Abstract
This paper focus on a neural network classification model to estimate the association among gender, race, BMI, age, smoking, kidney disease and diabetes in hypertensive patients. It also shows that artificial neural network techniques applied to large clinical data sets may provide a meaningful data-driven approach to categorize patients for population health management, and support in the control and detection of hypertensive patients, which is part of the critical factors for diseases of the heart. Data was obtained from the National Health and Nutrition Examination Survey from 2007 to 2016. This paper utilized an imbalanced data set of 24,434 with (69.71%) non-hypertensive patients, and (30.29%) hypertensive patients. The results indicate a sensitivity of 40%, a specificity of 87%, precision of 57.8% and a measured AUC of 0.77 (95% CI [75.01–79.01]). This paper showed results that are to some degree more effectively than a previous study performed by the authors using a statistical model with similar input features that presents a calculated AUC of 0.73. This classification model can be used as an inference agent to assist the professionals in diseases of the heart field, and can be implemented in applications to assist population health management programs in identifying patients with high risk of developing hypertension.
Subject terms: Computer science, Information technology, Medical research
Introduction
Currently, the use of neural network models for disease classification is increasing rapidly, not only because of a significant amount of data available that is being generated by healthcare devices and systems but also for the magnitude of computational resources available for data calculation and processing1, 2. This immense volume of data is utilized to train models importantly, and facilitates the use of expert systems, NLP techniques3, 4 and classification techniques for finding trends and patterns in the evaluation and classification of several diseases. Hypertension is considered in the group of risk factors for cardiovascular disease that caused 17.7 million deaths in the world in 20155–7. In the United States, hypertension is contemplated as the primary determinant of decease among U.S. adults even with the existence of practical and low-cost treatments8–10, with significant public health risks and economic implications for U.S. population. The National Health and Nutrition Examination Survey (NHANES) conducted by the National Center for Health Statistics is one of the principal sources for tracking hypertension in the U.S. population11 with vast amounts of features and data related to cardiovascular diseases.
In this paper, we develop a neural network classification model to predict hypertension with non-invasive risk factors applying healthcare data from the NHANES. A multi-layer neural network architecture was used to identify hypertensive patients at risk of developing hypertension. Our primary goal in this paper was to train a classifier that will identify hypertensive patients in a highly imbalanced NHANES data set. Additionally, we aspire to achieve lower error rate with a neural network architecture compared to the logistic regression model developed in a previous paper12 by using the same set of input features. The motivation to develop a new model was the non-linearity of the input features, and neural networks are usually trained to treat non-linearity due to the non-linear nature of them13, making the model more flexible compared to logistic regression.
This paper is organized along these sections. Second section describes related work and literature research of various models that implemented neural networks for cardiovascular disease classification. Third section introduces the elaboration of the model, population, data source, and validation. Fourht section combines statistical and clinical analysis. Fifth section presents our results and limitations. Finally, sixth section presents conclusions and future work.
Related work
We reviewed a few published papers adopting neural networks models to infer hypertension, and some other studies that compared classification performance and accuracy with logistic regression14–16. In every paper, the development process, feature selection, ground truth definition, training data sets, test data sets, overfitting prevention, error assessment, and accuracy information were reviewed. We also reviewed if the models were validated or not, either by an unseen data set or by a panel of experts in the domain17–20.r Some of the models are shown in Table 1.
Table 1.
Related work.
| Author | Input features | n Total | Type of model | AUC (%) |
|---|---|---|---|---|
| Artifical neural network models comparison | ||||
| LaFreniere et al.21 | Age, gender, BMI, sys/diast BP, high and low density lipoproteins, triglycerides, cholesterol, microalbumin, and urine albumin creatinine ratio | 379,027 | Backpropagation neural network | 82 |
| Polak and Mendyk22 | Age, sex, diet, smoking and drinking habits, physical activity level and BMI | 159,989 | backpropagation (BP) and fuzzy network | 75 |
| Tang et al.23 | Sys/diast BP, fasting plasma glucose, age, BMI, heart rate, gender, WC, diabetes, renal profile | 2,092 | Feed-forward, back-propagation neural network | 76 |
| Ture et al.24 | Age, sex, hypertension, smoking, lipoprotein, triglyceride, uric acid, total cholesterol, BMI | 694 | Feed-forwardneural network | 81 |
| Lynn et al.25 | Sixteen genes, age, BMI, fasting blood sugar, hypertension medication, no history of cancer, kidney, liver or lung | 22,184 genes, 159 cases | One-hidden-layer neural network | 96.72 |
| Sakr et al.6 | Age, gender, race, reason for test, stress, medical history | 23,095 | Backpropagation neural network | 64 |
| López-Martínez et al.12 | Age, gender, ethnicity, BMI, smoking history, kidney disease, diabetes | 24,434 | Three-hidden layer neural network | 77 |
LaFreniere et al.21 presented an artificial neural network (ANN) to predict hypertensive patients utilizing the Canadian Primary Care Sentinel Surveillance Network (CPCSSN) data set. The independent features used were age, gender, BMI, systolic and diastolic blood pressure, high and low-density lipoproteins, triglycerides, cholesterol, microalbumin, and urine albumin–creatinine ratio. Confusion matrix and Receiver Operating Characteristic (ROC) curve were utilized to measure the accurateness of the model. This paper used an extensive data set to train the model compared with other studies.
Polak and Mendyk22 improve and validate an artificial neural network for high blood pressure risk, using data from the Center for Disease Control and the National Center for Health Statistics (CDC-NCHS). The independent features used in this model were age, sex, diet, smoking and drinking habits, physical activity level and BMI index. ROC curve was utilized to measure the accurateness of the model, and they performed a comparison with a logistic regression classification model.
Tang et al.23 presented an artificial neural network for classification of cardiovascular disease including hypertension; this paper used a Chinese population. Statistical analysis indicated that 14 risk factors showed statistical importance with cardiovascular disease. The ROC curve is utilized to measure the performance of the model.
Ture et al.24 implemented a multilayer perceptron for the classification of hypertensive patients. The independent features utilized were age, sex, family history of hypertension, smoking habits, lipoprotein, triglyceride, uric acid, total cholesterol, and body mass index (BMI). ROC curve is utilized to measure the accuracy of the model.
Lynn et al.25 constructed a neural network model to simulate the geneendophenotype-disease relationship for Taiwanese hypertensive males. Sixteen genes, age, BMI, fasting blood sugar, hypertension medication, no history of cancer, kidney, liver or lung. Classification accuracy is utilized to measure the performance of the model.
Sakr et al.6 built an artificial neural network to compares the performance of it with different machine learning techniques on predicting the risk of developing hypertension. Age, gender, race, a reason for the test, stress tests and medical history used for classification. ROC curve is utilized to measure the accuracy of the model.
We identified other studies for predicting hypertension using ANN, and all of them have advantages and disadvantages. However, the above mentioned are the most relevant. Our paper did not use data from any medical facility as the studies mentioned earlier. However, our model used a data sample more significant that the majority of them, collected from a national examination survey. The number of predictors was small and non-invasive, in comparison with the cited studies that used lab and exam data.
The national examination survey was designed to assess the health and nutritional status of adults and children in the United States, the data on this survey is unique because it combines social determinants of health data such as smoking, alcohol consumption and dietary habits, and physical examinations. This survey emphasized data regarding the prevalence of major diseases and risk factors for diseases for a broader population than just data from a medical facility, which contains only data for a small subset of the population that does not represent the entire picture of significant disease. In addition, historically, disease trends in the United States have been assessed by surveys.
We achieve an AUC of 0.77 which is acceptable and close to all the studies, considering the imbalanced data used in our paper. The results in our paper and the cited studies could be successfully utilized in hypertension classification, and can be included as inference engines in expert systems for hypertension screening tools. Our paper also includes more hidden layers that the others and we determined the number of hidden layers through cross-validation experiments. Not evidence of the number of layers and hidden nodes selection techniques are present in the studies.
Materials and methods
We present and discuss an alternative approach, using artificial neural network to classify hypertensive patients. We build, trained, and evaluated the model with the Sklearn of Python programing language package26, Microsoft Cognitive Toolkit (CNTK) from Microsoft, and Azure Machine Learning Studio27, 28.
A cross-sectional analysis comes from the collection of health examination data for a representative sample of noninstitutionalized U.S. residents, questionnaires administered in the home of the residents. The interview collects demographic, health, and nutrition information, as well as information about the household. This examination includes physical measurements such as blood pressure, dental examination, and the collection of blood and urine specimens for lab testing.
Data source
We collected NHANES data sets from NHANES 2007–2008 to NHANES 2015–2016. This dataset is intended for public access and health care utilization. This datasets are prepared and published through the Centers for Disease Control and Prevention (CDC) to provide full access. Statistic characterizing human populations, laboratory data, blood pressure, body measures data and questionnaires linked to diabetes, smoking, and kidney conditions are part of the data set. The original data set consists of five folders from 2007 to 2016, each one of them contains a pdf file with statistics of the response rates of the NHANES survey and the SAS Transport files for all the survey measure variables. After imported the original data sets in python, data extraction and transformation were necessary to select and categorize the input features. We created a repository in Github with the original files from NHANES, the final data set used to run the model and the notebooks used for the data preparation29.
Ethic review board approval
For the use of NHANES data, the Institutional Review Board (IRB) approval and documented consent was obtained from participants. The description of the survey name and data, and the NCHS IRB/ERB Protocol Number or Description can be found in Centers for Disease Control and Prevention30. In 2003, the NHANES Institutional Review Board (IRB) changed its name to the NCHS Research Ethics Review Board (ERB). The National Center for Health Statistics (NCHS) offered downloadable public-use data files through the Centers for Disease Control and Preventions (CDC) FTP file server. NHANES survey is a public-use data files prepared and disseminated to provide access to the full scope of the data. This allows researchers to manipulate the data in a format appropriate for their analyses. In our study, by using these data we signify our agreement to comply with the data use restrictions to ensure that the information will be used solely for statistical analysis or reporting purposes. The data use restrictions can be found in National Center for Health Statistics31. In this study, all experiments were performed in accordance with relevant guidelines and regulations.
Study population and analysis
Healthcare survey data collected in the course of 2007–2016 was used to train and evaluate the classification model. A neural network model was developed to assess the importance of several factors and their relation with prevalence of hypertension with a symbolical sampling of adults 20 years in the United States (n = 24,434). Table 2 shows the grouping of the hypertensive patients by race and gender.
Table 2.
n samples by hypertensive class, gender and race.
| Class | Gender | Race | n |
|---|---|---|---|
| Hypertension, adults 20 and over: 2007–2016 | |||
| Hypertensive | Female | Mexican American | 464 |
| Non-Hispanic black | 925 | ||
| Non-Hispanic white | 1,433 | ||
| Other Hispanic | 368 | ||
| Other race—including multi-racial | 277 | ||
| Male | Mexican American | 575 | |
| Non-Hispanic black | 1,039 | ||
| Non-Hispanic white | 1,582 | ||
| Other Hispanic | 371 | ||
| Other race—including multi-racial | 365 | ||
| Non-hypertensive | Female | Mexican American | 1,461 |
| Non-Hispanic black | 1,676 | ||
| Non-Hispanic white | 3,663 | ||
| Other Hispanic | 1,084 | ||
| Other race—including multi-racial | 1,038 | ||
| Male | Mexican American | 1,275 | |
| Non-Hispanic black | 1,465 | ||
| Non-Hispanic white | 3,585 | ||
| Other Hispanic | 820 | ||
| Other race—including multi-racial | 968 | ||
| Total | 24,434 | ||
Input features
Several studies in the US integrated healthcare system in cardiovascular research with incident hypertension showed association between race, age, smoking, BMI, diabetes, and kidney conditions with hypertension32–34. Among different cohorts of patients with hypertension, during the follow up, individuals present more kidney disease, diabetes problems and remarkable association with smoking habits. In addition, these studies shown that effective BMI management decrease the incidence of hypertension, hypertension prevalence increase with age, and race is a significant factor of prevalence of hypertension.
For this paper, and based on the previous analysis, the selected input features are race, age, smoking, body mass index (BMI), diabetes, and kidney conditions. Participants admit to have diabetes if the answer presents “Yes or “Borderline” to the question “Doctor told that you have diabetes?”35. Smokers defined as individuals having smoked 100 cigarettes during their lifetime, and currently smoke some days or every day36. Chronic kidney disease (CKD) defined as “yes” response to the question “Have you ever told by a health care provider you have weak or failing kidneys?” during the interview, and for NHANES 2015–2016, CKD defined as a glomerular filtration rate (GFR) 60 ml/min/1.73 37 and albumin–creatinine ratio 30 mg/g38. Body mass index and age transformed from continues features to Categorical features to understand the relationship among the features. Blood pressure is utilized to generate the dependent feature.Hypertension category designated as systolic blood pressure of 130 mmHg, previously define as 140 mmHg by the American Heart Association39. Table 3 show the independent features and the dependent feature.
Table 3.
Variables included in the model.
| Variable name | Description | Code | Meaning |
|---|---|---|---|
| Gender | Gender | 1 | Male |
| 2 | Female | ||
| Agerange | Age at screening adjudicated—date of birth was used to calculate AGE | 1 | 20-30 |
| 2 | 31–40 | ||
| 3 | 41–50 | ||
| 4 | 51–60 | ||
| 5 | 61–70 | ||
| 6 | 71–80 | ||
| Race | Race/Hispanic origin | 1 | Mexican American |
| 2 | Other Hispanic | ||
| 3 | Non-Hispanic white | ||
| 4 | Non-Hispanic black | ||
| 5 | Other race—including multi-racial | ||
| BMXBMI | Body mass index (kg/m) | 1 | Underweight = |
| 2 | Normal weight = 18.5–24.9 | ||
| 3 | Overweight = 25–29.9 | ||
| 4 | Obesity = BMI of 30 or greater | ||
| Kidney | Ever told you had weak/failing kidneys | 1 | Yes |
| 2 | No | ||
| Smoke | Smoked at least 100 cigarettes in life | 1 | Yes |
| 2 | No | ||
| Diabetes | Doctor told you have diabetes | 1 | Yes |
| 2 | No | ||
| 3 | Borderline | ||
| Hypclass | Systolic: blood pres (mean) mm Hg | 0 | Non-hypertensive |
| 1 | Hypertensive |
Features selection
Clinical importance was pertinent plus the statistical significance of the features to choose the final inputs. For this paper, we utilized chi-square because previous work indicates that this statistical test performs well to evaluate sets of categorical features40–43. Some heuristic methods were investigated to confirm the usefulness and relevance of the features. Genetic algorithm with other machine learning methods probably generates better results42, and produce adequate time complexity to find optimal solutions44, 45. We will consider it and discuss it in forthcoming studies. For this paper, we utilized statistical methods between each feature due to the nature of the inputs. Table 4 shows all features with their p values and scores. Based on the clinical significance of all input features, our clinical expert decided not to exclude any variable from the paper due to the relationship among features in previous studies. In addition, one of the options we used to get the feature importance or the influence of a given parameter in the classification model is to obtain and examine the coefficient of the parameters with the provided dataset as shown in López-Martínez et al.12 Coefficients and Odds Ratio. Features using the same scale with larger coefficients are more important because they represent more significant changes in the dependent variable.
Table 4.
Chi-squared between each variable.
| Feature | p value | Score |
|---|---|---|
| Gender | 0.3988107 | 0.711909 |
| Agerange | 0.000000 | 1,965.607023 |
| Race | 0.008822 | 6.858521 |
| BMIrange | 0.0172385 | 5.67193 |
| Kidney | 0.3546428 | 0.856775 |
| Smoke | 0.0975246 | 2.745566 |
| Diabetes | 0.0012164 | 10.465222 |
Neural network model
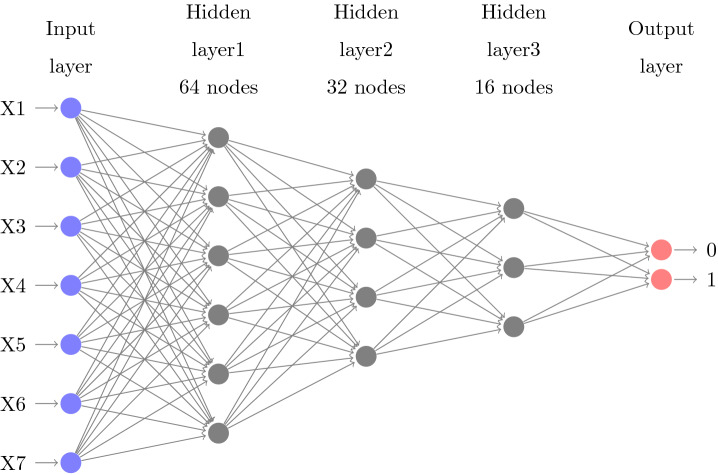
An artificial neural network describes a machine learning algorithms that are made of layers of nodes, usually, an input layer, hidden layers46 and an output layer47. Figure 4 shows the form of the neural network architecture developed for this paper. The input nodes values are encoded and normalized with gaussian normalization48 in order to improve the computation.
Fig. 4.
Multilayer perceptron architecture.
The non-linearity of the model
The motivation of developing this neural networks model is the ability to use non-linear activation functions to eliminate the non-linearity of the input features. The data used to train the model is not linearly separable, this means that there is no line that separates the data points easily as shown in Figs. 1 and 2 where we plot several input variables and the decision boundary using logistic regression.
Fig. 1.
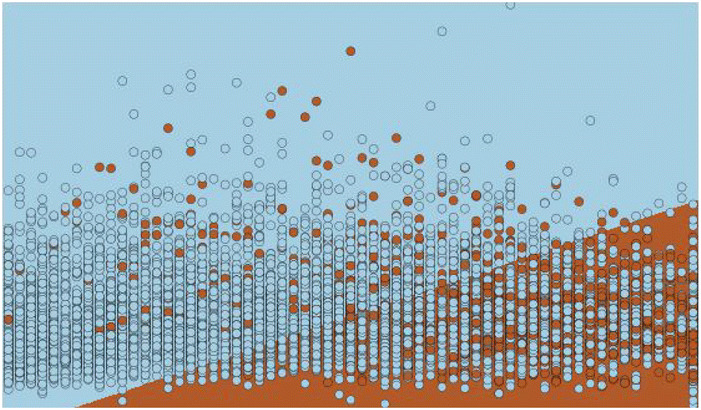
Decision boundary.
Fig. 2.
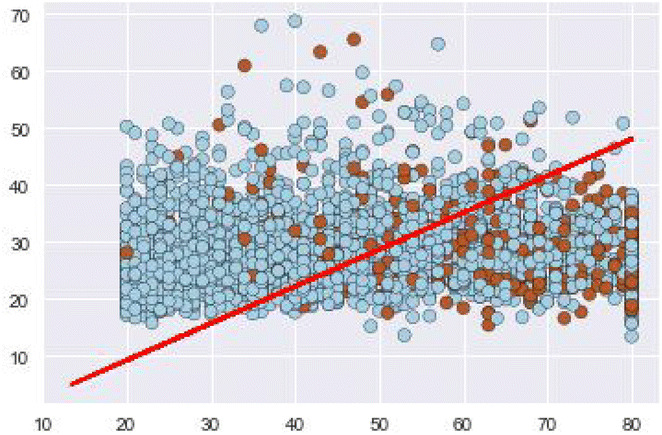
Draw test points.
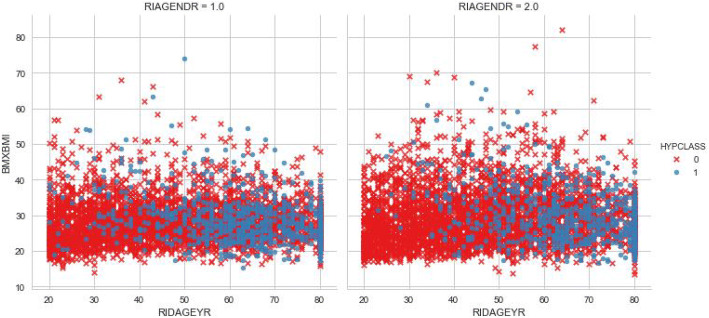
This non separability can be seen also if we plot three input variables such as gender, age and bmi as shown in Fig. 3. The Neural network model learn a new representations of the data which makes the classification more approachable with respect to this representation. A non-linear activation function allows non-linear classification with a non-linear decision boundary which will be a hyperplane that is orthogonal to the line.
Fig. 3.
Relation between BMI and age by gender and hypertension class.
Model training
The generated probabilities need to be as proximate as possible to the observed features. The loss function calculated as the difference between the learned model against the generated by the training set49. Cross-entropy with Softmax is utilized, but the mathematical computation of the derivative is not presented in this paper50 (Fig. 4).
Random initialization of the parameters is the first step, and the network produces a new set of parameters after each evaluation. In this network, He initialization51 is utilized. This type of initialization is comparable with the Xavier initialization excepting Xavier uses a different weights scaling factors W in layer l, and the author recommended for layers with ReLU activation. Mini-batches are utilized to train the model. Learning rate52 is a factor that balances how much the parameters change in every iteration. Each iteration works on ten samples, and the model is trained on 70% (17,104) of the data set. Table 5 presents the parameters of the architecture, and Figs. 5 and 6 present the training loss and classification error of the mini-batch run for the model.
Table 5.
Model architecture parameters.
| Parameter | Value | |
|---|---|---|
| Input dimension | 7 | |
| Num output classes | 2 | |
| Num hidden layers | 3 | |
| Hidden layer1 dimension | 64 | |
| Activation func layer1 | Relu | |
| Hidden layer2 dimension | 32 | |
| Activation func layer2 | Relu | |
| Hidden layer3 dimension | 16 | |
| Activation func layer3 | Relu | |
| Minibatch size | 10 | |
| Num samples to train | 17,104 | |
| Num minibatches to train | 1,710 | |
| Loss function | Cross entropy with softmax | |
| Eval error | Classification error | |
| Learner for parameters | Momentum sgd | |
| Learning rate | 0.01 | |
| Momentum | 0.9 | |
| Eval metrics | Confusion matrix, AUC | |
Fig. 5.
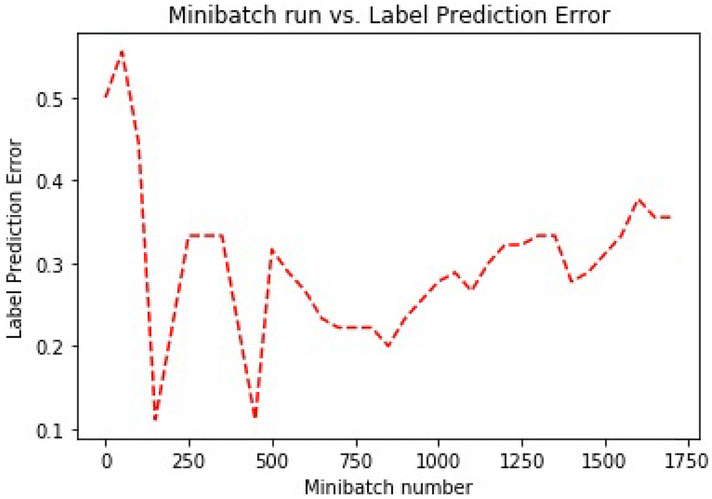
Training error.
Fig. 6.
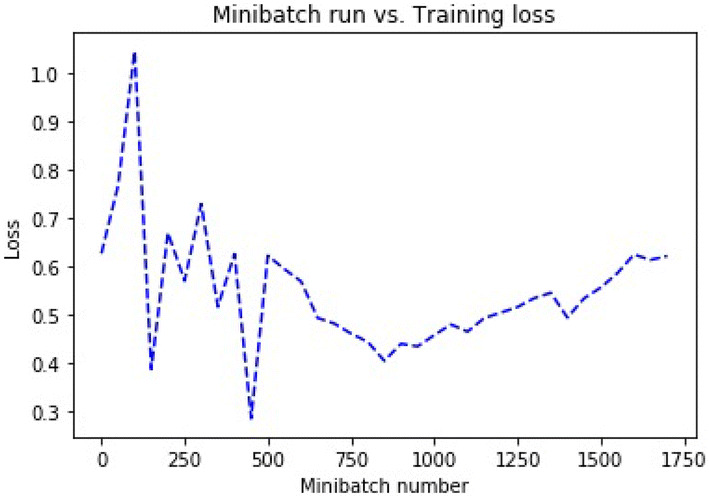
Loss error.
Model evaluation
To evaluate the classification model, computation of the average test error is utilized. The algorithm finds the position of the highest value in the output array, and compares it to the actual label. The evaluation of the network is performed on data never used for training, and coincide with the 30% (7,331) of the data set. The resulting error is compared with the training error and the results indicates that the model presents a useful generalization error. Our model can meritoriously deal with unseen observations, and this is one of the keys to avoiding overfitting53.
For each observation, our model use softmax as the evaluation function that returns the probability distribution across all the classes. In our paper, it would be a vector of 2 elements per observation. The output nodes in our model convert the activations into a probability and map the aggregated activations across the network to probabilities across the two classes. Figure 7 shows the test classification error for our model.
Fig. 7.
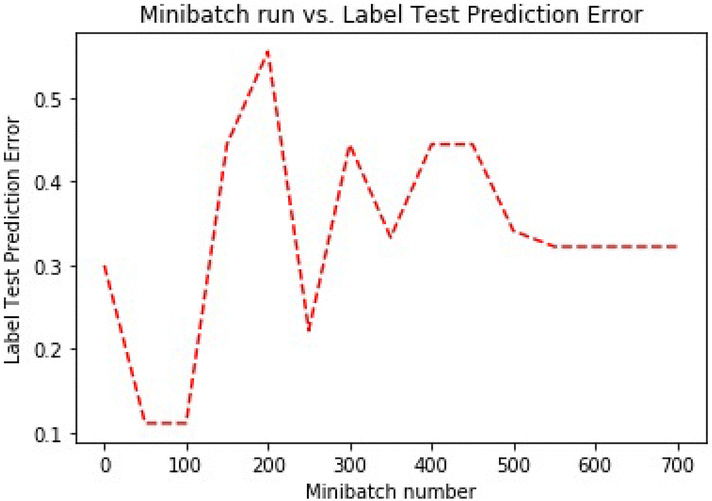
Test prediction error.
In this paper, we utilized a few evaluation metrics to evaluate the model. Results are show in Table 6. Summary of the actual label versus the predicted. True positive value (887), True Negative value (4,477), False Negative (1,318) and False Positive value (648).
Table 6.
Confusion matrix.
| Predicted | ||
|---|---|---|
| Non-hypertensive | Hypertensive | |
| True | ||
| Non-hypertensive | 4,477 | 648 |
| Hypertensive | 1,318 | 887 |
Table 7, shows the classification report with the sensitivity, precision, and the harmonic mean. The low precision and sensitivity on the hypertensive label is caused because of the large presence of false positives, and imbalanced of the testing data set.
Table 7.
Classification report.
| True positive | False negative | Precision | Accuracy |
| 887 | 1,318 | 0.578 | 0.732 |
| False positive | True negative | Recall | f1-score |
| 648 | 4,477 | 0.402 | 0.474 |
| Positive label: 1 | Negative label: 0 | ||
The sensitivity of the model moderately acceptable due to the imbalanced testing data set, and this reveals a high number of false negatives.
Comparison of the model with alternative techniques
Conducting data analysis in a highly imbalanced data set is not trivial and often leads to obtain low sensitivity results. A comparison of our proposed ANN with other highly interpretable methods will allow us to compare the AUC curves of the models and to validate the performance and sensitivity of disease diagnostic. Five machine learning algorithms were identified and compared using the NHANES data. These algorithms not only accurately classify hypertensive patients, but were able to identify key features useful for hypertension diagnostic. A Two-Class Decision Jungle that represents an ensemble of decision directed acyclic graphs (DAGs), a Two-Class Boosted Decision Tree, an ensemble learning method. A Two-Class Bayes Point Machine, that efficiently approximates the Bayesian average of linear classifiers by choosing one “average” classifier, the Bayes Point. A Two-Class Support Vector Machine, and a Two-Class Logistic Regression.
Two-class decision jungle
This algorithm is a development of decision forest that lie on ensemble of decision directed acyclic graphs (DAGs), used to obtain accurate classifiers54. Decision jungles are very comparable to random forests, but it uses DAGs instead of trees as base learners. This structure is more memory-efficient because it eliminates the need for repeating leaf nodes, but it needs more computing time.
This method is selected because decision jungles are models that can express non-linear selection boundaries, and they are strong in the existence of noisy features. Table 8 shows the parameters and Table 14 shows the classification report.
Table 8.
Decision jungle parameters.
| Parameter | Value | |
|---|---|---|
| Two-class decision jungle parameters | ||
| Resampling method | Bagging | |
| Trainer mode | Single parameter | |
| Number of decision DAGs | 8 | |
| Maximum depth of the decision DAGs | 32 | |
| Maximum width of the decision DAGs | 128 | |
| Number of optimization steps per layer | 2,048 | |
Table 14.
Classification report.
| Method | True positive | False negative | False positive | True negative | Precision | Accuracy | Recall | f1-score |
|---|---|---|---|---|---|---|---|---|
| Our model | 887 | 1,318 | 648 | 4,477 | 0.578 | 0.732 | 0.402 | 0.474 |
| Decision jungle | 540 | 912 | 390 | 3,045 | 0.581 | 0.734 | 0.372 | 0.453 |
| Logistic regression | 557 | 895 | 389 | 3,046 | 0.589 | 0.737 | 0.384 | 0.465 |
| Support vector machine | 556 | 896 | 387 | 3,048 | 0.59 | 0.737 | 0.383 | 0.464 |
| boosted decision tree | 568 | 884 | 439 | 2,996 | 0.564 | 0.729 | 0.391 | 0.462 |
| Bayes point machine | 543 | 909 | 388 | 3,047 | 0.583 | 0.735 | 0.374 | 0.456 |
| Synthetic minority oversampling | 3,645 | 789 | 1,326 | 2,086 | 0.73 | 0.73 | 0.82 | 0.77 |
| Positive label: 1 | Negative label: 0 | |||||||
Two-class logistic regression
Logistic regression is a well-known classification technique especially used for classification tasks. The algorithm tries to find the optimal values by maximizing the log probability of the parameters given the inputs. Maximization is performed by using a method for parameter estimation called Limited Memory BFGS55. Table 9 shows the parameters and Table 14 shows the classification report.
Table 9.
Logistic regression parameters.
| Parameter | Value | |
|---|---|---|
| Two-class logistic regression parameters | ||
| Optimization tolerance | 1.00E−07 | |
| L1 regularization weight | 1 | |
| L2 regularization weigh | 1 | |
| Memory size for L-BFGS | 20 | |
Two-class support vector machine
The Support Vector Machine algorithm is a supervised learning model that evaluates input data in a multi-dimensional label zone called the hyperplane. The inputs are points in this zone or space, and are mapped to outputs that are divided as clear as possible56. Table 10 shows the parameters and Table 14 shows the classification report.
Table 10.
Support vector machine parameters.
| Parameter | Value | |
|---|---|---|
| Two-class support vector machine parameters | ||
| Lambda—weight for L1 regularization | 1.00E−03 | |
| normalize features before training | Yes | |
Two-class boosted decision tree
A boosted decision tree, ensemble learning method where the trees corrects for the errors of the previous trees. Predictions are established on the full ensemble of trees57. Table 11 shows the parameters and Table 14 shows the classification report.
Table 11.
Boosted decision tree parameters.
| Parameter | Value | |
|---|---|---|
| Two-class boosted decision tree parameters | ||
| Maximum number of leaves per tree | 20 | |
| Minimum number of training instances | 10 | |
| Learning rate | 0.2 | |
| Number of trees constructed | 100 | |
Two-class Bayes point machine
This method approximates the optimal Bayesian average of linear classifiers by choosing the Bayes Point. This method created by Microsoft Research has shown that no external hyper-parameters are needed and the model can be trained in a single pass, without over-fitting, and without needing pre-processing steps such as data re-scaling58. Table 12 shows the parameters and Table 14 shows the classification report.
Table 12.
Bayes point machine parameters.
| Parameter | Value | |
|---|---|---|
| Number of training iterations | 30 | |
| bias to be added to each instance in training | Yes | |
Synthetic minority oversampling technique
In addition to the previous methods, we have decided to solve the imbalance problem by using the Synthetic Minority Oversampling Technique (SMOTE) and compare the performances with all the methods. This statistical technique increase the number of underrepresented cases in the dataset used in the study. This method returns a dataset that contains the original samples, plus an additional number of synthetic minority samples. In our case we have increase the number of cases 200% (module doubles the percentage of minority cases compared to the original dataset) and the number of nearest neighbors used was 5 (A nearest neighbor is a a case that is very similar to some target case. The distance between any two cases is measured by combining the weighted vectors of all features)59. Increasing the number of cases using this technique is not guaranteed to produce more accurate results. We experimented with different percentages, different feature sets, and different numbers of nearest neighbors to find the best results. Table 13 shows the parameters and Table 14 shows the classification report.
Table 13.
Synthetic minority oversampling parameters.
| Parameter | Value | |
|---|---|---|
| SMOTE percentage | 200 | |
| Number of nearest neighbors | 5 | |
Table 15 presents the result of comparing six methods with our ANN proposed method. We presented the AUC and the corresponding accuracy rates. We observed that the accuracy of the methods are very similar with imbalanced dataset, but the AUC and f1-score of our method are slightly higher and competitive; except for the technique used to solve the imbalance of the dataset which shows a higher AUC. however, no one is statistically better than the others. We utilized cross-validation to measure the performance of the models, and performed several train-score-evaluate operations (10 folds) on different subsets of the input data. An statistical significance test, proposed by Giacomini and White60 was utilized. Where the predictive ability of the presented model for the Cross-entropy loss function showed better performance than the others. We performed a pairwise test of predictive ability of the five models using the Cross-entropy loss function. Table 16 shows the results for the cases. The minus sign indicates that the model under performs the method in the row at the 5% significance level, evidenced by the value in parenthesis (critical value) greater than 1.
Table 15.
Classification methods comparison.
| Method | Precision | Accuracy | f1-score | AUC |
|---|---|---|---|---|
| Our model | 0.578 | 0.732 | 0.474 | 0.77 |
| Decision jungle | 0.581 | 0.734 | 0.453 | 0.769 |
| Logistic regression | 0.589 | 0.737 | 0.465 | 0.764 |
| Support vector machine | 0.59 | 0.737 | 0.464 | 0.759 |
| Boosted decision tree | 0.564 | 0.729 | 0.462 | 0.765 |
| Bayes point machine | 0.583 | 0.735 | 0.456 | 0.763 |
| Synthetic minority oversampling | 0.73 | 0.73 | 0.77 | 0.8 |
Table 16.
Predictive ability tests.
| DJ | LR | SVM | BDT | BPM | |
|---|---|---|---|---|---|
| ANN | 0.001- (3.65) | 0.035- (1.80) | 0.001- (4.03) | 0.036- (1.80) | 0.011- (1.67) |
Results and analysis
The classification of hypertensive patients was carried out by using artificial neural network with back-propagation. Statistical and clinical analysis were performed to explain the results.
Statistical analysis
In our paper, we used a non-linear model to determine the unidentified non-linearity of the input. The test data set of 7,330 includes 5,125 (69.9%) non-hypertensive samples and 2,205 (30.,1%) hypertensive samples. The model shows a sensitivity of 887/2,205 = 40% (positives properly classified), and a specificity of 4,477/5,125 = 87% (negatives properly classified).
A positive predicted value of 887/1,535 = 57%, and a negative predicted value of 4,477/5,795 = 77%. A false negative rate of 1,318/2,205 = 59%, and a false positive rate of 648/5,125 = 12%. A false alarm of 12%, and a likelihood ratio for negative patients of 0.68%. In this paper, the multi-layer neural network model exceed at classifying patients who will not develop hypertension than those that will develop hypertension. The area under the curve is shown in Fig. 8. Figure 9 shows the proportion of the true results of overall positives results in contrast with the fraction of all correct results.
Fig. 8.
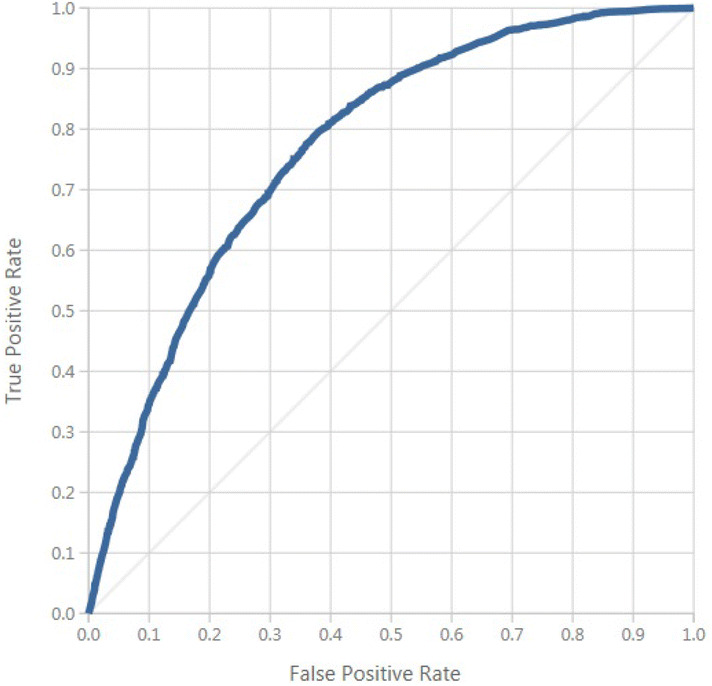
ROC curve.
Fig. 9.
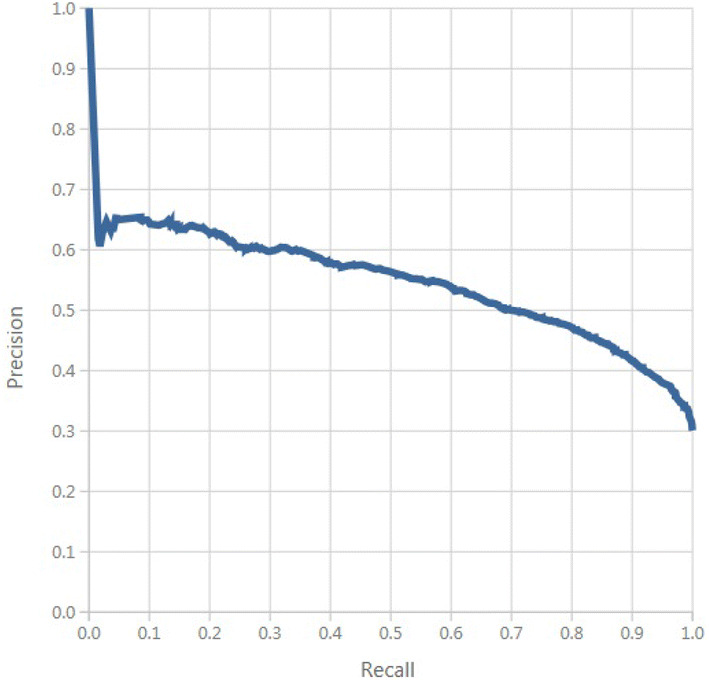
Precision/recall.
Clinical analysis
With a sensitivity of 40%, and specificity of 87%, the artificial neural network model demonstrates that it might be ineffective for healthcare diagnosis in detecting positive occurrences, but the true negative rate demonstrates that the model is effective at finding non-hypertensive patients. The high negative predicted value of 77% shows that our model can be used as an examination tool. The positive cases of 57% shows that our model is superior to a random inference with a low probability for negative test results. This model correctly identifies non-hypertensive patients with an accuracy of 73%.
Discussion and limitations
Even though a multi-layer neural network with one layer can model a vast variety of problems in the clinical domain, in our paper, a model with three hidden layers was advantageous to approximate the highly non-linear behavior of the input features. The result of the model was affected by the imbalanced data set, but we did not balance it to maintain the real distribution of the samples.
The current model configuration and size of the data source captured the complexity of the data. We used data augmentation to generate more input data from the already collected data, but the model was over-fitting and learned too many specific details about the training set. We reduced the number of training iterations to prevent over-fitting, and the accuracy was the same as the model without data augmentation.
The paper has shown that the classification capability of the model improved (AUC—0.77), based on the results of the statistical model utilized in a previous paper (AUC—0.73) when applied to the input features gender, race, BMI, age, smoking, kidney conditions and diabetes. However, challenges in applying artificial neural networks to the clinical domain remain. The use of deep learning to analyze hypertension risk features can be considered as complementary for the traditional approach and might be considered to validate other statistical models.
Our model achieved an AUC of 0.77 and used a smaller network architecture than the architecture used by Polak and Mendyk22 obtaining an AUC of 0.73 and23 that achieves an AUC of 0.76. However, our model presents a bigger network than24 that developed a network that achieves an AUC of 0.81, LaFreniere et al.21 achieves an AUC of 0.82 and Lynn et al.25 achieves an AUC of 0.96.
One of the significant limitations of our model is that it was developed using a highly imbalanced data set from the CDC to which a high prevalence of non-hypertensive patients was observed. There was no significant increase in accuracy. And we are not relying on this measure. We have a severe class imbalance, and the model will maximize accuracy by simply always picking the most common class.
Therefore, our model must be validated in other clinical settings, and further studies should include other neural network architectures and socio-demographic information61 to improve the precision and recall of the model, and to consider the integration of this model to the clinical diagnostic scheme. Also, adequate training data volume will be needed to train a bigger model to improve the classification results.
Conclusions and future work
This paper presents a neural network approach to overthrown the non-linearity problem with the risk factors utilized as inputs for the model. This paper shows that the proposed model improves the accuracy and performance of a previous paper that used the same input features and the results were better than logistic regression in a small percentage. The main contribution of this paper is the developed model and based on results showed that ANN was the proper model compared with the previously developed LR model.
Our multi-layer model confirmed the influence of the imbalanced data set to the class with more presence in the data. This paper showed that the proposed model could be a guide to the design of other models and inference engines for expert systems. This model cannot be used yet to provide the final diagnosis in developing hypertension in patients due that it requires clinician’s involvement for validation with real patients. However, this model can be used to make them aware that there is a probability that the patients could be developing hypertension.
Knowledge of the current model, and their parameters on risk-prediction models in general, is constructive to determine how to best approach the build of prediction models for hypertension, design the study, and interpret its results. When there is a realistic chance to find an applicable positive effect on decision-making and patient outcome, this model on a new setting could be potentially useful. This paper outlines the process for the development of a neural network risk prediction model, from choosing a data source and selecting features to assess model performance, performing validation, and assessing the impact of the model outcomes.
For future work, this model will be apply and re-train if necessary to a real balanced data set, and bigger network architectures will be considered. Also, new risk factors can be used to better handle the distribution and behavior of the input features for the model, and a sensitivity analysis will be performed to determine which inputs in our ANN model are significant with respect to the output. This paper will be the ground for the construction of a decision supporting tool that may be useful to healthcare practitioners for contributing to decision making about the risk of developing hypertension in typical or atypical patient screening circumstances.
Acknowledgements
This document presents independent study funded by Sanitas USA. The points of view expressed are those of the authors and not necessarily those of the NIHR, the NHS, the NHANES or the department of health. We thank Ivan Javier Murcia Muõz, Healthcare Services Director at Sanitas USA and Martha Duarte, Epidemiologist at Sanitas USA. Their skills and competence remarkably support the study.
Author contributions
R.G.C.: worked on the global and methodological review of the paper. F.L.M.: worked on the implementation, research and eld tests. E.R.N.V.: worked on methodological part. V.G.D.: worked in the development and field tests.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vijayarani M. Liver disease prediction using SVM and Naïve Bayes algorithms. Int. J. Sci. Eng. Technol. Res. 2015;4:816–820. [Google Scholar]
- 2.Lakshmanaprabu SK, et al. Online clinical decision support system using optimal deep neural networks. Appl. Soft Comput. 2019;81:105487. doi: 10.1016/j.asoc.2019.105487. [DOI] [Google Scholar]
- 3.Sandoval AM, Díaz J, Llanos LC, Redondo T. Biomedical term extraction: NLP techniques in computational medicine. Int. J. Interact. Multimed. Artif. Intell. 2018 doi: 10.9781/ijimai.2018.04.001. [DOI] [Google Scholar]
- 4.Bobak CA, Titus AJ, Hill JE. Comparison of common machine learning models for classification of tuberculosis using transcriptional biomarkers from integrated datasets. Appl. Soft Comput. J. 2019;74:264–273. doi: 10.1016/j.asoc.2018.10.005. [DOI] [Google Scholar]
- 5.World Health Organization. World Health Statistics 2017: Monitoring Health for The SDGs. arXiv:1011.1669v3 (2017).
- 6.Sakr S, et al. Using machine learning on cardiorespiratory fitness data for predicting hypertension: The Henry Ford exercise testing (FIT) Project. PLoS ONE. 2018;13:e0195344. doi: 10.1371/journal.pone.0195344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park J, et al. Patient-level prediction of cardio-cerebrovascular events in hypertension using nationwide claims data. J. Med. Internet Res. 2019;21:e11757. doi: 10.2196/11757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Center for Health Statistics. Health, United States, 2016: With Chartbook on Long-term Trends in Health. Technical Report (2017). [PubMed]
- 9.Gu A, Yue Y, Kim J, Argulian E. The burden of modifiable risk factors in newly defined categories of blood pressure. Am. J. Med. 2018;131:1349–1358.e5. doi: 10.1016/j.amjmed.2018.06.030. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, et al. Impact of healthy lifestyle factors on life expectancies in the us population. Circulation. 2018;138:345–355. doi: 10.1161/CIRCULATIONAHA.117.032047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.David F, Howard K, Roux Ana D, Jiang H. A Population-Based Policy and Systems Change Approach to Prevent and Control Hypertension. Washington, DC: National Academies Press; 2010. [PubMed] [Google Scholar]
- 12.López-Martínez F, Schwarcz MDA, Núñez-Valdez ER, García-Díaz V. Machine learning classification analysis for a hypertensive population as a function of several risk factors. Expert Syst. Appl. 2018;110:206–215. doi: 10.1016/j.eswa.2018.06.006. [DOI] [Google Scholar]
- 13.Dreiseitl S, Ohno-Machado L. Logistic regression and artificial neural network classification models: a methodology review. J. Biomed. Inform. 2002 doi: 10.1016/S1532-0464(03)00034-0. [DOI] [PubMed] [Google Scholar]
- 14.Seidler T, et al. A machine learning approach for the prediction of pulmonary hypertension. J. Am. Coll. Cardiol. 2019;73:1589. doi: 10.1016/s0735-1097(19)32195-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ambale-Venkatesh B, et al. Cardiovascular event prediction by machine learning: the multi-ethnic study of atherosclerosis. Circ. Res. 2017;121:1092–1101. doi: 10.1161/CIRCRESAHA.117.311312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mortazavi B. J, et al. Analysis of machine learning techniques for heart failure readmissions. Circ. Cardiovasc. Qual. Outcomes. 2016;9:629–640. doi: 10.1161/CIRCOUTCOMES.116.003039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Debray TPA, et al. A new framework to enhance the interpretation of external validation studies of clinical prediction models. J. Clin. Epidemiol. 2015;68:279–289. doi: 10.1016/j.jclinepi.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 18.Tengnah, M. A. J., Sooklall, R. & Nagowah, S. D. A predictive model for hypertension diagnosis using machine learning techniques. In Telemedicine Technologies (eds Jude, H. D. & Balas, V. E.) 139–152 (Academies Press, Elsevier, 2019). 10.1016/b978-0-12-816948-3.00009-x.
- 19.Clim A, Zota RD, Tinica G. The Kullback–Leibler divergence used in machine learning algorithms for health care applications and hypertension prediction: a literature review. Procedia Comput. Sci. 2018;141:448–453. doi: 10.1016/j.procs.2018.10.144. [DOI] [Google Scholar]
- 20.Singh N, Singh P, Bhagat D. A rule extraction approach from support vector machines for diagnosing hypertension among diabetics. Expert Syst. Appl. 2019;130:188–205. doi: 10.1016/j.eswa.2019.04.029. [DOI] [Google Scholar]
- 21.LaFreniere, D., Zulkernine, F., Barber, D. & Martin, K. Using machine learning to predict hypertension from a clinical dataset. In 2016 IEEE Symposium Series on Computational Intelligence (SSCI), 1–7. 10.1109/SSCI.2016.7849886 (2016).
- 22.Polak S, Mendyk A. Artificial neural networks based Internet hypertension prediction tool development and validation. Appl. Soft Comput. 2008;8:734–739. doi: 10.1016/j.asoc.2007.06.001. [DOI] [Google Scholar]
- 23.Tang Z-H, et al. Comparison of prediction model for cardiovascular autonomic dysfunction using artificial neural network and logistic regression analysis. PLoS ONE. 2013;8:e70571. doi: 10.1371/journal.pone.0070571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ture M, Kurt I, Turhan Kurum A, Ozdamar K. Comparing classification techniques for predicting essential hypertension. Expert Syst. Appl. 2005;29:583–588. doi: 10.1016/j.eswa.2005.04.014. [DOI] [Google Scholar]
- 25.Lynn KS, et al. A neural network model for constructing endophenotypes of common complex diseases: an application to male young-onset hypertension microarray data. Bioinformatics. 2009;25:981–988. doi: 10.1093/bioinformatics/btp106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pedregosa F, et al. Scikit-learn: machine learning in python. J. Mach. Learn. Res. 2012;12:2825–2830. doi: 10.1007/s13398-014-0173-7.2. [DOI] [Google Scholar]
- 27.Team A, Dorard L, Reid M. D, Martin F. J. AzureML: anatomy of a machine learning service. JMLR Workshop Conf. Proc. 2016;50:1–13. [Google Scholar]
- 28.Seide, F. & Agarwal, A. Cntk. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining-KDD ’16, 2135–2135. 10.1145/2939672.2945397 (ACM, New York, NY, USA, 2016).
- 29.López-Martínez, F. Deep learning hypertension model repository. https://github.com/sysdevelopment/phd (2018). Accessed July 2019.
- 30.Centers for Disease Control and Prevention. NHANES—NCHS Research Ethics Review Board Approval.
- 31.National Center for Health Statistics, C. Data Access—Data User Agreement (2017).
- 32.Daugherty SL, et al. Age-dependent gender differences in hypertension management. J. Hypertens. 2011;29:1005–1011. doi: 10.1097/HJH.0b013e3283449512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dye, B. A., Thornton-Evans, G., Li, X. & Iafolla, T. J. Key findings Data from the National Health and Nutrition Examination Survey, 2011–2012. Technical Report, Vol. 197 (2011).
- 34.Ong KL, Tso AW, Lam KS, Cheung BM. Gender difference in blood pressure control and cardiovascular risk factors in Americans with diagnosed hypertension. Hypertension. 2008;51:1142–1148. doi: 10.1161/HYPERTENSIONAHA.107.105205. [DOI] [PubMed] [Google Scholar]
- 35.HSS. Awareness of Prediabetes—United States, 2005–2010. Centers for Disease Control & Prevention Source: Morbidity and Mortality Weekly Report Centers for Disease Control & Prevention, Vol. 62, 209–212 (2005).
- 36.CDC. Current Cigarette Smoking Prevalence Among Working Adults–United States , 2004–2010. Technical Report. Morbidity and Mortality Weekly Report (MMWR) (2016). [PubMed]
- 37.Miller WG. Estimating glomerular filtration rate. Clin. Chem. Lab. Med. 2009;47:1017–1019. doi: 10.1515/CCLM.2009.264. [DOI] [PubMed] [Google Scholar]
- 38.CDC. Percentage with CKD stage 3 or 4 who were aware of their disease by stage and age 1999–2012. Technical Report (2015).
- 39.Whelton PK, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults. J. Am. Coll. Cardiol. 2017 doi: 10.1016/j.jacc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 40.Feizi-Derakhshi, M.-R. & Ghaemi, M. Classifying different feature selection algorithms based on the search strategies. In International Conference on Machine Learning, Electrical and Mechanical Engineering (ICMLEME’2014 17–21. 10.15242/IIE.E0114032 (2014).
- 41.Razmjoo A, Xanthopoulos P, Zheng QP. Online feature importance ranking based on sensitivity analysis. Expert Syst. Appl. 2017;85:397–406. doi: 10.1016/j.eswa.2017.05.016. [DOI] [Google Scholar]
- 42.Uysal AK, Gunal S. Text classification using genetic algorithm oriented latent semantic features. Expert Syst. Appl. 2014;41:5938–5947. doi: 10.1016/j.eswa.2014.03.041. [DOI] [Google Scholar]
- 43.Seret A, Maldonado S, Baesens B. Identifying next relevant variables for segmentation by using feature selection approaches. Expert Syst. Appl. 2015;42:6255–6266. doi: 10.1016/j.eswa.2015.01.070. [DOI] [Google Scholar]
- 44.Jiang S, Chin KS, Wang L, Qu G, Tsui KL. Modified genetic algorithm-based feature selection combined with pre-trained deep neural network for demand forecasting in outpatient department. Expert Syst. Appl. 2017;82:216–230. doi: 10.1016/j.eswa.2017.04.017. [DOI] [Google Scholar]
- 45.Wu Y-L, Tang C-Y, Hor M-K, Wu P-F. Feature selection using genetic algorithm and cluster validation. Expert Syst. Appl. 2011;38:2727–2732. doi: 10.1016/j.eswa.2010.08.062. [DOI] [Google Scholar]
- 46.Huang G.-B, et al. Extreme learning machine: theory and applications. Neurocomputing. 2006;70:489–501. doi: 10.1016/j.neucom.2005.12.126. [DOI] [Google Scholar]
- 47.Ching T, et al. Opportunities and obstacles for deep learning in biology and medicine. J. R. Soc. Interface. 2018;15:20170387. doi: 10.1098/rsif.2017.0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jain S, Shukla S, Wadhvani R. Dynamic selection of normalization techniques using data complexity measures. Expert Syst. Appl. 2018;106:252–262. doi: 10.1016/j.eswa.2018.04.008. [DOI] [Google Scholar]
- 49.Singh Gill H, Singh Khehra B, Singh A, Kaur L. Teaching–learning-based optimization algorithm to minimize cross entropy for selecting multilevel threshold values. Egypt. Inform. J. 2018 doi: 10.1016/j.eij.2018.03.006. [DOI] [Google Scholar]
- 50.Bendersky, E. The Softmax Function and Its Derivative 1–9. https://eli.thegreenplace.net/2016/the-softmax-function-and-its-derivative/ (2018). Accessed November 2018.
- 51.He, K., Zhang, X., Ren, S. & Sun, J. Delving deep into rectifiers: Surpassing human-level performance on imagenet classification. Technical Report 10.1109/ICCV.2015.123 (2015). arXiv:1502.01852.
- 52.Takase T, Oyama S, Kurihara M. Effective neural network training with adaptive learning rate based on training loss. Neural Netw. 2018;101:68–78. doi: 10.1016/j.neunet.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 53.Subramanian J, Simon R. Overfitting in prediction models—is it a problem only in high dimensions? Contemp. Clin. Trials. 2013;36:636–641. doi: 10.1016/j.cct.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 54.Shotton J, Sharp T, Kohli P. Decision jungles: compact and rich models for classification. Adv. Neural Inf. Process. Syst. 2013;26:234–242. [Google Scholar]
- 55.Asl, A. & Overton, M. L. Analysis of Limited-Memory BFGS on a Class of Nonsmooth Convex Functions. arXiv:1810.00292 (2018).
- 56.Son YJ, Kim HG, Kim EH, Choi S, Lee SK. Application of support vector machine for prediction of medication adherence in heart failure patients. Healthc. Inform. Res. 2010;16:253–259. doi: 10.4258/hir.2010.16.4.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Friedman J. H. Greedy function approximation : a gradient boosting machine 1 function estimation 2 numerical optimization in function space. North. 1999;1:1–10. doi: 10.2307/2699986. [DOI] [Google Scholar]
- 58.Lazic, N., Bishop, C. & Winn, J. Structural Expectation Propagation (SEP): Bayesian structure learning for networks with latent variables. In 16th International Conference on Artificial Intelligence and Statistics Vol. 31, 379–387 (2013).
- 59.Barua, S., Islam, M. M. & Murase, K. A novel synthetic minority oversampling technique for imbalanced data set learning. In Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics), Vol. 7063 LNCS, 735–744. 10.1007/978-3-642-24958-7_85 (2011).
- 60.Giacomini, R. & White, H. Tests of conditional predictive ability. 10.1111/j.1468-0262.2006.00718.x (2006).
- 61.Elvira C, Ochoa A, Gonzalvez JC, Mochon F. Machine-learning-based no show prediction in outpatient visits. Int. J. Interact. Multimed. Artif. Intell. 2018 doi: 10.9781/ijimai.2017.03.004. [DOI] [Google Scholar]