Abstract
About 15–40% India is Vitamin B12 deficient (commonly diagnosed by total Vitamin B12) but, as only holoTC (active form) is taken up by body cells, thus measuring holoTC is more reflective of Vitamin B12 status than the former. We aimed to assess diagnostic accuracy of serum holoTC in comparison with total Vitamin B12 and total Homocysteine (HCY) as indicator of serum Vitamin B12 status. 217 human subjects (99 males and 118 females) ranging from 17 to 83 years were divided into Vitamin B12 deficient (n = 70), borderline (n = 100) and sufficient groups (n = 47) who were further assessed for markers of Vitamin B12 deficiency—holoTC, HCY, Mean Corpuscular Volume (MCV), Folate, heamoglobin and creatinine. Samples were analysed using Siemens Advia Centaur Xpi. Total Vitamin B12 deficient group had − 84.3% holoTC deficient; 15.7% holoTC sufficient; 72.9% with elevated HCY; 27.1% with normal HCY; 11.4% with megaloblastic anaemia. Borderline group had − 34% holoTC deficient; 28% elevated HCY. A strong positive correlation was found between Total Vitamin B12 and holoTC (r = 0.754, p = <0.001) but strong negative correlation existed between holoTC and HCY (r = − 0.471, p = <0.001). Concordance between Total Vit B12 and HCY (Kappa index = 0.51, p < 0.001); between holoTC and HCY (Kappa index = 0.52, p = <0.001) were statically significant but the latter had a better sensitivity and specificity. Also, statically significant association exists between Total Vitamin B12 and holoTC with HCY (p = <0.001). Therefore, it is ascertained that Active Vitamin B12 assay is a better test and can be considered as an early marker of vitamin B12 deficiency.
Keywords: Vitamin B12 deficiency, Holotranscobalamin (holoTC), Homocystine (HCY), Vitamin B12, Active vitamin B12
Introduction
Vitamin B12 deficiency is known to result from malabsorption from the GI tract or inadequate dietary intake of Vitamin B12 i.e. in vegan and vegetarian diets [1–3]. As humans do not synthesize Vitamin B12, the main source is diet.
In early stages, Vitamin B12 deficiency is asymptomatic but then progresses to be associated with macrocytic anaemia and/or causes typical neurological symptoms which may vary in severity [4]. Thus, objectively assessing for Vitamin B12 deficiency poses a major challenge. Many studies have shown that an increase in methylmalonic acid (MMA) and Homocystine (HCY) co-exist with Vitamin B12 deficiency as its metabolic outcomes [5, 6]. Vitamin B12 deficiency reduces the activity of enzyme methylmalonyl-CoA mutase, thus MMA, its substrate, accumulates in the body. Presently, MMA is accepted as gold standard for diagnosing metabolic Vitamin B12 deficiency [7]. But, MMA is exclusively measured using complex, specialized methods which are expensive [8]. On the other hand, Vitamin B12 deficiency may be also detected by elevated HCY levels, as Vitamin B12 plays a role in synthesis of methionine from HCY [1, 2].
Serum total Vitamin B12 assay is performed primarily to assess for Vitamin B12 deficiency. But Vitamin B12 (Cobalamin) exists bound to two proteins: transcobalamin (TC) and hepatocorrin (HC). The TC-Vitamin B12 complex is known as holotranscobalamin (holoTC) which is the biologically active form and allows uptake of cobalamin from cells through specific receptors. Unfortunately, the major portion (about 80%) of cobalamin is carried by HC which is metabolically inert as it lacks cellular receptors except in the liver. This is the major limitation of detection of Vitamin B12 deficiency using total Vitamin B12 assay. Additionally, it has a longer half-life when compared with holoTC, thereby reducing the chances of detection of Vitamin B 12 deficiency in early stages [9–11]. Nevertheless, numerous studies have assessed the diagnostic accuracy of holoTC in Vitamin B12 deficiency and have found it to be a better indicator than total Vitamin B12 [11–14].
Early diagnosis of cobalamin deficiency is crucial, owing to the dormant nature of this disorder and the resultant possibly irreversible neurological damage [15]. A normal serum cobalamin concentration does not necessarily rule out a functional cobalamin deficiency and there isn’t any single diagnostic approach to achieve this diagnosis [16]. Furthermore, at this juncture, there is no gold standard or accurate reference method to diagnose cobalamin deficiency in its subtle form, which makes evaluation of the clinical significance of holoTC and the estimation of sensitivity and specificity more important [1]. HoloTC was not available till recent times as a suitable method for routine diagnosis. Thus clinical value of low holoTC is still uncertain [17].
In this study, we assessed the diagnostic accuracy of holoTC as a diagnostic test for metabolic vitamin B12 deficiency in patients with suspected Vitamin B12 deficiency. This was further compared with other markers of vitamin B12 deficiency to evaluate the method sensitivity and specificity.
Materials and Methods
217 patients attending the outpatient department at the Christian Medical College, Vellore and with a total Vitamin B12 lab request were recruited as study subjects. This included 118 female and 99 male subjects ranging from 17 to 83 years of age. The subjects were further divided into 3 major groups based on the serum total Vitamin B12 levels:
The total Vitamin B12 sufficient group with serum total Vitamin B12 levels > 350 pg/mL (n = 47)
The borderline group with serum Vitamin B12 levels ranging from 200 to 350 pg/mL (n = 100)
The deficient group with serum Vitamin B12 levels below 200 pg/mL (n = 70)
All the subjects were assessed for renal function and folate deficiency by analysing serum creatinine and folate levels respectively.
Blood samples were collected and sera were separated. The separated serum was then frozen and stored at − 70 °C for further testing. Markers of Vitamin B12 deficiency assessed were: serum total Vitamin B12; serum active vitamin B12; total Homocystine (HCY); Mean Corpuscular Volume (MCV). Total Vitamin B12, active Vitamin B12 and HCY were determined by chemiluminescence microparticle automated immunoassay on Siemens ADVIA Centaur xpi system. Serum Folate was assessed using electochemiluminescence microparticle immunoassay on Roche cobas e602 to rule out folate deficiency. Serum creatinine was measured using creatinine Jaffe’s kinetic method on Roche Cobas c702 automated chemistry analyzer.
Statistical Analysis
Results were expressed as mean with standard deviation. Values with a 95% confidence interval were recorded. Pearson’s correlation coefficient and Chi square were used to assess the relationship between variables. Concordance analysis was performed using the kappa index between the groups. Statistical analyses were performed with SPSS version 19.0 software.
Results
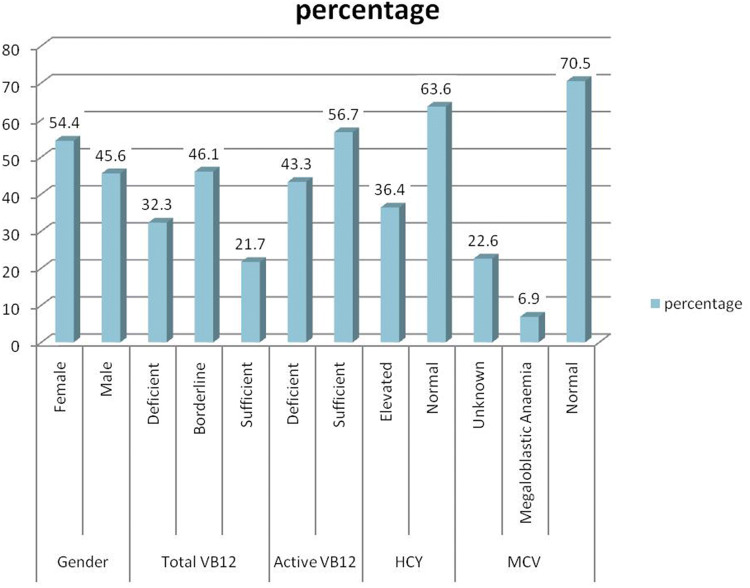
Figure 1 depicts that majority (54.4%) of the subjects studied were female with a mean age of 44.5 (± 13.7). Total Vit B12 borderline group consisted of 46.1% subjects when compared with deficient and sufficient group which were 32.3% and 21.7% respectively. 56.7% of the subjects had sufficient Active Vit B12 while 43.3% of the subjects were deficient. HCY was elevated among 36.4% cases and normal in the rest. Furthermore MCV was normal in 70.5% subjects and elevated in 6.9% subjects while in 22.6% subjects MCV was unknown.
- Total Vit B12 Mean of Total Vit B12 was 347.6 pg/mL, minimum = 60 pg/mL and maximum = 1923 pg/mL. Out of the 217 subjects that were assessed 32.3% (n = 70) were found to be Total Vit B12 deficient; 46.1% (n = 100) of the subjects were found to have borderline levels of Vit B12 whereas only 21.7% (n = 47) subjects were with sufficient Vit B12.
-
i.Total Vit B12 deficient Among the deficient group 84.3% subjects were Active B12 deficient and 15.7% subjects were Active B12 sufficient. HCY was found to be elevated among 72.9% subjects and was normal in 27.1% of the subjects studied. Elevated MCV was detected in only 11.4% whereas 62.9% subjects had normal MCV.
-
ii.Total Vit B12 borderline Among the borderline group 34% were Active B12 deficient and 66% were Active B12 sufficient. HCY was found to be elevated among 28% and was normal among 72% cases. MCV was elevated among 7% whereas 77% had normal MCV.
-
iii.Total Vit B12 sufficient Among the sufficient group 2.1% were Active B12 deficient and 97.9% were Active B12 sufficient. HCY was not found to be elevated any cases and none had elevated MCV.
-
i.
- Active Vit B12 Mean of Active Vit B12 was 54.3 pmol/L (± 43.5), Range = 5.3 pmol/L to > 146 pmol/L. In this group 43.3% (n = 94) were found to be Active Vit B12 deficient; 56.7% (n = 123) were found to have sufficient levels of Active Vit B12.
-
i.Active Vit B12 deficient Among the deficient group 62.8% were Total B12 deficient; 36.2% had borderline total Vit B12 levels whereas only 1.1% were Total B12 sufficient cases. HCY was found to be elevated among 64.9% and was normal among 35.1% cases. Elevated MCV was found among 11.7% whereas 66% had normal MCV.
-
ii.Active Vit B12 sufficient In this group only 8.9% were total B12 deficient while 53.7% cases were total vit B12 boderline and 37.4% were Total Vit B12 sufficient. HCY was found to be elevated in 14.6% cases and majority (85.4%) had normal HCY levels. MCV was elevated in 3.3% cases only while 74% had normal MCV.
-
i.
-
Correlation
Fig 2 the scatter plot illustrates strong positive correlation between Total Vit B12 and Active Vit B12 (r = 0.75, p < 0.001). On correlation analysis, there is also a strong negative correlation between Active Vit B12 and HCY (r = − 0.47, p < 0.001) as well as Total Vit B12 and HCY (r = − 0.322, p < 0.001).
-
Concordance analysis
Table 1 describes Concordance (using Kappa index) between Total Vitamin B12 and HCY was 51% which was statistically significant (p < 0.001) with sensitivity = 0.64 and specificity = 0.86.
On the other hand, Table 2 shows that Active Vit B12 when compared with HCY showed 52% agreement with sensitivity = 0.78 and specificity = 0.77. Moreover, there is a significant Kappa agreement between Total Vit B12 and MCV (p = 0.025) or of Active Vit B12 with MCV (p = 0.007).
Association analysis had similar findings where Total Vitamin B12 when compared to HCY and Active Vit B12 on comparison to HCY both were highly significant (p < 0.001). But, there was no association found between Total Vit B12 or Active Vit B12 and MCV (p = 0.076 and p = 0.014 respectively) Figures 3 and 4.
Fig. 1.
Descriptive statistics of the data (n = 217)
Fig. 2.

Scatter plot of the correlation between Total Vitamin B12 and Active Vitamin B12 (n = 217)
Table 1.
Kappa agreement analysis of total vitamin B12 with HCY
| VB12 | HCY | Kappa (95% CI) | p value | |
|---|---|---|---|---|
| Deficient | Normal | |||
| Deficient | 51 | 19 | 0.51 (0.39, 0.63) | < 0.001 |
| Borderline/sufficient | 29 | 118 | ||
Sensitivity: 0.64 (0.52, 0.74), Specificity: 0.86 (0.79, 0.91), PPV: 0.73 (0.61, 0.83), NPV: 0.80 (0.73, 0.86)
Table 2.
Kappa agreement analysis of Active vitamin B12 with HCY
| Active VB12 | HCY | Kappa (95% CI) | p value | |
|---|---|---|---|---|
| Deficient | Normal | |||
| Deficient | 62 | 32 | 0.52 (0.41, 0.64) | < 0.001 |
| Sufficient | 18 | 105 | ||
Sensitivity: 0.78 (0.67, 0.86), Specificity: 0.77 (0.69, 0.83), PPV: 0.66 (0.56, 0.75), NPV: 0.85 (0.78, 0.91)
Fig. 3.
Active vitamin B12 levels in total Vitamin B12 deficient samples
Fig. 4.
Active Vitamin B12 levels in total Vitamin B12 boardline samples
Discussion
In this study, we have validated the diagnostic accuracy of the Active Vit B12 and HCY in comparison to the traditionally used Total Vitamin B12 along with other markers among the patients attending the outpatient department at a tertiary care hospital in South India. We confirmed that Active Vitamin B12 has a better sensitivity when compared to Total Vitamin B12 assay for screening of metabolic Vitamin B12 deficiency.
Vitamin B12 deficiency may result of inadequate absorption of Vitamin B12 from the GI tract or inadequate intake of the vitamin through diet. This is seen to exist among people ingesting vegan diet [1, 18]. Vitamin B12 deficiency is a serious condition that might result in severe irreversible neurological complications. Therefore, there is a need to have high sensitivity of detection methods (low amount of missed cases). A study [12] suggests that diagnostic accuracy is enhanced by combining tests of Total Vitamin B12 and Active Vitamin B12. Many studies prove that Active Vitamin B12 has a better performance than Total Vitamin B12 by using a ROC analysis in detecting metabolic Vitamin B12 deficiency [5, 12].
In our study, we evaluated whether combined testing with vitamin B12 and holoTC would result in identification of a higher number of true positives. From the 217 randomly selected patients, 54.4% were female and 45.6% were male. The previous medical history was unknown for these patients but all had a laboratory request for Total Vitamin B12 assay. The patients were ruled out for anaemia and renal problems. The cut-off for the assessment however, was according to the standardised normal value of 32 pmol/l for Active Vitamin B12 assay where subjects with values < 32 pmol/l were determined to be Vitamin B12 deficient. Results from our study have shown an enhancement of 14–15% sensitivity with Active Vit B12 assay which correlates with another study where about 10–20% improvement of sensitivity was achieved [19].
Among the total Vitamin B12 deficient subjects 84.2% (n = 59) were Active Vit B12 deficient of which 47 had elevated HCY and 12 had normal HCY. This signifies that 15.7% subjects had false positive results using the Total Vit B12 assay. Additionally, on correlation analysis, there was a strong negative correlation between Active Vit B12 and HCY (r = − 0.47, p < 0.001). Therefore, on combining Active Vit B12 and HCY, 79% (n = 47) true positive cases can be detected. On the other hand, Total Vitamin B12 borderline group had 34% Active B12 deficient cases among these 41.2% had elevated HCY. Thus it may be specified that there is a higher possibility of missing true positive results with Total Vit B12 assay.
As mentioned earlier, a study has suggested that combining test results of Total Vitamin B12, Active Vitamin B12 and HCY improves diagnostic accuracy. In our study, the concordance analysis revealed higher agreement (52%) of Active Vit B12 with HCY (p < 0.001). It may be interpreted that, though Active Vit B12 and Total Vitamin B12 assay have not much difference in predictive values but as Active Vit B12 assay has higher sensitivity, it can preferred assay compare to the Total Vitamin B12 assay.
Conclusion
Our study has shown that, Active Vit B12 levels were decreased in both Total Vit B12 deficient and borderline groups. Owing to the higher concordance of Active Vit B12 with HCY and the higher sensitivity of the test, we confirm that Active Vit B12 is a better test and can be considered as an early marker of vitamin B12 deficiency.
Acknowledgements
We would like to thank Siemens healthineers for their support.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Devalia V, Hamilton MS, Molloy AM. British committee for standards in haematology. Guidelines for the diagnosis and treatment of cobalamin and folate disorders. Br J Haematol. 2014;166(4):496–513. doi: 10.1111/bjh.12959. [DOI] [PubMed] [Google Scholar]
- 2.Herrmann W, Geisel J. Vegetarian lifestyle and monitoring of vitamin B-12 status. Clin Chim Acta Int J Clin Chem. 2002;326(1–2):47–59. doi: 10.1016/S0009-8981(02)00307-8. [DOI] [PubMed] [Google Scholar]
- 3.Clarke R, Refsum H, Birks J, Evans JG, Johnston C, Sherliker P, et al. Screening for vitamin B-12 and folate deficiency in older persons. Am J Clin Nutr. 2003;77(5):1241–1247. doi: 10.1093/ajcn/77.5.1241. [DOI] [PubMed] [Google Scholar]
- 4.Carmel R. Current concepts in cobalamin deficiency. Annu Rev Med. 2000;51:357–375. doi: 10.1146/annurev.med.51.1.357. [DOI] [PubMed] [Google Scholar]
- 5.Clarke R, Sherliker P, Hin H, Nexo E, Hvas AM, Schneede J, et al. Detection of vitamin B12 deficiency in older people by measuring vitamin B12 or the active fraction of vitamin B12, holotranscobalamin. Clin Chem. 2007;53(5):963–970. doi: 10.1373/clinchem.2006.080382. [DOI] [PubMed] [Google Scholar]
- 6.Herrmann W, Obeid R, Schorr H, Geisel J. Functional vitamin B12 deficiency and determination of holotranscobalamin in populations at risk. Clin Chem Lab Med. 2003;41(11):1478–1488. doi: 10.1515/CCLM.2003.227. [DOI] [PubMed] [Google Scholar]
- 7.Allen RH, Stabler SP, Savage DG, Lindenbaum J. Diagnosis of cobalamin deficiency I: usefulness of serum methylmalonic acid and total homocysteine concentrations. Am J Hematol. 1990;34(2):90–98. doi: 10.1002/ajh.2830340204. [DOI] [PubMed] [Google Scholar]
- 8.Schmedes A, Brandslund I. Analysis of methylmalonic acid in plasma by liquid chromatography-tandem mass spectrometry. Clin Chem. 2006;52(4):754–757. doi: 10.1373/clinchem.2005.058586. [DOI] [PubMed] [Google Scholar]
- 9.Carmel R. Measuring and Interpreting Holo-Transcobalamin (Holo-Transcobalamin II) Clin Chem. 2002;48(3):407–409. doi: 10.1093/clinchem/48.3.407. [DOI] [PubMed] [Google Scholar]
- 10.Hunt A, Harrington D, Robinson S. Vitamin B12 deficiency. BMJ. 2014;349:g5226. doi: 10.1136/bmj.g5226. [DOI] [PubMed] [Google Scholar]
- 11.Nexo E, Hoffmann-Lücke E. Holotranscobalamin, a marker of vitamin B-12 status: analytical aspects and clinical utility. Am J Clin Nutr. 2011;94(1):359S–365S. doi: 10.3945/ajcn.111.013458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hvas A-M, Nexo E. Holotranscobalamin—a first choice assay for diagnosing early vitamin B deficiency? J Intern Med. 2005;257(3):289–298. doi: 10.1111/j.1365-2796.2004.01437.x. [DOI] [PubMed] [Google Scholar]
- 13.Obeid R, Herrmann W. Holotranscobalamin in laboratory diagnosis of cobalamin deficiency compared to total cobalamin and methylmalonic acid. Clin Chem Lab Med. 2007;45(12):1746–1750. doi: 10.1515/CCLM.2007.361. [DOI] [PubMed] [Google Scholar]
- 14.Herbert V, Fong W, Gulle V, Stopler T. Low holotranscobalamin II is the earliest serum marker for subnormal vitamin B12 (cobalamin) absorption in patients with AIDS. Am J Hematol. 1990;34(2):132–139. doi: 10.1002/ajh.2830340210. [DOI] [PubMed] [Google Scholar]
- 15.Harrington DJ. Laboratory assessment of vitamin B12 status. J Clin Pathol. 2017;70(2):168–173. doi: 10.1136/jclinpath-2015-203502. [DOI] [PubMed] [Google Scholar]
- 16.Carmel R, Agrawal YP. Failures of cobalamin assays in pernicious anemia. N Engl J Med. 2012;367(4):385–386. doi: 10.1056/NEJMc1204070. [DOI] [PubMed] [Google Scholar]
- 17.Brady J, Wilson L, McGregor L, Valente E, Orning L. Active B12: a rapid, automated assay for holotranscobalamin on the Abbott AxSYM analyzer. Clin Chem. 2008;54(3):567–573. doi: 10.1373/clinchem.2007.096784. [DOI] [PubMed] [Google Scholar]
- 18.Herrmann W, Schorr H, Obeid R, Geisel J. Vitamin B-12 status, particularly holotranscobalamin II and methylmalonic acid concentrations, and hyperhomocysteinemia in vegetarians. Am J Clin Nutr. 2003;78(1):131–136. doi: 10.1093/ajcn/78.1.131. [DOI] [PubMed] [Google Scholar]
- 19.Heil SG, de Jonge R, de Rotte MCFJ, van Wijnen M, Heiner-Fokkema RMR, Kobold ACM, et al. Screening for metabolic vitamin B12 deficiency by holotranscobalamin in patients suspected of vitamin B12 deficiency: a multicentre study. Ann Clin Biochem. 2012;49(Pt 2):184–189. doi: 10.1258/acb.2011.011039. [DOI] [PubMed] [Google Scholar]