Abstract
Overuse of fungicides and fertilizers has resulted in copper (Cu) contamination of soils and toxic levels of Cu in apple fruits. To breed Cu-resistant apple (Malus domestica) cultivars, the underlying molecular mechanisms and key genes involved in Cu resistance must be identified. Here, we show that MdWRKY11 increases Cu tolerance by directly promoting the transcription of MdHMA5. MdHMA5 is a Cu transporter that may function in the storage of excess Cu in root cell walls and stems for Cu tolerance in apple. The transcription factor MdWRKY11 is highly induced by excess Cu. MdWRKY11 overexpression in transgenic apple enhanced Cu tolerance and decreased Cu accumulation. Apple calli transformed with an MdWRKY11-RNAi construct exhibited the opposite phenotype. Both an in vivo chromatin immunoprecipitation assay and an in vitro electrophoretic mobility shift assay indicated that MdWRKY11 binds to the promoter of MdHMA5. Furthermore, MdWRKY11 promoted MdHMA5 expression in transgenic apple plants, as revealed by quantitative PCR. Moreover, inhibition of MdWRKY11 expression by RNA interference led to a significant decrease in MdHMA5 transcription. Thus, MdWRKY11 directly regulates MdHMA5 transcription. Our work resulted in the identification of a novel MdWRKY11-MdHMA5 pathway that mediates Cu resistance in apple.
Subject terms: Plant molecular biology, Plant stress responses
Introduction
Apple (Malus domestica) is one of the four most widely cultivated fruit crop species, and ensuring that apples do not accumulate toxic levels of metals from the soil is important for public health1,2. The widespread use of the Bordeaux fungicide mixture, farmyard manure containing Cu as fertilizer, and wastewater for irrigation has led to the accumulation of excess Cu in the soil and in apple fruits1. Indeed, the Cu levels of apples in many orchards have been reported to be ten times higher than safe limits3,4, and the problem is getting worse. The threat to human health from toxic, Cu-contaminated apple fruits is a long-term problem because it removing excess Cu already present in soils is challenging5.
An extreme excess amount of Cu in the soil leads to leaf chlorosis, limits apple tree growth, and greatly reduces yield6; however, light-to-moderate Cu pollution of orchard soils, which does not cause these symptoms, probably poses a greater threat to human health, as toxic, Cu-contaminated apple fruits can continue to be produced by trees that show no signs of Cu stress, causing the problem to go undetected1,7. Therefore, it is important to elucidate the molecular mechanisms underlying the response to excess Cu in apple both for monitoring Cu contamination and for molecular breeding of Cu-resistant apple cultivars.
Excess Cu inhibits photosystem II activity and photosynthesis, impairs the elongation of roots and shoots, reduces fruit quality and yield, and can trigger senescence and death8–11. To withstand excess Cu in the soil, plants have developed two strategies for maintaining normal Cu levels in their tissues: Cu efflux and Cu sequestration12. When excess Cu enters root epidermal cells, the first strategy is to export Cu back outside the plant cytoplasm, possibly via storage in the root cell wall12,13. The second strategy is to store excess Cu in tissues that are less sensitive to the toxic effects of Cu, such as stem tissue12. In this second case, in chelated form, Cu moves up the stem through the transpiration stream after being transported into xylem14. Chelation not only decreases the cytosolic free Cu concentration, thereby reducing photosystem II damage, but also facilitates Cu transport through the plant15. Once free Cu is chelated by metallothionein or phytochelatin proteins, metallochaperones deliver the Cu–ligand complexes directly to P1B-type ATPases for transport16,17.
Heavy Metal ATPase 5 (HMA5) is a Cu-specific P1B-type ATPase that transports chelated Cu across membranes18. HMA5 is involved in Cu tolerance in two ways: it transports Cu out of the root, and it mediates Cu uploading for long-distance transport and redistribution within the plant. In the Cu export strategy, HMA5 in the plasma membrane of root epidermal cells transports excess Cu out of the cytoplasm to maintain proper Cu levels in the plant19. AtHMA5, which has been identified as component of a QTL in Arabidopsis thaliana, transports chelated Cu outside root epidermal cells under Cu excess stress20,21. The Arabidopsis hma5 mutant is hypersensitive to excess Cu and accumulates relatively large amounts of Cu in its roots16. A similar function was reported for SvHMA5II in Silene vulgaris21. With respect to the redistribution strategy, chelated Cu moves laterally from cell to cell via HMA5 transporters and is ultimately uploaded to the xylem for transport from the roots to the stem22,23. The Cu insensitivity of the stem makes this tissue an ideal place to sequester excess Cu away from Cu-sensitive organs such as roots and leaves19,23. In rice (Oryza sativa), OsHMA5 is localized in the plasma membrane of root pericycle cells, where it loads Cu into the xylem for long-distance transport to stems22,24. However, there have not been any reports of HMA5 genes in woody plant species, in which the Cu resistance mechanism is expected to be even more complex.
Transcription factors (TFs) play a central role in the response to excess heavy metal by orchestrating several physiological processes25–28. There have been several reports on the transcriptional regulation of the Cu response in multicellular eukaryotes. The transcription factors SPL7, CRR1, and Ace1-like protein regulate the Cu chaperones CCH (involved in Cu chelation and detoxification), COPT1 (involved in Cu absorption), and FeSOD and Cu/ZnSOD (involved in reactive oxygen species mitigation), respectively29–31. However, the transcriptional regulation of HMA5 under excess Cu remains unknown.
WRKY TFs play a critical role in the response to excess heavy metals (iron, cadmium, and aluminum) by regulating their chelation and translocation of the metals and by reducing secondary oxidative damage32–34. WRKYs belong to one of the largest TF families in plants and are named for their highly conserved WRKYGQK heptapeptide at the N-terminus, which specifically binds to W-box cis-elements (containing a TTGACC/T core sequence) in the promoters of downstream target genes35–37. However, it is not known whether WRKY TFs are involved in the response to excess Cu or what regulatory pathways might be involved.
In this study, we isolated MdWRKY11, which is significantly induced in response to Cu stress, in apple. Overexpression of MdWRKY11 conferred increased Cu tolerance to transgenic apple trees. Furthermore, we demonstrated that MdWRKY11 directly binds to the promoter of MdHMA5, which encodes a P1B ATPase, and activates its expression. MdHMA5 functions in Cu transport and decreases Cu accumulation in apple plants. In addition to isolating a novel transcriptional regulatory pathway of Cu tolerance in plants, this study provides marker genes for monitoring Cu contamination in orchards.
Results
Expression of MdWRKY11 in response to CuSO4 treatment
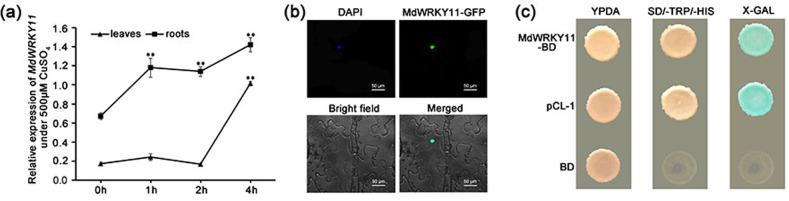
To identify WRKY genes that might be involved in the response to excess Cu, we screened the expression of 29 candidate MdWRKYs in the leaves and roots of hydroponic plants treated with 500 μM CuSO4. Among these MdWRKYs, MdWRKY11 expression was significantly induced in response to CuSO4 treatment in both the roots and the leaves (Fig. 1a), suggesting that this gene has an important role in the response to excess Cu. Therefore, we selected MdWRKY11 for further study.
Fig. 1. Expression, subcellular localization, and transcriptional activity of MdWRKY11.
aMdWRKY11 expression in the leaves and roots under excess Cu stress, as detected by qPCR. The MdWRKY11 expression level was normalized to the internal MdActin expression level. The apple plants were treated with 500μM CuSO4 for 0, 1, 2, and 4h. The data are the means±SDs of triplicate experiments for each time point. The asterisks indicate values that are significantly different from those of the control (Student’s t-test): *P < 0.05; **P < 0.01. b MdWRKY11-GFP is localized to the nucleus of Nicotiana benthamiana cells. 35S::MdWRKY11-GFP was transiently expressed in epidermal cells of N. benthamiana leaves and visualized by confocal microscopy (×40). The nucleus was dyed with 4,6-diamidino-2-phenylindole (DAPI). c Transcriptional activation of MdWRKY11 in yeast cells. Yeast AH109 strains expressing pCL-1, binding domain (BD), and pBD-MdWRKY11 were cultured on yeast peptone dextrose adenine agar (YPDA) or selective SD-His-Trp media. pCL-1 encoding the GAL4 protein and the empty vector pGBKT7 (BD) were used as the positive and negative controls, respectively
Subcellular localization of MdWRKY11
To examine the subcellular localization of MdWRKY11, 35S::MdWRKY11-GFP was infiltrated into N. benthamiana leaves via Agrobacterium-mediated transient transformation. The MdWRKY11-GFP fluorescence was localized exclusively to the nucleus (Fig. 1b).
Transcriptional activity of MdWRKY11
The transcriptional activation activity of MdWRKY11 was assayed in a yeast system. Yeast cells transformed with pBD-MdWRKY11 or the positive control construct pCL-1 grew well on SD-Trp-His selective media and displayed α-galactosidase activity, whereas yeast cells carrying the negative control construct pGBKT7 were unable to grow on the selective medium (Fig. 1c). These results indicate that MdWRKY11 is a transcriptional activator in the yeast system.
Cu tolerance of transgenic apple plants overexpressing MdWRKY11
To investigate the potential function of MdWRKY11 in Cu tolerance, transgenic apple plants overexpressing MdWRKY11 were generated via Agrobacterium-mediated transformation. The expression of MdWRKY11 in OEWRKY11-1, OEWRKY11-2, and OEWRKY11-3 transgenic apple lines was significantly higher than that in the untransformed controls (Fig. S1a). Therefore, we selected these three lines for further analysis.
The control apple plants grew slowly under excess Cu conditions. After thirty days of Cu treatment, the older leaves displayed chlorosis and brown spots, and the newer leaves turned yellow. However, these toxic symptoms were not observed in the transgenic plants (Fig. 2a). Therefore, the overexpression of MdWRKY11 conferred enhanced Cu tolerance to the transgenic apple plants.
Fig. 2. Assessment of Cu tolerance of transgenic apple plants and calli subjected to CuSO4 treatment.
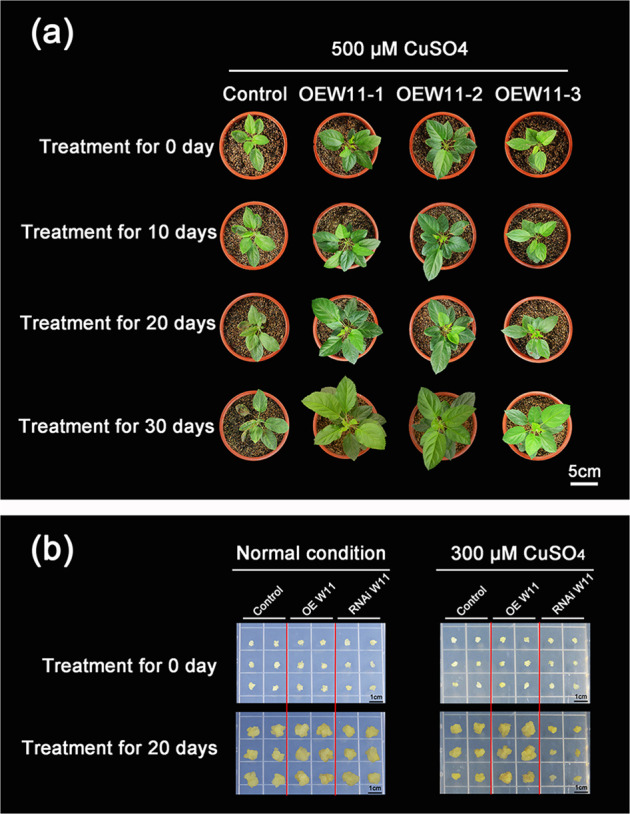
a Phenotypes of three transgenic apple lines overexpressing MdWRKY11 and an untransformed control plant treated with 500μM CuSO4 for 10, 20, and 30 days. b Cu tolerance of transgenic MdWRKY11-overexpressing and MdWRKY11 RNAi calli and control calli cultured on media supplemented with excess Cu (300μM CuSO4) or normal Cu concentrations for 20 days
We also examined MdWRKY11 expression and Cu tolerance in transgenic apple calli harboring either the overexpression construct or an MdWRKY11 RNA interference construct. MdWRKY11 overexpression or underexpression was confirmed by qPCR (Fig. S1b). Similar to that which occurred for the plants transformed with the overexpression construct, transgenic apple calli overexpressing MdWRKY11 presented enhanced Cu tolerance. Calli in which MdWRKY11 expression had been decreased by the RNAi construct presented decreased Cu tolerance (Fig. 2b). Under normal conditions, the control calli and both types of transgenic calli appeared to grow at similar rates. In the presence of CuSO4, however, calli overexpressing MdWRKY11 grew better than the control, whereas calli carrying the RNAi construct grew more slowly. Overall, MdWRKY11 overexpression resulted in increased Cu tolerance, while decreased MdWRKY11 expression resulted in decreased Cu tolerance.
Effects of MdWRKY11 overexpression on Cu accumulation in the roots and leaves of transgenic apple plants
To further investigate the role of MdWRKY11 in Cu tolerance, we used X-ray fluorescence (XRF) microtomography to analyze the content and distribution of Cu in control plants and MdWRKY11-overexpressing plants treated with excess Cu. The same pattern of Cu distribution was observed in both the control and transgenic apple plants. The highest Cu level was in the vascular cylinder (VC). The Cu level decreased with increasing distance from the VC, being highest in the endodermis (EN) and lowest in the epidermis (EP). Consistent with their Cu-tolerant phenotype, the MdWRKY11-overexpressing plants had significantly less Cu than did the control plants in their roots and leaves (Fig. 3).
Fig. 3. Cu levels and distribution shown as μ-XRF elemental maps of the roots and leaves of transgenic plants overexpressing MdWRKY11 and an untransformed control plant treated with 500 μM CuSO4 for 24 h.
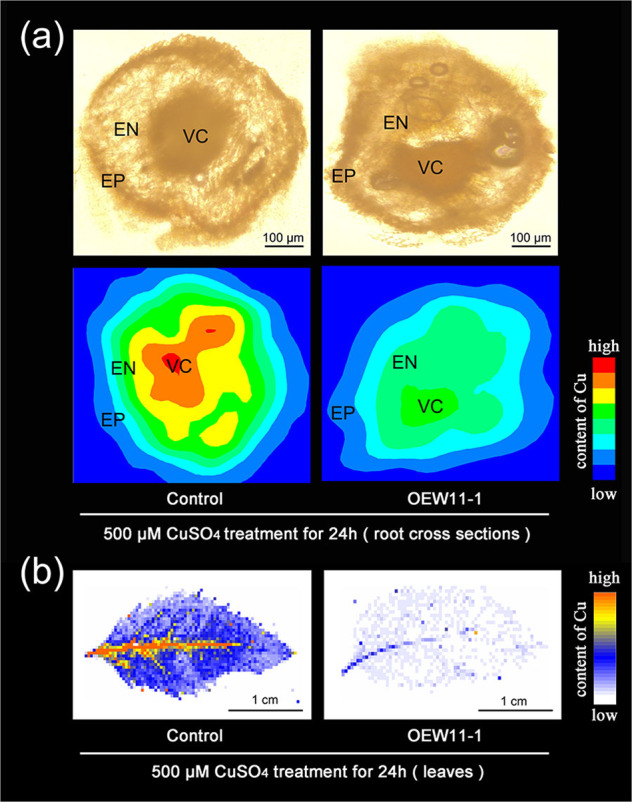
a Cu level and distribution depicted as μ-XRF elemental maps of the roots. VC vascular cylinder, EN endodermis, EP epidermis. b Cu level and distribution shown as μ-XRF elemental maps of leaves
Binding of MdWRKY11 to the MdHMA5 promoter and its effect on MdHMA5 transcription
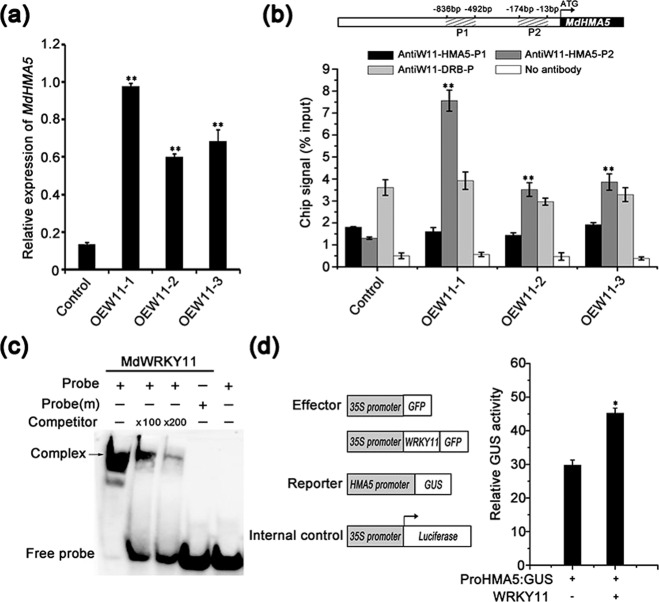
To determine how MdWRKY11 overexpression provides increased Cu tolerance, we analyzed the expression of key genes involved in Cu absorption and transport (Fig. 4a and S2). Among these genes, the expression of MdHMA5, which encodes a Cu-specific transporter, was significantly higher in the MdWRKY11-overexpressing lines than in untransformed control plants (Fig. 4a). Similarly, MdHMA5 expression in transgenic calli overexpressing MdWRKY11 was nearly 50% higher than that in control calli. Conversely, MdHMA5 expression decreased to nearly half the control levels in calli transformed with the MdWRKY11 RNAi construct (Fig. S4a). These results suggest that MdWRKY11 positively regulates the expression of MdHMA5.
Fig. 4. MdWRKY11 binds to the promoter and activates the expression of MdHMA5, which encodes a Cu-transporting P1B-type ATPase.
aMdHMA5 expression in transgenic and control apple plants. b Binding of MdWRKY11 to the W-box of the MdHMA5 promoter indicated by chromatin immunoprecipitation (ChIP)-qPCR. The position of the W-box is indicated by the gray bar. The ChIP signal was quantified as the percentage of immunoprecipitated DNA out of the total input DNA, as determined by qPCR. A parallel experiment without antibodies and the promoter of MdDRB served as the negative control. c Electrophoretic mobility shift assay (EMSA) results confirming the in vitro binding of MdWRKY11 to the MdHMA5 promoter fragment. The arrow indicates the position of a protein–DNA complex after incubation with GST-MdWRKY11 and the biotin-labeled DNA probe MdHMA5. Both the probe containing a W-box and the probe (m) containing a mutated W-box were synthesized according to the sequence of the MdHMA5 promoter. d Relative GUS activity normalized with respect to luciferase (LUC) activity in transiently transformed apple calli expressing 35S::MdWRKY11-GFP, proMdHMA5::GUS, and 35S::LUC; the relative GUS activity in transiently transformed apple calli expressing 35S::GFP, proMdHMA5::GUS, and 35S::LUC served as the control. The data are the means±SDs of triplicate experiments. The asterisks indicate values that are significantly different from those of the control (Student’s t-test): *P < 0.05; **P < 0.01
To test whether MdHMA5 is directly regulated by MdWRKY11, we examined whether MdWRKY11 binds to the MdHMA5 promoter both in vivo and in vitro using chromatin immunoprecipitation (ChIP)-qPCR and EMSAs, respectively. ChIP-qPCR analysis showed that the P2 fragment, which contains the W-box motif of the MdHMA5 promoter, was enriched in samples from the transgenic lines (Fig. 4b), confirming that MdWRKY11 binds specifically to the MdHMA5 promoter in vivo. EMSAs demonstrated the binding of MdWRKY11 to the P2 fragment of the MdHMA5 promoter in vitro. This binding was reduced in a dose-dependent manner with the addition of a 100-fold or 200-fold excess of unlabeled competitor. In addition, the binding was completely abolished when the probe contained a mutated W-box element, further confirming that the W-box of the P2 fragment of the MdHMA5 promoter is the binding site for MdWRKY11 (Fig. 4c).
We further tested the effects of MdWRKY11 on MdHMA5 expression in apple calli transiently cotransformed with an MdWRKY11 overexpression construct and with a construct in which GUS expression was driven by the MdHMA5 promoter. The ratio of GUS to LUC activity was significantly higher in calli expressing proMdHMA5::GUS, 35S::MdWRKY11-GFP, and 35S::LUC than in control calli without the 35S::MdWRKY11-GFP construct (Fig. 4d). Taken together, these results indicate that MdWRKY11 specifically binds to the MdHMA5 promoter and activates its expression.
Phylogenetic analysis, subcellular localization, and functional analysis of MdHMA5
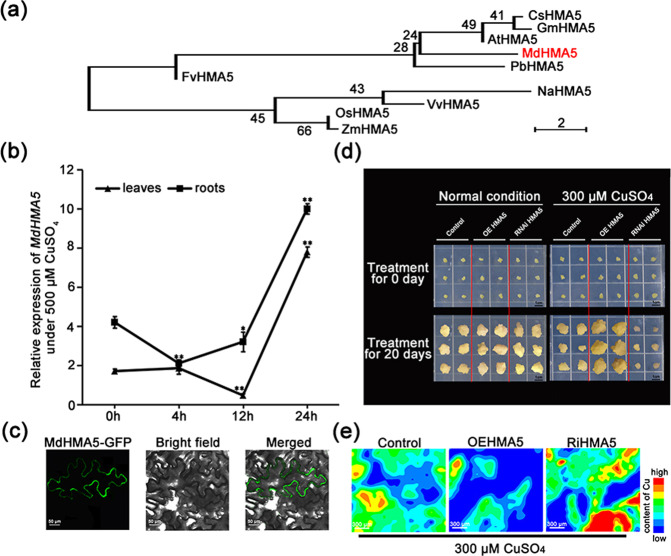
Phylogenetic analysis showed that MdHMA5 is more closely related to AtHMA5 (Fig. 5a), which plays an important role in Cu homeostasis and detoxification in Arabidopsis16,21, than to other HMA5s from other species. MdHMA5 expression was significantly induced by excess CuSO4 treatment in both the roots and leaves of hydroponic plants (Fig. 5b). Similar to AtHMA5, MdHMA5 was localized to the plasma membrane16,21 (Fig. 5c). These results suggest that, like AtHMA5, MdHMA5 may be involved in the detoxification of excess Cu.
Fig. 5. Phylogenetic analysis, subcellular localization, and functional analysis of MdHMA5.
a Phylogenetic tree of the HMA5 subgroup of heavy metal ATPases from select plant species. The species and the accession numbers of the amino acid sequences used in the analysis are as follows: Arabidopsis thaliana (AT1G63440), Oryza sativa (OS04G46940), Fragaria vesca (FvH4_5g02091), Vitis vinifera (XP_010651259.1), Cucumis sativus (CSPI05G05530), Zea mays (ZM2G143512), Nicotiana attenuata (XP_019241377.1), Glycine max (GM11G024400), and Pyrus x bretschneideri (XP_018506630.1). bMdHMA5 expression in the leaves and roots under excess Cu treatment measured by qPCR and normalized according to the MdActin expression level. Hydroponic apple plants under excess Cu stress were sampled at 0, 4, 12, and 24h of treatment. The data are the means±SDs of triplicate experiments for each time point. The asterisks indicate the values that are significantly different from the controls (Student’s t-test): *P < 0.05; **P < 0.01. c MdHMA5-GFP is localized to the plasma membrane of Nicotiana benthamiana leaf epidermal cells. 35S::MdHMA5-GFP was transiently expressed in epidermal cells of N. benthamiana leaves and visualized by confocal microscopy (×40). d Cu tolerance of transgenic MdHMA5-overexpressing and MdHMA5-RNAi calli and untransformed control calli treated with excess Cu (300μM) for 20 days. e Cu levels, shown as μ-XRF elemental maps, in transgenic MdHMA5-overexpressing and MdHMA5-RNAi calli and control calli under excess Cu stress for 20 days
To investigate the function of MdHMA5 in Cu detoxification, apple calli were transformed with MdHMA5 overexpression or MdHMA5-RNAi constructs and cultured on media containing normal or excess amounts of Cu (Fig. 5d). All of the calli grew well under normal conditions, with no significant differences. On media supplemented with excess Cu, however, the MdHMA5-overexpressing calli grew markedly better than did the controls, while the growth of the MdHMA5-RNAi calli was more severely inhibited. Furthermore, XRF microtomography analysis showed that the highest Cu level occurred in MdHMA5-RNAi calli and that the lowest Cu level occurred in the MdHMA5-overexpressing calli (Fig. 5e). These results indicate that the overexpression of MdHMA5 confers increased Cu tolerance to apple calli by maintaining the Cu level, while MdHMA5-RNAi calli exhibited the opposite phenotype. These results are in good agreement with the results of our analysis of transgenic apple plants overexpressing MdWRKY11.
Discussion
Cu contamination has become a severe problem in apple orchards by impairing growth and reducing apple yield. Importantly, human health may be threatened by toxic apple fruit production1. Understanding the mechanisms underlying Cu resistance in apple is the basis for molecular breeding of Cu-resistant apple cultivars.
We screened apple WRKY genes to isolate the key transcription factor involved in Cu resistance. MdWRKY11 expression was significantly induced by excess Cu (Fig. 1a) and was chosen for subsequent research. MdWRKY11 is a typical Group II WRKY transcription factor; it is located in the nucleus and functions as a transcriptional activator in a yeast system (Fig. 1b, c). We overexpressed MdWRKY11 in Gala, a popular apple cultivar grown worldwide38, which conferred increased Cu tolerance to the transgenic plants. Moreover, a similar Cu-tolerant phenotype was observed for transgenic apple calli overexpressing MdWRKY11, while calli transformed with an MdWRKY11 RNAi construct were less tolerant to excess Cu (Fig. 2b). These results imply that MdWRKY11 plays an important role in Cu resistance and constitute the first line of evidence that WRKY TFs are involved in regulating the response of apple to excess Cu.
To isolate the genes regulated by MdWRKY11 as part of the Cu response, we measured the expression of key genes involved in Cu absorption and transport in control plants and MdWRKY11-overexpressing apple plants. MdHMA5 expression significantly increased in the plants overexpressing MdWRKY11 (Fig. 4a). The direct regulation of MdHMA5 was indicated by in vivo ChIP-qPCR (Fig. 4b), by in vitro EMSAs (Fig. 4c) and by additional in vivo transgenic tests (Fig. 4d). In addition, MdHMA5 expression was relatively low in calli transformed with an MdWRKY11 RNAi construct (Fig. S2a), suggesting that MdWRKY11 plays a critical role in regulating the expression of MdHMA5. Taken together, these results demonstrate that MdWRKY11 binds to the MdHMA5 promoter to activate its transcription under excess Cu stress.
Given that the role of HMA5 in woody plant species is not known, we investigated its involvement in Cu detoxification in apple calli transformed with MdHMA5-overexpression or MdHMA5-RNAi constructs. Under excess Cu conditions, overexpression of MdHMA5 provided enhanced Cu tolerance, while RNAi of MdHMA5 expression decreased Cu tolerance (Fig. 5d). This confirmed the important role of MdHMA5 in Cu detoxification.
To clarify how the MdWRKY11-MdHMA5 pathway functions in Cu resistance, we used XRF to analyze the concentration and distribution of Cu in transgenic apple plants and calli presenting altered MdWRKY11 or MdHMA5 expression (Fig. 5e). Under excess Cu conditions, transgenic apple plants overexpressing MdWRKY11 had markedly lower concentrations of Cu in both their roots and leaves compared with those of the controls (Fig. 3). The Cu level of the roots was much higher than that of the leaves in both the control plants and transgenic plants (Fig. S3b, d). Compared with those of the control plants, the roots of the transgenic plants had less Cu in every tissue layer, indicating that roots of the transgenic plants accumulate less Cu than do those of the control plants, which suggests that epidermal root cells might transport Cu outside the cytoplasm more efficiently, possibly through upregulated MdHAM5. In root cross-sections, the Cu level was highest in the VC and decreased from the endodermis to the epidermis in both the transgenic plants and control plants. These results imply that the MdWRKY11-induced MdHMA5 pathway may also be involved in Cu loading, as has been reported in rice24. Indeed, physiological studies showed that, compared with Cu-sensitive apple rootstocks, Cu-tolerant apple rootstocks had lower levels of Cu in their roots, possibly the result of more efficient Cu export or redistribution2.
Our observations of transgenic plants suggest that apple employs a redistribution strategy to detoxify excess Cu. Furthermore, Cu levels were dramatically lower in transgenic calli overexpressing MdHMA5 and higher in MdHMA5-RNAi calli, indicating that MdHMA5 is also involved in Cu export. Therefore, MdHMA5, which is directly regulated by MdWRKY11 and functions in transmembrane Cu transport, is possibly required for the extrusion or redistribution of Cu in apple. This would explain the significantly lower Cu levels in the roots and leaves of the transgenic apple plants overexpressing MdWRKY11 compared with those of the controls. Together, our findings suggest that the use of the MdWRKY11-MdHMA5 pathway is a key strategy for Cu detoxification in apple.
Based on these observations, we present a model for Cu detoxification in apple (Fig. 6): excess Cu induces MdWRKY11, which directly binds to the promoter of MdHMA5 (a Cu-transporting P1B-type ATPase gene), increasing its transcription. MdHMA5 then reduces Cu levels in the cytoplasm by increasing Cu transmembrane transport in root cells. Our work identified a novel MdWRKY11-MdHMA5 pathway that mediates Cu resistance in apple. This study not only contributes to the molecular breeding of Cu-resistant apple cultivars but also provides marker genes to monitor Cu contamination.
Fig. 6. Model of the role of the MdWRKY11-MdHMA5 pathway in the response to excess Cu in apple root cells.
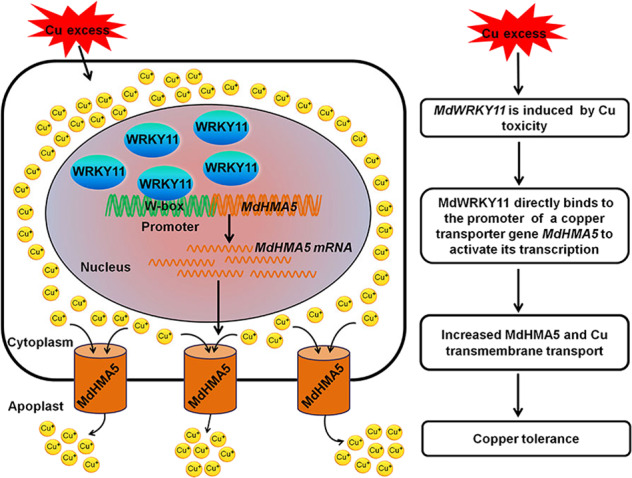
Excess Cu-inducible MdWRKY11 binds to the promoter of MdHMA5, increased MdHMA5 transcription. Increased MdHMA5 promotes transmembrane transport Cu, which more efficiently decreases Cu levels in the cytoplasm
Materials and methods
Plant materials and growth conditions
Gala 3 (Malus x domestica Borkh. cv. Royal Gala) plants were cultured on MS media containing 0.2 mg l−1 indole-3-acetic acid (IAA), 0.3 mg l−1 6-benzylaminopurine (6-BA), and 0.1 mg l−1 gibberellin 3 (GA3) at 23 °C under a 16 h:8 h (light/dark) photoperiod with a light intensity of 100 μmol m−2s−1, after which they were subcultured every four weeks. The Gala 3 plants were rooted and transplanted as described by Zheng et al.39. Apple callus induction and culture were conducted as described by Zheng et al.39.
Quantitative reverse transcription PCR (qPCR)-based analysis
qPCR-based analysis was used to measure MdWRKY11 and MdHMA5 expression in apple plants exposed to excess Cu. Hydroponically cultivated apple plants that had 4–6 leaves and were approximately 10 cm tall were treated with Hoagland nutrient solution supplemented with 500 μM CuSO4. Root and leaf samples were collected at 0, 1, 2, 4, 12, and 24 h. Total RNA isolation, reverse transcription, and qPCR were performed as described by Zheng et al.39. MdActin was used as an internal control. MdWRKY11 and MdHMA5 expression in transgenic plants and control plants or calli was assessed similarly. All the primers used are listed in Supporting Information Table S1.
Determination of MdWRKY11 subcellular localization
The coding sequence of MdWRKY11 without the stop codon was amplified and subcloned into a pMDC83 vector to create a 35S::MdWRKY11-GFP fusion construct, which was subsequently introduced into Agrobacterium tumefaciens strain GV3101. The primers used to amplify the construct are listed in Supporting Information Table S2. The construct was infiltrated into N. benthamiana leaves, and GFP fluorescence in the transgenic leaves was observed by confocal microscopy (×40) after staining with the nucleus-specific dye DAPI, as previously described by Zheng et al.39.
Transcriptional activation assays in yeast
With respect to transcriptional activation assays in yeast, the MdWRKY11 coding region without the stop codon was amplified and inserted into a pGBKT7 vector to generate a pBD-MdWRKY11 construct for MdWRKY11 expression as a fusion protein with the GAL4-binding domain (BD). The primers used are listed in Table S2. The transcriptional activation assay of MdWRKY11 was performed as described previously39. pCL-1 and pGBKT7 vectors were used as positive and negative controls, respectively.
Generating transgenic Gala 3 apple plants and calli presenting altered MdWRKY11 and MdHMA5 expression
The MdWRKY11 coding region was amplified and inserted into a pBI121 vector to generate a 35S::MdWRKY11-GUS overexpression construct. The plasmid was then introduced into Agrobacterium tumefaciens strain EHA105 for subsequent Agrobacterium-mediated transformation of Gala3 according to the method of Dai et al.40. Transgenic plants were confirmed by PCR analysis, while the mRNA abundance of MdWRKY11 in all transgenic apple lines and control plants was determined by qPCR-based analysis. Each experiment was independently repeated three times.
To generate transgenic calli overexpressing MdWRKY11 or MdHMA5, the coding sequence of MdWRKY11 or MdHMA5 was subcloned into a pMDC83 vector to generate 35S::MdWRKY11 and 35S::MdHMA5 plasmids, respectively. To reduce MdWRKY11 and MdHMA5 expression in apple calli, the sense and antisense fragments of the two genes were inserted into a pZH01 RNA interference (RNAi) vector, yielding RNA interference constructs pZH01-MdWRKY11-RNAi and pZH01-MdHMA5-RNAi, respectively. The primers used in this experiment are listed in Supporting Information Table S2. The plasmids were introduced into Agrobacterium tumefaciens strain EHA105. Apple calli were transformed using the method described by An et al.41. Transgenic calli with altered expression levels of MdWRKY11 and MdHMA5 were confirmed by PCR-based analysis, and the expression levels of MdWRKY11 or MdHMA5 in all the transgenic lines and control calli were quantified via qPCR. Each experiment was independently repeated three times.
Determination of the Cu tolerance of transgenic apple plants or calli presenting altered levels of MdWRKY11 or MdHMA5 expression
To determine the Cu tolerance of transgenic apple plants, transgenic apple plants overexpressing MdWRKY11 and control plants were watered with full-strength Hoagland nutrient solution supplemented with 500 μM CuSO4 every three days, and the pH of the nutrient solution was adjusted to 5.6. To apply the excess Cu treatment, apple calli were grown on proliferation media that consisted of 300 μM CuSO4 for 20 days. Images of the plants and calli were taken before and after treatment.
Detection of Cu content via micro-X-ray fluorescence (μ-XRF) microspectroscopy
The micro-X-ray fluorescence (μ-XRF) microspectroscopy experiment was performed via a 4W1B beamline system at the Beijing Synchrotron Radiation Facility (BSRF), Institute of High Energy Physics, Chinese Academy of Sciences, which runs 2.5 GeV electrons with current from 150 to 250 mA. The incident X-ray energy was monochromatized via a W/B4C double-multilayer-monochromator (DMM) at 15 keV and was narrowed to 50 μm in diameter by a polycapillary lens. After being treated with a 500 μM CuSO4 solution for 24 h, the roots and leaves of Gala 3 plants overexpressing MdWRKY11 and those of control plants were sampled for Cu detection. Apple calli with altered levels of MdHMA5 expression were collected after excess Cu treatment on media consisting of 300 μM CuSO4 for 20 days. A cryotome was used to obtain 200-mm thick root cross-sections and calli sections, and the sections were placed on Kapton tape and freeze-dried in a vacuum freeze dryer (LGJ-10B, Beijing Four-Ring Science Instrument Factory). The sample was held on a precision motor-driven stage and scanned at 60-μm intervals by two-dimensional mapping. A Si (Li) solid-state detector was used to detect XRF emission lines with a live time of 60 s. The data were processed using the PyMCA package42,43.
Heterologous MdWRKY11 expression in E. coli and preparation of polyclonal antibodies
The coding sequence of MdWRKY11 was amplified and inserted into a pGEX-6p-1 vector. The GST-MdWRKY11 fusion protein was then expressed and purified as described by Zheng et al.44. Polyclonal anti-MdWRKY11 antibodies was prepared using the method approved by the Beijing Municipal Commission of Science and Technology44.
Chromatin immunoprecipitation-qPCR (ChIP-qPCR) assays
ChIP assays involving anti-MdWRKY11 polyclonal antibodies were performed as described by Zheng et al.39. The primers for ChIP-qPCR were designed to amplify regions in the promoter sequence of MdHMA5 (Table S2). The experiment was performed in triplicate.
Electrophoretic mobility shift assays (EMSAs)
EMSAs were performed using a LightShift Chemiluminescent EMSA Kit (Thermo Scientific, Waltham, MA, USA) as described by Zheng et al.39. The 5′ biotin-labeled MdHMA5 promoter DNA probe containing the W-box (proMdHMA5-F-biotin + proMdHMA5-R), mutated W-box probe (proMdHMA5 (m) -F-biotin + proMdHMA5 (m) -R), and corresponding competitor DNA sequences (proMdHMA5-F + proMdHMA5-R) are listed in Table S2.
Detection of gene expression in the transiently transformed apple calli
To determine the effects of MdWRKY11 overexpression on MdHMA5 expression, the promoter of MdHMA5 was amplified and inserted into a pCAMBIA1301 vector to generate a proMdHMA5::GUS construct. Apple calli were transiently cotransformed with Agrobacterium EHA105 strains carrying 35S7MdWRKY11-GFP, proMdHMA57GUS, and 35S7LUC. Apple calli cotransformed with Agrobacterium strains carrying 35S::GFP, proMdHMA5::GUS, and 35S::LUC were used as controls. The GUS and LUC activities were determined as described by Zheng et al.39. The GUS:LUC activity ratio was used as the ultimate quantification of GUS activity. Each experiment was independently repeated three times.
Phylogenetic analysis
With respect to the phylogenetic analysis of MdHMA5, previously annotated HMA5 homologs from Arabidopsis thaliana (AT1G63440), Oryza sativa (OS04G46940), Fragaria vesca (FvH4_5g02091), Vitis vinifera (XP_010651259.1), Cucumis sativus (CSPI05G05530), Zea mays (ZM2G143512), Nicotiana attenuata (XP_019241377.1), Glycine max (GM11G024400), and Pyrus x bretschneideri (XP_018506630.1) were retrieved from GenBank and aligned with MUSCLE in MEGA 7. A phylogenetic tree was then constructed using the neighbor-joining method with 1,000 bootstraps.
Supplementary information
Acknowledgements
This research was supported by the National Key Research and Development Program of China (SQ2018YFD100303 and 2019YFD1000104), National Natural Science Fund (No. 31772279) and the Construction of Beijing science and technology innovation and service capacity in top subjects (CEFF-PXM2019_014207_000032). The μ-XRF beam time was granted by the 4W1B beamline of the Beijing Synchrotron Radiation Facility, Institute of High Energy Physics, Chinese Academy of Sciences. The staff members of 4W1B are acknowledged for their support in the measurements and data reduction.
Author contributions
J.K. planned and designed the research. K.S., X.L., Y.Z., Y.B., D.S., X.Z., L.W., H.Z., C.W., T.Y., F.Z., Z.H., Y.S., Y.G., and J.K. performed experiments, conducted the fieldwork, analyzed the data, etc. K.S. and J.K. wrote the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41438-020-0326-0).
References
- 1.Li W, Zhang M, Shu H. Distribution and fractionation of copper in soils of apple orchards. Environ. Sci. Pollut. R. 2005;12:168–172. doi: 10.1065/espr2005.04.243. [DOI] [PubMed] [Google Scholar]
- 2.Liu CS, et al. Copper toxicity and accumulation in potted seedlings of three apple rootstock species: implications for safe fruit production on copper-polluted soils. J. Plant. Nutr. 2011;34:1268–1277. [Google Scholar]
- 3.Brun A, Maillet J, Hinsinger P, Pépina M. Evaluation of copper availability to plants in copper-contaminated vineyard soils. Environ. Pollut. 2001;111:293–302. doi: 10.1016/s0269-7491(00)00067-1. [DOI] [PubMed] [Google Scholar]
- 4.Wang QY, Liu JS, Cheng S. Heavy metals in apple orchard soils and fruits and their health risks in Liaodong peninsula, Northeast China. Environ. Monit. Assess. 2015;187:4178. doi: 10.1007/s10661-014-4178-7. [DOI] [PubMed] [Google Scholar]
- 5.Girotto E, et al. Copper availability assessment of cu-contaminated vineyard soils using black oat cultivation and chemical extractants. Environ. Monit. Assess. 2014;186:9051–9063. doi: 10.1007/s10661-014-4065-2. [DOI] [PubMed] [Google Scholar]
- 6.Adrees M, et al. The effect of excess copper on growth and physiology of important food crops: a review. Environ. Sci. Pollut. Res. 2015;22:8148–8162. doi: 10.1007/s11356-015-4496-5. [DOI] [PubMed] [Google Scholar]
- 7.Kan SH, Sun BY, Liu CS. Toxic effects of long-term low-dose Copper (Cu) stress on apple trees in brown soils. J. Agro-Environ. Sci. 2010;29:38–42. [Google Scholar]
- 8.De Forest DKC, Meyer JS. Critical review: toxicity of diet borne metals to aquatic organisms. Crit. Rev. Env. Sci. Tec. 2015;45:1176–1241. [Google Scholar]
- 9.Hippler FWR, et al. Citrus rootstocks regulate the nutritional status and antioxidant system of trees under copper stress. Environ. Exp. Bot. 2016;130:42–52. [Google Scholar]
- 10.Leng X, et al. Transporters, chaperones, and P-type ATPases controlling grapevine copper homeostasis. Funct. Integr. Genomics. 2015;15:673–684. doi: 10.1007/s10142-015-0444-1. [DOI] [PubMed] [Google Scholar]
- 11.Li Q, et al. Excess copper effects on growth, uptake of water and nutrients, carbohydrates, and PSII photochemistry revealed by OJIP transients in Citrus seedlings. Environ. Sci. Pollut. Res. 2019;26:30188–30205. doi: 10.1007/s11356-019-06170-2. [DOI] [PubMed] [Google Scholar]
- 12.Wang QY, Liu JS, Hu B. Integration of copper subcellular distribution and chemical forms to understand copper toxicity in apple trees. Environ. Exp. Bot. 2016;123:125–131. [Google Scholar]
- 13.Nishizono H, Ichikawa H, Suzikp S, Ishii F. The role of the root cell wall in the heavy metal tolerance of Athyrium yokoscense. Plant Soil. 1987;101:15–20. [Google Scholar]
- 14.Burkhead JL, Reynolds KA, Abdel-Ghany SE, Cohu CM, Pilon M. Copper homeostasis. N. Phytol. 2009;182:799–816. doi: 10.1111/j.1469-8137.2009.02846.x. [DOI] [PubMed] [Google Scholar]
- 15.Kholodova, V. P., Ivanova, E. M., & Kuznetsov, V. V. Initial steps of copper detoxification: outside and inside of the plant cell. In Detoxification of Heavy Metals, Soil Biology 30 (eds. Sherameti, I. & Varma, A.) Ch. 8, 143–167 (Springer-Verlag Berlin Heidelberg, 2011).
- 16.Andres-Colas N, et al. The Arabidopsis heavy metal P-type ATPase HMA5 interacts with metallochaperones and functions in copper detoxification of roots. Plant J. 2006;45:225–236. doi: 10.1111/j.1365-313X.2005.02601.x. [DOI] [PubMed] [Google Scholar]
- 17.Cobbett CS, Hussain D, Haydon MJ. Structural and functional relationships between type 1B heavy metal-transporting P-type ATPases in Arabidopsis. N. Phytol. 2003;159:315–321. doi: 10.1046/j.1469-8137.2003.00785.x. [DOI] [PubMed] [Google Scholar]
- 18.Argüello JM. Identification of ion-selectivity determinants in heavy-metal transport P 1B-type ATPases. J. Membr. Biol. 2003;195:93–108. doi: 10.1007/s00232-003-2048-2. [DOI] [PubMed] [Google Scholar]
- 19.Yruela I. Copper in plants: acquisition, transport and interactions. Funct. Plant Biol. 2009;36:409–430. doi: 10.1071/FP08288. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi Y, et al. Amino acid polymorphisms in strictly conserved domains of a P-Type ATPase HMA5 are involved in the mechanism of copper tolerance variation in Arabidopsis. Plant Physiol. 2008;148:969–980. doi: 10.1104/pp.108.119933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li YB, et al. Two Silene vulgaris copper transporters residing in different cellular compartments confer copper hypertolerance by distinct mechanisms when expressed in Arabidopsis thaliana. N. Phytol. 2017;215:1102–1114. doi: 10.1111/nph.14647. [DOI] [PubMed] [Google Scholar]
- 22.Zhang YY, et al. OsATX1 interacts with heavy metal P1B-Type ATPases and affects copper transport and distribution. Plant Physiol. 2018;178:329–344. doi: 10.1104/pp.18.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lange B, et al. Copper and cobalt accumulation in plants: a critical assessment of the current state of knowledge. N. Phytol. 2017;213:537–551. doi: 10.1111/nph.14175. [DOI] [PubMed] [Google Scholar]
- 24.Deng FL, Yamaji N, Xia JX, Ma JF. A member of the heavy metal P-type ATPase OsHMA5 is involved in xylem loading of copper in rice. Plant Physiol. 2013;163:1353–1362. doi: 10.1104/pp.113.226225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He L, et al. Maize OXIDATIVE STRESS2 homologs enhance cadmium tolerance in Arabidopsis through activation of a putative SAM-dependent methyltransferase gene. Plant Physiol. 2016;171:1675–1685. doi: 10.1104/pp.16.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khare D, et al. Root avoidance of toxic metals requires the GeBP-LIKE 4 transcription factor in Arabidopsis thaliana. N. Phytol. 2017;213:1257–1273. doi: 10.1111/nph.14242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin TT, Yang WN, Lu W, Wang Y, Qi XT. Transcription factors PvERF15 and PvMTF-1 form a cadmium stress transcriptional pathway. Plant Physiol. 2017;173:1565–1573. doi: 10.1104/pp.16.01729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang G, et al. Overexpression of ThVHAc1 and its potential upstream regulator, ThWRKY7, improved plant tolerance of cadmium stress. Sci. Rep. 2016;6:18752. doi: 10.1038/srep18752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kropat J, et al. A regulator of nutritional copper signaling in Chlamydomonas is an SBP domain protein that recognizes the GTAC core of copper response element. Proc. Natl Acad. Sci. USA. 2005;102:18730–18735. doi: 10.1073/pnas.0507693102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruzsa SM, Scandalios JG. Altered Cu metabolism and differential transcription of Cu/ZnSod genes in a Cu/ZnSOD-deficient mutant of maize: evidence for a Cu-responsive transcription factor. Biochemistry. 2003;42:1508–1516. doi: 10.1021/bi020551x. [DOI] [PubMed] [Google Scholar]
- 31.Yamasaki H, Hayashi M, Fukazawa M, Kobayashi Y, Toshiharu Shikanai T. SQUAMOSA promoter-binding protein-like 7 is a central regulator for copper homeostasis in Arabidopsis. Plant Cell. 2009;21:347–361. doi: 10.1105/tpc.108.060137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ding ZJ, Yan JY, Xu XY, Li GX, Zheng SJ. WRKY46 functions as a transcriptional repressor of ALMT1, regulating aluminum-induced malate secretion in Arabidopsis. Plant J. 2013;76:825–835. doi: 10.1111/tpj.12337. [DOI] [PubMed] [Google Scholar]
- 33.Hong CY, et al. The role of ZmWRKY4 in regulating maize antioxidant defense under cadmium stress. Biochem. Bioph. Res. Commun. 2017;482:1504–1510. doi: 10.1016/j.bbrc.2016.12.064. [DOI] [PubMed] [Google Scholar]
- 34.Yan JY, et al. A WRKY transcription factor regulates Fe translocation under Fe deficiency in Arabidopsis. Plant Physiol. 2016;171:2017–2027. doi: 10.1104/pp.16.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dai X, Wang Y, Zhang WH. OsWRKY74, a WRKY transcription factor, modulates tolerance to phosphate starvation in rice. J. Exp. Bot. 2016;67:947–960. doi: 10.1093/jxb/erv515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang Y, Liang G, Yang S, Yu D. Arabidopsis WRKY57 functions as a node of convergence for jasmonic acid- and auxin-mediated signaling in jasmonic acid-induced leaf senescence. Plant Cell. 2014;26:230–245. doi: 10.1105/tpc.113.117838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li W, Wang H, Yu D. Arabidopsis WRKY transcription factors WRKY12 and WRKY13 oppositely regulate flowering under short-day conditions. Mol. Plant. 2016;9:1492–1503. doi: 10.1016/j.molp.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 38.Iglesias I, Echeverría G, Soria Y. Differences in fruit colour development, anthocyanin content, fruit quality and consumer acceptability of eight ‘Gala’ apple strains. Sci. Hortic.-Amst. 2008;119:32–40. [Google Scholar]
- 39.Zheng XD, et al. MdWRKY9 overexpression confers intensive dwarfing in the M26 rootstock of apple by directly inhibiting brassinosteroid synthetase MdDWF4 expression. N. Phytol. 2018;217:1086–1098. doi: 10.1111/nph.14891. [DOI] [PubMed] [Google Scholar]
- 40.Dai H, et al. Development of a seedling clone with high regeneration capacity and susceptibility to Agrobacterium in apple. Sci. Hortic.-Amst. 2013;164:202–208. [Google Scholar]
- 41.An XH, et al. MdMYB9 and MdMYB11 are involved in the regulation of the JA-induced biosynthesis of anthocyanin and proanthocyanidin in apples. Plant Cell Physio. 2015;56:650–662. doi: 10.1093/pcp/pcu205. [DOI] [PubMed] [Google Scholar]
- 42.Kim SA, et al. Localization of iron in Arabidopsis seed requires the vacuolar membrane transporter VIT1. Science. 2006;314:1295–1298. doi: 10.1126/science.1132563. [DOI] [PubMed] [Google Scholar]
- 43.Solé VA, Papillon E, Cotte M, Walter P, Susini J. A multiplatform code for the analysis of energy-dispersive X-ray fluorescence spectra. Spectrochim. Acta B. 2007;62:63–68. [Google Scholar]
- 44.Zheng XD, et al. Chloroplastic biosynthesis of melatonin and its involvement in protection of plants from salt stress. Sci. Rep. 2017;7:41236. doi: 10.1038/srep41236. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.