Introduction
To achieve the benefits of antiretroviral therapy (ART), people living with HIV (PLWH) need to be diagnosed in a timely manner, to be engaged in HIV care, and to initiate and adhere to potentially lifelong ART. In Los Angeles County (LAC), California, 51,438 persons were living with a confirmed HIV diagnosis in 2017, and the number of new HIV diagnoses has remained relatively steady from 2,176 in 2012 to 1,949 in 20161. Based on the local HIV care continuum, the percentage of PLWH who were virally suppressed (viral load [VL]<200 copies/mL at last test in the past 12 months) increased from 53% in 2010 to 60% in 2016. Additionally, the percentage of PLWH who were retained in care (≥2 CD4, VL, or genotyping HIV laboratory tests within the past 12 months) remained relatively stable at around 56% from 2010 to 20161. These data highlight some modestly improved continuum outcomes, but continued efforts to comprehensively address the health and psychosocial needs of PLWH are needed to reach local and national targets.
While cross-sectional assessment can estimate the proportion of PLWH in care or virally suppressed at any single time point, longitudinal data can capture important clinical trajectories following initial engagement in care, such as changes in the proportion of people achieving VS over follow-up periods of months or even years2. This is important information for public health professionals given that late linkage to HIV care, late ART initiation, and in turn, delayed VS have been shown to contribute to increased rates of transmission and poorer clinical outcomes3–7. Also, a previously suppressed individual can become virally unsuppressed over time due to challenges with treatment adherence, new barriers to prescription access, or competing life needs that interrupt in care8. These population-level trajectories are not evident, however, in the typical cross-sectional analysis. Understanding the factors that contribute to these transitions is vital to attain national and local HIV/AIDS strategy benchmarks.
Syndemic structural, behavioral, and psychosocial conditions linked to HIV infection are associated with poorer management of the virus among PLWH along the HIV care continuum9–11. From 2010 to 2015, homelessness in PLWH increased from 7.7% to 9% nationally12, and has been a structural barrier to accessing HIV medical care, obtaining and adhering to ART, and achieving VS1,13,14. Multisite estimates in the U.S. indicate that about 13% and 11% of PLWH meet the diagnostic criteria for methamphetamine use disorder and cocaine use disorder, respectively15. Use of such stimulants can contribute to unsuppressed VL and coinfections due to disengagement from HIV care, reduced ART adherence, and direct toxic effects on the immune system10,16–18. Depression has been identified as the most common mental health issue among PLWH at rates ranging from 30 to 42%, and is associated with reduced ART adherence, poorer quality of life, unsuppressed VL, and more rapid disease progression, all of which result in negative clinical outcomes19–22. Adequate management of HIV requires a holistic approach to care that addresses both HIV directly and the comorbid conditions that perpetuate disease progression12,23,24, and longitudinal evaluation is necessary to quantify how treatment progression is impacted by multiple comorbidities.
In March of 2013, the LAC Department of Public Health Division of HIV and STD Programs (DHSP) developed and implemented a clinic-based Medical Care Coordination (MCC) Program to increase retention in care and VS at 35 Ryan White Program (RWP)-funded HIV clinics25. Patients at risk for poor health outcomes (e.g., diagnosed with HIV in the past six months, on ART without VS, out of care for more than six months) were offered the MCC Program. Patients enrolled in MCC during its first year of implementation had complex needs that included comorbid depressive, anxiety, bipolar and/or schizophrenia disorders (51%), history of incarceration (38%), drug/alcohol use in the past six months (66%), and homelessness in the past six months (14%). Results from DHSP’s first-year evaluation suggested that MCC was an effective program to improve VS after 12 months among PLWH at risk for poor health outcomes25. To better understand whether the MCC program improved VS among patients with complex comorbid conditions, we used a longitudinal evaluation design to account for longer follow-up and changes in VS over multiple years of program implementation. We estimate trajectories of VS from 12-months prior to MCC enrollment to 36 months following MCC enrollment, and assess whether these trajectories differed by stimulant use, housing instability, and depressive symptom severity as reported by patients at MCC enrollment.
Methods
Medical Care Coordination (MCC) Program
Multidisciplinary MCC teams consisting of a registered nurse, a Master’s level social worker and a case worker were integrated in the clinical care teams at LAC RWP clinics. Patients were offered MCC services if they: 1) were newly diagnosed with HIV in the past six months; 2) had not seen an HIV medical provider in 7 months or more; 3) were not on ART despite meeting current clinical guidelines for treatment; 4) were on ART but did not have suppressed VL (>200 copies/mL); or 5) were diagnosed with a sexually transmitted infection (STI) in the past six months. These indicators were used for initial identification of individuals who require rapid linkage to care, as delayed linkage to care in newly diagnosed PLWH, disengagement from treatment, and/or co-infection with an STI increase risk of HIV disease progression26–29 and transmission30–32. To identify and characterize the severity of medical and psychosocial needs, MCC teams conducted standardized assessments. These assessments were used to score needs around physical health, mental health, substance use, and socioeconomic factors, which mapped to a decision tree that guided integrated care plans, the type and frequency of brief interventions delivered, and the provision of support service referrals25,33. The MCC services include outreach to those recently diagnosed or out-of-care, linkage to HIV care providers, assistance with healthcare navigation including accompaniment to HIV care appointments if needed, ART adherence counseling, risk reduction counseling, and warm hand-off referrals to psychosocial health services such as addiction counseling/treatment, mental health care, and housing services. The MCC assessment and service components are described in greater detail in LAC’s evaluation of the first year of MCC implementation25 and in the most recent LAC MCC guidelines33.
Program Evaluation
This evaluation reports on a longitudinal, secondary data analysis of de-identified programmatic and surveillance data from LAC DHSP. We estimated changes in VS among 6,408 PLWH in the MCC Program during the 12 months prior to MCC enrollment through 36 months post-enrollment, as functions of PLWH’s housing status, stimulant use and depressive symptoms reported at enrollment. The Los Angeles County Department of Public Health Institutional Review Board and the Office of the Human Research Protection Program at the University of California, Los Angeles approved this secondary data analysis for evaluation of the MCC Program.
Data Sources
We analyzed data for all PLWH who enrolled in MCC from January 1, 2013 through September 31, 2017. Socio-demographic, assessment, and service data were entered by MCC teams in HIV Casewatch, the local data reporting system system for RWP- contracted providers. HIV Casewatch data were matched with the LAC HIV surveillance database, the Enhanced HIV/AIDS Reporting System (eHARS), to obtain VL testing data and HIV diagnosis dates reported as of December 2018. VL data were based on laboratory results reported between 12 months prior to the patient’s MCC enrollment date and 36 months post-enrollment. These laboratory results are routinely reported to the eHARS surveillance system as part of mandated reporting requirements (separate from MCC) for all HIV care patients in the LAC jurisdiction.
Measures
HIV Measures.
The outcome is viral suppression (VS) defined as a VL test less than 200 copies/mL. We defined the baseline VS measure as the most recent VS measurement within 12 months prior to MCC enrollment. The time since HIV diagnosis was calculated as the number of years from date of HIV diagnosis to date of MCC enrollment and coded both as a continuous variable and also rescaled in decades for regression analysis.
Assessment measures.
Programmatic data on comorbidities and patient characteristics were obtained by MCC staff at enrollment using a standardized assessment tool developed by LAC DHSP. The comorbidities of interest were stimulant use, housing instability, and depressive symptoms, and were assessed as follows. Patients were asked if they had ever used substances and if so, whether they had any substance use in the past six months. Those reporting any substance use in the past six months were then asked about specific substances used in the past six months (no/yes), including methamphetamine, cocaine, and crack. Patients who reported no history of substance use were included with patients who reported no specific substance use in the past six months. Those who reported methamphetamine, cocaine, and/or crack were categorized as having used stimulants. To assess housing instability, patients were asked, “Have you been homeless in the past six months?” with response options of No or Yes. The PHQ-9 score was used to assess severity of depressive symptoms on a continuous scale ranging from 0 to 24. Responses to individual items ranged from 0 (Not At All) to 3 (Nearly Every Day) for each depressive symptom described (e.g., Feeling down, depressed, or hopeless), and summed to create the final score34. This PHQ-9 score was modeled as continuous in longitudinal regression analyses, then in post hoc analyses, fixed at a score of 10 for estimation of marginal probabilities of VS by comorbidity. We specifically fixed the PHQ-9 score at 10 because this has been shown to be the optimal cut-point for likely major depression35.
Sociodemographic information included age, gender, race/ethnicity, education, income, and birth outside of the U.S. Age at enrollment was coded in years and rescaled in decades for regression analysis. Gender included the categories cisgender male, cisgender female, and transgender. Race/ethnicity included the categories White, Black, Latinx, and other. Education was categorized as less than high school, completion of high school/GED, and more than high school. Income was measured by federal poverty level (FPL) and categorized as cut-offs of at or below FPL, 101–200% FPL, and 201% FPL or greater. Patients reported whether or not they were born outside the U.S. Baseline behavioral measures included self-reported history of incarceration, cannabis use, and injection drug use in the past six months, self-reported experience of interpersonal violence in the past three months, and any STI diagnosis in the past 6 months reported to LAC public health surveillance. These measures were pre-specified for inclusion in the analyses, having been identified in prior research to be possible barriers to accessing care and ART adherence36–38, or factors influencing viral replication29,39.
Statistical Analysis
Cross-tabulations and chi-squared tests or t-tests were used to assess the association between categorical or continuous variables and baseline VS, and are reported in Table 1.
Table 1.
Viral suppression at enrollment by patient characteristics among HIV-positive persons in the Medical Care Coordination Program (n=6,408), Los Angeles County, 2013–2018.
| Viral Suppression | |||||||
|---|---|---|---|---|---|---|---|
| All patients | <200 c/mL | ≥200 c/mL | |||||
| n = 6,408 | n = 2,734 | n = 3,674 | |||||
| M | SD | M | SD | M | SD | p | |
| Age (years) at MCC enrollment | 40.5 | 11.9 | 43.2 | 12.0 | 38.6 | 11.4 | < .001 |
| Time (years) since HIV diagnosis | 8.3 | 7.9 | 10.0 | 7.9 | 7.0 | 7.7 | < .001 |
| PHQ-9 score | 7.1 | 6.2 | 6.7 | 6.1 | 7.3 | 6.4 | < .001 |
| n | column % | n | row % | n | row % | p | |
| Gender | .012 | ||||||
| Cisgender male | 5,406 | 84.4 | 2,266 | 41.9 | 3,140 | 58.1 | |
| Cisgender female | 854 | 13.3 | 404 | 47.3 | 450 | 52.7 | |
| Transgender† | 148 | 2.3 | 64 | 43.2 | 84 | 56.8 | |
| Men who have sex with men (MSM)†† | < .001 | ||||||
| No | 1,088 | 20.1 | 525 | 48.3 | 563 | 51.7 | |
| Yes | 4,318 | 79.9 | 1,741 | 40.3 | 2,577 | 59.7 | |
| Race/Ethnicity | .609 | ||||||
| White | 1,238 | 19.3 | 545 | 44.0 | 693 | 56.0 | |
| Latino/a | 3,104 | 48.4 | 1,300 | 41.9 | 1,804 | 58.1 | |
| Black | 1,807 | 28.2 | 778 | 43.1 | 1,029 | 56.9 | |
| Other | 259 | 4.0 | 111 | 42.9 | 148 | 57.1 | |
| Education | .222 | ||||||
| Less than high school | 1,866 | 29.1 | 779 | 41.7 | 1,087 | 58.3 | |
| High school/GED | 2,107 | 32.9 | 931 | 44.2 | 1,176 | 55.8 | |
| Some college or higher | 2,435 | 38.0 | 1,024 | 42.1 | 1,411 | 57.9 | |
| Income | .098 | ||||||
| ≤ Federal poverty level (FPL) | 4,911 | 76.6 | 2,064 | 42.0 | 2,847 | 58.0 | |
| 101–200% FPL | 1,056 | 16.5 | 482 | 45.6 | 574 | 54.4 | |
| > 201% FPL | 441 | 6.9 | 188 | 42.6 | 253 | 57.4 | |
| Born outside of U.S. | .039 | ||||||
| No | 4,051 | 63.2 | 1,689 | 41.7 | 2,362 | 58.3 | |
| Yes | 2,357 | 36.8 | 1,045 | 44.3 | 1,312 | 55.7 | |
| Housing instability (past 6 months) | .023 | ||||||
| No | 4,879 | 76.1 | 2,120 | 43.5 | 2,759 | 56.5 | |
| Yes | 1,529 | 23.9 | 614 | 40.2 | 915 | 59.8 | |
| Methamphetamine use (past 6 months) | < .001 | ||||||
| No | 5,158 | 80.5 | 2,299 | 44.6 | 2,859 | 55.4 | |
| Yes | 1,250 | 19.5 | 435 | 34.8 | 815 | 65.2 | |
| Cocaine/crack use (past 6 months) | < .001 | ||||||
| No | 5,929 | 92.5 | 2,569 | 43.3 | 3,360 | 56.7 | |
| Yes | 479 | 7.5 | 165 | 34.4 | 314 | 65.6 | |
| Cannabis use (past 6 months) | < .001 | ||||||
| No | 4,605 | 71.9 | 2,072 | 45.0 | 2,533 | 55.0 | |
| Yes | 1,803 | 28.1 | 662 | 36.7 | 1,141 | 63.3 | |
| Injection drug use (IDU) (past 6 months) | .005 | ||||||
| No | 6,017 | 93.9 | 2,594 | 43.1 | 3,423 | 56.9 | |
| Yes | 391 | 6.1 | 140 | 35.8 | 251 | 64.2 | |
| Experienced violence (past 3 months) | .509 | ||||||
| No | 4,647 | 72.5 | 1,971 | 42.4 | 2,676 | 57.6 | |
| Yes | 1,761 | 27.5 | 763 | 43.3 | 998 | 56.7 | |
| Incarcerated (past 6 months) | < .001 | ||||||
| No | 5,832 | 91.0 | 2,430 | 41.7 | 3,402 | 58.3 | |
| Yes | 576 | 9.0 | 304 | 52.8 | 272 | 47.2 | |
| Sexually transmitted infection (STI) (past 6 months) | < .001 | ||||||
| No | 5,103 | 79.6 | 2,239 | 43.9 | 2,864 | 56.1 | |
| Yes | 1,305 | 20.4 | 495 | 37.9 | 810 | 62.1 | |
Includes 146 persons who identify as transgender women and 2 as transgender men
For cisgender men only
We analyzed VS longitudinally with a generalized linear mixed model (GLMM) fit in Stata 15 using the mixed command40. For the GLMM, we selected a logistic random effects model41 with a random intercept. MCC enrollment was set as the zero time. Because timing of VL tests varied by patient, depending on degree of engagement care and medical provider practices, time was modeled continuously. The time trend was modeled as piecewise linear from 12 months before MCC enrollment to 36 months after enrollment with slope change points at enrollment and six months post-enrollment. Change points were determined by inspecting LOWESS curves (locally weighted scatterplot smoothing) of VS as functions of time. We allowed for a jump in the logit of VS probability at enrollment to account for the likely possibility of rapid change in VS probability at enrollment. Estimates of VS just before and just after the jump at enrollment are denoted −0 months and +0 months.
Demographic covariates included in the model were age in decades, gender, race, income, education, foreign born. We also adjusted for pre-specified baseline covariates for report of incarceration, cannabis use, injection drug use, and STI diagnosis in the past six months, and experience of interpersonal violence in the past three months. We included time by covariate interactions for three reported comorbid conditions: stimulant use, housing instability, and depressive symptoms (based on continuous PHQ-9 scores).
Using the Stata margins function42, we then conducted post hoc estimation of marginal probabilities of VS every 6 months from −12 months pre-enrollment to 36 months post-enrollment including separate estimates for just before (−0) and after (+0) enrollment for the entire population and for five archetypes: 1) no comorbidities, 2) high PHQ-9 only, 3) stimulant use only, 4) housing instability only, and 5) all comorbidities. Probabilities of VS are estimated at fixed time points and at fixed values of PHQ-9 score, stimulant use, and housing instability, with other covariates set to their observed values42 for all subjects in the data set; estimates are then averaged over all subjects and averages and associated standard errors are reported. We defined the no comorbidities archetype as having a PHQ-9 score fixed at 0 points, no stimulant use, and no reported housing instability. The high PHQ-9 only archetype was fixed at a PHQ-9 score of 10 points35, as well as no stimulant use and no reported housing instability. The stimulant use only archetype used stimulants, had a PHQ-9 score of 0 and did not report housing instability. The housing instability only archetype had housing instability, a PHQ-9 score of 0 and no stimulant use. The all comorbidities group had a PHQ-9 score fixed at 10 and both stimulant use and housing instability. We then plotted the estimated probabilities of VS for these five comorbidity archetypes as functions of time.
Results
Baseline patient characteristics and bivariate associations with VS
Table 1 reports HIV-related, psychosocial, and behavioral characteristics and demographics of 6,408 MCC patients overall and by viral suppression at time of enrollment. Patients in MCC had a mean age at enrollment of 40.5 years (SD = 11.9). MCC patients were 19% White, 48% Latinx, 28% Black, and 4% other. The majority of MCC patients were cisgender male (84%), while the remainder were cisgender female (13%) and transgender (2%).
At enrollment, on average, people who were virally suppressed were older and had been diagnosed with HIV longer time ago. Cisgender female patients were more likely (47.3%) to be virally suppressed than cisgender male patients (41.9%) and transgender patients (43.2%) (p<.012). Cisgender men who reported being were exposed to HIV through sex with men (MSM) were less likely (40.3%) to be virally suppressed than those who did not (48.3%) (p<.001). Patients who were born outside the U.S. were more likely (44.3%) to be virally suppressed compared to those born in the U.S. (41.7%) (p=.039), and those who were incarcerated in the past six months were more likely (52.8%) to be suppressed than those who were not incarcerated (41.7%) (p<.001). Patients who reported housing instability (40.2%) (p=.023), methamphetamine use (34.8%) (p<.001), cocaine/crack use (34.4%) (p<.001), cannabis use (36.7%) (p<.001), and injection drug use (35.8%) (p=.005) in the past six months, as well as those who had an STI in the past six months (37.9%), were less likely to be virally suppressed than those with no housing instability (43.5%), no meth (44.6%), cocaine (43.3%), or cannabis use (45.0%), and no STIs (43.9%). Patients with a PHQ-9 score ≥10 were less likely (40.5%) to be virally suppressed at enrollment (p=.025) than those with lower scores (42.8% for scores 1–9 and 46.0% for scores of 0).
Modeling VS trajectories by comorbid conditions
Figure 1 plots the time trends of the estimated VS probabilities for the five comorbidity archetypes—no comorbidities, high PHQ-9 only, stimulant use only, housing instability only, and all comorbidities. VS probabilities and changes from previous time points are presented in Table 2, and differences in VS probabilities between archetypes are in Table 3. The time trend for the probability of VS was similar across all five comorbidity archetypes (Figure 1) though levels differ significantly. The overall probability of VS declined rapidly over the 12 months prior to MCC enrollment, from 0.64 (95% CI [0.62, 0.65]) to 0.35 (95% CI [0.34, 0.36]) (p<.001). Probability of VS increased substantially (p<.001) to 0.57 (95% CI [0.56, 0.58]) immediately after MCC enrollment (+0 months), then increased again (p<.001) to 0.77 (95% CI [0.76, 0.78] at six months post-enrollment. By six months post-enrollment, those who reported no comorbidities had a significantly greater VS probability than those who reported using stimulants only, housing instability only, high PHQ-9 only, and all three comorbidities. By 36 months post-enrollment, those with a high PHQ-9 increased to a similar VS probability level as those who reported no comorbidities. However, those in any of the other comorbid archetypes continued to have lower probabilities of VS than those with no comorbidities by 36 months post-enrollment, with the lowest VS probability in those with all three comorbidities.
Figure 1.
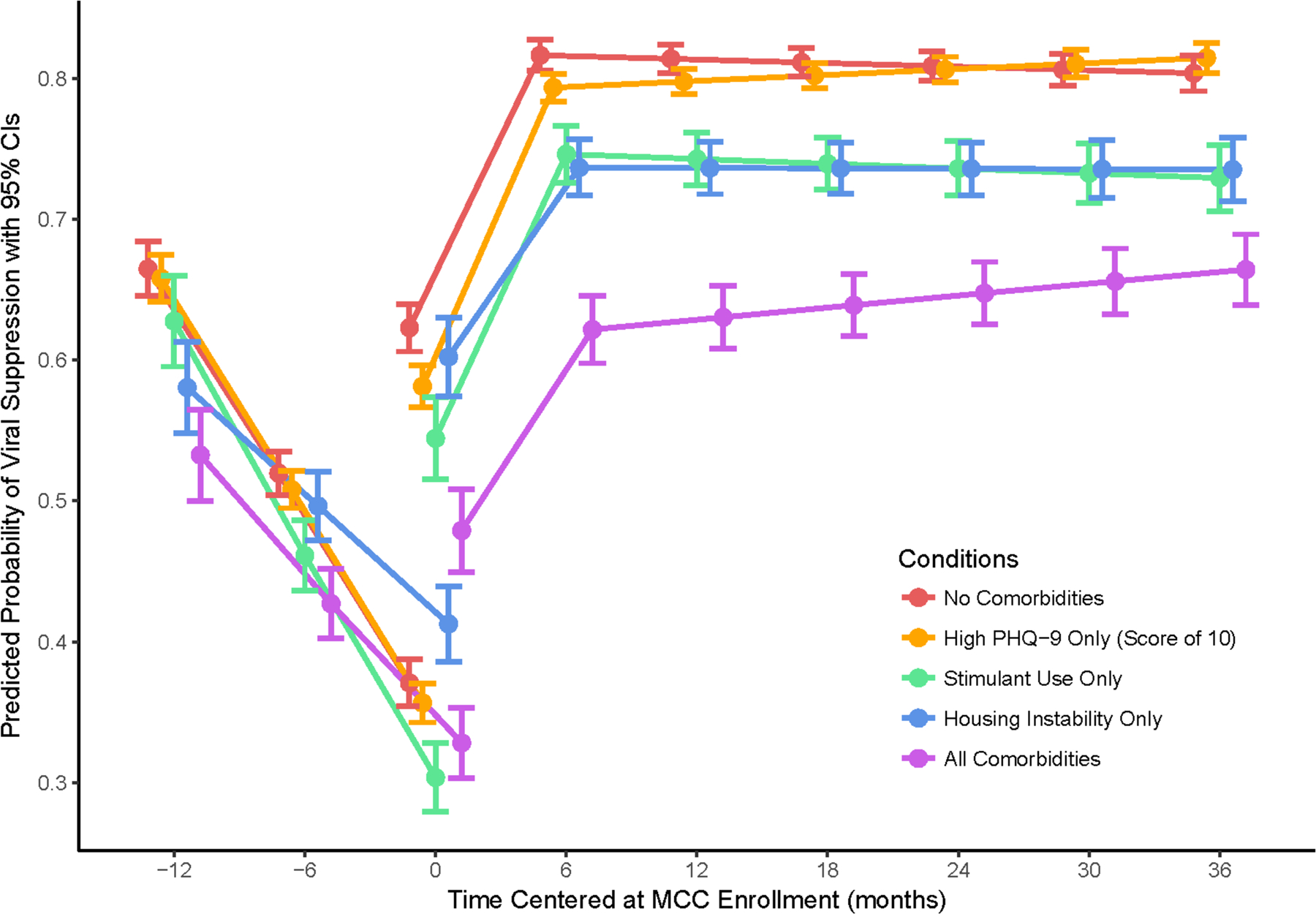
Probabilities of VS by comorbid condition over time among Medical Care Coordination patients, Los Angeles County, 2013–2018.
Table 2.
Changes in estimated probability of VS over time, by comorbidity archetype with 95% CI
| Archetype | Time, mo* | Probability | 95% CI | Change from previous time | 95% CI | p |
|---|---|---|---|---|---|---|
| Overall trend | −12 | 0.635 | 0.622, 0.647 | |||
| −0 | 0.351 | 0.341, 0.361 | −0.284 | −0.298, −0.269 | <.001 | |
| +0 | 0.572 | 0.561, 0.583 | 0.221 | 0.211, 0.232 | <.001 | |
| 6 | 0.768 | 0.761, 0.776 | 0.196 | 0.186, 0.206 | <.001 | |
| 36 | 0.783 | 0.775, 0.791 | 0.015 | 0.007, 0.023 | <.001 | |
| No comorbidities | −12 | 0.665 | 0.645, 0.684 | |||
| −0 | 0.371 | 0.354, 0.387 | −0.294 | −0.316, −0.271 | <.001 | |
| +0 | 0.623 | 0.606, 0.640 | 0.252 | 0.236, 0.268 | <.001 | |
| 6 | 0.816 | 0.805, 0.827 | 0.193 | 0.179, 0.208 | <.001 | |
| 36 | 0.804 | 0.791, 0.816 | −0.013 | −0.025, −0.001 | 0.035 | |
| High PHQ only | −12 | 0.658 | 0.641, 0.675 | |||
| −0 | 0.357 | 0.343, 0.370 | −0.324 | −0.359, −0.288 | <.001 | |
| +0 | 0.581 | 0.566, 0.596 | 0.241 | 0.215, 0.267 | <.001 | |
| 6 | 0.793 | 0.783, 0.803 | 0.202 | 0.177, 0.227 | <.001 | |
| 36 | 0.814 | 0.804, 0.825 | −0.017 | −0.039, 0.005 | 0.128 | |
| Stimulant use only | −12 | 0.627 | 0.595, 0.660 | |||
| −0 | 0.304 | 0.279, 0.328 | −0.168 | −0.205, −0.131 | <.001 | |
| +0 | 0.544 | 0.515, 0.574 | 0.189 | 0.163, 0.216 | <.001 | |
| 6 | 0.746 | 0.726, 0.766 | 0.134 | 0.110, 0.159 | <.001 | |
| 36 | 0.729 | 0.706, 0.752 | −0.001 | −0.023, 0.020 | 0.897 | |
| Housing instability only | −12 | 0.580 | 0.548, 0.613 | |||
| −0 | 0.413 | 0.386, 0.440 | −0.301 | −0.321, −0.282 | <.001 | |
| +0 | 0.602 | 0.574, 0.630 | 0.225 | 0.211, 0.239 | <.001 | |
| 6 | 0.737 | 0.717, 0.756 | 0.212 | 0.199, 0.225 | <.001 | |
| 36 | 0.735 | 0.712, 0.758 | 0.021 | 0.011, 0.031 | <.001 | |
| All comorbidities | −12 | 0.533 | 0.500, 0.565 | |||
| −0 | 0.328 | 0.303, 0.353 | −0.204 | −0.237, −0.171 | <.001 | |
| +0 | 0.479 | 0.450, 0.508 | 0.151 | 0.126, 0.175 | <.001 | |
| 6 | 0.622 | 0.598, 0.646 | 0.143 | 0.118, 0.167 | <.001 | |
| 36 | 0.664 | 0.639, 0.689 | 0.043 | 0.020, 0.065 | <.001 |
−0 months denotes time before MCC enrollment, while +0 months denotes time right after MCC enrollment.
Table 3.
Difference in estimated VS probability for each comorbidity archetype minus the archetype with no comorbidities at the given time point with 95% CI
| Comorbidity archetypea | Time pointb | Difference | 95% CI | p |
|---|---|---|---|---|
| Stimulant use only | −12 months | −0.037 | −0.067, −0.007 | .015 |
| Housing instability only | −12 months | −0.084 | −0.114, −0.054 | <.001 |
| High PHQ-9 | −12 months | −0.007 | −0.026, 0.013 | .500 |
| All comorbidities | −12 months | −0.132 | −0.174, −0.091 | <.001 |
| Stimulant use only | −0 months | −0.067 | −0.091, −0.044 | <.001 |
| Housing instability only | −0 months | 0.042 | 0.017, 0.067 | .001 |
| High PHQ-9 | −0 months | −0.014 | −0.031, 0.002 | .091 |
| All comorbidities | −0 months | −0.043 | −0.075, −0.010 | .011 |
| Stimulant use only | +0 months | −0.078 | −0.106, −0.051 | <.001 |
| Housing instability only | +0 months | −0.021 | −0.047, 0.005 | .118 |
| High PHQ-9 | +0 months | −0.041 | −0.059, −0.024 | <.001 |
| All comorbidities | +0 months | −0.144 | −0.181, −0.107 | <.001 |
| Stimulant use only | 6 months | −0.070 | −0.089, −0.051 | <.001 |
| Housing instability only | 6 months | −0.080 | −0.098, −0.062 | <.001 |
| High PHQ-9 | 6 months | −0.023 | −0.034, −0.012 | <.001 |
| All comorbidities | 6 months | −0.195 | −0.223, −0.166 | <.001 |
| Stimulant use only | 36 months | −0.074 | −0.096, −0.053 | <.001 |
| Housing instability only | 36 months | −0.068 | −0.089, −0.048 | <.001 |
| High PHQ-9 | 36 months | 0.011 | −0.001, 0.023 | .083 |
| All comorbidities | 36 months | −0.139 | −0.170, −0.109 | <.001 |
The reference archetype is those with no comorbidities
−0 months denotes time immediately before MCC enrollment, and +0 months denotes the time right after MCC enrollment.
Odds ratios of VS at different levels of each covariate as measured at enrollment are reported in Appendix 1. Those who were Black had 0.68 times (95% CI: 0.57–0.81), p<.001) lower odds of VS than White patients. Each decade since an HIV diagnosis was associated with 0.91 times (95% CI (0.84, 1.00), p =.043) lower odds of VS. Every decade of age (OR=1.44, 95% CI (1.35, 1.53), p<.001), having a higher income (OR=1.62, 95% CI (1.27, 2.05), p<.001), having a higher education (OR=1.59, 95% CI (1.36, 1.87), p<.001), being born outside the U.S. rather than within the U.S. (OR=1.36, 95% CI (1.17, 1.59), p<.001), and having a positive STI diagnosis in the past six months rather than no diagnosis (OR=1.26, 95% CI (1.08, 1.47) were associated with greater odds of VS.
Discussion
The present study shows near immediate improvements in rates of VS in PLWH participating in the LAC MCC Program. Findings also show important lower rates of VS for those living with any of three comorbid conditions—stimulant use, housing instability, and depressive symptoms. Across all comorbidity groups, probability of VS significantly increased with the greatest increase occurring from MCC enrollment to six months later, and this probability remained stable or increased up to 36 months post-enrollment. The significant increase in probability of VS at MCC enrollment (i.e., the jump discontinuity) suggests that the most rapid improvement occurred soon after enrollment, followed by a less rapid improvement for the remainder of the six months post-enrollment. Another explanation is that some patients who were previously non-adherent to their ART regimens resumed taking their medications in the timeframe between first being contacted to participate in MCC and their first actual MCC appointment.
The distinct trajectories in people with different morbidities show differential responses to this widely used model to improve relevant HIV outcomes. These results highlight characteristics of people who may require more intensive services. Patients enrolled in MCC who reported all three comorbidities improved as measured by probability of VS over time, but that improvement progressed more slowly compared to those MCC patients with no comorbid conditions. It is worth noting that after three years of enrollment in MCC, patients who reported high depressive symptoms, but no stimulant use or homelessness, had a similar probability of VS as those with none of the comorbid conditions. This suggests that patients with depressive symptoms may face fewer barriers adhering to HIV treatment than those with chronic problems with stimulant use and homelessness.
Generally, these findings are consistent with evaluation of New York City’s HIV Care Coordination Program, which demonstrated increased odds of VS over the course of receiving coordinated care, but also showed that baseline housing instability and substance use decreased these odds9,43. Our study expands upon prior program evaluations and research by modeling temporal trends showing when and how VS probabilities improved and stabilized, as well as elucidating disparities in VS by comorbid condition. Even with additional resources, these individuals still endure greater challenges to achieving VS than those without these comorbid conditions43,44, warranting specific attention to available services and local resources to address substance use and homeless. However, increasing VS from 86% nationally—the current rate under the Ryan White HIV/AIDS Program—to the Ending the HIV Epidemic (EHE) target of 90%45, will require further expansion of healthcare resources to reduce comorbidities in PLWH.
Ultimately, investing in HIV coordinated care models like MCC may be more cost-effective than the standard of care if such programs are clinically effective and targeted to PLWH with the greatest needs46. Therefore, it will be especially important to leverage the EHE initiative towards supporting local health jurisdictions that currently lack the necessary resources for successful implementation of coordinated care programs45,47. A key focus of the EHE initiative is to provide additional funding and support for medical case management across Ryan White Clinics and programs like MCC throughout the U.S., and to partner with local healthcare agencies and other institutions in this effort45,47. As such, support from the EHE may open opportunities for other local health agencies to implement new programs, collaborate and coordinate care across agencies at the regional level48, share programmatic data, and implement and evaluate novel, evidence-based intervention components for substance use and homelessness in comprehensive HIV care programs like MCC.
Our findings have some limitations. There is intentional selection in enrollments into MCC; PLWH in LAC who have characteristics associated with successful VS are not enrolled in MCC and thus are not included in our analysis. In fact, conditions associated with worsening probability of VS are what gets PLWH enrolled in MCC, as reflected in the decrease in VS prior to MCC enrollment. Because the MCC Program serves PLWH the greatest challenges managing HIV, our analysis cannot control for an equivalent comparison group who did not partake in MCC. Still, modeling VS over time allowed us to show that there was meaningful change in the probability of VS at and following MCC enrollment49. Even more encouraging, after only six months in MCC, the probability of VS surpassed that at 12 months before MCC participation. Thus, we believe these trends were likely the result of improved access to care. Another limitation is that it is not clear what MCC program components and services were the most effective (or the least effective) at supporting patients in achieving VS, particularly because comprehensive care plans were individually tailored to patients’ needs assessments. In other words, MCC patients did not all receive the same services. Still, based on our estimated disparities in VS, we infer that those with stimulant use and housing instability require additional support for those challenges despite significant improvement over the 36-month evaluation period.
The present study suggests that MCC improved probability of VS for all groups of patients regardless of the presence of comorbidities, but as VS is not nearly 100%, further improvements in VS are possible. Expanding resources for and tailoring services to meet the needs of PLWH with complex comorbidities are critical to reaching local and national HIV strategy targets, such as increasing the percentage of PLWH with VS to least 90% and increasing retention in HIV medical care to at least 90%50, and in turn, decreasing HIV transmission in the era of Undetectable Equals Untransmittable31. Future longitudinal analyses are needed to evaluate MCC on other outcomes, specifically, the degree to which comorbid stimulant use, housing instability, depressive symptoms, and other psychosocial challenges improved among MCC patients following enrollment. Such investigation would help to identify additional program components needed to better address these comorbid conditions, retention in care and treatment, and in turn, VS.
Supplementary Material
Acknowledgements
This work was supported by the Center for HIV Identification, Prevention, and Treatment Services (CHIPTS) NIMH grant P30MH58107, the California HIV/AIDS Research Grants Program Office of the University of California grant MH10-LAC-610, the University of California, Los Angeles Postdoctoral Fellowship Training Program in Global HIV Prevention Research NIMH grant 5T32MH080634-13. The authors would like to thank the Los Angeles County Medical Care Coordination teams and clients, as well our partners and colleagues at the Los Angeles County Department of Public Health for their support and contributions: Mario Perez MPH, Sonali Kulkarni MD, MPH and Angela Boger.
Conflicts of interest and Source of Funding
The authors report no conflicts of interest.
References
- 1.Division of HIV and STD Programs. 2017. Annual HIV Surveillance Report. Los Angeles, CA: Los Angeles County Department of Public Health; 2018: http://publichealth.lacounty.gov/dhsp/Reports.htm. [Google Scholar]
- 2.Colasanti J, Kelly J, Pennisi E, et al. Continuous retention and viral suppression provide further insights into the HIV care continuum compared to the cross-sectional HIV care cascade. Clin Infect Dis. 2015;62(5):648–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siedner MJ, Ng CK, Bassett IV, Katz IT, Bangsberg DR, Tsai AC. Trends in CD4 count at presentation to care and treatment initiation in sub-Saharan Africa, 2002–2013: a meta-analysis. Clin Infect Dis. 2015;60(7):1120–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phillips AN, Gazzard BG, Clumeck N, Losso MH, Lundgren JD. When should antiretroviral therapy for HIV be started? BMJ. 2007;334(7584):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cope AB, Powers KA, Kuruc JD, et al. Ongoing HIV Transmission and the HIV Care Continuum in North Carolina. PLoS ONE. 2015;10(6):e0127950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skarbinski J, Rosenberg E, Paz-Bailey G, et al. Human immunodeficiency virus transmission at each step of the care continuum in the United States. JAMA Internal Medicine. 2015;175(4):588–596. [DOI] [PubMed] [Google Scholar]
- 7.Croxford S, Kitching A, Desai S, et al. Mortality and causes of death in people diagnosed with HIV in the era of highly active antiretroviral therapy compared with the general population: an analysis of a national observational cohort. The Lancet Public Health. 2017;2(1):e35–e46. [DOI] [PubMed] [Google Scholar]
- 8.Lesko CR, Edwards JK, Moore RD, Lau B. A longitudinal, HIV care continuum: 10-year restricted mean time in each care continuum stage after enrollment in care, by history of IDU. AIDS. 2016;30(14):2227–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Irvine MK, Chamberlin SA, Robbins RS, Kulkarni SG, Robertson MM, Nash D. Come as You Are: Improving Care Engagement and Viral Load Suppression Among HIV Care Coordination Clients with Lower Mental Health Functioning, Unstable Housing, and Hard Drug Use. AIDS Behav. 2017;21(6):1572–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedman MR, Stall R, Silvestre AJ, et al. Effects of syndemics on HIV viral load and medication adherence in the multicentre AIDS cohort study. AIDS. 2015;29(9):1087–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma A, Chambers BD, Jenkins Hall W, Tanner AE, Piper CN. Individual and structural factors influencing HIV care linkage and engagement: Perceived barriers and solutions among HIV-positive persons. J HIV/AIDS Soc Serv. 2017;16(1):34–42. [Google Scholar]
- 12.Office of HIV/AIDS. 2017. Progress Report National HIV/AIDS Strategy for the United States: Updated to 2020. Washington, D.C: 2020: https://www.hiv.gov/federal-response/national-hiv-aids-strategy/nhas-update. [Google Scholar]
- 13.Aidala AA, Wilson MG, Shubert V, et al. Housing Status, Medical Care, and Health Outcomes Among People Living With HIV/AIDS: A Systematic Review. Am J Public Health. 2015;106(1):e1–e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris RA, Xue X, Selwyn PA. Housing Stability and Medication Adherence among HIV-Positive Individuals in Antiretroviral Therapy: A Meta-Analysis of Observational Studies in the United States. J Acquir Immune Defic Syndr. 2017;74(3):309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartzler B, Dombrowski JC, Crane HM, et al. Prevalence and Predictors of Substance Use Disorders Among HIV Care Enrollees in the United States. AIDS Behav. 2017;21(4):1138–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aralis HJ, Shoptaw S, Brookmeyer R, Ragsdale A, Bolan R, Gorbach PM. Psychiatric Illness, Substance Use, and Viral Suppression Among HIV-Positive Men of Color Who Have Sex with Men in Los Angeles. AIDS Behav. 2018;22(10):3117–3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silverstein PS, Shah A, Gupte R, et al. Methamphetamine toxicity and its implications during HIV-1 infection. J Neurovirol. 2011;17(5):401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salamanca SA, Sorrentino EE, Nosanchuk JD, Martinez LR. Impact of methamphetamine on infection and immunity. Frontiers in Neuroscience. 2015;8:445–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nanni MG, Caruso R, Mitchell AJ, Meggiolaro E, Grassi L. Depression in HIV Infected Patients: a Review. Curr Psychiatry Rep. 2015;17(1):530. [DOI] [PubMed] [Google Scholar]
- 20.Bengtson AM, Pence BW, Mimiaga MJ, et al. Depressive Symptoms and Engagement in Human Immunodeficiency Virus Care Following Antiretroviral Therapy Initiation. Clin Infect Dis. 2018:ciy496–ciy496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ironson G, O’Cleirigh C, Kumar M, et al. Psychosocial and Neurohormonal Predictors of HIV Disease Progression (CD4 Cells and Viral Load): A 4 Year Prospective Study. AIDS Behav. 2015;19(8):1388–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pence BW, Mills JC, Bengtson AM, et al. Association of Increased Chronicity of Depression With HIV Appointment Attendance, Treatment Failure, and Mortality Among HIV-Infected Adults in the United StatesAssociation Between Depression and HIV Treatment Failure and MortalityAssociation Between Depression and HIV Treatment Failure and Mortality. JAMA Psychiatry. 2018;75(4):379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bengtson AM, Pence BW, Gaynes BN, et al. Improving Depression Among HIV-Infected Adults: Transporting the Effect of a Depression Treatment Intervention to Routine Care. Journal of acquired immune deficiency syndromes (1999). 2016;73(4):482–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization. Consolidated guidelines on HIV prevention, diagnosis, treatment and care for key populations. Geneva, Switzerland: World Health Organization; 2017: https://www.who.int/hiv/pub/toolkits/keypopulations-2016-update/en/. [Google Scholar]
- 25.Garland WH, Oksuzyan S, Mejia M, Kulkarni S. Medical Care Coordination Services for Persons Living with HIV in Los Angeles County: A Robust Strategy to Strengthen the HIV Care Continuum. Los Angeles, CA: Division of HIV and STD Programs, Los Angeles County Department of Public Health; 2017: http://publichealth.lacounty.gov/dhsp/Reports/HIV/MCC_Year-1_EvaluationReport-FINAL.pdf. [Google Scholar]
- 26.Mugavero MJ, Davila JA, Nevin CR, Giordano TP. From access to engagement: measuring retention in outpatient HIV clinical care. AIDS Patient Care STDs. 2010;24(10):607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zolopa AR, Andersen J, Komarow L, et al. Early antiretroviral therapy reduces AIDS progression/death in individuals with acute opportunistic infections: A multicenter randomized strategy trial. PLoS ONE. 2009;4(5):e5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.May M, Gompels M, Delpech V, et al. Impact of late diagnosis and treatment on life expectancy in people with HIV-1: UK Collaborative HIV Cohort (UK CHIC) Study. BMJ. 2011;343:d6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Modjarrad K, Vermund SH. Effect of treating co-infections on HIV-1 viral load: a systematic review. The Lancet Infectious Diseases. 2010;10(7):455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller WC, Rosenberg NE, Rutstein SE, Powers KA. The role of acute and early HIV infection in the sexual transmission of HIV. Current Opinion in HIV and AIDS. 2010;5(4):277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eisinger RW, Dieffenbach CW, Fauci AS. HIV Viral Load and Transmissibility of HIV Infection: Undetectable Equals UntransmittableHIV Viral Load and Transmissibility of HIV InfectionHIV Viral Load and Transmissibility of HIV Infection. JAMA. 2019;321(5):451–452. [DOI] [PubMed] [Google Scholar]
- 32.Galvin SR, Cohen MS. The role of sexually transmitted diseases in HIV transmission. Nature Reviews Microbiology. 2004;2(1):33–42. [DOI] [PubMed] [Google Scholar]
- 33.Division of HIV and STD Programs. Guidelines for the Provision of HIV/AIDS Medical Care Coordination Services in Los Angeles County. Los Angeles, CA: Los Angeles County Department of Public Health; 2017: http://publichealth.lacounty.gov/dhsp/Contractors/MCC/MCCGuidelinesRevised2017.pdf. [Google Scholar]
- 34.Kroenke K, Spitzer RL, Williams JBW. The PHQ‐9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manea L, Gilbody S, McMillan D. Optimal cut-off score for diagnosing depression with the Patient Health Questionnaire (PHQ-9): a meta-analysis. CMAJ. 2012;184(3):E191–E196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iroh PA, Mayo H, Nijhawan AE. The HIV Care Cascade Before, During, and After Incarceration: A Systematic Review and Data Synthesis. Am J Public Health. 2015;105(7):e5–e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Westergaard RP, Hess T, Astemborski J, Mehta SH, Kirk GD. Longitudinal changes in engagement in care and viral suppression for HIV-infected injection drug users. AIDS (London, England). 2013;27(16):2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonn-Miller MO, Oser ML, Bucossi MM, Trafton JA. Cannabis use and HIV antiretroviral therapy adherence and HIV-related symptoms. J Behav Med. 2014;37(1):1–10. [DOI] [PubMed] [Google Scholar]
- 39.Palepu A, Tyndall M, Yip B, O’Shaughnessy MV, Hogg RS, Montaner JSG. Impaired virologic response to highly active antiretroviral therapy associated with ongoing injection drug use. JAIDS J Acquired Immune Defic Syndromes. 2003;32(5):522–526. [DOI] [PubMed] [Google Scholar]
- 40.Stata Statistical Software [computer program]. Version 15 College Station, TX: StataCorp LLC; 2017. [Google Scholar]
- 41.Weiss RE. Modeling longitudinal data. New York, NY: Springer Science & Business Media; 2005. [Google Scholar]
- 42.StataCorp. Obtaining marginal means, adjusted predictions, and predictive margins Stata 15 Base Reference Manual. 15 ed. TX: College Station; 2017:302–311. [Google Scholar]
- 43.Robertson MM, Penrose K, Irvine MK, et al. Impact of an HIV Care Coordination Program on Durable Viral Suppression. JAIDS J Acquired Immune Defic Syndromes. 2019;80(1):46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bove JM, Golden MR, Dhanireddy S, Harrington RD, Dombrowski JC. Outcomes of a Clinic-Based Surveillance-Informed Intervention to Relink Patients to HIV Care. Journal of acquired immune deficiency syndromes (1999). 2015;70(3):262–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.US Department of Health and Human Services. What is ‘Ending the HIV Epidemic: A Plan for America’? 2019; https://www.hiv.gov/federal-response/ending-the-hiv-epidemic/overview.
- 46.Flash MJE, Garland WH, Martey EB, et al. Cost-effectiveness of a medical care coordination program for people with HIV in Los Angeles County. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fauci AS, Redfield RR, Sigounas G, Weahkee MD, Giroir BP. Ending the HIV Epidemic: A Plan for the United States. JAMA. 2019;321(9):844–845. [DOI] [PubMed] [Google Scholar]
- 48.Center for HIV Identification, Prevention and Treatment Services. A Regional Response to End the HIV Epidemic in CA. 2020; http://chipts.ucla.edu/features/a-regional-response-to-end-the-hiv-epidemic-in-ca/.
- 49.Chou C-P, Yang D, Pentz MA, Hser Y-I. Piecewise growth curve modeling approach for longitudinal prevention study. Comput Stat Data Anal. 2004;46(2):213–225. [Google Scholar]
- 50.Office of HIV/AIDS. Indicator Supplement National HIV/AIDS Stategy for the United States: Updated to 2020. Washington, D.C.: Department of Health and Human Services; 2016: https://www.hiv.gov/federal-response/national-hiv-aids-strategy/overview. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


