Abstract
The function of serrate (SE) in miRNA biogenesis in Arabidopsis is well elucidated, whereas its role in plant drought resistance is largely unknown. In this study, we report that MdSE acts as a negative regulator of apple (Malus × domestica) drought resistance by regulating the expression levels of MdMYB88 and MdMYB124 and miRNAs, including mdm-miR156, mdm-miR166, mdm-miR172, mdm-miR319, and mdm-miR399. MdSE interacts with MdMYB88 and MdMYB124, two positive regulators of apple drought resistance. MdSE decreases the transcript and protein levels of MdMYB88 and MdMYB124, which directly regulate the expression of MdNCED3, a key enzyme in abscisic acid (ABA) biosynthesis. Furthermore, MdSE is enriched in the same region of the MdNECD3 promoter where MdMYB88/MdMYB124 binds. Consistently, MdSE RNAi transgenic plants are more sensitive to ABA-induced stomatal closure, whereas MdSE OE plants are less sensitive. In addition, under drought stress, MdSE is responsible for the biogenesis of mdm-miR399, a negative regulator of drought resistance, and negatively regulates miRNAs, including mdm-miR156, mdm-miR166, mdm-miR172, and mdm-miR319, which are positive regulators of drought resistance. Taken together, by revealing the negative role of MdSE, our results broaden our understanding of the apple drought response and provide a candidate gene for apple drought improvement through molecular breeding.
Subject terms: Drought, Plant molecular biology
Introduction
Serrate (SE) in Arabidopsis is a conserved eukaryotic RNA processing factor that was first reported to mediate the formation of early juvenile leaves and phase length1. Encoding a C2H2 zinc-finger protein, SE is required for normal shoot development2. Moreover, SE influences the alternative splicing of pre-mRNAs that primarily affect the selection of alternative 5′ splice sites of first introns3. Other genes with alternative splicing affected by SE encode transcription factors, splicing factors, and stress-related proteins3. SE also functions in intron splicing and the transcription of intronless genes by pausing and elongating polymerase II complexes to promote their association with these intronless target genes4,5. Moreover, label-free quantitative proteomic analysis has revealed that SE is regulated by abscisic acid (ABA) under flooding stress6. In addition to these functions, SE has a role in microRNA (miRNA) biogenesis7,8, and previous studies report that SE and Hyponastic Leaves1 (HYL1) form a complex with DICER1 to achieve efficient and precise processing of pri-miRNAs9.
Drought stress is a major limiting factor that affects the yield and quality of apple. Researchers have long sought to increase the drought resistance of apple trees using molecular tools, such as genetic transformation and QTL mapping of loci associated with water use efficiency10–14. To date, a number of genes have been reported to play positive or negative roles in apple drought resistance. For example, MdMYB88 and MdMYB124 are proven to be two positive regulators of apple drought stress that influence xylem formation and secondary cell wall deposition11. In addition, MdMYB88 and MdMYB124 bind to gene promoters containing the cis-element AACCG11,13 to regulate expression.
miRNAs are ~20–25 nucleotide (nt) endogenous small molecules involved in various plant processes, including development and environmental stresses15–17. For example, the overexpression of miR156 improves the drought tolerance of alfalfa (Medicago sativa) by silencing SPL1318, a squamosa promoter-binding-like protein that binds to a core GTAC sequence in the promoter region of dihydroflavonol-4-reductase (DFR) to induce anthocyanin biosynthesis in response to drought stress18,19. miR393 is involved in the rice response to stress by targeting auxin receptors and plays negative roles in drought and salt stress20. In addition, transgenic Arabidopsis plants overexpressing miR399 exhibit hypersensitivity to drought but enhanced tolerance to salt stress and exogenously applied ABA21. In the apple genome, 23 conserved, 10 less conserved, and 42 apple-specific miRNAs or families with distinct expression patterns have been identified; these miRNAs target various genes and represent a wide range of enzymatic and regulatory activities22. Genome-wide miRNA analysis has revealed that 61 and 35 miRNAs are differentially expressed in drought-tolerant and drought-sensitive apple hybrid progeny, respectively, under drought stress23. Among these mdm-miRNAs, mdm-miR156 and mdm-miRn249 are two positive regulators of apple osmotic stress23.
ABA is a drought-induced phytohormone that plays important roles in plant responses to environmental stresses. Upon drought stress, ABA accumulates rapidly to promote stomatal closure and avoid water loss24,25. Exogenous ABA treatment effectively and sufficiently upregulates many stress-marker proteins in wheat and maize that are indicated to enhance drought tolerance26,27. ABA also acts as a signaling molecule in response to drought stress. Rice (Oryza sativa) OsPM1 (PLASMA MEMBRANE PROTEIN1) encodes an ABA influx carrier that mediates the movement of ABA across the plasma membrane and plays important roles in drought responses28. Under drought conditions, elevated ABA induces the production of H2O2 in guard cells, and subsequent H2O2-activated Ca2+ channels mediate the influx of Ca2+ in intact guard cells to close stomata29,30.
In the current study, we provide evidence that MdSE participates in the drought resistance of apple by negatively regulating MdMYB88- and MdMYB124-mediated ABA homeostasis. MdSE also regulates the expression of miRNAs that play critical roles in drought resistance in apple. Our results highlight the roles of MdSE in the drought tolerance of apple and thereby provide genetic determinants for apple breeding.
Results
MdSE interacts with and reduces the transcript and protein levels of MdMYB88 and MdMYB124
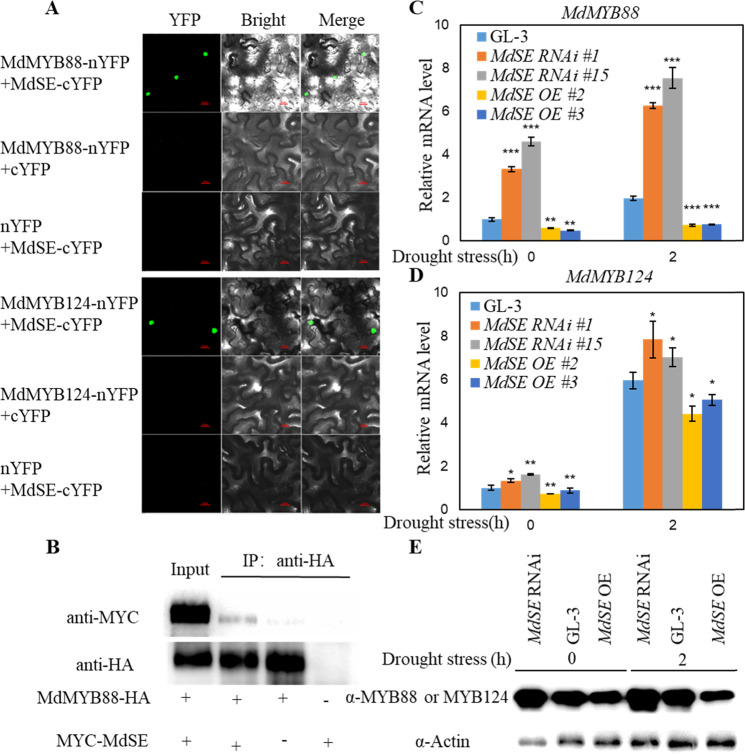
When applying affinity-purified mass spectrometry analysis to ascertain the interacting partners of MdMYB88 and MdMYB124, the SERRATE protein, which usually participates in miRNA biogenesis, pri-miRNA, and pre-mRNA splicing in Arabidopsis, was identified3. The interaction between MdSE and MdMYB88 or MdMYB124 was confirmed by BiFC analysis (Fig. 1a), which was further verified by a Co-IP assay (Fig. 1b). However, MdSE did not interact with MdMYB88 in yeast, as demonstrated by a yeast two-hybrid analysis (Fig. S1), indicating that the physical interaction of MdSE with MdMYB88 or MdMYB124 may require another component.
Fig. 1. MdSE reduces the transcript and protein levels of MdMYB88 and MdMYB124 by interacting with them.
a MdSE interacts with MdMYB88 and MdMYB124 in tobacco leaves by BiFC analysis. Bars = 20μm. b MdSE interacts with MdMYB88 in tobacco by Co-IP analysis. MdMYB88-HA was coinfiltrated with MYC-MdSE in tobacco leaves. Proteins were extracted and immunoprecipitated with an anti-HA antibody. The immunocomplex was then detected with western blot using anti-MYC or anti-HA. c, d Expression level of MdMYB88 or MdMYB124 in GL-3, MdSE RNAi, and MdSE OE plants under control or drought conditions. Data are means ± SD (n = 3). Student’s t test was performed, and statistically significant differences are indicated by *P < 0.05, **P < 0.01, or ***P < 0.001. e Protein level of MdMYB88 or MdMYB124 in GL-3, MdSE RNAi, and MdSE OE plants under control or drought conditions
The expression of MdSE was examined in MdMYB88 and MdMYB124 transgenic plants, which were generated previously13. qRT-PCR analysis revealed no regulation of MdSE by MdMYB88 or MdMYB124 under control or dehydration conditions (Fig. S2). To assess whether MdMYB88 or MdMYB124 expression levels are regulated by MdSE, MdSE RNAi and MdSE OE plants were generated. The transgenic plants were verified at the DNA and RNA levels (Fig. S3). After air dehydration for 2 h, transcripts of MdMYB88 or MdMYB124 were reduced dramatically in MdSE OE plants but increased in MdSE RNAi plants (Fig. 1c, d). Western blot analysis confirmed the downregulation of MdMYB88 and MdMYB124 by MdSE under drought (Fig. 1e), indicating that under drought conditions, MdSE decreases levels of MdMYB88 and MdMYB124 proteins.
Since SE is responsible for the alternative splicing of pre-mRNAs in Arabidopsis, we then examined the transcripts of MdMYB88 and MdMYB124 in MdSE RNAi plants by a RT-PCR assay. We found that decreased MdSE levels did not affect splicing of MdMYB88 and MdMYB124 in apple under control or drought conditions (Fig. S4).
MdSE subcellular localization and expression pattern
Protein alignment demonstrated that MdSE shares 67.2% sequence similarity with Arabidopsis SE and is more closely related to SERRATE from Prunus persica (Fig. S5). Based on a transient expression assay, the YFP–MdSE fusion protein was present in the nucleus of tobacco cells (Fig. 2a), consistent with the nuclear localization of SE in Arabidopsis31. SE from M. prunifolia was found to be expressed predominantly in flowers, followed by stems, leaves, and roots (Fig. 2b). The MdSE expression level was reduced in response to drought stress (Fig. 2c).
Fig. 2. MdSE localization and expression patterns.
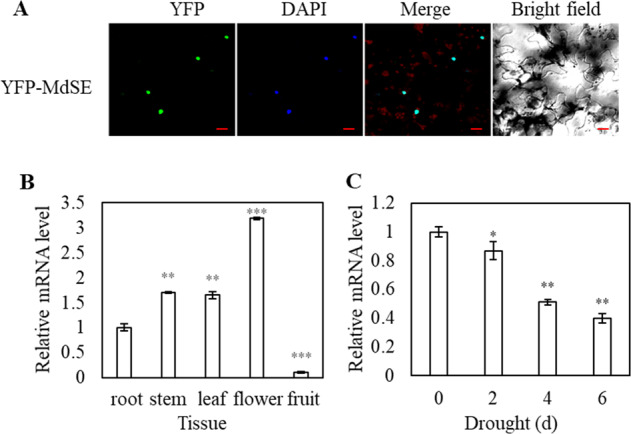
a MdSE is localized in the nucleus. Bars = 20μm. b Expression of MdSE in different organs in M. prunifolia. c Transcript level of MdSE in response to drought. Error bars indicate the standard deviation (n = 3 in b and c). Student’s t test was performed, and statistically significant differences are indicated by *P < 0.05, **P < 0.01, or ***P < 0.001
MdSE is a negative regulator of drought tolerance
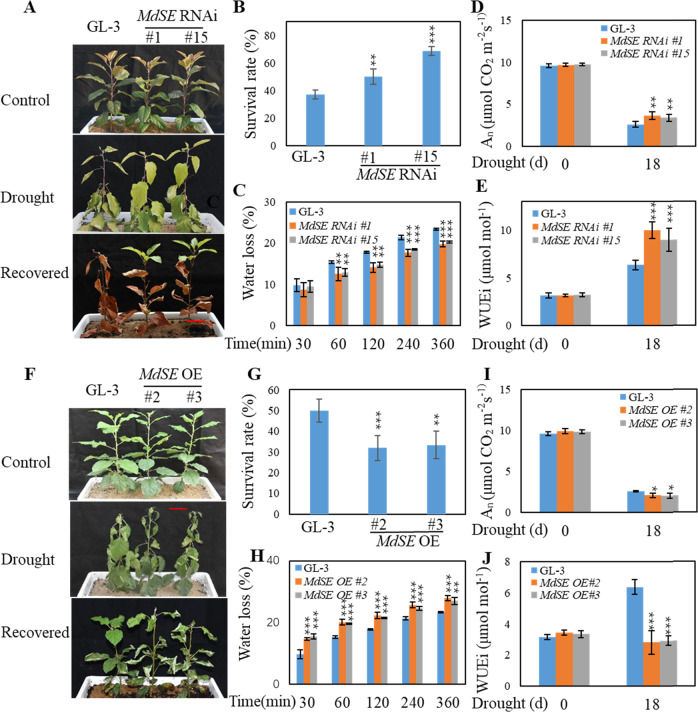
To understand the biological function of MdSE in the drought response of apple, the drought tolerance of 5-month-old MdSE transgenic plants and nontransgenic GL-3 plants was examined. After withholding water for 30 days, and then rewatering for 7 days, 50–70% of MdSE RNAi plants survived, whereas only 40% of the control plants were alive (Fig. 3a, b). Water loss from MdSE RNAi plants was much lower than loss from GL-3 plants (Fig. 3c). Compared with GL-3 plants under drought for 18 days, MdSE RNAi plants had higher photosynthesis rates and water use efficiency (Fig. 3d, e).
Fig. 3. MdSE transgenic plant response to drought stress.
a, f Drought tolerance of GL-3 and transgenic plants. Bars = 5cm. Five-month-old MdSE OE and GL-3 plants were treated with drought for 24 days, and then rewatered for 7 days. MdSE RNAi and GL-3 plants were treated with drought for 30 days, and then rewatered for 7 days. b, g Survival rate of GL-3 and transgenic plants shown in a and f. Data are means ± SD (n = 36). c, h Water loss of GL-3 and MdSE OE or RNAi plants under dehydration conditions for up to 360 min. Data are means ± SD (n = 10). d, i The rate of photosynthesis (AN). e, j Intrinsic water use efficiency (WUEi). Data are means ± SD (n = 15). Student’s t test was performed, and statistically significant differences are indicated by *P < 0.05, **P < 0.01, or ***P < 0.001
When exposed to drought for 24 days and rewatered for 7 days, ~30% of 5-month-old MdSE OE plants survived; in contrast, 50% of GL-3 plants remained alive (Fig. 3f, g), which suggested that MdSE OE plants are more sensitive to drought stress. In addition, MdSE OE plants lost significantly more water under dehydration (Fig. 3h), and MdSE OE plants had significantly lower photosynthesis rates and water use efficiency than GL-3 plants under drought (Fig. 3i, j). Together, these data suggest that MdSE negatively regulates apple drought resistance.
MdSE regulates stomatal aperture and ABA accumulation under drought
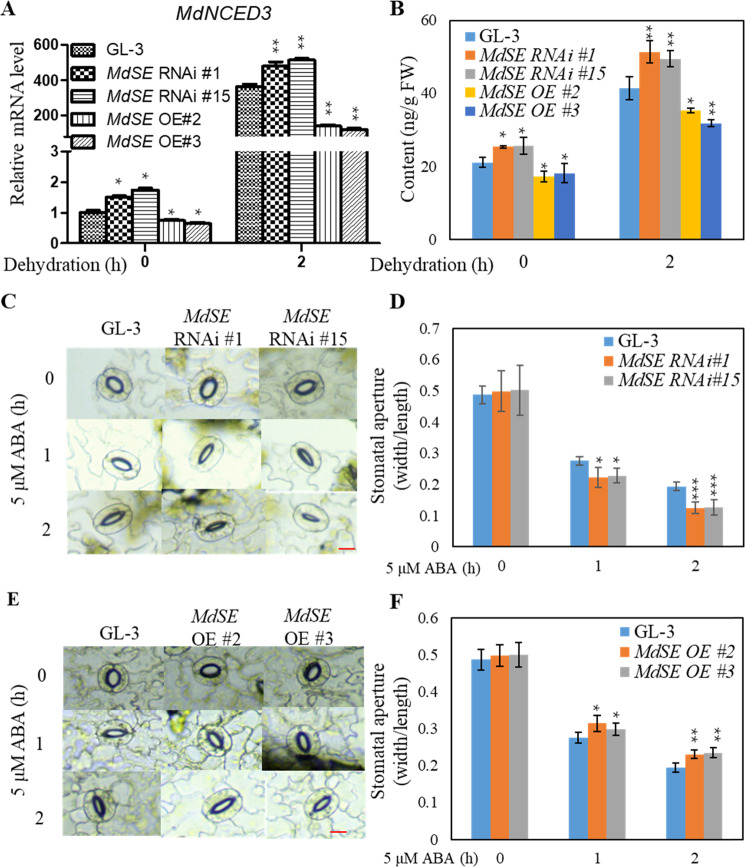
Because MdSE interacts with MdMYB88 and MdMYB124 in vivo (Fig. 1) and MdMYB88 and MdMYB124 directly regulate 9-cis-epoxycarotenoid dioxygenase 3 (MdNCED3) expression and ABA content under drought conditions (unpublished), MdSE regulation of MdNCED3 transcripts and ABA content under drought was investigated. According to qRT-PCR analysis, drought-induced MdNCED3 expression was significantly higher in MdSE RNAi plants but lower in MdSE OE plants than in GL-3 plants (Fig. 4a). The ABA content was then measured in MdSE transgenic and GL-3 plants under control and drought conditions. LC-MS analysis showed that MdSE RNAi plants contained significantly more ABA but that MdSE OE plants contained less ABA in response to drought than GL-3 plants (Fig. 4b). Consistently, MdSE RNAi plants were hypersensitive to ABA-induced stomatal closure, whereas MdSE OE plants were less sensitive (Fig. 4c–f).
Fig. 4. ABA response and content in MdSE RNAi and MdSE OE plants.
a Expression of MdNCED3 in GL-3, MdSE RNAi, or MdSE OE plants under control or dehydration conditions. Error bars indicate standard deviation (n = 3). Student’s t test was performed, and statistically significant differences are indicated by *P < 0.05 or **P < 0.01. b The ABA content in GL-3, MdSE RNAi, or MdSE OE plants under control or dehydration conditions. Error bars indicate standard deviation (n = 5). c, e Representative images of stomata of GL-3 and MdSE transgenic plants in response to ABA treatment. Bars = 10 μm. d, f Stomatal aperture of GL-3 and MdSE transgenic plants under ABA treatment. Data are the means ± SD; 5 leaves were used, and at least 80 stomatal apertures were measured for each treatment. Student’s t test was performed, and statistically significant differences are indicated by *P < 0.05, **P < 0.01, or ***P < 0.001
MdSE is enriched in the MdNCED3 promoter
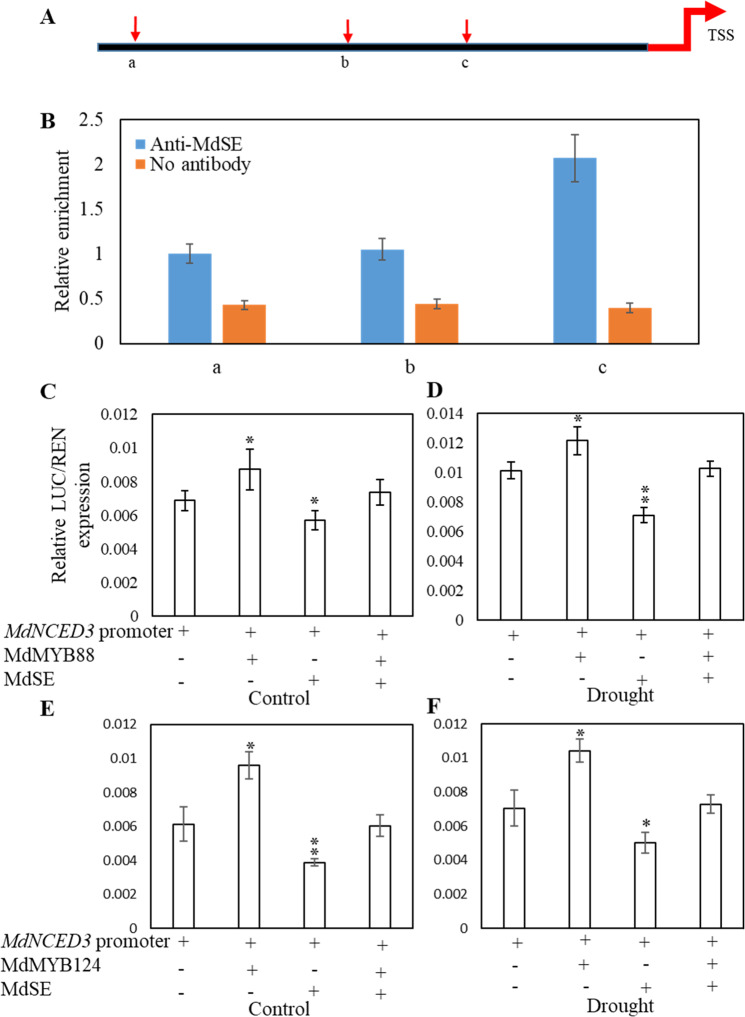
Regulation of the main biosynthetic pathway of ABA is mediated by NCED3, which cleaves 9-cis-epoxy-carotenoids and produces in the active isomer of ABA32. Because the ABA content of MdSE transgenic lines was affected under drought conditions, we investigated whether MdSE influences the MdNECD3 expression level by associating with the MdNCED3 promoter. Chromatin immunoprecipitation (ChIP) experiments were carried out using an SE-specific antibody, followed by qRT-PCR with the same primers used to assess MdMYB88 and MdMYB124 binding activity to MdNCED3. The ChIP-qPCR results showed enrichment of MdSE at the MdNCED3 promoter in the same region where MdMYB88 and MdMYB124 bind (Fig. 5a, b). A dual luciferase reporter assay was also used to detect the influence of MdSE on MdNCED3 expression. The results showed that MdMYB88 and MdMYB124 enhanced MdNCED3 expression under both normal and dehydration conditions, whereas MdSE reduced the expression of MdNCED3. When MdSE was present, the expression of MdNCED3 induced by MdMYB88 or MdMYB124 was attenuated under control and drought conditions (Fig. 5c–f). These results suggest that the regulation of MdNCED3 by MdSE depends on its association with MdMYB88 and MdMYB124.
Fig. 5. MdSE is enriched in the MdNCED3 promoter and decreases MdNCED3 activity.
a Diagram of MdNCED3 promoter regions. a–c Represent fragments containing two cis-elements of AGCCG from −1830 to −1826 bp, −1368 to −1364 bp and one cis-element of CGCGG from −880 to −876 bp, respectively. For the negative control, no antibody was added. TSS transcription start site. b MdSE enrichment in the MdNCED3 promoter determined by ChIP-qPCR analysis. c–f Relative luciferase activity from dual luciferase reporter assays in N. benthamiana leaves. Pro35S::REN was used as an internal control. Quantification was performed by normalizing firefly luciferase activity to that of Renilla luciferase. Leaves of N. benthamiana were coinfiltrated and grown for 72 h, and then leaves were collected or dehydrated for an additional 2 h. Error bars indicate standard deviation (n = 10). Student’s t test was performed, and statistically significant differences are indicated by *P < 0.05 or **P < 0.01
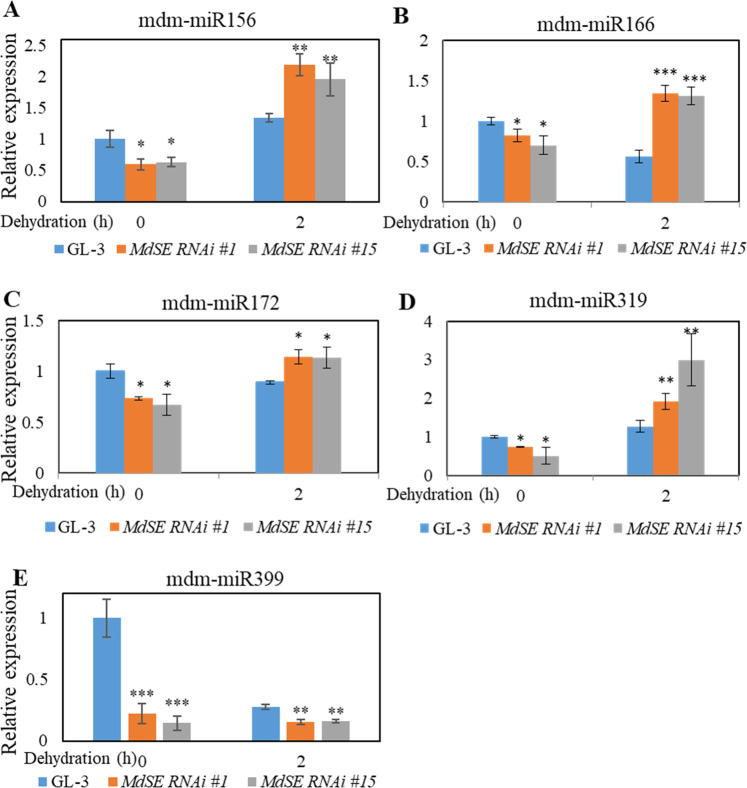
MdSE regulates the biogenesis of miRNAs in apple under drought
Arabidopsis SE is required for the biogenesis of miRNA33,34. Loss-of-function mutant plants of SE have upward curling and radialized leaves35; secondary inflorescences lack an associated cauline leaf, and inflorescences often produce siliques that emerge from the same node on the stem34. Curly leaves on MdSE RNAi plants were not observed (Fig. 3), which may be due to ~30–50% MdSE being maintained in MdSE RNAi plants (Fig. S3A). To understand whether MdSE has a similar function to Arabidopsis SE in miRNA biogenesis, we analyzed the expression of drought-responsive miRNAs, including mdm-miR156, mdm-miR166, mdm-miR172, mdm-miR319, and mdm-miR399, using stem-loop qPCR. Mdm-miR156, miR166, miR172, and miR319 are positive regulators of apple osmotic stress36 and drought resistance in alfalfa18, Arabidopsis37,38, and creeping bentgrass39, though miR399 is a negative regulator of drought resistance21. The expression levels of these miRNAs were reduced in MdSE RNAi plants under control conditions (Fig. 6), suggesting a similar role for MdSE and SE in miRNA biogenesis. Under drought stress, compared with GL-3 plants, the expression levels of mdm-miR156, mdm-miR166, mdm-miR172, and mdm-miR319 increased in MdSE RNAi plants, whereas mdm-miR399 expression was reduced (Fig. 6), consistent with the drought tolerance phenotype of MdSE RNAi plants. These data indicate that MdSE has a similar function in miRNA biogenesis as Arabidopsis SE and negatively modulates drought through regulation of drought-responsible miRNAs.
Fig. 6. MdSE affects the biogenesis of drought-responsive miRNAs in apple in response to drought.
Mdm-miR156 (a), mdm-miR166 (b), mdm-miR172 (c), mdm-miR319 (d) and mdm-miR399 (e) were determined by stem-loop qPCR. Error bars indicate standard deviation (n = 3). Student’s t test was performed, and statistically significant differences are indicated by *P < 0.05, **P < 0.01, or ***P < 0.001
Discussion
In this study, we characterized a protein interacting with MdMYB88 and MdMYB124, MdSE, in response to drought. MdSE plays a negative role in apple drought resistance by negatively modulating the expression of MdMYB88 and MdMYB124, leading to the downregulation of MdNCED3 and reduced ABA levels. Furthermore, MdSE regulates the expression of drought-responsive miRNAs under drought stress, which may contribute to its negative role in drought resistance.
The interaction between MdSE and MdMYB88 or MdMYB124 was confirmed by BiFC and Co-IP analyses. However, an in vitro interaction was not verified by Y2H analysis, indicating the possibility of a bridge between MdSE and MdMYB88. For example, the interaction of Arabidopsis C-terminal domain phosphatase-like 1 (CPL1) and HYL1 requires SE as a bridge31. MdHYL1 is a putative candidate for bridging MdSE and MdMYB88 or MdMYB124. Indeed, we found that MdHYL1 interacts with MdSE in Y2H analysis (Fig. S6). The interaction between SE and HYL1 was also observed in Arabidopsis33, indicating that the functions of SE and HYL1 in some plant processes are conserved among plant species. Factors other than MdHYL1 might act as a bridge between MdSE and MdMYB88 or MdMYB124, but further study is necessary.
SE is responsible for alternative splicing of pre-mRNAs3. In addition to the regulation of MdMYB88 and MdMYB124 transcripts by MdSE (Fig. 1), we hypothesized that MdSE might modulate the alternative splicing of MdMYB88 and MdMYB124. However, RT-PCR results showed that reduced MdSE levels did not affect splicing of MdMYB88 and MdMYB124 in apple under control and drought conditions (Fig. S4). It is possible that MdSE can affect alternative splicing of other genes under control or drought conditions. The only challenge of alternative splicing detection in apple is that 30–50% of MdSE was still functional in MdSE RNAi plants (Fig. S3A), which might affect the accuracy of analyses.
We demonstrated that MdSE is a negative regulator of drought resistance. First, survival ability analysis suggested that MdSE RNAi plants were more tolerant to drought stress but that MdSE OE plants were more sensitive (Fig. 3). Second, under dehydration conditions, MdSE OE plants lost water more quickly than MdSE RNAi plants (Fig. 3c, h). Third, under drought stress, the cell membranes of MdSE RNAi plants were less damaged, as indicated by ion leakage (Fig. 3). Fourth, MdSE negatively regulated ABA accumulation and some drought-positive miRNAs (Figs. 4 and 6a–d). In addition, MdSE positively regulated drought-negative mdm-miR399. Fifth, Arabidopsisse-1 mutants were also more tolerant to drought stress (Fig. S7). All these data support that MdSE plays a negative role in apple drought resistance and that the role of SE under drought might be conserved among plant species.
ABA is a plant stress hormone that regulates stomatal closure within and outside guard cells through combinational mechanisms40. In Arabidopsis, maize, wheat, rice, and apple, elevated ABA content contribute to plant drought tolerance by inducing stomatal closure and stress-related signal transduction21,23,24,26,27,41,42. NCED3 is considered to be the key contributor to ABA production under water deficit conditions, and ZEP and AAO3 play minor roles43,44. In Arabidopsis, short vegetative phase (SVP) is able to bind to the promoters of the ABA catabolism pathway genes CYP707A1, CYP707A3, and AtBG1, and thus contributes to ABA homeostasis45. Homeostasis of ABA was also regulated by reversible glycosylation mediated by ABA-UGTs (uridine diphosphate glucosyltransferases) to affect ABA bioactivity (Fig. S8)46,47. In our study, more ABA accumulated in MdSE RNAi transgenic lines compared with MdSE OE and GL-3 plants (Fig. 4b). Such negative regulation of ABA content should contribute to the negative role of MdSE in drought resistance. We also found that MdSE was enriched at the promoter region of MdNCED3, which is the same region bound by MdMYB88 and MdMYB124 (Fig. 5). Considering the in vivo interaction between MdSE and MdMYB88 or MdMYB124, we conclude that the enrichment of MdSE at the MdNCED3 promoter was due to recruitment by MdMYB88 or MdMYB124 instead of direct binding.
Arabidopsis SE is a critical component required for pri-miRNA processing and miRNA biogenesis33,34. The results of microarray analysis showed that numerous miRNAs and their target genes are misexpressed in se-1, including miR156, miR165, miR167, miR163, miR164, miR168, and miR1715,33. In our study, stem-loop qPCR analysis demonstrated reduced levels of mdm-miR156, mdm-miR166, mdm-miR172, mdm-miR319, mdm-miR399, and mdm-miR398 transcripts in MdSE RNAi transgenic lines under normal environmental conditions (Fig. 6), indicating a conserved role for SE in miRNA biogenesis among plant species.
miRNAs participate in various plant processes, including root development48, flowering time49, apical dominance35, and plant architecture16, and are also associated with tolerance to environmental stresses, including salt50, drought18,21, cold51, and bacterial infection36. The overexpression of mdm-miR156 in apple calli enhances osmotic stress22 and drought stress tolerance in alfalfa (Medicago sativa)18; miR166, miR172, and miR319 are also reported to act as positive regulators of drought tolerance in rice38, soybean52, and creeping bentgrass (Agrostis stolonifera)39, and miR399 plays a negative role in Arabidopsis drought resistance21. In our study, mdm-miR156, mdm-miR166, mdm-miR172, and mdm-miR319 were induced in MdSE RNAi transgenic plants after drought exposure, whereas mdm-miR399 was reduced, suggesting that these factors might contribute to the drought tolerance of MdSE RNAi plants.
In summary, our study elucidated the roles of MdSE in drought stress resistance. MdSE plays a negative role in drought resistance by affecting miRNA biogenesis and negatively regulating protein accumulation of MdMYB88 and MdMYB124, which results in negative regulation of ABA accumulation. Our study provides a deeper understanding of the complex mechanism of MdSE in response to drought stress and identifies a candidate gene for drought improvement through molecular breeding.
Materials and methods
Plant materials and growth conditions
For gene cloning, “Golden delicious” (Malus × domestica) grown in a greenhouse was used for RNA extraction. GL-3, a genotype selected from seedlings of “Royal Gala” (Malus × domestica), was used for genetic transformation53. GL-3 grown on Murashige and Skoog (MS) medium (4.43 g/L MS salts, 30 g/L sucrose, and 7 g/L agar, pH 5.8) supplemented with 0.2 mg/L 6-benzylaminopurine and 0.2 mg/L indoleacetic acid (IAA) under long-day conditions (14 h light/10 h dark cycle) for 4 weeks at 25 °C were used for gene transformation. MdMYB88/124 RNAi plants and MdMYB88 or MdMYB124 overexpression plants were produced in a previous study13. The transgenic plants were rooted in MS medium (2.22 g/L MS salts, 20 g/L sucrose, 7.5 g/L agar, 0.5 mg/L IAA, 0.5 mg/L indolebutyric acid (IBA), pH 5.8) for 2 months, and then transplanted to substrate (Pindstrup, Denmark). se-1 was obtained from ABRC.
RNA extraction and qRT-PCR analysis
Detailed methods for RNA extraction and qRT-PCR analysis are provided in ref. 13. The primers used for qRT-PCR analysis are listed in Supplementary Table 1.
Generation of transgenic apple
To generate a construct for MdSE overexpression, the coding region (CDS) of MdSE was cloned into pGWB414 to produce MdSE-pGWB414. To knock down MdSE, a 292-bp fragment of MdSE was introduced into pK7WIWG2D, resulting in MdSE-pK7WIWG2D. Both plasmids were transformed into Agrobacterium strain EHA105. For genetic transformation, we used an Agrobacterium-mediated transformation method. Plant transformation was carried out according to Dai et al.53. Briefly, 4-week-old GL-3 leaves were cut into strips in liquid MS medium (4.43 g/L MS salts and 30 g/L sucrose, pH 5.2) with EHA105 (OD600 = 0.6–0.9) carrying the relevant plasmid for 15 min. Then, the leaf strips were transferred into maintenance medium (4.43 g/L MS + 2 mg/L TDZ + 0.5 mg/L NAA + 100 μM acetosyringone +1 mM betaine +7.5 g/L agar +30 mg/L sugar, pH = 5.8). After 3 days, leaf strips were transferred into selection medium (4.43 g/L MS + 2 mg/L TDZ + 0.5 mg/L NAA + 250 mg/L cefotaxime +50 mg/L kanamycin +7.5 g/L agar +30 mg/L sugar, pH = 5.8) for 4 weeks in the dark, and then incubated for 6 weeks under light. The transgenic buds that stayed green on selection medium were grown for ~4 weeks. DNA and RNA were extracted from the transgenic plants and GL-3 and used to detect transgene insertion and MdSE expression levels by PCR and RT-qPCR, respectively. Transgenic plants with transgene insertion, as well as altered expression levels of MdSE, were selected for further experiments. The primers used are listed in Supplementary Table 1.
Drought treatment
Drought treatment was carried out by withholding water for a certain number of days, and then rewatering for 7 days, followed by calculation of the survival rate. Specifically, 5-month-old MdSE OE and GL-3 plants were treated with drought for 24 days, and then rewatered for 7 days. MdSE RNAi and GL-3 plants were treated with drought for 30 days, and then rewatered for 7 days. To obtain photosynthesis data (the rate of photosynthesis and intrinsic water use efficiency), a LiCor-6400 portable photosynthesis system (LiCor) was used.
Detached leaves from 5-month-old MdSE OE, MdSE RNAi, and GL-3 were used for the water loss assay.
Stomatal aperture measurements
For stomatal aperture measurements, we used leaves of 2-month-old soil-grown transgenic apple and GL-3 plants. Leaves were cut off and plunged into stomatal opening solution (30 mM KCl, 0.1 mM CaCl2, and 10 mM MES-KOH, pH 6.15) under light (120 μmol m−2 s−1) for 2 h to induce stomatal opening as described54. Then, ABA was added to the stomatal opening solution to a final concentration of 5 μM. The leaf epidermis was observed for stomatal aperture with an EX30 microscope (SDPTOP) after ABA treatment for 1 or 2 h. Stomatal length and width were measured by ImageJ software, and stomatal aperture was then calculated.
Western blot
Proteins were extracted from leaf samples with extraction buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 2 mM EDTA, 10% glycerol, 1% NP-40, 1 mM phenylmethylsulfonyl fluoride). Twenty micrograms of protein was separated by 10% SDS–PAGE and blotted onto PVDF membranes (Millipore) using standard methods. The blots were blocked for 2 h in PBS (50 mM Na2HPO4, pH 7.4) with 5% nonfat milk, after which anti-MdMYB88 and MdMYB124 or anti-actin (ABclonal, AC009) antibodies were added. After 2 h, the blots were washed twice in PBS milk, and a secondary antibody (goat anti-rabbit horseradish peroxidase-conjugated, 1 mg/mL; catalog no. HS101; Transgen Biotech) was added. After washing, the blots were treated with Bio-Rad ChemiDoc XRS+ to visualize the signals.
Yeast two-hybrid assay
A yeast two-hybrid assay was carried out according to the manufacturer’s manuals (Clontech, 630439, 630489). MdMYB88–155 aa and CDS of MdSE were introduced into pGBDT7. CDS of MdMYB88 or MdHYL1 was cloned into pGADT7. MdSE-pGBDT7 and MdMYB88-pGADT7 or MdHYL1-pGADT7 were cotransformed into yeast strain AH109. MdMYB88–155 aa-pGBDT7 and MdHYL1-pGADT7 were also cotransformed into yeast strain AH109. Positive clones were selected on SD/-Leu-Trp, and then on SD/-Leu-Trp-His-Ade + x-α-gal plates for the x-α-gal assay. The primers used are listed in Supplementary Table 1.
Subcellular localization, BiFC, and Co-IP assays
To generate constructs for BiFC assays, we cloned the CDS of MdMYB88 and its paralog gene MdMYB124 into pSPYNE-35S; the CDS of MdSE was cloned into the pSPYCE-35S vector. For subcellular localization, the CDS of MdSE was cloned into the pEearleyGate104 vector. Transient expression assays were performed according to Xie et al.13. After 3 days, fluorescent signals in transformed tobacco leaves were then detected using a Nikon A1R/A1 confocal microscope (Nikon).
For Co-IP analysis, the CDS of MdMYB88 was amplified by PCR and cloned into pEarleyGate 101; the CDS of MdSE was cloned into pEarleyGate 203. Co-IP analyses were performed as described previously55.
The primers used are listed in Supplementary Table 1.
ChIP-qPCR
ChIP-qPCR assays were performed as described previously13. Tissue-cultured GL-3 was used for crosslinking, and the ChIP assay was performed with an anti-SE antibody (Agrisera, AS09 532A). Three regions of the MdNCED3 promoter were examined by qPCR, with no antibody ChIP samples serving as the reference. The primers used for ChIP-qPCR are listed in Supplementary Table 1.
ABA measurement
ABA was extracted as described56. Frozen apple leaf samples (about 100 mg fresh weight) was ground in liquid nitrogen, and then extracted with 1 ml of cold extraction buffer (methanol:isopropanol:acetic acid = 20:79:1, v/v/v). After centrifugation at 4 °C and 12,000 rpm for 10 min, the supernatant was transferred into a 2 mL tube, and 500 μL cold extraction buffer was added followed by vortexing for 5 min. The extraction process was repeated three times followed by centrifugation at 4 °C and 12,000 rpm for 10 min. The supernatant was filtered through a 0.22 μm PTFE filter (Waters, Milford, MA, USA). GC-MS analysis was carried out using a QTRAP® 5500 LC-MS/MS (AB SCIEX, Redwood City, USA).
Accession numbers
Sequence data can be found in NCBI under the following numbers: MdMYB88 (KY569647), MdMYB124 (KY569648), MdSE (KY568649), and MdNCED3 (XM_008380174.2).
Supplementary information
Acknowledgements
We thank Dr Zhihong Zhang from Shenyang Agricultural University for providing tissue-cultured GL-3 plants. This work was supported by the National Key Research and Development Program of China (2019YFD1000100) and the National Natural Science Foundation of China (31622049 and 31872080).
Author contributions
Q.G. designed the project. X.L., P.C., Y.Y., L.W., H.D., and J.Z. performed the experiments. Y.Y., L.W., H.D., Y.X., L.X., and F.M. analyzed the data. L.X. and P.C. wrote the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
These authors contributed equally: Xuewei Li, Pengxiang Chen
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41438-020-0320-6).
References
- 1.Clarke JH, Tack DF, Findlay KM, Van Montagu M, Van Lijsebettens M. The SERRATE locus controls the formation of the early juvenile leaves and phase length in Arabidopsis. Plant J. 1999;20:493–501. doi: 10.1046/j.1365-313x.1999.00623.x. [DOI] [PubMed] [Google Scholar]
- 2.Prigge MJ, Wagner DR. The Arabidopsis SERRATE gene encodes a zinc-finger protein required fornormal shoot development. Plant Cell. 2001;13:1263–1279. doi: 10.1105/tpc.13.6.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raczynska KD, et al. The SERRATE protein is involved in alternative splicing in Arabidopsisthaliana. Nucleic Acids Res. 2014;42:1224–1244. doi: 10.1093/nar/gkt894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christie M, Carroll BJ. SERRATE is required for intron suppression of RNA silencing in Arabidopsis. Plant Signal Behav. 2011;6:2035–2037. doi: 10.4161/psb.6.12.18238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Speth C, et al. Arabidopsis RNA processing factor SERRATE regulates the transcription of intronless genes. Elife. 2018;7:e37078. doi: 10.7554/eLife.37078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Komatsu S, et al. Label-free quantitative proteomic analysis of abscisic acid effect in early-stage soybean under flooding. J. Proteome Res. 2013;1:4769. doi: 10.1021/pr4001898. [DOI] [PubMed] [Google Scholar]
- 7.Dong Z, Han MH, Fedoroff N. The RNA-binding proteins HYL1 and SE promote accurate in vitro processing of pri-miRNA by DCL1. Proc. Natl Acad. Sci. USA. 2008;105:9970–9975. doi: 10.1073/pnas.0803356105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sascha L, et al. Dual roles of the nuclear cap-binding complex and SERRATE in pre-mRNA splicing and microRNA processing in Arabidopsis thaliana. Proc. Natl Acad. Sci. USA. 2008;105:8795–8800. doi: 10.1073/pnas.0802493105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sabin LR, et al. Ars2 regulates both miRNA- and siRNA- dependent silencing and suppresses RNA virus infection in Drosophila. Cell. 2009;138:340–351. doi: 10.1016/j.cell.2009.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.An JP, et al. Apple MdMYC2 reduces aluminum stress tolerance by directly regulating MdERF3 gene. Plant Soil. 2017;418:255–266. [Google Scholar]
- 11.Geng DL, et al. MdMYB88 and MdMYB124 enhance drought tolerance by modulating root vessels and cell walls in apple. Plant Physiol. 2018;178:1296–1309. doi: 10.1104/pp.18.00502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun X, et al. Improvement of drought tolerance by overexpressing MdATG18a is mediated by modified antioxidant system and activated autophagy in transgenic apple. Plant Biotechnol. J. 2018;16:545–557. doi: 10.1111/pbi.12794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie YP, et al. An atypical R2R3 MYB transcription factor increases cold hardiness by CBF-dependent and CBF-independent pathways in apple. N. Phytol. 2018;218:201–218. doi: 10.1111/nph.14952. [DOI] [PubMed] [Google Scholar]
- 14.Wang HB, et al. Mapping QTLs for water-use efficiency reveals the potential candidate genes involved in regulating the trait in apple under drought stress. BMC Plant Biol. 2018;18:136. doi: 10.1186/s12870-018-1308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer JJ, Beatty PH, Good AG, Muench DG. Manipulation of microRNA expression to improve nitrogen use efficiency. Plant Sci. 2013;210:70–81. doi: 10.1016/j.plantsci.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Jiao Y, et al. Regulation of OsSPL14 by OsmiR156 defines ideal plant architecture in rice. Nat. Genet. 2010;42:541–544. doi: 10.1038/ng.591. [DOI] [PubMed] [Google Scholar]
- 17.Zeng Z, Liu Z, Xia R. Small RNAs, emerging regulators critical for the development of horticultural traits. Hortic. Res. 2018;5:63. doi: 10.1038/s41438-018-0072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arshad M, Feyissa BA, Amyot L, Aung B, Hannoufa A. MicroRNA156 improves drought stress tolerance in alfalfa (Medicago sativa) by silencing SPL13. Plant Sci. 2017;258:122–136. doi: 10.1016/j.plantsci.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 19.Feyissa BA, Arshad M, Gruber MY, Kohalmi SE, Hannoufa A. The interplay between miR156/SPL13 and DFR/WD40-1 regulate drought tolerance in alfalfa. BMC Plant Biol. 2019;19:434. doi: 10.1186/s12870-019-2059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xia KF, et al. OsTIR1 and OsAFB2 downregulation via OsmiR393 overexpression leads to more tillers, early flowering and less tolerance to salt and drought in rice. PLoS ONE. 2012;7:e30039. doi: 10.1371/journal.pone.0030039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baek D, et al. A role for Arabidopsis miR399f in salt, drought, and ABA signaling. Mol. Cells. 2016;39:111–118. doi: 10.14348/molcells.2016.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xia R, Hong Z, An YQ, Eric PB, Liu Z. Apple miRNAs and tasiRNAs with novel regulatory networks. Genome Biol. 2012;13:R47–R47. doi: 10.1186/gb-2012-13-6-r47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niu C, et al. Genome-wide identification of drought-responsive microRNAs in two sets of Malus from interspecific hybrid progenies. Hortic. Res. 2019;6:75. doi: 10.1038/s41438-019-0157-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim CW, Baek W, Jung J, Kim JH, Lee SC. Function of ABA in stomatal defense against biotic and drought stresses. Int J. Mol. Sci. 2015;16:15251–15270. doi: 10.3390/ijms160715251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shinozaki K, Yamaguchi-Shinozaki K. Molecular responses to dehydration and low temperature: differences and cross-talk between two stress signaling pathways. Curr. Opin. Plant Biol. 2000;3:217–223. [PubMed] [Google Scholar]
- 26.Alvarez S, Roy CS, Pandey S. Comparative quantitative proteomics analysis of the ABA response of roots of drought-sensitive and drought-tolerant wheat varieties identifies proteomic signatures of drought adaptability. J. Proteome Res. 2014;13:1688–1701. doi: 10.1021/pr401165b. [DOI] [PubMed] [Google Scholar]
- 27.Zamora-Briseno JA, de Jimenez ES. A LEA 4 protein up-regulated by ABA is involved in drought response in maize roots. Mol. Biol. Rep. 2016;43:221–228. doi: 10.1007/s11033-016-3963-5. [DOI] [PubMed] [Google Scholar]
- 28.Yao L, et al. The AWPM-19 family protein OsPM1 mediates abscisic acid influx and drought response in rice. Plant Cell. 2018;30:1258–1276. doi: 10.1105/tpc.17.00770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pei ZM, et al. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature. 2000;406:731–734. doi: 10.1038/35021067. [DOI] [PubMed] [Google Scholar]
- 30.Schroeder JI, Kwak JM, Allen GJ. Guard cell abscisic acid signalling and engineering drought hardiness in plants. Nature. 2001;410:327–330. doi: 10.1038/35066500. [DOI] [PubMed] [Google Scholar]
- 31.Manavella PA, et al. Fast-forward genetics identifies plant CPL phosphatases as regulators of miRNA processing factor HYL1. Cell. 2012;151:859–870. doi: 10.1016/j.cell.2012.09.039. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz SH, Tan BC, Gage DA, Zeevaart JA, Mccarty DR. Specific oxidative cleavage of carotenoids by VP14 of maize. Science. 1997;276:1872–1874. doi: 10.1126/science.276.5320.1872. [DOI] [PubMed] [Google Scholar]
- 33.Lobbes D, Rallapalli G, Schmidt DD, Martin C, Clarke J. SERRATE: a new player on the plant microRNA scene. EMBO Rep. 2006;7:1052–1058. doi: 10.1038/sj.embor.7400806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang L, Liu Z, Lu F, Dong A, Huang H. SERRATE is a novel nuclear regulator in primary microRNA processing in Arabidopsis. Plant J. 2010;47:841–850. doi: 10.1111/j.1365-313X.2006.02835.x. [DOI] [PubMed] [Google Scholar]
- 35.Grigg SP, Claudia C, Angela H, Miltos T. SERRATE coordinates shoot meristem function and leaf axial patterning in Arabidopsis. Nature. 2005;437:1022–1026. doi: 10.1038/nature04052. [DOI] [PubMed] [Google Scholar]
- 36.Niu D, et al. miRNA863-3p sequentially targets negative immune regulator ARLPKs and positive regulator SERRATE upon bacterial infection. Nat. Commun. 2016;7:11324. doi: 10.1038/ncomms11324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang T, et al. The interaction between miR160 and miR165/166 in the control of leaf development and drought tolerance in Arabidopsis. Sci. Rep. 2019;9:2832. doi: 10.1038/s41598-019-39397-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang J, et al. Knockdown of rice microRNA166 confers drought resistance by causing Leaf rolling and altering stem xylem development. Plant Physiol. 2018;176:2082. doi: 10.1104/pp.17.01432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou M, et al. Constitutive expression of a miR319 gene alters plant development and enhances salt and drought tolerance in transgenic creeping bentgrass. Plant Physiol. 2014;161:1375–1391. doi: 10.1104/pp.112.208702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuromori T, Seo M, Shinozaki K. ABA transport and plant water stress responses. Trends Plant Sci. 2018;23:513–522. doi: 10.1016/j.tplants.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 41.Seiler C, et al. ABA biosynthesis and degradation contributing to ABA homeostasis during barley seed development under control and terminal drought-stress conditions. J. Exp. Bot. 2011;62:2615–2632. doi: 10.1093/jxb/erq446. [DOI] [PubMed] [Google Scholar]
- 42.Zhang L, et al. Role of abscisic acid (ABA) in modulating the responses of two apple rootstocks to drought stress. Pak. J. Bot. 2014;46:117–126. [Google Scholar]
- 43.Endo A, et al. Drought induction of Arabidopsis 9-cis-epoxycarotenoid dioxygenase occurs in vascular parenchyma cells. Plant Physiol. 2008;147:1984–1993. doi: 10.1104/pp.108.116632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seo M, et al. The Arabidopsis aldehyde oxidase 3 (AAO3) gene product catalyzes the final step in abscisic acid biosynthesis in leaves. Proc. Natl Acad. Sci. USA. 2000;97:12908–12913. doi: 10.1073/pnas.220426197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Z, et al. The flowering repressor SVP confers drought resistance in Arabidopsis by regulating abscisic acid catabolism. Mol. Plant. 2018;11:1184–1197. [Google Scholar]
- 46.Bowles D, Isayenkova J, Lim EK, Poppenberger B. Glycosyltransferases: managers of small molecules. Curr. Opin. Plant Biol. 2005;8:254–263. doi: 10.1016/j.pbi.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 47.Yonekura-Sakakibara K, Hanada K. An evolutionary view of functional diversity in family 1 glycosyltransferases. Plant J. 2011;66:182–193. doi: 10.1111/j.1365-313X.2011.04493.x. [DOI] [PubMed] [Google Scholar]
- 48.Bazin J, Bustos-Sanmamed P, Hartmann C, Lelandais-Briere C, Crespi M. Complexity of miRNA-dependent regulation in root symbiosis. Philos. Trans. R. Soc. Lond. B. 2012;367:1570–1579. doi: 10.1098/rstb.2011.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gandikota M, et al. The miRNA156/157 recognition element in the 3’ UTR of the Arabidopsis SBP box gene SPL3 prevents early flowering by translational inhibition in seedlings. Plant J. 2007;49:683–693. doi: 10.1111/j.1365-313X.2006.02983.x. [DOI] [PubMed] [Google Scholar]
- 50.Sun X, et al. Identification of novel and salt-responsive miRNAs to explore miRNA-mediated regulatory network of salt stress response in radish (Raphanus sativus L.) BMC Genomics. 2015;16:1–16. doi: 10.1186/s12864-015-1416-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kis A, et al. Polycistronic artificial miRNA‐mediated resistance to wheat dwarf virus in barley is highly efficient at low temperature. Mol. Plant Pathol. 2016;17:427–437. doi: 10.1111/mpp.12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li W, Wang T, Zhang Y, Li Y. Overexpression of soybean miR172c confers tolerance to water deficit and salt stress, but increases ABA sensitivity in transgenic Arabidopsis thaliana. J. Exp. Bot. 2016;67:175. doi: 10.1093/jxb/erv450. [DOI] [PubMed] [Google Scholar]
- 53.Dai H, et al. Development of a seedling clone with high regeneration capacity and susceptibility to Agrobacterium in apple. Sci. Hortic. 2013;164:202–208. [Google Scholar]
- 54.Kwak JM, et al. Dominant negative guard cell K+ channel mutants reduce inward-rectifying K+ currents and light-induced stomatal opening in Arabidopsis. Plant Physiol. 2001;127:473–485. [PMC free article] [PubMed] [Google Scholar]
- 55.Guan Q, et al. The protein phosphatase RCF2 and its interacting partner NAC019 are critical for heat stress-responsive gene regulation and thermotolerance in Arabidopsis. Plant Cell. 2014;26:438–453. doi: 10.1105/tpc.113.118927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Müller M, Munné-Bosch S. Rapid and sensitive hormonal profiling of complex plant samples by liquid chromatography coupled to electrospray ionization tandem mass spectrometry. Plant Methods. 2011;7:37. doi: 10.1186/1746-4811-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.