKI-stopping trials [18].
DRAM for TKI Resistance
DRAM can been used for timely identification of TKI failure. Primary TKI failure is defined as the “failure” to achieve specified milestones after 3,6 and 12 months of therapy, while secondary TKI failure is defined as loss of previously achieved response i.e. CHR, CCyR or molecular remission, latter being defined as > 1 log increase in BCR-ABL1 levels confirmed on 2 occasions, 4–6 weeks apart [11]. TKI failure is either a result of clonal evolution, acquisition of KD Mutation or consequent to lack of compliance or intolerance to TKI [19]. Accordingly, WHO 2016 included “the emergence of clonal evolution or > 2 mutations in KD while on frontline TKI” in the provisional definition of CML-AP [9]. Overall, Imatinib failure has been reported in 26–45% patients while 37–52% patients fail to have a response with Dasatinib and Nilotinib [17]. A diagnosis of primary TKI failure confers an inferior prognosis and warrants repeat bone marrow aspiration with cytogenetics, KD mutation testing with subsequent change in therapy and HLA-typing of patient and siblings [17].
DRAM to Consider TKI Discontinuation
DRAM help in the identification of patients attaining sustained deep molecular responses (DMR, > MR4.5) on TKI. Such patients are eligible for a potential trial of TKI discontinuation known as Treatment Free Remission (TFR). The upfront use of 2nd Gen-TKIs (Nilotinib, Dasatinib) in CML-CP has failed to demonstrate an OS benefit compared to frontline imatinib, however, their use leads to earlier attainment of DMR, and consequently increases the eligibility for TFR trial [20, 21]. Given the possible detrimental effects of TKI’s on foetus, a TFR trial could allow for the safe continuation of pregnancy. DRAM based intensive molecular monitoring (monthly for the first year, 2-monthly for 2nd year and 3- monthly after that) forms the basis of TFR.TFR rates vary between 40 and 50%, if a loss of DMR is used to define recurrence and up to 60% at 12 months, if recurrence is defined as MMR loss [22–24]. Besides identifying eligible candidates for a TFR trial, DRAM could help predict the success of TFR. The ability to maintain DMR, in the first 6-months after TFR initiation is an important predictor of the TFR success, as most of the recurrences occur in that period, and late ‘relapses’ are unusual. In case of recurrence, MMR is successfully achieved in almost all the patients following TKI re-initiation, and disease progression is rare [25]. High TFR rates have been recorded in patients on frontline 2nd -generation TKI's [26–29]. Loss of MR4.5 after 3-months of TKI cessation predicted for TFR failure in the STOP-2G TKI study [26]. Besides, DRAM has been utilised in the setting of second TFR trial following recurrence on the first trial. RE-STIM trial attempted a second TFR (recurrence defined as loss of MMR) and showed that persistence of undetectable molecular disease (> MR4.5) at 6-months after the first TKI discontinuation was associated with a longer second TFR versus those who had an early recurrence within 6-months (24-month TFR probabilities: 72% and 36%, respectively) [30]. As the confidence in TFR is increasing, de-escalation of therapy (either dose-reduction or switch from 2nd -generation TKI to imatinib) may also be feasible at responses deeper than MMR [26].
Learning Point
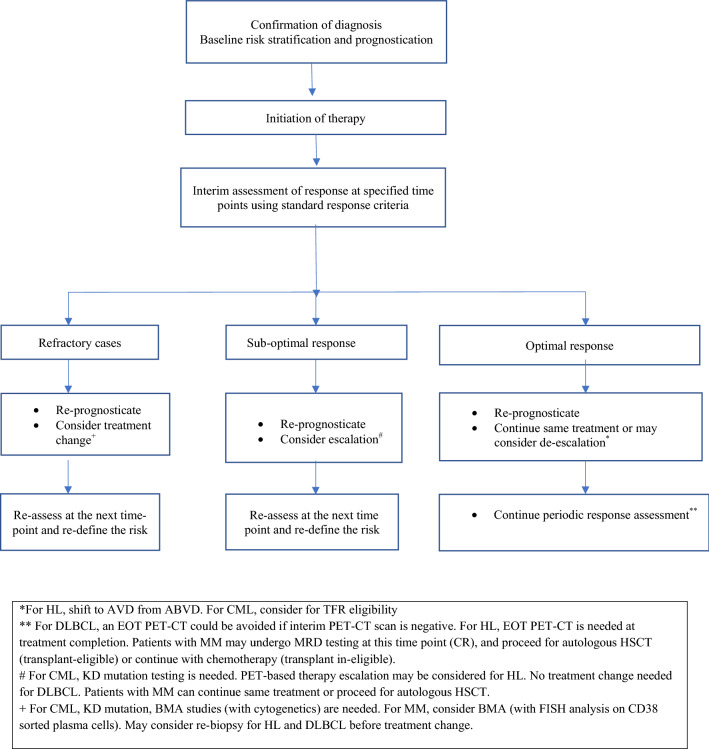
Baseline risk models provide useful information for initial prognostication of patients with CML. There is robust evidence to use DRAM for tailoring therapy and identifying both poor-risk patients with early treatment failures, who need treatment intensification; and good-risk patients, who could be offered a TFR trial. We re-inforce DRAM-based periodic counselling and therapy modification in patients of CML in-keeping with the current NCCN and ELN recommendations [11, 12] (Table 1, Fig. 1).
Table 1.
Application of DRAM in management of hematologic malignancies
| Disease | Suggested DRAM | Time points for DRAM | DRAM based treatment change | Remarks |
|---|---|---|---|---|
|
1a. Early HL |
FDG18 PET-CT |
Interim scan (PET-2) |
Escalation (PET-2 + ve): ABVD to BEACOPPescalated De-escalation (PET-2–ve): omit consolidative IFRT |
1. Improved PFS (77% to 91%) and OS with escalation 2. OS same, 3–4% reduced PFS with RT omission |
| 1b. Advanced HL | FDG18 PET-CT |
Interim scan (PET-2) |
Escalation (PET-2 + ve): ABVD to BEACOPPescalated De-escalation (PET-2–ve): 1. ABVD to AVD 2. BEACOPPescalated to ABVD |
1. Improved PFS (3-year-68%, 2-year PFS-66%) compared to historic controls (PFS-15%) with escalation 2. No difference in OS with de-escalation 3. Similar PFS (94% vs. 92%) with de-escalation |
| 1c.Pre-ASCTHL | FDG18 PET-CT | At relapse and after 2–3 cycles of ST | Brentuximab consolidation post-ASCT | 3-year PFS benefit (68% vs. 41%), OS same |
| 3. CML | Haematological, cytogenetic (karyotype and FISH) & molecular remission assessment (measurement of BCR-ABL1protein), KD mutation | 3 monthly till MMR then 3–6 monthly |
1. 3-month: > 95%Ph + or no CHR- 10 TKI failure 2. 6-month: BCR-ABL1 > 10%- 10 TKI failure 3. 12-month: No CCyR- 10 TKI failure 4. loss of previous response/> 1 log increase in BCR-ABL1 (2-occasions)- 20 TKI failure 10 or 20 TKI failure: BM, CG and KD mutation 5. sustained DMR (variable defined): TFR trial 6. stable MMR: de-escalation (dose-reduction or switch from 2nd-line TKI to imatinib) |
Within 3-month time zone 1. EMR failure (3-month BCR-ABL1 > 10%): may change TKI (NCCN, not ELN), shorter PFS 2. EMR with halving time (> 76 days): shorter PFS |
| 4. DLBCL | FDG18 PET-CT |
1. After 4 cycles 2. End of treatment (after 6 cycles) 3. Pre-transplant in R/R cases |
1. Negative iPET or EOT PET confers good prognosis 2. EOT PET may be omitted in patients with negative iPET |
1. Therapy escalation based on iPET positivity doesn’t transform into survival benefit |
| 5. Multiple Myeloma |
1. Monoclonal Protein assessment (SPEP,SIFE,SFLC) 2. MRD using Bone Marrow NGF or sequencing 3. FDG18PET-CT |
1. Monoclonal protein assessment every 2 months 2. MRD assessment pre-transplant 3. WB PET CT scan pre transplant 4. Monoclonal protein assessment, MRD and PET-CT at day + 100 post- transplant |
1. Response better than PR are eligible for transplant after 4–6 cycles 2. Response less than PR at 4 cycles warrant therapy change 3. Response CR or better guide shift to maintenance post-transplant |
1. MRD negativity is strongest predictor of PFS |
Fig. 1.
Proposed Algorithm for use of DRAM in the management of Hematologic Malignancies
Hodgkin Lymphoma
HL is considered a potentially curable haematological malignancy with a cure rate of 90–95%. Five to ten percent of HL patients have a relapsed/refractory (RR) disease [31]. In patients with HL, DRAM predominantly rely on demonstration of chemo-sensitivity through 18[F] FDG PET-CT based 5-point Deauville score (DS). Such a strategy has been proven to overcome the prognostic impact of conventional imaging as well as baseline prognostic markers like International Prognostic score (IPS). Although efforts have been made to improve the negative predictive value (NPV) of PET-CT by performing it after the first cycle, PET-2 remains the most validated approach [32]. Retrospective studies show a significant difference in the PFS (95% vs. 13%) in patients of HL based on PET-2 negativity and positivity, respectively [33]. Treatment modifications based on PET-2 have been tested in randomized studies in both early and advanced HL, and are discussed below.
Early HL
Traditionally, combined modality treatment has been the standard therapy for early HL [34]. Given the concern of long-term radiation toxicity, de-escalation of therapy based on an interim PET scan (iPET) has been attempted in early HL by omitting consolidation radiotherapy (RT). RAPID trial randomized early favourable HL to receive or omit involved field radiotherapy (IFRT) based on PET-3 negativity [35]. Similarly, EORTC HD10 study randomized favourable and unfavourable early HL patients to omit IFRT after therapy completion (4 cycles of ABVD for favourable and 6 cycles for unfavourable HL) based on a negative PET-2 [36]. Both the trials demonstrated a small reduction of PFS with the omission of IFRT with no difference in the OS. The lack of difference in OS was likely due to the availability of effective salvage regimens and autologous stem cell transplantation (ASCT). Patients must, therefore, be re-counselled after a negative iPET regarding the risks and benefits of omitting RT and the decision must be individualized. PET 2 positivity is seen in ~ 19% of early HL patients. DRAM based escalation approach after a positive PET-2 is based on the fact that early treatment intensification after demonstration of chemo-refractoriness can reduce the treatment-related mortality (TRM) associated with the salvage regimens and ASCT. In the randomized EORTC HD10 study, intensification to 2 cycles of BEACOPPescalated after a positive PET-2 following standard ABVD led to an increase in 5-year PFS from 77 to 91% as compared to continuation of ABVD [37]. This implies that although early HL has a good prognosis, about 19% of them who are PET 2 positive after 2 cycles of ABVD regimen need a risk re-stratification, re-prognostication, as well as treatment escalation based on iPET result.
Advanced HL
Chemotherapy alone is the therapy of choice in advanced HL with a cure rate of 90% [34]. The use of DRAM to tailor therapy based on pre-defined risk and iPET results has proven to be useful in advanced HL. De-escalation strategies based on PET-2 results aim to reduce the chemo-toxicity of treatment without compromising the overall outcome. Two approaches in this context have been evaluated in randomized trials. The first approach (RATHL study) randomized patients of advanced HL to AVD arm (4 cycles) or ABVD arm (total 6 cycles) after a negative PET-2 following 2 cycles of ABVD. Omission of bleomycin (AVD arm) did not affect OS in these patients [38]. Second approach (LYSA AHL 2011 study) randomized patients of advanced HL to ABVD (4 cycles) or continuation of BEACOPPescalated (total 6 cycles) after a negative iPET following 2 cycles of frontline BEACOPPescalated and demonstrated a similar 2-year PFS with either arm (92% and 94% respectively) [39]. In the Israeli H2 Study, no difference in PFS was found as per IPS score while 3 years PFS varied significantly in PET 2 positive versus PET 2 negative patients (75% vs. 86%; p = 0.012) with advanced HL. Again de-escalation from BEACOPPescalated to ABVD in PET 2 negative patients was safe and did not affect the outcome [40].
These data illustrate that therapy de-escalation is feasible in advanced HL based on the negative iPET. Patients need to be re-counselled after interim DRAM results regarding the possible benefit of reduced chemo-toxicity without compromising the long-term outcome using this approach. Historically, the outlook of ~ 16% of patients who have a positive iPET is poor (PFS-15%), therefore therapy escalation from ABVD to BEACOPP has not been evaluated in randomized trials [33]. Studies have shown an improvement in PFS with therapy escalation in patients with advanced HL who have a positive PET-2. RATHL study demonstrated a 3-year PFS of 68% with escalation to BEACOPPescalated after 2 cycles of ABVD and GITIL/FIL 0607 trial showed a 2-year FFS of 66% with escalation to 4 cycles of BEACOPPescalated + 4 cycles of BEACOPP baseline after 2 cycles of ABVD [38].
Pre-transplant 18FDG PET-CT
Salvage Therapy (ST) followed by ASCT remains the standard of care for RR HL patients with an OS ranging from 25 to 75% depending on the pre-transplant PET-CT scan result [41]. Pre-ASCT PET-CT remains the most important prognostic marker for PFS as well as OS due to its ability to demonstrate chemo-sensitivity to the ST [42]. Moreover, PET-CT pre-ASCT may be used to guide the decision regarding post-ASCT consolidation. Aethera Trial showed that Brentuximab Vedotin (BV) consolidation (1.8 mg/kg every 3 weeks for 16 cycles) post-ASCT in patients with extranodal disease at relapse or less than complete remission pre-ASCT improves 3-year PFS compared to placebo (61% vs. 43%), albeit without any OS benefit [43].Therefore, patients of RR-HL planned for an autograft may be re-stratified again based on the result of the pre-transplant PET-CT scan. A positive scan in such a case would place these patients at a higher risk of relapse post-ASCT and would justify the use of BV consolidation post-ASCT.
Learning Point
Outcomes of HL can be further improved by using DRAM-tailored approach. Interim imaging using PET-CT scan done after 2 cycles of ABVD, and before autologous HSCT in RR HL redefine the prognosis and also guide therapy modification independent of the IPS score at the baseline and is currently the preferred approach for DRAM in patients with HL (Fig. 1, Table 1).
Diffuse Large B Cell Lymphoma
DLBCL is the most common subtype of Non Hodgkin lymphoma and is potentially curable in about 60–70% cases with the frontline chemo-immunotherapy, while ~ 40% of cases have relapse/refractory disease [44]. Several baseline prognostic markers have been validated in DLBCL to predict the clinical outcome, both in the pre-Rituximab era (IPI) as well as in the Rituximab era (R-IPI, NCCN-IPI). Prognostic models based on gene-expression profile and immune-histochemistry have identified subsets of DLBCL (cell-of-origin subtypes- activated B-cell type, germinal centre type as well as double-hit/expressers and triple-hit/expressers) to account for the varied response to R-CHOP therapy [45]. However, responses continue to be heterogeneous within baseline risk groups. While IPI, R-IPI, and NCCN-IPI are clinically useful prognostic indices, incorporation of iPET may improve their prognostic value in predicting 2 year PFS and OS, particularly in patients with a high IPI score [46]. The use of DRAM in DLBCL is a promising approach to identify patients with early treatment failure who are likely to have a worse outcome than those who show an early favourable response.
Interim PET-CT
18[F] FDG PET-CT is used extensively in DLBCL, both for baseline staging as well as a DRAM after 2–4 cycles of chemotherapy and at the end-of-treatment (EOT) [47]. While iPET has proven to be of significant prognostic value, its clinical utility in therapy modification is debatable. In a prospective study (n = 203) analysing the utility of PET-CT in DLBCL performed at baseline, after every 2 cycles, and at the EOT, PET-2 positive patients had a poorer outcome as compared to PET-2 negative patients, and PET-4 positive patients had a poorer outcome as compared to PET-4 negative patients in terms of both 3-year PFS and OS. The outcome of PET-2 negative (early responders) and PET-4 negative patients (late responders) was similar, and about 50% of PET-2 positive patients became PET-4 negative. PET-4 positivity was associated with the worst prognosis and was an independent prognostic factor indicating that a positive PET-4 is associated with an inferior outcome, and identifies a subgroup of high-risk patients with a greater risk of relapse [48]. However, the strategy of early-intensification based on a positive iPET has not translated into improved clinical outcome and remains a research question. In a multicentre phase-II study of 162 patients, treatment intensification (3 cycles of RICE followed by autologous HSCT with Zevalin-BEAM) in PET-4 positive patients after RCHOP-14 led to a similar PFS and OS compared to PET-4 negative patients [49]. Many other studies have also consistently demonstrated the futility of changing therapy based on a positive iPET, unless there is a clear evidence of disease progression [50]. This is because half of the iPET-positive patients achieve EOT PET-negativity with continuation of frontline R-CHOP. Treatment of all iPET positive cases with an intensive chemotherapy regimens would not only result in over-treatment of a significant percentage of patients, but also increases treatment toxicity without an appreciable improvement in the clinical outcomes [50]. Since, most of the iPET-negative patients remain PET-negative at the EOT, EOT-PET can be avoided for patients with a negative interim scan. A combined study of iPET and EOT PET-CT showed that patients with negative iPET and EOT PET-CT have the best outcome (2-year EFS- 97%), whereas those in whom both scans are positive have the worst outcome (2-year EFS-35%) [51]. In a recent meta-analysis aimed to assess the prognostic impact of iPET on PFS or EFS in DLBCL patients treated with first-line immuno- chemotherapy regimens, the negative predictive value for progression generally exceeded 80% (64–95%), but sensitivity (33–87%), specificity (49–94%), and positive predictive values (20–74%) ranged widely [52].
End of Treatment PET-CT
EOT PET-CT remains the most important landmark for dynamic risk-stratification of patients with DLBCL. A positive EOT PET-CT (Deauville Score 4 or 5) confers a poor 2 year PFS (24–35%), whereas a negative scan has a high negative predictive value, with most of the EOT PET-CT negative patients enjoying a long-term remission or cure [53]. However, certain patients with a negative EOT PET-CT still relapse, suggesting that baseline NCCN-IPI and R-IPI remain prognostic in DLBCL, irrespective of the result of EOT PET-CT scan [54].
Pre-transplant PET-CT
Response to ST assessed with PET-CT pre-ASCT has been shown to predict the post-ASCT outcome. A positive PET-CT pre-ASCT (DS 4 or more) has been associated with a poorer outcome (3-year PFS-0% and 3-year OS-25%) compared to a negative scan (3-year PFS-64% and 3-year OS-84%) [55]. Dynamic response assessment based on pre-transplant PET-CT scan can identify a subgroup of high-risk patients who could potentially benefit from novel therapeutic approaches rather than HSCT.
Learning Point
DLBCL constitutes a heterogeneous disease with variable outcomes based on several baseline parameters. Using iPET after 4 cycles as a DRAM, a subgroup of iPET 4 negative patients who are likely to have a favourable outcome can be identified. However, considering the poor positive predictive value and lack of proven survival benefit, therapy escalation based on iPET should be considered only under a clinical trial setting or clear evidence of progression. A negative iPET may obviate the need of an EOT PET-CT scan, especially in resource constraint settings. While EOT PET-CT remains a strong DRAM, it doesn’t completely negate the significance of baseline prognostic markers (R-IPI, NCCN-IPI), unlike the case with HL. Until further research identifies a better methods to assess response changes in treatment strategy based on interim imaging are highly contentious (Fig. 1, Table 1).
Multiple Myeloma
The current treatment algorithm in MM relies on baseline risk-stratification based on patient-related and disease-related factors incorporated in the RISS: serum albumin, beta 2-microglobulin, lactate dehydrogenase, and high-risk cytogenetic abnormalities, in addition to others [56]. Quantification of monoclonal protein in response to therapy is a strong prognostic factor in MM and forms the conventional DRAM. Primary refractory disease is defined as the attainment of less than partial response (PR) after 4 cycles of triplet-based induction therapy, and is seen in ~ 17% cases. Patients with primary refractory MM have a significantly inferior OS and PFS as compared to those who attain CR [57]. Since none of the traditional baseline risk factors used to define high-risk MM can identify primary refractoriness, and the majority of patients in CR and stringent CR eventually relapse, techniques of dynamic response assessment have resulted in a paradigm shift in patients with MM over the last decade. Conventional DRAM milestones namely, CR and stringent CR used to guide treatment decisions are far from perfect in prognosticating patients with MM as they fail to detect minimal residual disease (MRD). Around 15–30% of patients in CR are MRD-positive by multi-colored flow cytometry (MFC). Patients with ‘stringent’ CR could still harbour about 1 × 108 myeloma cells [58]. Multiple studies including meta-analysis have consistently shown that MRD-negativity strongly correlates with an improved PFS and OS, while MRD-positivity is associated with an un-sustained CR [59]. Considering the strong prognostic impact of depth of response on outcome, International Myeloma Working Group (IMWG) updated the definitions of response, progression, relapse, MRD-negativity and relapse from MRD-negative state in MM [60]. Moreover, functional imaging using PET-CT or diffusion weighted Magnetic Resonance Imaging has been shown to be complimentary to MFC in MRD assessment, with MRDnegative/PETnegative patients having the best clinical outcome [61]. Since the therapeutic armamentarium of MM is expanding, it is now possible to obtain deeper and sustained responses in MM in a significant proportion of patients. Therefore, the current therapeutic strategies in MM aim at achieving deeper responses- immunophenotypic/molecular CR, and image CR (negative PET-CT) representing eradication of the residual disease in medullary and extramedullary compartments, respectively and form basis for the recent additions in the DRAM for MM [62].
Using DRAM for Current Therapy in MM
Primary refractoriness after 4 cycles of triplet-based induction therapy merits a change in therapy, while patients with PR or better response may continue with same treatment or proceed to auto HSCT [57]. In patients who undergo auto-HSCT, Day + 100 is the best validated time point for the assessment of depth of response using conventional and MRD-driven techniques, including PET-CT and correlates with survival [58]. Previously three trials showed improvement in PFS with post-ASCT consolidation in patients with < CR at Day + 100 [63]. However, recent results of the BMT-CTN 0702 trial suggested that the addition of consolidation or a second tandem auto-HSCT was not superior to a single ASCT followed by lenalidomide maintenance in the upfront treatment of MM for both PFS and OS [64]. In transplant-ineligible patients, continuous therapy until the development of dose limiting toxicity/progression is known to improve outcomes. However, the results of DRAM after every 2–4 cycles could help guide the detecting loss of response or progression and subsequent change of therapy while sustained response guides shift to maintenance [65, 66].
Learning Point
DRAM in MM have evolved from an era of using conventional criteria to define CR and stringent CR to the inclusion of MRD for prognostication. It is likely that the combination of MFC or next-generation sequencing (NGS), and PET-CT scan that is reflective of a state of complete eradication of myeloma cells from all the compartments would be the preferred DRAM in identifying patients with excellent prognosis in near future. However the use of these sensitive techniques for therapy escalation, continuation or de-escalation (including omission of ASCT in MRD negative patients) needs further prospective validation and should be considered experimental at present (Fig. 1, Table 1).
Cost Considerations in Deciding DRAM
Routine incorporation of any novel DRAM requires a careful consideration of the cost versus benefit assessment in comparison to the conventional techniques, particularly in the resource constraint setting. Lifelong dynamic response assessment using molecular monitoring is advocated for routine clinical care in patients with CML. Beyond the initial 6 months period,the frequency of monitoring of BCR-ABL1 by RQ-PCR on international scale can be reduced from 3 to 6 months once patient attains MMR thereby reducing the costs [67]. Cost effectiveness of TFR approach in CML needs prospective validation. Cost effectiveness of PET/CT in patients with HL for patients with unconfirmed CR or PR at the end of first line therapy has been proven in resource constraint settings [68]. It is possible that the use of interim PET/CT for therapy escalation or de-escalation in HL would prove cost effective as well. In patients with DLBCL, the strategy of avoiding EOT PET/CT in patients with negative iPET reduces the PET/CT utilization rate by 30%, without impacting the clinical outcome, and appears to be cost effective [69]. Recently, annual MRD monitoring in maintenance phase of MM was predicted to be a cost conservative strategy if patients were assumed to stop therapy based on a negative MRD result [70]. Further prospective studies are needed to determine the cost effectiveness as well as health outcomes of using MRD as DRAM in patients with MM.
Conclusion
The results of DRAM have a major clinical implication in the treatment and prognosis of hematological malignancies. Newer technologies have allowed for better and deeper response assessment. It is still unclear if currently available therapies can be modified as per the results of DRAM in all the hematological malignancies, as robust data are available only for some diseases. Whilst the results of DRAM often alter the baseline risk-stratification in hematologic malignancies, the use of DRAM for counselling, prognostication and therapy modification varies according to the disease, methodology of investigation and the available clinical evidence.
Author Contributions
AJ and AJ contributed equally to the draft. PM was involved in draft conception and supervision. All the authors reviewed the manuscript before submission.
Funding
None.
Compliance with Ethical Standards
Conflict of interest
Authors have no conflict of interests to declare.
Ethical Approval
The authors state that the study did not involve any patient or animal participation.
Human and Animal Rights
The article does not involve any study with participation of humans or animals by the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.El-Jawahri A, Traeger L, Kuzmuk K, Eusebio J, Vandusen H, Keenan T, et al. Prognostic understanding, quality of life and mood in patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant. 2015;50(8):1119–1124. doi: 10.1038/bmt.2015.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sokal J, Cox E, Baccarani M, Tura S, Gomez G, Robertson J, et al. Prognostic discrimination in chronic granulocytic leukemia. Blood. 1984;63(4):789–799. [PubMed] [Google Scholar]
- 3.Hasford J, Pfirrmann M, Hehlmann R, Allan NC, Baccarani M, Kluin-Nelemans JC, et al. A new prognostic score for survival of patients with chronic Myeloid Leukemia treated with interferon alfa writing committee for the collaborative CML prognostic factors project group. JNCI J Natl Cancer Inst. 1998;90(11):850–859. doi: 10.1093/jnci/90.11.850. [DOI] [PubMed] [Google Scholar]
- 4.Hasford J, Baccarani M, Hoffmann V, Guilhot J, Saussele S, Rosti G, et al. Predicting complete cytogenetic response and subsequent progression-free survival in 2060 patients with CML on imatinib treatment: the EUTOS score. Blood. 2011;118(3):686–692. doi: 10.1182/blood-2010-12-319038. [DOI] [PubMed] [Google Scholar]
- 5.Jabbour E, Cortes J, Nazha A, O’Brien S, Quintas-Cardama A, Pierce S, et al. EUTOS score is not predictive for survival and outcome in patients with early chronic phase chronic myeloid leukemia treated with tyrosine kinase inhibitors: a single institution experience. Blood. 2012;119(19):4524–4526. doi: 10.1182/blood-2011-10-388967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marin D, Ibrahim AR, Goldman JM. European treatment and outcome study (EUTOS) Score for chronic Myeloid Leukemia still requires more confirmation. J Clin Oncol. 2011;29(29):3944–3945. doi: 10.1200/JCO.2011.37.6962. [DOI] [PubMed] [Google Scholar]
- 7.Hu B, Savani BN. Impact of risk score calculations in choosing front-line tyrosine kinase inhibitors for patients with newly diagnosed chronic myeloid leukemia in the chronic phase. Eur J Haematol. 2014;93(3):179–186. doi: 10.1111/ejh.12356. [DOI] [PubMed] [Google Scholar]
- 8.Mahon F-X. Treatment-free remission in CML: who, how, and why? Hematology. 2017;2017(1):102–109. doi: 10.1182/asheducation-2017.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 10.Bower H, Björkholm M, Dickman PW, Höglund M, Lambert PC, Andersson TM-L. Life expectancy of patients with chronic Myeloid Leukemia approaches the life expectancy of the general population. J Clin Oncol. 2016;34(24):2851–2857. doi: 10.1200/JCO.2015.66.2866. [DOI] [PubMed] [Google Scholar]
- 11.Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122(6):872–884. doi: 10.1182/blood-2013-05-501569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Brien S, Radich JP, Abboud CN, Akhtari M, Altman JK, Berman E, et al. Chronic Myelogenous Leukemia, version 1.2014. J Natl Compr Canc Netw. 2013;11(11):1327–1340. doi: 10.6004/jnccn.2013.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hughes TP, Saglio G, Kantarjian HM, Guilhot F, Niederwieser D, Rosti G, et al. Early molecular response predicts outcomes in patients with chronic myeloid leukemia in chronic phase treated with frontline nilotinib or imatinib. Blood. 2014;123(9):1353–1360. doi: 10.1182/blood-2013-06-510396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrington P, Kizilors A, de Lavallade H. The role of early molecular response in the management of chronic phase CML. Curr Hematol Malig Rep. 2017;12(2):79–84. doi: 10.1007/s11899-017-0375-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Branford S, Yeung DT, Parker WT, Roberts ND, Purins L, Braley JA, et al. Prognosis for patients with CML and & < 10% BCR-ABL1 after 3 months of imatinib depends on the rate of BCR-ABL1 decline. Blood. 2014;124(4):511–518. doi: 10.1182/blood-2014-03-566323. [DOI] [PubMed] [Google Scholar]
- 16.Malhotra P, Chikkodi SV, Naseem S, Khadwal A, Prakash G, Kumari S, et al. CHR Velocity as a predictor of early molecular response in patients with chronic myeloid leukemia. J Clin Oncol. 2015;33(15_suppl):e18038. doi: 10.1016/j.clml.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 17.Barnes G. Burden of tyrosine kinase inhibitor failure in patients with chronic Myeloid Leukemia. J Leuk. 2015;3(1):170. [Google Scholar]
- 18.Hughes TP, Lipton JH, Spector N, Cervantes F, Pasquini R, Clementino NCD, et al. Deep molecular responses achieved in patients with CML-CP who are switched to nilotinib after long-term imatinib. Blood. 2014;124(5):729–736. doi: 10.1182/blood-2013-12-544015. [DOI] [PubMed] [Google Scholar]
- 19.Barnes G. Burden of tyrosine kinase inhibitor failure in patients with chronic Myeloid Leukemia. J Leuk. 2015;3(1):170. [Google Scholar]
- 20.Cortes JE, Saglio G, Kantarjian HM, Baccarani M, Mayer J, Boqué C, et al. Final 5-year study results of DASISION: the dasatinib versus imatinib study in treatment-naïve chronic Myeloid Leukemia patients trial. J Clin Oncol. 2016;34(20):2333–2340. doi: 10.1200/JCO.2015.64.8899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hochhaus A, Saglio G, Hughes TP, Larson RA, Kim D-W, Issaragrisil S, et al. Long-term benefits and risks of frontline nilotinib vs. imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30(5):1044–1054. doi: 10.1038/leu.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Etienne G, Guilhot J, Rea D, Rigal-Huguet F, Nicolini F, Charbonnier A, et al. Long-term follow-up of the french stop imatinib (STIM1) study in patients with chronic Myeloid Leukemia. J Clin Oncol. 2017;35(3):298–305. doi: 10.1200/JCO.2016.68.2914. [DOI] [PubMed] [Google Scholar]
- 23.Ross DM, Branford S, Seymour JF, Schwarer AP, Arthur C, Yeung DT, et al. Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: results from the TWISTER study. Blood. 2013;122(4):515–522. doi: 10.1182/blood-2013-02-483750. [DOI] [PubMed] [Google Scholar]
- 24.Campiotti L, Suter MB, Guasti L, Piazza R, Gambacorti-Passerini C, Grandi AM, et al. Imatinib discontinuation in chronic myeloid leukaemia patients with undetectable BCR-ABL transcript level: a systematic review and a meta-analysis. Eur J Cancer. 2017;77:48–56. doi: 10.1016/j.ejca.2017.02.028. [DOI] [PubMed] [Google Scholar]
- 25.Mahon F, Richter J, Guilhot J, Hjorth-Hansen H, Almeida A, Janssen JJWMJ, et al. Cessation of tyrosine kinase inhibitors treatment in chronic Myeloid Leukemia patients with deep molecular response: results of the Euro-Ski trial. Blood. 2016;128(22):787. [Google Scholar]
- 26.Rea D, Nicolini FE, Tulliez M, Guilhot F, Guilhot J, Guerci-Bresler A, et al. Discontinuation of dasatinib or nilotinib in chronic myeloid leukemia: interim analysis of the STOP 2G-TKI study. Blood. 2017;129(7):846–854. doi: 10.1182/blood-2016-09-742205. [DOI] [PubMed] [Google Scholar]
- 27.Imagawa J, Tanaka H, Okada M, Nakamae H, Hino M, Murai K, et al. Discontinuation of dasatinib in patients with chronic myeloid leukaemia who have maintained deep molecular response for longer than 1 year (DADI trial): a multicentre phase 2 trial. Lancet Haematol. 2015;2(12):e528–e535. doi: 10.1016/S2352-3026(15)00196-9. [DOI] [PubMed] [Google Scholar]
- 28.Hochhaus A, Masszi T, Giles FJ, Radich JP, Ross DM, Gómez Casares MT, et al. Treatment-free remission following frontline nilotinib in patients with chronic myeloid leukemia in chronic phase: results from the ENESTfreedom study. Leukemia. 2017;31(7):1525–1531. doi: 10.1038/leu.2017.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hughes TP, Boquimpani C, Kim D-W, Benyamini N, Clementino NCD, Shuvaev V, et al. Treatment-free remission (TFR) in patients (pts) with chronic myeloid leukemia in chronic phase (CML-CP) treated with second-line nilotinib (NIL): first results from the ENESTop study. J Clin Oncol. 2016;34(15_suppl):7054. [Google Scholar]
- 30.Legros L, Nicolini FE, Etienne G, Rousselot P, Rea D, Giraudier S, et al. Second tyrosine kinase inhibitor discontinuation attempt in patients with chronic myeloid leukemia. Cancer. 2017;123(22):4403–4410. doi: 10.1002/cncr.30885. [DOI] [PubMed] [Google Scholar]
- 31.Johnson PWM. Response-adapted frontline therapy for Hodgkin lymphoma: are we there yet? Hematology. 2016;2016(1):316–322. doi: 10.1182/asheducation-2016.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mamot C, Klingbiel D, Hitz F, Renner C, Pabst T, Driessen C, et al. Final results of a prospective evaluation of the predictive value of interim positron emission tomography in patients with diffuse large B-cell lymphoma treated with R-CHOP-14 (SAKK 38/07) J Clin Oncol. 2015;33(23):2523–2529. doi: 10.1200/JCO.2014.58.9846. [DOI] [PubMed] [Google Scholar]
- 33.Gallamini A, Hutchings M, Rigacci L, Specht L, Merli F, Hansen M, et al. Early Interim 2-[18 F]Fluoro-2-Deoxy-d-Glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage hodgkin’s lymphoma: a report from a joint italian-danish study. J Clin Oncol. 2007;25(24):3746–3752. doi: 10.1200/JCO.2007.11.6525. [DOI] [PubMed] [Google Scholar]
- 34.Hoppe RT, Advani RH, Ai WZ, Ambinder RF, Aoun P, Armand P, et al. NCCN guidelines insights: hodgkin lymphoma, Version 1.2018. J Natl Compr Cancer Netw. 2018;16(3):245–254. doi: 10.6004/jnccn.2018.0013. [DOI] [PubMed] [Google Scholar]
- 35.Radford J, Illidge T, Counsell N, Hancock B, Pettengell R, Johnson P, et al. Results of a trial of PET-directed therapy for early-stage hodgkin’s lymphoma. N Engl J Med. 2015;372(17):1598–1607. doi: 10.1056/NEJMoa1408648. [DOI] [PubMed] [Google Scholar]
- 36.Raemaekers JMM, André MPE, Federico M, Girinsky T, Oumedaly R, Brusamolino E, et al. Omitting radiotherapy in early positron emission tomography-negative stage I/II hodgkin lymphoma is associated with an increased risk of early relapse: clinical results of the preplanned interim analysis of the randomized EORTC/LYSA/FIL H10 trial. J Clin Oncol. 2014;32(12):1188–1194. doi: 10.1200/JCO.2013.51.9298. [DOI] [PubMed] [Google Scholar]
- 37.André MPE, Girinsky T, Federico M, Reman O, Fortpied C, Gotti M, et al. Early positron emission tomography response-adapted treatment in stage i and ii hodgkin lymphoma: final results of the randomized EORTC/LYSA/FIL H10 trial. J Clin Oncol. 2017;35(16):1786–1794. doi: 10.1200/JCO.2016.68.6394. [DOI] [PubMed] [Google Scholar]
- 38.Johnson P, Federico M, Kirkwood A, Fosså A, Berkahn L, Carella A, et al. Adapted treatment guided by interim PET-CT scan in advanced hodgkin’s lymphoma. N Engl J Med. 2016;374(25):2419–2429. doi: 10.1056/NEJMoa1510093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Casasnovas O, Brice P, Bouabdallah R, Salles GA, Stamatoullas A, Dupuis J, et al. Randomized phase III study comparing an early pet driven treatment de-escalation to a not pet-monitored strategy in patients with advanced stages hodgkin lymphoma: interim analysis of the AHL2011 Lysa study. Blood. 2015;126(23):577. [Google Scholar]
- 40.Dann EJ, Bairey O, Bar-Shalom R, Sabbag E, Izak M, Korenberg A, et al. Tailored therapy in hodgkin lymphoma, based on predefined risk factors and early interim PET/CT: Israeli H2 study. Blood. 2014;124(21):4409. [Google Scholar]
- 41.Moskowitz C. Novel agents and strategies in transplant-eligible patients with relapsed and refractory Hodgkin lymphoma. Hematology. 2016;2016(1):331–338. doi: 10.1182/asheducation-2016.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smeltzer JP, Cashen AF, Zhang Q, Homb A, Dehdashti F, Abboud CN, et al. Prognostic Significance of FDG-PET in Relapsed or Refractory Classical Hodgkin Lymphoma Treated with Standard Salvage Chemotherapy and Autologous Stem Cell Transplantation. Biol Blood Marrow Transplant. 2011;17(11):1646–1652. doi: 10.1016/j.bbmt.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moskowitz CH, Nademanee A, Masszi T, Agura E, Holowiecki J, Abidi MH, et al. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin’s lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;385(9980):1853–1862. doi: 10.1016/S0140-6736(15)60165-9. [DOI] [PubMed] [Google Scholar]
- 44.Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, et al. Long-term outcome of patients in the LNH-985 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood. 2010;116(12):2040–2045. doi: 10.1182/blood-2010-03-276246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coiffier B, Sarkozy C. Diffuse large B-cell lymphoma: r-CHOP failure–what to do? Hematology. 2016;2016(1):366–378. doi: 10.1182/asheducation-2016.1.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang M, Chen P, Ruan X, Ye X, Pan Y, Zhang J, et al. Interim 18F-FDG PET/CT improves the prognostic value of S-IPI, R-IPI and NCCN-IPI in patients with diffuse large B-cell lymphoma. Oncol Lett. 2017;14(6):6715–6723. doi: 10.3892/ol.2017.7093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weiler-Sagie M, Bushelev O, Epelbaum R, Dann EJ, Haim N, Avivi I, et al. 18F-FDG avidity in lymphoma readdressed: a study of 766 patients. J Nucl Med. 2010;51(1):25–30. doi: 10.2967/jnumed.109.067892. [DOI] [PubMed] [Google Scholar]
- 48.Huang H, Lin J, Guo C, Li S, Hong H, Li X, et al. Predictive value of interim 18 f-FDG PET-CT scans on diffuse large B-cell lymphoma treated with R-CHOP: a prospective study. Blood. 2015;126(23):1458. [Google Scholar]
- 49.Hertzberg MS, Gandhi MK, Butcher B, Columbus R, Taper J, Trotman J, et al. Early treatment intensification with R-ICE chemotherapy followed by autologous stem cell transplantation (ASCT) using zevalin-beam for patients with poor risk diffuse large B-cell lymphoma (DLBCL) as identified by interim PET/CT scan performed after four. Blood. 2015;126(23):815. [Google Scholar]
- 50.Carr R, Fanti S, Paez D, Cerci J, Gyorke T, Redondo F, et al. Prospective international cohort study demonstrates inability of interim pet to predict treatment failure in diffuse large B-Cell lymphoma. J Nucl Med. 2014;55(12):1936–1944. doi: 10.2967/jnumed.114.145326. [DOI] [PubMed] [Google Scholar]
- 51.Huntington SF, Nasta SD, Schuster SJ, Doshi JA, Svoboda J. Utility of interim and end-of-treatment fluorodeoxyglucose positron emission tomography–computed tomography in frontline therapy of patients with diffuse large B-cell lymphoma. Leuk Lymphoma. 2015;56(9):2579–2584. doi: 10.3109/10428194.2015.1007506. [DOI] [PubMed] [Google Scholar]
- 52.Burggraaff CN, de Jong A, Hoekstra OS, Hoetjes NJ, Nievelstein RAJ, Jansma EP, et al. Predictive value of interim positron emission tomography in diffuse large B-cell lymphoma: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2019;46(1):65–79. doi: 10.1007/s00259-018-4103-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mamot C, Klingbiel D, Hitz F, Renner C, Pabst T, Driessen C, et al. Final results of a prospective evaluation of the predictive value of interim positron emission tomography in patients with diffuse large B-cell lymphoma treated With R-CHOP-14 (SAKK 38/07) J Clin Oncol. 2015;33(23):2523–2529. doi: 10.1200/JCO.2014.58.9846. [DOI] [PubMed] [Google Scholar]
- 54.Safar V, Dupuis J, Itti E, Jardin F, Fruchart C, Bardet S, et al. Interim [18]Fluorodeoxyglucose positron emission tomography scan in diffuse large B-cell lymphoma treated with anthracycline-based chemotherapy plus rituximab. J Clin Oncol. 2012;30(2):184–190. doi: 10.1200/JCO.2011.38.2648. [DOI] [PubMed] [Google Scholar]
- 55.Winter A, Rybicki L, Shah SN, Jagadeesh D, Gerds AT, Hamilton BK, et al. Prognostic value of pre-transplant PET/CT in patients with diffuse large B-cell lymphoma undergoing autologous stem cell transplantation. Leuk Lymphoma. 2018;59(5):1195–1201. doi: 10.1080/10428194.2017.1369065. [DOI] [PubMed] [Google Scholar]
- 56.Palumbo A, Avet-Loiseau H, Oliva S, Lokhorst HM, Goldschmidt H, Rosinol L, et al. Revised international staging system for multiple myeloma: a report from international myeloma working group. J Clin Oncol. 2015;33(26):2863–2869. doi: 10.1200/JCO.2015.61.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Majithia N, Vincent Rajkumar S, Lacy MQ, Buadi FK, Dispenzieri A, Gertz MA, et al. Outcomes of primary refractory multiple myeloma and the impact of novel therapies. Am J Hematol. 2015;90(11):981–985. doi: 10.1002/ajh.24131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yanamandra U, Kumar SK. Minimal residual disease analysis in myeloma—when, why and where. Leuk Lymphoma. 2018;59(8):1772–1784. doi: 10.1080/10428194.2017.1386304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Landgren O, Devlin S, Boulad M, Mailankody S. Role of MRD status in relation to clinical outcomes in newly diagnosed multiple myeloma patients: a meta-analysis. Bone Marrow Transplant. 2016;51(12):1565–1568. doi: 10.1038/bmt.2016.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International myeloma working group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17(8):e328–e346. doi: 10.1016/S1470-2045(16)30206-6. [DOI] [PubMed] [Google Scholar]
- 61.Rasche L, Alapat D, Kumar M, Gershner G, McDonald J, Wardell CP, et al. Combination of flow cytometry and functional imaging for monitoring of residual disease in myeloma. Leukemia. 2019;33(7):1713–1722. doi: 10.1038/s41375-018-0329-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mailankody S, Korde N, Lesokhin AM, Lendvai N, Hassoun H, Stetler-Stevenson M, et al. Minimal residual disease in multiple myeloma: bringing the bench to the bedside. Nat Rev Clin Oncol. 2015;12(5):286–295. doi: 10.1038/nrclinonc.2014.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gandolfi S, Prada CP, Richardson P, Paba-Prada C. How I treat the young patient with multiple myeloma. Blood First Ed Pap. 2018 [DOI] [PubMed]
- 64.Stadtmauer EA, Pasquini MC, Blackwell B, Hari P, Bashey A, Devine S, et al. Autologous transplantation, consolidation, and maintenance therapy in multiple myeloma: results of the BMT CTN 0702 trial. J Clin Oncol. 2019;37(7):589–597. doi: 10.1200/JCO.18.00685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Palumbo A, Gay F, Cavallo F, Di Raimondo F, Larocca A, Hardan I, et al. Continuous therapy versus fixed duration of therapy in patients with newly diagnosed multiple myeloma. J Clin Oncol. 2015;33(30):3459–3466. doi: 10.1200/JCO.2014.60.2466. [DOI] [PubMed] [Google Scholar]
- 66.Wildes TM, Anderson KC. Approach to the treatment of the older, unfit patient with myeloma from diagnosis to relapse: perspectives of a US hematologist and a geriatric hematologist. Hematology. 2018;2018(1):88–96. doi: 10.1182/asheducation-2018.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Radich JP. How I monitor residual disease in chronic myeloid leukemia. Blood. 2009;114(16):3376–3381. doi: 10.1182/blood-2009-02-163485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cerci JJ, Trindade E, Pracchia LF, Pitella FA, Linardi CCG, Soares J, et al. Cost effectiveness of positron emission tomography in patients with hodgkin’s lymphoma in unconfirmed complete remission or partial remission after first-line therapy. J Clin Oncol. 2010;28(8):1415–1421. doi: 10.1200/JCO.2009.25.4367. [DOI] [PubMed] [Google Scholar]
- 69.Huntington SF, Nasta SD, Schuster SJ, Doshi JA, Svoboda J. Utility of interim and end-of-treatment fluorodeoxyglucose positron emission tomography–computed tomography in frontline therapy of patients with diffuse large B-cell lymphoma. Leuk Lymphoma. 2015;56(9):2579–2584. doi: 10.3109/10428194.2015.1007506. [DOI] [PubMed] [Google Scholar]
- 70.Carlson JJ, Eckert B, Zimmerman M. Cost-effectiveness of next-generation sequencing minimal residual disease testing during maintenance treatment for multiple myeloma. Presented at: 2019 American Society of Clinical Oncology Annual Meeting; May 31-June 4, 2019; Chicago, Illinois. Abstract E19529