Abstract
It has been suggested that an age-related loss of cognitive function might be driven by atherosclerotic effects associated with altered lipid patterns. However, the relationship between Lipoprotein (a) [Lp(a)] and healthy cognitive aging has not yet been sufficiently investigated. For the current analysis we used the cross-sectional data of 1,380 Berlin Aging Study II (BASE-II) participants aged 60 years and older (52.2% women, mean age 68 ± 4 years). We employed the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD)-Plus test battery to establish latent factors representing continuous measures of domain specific cognitive functions. Regression models adjusted for APOE genotypes, lipid parameters and other risk factors for cognitive impairment were applied to assess the association between Lp(a) and performance in specific cognitive domains. Men within the lowest Lp(a)-quintile showed better cognitive performance in the cognitive domain executive functions and processing speed (p = 0.027). No significant results were observed in women. The results of the current analysis of predominantly healthy BASE-II participants point towards an association between low Lp(a) concentrations and better cognitive performance. However, evidence for this relationship resulting from the current analysis and the employment of a differentiated cognitive assessment is rather weak.
Subject terms: Psychology, Diseases, Health care, Medical research, Pathogenesis, Risk factors
Introduction
The prevalence and incidence of dementia and cognitive impairment are increasing with advancing age1,2. Decline of cognitive functioning is a health risk, personally resulting in significant socio-economic and medical consequences1,2. The trajectories of cognitive decline in healthy aging can vary, affecting cognitive domains differently. The best studied and presumably most relevant modifiable risk factors for cognitive decline are e.g. hypertension, diabetes mellitus, obesity, and smoking habits3. To date, early detection and treatment of these specific risk profiles seem to be the most promising approach so far to prevent or delay age-related cognitive decline or dementia2,4. Moreover, an association between plasma lipid levels (low-density-lipoprotein (LDL) cholesterol, high-density-lipoprotein (HDL) cholesterol, apolipoprotein E4 (APOE4) and Lipoprotein(a) [Lp(a)]) with cognitive function has been reported. In particular, atherosclerosis and cerebrovascular diseases which are favored by altered lipid concentrations are discussed to promote cognitive impairment, including e.g. Alzheimer´s disease or vascular dementia [reviewed in5]3. So far, findings regarding the association between blood lipid levels and cognition are contradictory. Besides that, there is no yet common or internationally standardized assessment used within these analyses to define and evaluate different domains of cognitive function.
With regard to the relationship between metabolic markers and cognitive functioning, Van den Kommer and colleagues analyzed data from the Longitudinal Aging Study Amsterdam and reported a link between low LDL-C and worse cognitive performance in general. They also found a faster decline in processing speed in subjects with low LDL-C. Additionally, high HDL-C concentrations were found to be associated with better memory performance6. Ancelin et al. defined specific cognitive domains (visual memory, verbal fluency, psychomotor speed and executive abilities) and found associations between high total cholesterol, low HDL-C, high LDL-C and the risk of cognitive decline in psychomotor speed, executive abilities, and verbal fluency, but in men only7. In contrast, lower performance in motor speed was found to be associated with high HDL-C. Lower executive abilities were linked to low LDL-C and triglycerides in women7.
With respect to the relationship between Lp(a) and cognitive function, current studies reported conflicting results.
Lp(a) consists of an LDL-like particle, which is covalently attached to the apo(a) protein and differs structurally from other apolipoproteins. Its serum concentration is mainly genetically determined and its physiological function is still relatively unknown8,9.
Results from observational studies and studies employing mendelian randomization suggest a causal association between Lp(a) and cardiovascular diseases, e.g. myocardial infarction, stroke, and aortic valve stenosis and metabolic health10–12.
Notably, higher Lp(a) concentrations have also been linked to positive outcomes such as lower incidence of diabetes or better pulmonary function13–17.
A majority of studies found a link between higher Lp(a) concentrations and vascular dementia (VD)18,19 or Alzheimer’s disease (AD)20, thus interpreting an increased occurrence of cardiovascular diseases, ischemia and inflammation promoted by Lp(a) as a possible mechanism for cerebrovascular disease and cognitive decline. Solfrizzi et al.20 discovered a non-linear relationship between Lp(a) and AD, whilst study participants over 72 years and high Lp(a) levels showing a reduced risk to develop AD. Iwamoto et al. suggested an inverse effect were high Lp(a) levels being associated to an increased risk of VD but an decreased occurrence of AD18. In contrast, Kunutsor et al. even reported evidence for a protective effect of Lp(a) on cognitive decline in general21. Other authors found no association of elevated Lp(a) levels with poorer cognitive performances at all22.
It also has been shown that the combination of high Lp(a) plasma level and carrier status of the apolipoprotein E (APOE) epsilon 4 allele increases the risk for late-onset Alzheimer´s disease, while Lp(a) might protect against this decline in APOE-4 non-carriers23.
Taken together, the mechanisms for conflicting cross-sectional results on the relationship between Lp(a) and cognitive performance are still unclear. Existing studies mainly focus on advanced stages of cognitive decline, e.g. dementia or AD, and mostly not differentiate between cognitive domains. Moreover, studies investigating sex-specific links and differences between Lp(a) and cognitive performance are sparse.
To address these limitations and shed more light in investigating the associations of Lp(a) on different cognitive abilities, we employed the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD)-Plus test battery24. The German CERAD-Plus test battery contains various tasks for assessing different cognitive abilities. Existing studies that investigated the factor structure of the CERAD test battery using exploratory factor analyses (EFA) show inconsistent results. While Strauss and Fritsch found a general factor, Morris et al. (1989) reported a three factor structure, whereas Collie et al. (1999) found even five factors of selected CERAD subtest25. It is important to mention that all studies differ in the samples selected (Alzheimer’s patients and healthy adults) and in the selection of CERAD subtest which makes it hard to compare the resulting factor structures. While Morris et al. (1989), for example, included the total score of MMST and the word list learning, Collie et al. (1999) only chose individual subtests of single tests of the MMST25. Strauss and Fritsch (2004) also used subtests of the MMST, whereby the composition of the subtests in Collie et al. (1999) differs25,26. In addition, the CERAD items have so far only been examined using EFA but the theoretical assumptions about different cognitive domains or specific relationships between factors have not taken into account. In addition, the German version of CERAD Plus includes novel sub-tests, namely trail making test A & B and phonemic word fluency test. These tasks have not yet been included in the factor analyses mentioned above. Taken together, the factor structure of the complete CERAD items in a large sample of healthy older adults remains unclear.
To this end, we carried out exploratory and confirmatory factor analyses which resulted in establishing four latent cognitive factors representing different domain specific cognitive functions in a large sample of 1,380 older and generally healthy Berlin Aging Study II (BASE-II) participants. We hypothesized that elevated Lp(a) levels are associated with poorer cognitive performance in specific domains that might be vulnerable to unfavorable metabolic and cardiovascular risk profile in older males and females, such as executive functions working memory and episodic memory.
Methods
Participants
The Berlin Aging Study II (BASE-II) aims to identify factors involved in ‘healthy’ and ‘unhealthy’ aging and collected medical baseline data of 2,171 participants (≈75% aged 60–84 years and ≈25% aged 20–37 years) between 2009 and 2014. The participants comprise a convenient sample of community-dwelling participants, living in the greater metropolitan area of Berlin, Germany, and the collected data cover numerous ageing-relevant variables27,28. All participants scored more than 27 points on the MMSE29. All participants gave written informed consent to participation and the Ethics Committee of the Charité-Universitätsmedizin Berlin approved this study (approval number EA2/029/09) and all research was performed in accordance with relevant guidelines/regulations.
Laboratory tests
Blood samples were drawn after a fasting period of at least 8 hours. Plasma concentration of Lp(a) was assessed using an enzyme-linked immunosorbent assay. Triglycerides were quantitatively acquired photometrically by an enzymatic in vitro test using the analyses instrument of Roche. Total cholesterol (TC), low density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) were determined in the same way. HbA1c was measured using high-performance chromatography (Variant II Turbo HbA1c Kit- 2.0, Bio-Rad). The thyroid stimulating hormone (TSH), Vitamin B12, folic acid and the concentration of C-reactive protein (CRP) were measured by an electrochemiluminescence immunoassay. Magnesium was measured photometrically by using a xylidyl blue complex. Sodium and potassium were determined by an ion-selective electrode. Homocystein was measured photometrically by an enzyme-cycling assay. APOE genotyping was performed for two polymorphisms defining the epsilon 2/3/4 haplotype from blood-derived DNA samples either by direct sequencing (performed at LGC Genomics, Berlin, Germany) or by targeted genotyping using TaqMan assays (ThermoFisher Scientific, Foster City, CA) on a QuantStudio-12K-Flex system in 384-well format. APOE haplotypes were then classified according to the “epsilon 2/3/4” allele designation derived from polymorphisms rs7412 [a.k.a. as “epsilon 2-allele”] and rs429358 [a.k.a. “epsilon 4-allele]).
Co-variables
Regular alcohol intake (yes/no) and current smoking status (yes/no) were evaluated by standardized questions. Information on past and current diseases was obtained from participant reports, clinical examinations and laboratory tests. Diagnoses were used to compute a morbidity index largely based on the categories of the Charlson index, which is a weighted sum of moderate to severe, mostly chronic physical illnesses, including cardiovascular (e.g., congestive heart failure), cancer (e.g., lymphoma), and metabolic diseases (e.g., diabetes mellitus)30,31. We used the Rapid Assessment of Physical Activity (RAPA) questionnaire to assess physical activity of the study participants32.
As a screening for depression we employed the geriatric depression scale (GDS)33.
Neuropsychological assessment
To assess cognitive performance the German version of the neuropsychological test battery CERAD-Plus (Consortium to Establish a Registry for Alzheimer’s Disease) was applied24. The complete test battery was administered in individual sessions to all BASE-II participants of the older group studied here. The following nine CERAD-Plus (age, gender, education adjusted z-values) scores were finally used to evaluate the cognitive performance of the subjects: Word list learning, word list recall, word list recognition, recall the figure, copying a figure, responses of semantic fluency, phonemic fluency, Trail Making Test A and Trail Making Test B.
Behavioral data analysis
Exploratory factor analyses (EFA) of CERAD-Plus test
In a first set of analyses we aimed to explore the factor structure of CERAD-Plus in our large BASE-II sample consisting of healthy older adults by applying EFA. In this first set of analyses we carried out a principal component analyses (PCA) in SPSS based on the age, education and gender-corrected z-values of the following eleven CERAD-Plus tests: word list learning, word list recall, word list recognition, recall the figure, copying a figure, semantic fluency, phonemic fluency, Trail Making Test A and Trail Making Test B, Boston naming test, and word list intrusions.
Confirmatory factor analyses (CFA) of CERAD test
In order to investigate whether CERAD tests form the hypothezised specific cognitive domains we selected the remaining nine CERAD subtest and covariates according to our hypothesis and applied CFA before using the extracted factor scores for conducting regression analyses. CFA allows testing structural hypotheses about associations among multiple variables by examining how well a given model is able to reproduce the variance–covariance matrix of a set of observed variables. Factor analysis represents the variance shared by the observed (measured) variables. The latent variables can be assumed to be free of task-specific sources of variance as well as measurement error. Previous studies demonstrated specific factor structures of the CERAD tests24,26.
In the current study, latent factor models were established by using MPlus v6.134 We relied on standard indices such as the Root Mean Square Error of Approximation (RMSEA) and the Comparative Fit Index (CFI) for evaluation of model fit. Commonly accepted thresholds indicating good model fit are 0 < =RMSEA < = 0.05 and 0.97 < =CFI < = 135.
We performed CFA to tests weather previous suggested factor models fit to our data: (A) a general latent factor model on which all nine CERAD-Plus tasks simultaneously loaded on24,26, (B) a two latent factor model which is based on the theoretical differentiation of MCI in amestic (word list learning, word list recall, word list recognition, recall the figure) and non-amnestic (semantic fluency, phonemic fluency, Trail Making Test A and Trail Making Test B) state, (C) a three latent factor model (verbal memory, visuo-construction, executive functions and processing speed) based on the results of Morris et al.24 and (D) in a four latent factor (verbal memory, visuo-construction, executive functions and processing speed, verbal fluency) model that was suggested by our exploratory factor analyses (PCA, Supplementary Table 7). For details regarding model comparisons and fit indices, see Table 1.
Table 1.
Communalities (h²) of the nine CERAD subtests of CFA models 1–4.
| domain/test | factor loadings (standardized) | |||
|---|---|---|---|---|
| general factor model | 2 factor model | 3 factor model | 4 factor model | |
| verbal memory/word list learning | 0.771** | 0.749** | 0.732** | 0.730** |
| verbal memory/word list recall | 0.823** | 0.889** | 0.915** | 0.905** |
| verbal memory/word list recognition | 0.422** | 0.432** | 0.428** | 0.424** |
| visuo-construction/copying a figure | 0.091** | −0.004 | 0.466** | 0.491** |
| visuo-construction/recall the figure | 0.306** | 0.272** | 0.980** | 0.932** |
| verbal fluency/semantic fluency | 0.371** | 0.497** | 0.477** | 0.613** |
| verbal fluency/phonemic fluency | 0.349** | 0.486** | 0.460** | 0.491** |
| executive functions & processing speed/Trail Making Test part A | −0.210** | −0.449** | −0.517** | 0.526** |
| executive functions & processing speed/Trail Making Test part B | −0.340** | −0.648** | −0.647** | 0.847** |
CERAD subtests: encode = word list learning; recall = word list recall; recogn = word list recognition; frecall = recall the figure; fcopy = copying a figure; semantic = responses of the semantic fluency; phonem = phonemic fluency; TMTA = Trail Making Test part A; TMTB Trail Making Test part B, **p < 0.001.
Each latent factor was defined by at least two indicators, namely the sum of correct responses from word list learning, word list recall, word list recognition (3 indicators) for the verbal memory factor, the sum of correct responses of copying a figure and recall the figure (2 indicators) for the visuo-construction factor, the number of correct responses of the semantic fluency and the phonemic fluency task (2 indicators) for defining the verbal fluency factor, and the score of time in seconds spend to finish the Trail Making Test part A and B (2 indicators) for the executive functions and processing speed factor.
Factor scores of the four factor model (D)
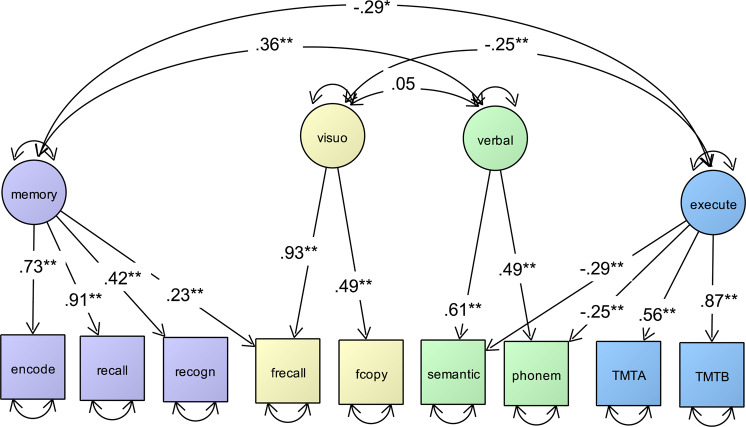
For each participant the four individual intercorrelated factor scores of the best fitting model (D) were estimated using the regression method (modal posterior estimator) in Mplus v6.1 (Muthén & Muthén, 2007) on the basis of the final selected model D (Fig. 1)34.
Figure 1.
Depiction of a simplified CFA of the final selected four latent factor model of CERAD with good model fit (χ² 71.102, DF = 20; RMSEA = 0.031; CFI = 0.979) including standardized factor loadings (single headed arrows) and covariances between latent factors (double headed arrows). Circles represent latent factors and squares represent manifest variables. Double-headed arrows with both heads pointing on a manifest variable represent the variance of this variable. Latent factors: memory = verbal memory; visuo = visuo-construction; verbal = verbal fluency; execute = executive functions and processing speed. Manifest variables: encode = word list learning; recall = word list recall; recogn = word list recognition; frecall = recall the figure; fcopy = copying a figure; semantic = responses of the semantic fluency; phonem = phonemic fluency; TMTA = Trail Making Test part A; TMTB Trail Making Test part B, **p < 0.001.
We applied the extracted individual four factor scores as independent variables for subsequent regression analyses within our sample.
The data was analyzed using the Statistical Package for Social Science (SPSS, IBM Analytics) version 25. Mann-U-test was performed to assess differences between continuous data. Comparisons between groups were performed applying Chi2-test. Adjusted regression models were performed to estimate whether Lp(a) is associated with four cognitive factor scores. According to the EAS consensus panel Lp(a) levels in the upper quintile can be considered as elevated with respect to its role as a cardiovascular risk factor11 and it has become current practice in the field to examine Lp(a) by quintiles. Following this, we divided Lp(a) in quintiles and low Lp(a)-concentrations (Lp(a) quintile 1) were used as an independent variable. During the data quality check less than 15 unrealistically high or low values, especially for potassium, were excluded from the analyses (for example attributed to preanalytical problems and due to low plausibility).
All reported associations were stepwise controlled for age, weight, height, HbA1c and APOE epsilon 4 carrier status (i.e. participants carrying the APOE 24, APOE 34 or APOE 44) (model1), additionally for regular alcohol intake, current smoking, physical activity level (RAPA), depression (GDS-Score), TSH, homocysteine and CRP (model 2) as depression, endocrinopathies and inflammation are known to mimic or rather promote cognitive deficits. Since vitamin deficiencies, and shifted electrolytes are also known to affect cognitive performance we additionally considered vitamin B12, folic acid, sodium, potassium and magnesium as covariates in model 3, also adjusting for the morbidity burden (morbidity index). Due to the genetic determination of Lp(a), which can hardly be influenced by other factors – and which has no influence on the co-variates used here -, we considered all the co-variables used as confounders and assumed no mediation effect here.
P-values of <0.05 were considered to indicate statistical significance. Additionally, we applied Bonferroni correction to account for multiple testing (four latent factors of cognitive performance), therefore p-values below 0.05/4 = 0.0125 were considered to be statistically significant.
Results
Cross-sectional data for Lp(a), APOE epsilon 4 carrier status and the four latent factor scores estimated here and reflecting cognitive functions of different domains (verbal memory, visuo-construction, executive functions and processing speed, verbal fluency) were available for 1,380 old BASE-II participants (mean age 68 ± 4 years; 52.2% women).
Baseline characteristics of the study population are displayed in Table 2, divided by sex. Due to known sex-specific differences in Lp(a) levels,36–38, in brain development39 and metabolism40,41, we performed sex-specific analyses. Men were significantly more frequently current smokers, reported regular alcohol intake more often, and were less frequently physically active. Regarding lipid patterns, men had significantly lower concentrations of HDL-C, LDL-C and Lp(a), but increased levels of serum triglycerides, when compared to women.
Table 2.
Characteristics of the BASE-II study population divided by sex.
| Men (n = 659) | Women (n = 721) | p-value | |
|---|---|---|---|
| Age [years] | 68 ± 3.6 | 68 ± 3.4 | <0.001 |
| Weight [kg] | 83.95 ± 12.27 | 69.77 ± 12.34 | <0.001 |
| Height [cm] | 175.55 ± 6.21 | 162.83 ± 6.02 | <0.001 |
| BMI [kg/m2] | 27.23 ± 3.65 | 26.34 ± 4.62 | <0.001 |
| Regular alcohol intake a | 598 (91.0) | 633 (87.8) | 0.055 |
| Current smoking a | 73(11.1) | 63 (8.8) | <0.001 |
| Physically inactive a | 73 (11.1) | 56 (7.8) | 0.04 |
| GDS score | 24.61 (1.04) | 24.63 (1.05) | n.s. |
| Cognitive function (factor scores). | |||
| Verbal memory | −0.1805 ± 0.9406 | 0.2259 ± 0.8751 | <0.001 |
| Visuo-construction | 0.1460 ± 0.9589 | −0.1276 ± 0.9381 | <0.001 |
| Executive functions and processing speed | 0.0505 ± 0.9427 | −0.0708 ± 0.8245 | 0.032 |
| Verbal fluency | −0.1015 ± 0.7355 | 0.1018 ± 0.7065 | <0.001 |
| Lipid profile | |||
| Total Cholesterol [mg/dL] | 202 ± 38 | 226 ± 38 | <0.001 |
| HDL-C [mg/dL] | 55 ± 14 | 69 ± 16 | <0.001 |
| LDL-C [mg/dL] | 123 ± 34 | 136 ± 35 | <0.001 |
| TG [mg/dL] | 122 ± 77 | 104 ± 51 | <0.001 |
| Lp(a) [mg/L] | 230.2 ± 320.6 | 265 ± 338.5 | 0.004 |
| APOE genotypesa | |||
| 22 | 5 (0.8) | 3 (0.4) | n.s. |
| 23 | 89 (13.5) | 102 (14.1) | n.s. |
| 24 | 15 (2.3) | 25 (3.5) | n.s. |
| 33 | 394 (59.8) | 441 (61.2) | n.s. |
| 34 | 146 (22.2) | 138 (19.1) | n.s. |
| 44 | 10 (1.5) | 12 (1.7) | n.s. |
| Vitamin B12 [ng/L] | 371.8 ± 213.5 | 423.1 ± 293.8 | <0.001 |
| Homocysteine [µmol/L] | 14.29 ± 3.99 | 12.49 ± 3.44 | <0.001 |
| CRP [mg/L] | 1.8 ± 2.4 | 2.2 ± 3.4 | 0.015 |
| Folic acid [µg/L] | 10.88 ± 5.34 | 11.97 ± 5.77 | <0.001 |
| HbA1c [%] | 5.7 ± 0.6 | 5.6 ± 0.5 | 0.012 |
| TSH basal [mU/L] | 2.10 ± 3.07 | 2.29 ± 4.2 | 0.034 |
| Sodium [mmol/L] | 139 ± 3 | 140 ± 3 | 0.005 |
| Potassium [mmol/L] | 4.5 ± 0.4 | 4.5 ± 0.4 | n.s. |
| Magnesium [mmol/L] | 0.82 ± 0.07 | 0.81 ± 0.07 | n.s. |
BMI = body mass index; GDS = geriatric depression scale; HDL-C = high density lipoprotein cholesterol; LDL-C = low density lipoprotein cholesterol; Lp(a) = lipoprotein(a), APOE = apolipoprotein E; CRP = C-reactive protein; HbA1c =hemoglobin A 1c; TSH = thyroid stimulating hormone.
Mann-U-test was performed to assess differences between continuous data. Comparisons between groups were performed applying Chi-squared- test (a).
The exploratory PCA including all CERAD subtests resulted in one to five factor model solutions. Only the three to five factor models showed a good model fit (see supplementary table 6). By inspecting the factor loadings, the indicators Boston naming test, and word list intrusion did not load significantly (all p’s > 0.05) on the factors. As a consequence we excluded the two tests from the PCA, resulting in one-four factor model solutions which are summarized in supplementary table 7. The best model fit showed model 4 consisting of a verbal memory factor, a visuo-construction factor, an executive functions and processing speed factor and a verbal fluency factor.
Next, by applying CFA we established four different latent factor models based on previous PCA findings and theoretical assumptions reported in the literature. Model D provided the best fit to the data [χ20 = 71.102, CFI = 0.979, RMSEA = 0.031], relative to the global (model A), two (model B) and three-factor models (Model C; see Table 4 for fit indices of model comparison).
Table 4.
CFA results of the model comparisons and fit indices testing different latent factor solutions of the 11 CERAD-Plus indices.
| Model | χ² | df | p | RMSEA | CFI |
|---|---|---|---|---|---|
| A (1 factor) | 954.792 | 27 | <0.001 | 0.149 | 0.613 |
| B (2 factors) | 708.014 | 25 | <0.001 | 0.132 | 0.715 |
| C (3 factors) | 271.416 | 23 | <0.001 | 0.083 | 0.897 |
| D (4 factors) | 71.102 | 20 | <0.001 | 0.031 | 0.979 |
CFA = Confirmatory factor analyses; CFI = comparative fit index; RMSEA = root mean square error of approximation.
We used model D subsequent analyses. Core results of the CFA for model D are shown in Fig. 1 were we specified four intercorrelated latent variables, each with unique loadings from the corresponding nine CERAD-Plus test scores (Fig. 1). All items loaded reliably on the postulated latent factors (p < 0.001).
Next, we extracted the four factor scores from model D and used them as independent variables for subsequent analyses. Factor scores are z-standardized composite variables which provide information about an individual’s placement on the factors. For factor 1 (verbal memory; mean = 0.00; SD = 0.91; range = −3.69–2.46) the higher the standardized score the better is the performance within this domain. The same direction applies for factor 2 (visuo-construction; mean = 0.00, SD = 0.86, range = −2.45–2.15), and factor 4 (verbal fluency; mean = 0.00, SD = 0.73, range = −2.51–2.45). In contrast, a high factor score on factor 3 (executive functions and processing speed; mean = 0.00, SD = 0.93, range = −2.53–3.82), reflects a lower performance.
By inspecting the extracted individual four cognitive factors scores, women performed significantly better in tests related to verbal memory, executive functions and processing speed and verbal fluency, while men showed better results in tests representing visuo-construction (see Table 2). As shown in supplementary tables 1 and 2, we found no significant difference between cognitive functions and Lp(a) levels in men and women in unadjusted calculations.
With respect to other covariates, which may be linked to cognitive performance, men had lower concentrations of vitamin B12 and folic acid. Moreover, men showed higher levels of homocysteine, higher concentrations of HbA1c and higher BMI. Electrolyte levels and TSH concentrations were equally distributed, independent of sex. Notably, CRP concentrations were higher in women.
Next, we set up different linear regression models assessing the association between Lp(a) and the four latent factors for cognitive functions, namely verbal memory, visuo-construction, executive functions and processing speed, and verbal fluency. Lp(a) was divided in quintiles and low Lp(a)-concentrations (Lp(a) quintile 1) were used as an independent variable. Table 3 shows the results of three linear regression models adjusted for an increasing number of confounders with the factor scores for executive functions and processing speed as the dependent variable.
Table 3.
Association between Lp(a) and the latent factor score reflecting executive functions and processing speed divided by sex including lipid-lowering agents.
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| beta | SE | p-value | R2* | beta | SE | p-value | R2* | |
| Model 1 | −0.178 | 0.088 | 0.043 | 0.049 | −0.093 | 0.080 | 0.246 | 0.045 |
| Model 2 | −0.202 | 0.092 | 0.029 | 0.056 | −0.087 | 0.084 | 0.301 | 0.040 |
| Model 3 | −0.217 | 0.098 | 0.027 | 0.050 | −0.110 | 0.089 | 0.215 | 0.039 |
| Model 4 | −0.223 | 0.098 | 0.023 | 0.048 | −0.110 | 0.088 | 0.211 | 0.045 |
| Model 5 | −0.221 | 0.098 | 0.025 | 0.047 | −0.124 | 0.087 | 0.155 | 0.057 |
| Model 6 | −0.215 | 0.098 | 0.028 | 0.048 | −0.103 | 0.088 | 0.241 | 0.049 |
Model 1: age, weight, height, HbA1c, APOE genotype.
Model 2: Model 1 + regular alcohol intake, current smoking, physical inactivity, GDS score, TSH, homocysteine, CRP.
Model 3: Model 2 +vitamin B12, magnesium, potassium, sodium, folic acid, morbidity index.
Model 4: Model 3 + HDL-C + lipid-lowering agents.
Model 5: Model 3 + LDL –C + lipid-lowering agents.
Model 6: Model 3 + TG + lipid-lowering agents.
GDS = geriatric depression scale; HDL-C = high density lipoprotein cholesterol; LDL-C = low density lipoprotein cholesterol; TG = triglycerides, Lp(a) = lipoprotein(a), APOE = apolipoprotein E; CRP = C-reactive protein; HbA1c = hemoglobin A 1c; TSH = thyroid stimulating hormone,*= R2 corrected, p-values <0.05 = statistical significant, corrected p-value (Bonferroni).
<0.0125 = statistical significant.
In the fully adjusted model 3 we found a nominal statistically significant association between low Lp(a) levels and the factor score of executive functions and processing speed (beta: −0.217; SE: 0.098; p = 0.027, R2 = 0.050) in men; in women, however, this association was not detected. Following correction for multiple testing (testing the Lp(a) association with each of the four latent factors of cognitive performance), this association found in men was no longer statistically significant. A recalculation of this model including sedatives as an additional co-variable did not change these results significantly (0.220 SE: 0.096; p = 0.023, R2 = 0.052).
We recalculated model 3 adding HDL-C (model 4), LDL-C (model 5) and triglycerides (model 6) separately as independent variables, and also included the use of lipid lowering medication as a co-variable in the regression analyses. Adjustment for these other lipid parameters did not change the results significantly. The supplementary table 3 summarizes the results of the calculated and fully adjusted models investigating the relationship between Lp(a) and the four latent factor scores for cognitive function established in the current study. Except the above described association between Lp(a) and executive functions and processing speed in older men, no nominal association was found between Lp(a) and the three other cognitive domains studied here. As the APOE epsilon 4 allele is a risk factor for the development of AD, we additionally recalculated model 3 (see supplementary table 4) excluding subjects with APOE epsilon 4 alleles, this, however, did not change the results significantly.
Discussion
In the current analysis of 1,380 older community-dwelling participants of BASE-II, low plasma concentrations of Lp(a) were associated with better cognitive performance in executive functions and processing speed in men only at nominal significance. This association was not found in women. This association was independent of other lipid parameters, APOE genotype and potential confounders. Moreover, there was no association between single markers of LDL-C, HDL-C and triglycerides with cognitive function.
This is to our knowledge the first study that established a four latent factor-structure including all CERAD-Plus based tests and investigated the associations between the four resulting cognitive domains to Lp(a) in a predominantly healthy large sample of older adults. Albeit after correcting for multiple testing none of the results remained significant, our analyses point towards a possible sex-specific link between Lp(a) and executive functions and processing speed.
Our finding is in line with other studies that have been shown that an optimal control of cardiovascular risk factors may reduce the risk of cognitive decline42, whilst high levels of Lp(a) increase the atherothrombotic risk by various mechanisms such as impaired fibrinolysis, increased cholesterol deposition in arterial walls and by stimulating inflammatory processes at the vascular walls43,44. From this pathogenic viewpoint, a link between Lp(a) and impaired cognitive performance remains plausible. In addition, stroke is an established risk factor for all-cause dementia45 and elevated Lp(a) is an independent risk factor for stroke46.
Further, Lp(a) levels seem to be significantly higher in both men and women with coronary artery disease compared to those without47 and small-vessel disease promoted by elevated Lp(a) may induce microstructural alterations leading to brain damage and poorer cognitive function9–12.
Thus, the current results may indicate a preclinical/prodromal stage of sex-specific vascular-related cognitive decline. A sex-specific difference seems plausible, as atherosclerotic manifestations affect men earlier in life, although increased Lp(a) concentrations are observed in postmenopausal women due to hormonal changes36–38.
Premenopausal women commonly have a less proatherogenic plasma lipid pattern than men, although physiological alterations regarding hormones (due to the menstrual cycle or menopause) do not affect lipid homeostasis significantly48. Moreover, there are sex differences in insulin sensitivity of glucose metabolism in the liver and muscle and insulin is an important regulator of lipid metabolism48. Women seem to be more sensitive to insulin regarding to glucose metabolism, whereas there are no differences between men and women in lipolysis49.
The generalizability of the results to the general population is limited by the convenient sampling of the BASE-II participants. This led to a bias of recruiting cognitively and physically high functioning individuals with relatively low incidence of comorbidity, especially cardiovascular diseases27 This may also drive the weak significant associations found in this study between cognition and Lp(a).
Additionally, the comparability of our results to previous studies is limited due to the differences in measures of cognitive functioning and decline. We aimed to investigate cognitive domains in a widely used measure such as CERAD. To our knowledge there is no internationally standardized assessment of cognitive function. Moreover, assessment of e.g. smoking habits or physical activity are mainly based on questionnaires, which might have led to over-or underreporting.
On the other hand the measurement of cognitive function employing the CERAD-Plus test battery followed by extracting latent factor scores representing different cognitive functions is a strength of the current study, however, this also raises the question about the comparability of the cognitive variables assessed in other studies, because the relations might depend on the selected cohort tests and cohorts.
Executive functions (EF) include a range of cognitive skills that facilitate purposeful, goal – directed and socially-competent behavior50. The three core EFs are “inhibition (inhibitory control, including self-control) and interference control” (selective attention and cognitive inhibition), “working memory” (holding information in mind and mentally working with; includes verbal working memory and visual-spatial working memory) and “cognitive flexibility” (changing perspectives spatially or interpersonally)50. Processing speed describes the efficiency an individual is able to perceive and act upon a stimulus51,52. Six variables have been described to assess the processing speed: “decision speed” (time to respond in cognitive tests with complex content), “perceptual speed” (time to respond in cognitive tests with simple content), “psychomotor speed” (simple tasks like drawing lines), “reaction time” (choice reaction time with visual stimuli and manual keypress responses), “psycho-physical speed” (decision accuracy with visual or auditory stimuli) and “time course of internal responses” (event-related potential)52.
The association (at nominal significance) between Lp(a), executive functions and processing speed found in the male group only of our dataset is in line with two theories with respect to deterioration of processing speed in older individuals the so-called “processing speed theory”53 and the “prefrontal-executive theory”54. On the one hand, diffuse age-related deterioration of white matter may result in the slowing of processing speed, on the other hand, structural age-related alterations of grey and white matter in the prefrontal cortex, which also has been associated with executive functions and processing speed might be responsible for worse performance in this cognitive domain. Both theories have been supported by neuroimaging studies55. An exact mechanism such as brain integrity through which Lp(a) may effect this specific cognitive domain has not been in the focus of the current study. However, an association between white matter lesions and atherosclerosis has been described elsewhere56. We think that white and gray matter integrity should be subject of further research, when investigating the association between Lp(a) and cognitive performance, although, it is currently unknown if Lp(a) can cross the blood brain barrier57, which may enable a direct influence of elevated Lp(a) blood levels on cognitive functioning.
Considering the inconsistent study results with respect to the association between Lp(a) and cognitive function (see supplementary table 5) the approach of investigating separate cognitive domains rather than cognitive performance in general could be useful to shed light on the relationship between Lp(a) and cognition.
Worse performance in tests assessing executive functions and processing speed have already been found to be related to vascular disease in patients with mild cognitive impairment (MCI) when compared to patients without vascular disease58. Notably, executive functions and processing speed have also been shown to be associated with inflammatory markers in BASE-II59. Pro-inflammatory effects of Lp(a) have been reported before49,60, which a mechanism that may also influence cognition, regarding the association between Lp(a) and the domains of executive functions and processing speed found in the current study48,59.
Similar to our approach, by examining the relationships between serum lipids and cognitive functions, Ancelin et al. defined four cognitive domains, visual memory, verbal fluency, psychomotor speed and executive abilities7 and demonstrated a poorer cognitive performance in men and women with a hypercholesterolemic pattern in general. In contrast to our study they did not specifically test for the effect of Lp(a).
Further research of the links and mechanisms between Lp(a) and cognition is desirable, because a medical Lp(a) modulation could be used as a treatment to prevent or decelerate age-related cognitive decline. With new lipid lowering approaches treatments such as PCSK9 inhibitors (Protein convertase subtilisin/kexin type 9) or antisense oligonucleotides that block the production of Lp(a) in the liver, the reduction of Lp(a) might result in better cognitive function61–63. Two studies investigated the relationship between AD or cognitive decline and their putative risk associated with PCSK9 inhibitors. However, no effect on cognitive performance was detected in these studies50,51,64,65. Sub-analysis in such large-scale studies might be of interest to detect Lp(a)-cognition association.
Conclusion
In conclusion, we found a weak evidence for an association between Lp(a) plasma concentrations and cognitive performance in the male group only. However, our results suggest, that the cognitive domain of executive functions and processing speed might be of interest for future research based on the observed association with Lp(a) found here, e.g. in larger cohorts with longitudinal data. With respect to the current literature, the results of our study are plausible and controversial at the same time. Future studies using a longitudinal design and new Lp(a)-lowering target drugs may shed light on the potential mechanisms. Assessment of specific cognitive domains in vascular dementia research could represent a promising approach to detect specific and cognitive changes at an early stage of disease development.
Supplementary information
Acknowledgements
The BASE-II research project (Co-PIs are Lars Bertram, Ilja Demuth, Denis Gerstorf, Ulman Lindenberger, Graham Pawelec, Elisabeth Steinhagen-Thiessen, and Gert G. Wagner) is supported by the German Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung, BMBF) under grant numbers #16SV5536K, #16SV5537, #16SV5538, #16SV5837, #01UW0808, 01GL1716A and 01GL1716B. Another source of funding is the Max Planck Institute for Human Development, Berlin, Germany. Additional contributions (e.g., equipment, logistics, personnel) are made from each of the other participating sites.
Author contributions
F.R. and N.B. analyzed the data and wrote the first manuscript draft. S.T. prepared data on medication use. S.D. and Nina B. established latent factors representing continuous measures of domain specific cognitive functions. C.L. and L.B. provided genetic data. K.N. and D.S. discussed results. I.D. and E.S.T. provided phenotypic data. I.D. conceived the study, interpreted and discussed data, edited the manuscript, is the guarantor of this work, had full access to all data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors reviewed the manuscript.
Competing interests
EST received research grants and honoraria for lectures, consultancy and for being a member of advisory boards from the following companies (within the past three years): Sanofi, Amgen, MSD, Fresenius Medical Care and Chiesi. ID received a research grant from Sanofi and honorary for consultancy from uniQure biopharma B.V.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Nikolaus Buchmann and Sandra Düzel.
Supplementary information
is available for this paper at 10.1038/s41598-020-66783-3.
References
- 1.Schaller S, Mauskopf J, Kriza C, Wahlster P, Kolominsky‐Rabas PL. The main cost drivers in dementia: a systematic review. International journal of geriatric psychiatry. 2015;30(2):111–29. doi: 10.1002/gps.4198. [DOI] [PubMed] [Google Scholar]
- 2.Livingston G, et al. Dementia prevention, intervention, and care. The Lancet. 2017;390(10113):2673–734. doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- 3.Etgen T, Sander D, Bickel H, Förstl H. Mild cognitive impairment and dementia: the importance of modifiable risk factors. Deutsches Ärzteblatt International. 2011;108(44):743. doi: 10.3238/arztebl.2011.0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deckers K, et al. Target risk factors for dementia prevention: a systematic review and Delphi consensus study on the evidence from observational studies. International journal of geriatric psychiatry. 2015;30(3):234–46. doi: 10.1002/gps.4245. [DOI] [PubMed] [Google Scholar]
- 5.Cooper C, Sommerlad A, Lyketsos CG, Livingston G. Modifiable predictors of dementia in mild cognitive impairment: a systematic review and meta-analysis. American Journal of Psychiatry. 2015;172(4):323–34. doi: 10.1176/appi.ajp.2014.14070878. [DOI] [PubMed] [Google Scholar]
- 6.van den Kommer, T. N., Dik, M. G., Comijs, H. C., Jonker C. & Deeg, D. J. The role of lipoproteins and inflammation in cognitive decline: Do they interact? Neurobiology of aging33(1), 196. e1–e12. (2012). [DOI] [PubMed]
- 7.Ancelin M-L, et al. Gender-specific associations between lipids and cognitive decline in the elderly. European Neuropsychopharmacology. 2014;24(7):1056–66. doi: 10.1016/j.euroneuro.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Utermann G, et al. Lp (a) glycoprotein phenotypes. Inheritance and relation to Lp (a)-lipoprotein concentrations in plasma. The Journal of clinical investigation. 1987;80(2):458–65. doi: 10.1172/JCI113093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kronenberg F, Utermann G. Lipoprotein (a): resurrected by genetics. Journal of internal medicine. 2013;273(1):6–30. doi: 10.1111/j.1365-2796.2012.02592.x. [DOI] [PubMed] [Google Scholar]
- 10.Bennet A, et al. Lipoprotein (a) levels and risk of future coronary heart disease: large-scale prospective data. Archives of internal medicine. 2008;168(6):598–608. doi: 10.1001/archinte.168.6.598. [DOI] [PubMed] [Google Scholar]
- 11.Nordestgaard BG, et al. Lipoprotein (a) as a cardiovascular risk factor: current status. European heart journal. 2010;31(23):2844–53. doi: 10.1093/eurheartj/ehq386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamstrup PR, Tybjærg-Hansen A, Nordestgaard BG. Extreme lipoprotein (a) levels and improved cardiovascular risk prediction. Journal of the American College of Cardiology. 2013;61(11):1146–56. doi: 10.1016/j.jacc.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 13.Buchmann N, et al. Higher Lipoprotein (a) Levels Are Associated with Better Pulmonary Function in Community-Dwelling Older People–Data from the Berlin Aging Study II. PloS one. 2015;10(9):e0139040. doi: 10.1371/journal.pone.0139040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buchmann N, et al. Association between lipoprotein (a) level and type 2 diabetes: no evidence for a causal role of lipoprotein (a) and insulin. Acta diabetologica. 2017;54(11):1031–8. doi: 10.1007/s00592-017-1036-4. [DOI] [PubMed] [Google Scholar]
- 15.Ye Z, et al. The association between circulating lipoprotein (a) and type 2 diabetes: is it causal? Diabetes. 2014;63(1):332–42. doi: 10.2337/db13-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding L, et al. Serum lipoprotein (a) concentrations are inversely associated with type 2 diabetes, prediabetes, and insulin resistance in a middle-aged and elderly Chinese population. Journal of lipid research. 2015;jlr:P049015. doi: 10.1194/jlr.P049015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mora S, et al. Lipoprotein (a) and risk of type 2 diabetes. Clinical chemistry. 2010;56(8):1252–60. doi: 10.1373/clinchem.2010.146779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwamoto, T. et al. Dual inverse effects of lipoprotein (a) on the dementia process in Japanese late‐onset Alzheimer’s disease. Psychogeriatrics4(3), 64–71 (2004).
- 19.Urakami K, et al. Lipoprotein (a) phenotypes in patients with vascular dementia. Dementia and geriatric cognitive disorders. 2000;11(3):135–8. doi: 10.1159/000017226. [DOI] [PubMed] [Google Scholar]
- 20.Solfrizzi, V. et al. Lipoprotein (a), apolipoprotein E genotype, and risk of Alzheimer’s disease. Journal of Neurology, Neurosurgery & Psychiatry72(6), 732–6 (2002). [DOI] [PMC free article] [PubMed]
- 21.Kunutsor SK, Khan H, Nyyssönen K, Laukkanen JA. Is lipoprotein (a) protective of dementia? European journal of epidemiology. 2016;31(11):1149–52. doi: 10.1007/s10654-016-0184-0. [DOI] [PubMed] [Google Scholar]
- 22.Sarti C, et al. Lipoprotein (a) and Cognitive Performances in an Elderly White Population: Cross-sectional and Follow-up Data. Stroke: Journal of the American Heart Association. 2001;32(7):1678–83. doi: 10.1161/01.str.32.7.1678. [DOI] [PubMed] [Google Scholar]
- 23.Mooser V, et al. Interactions between apolipoprotein E and apolipoprotein (a) in patients with late-onset Alzheimer disease. Annals of internal medicine. 2000;132(7):533–7. doi: 10.7326/0003-4819-132-7-200004040-00004. [DOI] [PubMed] [Google Scholar]
- 24.Morris, J. C. et al. The consortium to establish a registry for Alzheimer’s disease (CERAD): I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology (1989). [DOI] [PubMed]
- 25.Collie A, et al. Psychiatry NZJo. Norms and the effects of demographic variables on a neuropsychological battery for use in healthy ageing Australian populations. 1999;33(4):568–75. doi: 10.1080/j.1440-1614.1999.00570.x. [DOI] [PubMed] [Google Scholar]
- 26.Strauss ME, Fritsch T. Factor structure of the CERAD neuropsychological battery. Journal of the international Neuropsychological Society. 2004;10(4):559–65. doi: 10.1017/S1355617704104098. [DOI] [PubMed] [Google Scholar]
- 27.Bertram L, et al. Cohort profile: the Berlin aging study II (BASE-II) International journal of epidemiology. 2013;43(3):703–12. doi: 10.1093/ije/dyt018. [DOI] [PubMed] [Google Scholar]
- 28.Gerstorf D, et al. The Berlin Aging Study II: An overview. Gerontology. 2016;62(3):311–62. [Google Scholar]
- 29.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 30.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 31.Meyer A, et al. Leukocyte telomere length is related to appendicular lean mass: cross-sectional data from the Berlin Aging Study II (BASE-II), 2. The American journal of clinical nutrition. 2015;103(1):178–83. doi: 10.3945/ajcn.115.116806. [DOI] [PubMed] [Google Scholar]
- 32.Topolski TD, et al. Peer reviewed: the Rapid Assessment of Physical Activity (RAPA) among older adults. Preventing chronic disease. 2006;3:4. [PMC free article] [PubMed] [Google Scholar]
- 33.Yesavage JA, et al. Development and validation of a geriatric depression screening scale: a preliminary report. Journal of psychiatric research. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 34.Muthen, L. K. & BO, M. Mplus User’s Guide, Seventh edn, Los Angeles, CA: Muthén & Muthé n. Google Scholar. 1998.
- 35.Schermelleh-Engel K, Kerwer M, Klein AG. Evaluation of model fit in nonlinear multilevel structural equation modeling. Frontiers in Psychology. 2014;5:181. doi: 10.3389/fpsyg.2014.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phan, B. A. P. & Toth, P. P. Dyslipidemia in women: etiology and management. International journal of women’s health6, 185 (2014). [DOI] [PMC free article] [PubMed]
- 37.Shenoy R, Vernekar P. Fasting lipid profile in pre-and postmenopausal women: a prospective study. International Journal of Scientific Study. 2015;3(9):116–9. [Google Scholar]
- 38.Derby, C. A. et al. Lipid changes during the menopause transition in relation to age and weight: the Study of Women’s Health Across the Nation. American journal of epidemiology169(11), 1352–61 (2009). [DOI] [PMC free article] [PubMed]
- 39.Gennatas, E. D. et al. Age-related effects and sex differences in gray matter density, volume, mass, and cortical thickness from childhood to young adulthood. Journal of Neuroscience, 3550–16 (2017). [DOI] [PMC free article] [PubMed]
- 40.Satterthwaite, T. D. et al. Impact of puberty on the evolution of cerebral perfusion during adolescence. Proceedings of the National Academy of Sciences 201400178 (2014). [DOI] [PMC free article] [PubMed]
- 41.Jack CR, et al. Age, sex, and APOE ε4 effects on memory, brain structure, and β-amyloid across the adult life span. JAMA neurology. 2015;72(5):511–9. doi: 10.1001/jamaneurol.2014.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baumgart, M. et al. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheimer’s & Dementia11(6), 718–26 (2015). [DOI] [PubMed]
- 43.Malaguarnera, M. et al. Lipoprotein (a) in cardiovascular diseases. BioMed research international 2013, (2013).
- 44.Bucci M, Tana C, Giamberardino M, Cipollone F. Lp (a) and cardiovascular risk: Investigating the hidden side of the moon. Nutrition, Metabolism and Cardiovascular Diseases. 2016;26(11):980–6. doi: 10.1016/j.numecd.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 45.Kuźma, E. et al. Stroke and dementia risk: A systematic review and meta-analysis. Alzheimer’s & Dementia14(11), 1416–26 (2018). [DOI] [PMC free article] [PubMed]
- 46.Nave AH, et al. Lipoprotein (a) as a risk factor for ischemic stroke: a meta-analysis. Atherosclerosis. 2015;242(2):496–503. doi: 10.1016/j.atherosclerosis.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 47.Frohlich J, Dobiášová M, Adler L, Francis M. Gender Differences in Plasma Levels of Lipoprotein (a) in Patients with Angiographically Proven Coronary Artery. Physiol Res. 2004;53:481–6. [PubMed] [Google Scholar]
- 48.Wang, X., Magkos, F. & Mittendorfer, B. Sex differences in lipid and lipoprotein metabolism: it’s not just about sex hormones. The Journal of Clinical Endocrinology & Metabolism96(4), 885–93 (2011). [DOI] [PMC free article] [PubMed]
- 49.Magkos F, Wang X, Mittendorfer B. Metabolic actions of insulin in men and women. Nutrition. 2010;26(7-8):686–93. doi: 10.1016/j.nut.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Diamond A. Executive functions. Annual review of psychology. 2013;64:135–68. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kail R, Salthouse TA. Processing speed as a mental capacity. Acta psychologica. 1994;86(2-3):199–225. doi: 10.1016/0001-6918(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 52.Salthouse TA. Aging and measures of processing speed. Biological psychology. 2000;54(1-3):35–54. doi: 10.1016/s0301-0511(00)00052-1. [DOI] [PubMed] [Google Scholar]
- 53.Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychological review. 1996;103(3):403. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- 54.West RL. An application of prefrontal cortex function theory to cognitive aging. Psychological bulletin. 1996;120(2):272. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- 55.Gunning‐Dixon FM, Brickman AM, Cheng JC, Alexopoulos GS. Aging of cerebral white matter: a review of MRI findings. International Journal of Geriatric Psychiatry: A journal of the psychiatry of late life and allied sciences. 2009;24(2):109–17. doi: 10.1002/gps.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bots ML, et al. Cerebral white matter lesions and atherosclerosis in the Rotterdam Study. The Lancet. 1993;341(8855):1232–7. doi: 10.1016/0140-6736(93)91144-b. [DOI] [PubMed] [Google Scholar]
- 57.Wang, H. & Eckel, R. H. What are lipoproteins doing in the brain? Trends in Endocrinology & Metabolism.25(1), 8–14 (2014). [DOI] [PMC free article] [PubMed]
- 58.Nordlund A, et al. Cognitive profiles of mild cognitive impairment with and without vascular disease. Neuropsychology. 2007;21(6):706. doi: 10.1037/0894-4105.21.6.706. [DOI] [PubMed] [Google Scholar]
- 59.Tegeler C, et al. The inflammatory markers CRP, IL-6, and IL-10 are associated with cognitive function—data from the Berlin Aging Study II. Neurobiology of aging. 2016;38:112–7. doi: 10.1016/j.neurobiolaging.2015.10.039. [DOI] [PubMed] [Google Scholar]
- 60.Orsó E, Schmitz G. Lipoprotein (a) and its role in inflammation, atherosclerosis and malignancies. Clinical research in cardiology supplements. 2017;12(1):31–7. doi: 10.1007/s11789-017-0084-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.He N-y LiQ, et al. Lowering serum lipids via PCSK9-targeting drugs: current advances and future perspectives. Acta Pharmacologica Sinica. 2017;38(3):301. doi: 10.1038/aps.2016.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Raal FJ, et al. Reduction in lipoprotein (a) with PCSK9 monoclonal antibody evolocumab (AMG 145): a pooled analysis of more than 1,300 patients in 4 phase II trials. Journal of the American College of Cardiology. 2014;63(13):1278–88. doi: 10.1016/j.jacc.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 63.Yamamoto T, Wada F, Harada-Shiba M. Development of antisense drugs for dyslipidemia. Journal of atherosclerosis and thrombosis. 2016;23(9):1011–25. doi: 10.5551/jat.RV16001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Giugliano RP, et al. Cognitive function in a randomized trial of evolocumab. New England Journal of Medicine. 2017;377(7):633–43. doi: 10.1056/NEJMoa1701131. [DOI] [PubMed] [Google Scholar]
- 65.Schmidt, A. F. et al. Phenome-wide association analysis of LDL-cholesterol lowering genetic variants in PCSK9. bioRxiv:329052. 2018. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.