Graphical abstract
Keywords: Migraine, Medication overuse, Artificial intelligence, Machine learning, Decision support systems
Abbreviations: AI, Artificial Intelligence; AUC, Area Under the Curve; BMI, body mass index; CI, Confidence Interval; DBH 19-bp I/D polymorphism, Dopamine-Beta-Hydroxylase 19 bp insertion/deletion polymorphism; DSS, Decision Support System; ICT, Information and Communications Technology; KELP, Kernel-based Learning Platform; LRs, likelihood ratios; MKL, Multiple Kernel Learning; ML, Machine Learning; MO, Medication Overuse; NSAID, nonsteroidal anti-inflammatory drugs; PVI, Predictive Value Imputation; ROC, Receiver operating characteristic; SE, Standard Error; SVM, Support Vector Machine; RO, Random Optimization
Highlights
-
•
Medication overuse is related to chronicization and medication-overuse headache.
-
•
Prediction of medication overuse (MO) is a challenge in the management of migraine.
-
•
Machine learning and random optimization could help to estimate MO risk in migraine.
-
•
A customized decision support system was devised for migraine clinical management.
-
•
This approach may exploit significant patterns in data connoting causality.
Abstract
Machine learning (ML) is largely used to develop automatic predictors in migraine classification but automatic predictors for medication overuse (MO) in migraine are still in their infancy. Thus, to understand the benefits of ML in MO prediction, we explored an automated predictor to estimate MO risk in migraine. To achieve this objective, a study was designed to analyze the performance of a customized ML-based decision support system that combines support vector machines and Random Optimization (RO-MO). We used RO-MO to extract prognostic information from demographic, clinical and biochemical data. Using a dataset of 777 consecutive migraine patients we derived a set of predictors with discriminatory power for MO higher than that observed for baseline SVM. The best four were incorporated into the final RO-MO decision support system and risk evaluation on a five-level stratification was performed. ROC analysis resulted in a c-statistic of 0.83 with a sensitivity and specificity of 0.69 and 0.87, respectively, and an accuracy of 0.87 when MO was predicted by at least three RO-MO models. Logistic regression analysis confirmed that the derived RO-MO system could effectively predict MO with ORs of 5.7 and 21.0 for patients classified as probably (3 predictors positive), or definitely at risk of MO (4 predictors positive), respectively. In conclusion, a combination of ML and RO – taking into consideration clinical/biochemical features, drug exposure and lifestyle – might represent a valuable approach to MO prediction in migraine and holds the potential for improving model precision through weighting the relative importance of attributes.
1. Introduction
Migraine is a primary form of headache characterized by recurrent episodes of debilitating headache, sometimes preceded by transient neurological symptoms named aura. Its pathophysiology recognizes a unique mixture of bio-psycho-social aspects, which may all trigger the attack in susceptible individuals, unveiling a biological predisposition of a dysexcitable brain to convert non-painful stimulation into headache pain. This ultimately leads to impressive disability, significant productivity loss, huge economic burden and healthcare resource use.
In the last two decades, an unprecedented number of studies have contributed to substantial advances in abortive and preventive therapy, opening new and promising scenarios in the field of precision medicine. Nonetheless, migraine remains a major clinical challenge, as it is often under-diagnosed [1]. Current validated diagnostic criteria, in fact, distinguish migraine according to the attack frequency (episodic or chronic) or to the presence/absence of aura [2], but do not disentangle the different endophenotypes of this highly heterogeneous headache disorder [3]. Furthermore, preventative migraine medications are often underutilized, leaving patients at-risk of medication overuse (MO), disease progression, higher disability and increased healthcare costs [4]. MO affects approximately 15% of migraine patients [5] and is the most relevant risk factor for migraine chronicization [6] as well as the development of a secondary headache disorder known as medication-overuse headache (MOH) [2], [7], [8]. Daily or weekly analgesic use, in fact, disproportionately increases the risk for chronic migraine (RR = 13.3, 95% CI: 9.3–19.1) as documented by a large prospective population survey [9]. When considering a specialized headache center setting, the odds for developing chronic migraine 1 year later in patients with episodic migraine with MO is 19.4 times (95% CI: 8.7–43.2) higher than in those without [10]. The risk of chronic migraine progression is further incremented when overusing involves acute medications containing barbiturates (OR = 2.1; 95% CI: 1.3–3.1) or opioids (OR = 1.2; 95% CI: 1.4–2.2), compared with acetaminophen, as hinted by the American Migraine Prevalence and Prevention (AMPP) study [11]. This is also true in adolescents, in whom medication overuse is a predictor of migraine chronic evolution (HR = 2.5; 95% CI: 1.1–5.5) [12]. Once established, MOH is the epitome of hard to treat neurological disorders due to its disappointing response to treatment and frequent relapse.
Thus, the possibility of predicting MO, or estimating the efficacy of a given therapy, is a compelling challenge in the clinical management of migraine to set out prevention programs for patients prone to frequent headache.
Artificial intelligence (AI) with machine learning (ML) has shown great potential in building automatic predictors in the field of migraine [13], [14], [15], [16], [17], [18], [19], [20], [21], [22] but detectors for MO are still in their infancy [20]. In fact, many AI models have been applied for nearly 10 years to implement medical decision support systems for the diagnosis of migraine [13], [14], [15], [16], [17], [18], [19], [20] and for predicting migraine treatment outcomes [21], [22]. For example, artificial neural networks (ANNs), artificial immune systems (AIS) and support vector machines (SVM) have shown encouraging results. Some systems were built on clinical dataset derived from patients’ medical records [13], [14], [15], [16], [17], while others analyzed data from resting-state functional magnetic resonance neuroimaging (rs-fMRI) [18], [19], [20]. All these studies demonstrated that intelligent systems represent a promising approach for migraine classification, holding potential to revolutionize conventional models of diagnostic deliverance in a contest of personalized medicine. However, To the best of our knowledge, there is only one study studying the potential of AI in predicting MO [20]. In their study, Garcia-Chimeno et al. reported the possibility to distinguish between patients with episodic or chronic migraine with MO via feature selection techniques and machine learning [20]. Using SVM, Boosting and Naive Bayes they obtained an overall classification with over 93% accuracy. However, the study had a big major limitation: due to the small number of participants (n = 18 for chronic migraine with MO), the testing was undertaken in the same dataset used for training and no holdout test set was used [20]. Moreover, the specific aim was not to predict MO risk, but to correctly classify between different migraine phenotypes [20]. Thus, there is still an unmet need to develop classifications models that embody the newest AI technologies and can be used to predict MO in individual migraine patients for a personalized patient care.
In this study, we present our approach RO-MO to use machine learning along with Random Optimization (RO) for predicting Medication Overuse (MO) in migraine patients. Our approach in this study solves the problems of previous studies [20] as testing has been carried out in a held-out set and RO-MO outperforms SVM as used in [20]. RO-MO derives from our previous studies. In a perspective of predictive medicine and tailored therapy, we have recently demonstrated that an approach based on AI methodologies holds the potential to devise decision support systems (DSSs) that can be adapted to different medical problems [23], [24], [25], [26]. Our model based on Multiple Kernel Learning (MKL) [27], combines SVM [28] algorithm and Random Optimization (RO) [29], offering the possibility to inspect the learned model and providing an estimate of the relative weight of routinely collected demographic, clinical and biochemical data in predictions. This model – originally developed for cancer-associated thrombosis risk assessment [23], [24], [25] and then adapted to estimate the risk of disease progression of breast cancer patients [26] – seeks not only decision, but also interpretability of the model itself, which represents the novel aspect of our research. Based on previous observations, we hypothesized that our previous approach could be of assistance in predicting the risk of MO in migraine patients. To achieve this objective, a proof-of-concept study was specifically designed produce MO risk predictors (RO-MO) using real world data from a large, well clinical characterized migraine outpatient population, detailing lifestyle, behavioral and socio-demographic factors and clinical phenotype. The performance of RO-MO was also compared with that of baseline SVM to assess the significance of optimizing the relative importance of groups of clinical attributes in the selection of MO risk predictors.
2. Patients and methods
2.1. Patient’s dataset
Starting from January 2008, the Headache and Pain Unit of the Department of Neurological, Motor and Sensorial Sciences and the InterInstitutional Multidisciplinary Biobank (BioBIM) of the IRCCS San Raffaele Pisana, Rome, Italy, are jointly involved in the recruitment of outpatients affected by headache, who are prospectively followed under the appropriate Institutional ethics approval and in accordance with the principles embodied in the Declaration of Helsinki [30]. All patients undergo a careful physical and neurological examination performed by trained neurologists (PB, GE, LF) and are screened with face-to-face interviews using a semi-structured questionnaire formulated to collect thorough data on lifestyle, behavioral and socio-demographic factors (age, sex, civil status, occupation, body mass index, arterial blood pressure, sport activity, use of coffee, alcohol, smoking, sleep disturbances, menopause, contraceptive use), comorbidities and concomitant medications and clinical features of migraine (disease duration, family history of migraine, presence, frequency, duration of attacks, location, accompanying symptoms, unilateral cranial autonomic symptoms, triggers and alleviating factors, prodromes, postdromes, allodynia, presence and duration of medication overuse, current acute or preventive medications, patient’s satisfaction with triptans) [31]. All patients provide written informed consent, previously approved by the local Institutional Review Board, to donate a blood sample to be used in the analysis of possible determinants of migraine outcome or response to treatment.
Blood samples are withdrawn in fasting conditions and processed using standard operating procedures, ICT tools and dedicated software to track the entire sample life, including elapsed time between blood withdrawal and storage [30]. Routine biochemical analyses are performed on fresh blood samples at time of enrolment. Thereafter, blood samples are processed, aliquoted, coded, and stored at −80 °C for subsequent batch analyses. Storage conditions are carefully monitored, and all aliquots are limited to one freeze–thaw cycle to ensure the best sample quality. For the present study, 777 consecutive migraine patients were analyzed.
2.2. Experimental settings and statistical analysis
Our MO risk predictor is based on multiple kernel learning over support vector machines and Random Optimization (RO-MO). We built RO-MO in KELP [32] as previously reported [23]. RO-MO was used to produce prognostic discriminators (referred as RO-MO-x) yielding the best classification performance over a training (3-fold cross validation) and test set. Twenty different learning sessions with 20 different RO initializations were performed. The dataset, consisting of 777 patients, was randomly divided into a training and a test set. The training set consisted of 543 migraine patients (70% of the dataset); the remaining 234 patients were allocated to the test set (30% of the cases).
Baseline SVM was run on weka platform and its performance was evaluated in comparison with RO-MO classifiers.
Demographic, clinical and biochemical characteristics of the training and test sets are summarized in Table 1, Table 2.
Table 1.
Clinical features of migraine patients in the training and test set.
| Training | Test | P value | ||
|---|---|---|---|---|
| Sex, N (%) | 0.513 | |||
| Male | 96 (17.7) | 46 (19.7) | ||
| Female | 447 (82.3) | 188 (80.3) | ||
| Menopausal status | 0.235 | |||
| Pre | 327 (73.2) | 146 (77.7) | ||
| Post | 120 (26.8) | 42 (22.3) | ||
| Age at menarche | 12.2 ± 1.2 | 12.2 ± 1.4 | 0.875 | |
| Age (Years), Mean ± SD (range) | 41.5 ± 13.2 (13–78) | 39.5 ± 12.5 (17–71) | 0.052 | |
| BMI, Mean ± SD (range) | 23.9 ± 3.8 (17.0–46.8) | 23.7 ± 3.6 (15.0–36.5) | 0.623 | |
| Age of onset (Years), Mean ± SD (range) | 19.9 ± 11.0 (3–73) | 19.9 ± 10.7 (4–62) | 0.970 | |
| Length of chronicization (Years), Median (IQR) | 2 (1–4) | 2 (1–5) | 0.768 | |
| Type of migraine, N (%) | 0.092 | |||
| Chronic | 139 (25.6) | 48 (20.5) | ||
| Episodic without aura | 287 (52.9) | 114 (48.8) | ||
| Menstrual migraine, N (%)* | 181 (40.5) | 92 (39.3) | ||
| Episodic with aura | 85 (15.6) | 54 (23.1) | ||
| Cluster headache | 11 (2.0) | 9 (3.8) | 0.811 | |
| Tension-type headache | 21 (3.9) | 9 (3.8) | 0.990 | |
| Familiarity, N (%) | 383 (70.8) | 168 (72.4) | 0.648 | |
| Frequency (days/months), Median (IQR) | 7 (3–15) | 5 (2–10) | 0.002 | |
| Pain Localization, N (%) | 0.377 | |||
| Unilateral | 357 (65.8) | 146 (62.4) | ||
| Unilateral or bilateral | 4 (0.7) | 2 (0.8) | ||
| Bilateral | 182 (33.5) | 86 (36.8) | ||
| Unilateral cranial autonomic symptoms, N (%) | 242 (44.6) | 103 (44.0) | 0.887 | |
| Dopaminergic symptoms, N (%) | 173 (31.9) | 63 (26.9) | 0.509 | |
| Comorbidities, N (%) | ||||
| neuropsychiatric | 167 (30.8) | 65 (27.8) | 0.397 | |
| cardiovascular | 61 (11.3) | 22 (9.4) | 0.439 | |
| endocrine-metabolic | 83 (15.4) | 30 (12.8) | 0.356 | |
| Treatment, N (%) | ||||
| Type of medication | 0.009 | |||
| Triptans | 140 (25.8) | 71 (30.3) | ||
| NSAID | 165 (30.4) | 74 (31.6) | ||
| Triptans + NSAID | 178 (32.8) | 51 (21.9) | ||
| Other | 60 (11.1) | 38 (16.2) | ||
| Response to triptans | 280 (88.0) | 104 (85.3) | 0.523 | |
| Use of prophylaxis | 304 (56.0) | 112 (47.9) | 0.087 | |
| Medication overuse | ||||
| Overusing patients, N (%) | 127 (23.4) | 35 (15.0) | 0.010 | |
| Abused Drug, N (%) | ||||
| Triptans | 38 (29.9) | 9 (25.7) | 0.437 | |
| NSAIDs ± combination medications | 19 (15.0) | 7 (20.0) | ||
| Combination medications | 64 (50.4) | 15 (42.9) | ||
| Others | 8 (6.3) | 2 (5.7) | ||
| Quantity of abused drug (tablets/month) | 34 ± 29 | 33 ± 26 | 0.724 | |
| Overuse duration (Years), Median (IQR) | 2 (1–4) | 2 (1–4) | 0.439 | |
BMI: body mass index; NSAID: nonsteroidal anti-inflammatory drugs; IQR: interquartile range.
*p = 0.847 for subgroup analysis.
Table 2.
Biochemical features of migraine patients in the training and test set.
| Training | Test | P value | |
|---|---|---|---|
| Blood cell counts | |||
| Red blood cells | 4.5 ± 0.5 | 4.4 ± 0.42 | 0.161 |
| Haematocrit | 39.4 ± 3.5 | 39.4 ± 3.4 | 0.811 |
| Hemoglobin | 13.3 ± 1.3 | 13.4 ± 1.2 | 0.502 |
| White blood cells | 6.6 ± 1.7 | 6.5 ± 1.6 | 0.581 |
| Neutrophils | 3.9 ± 1.4 | 3.8 ± 1.2 | 0.487 |
| Lymphocytes | 2.1 ± 0.6 | 2.6 ± 0.6 | 0.947 |
| Monocytes | 0.5 ± 0.1 | 0.5 ± 0.2 | 0.409 |
| Platelets | 230.2 ± 52.1 | 231.7 ± 51.4 | 0.818 |
| Mean Platelet Volume | 9.0 ± 1.0 | 8.9 ± 1.1 | 0.218 |
| Coagulative asset | |||
| International Normalized Ratio | 1.0 ± 0.1 | 1.0 ± 0. 1 | 0.984 |
| Prothrombin Time | 103.3 ± 13.2 | 104.1 ± 16.3 | 0.656 |
| aPTT | 27.9 ± 2.9 | 30.5 ± 30.5 | 0.228 |
| Fibrinogen | 282.1 ± 54.5 | 279.7 ± 47.6 | 0.757 |
| Glucose metabolic asset | |||
| Fasting blood glucose (mg/dl), Mean ± SD | 82.5 ± 11.5 | 84.2 ± 10.0 | 0.201 |
| Fasting insulin (µIU/ml), Mean ± SD | 9.6 ± 9.2 | 10.0 ± 9.8 | 0.677 |
| HbA1c (%), Mean ± SD | 5.5 ± 0.3 | 5.5 ± 0.4 | 0.595 |
| Blood lipids | |||
| Total cholesterol (mg/dl), Mean ± SD | 202.3 ± 35.7 | 195.8 ± 35.3 | 0.134 |
| HDL-cholesterol (mg/dl), Mean ± SD | 56.7 ± 14.0 | 56.9 ± 12.9 | 0.864 |
| LDL-cholesterol (mg/dl), Mean ± SD | 125.2 ± 31.4 | 120.3 ± 30.7 | 0.192 |
| Triglycerides (mg/dl), Mean ± SD | 102.2 ± 57.2 | 93.3 ± 56.5 | 0.196 |
| Blood lipids | |||
| Creatinine (mg/dl), Mean ± SD | 0.80 ± 0.13 | 0.78 ± 0.14 | 0.414 |
| Blood urea nitrogen (mg/dl), Median (IQR) | 31.5 ± 9.7 | 31.4 ± 9.3 | 0.897 |
| Alanine transaminase | 17.6 ± 9.2 | 18.5 ± 10.6 | 0.464 |
| Aspartate transaminase | 18.1 ± 4.6 | 18.0 ± 4.9 | 0.809 |
| Gamma-glutamyl transferase | 20.0 ± 22.0 | 18.2 ± 14.3 | 0.442 |
| Total bilirubin | 0.53 ± 0.30 | 0.59 ± 0.39 | 0.139 |
| DBH 19-bp I/D polymorphism* | 0.141 | ||
| II | 130 (46.3) | 45 (42.1) | |
| ID | 60 (21.4) | 33 (30.8) | |
| DD | 91 (32.4) | 29 (27.1) | |
*Available in 388 patients (training set, n = 281; test set, n = 107).
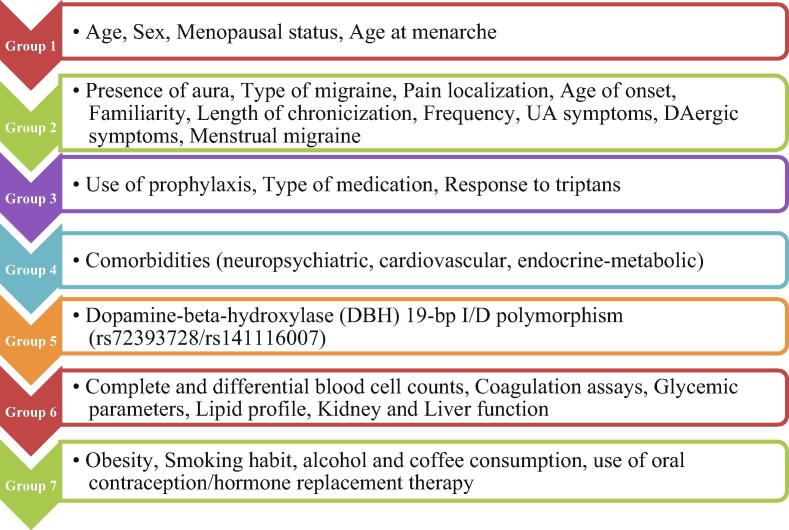
Numerical attributes were analyzed as continuous values. Missing clinical attribute values were treated according to Predictive Value Imputation (PVI) method by replacing missing values with the average of the attribute observed in the training set [33]. Group clustering was performed according to the clinical significance of the attributes included in the patient dataset. These included: demographic characteristics (group 1), migraine clinical features (group 2), treatment details (group 3), presence of co-morbidities (group 4), biochemical variables (group 6) and lifestyle information (group 7). Dopamine-beta-hydroxylase (DBH) 19-bp I/D polymorphism (rs72393728/rs141116007) was individually considered given its proposed association with MO [34]. A detailed list of all the features that have been applied to construct the predictor is reported in Fig. 1. RO was used to devise their relative weights in final prediction. In RO, relative weights are initialized with random number and estimated by maximizing performance in the 3-fold cross validation. These weights can be used to interpret the importance of the groups of features within the model. Thus, the final DSS is interpretable.
Fig. 1.
Features included in the model.
Receiver operating characteristic (ROC) curve, Bayesian analysis and logistic regression were performed by MedCalc Statistical Software version 13.1.2 (MedCalc Software bvba, Ostend, Belgium) to estimate the probability of medication overuse. All tests were two-tailed and only p-values lower than 0.05 were regarded as statistically significant.
3. Results
Overall, 21% (162 of 777) of the enrolled patients reported the presence of MO which had lasted for at least 2 years (Table 1). No substantial differences were observed for clinical and biomolecular variables between patients included in the training and test sets, with the exception of attack frequency, the use of triptans in combination with NSAID and the percentage of patients with MO, which were all slightly higher in the training compared with the test set.
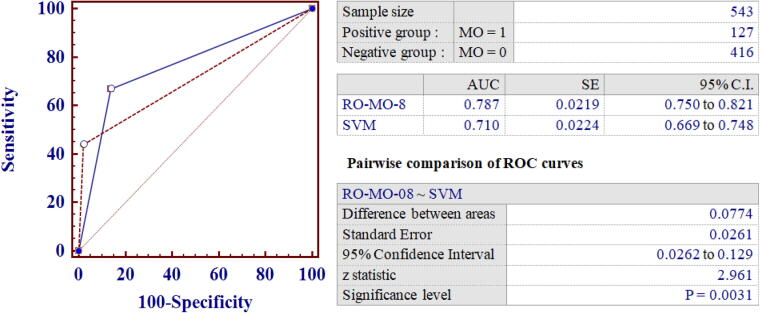
A set of predictors (named RO-MO-x) was identified using a 3-fold cross validation technique on a training set (n = 543). A test set (n = 234) was used to compute the final performance of risk predictors. As shown in Table 3, RO-MO was capable of improving MO risk prediction compared to baseline SVM as demonstrated by a substantial improvement of the f-measure. Thus, to better characterize the performance of the proposed method, the area under the ROC curve (AUC) and positive and negative likelihood ratios (LRs) were also calculated for all RO-MO models in comparison with baseline SVM. As shown in Table 3, RO-MO-08 was the best performing predictor in the training set with an AUC of 0.79, which was significantly higher than that observed for baseline SVM (p = 0.003) (Table 3, Fig. 2) and further increased to 0.81 in the test set (Table 4).
Table 3.
Analytical performance of machine learning with random optimization in the training set.
| Model | Precision | Recall | F-Measure | AUC (SE) | 95% CI | +LR | −LR |
|---|---|---|---|---|---|---|---|
| SVM Baseline | 0.862 | 0.441 | 0.583 | 0.710 (0.0224) | 0.669–0.748 | 20.4 | 0.57 |
| RO-MO-05 | 0.819 | 0.683 | 0.745 | 0.657 (0.0242) | 0.615–0.697 | 1.95 | 0.53 |
| RO-MO-12 | 0.704 | 0.754 | 0.728 | 0.737 (0.0223) | 0.697–0.773 | 2.77 | 0.35 |
| RO-MO-14 | 0.685 | 0.690 | 0.688 | 0.669 (0.0236) | 0.628–0.708 | 1.93 | 0.47 |
| RO-MO-13 | 0.691 | 0.675 | 0.683 | 0.610 (0.0248) | 0.567–0.651 | 1.57 | 0.64 |
| RO-MO-04 | 0.611 | 0.698 | 0.652 | 0.683 (0.0241) | 0.642–0.722 | 2.91 | 0.55 |
| RO-MO-19 | 0.753 | 0.532 | 0.623 | 0.599 (0.0246) | 0.557–0.641 | 1.80 | 0.73 |
| RO-MO-03 | 0.483 | 0.770 | 0.593 | 0.717 (0.0232) | 0.677–0.754 | 2.67 | 0.41 |
| RO-MO-08 | 0.474 | 0.786 | 0.591 | 0.787 (0.0219) | 0.750–0.821 | 5.26 | 0.34 |
| RO-MO-06 | 0.466 | 0.698 | 0.559 | 0.645 (0.0245) | 0.603–0.685 | 1.89 | 0.57 |
| RO-MO-09 | 0.420 | 0.794 | 0.549 | 0.656 (0.0236) | 0.614–0.696 | 1.80 | 0.49 |
| RO-MO-16 | 0.395 | 0.802 | 0.529 | 0.696 (0.0230) | 0.656–0.735 | 2.18 | 0.41 |
| RO-MO-11 | 0.435 | 0.643 | 0.519 | 0.663 (0.0242) | 0.622–0.703 | 2.77 | 0.60 |
| RO-MO-07 | 0.396 | 0.754 | 0.519 | 0.644 (0.0237) | 0.602–0.684 | 1.69 | 0.51 |
| RO-MO-17 | 0.381 | 0.746 | 0.504 | 0.639 (0.0233) | 0.598–0.680 | 3.28 | 0.68 |
| RO-MO-18 | 0.405 | 0.643 | 0.497 | 0.681 (0.0223) | 0.640–0.721 | 1.89 | 0.39 |
| RO-MO-15 | 0.465 | 0.532 | 0.496 | 0.666 (0.0230) | 0.624–0.705 | 1.81 | 0.44 |
| RO-MO-01 | 0.363 | 0.746 | 0.488 | 0.714 (0.0235) | 0.674–0.752 | 4.71 | 0.52 |
| RO-MO-02 | 0.339 | 0.651 | 0.446 | 0.726 (0.0235) | 0.687–0.763 | 3.94 | 0.47 |
| RO-MO-00 | 0.270 | 0.738 | 0.396 | 0.511 (0.0235) | 0.468–0.554 | 1.07 | 0.97 |
| RO-MO-10 | 0.276 | 0.643 | 0.387 | 0.589 (0.0250) | 0.546–0.631 | 1.57 | 0.74 |
| Average RO-MO | 0.509 | 0.699 | 0.560 | 0.664 | 0.705 – 0.690 | 3.32 | 0.54 |
AUC: Area Under the Curve; CI: Confidence Interval; LR: Likelihood Ratio; SE: Standard Error.
Fig. 2.
Comparison between the Receiver Operator Characteristics Curves for RO-MO-08 and SVM baseline predictor in the training set.
Table 4.
Analytical performance of machine learning with random optimization in the test set.
| Model | Precision | Recall | F-Measure | AUC (SE) | 95% CI | +LR | −LR |
|---|---|---|---|---|---|---|---|
| SVM Baseline | 0.957 | 0.629 | 0.759 | 0.812 (0.0414) | 0.760–0.863 | 15.0 | 0.34 |
| RO-MO-00 | 0.161 | 0.429 | 0.234 | 0.501 (0.0431) | 0.436–0.567 | 1.01 | 1.00 |
| RO-MO-01 | 0.302 | 0.457 | 0.364 | 0.625 (0.0439) | 0.559–0.687 | 2.65 | 0.71 |
| RO-MO-02 | 0.267 | 0.657 | 0.380 | 0.739 (0.0433) | 0.678–0.794 | 4.17 | 0.44 |
| RO-MO-03 | 0.382 | 0.743 | 0.505 | 0.746 (0.0405) | 0.685–0.800 | 2.96 | 0.34 |
| RO-MO-04 | 0.348 | 0.657 | 0.455 | 0.688 (0.0447) | 0.624–0.747 | 2.92 | 0.53 |
| RO-MO-05 | 0.806 | 0.714 | 0.758 | 0.675 (0.0439) | 0.611–0.735 | 2.14 | 0.49 |
| RO-MO-06 | 0.284 | 0.714 | 0.407 | 0.702 (0.0429) | 0.639–0.760 | 2.44 | 0.44 |
| RO-MO-07 | 0.231 | 0.686 | 0.345 | 0.683 (0.0412) | 0.619–0.742 | 1.97 | 0.41 |
| RO-MO-08 | 0.325 | 0.743 | 0.452 | 0.806 (0.0393) | 0.750–0.855 | 5.69 | 0.30 |
| RO-MO-09 | 0.245 | 0.714 | 0.365 | 0.649 (0.0434) | 0.585–0.710 | 1.77 | 0.51 |
| RO-MO-10 | 0.200 | 0.486 | 0.283 | 0.606 (0.0459) | 0.541–0.669 | 1.71 | 0.70 |
| RO-MO-11 | 0.429 | 0.429 | 0.429 | 0.571 (0.0431) | 0.505–0.635 | 1.71 | 0.82 |
| RO-MO-12 | 0.676 | 0.714 | 0.694 | 0.770 (0.0390) | 0.711–0.822 | 3.34 | 0.30 |
| RO-MO-13 | 0.446 | 0.714 | 0.549 | 0.597 (0.0444) | 0.532–0.661 | 1.42 | 0.64 |
| RO-MO-14 | 0.410 | 0.714 | 0.521 | 0.703 (0.0411) | 0.640–0.761 | 2.21 | 0.39 |
| RO-MO-15 | 0.232 | 0.629 | 0.338 | 0.685 (0.0400) | 0.621–0.744 | 1.92 | 0.38 |
| RO-MO-16 | 0.220 | 0.686 | 0.333 | 0.687 (0.0431) | 0.623–0.746 | 2.20 | 0.46 |
| RO-MO-17 | 0.400 | 0.457 | 0.427 | 0.603 (0.0435) | 0.537–0.666 | 2.24 | 0.75 |
| RO-MO-18 | 0.239 | 0.629 | 0.346 | 0.701 (0.0368) | 0.638–0.759 | 1.94 | 0.30 |
| RO-MO-19 | 0.479 | 0.657 | 0.554 | 0.632 (0.0455) | 0.567–0.694 | 1.86 | 0.62 |
| Average RO-MO | 0,382 | 0,631 | 0.452 | 0.812 | 0.728 | 3.01 | 0.52 |
AUC: Area Under the Curve; CI: Confidence Interval; LR: Likelihood Ratio; SE: Standard Error.
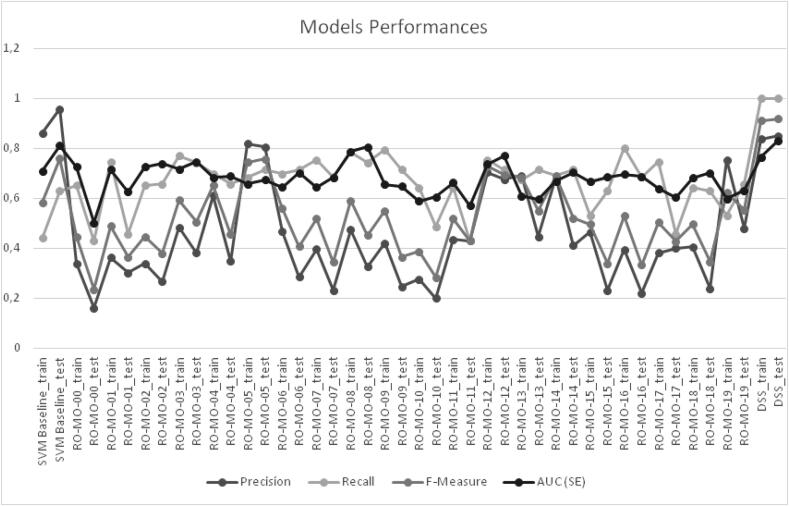
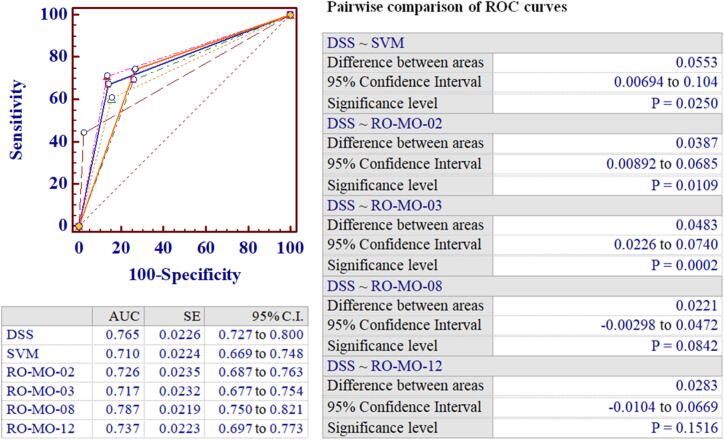
Overall, five RO-MO predictors showed an acceptable predictive performance with AUCs > 0.70 and significant positive and negative LRs (Table 3), but only RO-MO-02, RO-MO-03, RO-MO-08 and RO-MO-12 confirmed their predictive value in the test set (Table 4, Fig. 3).
Fig. 3.
Comparison of the predictive performance among the analyzed models.
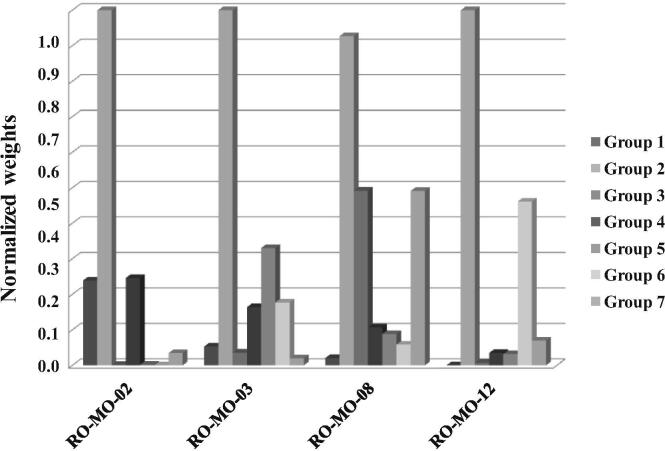
Of interest, all four predictors were not only clinically plausible – as demonstrated by the finding that group 2 variables (migraine clinical features) retained the strongest weight in all models (Table 5) – but they had also a complementary configuration of weights (Table 5, Fig. 4). In particular, RO-MO-02 was weighted on demographics and co-morbidities, RO-MO-03 on DBH polymorphism, RO-MO-08 on treatment details and lifestyle-related triggers and RO-MO-12 on the biochemical and metabolic asset. These findings are consistent with literature data showing that DBH polymorphism is associated with MO in chronic migraine patients [34] and suggest that patients’ metabolic asset, obesity, or lifestyle-related factors might contribute to drug overuse in this clinical setting.
Table 5.
Normalized weights of attribute groups in the training set.
| Model | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | Group 7 |
|---|---|---|---|---|---|---|---|
| RO-MO-00 | 0.05024 | 0.04528 | 0.36122 | 0.13728 | 0.41846 | 0.80842 | 0.05055 |
| RO-MO-01 | 0.18894 | 0.69045 | 0.17202 | 0.04681 | 0.04858 | 0.03836 | 0.14286 |
| RO-MO-02 | 0.23891 | 1.08083 | 0.00088 | 0.24596 | 0.00138 | 0.00011 | 0.03468 |
| RO-MO-03 | 0.05299 | 1.57451 | 0.03584 | 0.16382 | 0.33030 | 0.17691 | 0.02008 |
| RO-MO-04 | 0.00002 | 2.22696 | 0.07553 | 0.14644 | 0.12533 | 0.01231 | 0.04431 |
| RO-MO-05 | 0.01375 | 0.74568 | 0.01027 | 0.01685 | 0.01496 | 0.44050 | 0.03129 |
| RO-MO-06 | 0.00722 | 1.11237 | 0.08035 | 0.45895 | 0.11300 | 0.33577 | 0.23231 |
| RO-MO-07 | 0.00545 | 0.38898 | 0.01664 | 0.14503 | 0.34447 | 0.09276 | 0.19871 |
| RO-MO-08 | 0.02089 | 0.92704 | 0.49196 | 0.10673 | 0.08813 | 0.05844 | 0.49128 |
| RO-MO-09 | 0.18281 | 1.11588 | 0.08265 | 0.14504 | 0.40745 | 0.00791 | 0.09501 |
| RO-MO-10 | 0.42575 | 0.01383 | 0.03373 | 0.02243 | 0.34697 | 0.20611 | 0.38230 |
| RO-MO-11 | 0.11897 | 0.67353 | 1.02092 | 0.09301 | 0.05042 | 0.16503 | 0.01637 |
| RO-MO-12 | 0.00007 | 1.09627 | 0.00705 | 0.03511 | 0.03214 | 0.46090 | 0.06996 |
| RO-MO-13 | 0.00612 | 1.45488 | 0.03022 | 1.45488 | 0.02723 | 0.03022 | 0.15365 |
| RO-MO-14 | 0.23138 | 1.44378 | 0.01624 | 0.13616 | 0.02975 | 0.52446 | 0.12165 |
| RO-MO-15 | 0.41376 | 1.05772 | 0.48082 | 0.00006 | 0.21378 | 0.03978 | 0.02857 |
| RO-MO-16 | 0.00002 | 0.67413 | 0.04690 | 0.15811 | 0.54734 | 0.03346 | 0.65751 |
| RO-MO-17 | 0.22385 | 1.55356 | 0.24080 | 0.08292 | 0.07987 | 0.00019 | 0.09701 |
| RO-MO-18 | 0.02800 | 1.10822 | 0.08851 | 0.19543 | 0.17163 | 0.30326 | 0.00743 |
| RO-MO-19 | 0.42158 | 0.44897 | 0.03173 | 0.06303 | 0.02103 | 0.25017 | 0.01316 |
Fig. 4.
Normalized weights of groups of clinical attributes for the different models.
As the performance of RO-MO predictors could be further enhanced, we sought to investigate whether the combination of the best binary predictors (RO-MO-02, RO-MO-03, RO-MO-08 and RO-MO-12) into a decision support system (ML-based DSS) could be of advantage over the individual predictors, or baseline SVM. Using this combined model, we were able to classify migraine patients into five categories: definitely not likely to overuse (0, all predictors negative); probably not likely to overuse (1, at least one positive); possibly at risk of drug overuse (2, two of four positive); probably at risk of drug overuse (3, three of four positive); definitely at risk of drug overuse (4, all predictors positive). The discriminatory power of the ML-based DSS was first analyzed in the training set. The results obtained showed an overall improvement of MO risk prediction performance, with an AUC of 0.81 (95% CI: 0.78–0.84), which was higher than that observed with baseline SVM (AUC = 0.710; difference between areas: 0.099, p < 0.001), or each single predictor (RO-MO-2 AUC = 0.726, p < 0.001; RO-MO-3 AUC = 0.717, p < 0.001; RO-MO-8 AUC = 0.787, p = 0.05; RO-MO-12 AUC = 0.737, p < 0.001).
It should be noted that the adoption of a DSS incorporating multiple predictors implies that risk evaluation would be represented by a n-level stratification (generated in the event that risk estimate is achieved by all predictors, or by different combinations). In our model, the use of 4 predictors had led to a 5-level stratification, which was barely comparable with the binary classification of the original models or baseline SVM. Thus, instances were re-coded as 1/0 depending on whether they were positive or not to at least three of the RO-MO predictors (DSS predicted class 3 or 4) and ROC curves were re-analyzed. As reported in Fig. 5, the combined model resulted in an overall improvement of MO risk prediction performance, with a 0.765 AUC, which was higher than that observed with SVM, or each single predictor (Fig. 3).
Fig. 5.
Comparison between the Receiver Operator Characteristics Curves for ML-based decision support system (DSS) and SVM baseline predictor in the training set.
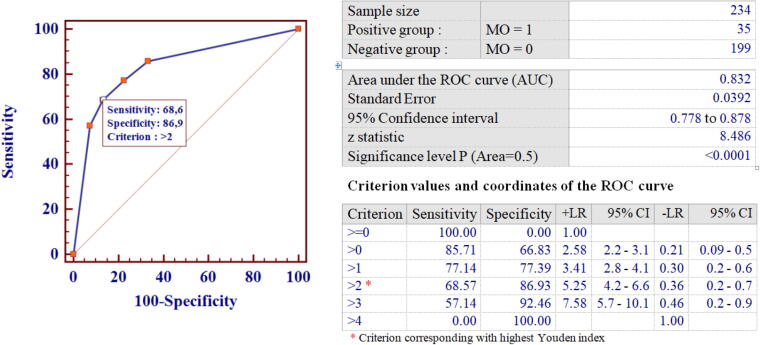
The improved predictive performance of the ML-based DSS was also confirmed in the test set. As shown in Fig. 6, in fact, the combined model resulted in an overall improvement of MO risk prediction performance, with an AUC of 0.83, a sensitivity and specificity of 0.69 and 0.87, respectively, and an accuracy of 0.87 when MO was predicted by at least three RO-MO models. Logistic regression analysis further confirmed that the ML-based DSS could effectively distinguish between MO and non-MO with ORs of 5.72 (95%CI: 1.56–20.9) and 21.0 (95%CI: 8.5–52) for patients classified as probably (3 RO-MO predictors positive), or definitely at risk of drug overuse (4 RO-MO predictors positive), respectively.
Fig. 6.
Receiver Operator Characteristics Curve for the combined Decision Support System in the test set.
4. Discussion
The present study was designed to investigate the performance of a novel ML-based methodological approach to derive an AI-based DSS for MO risk assessment in migraine patients.
MO is a challenging problem in migraine, mostly chronic migraine, in which is associated with higher pain intensity scores, symptoms severity and disability [5], [35], as well as the development of MOH [2], [7], [8]. MOH is defined as a headache for ≥15 days/month in a patient with a pre-existing primary headache (usually migraine or tension-type headache), and develops as a consequence of regular overuse (more than three months) of simple analgesics such as non-steroidal anti-inflammatory drugs, acetylsalicylic acid and paracetamol for ≥15 days/month, or analgesic combinations, triptans, ergotamine or opioid for ≥10 days/month [2].
Given the high disability rate of MOH and the treatment challenges it poses [7], prevention is especially important in patients prone to frequent headache and the possibility to predict MO is a compelling challenge in the clinical management of migraine.
Based on previous results [20], [23], [24], [25], [26], we hypothesized that a combined approach of kernel machines and RO of performance would have found a combination of attributes yielding the best classification performance of MO predictors over a test set. The results obtained demonstrated, for the first time to our knowledge, that this approach can be useful in performing an automated prediction for MO, holding the potential for improving model precision through weighting the relative importance of attributes. The clinical soundness of our approach was also supported by the finding that the best scoring models in terms of both f-measure and AUCs were also clinically plausible, as they were all strongly weighted on clinical features (Group 2), which represent some of the major determinants of MO development identified in epidemiological studies [5]. Furthermore, RO-MO-08, the best performing predictor, was substantially weighted on treatment information (use of prophylaxis, different drug classes, or response to triptans), whose role in the development of MO and MOH is also generally accepted [8]. On the other hand, the DBH 19-bp I/D polymorphism, showed irrelevant weights in three of the four models selected for DSS construction, with the exception of RO-MO-03, which may appear in partial disagreement with our previous finding of a significant association between DBH 19-bp I/D polymorphism and MO in inheritance models of genotype-variable association [34]. However, we must acknowledge that while the latter are designed for statistical inference, ML models are designed for prediction and not to prove relationships within the data.
As stated in Section 1, MO is strongly associated to MOH, although it does not necessarily represent its leading cause [2]. Of interest, some of the RO-MO predictors identified in our analysis also showed a discriminatory power for MOH. MOH was present in 9 of the 234 migraine patients included in the test set, 7 of whom (78%) had a positive RO-MO-12 predicted class. Hence, in this patient subset RO-MO-12 was capable of predicting MOH with a sensitivity and specificity of 89% and 71%, respectively, an AUC of 0.80 and a positive likelihood ratio of 3.1 (95% CI: 2.4–3.9) (data not shown). Nonetheless, whether this approach might be useful to identify individuals who will likely develop MOH still needs to be explored.
On the other hand, the analysis of clinical/biochemical variables identified other features possibly associated to MO, which were seldom considered before [36], [37], [38]. This is the case of biochemical features and lifestyle-related attributes, whose relative weight was considerably represented in RO-MO-08 and RO-MO-12, respectively. Previous studies, in fact, suggested that psychiatric co-morbidities (e.g. anxiety disorder or depression) [36], smoking [38], lack of exercise [38] or metabolic syndrome [37] could represent risk factors for the development of MOH. Here, we provide further evidence suggesting that an AI-based approach may exploit significant patterns in data – connoting causality between individual features and MO – that can be used to further explore the pathophysiology of MO and MOH in patients with migraine.
The availability of a set of predictive discriminators further allowed the design of a DSS based on a complementary set of four predictors, to investigate whether a combined approach may be of advantage over individual models. The adoption of a model incorporating four predictors allowed to perform a risk evaluation on a five-level stratification and resulted in an overall improvement of MO risk prediction performance over the single predictors, with an AUC of 0.83 and ORs of 5.7 and 21.0 for patients classified as probably (3 RO-MO predictors positive), or definitely at risk of drug overuse (4 RO-MO predictors positive), respectively. This result could be of considerable importance in migraine, where patient education and counseling to avoid MO remains the single most important way to prevent MOH. The possibility to predict those patients at risk for MO, indeed, would help the neurologist to advise people from an early stage and tailor treatment based on individual risk.
The results obtained in the present study are barely comparable with previous studies, as the application of ML to implement medical decision making in migraine has generally addressed the issue of disease classification [13], [14], [15], [16], [17], [18], [19], [20], [39] or treatment outcome prediction [21], [22]. All these studies demonstrated that intelligent systems represent a promising approach for migraine classification, holding potential to revolutionize conventional models of diagnostic deliverance in a contest of personalized medicine. Interestingly, using ML approaches based on magnetic resonance imaging resting-state data [19] or somatosensory evoked potentials [39] it was possible to discriminate migraine patients from healthy subjects with an accuracy of 81% and 88%, respectively. On the other hand, the issue of ML-based risk prediction of MO in migraine has never been addressed before. Even the study by Garcia-Chimeno et al. – reporting an accuracy >90% using SVM – analyzed the possibility to correctly classify between different migraine phenotypes (i.e. episodic vs. chronic migraine with MO) [20]. Conversely, in the present study we specifically addressed the issue of MO risk prediction in patients with various migraine phenotypes, demonstrating that the use of a combined approach of kernel machines and RO of performance outperforms SVM with an accuracy of 87%. This percentage is also higher than that reported by Maizels et al., who demonstrated that the use of a computerized headache assessment tool (CHAT) was able to correctly recognize MO in 82.7% of cases [40].
There are, of course, some limitations to acknowledge. First, the sample size was relatively small, ultimately leading to a small number of recorded events. Second, the model here reported was designed and validated on a dataset derived from a biobank/database project in which all patients are interviewed with a face-to-face semistructured questionnaire detailing in depth demographic and clinical characteristics. Therefore, the population enrolled is highly homogenous and well characterized. Nonetheless, the data here reported demonstrate that the use of ML algorithms and RO models might be of advantage in developing local classifiers capable of predicting MO in migraine, thus supporting the neurologist in the critical phase of migraine clinical and therapeutic decision making. Validation in multicenter prospective studies is needed before making any ML approach into the clinical practice available.
5. Conclusions
In conclusion, a combination of machine learning and random optimization – taking into consideration clinical and biochemical features, drug exposure and lifestyle – might represent a valuable approach to MO prediction in migraine. This is particularly appealing in a context of predictive medicine, in which attributes, routinely collected in electronic health records, may be all used to design new tools for clinical and therapeutic decision making. This approach holds the potential for improving model precision through weighting the relative importance of attributes and demonstrates that other variables must be considered in MO risk evaluation, thus strengthening the theory advocated by precision medicine that data should be considered in a more general association, rather than individually.
CRediT authorship contribution statement
Patrizia Ferroni: Conceptualization, Methodology, Writing - original draft. Fabio M. Zanzotto: Conceptualization, Methodology, Software. Noemi Scarpato: Data curation, Methodology, Software. Antonella Spila: Data curation, Visualization. Luisa Fofi: Data curation, Visualization. Gabriella Egeo: Data curation, Visualization. Alessandro Rullo: Visualization, Writing - review & editing. Raffaele Palmirotta: Data curation, Writing - review & editing. Piero Barbanti: Conceptualization, Methodology, Writing - original draft. Fiorella Guadagni: Conceptualization, Supervision, Writing - review & editing.
Declaration of Competing Interest
Piero Barbanti has received consultancy fees from Allergan, Bayer, electroCore, Lusofarmaco, Merck, Visufarma, and advisory fees from TEVA, Novartis and Eli-Lilly. Gabriella Egeo received travel grants and honoraria from Eli-Lilly, Novartis, New Penta and Ecupharma; Luisa Fofi received travel grants and honoraria from Teva, Eli-Lilly and Novartis; Patrizia Ferroni, Fabio M. Zanzotto, Noemi Scarpato, Antonella Spila, Alessandro Rullo, Raffaele Palmirotta and Fiorella Guadagni report no conflict of interest.
Role of the funding source
This work was partially supported by Grants from the European Social Fund, PNR 2015–2020 ARS01_01163 PerMedNet under the Italian Ministries of Education, University and Research (CUP B66G18000220005), POR Campania FESR 2014–2020 RARE.PLAT.NET (CUP B63D18000380007) and by the Italian Ministry of Health (institutional funding ricerca corrente). The funding sources had no involvement in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Contributor Information
Patrizia Ferroni, Email: patrizia.ferroni@sanraffaele.it.
Noemi Scarpato, Email: noemi.scarpato@uniroma5.it.
Antonella Spila, Email: antonella.spila@sanraffaele.it.
Luisa Fofi, Email: luisa.fofi@sanraffaele.it.
Gabriella Egeo, Email: gabriella.egeo@sanraffaele.it.
Alessandro Rullo, Email: a.rullo@neatec.it.
Raffaele Palmirotta, Email: raffaele.palmirotta@uniba.it.
Piero Barbanti, Email: piero.barbanti@sanraffaele.it.
Fiorella Guadagni, Email: fiorella.guadagni@sanraffaele.it.
References
- 1.Burch R. Migraine and tension-type headache: diagnosis and treatment. Med Clin North Am. 2019;103:215–233. doi: 10.1016/j.mcna.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Headache Classification Committee of the International Headache Society (IHS). The International classification of headache disorders, 3rd ed., 2018; Cephalalgia 38: 1–211. [DOI] [PubMed]
- 3.Barbanti P., Egeo G. Pharmacological trials in migraine: it's time to reappraise where the headache is and what the pain is like. Headache. 2015;55:439–441. doi: 10.1111/head.12498. [DOI] [PubMed] [Google Scholar]
- 4.Ford J.H., Schroeder K., Buse D.C., Joshi S., Gelwicks S. Predicting initiation of preventive migraine medications: exploratory study in a large U.S. medical claims database. Curr Med Res Opin. 2019 doi: 10.1080/03007995.2019.1657716. [DOI] [PubMed] [Google Scholar]
- 5.Schwedt T.J., Alam A., Reed M.L., Fanning K.M., Munjal S. Factors associated with acute medication overuse in people with migraine: results from the 2017 migraine in America symptoms and treatment (MAST) study. J Headache Pain. 2018;19:38. doi: 10.1186/s10194-018-0865-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.May A., Schulte L.H. Chronic migraine: risk factors, mechanisms and treatment. Nat Rev Neurol. 2016;12:455–464. doi: 10.1038/nrneurol.2016.93. [DOI] [PubMed] [Google Scholar]
- 7.Chen P.K., Wang S.J. Medication overuse and medication overuse headache: risk factors, comorbidities, associated burdens and nonpharmacologic and pharmacologic treatment approaches. Curr Pain Headache Rep. 2019;23:60. doi: 10.1007/s11916-019-0796-7. [DOI] [PubMed] [Google Scholar]
- 8.Wakerley B.R. Medication-overuse headache. Pract Neurol. 2019;19:399–403. doi: 10.1136/practneurol-2018-002048. [DOI] [PubMed] [Google Scholar]
- 9.Zwart J.A., Dyb G., Hagen K., Svebak S., Holmen J. Analgesic use: a predictor of chronic pain and medication overuse headache: the Head-HUNT Study. Neurology. 2003;61:160–164. doi: 10.1212/01.wnl.0000069924.69078.8d. [DOI] [PubMed] [Google Scholar]
- 10.Katsarava Z., Schneeweiss S., Kurth T. Incidence and predictors for chronicity of headache in patients with episodic migraine. Neurology. 2004;62:788–790. doi: 10.1212/01.wnl.0000113747.18760.d2. [DOI] [PubMed] [Google Scholar]
- 11.Bigal M.E., Serrano D., Buse D., Scher A., Stewart W.F., Lipton R.B. Acute migraine medications and evolution from episodic to chronic migraine: a longitudinal population-based study. Headache. 2008;48:1157–1168. doi: 10.1111/j.1526-4610.2008.01217.x. [DOI] [PubMed] [Google Scholar]
- 12.Wang S.J., Fuh J.L., Lu S.R., Juang K.D. Outcomes and predictors of chronic daily headache in adolescents: a 2-year longitudinal study. Neurology. 2007;68:591–596. doi: 10.1212/01.wnl.0000252800.82704.62. [DOI] [PubMed] [Google Scholar]
- 13.Krawczyk B., Simić D., Simić S., Woźniak M. Automatic diagnosis of primary headaches by machine learning methods. Cent Eur J Med. 2013;8:157–165. [Google Scholar]
- 14.Çelik U., Yurtay N., Koç E.R., Tepe N., Güllüoğlu H. Diagnostic accuracy comparison of artificial immune algorithms for primary headaches. Comput Math Methods Med. 2015;2015 doi: 10.1155/2015/465192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keight R, Aljaaf AJ, Al-Jumeily D, Hussain AJ, Özge A, et al. An intelligent systems approach to primary headache diagnosis. International conference on intelligent computing; 2017. Dordrecht: Springer
- 16.Vandewiele G., De Backere F., Lannoye K., Vanden Berghe M., Janssens O. A decision support system to follow up and diagnose primary headache patients using semantically enriched data. BMC Med Inform Decis Mak. 2018;18:98. doi: 10.1186/s12911-018-0679-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khayamnia M., Yazdchi M., Heidari A., Foroughipour M. Diagnosis of common headaches using hybrid expert-based systems. J Med Signals Sens. 2019;9:174–180. doi: 10.4103/jmss.JMSS_47_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang H., Zhang J., Liu Q., Wang Y. Multimodal MRI-based classification of migraine: using deep learning convolutional neural network. Biomed Eng Online. 2018;17:138. doi: 10.1186/s12938-018-0587-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chong C.D., Gaw N., Fu Y., Li J., Wu T. Migraine classification using magnetic resonance imaging resting-state functional connectivity data. Cephalalgia. 2017;37:828–844. doi: 10.1177/0333102416652091. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Chimeno Y., Garcia-Zapirain B., Gomez-Beldarrain M., Fernandez-Ruanova B., Garcia-Monco J.C. Automatic migraine classification via feature selection committee and machine learning techniques over imaging and questionnaire data. BMC Med Inform Decis Mak. 2017;17:38. doi: 10.1186/s12911-017-0434-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parrales Bravo F., Del Barrio García A.A., Gallego M.M., Gago Veiga A.B., Ruiz M. Prediction of patient's response to Onabotulinumtoxin A treatment for migraine. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2018.e01043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Athreya A., Iyer R., Neavin D., Wang L., Weinshilboum R. Augmentation of physician assessments with multi-omics enhances predictability of drug response: a case study of major depressive disorder. IEEE Comput Intell Mag. 2018;13:20–31. doi: 10.1109/MCI.2018.2840660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferroni P., Zanzotto F.M., Scarpato N., Riondino S., Nanni U. Risk assessment for venous thromboembolism in chemotherapy treated ambulatory cancer patients: a precision medicine approach. Med Decis Mak. 2017;37:234–242. doi: 10.1177/0272989X16662654. [DOI] [PubMed] [Google Scholar]
- 24.Ferroni P., Zanzotto F.M., Scarpato N., Riondino S., Guadagni F. Validation of a machine learning risk predictor for venous thromboembolism in oncology. Dis Markers. 2017;2017:8781379. doi: 10.1155/2017/8781379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferroni P., Roselli M., Zanzotto F.M., Guadagni F. Artificial Intelligence for cancer-associated thrombosis risk assessment. Lancet Haematol. 2018;5 doi: 10.1016/S2352-3026(18)30111-X. [DOI] [PubMed] [Google Scholar]
- 26.Ferroni P., Zanzotto F.M., Riondino S., Scarpato N., Guadagni F. Predicting breast cancer prognosis using a machine learning approach. Cancers (Basel) 2019;11 doi: 10.3390/cancers11030328. pii: E328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gönen M., Alpaydın E. Multiple kernel learning algorithms. J Mach Learn Res. 2011;12:2211–2268. [Google Scholar]
- 28.Cristianini N., Shawe-Taylor J. An Introduction to Support Vector Machines and other kernel based learning methods. Ai Mag. 2000;22:190. [Google Scholar]
- 29.Matyas J. Random optimization. Automat Rem Contr. 1965;26:246–253. [Google Scholar]
- 30.Palmirotta R., Barbanti P., Ludovici G., Egeo G., Aurilia C. Establishment of a biorepository for migraine research: the experience of Interinstitutional Multidisciplinary BioBank (BioBIM) Neurol Sci. 2013;34:1659–1663. doi: 10.1007/s10072-013-1308-x. [DOI] [PubMed] [Google Scholar]
- 31.Barbanti P., Aurilia C., Dall’Armi V., Egeo G., Fofi L. The phenotype of migraine with unilateral cranial autonomic symptoms documents increased peripheral and central trigeminal sensitization. A case series of 757 patients. Cephalalgia. 2016;36:1334–1340. doi: 10.1177/0333102416630579. [DOI] [PubMed] [Google Scholar]
- 32.Filice S, Castellucci G, Croce D, Basili R. KeLP: a Kernel-based learning platform for natural language processing. Proceedings of ACL-IJCNLP 2015 system demonstrations. Beijing, China, July 26–31; 2015. p. 19–24.
- 33.Saar-Tsechansky M., Provost F. Handling missing values when applying classification models. J Mach Learn Res. 2007;8:1623–1657. [Google Scholar]
- 34.Barbanti P., Guadagni F., De Marchis M.L., Ialongo C., Egeo G. Dopamine-beta-hydroxylase 19-bp insertion/deletion polymorphism affects medication overuse in patients with chronic migraine. Neurol Sci. 2019;40:1717–1724. doi: 10.1007/s10072-019-03865-9. [DOI] [PubMed] [Google Scholar]
- 35.Lipton R.B., Munjal S., Buse D.C., Fanning K.M., Bennett A. Predicting inadequate response to acute migraine medication: results from the American migraine prevalence and prevention (AMPP) study. Headache. 2016;56:1635–1648. doi: 10.1111/head.12941. [DOI] [PubMed] [Google Scholar]
- 36.Diener H.C., Dodick D., Evers S., Holle D., Jensen R.H. Pathophysiology, prevention, and treatment of medication overuse headache. Lancet Neurol. 2019;18:891–902. doi: 10.1016/S1474-4422(19)30146-2. [DOI] [PubMed] [Google Scholar]
- 37.He Z., Dong L., Zhang Y., Kong Q., Tan G. Metabolic syndrome in female migraine patients is associated with medication overuse headache: a clinic-based study in China. Eur J Neurol. 2015;22:1228–1234. doi: 10.1111/ene.12732. [DOI] [PubMed] [Google Scholar]
- 38.Hagen K., Linde M., Steiner T.J., Stovner L.J., Zwart J.A. Risk factors for medication-overuse headache: an 11-year follow-up study The Nord-Trøndelag Health Studies. Pain. 2012;153:56–61. doi: 10.1016/j.pain.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 39.Bingzhao Z., Coppola G., Shoaran M. Migraine classification using somatosensory evoked potentials. Cephalalgia. 2019;39:1143–1155. doi: 10.1177/0333102419839975. [DOI] [PubMed] [Google Scholar]
- 40.Maizels M., Wolfe W.J. An expert system for headache diagnosis: the Computerized Headache Assessment tool (CHAT) Headache. 2008;48:72–78. doi: 10.1111/j.1526-4610.2007.00918.x. [DOI] [PubMed] [Google Scholar]