Abstract
In current study, we aimed to investigate whether the gentiopicroside (GPS) derived from Gentiana manshurica Kitagawa could block the progression of alcoholic hepatic steatosis to fibrosis induced by chronic ethanol intake. C57BL/6 mice were fed an ethanol-containing Lieber-DeCarli diet for 4 weeks. LX-2 human hepatic stellate cells were treated with GPS 1 h prior to transforming growth factor-β (TGF-β) stimulation, and murine hepatocyte AML12 cells were pretreated by GPS 1 h prior to ethanol treatment. GPS inhibited the expression of type I collagen (collagen I), α-smooth muscle actin (α-SMA) and tissue inhibitor of metal protease 1 in ethanol-fed mouse livers with mild fibrosis. In addition, the imbalanced lipid metabolism induced by chronic ethanol-feeding was ameliorated by GPS pretreatment, characterized by the modulation of lipid accumulation. Consistently, GPS inhibited the expression of collagen I and α-SMA in LX-2 cells stimulated by TGF-β. Inhibition of lipid synthesis and promotion of oxidation by GPS were also confirmed in ethanol-treated AML12 cells. GPS could prevent hepatic steatosis advancing to the inception of a mild fibrosis caused by chronic alcohol exposure, suggesting GPS might be a promising therapy for targeting the early stage of alcoholic liver disease.
Keywords: Gentiopicroside, Hepatic fibrosis, Hepatic steatosis, Chronic alcohol intake
INTRODUCTION
Alcoholic liver disease (ALD) is caused by long-term alcohol abuse, leading to loss of liver function (Bruha et al., 2012). The disease mainly includes alcoholic liver steatosis, alcoholic hepatitis, alcoholic liver fibrosis, cirrhosis and liver cancer (Torruellas et al., 2014; Yang et al., 2016). To date, the progression of steatosis to liver fibrosis is still incurable and no suitable drug has been reported yet.
Currently, ALD mechanisms have not been completely identified (Ceni et al., 2014). It is well known that the initial stage of liver disease caused by chronic ethanol intake is steatosis. Lipid deposition results in steatosis, which is regulated by peroxisome proliferator-activated receptor α (PPARα) and sterol regulatory element binding protein-1 (SREBP1). Several studies have reported that AMP-activated protein kinase (AMPK) is an important lipid metabolism-regulating kinase and plays an important regulatory role in the pathogenesis of ALD (Lee et al., 2014; Mandal et al., 2014; Jiang et al., 2017a). Liver kinase 1 (LKB1), an upstream kinase of AMPK, promotes the phosphorylation of AMPK and the enhances phosphorylation of AMPK to accelerate fatty acid oxidation (Xiao et al., 2013). Phosphorylation of AMPK inactivates acetyl-CoA carboxylase (ACC). Herein, ethanol intake results in decrease of AMPK activity and increased of ACC activity and consequently, exacerbates the imbalance of lipid metabolism. Therefore, inhibiting lipid accumulation or promoting lipolysis may prevent liver steatosis from further developing into liver fibrosis.
The progression from steatosis to fibrosis is characterized by the activation of hepatic stellate cells (HSCs) and increased extracellular matrix (ECM) secretion (Pellicoro et al., 2014; Zhang et al., 2016). Hepatic stellate cells (HSCs) in the liver play a key role in the development of liver fibrosis (Wu and Zern, 2000). In addition, liver fibrosis is caused by chronic liver disease. Static HSCs are enriched for vitamin A. Once the liver stimulated by external factors, such as ethanol and viruses, HSCs lose vitamin A and are activated (Ezhilarasan et al., 2016). Activated HSCs secret large amounts of liver fibrosis markers, including extracellular matrix (ECM) components such as collagen I, smooth muscle alpha-actin (α-SMA) and transforming growth factor-β (TGF-β). Thus, controlling the activation of HSCs is an essential strategy for reversing liver fibrosis.
Gentiana manshurica Kitagawa (GM), which belongs to the gentian family, mainly is distributed in Northeastern China and is commonly used to treat jaundice and hepatitis (Lian et al., 2010b; Liu et al., 2016; Chen et al., 2018b). Gentiopicroside (GPS), found in the roots of GM, has hepatoprotective capacities against various liver injuries (Lian et al., 2010a, 2010b, 2018). Although we previously reported that GPS-containing GM or GPS could ameliorate hepatic steatosis induced by acute and subacute alcohol feeding (Lian et al., 2010b; Li et al., 2018), the possibility of GPS-induced improvement of chronic alcohol consumption-caused liver fibrosis remains unknown. Because long-term and repetitive damages to hepatocytes induced by ethanol exposure contributes to accelerating the development of fatty liver to liver fibrosis, we are interested in whether GPS could reverse hepatic fibrosis caused by chronic alcohol consumption. The current research was designed to investigate whether and how GPS could hold back the progression from liver steatosis to liver fibrosis during chronic ethanol consumption in the mice that suffered from alcoholic steatosis and confirmed the inhibitory capacity of HSC activation by GPS in a human HSC cell line, LX-2, and the amelioration of lipid accumulation in a mouse hepatocytes cell line, AML12. Our results indicated that GPS inhibited steatosis and blocked the development to mild liver fibrosis induced by chronic alcohol intake.
MATERIALS AND METHODS
Materials
GPS was purchased from the National Institute for the Control of Pharmaceutical and Biological Products (>99% purity; Beijing, China). Anti-phospho-AMPKα, anti-AMPKα, anti-phospho-LKB1, anti-LKB-1, and anti-P-ACC antibodies were purchase from Cell Signaling Technology (Beverly, MA, USA). Anti-SREBP1, anti-TIMP-1, anti-MMP13, anti-collagen I, anti-PPARα, anti-β-actin, anti-α-SMA and anti-GAPDH antibodies were purchased from Abcam (Cambridge, MA, USA).
Animal experiment
Male C57BL/6 mice (8-10 weeks, 20-22 g) were obtained from the Changchun Yisi Laboratory Animal Technology Co., Ltd (Jilin, China). Before experiments, five mice per cage were housed in a temperature/humidity-controlled environment with a 12-h light/dark cycle under specific-pathogen free conditions and provided free access to chow and water. All animal care and experimental procedures were carried out strictly according to the criteria of the “Guide for the Care and Use of Laboratory Animals” published by the US National Institutes of Health (National Research Council, 1996) and approved by the Ethics Committee on Animal Experiments of Yanbian University. All efforts were made to minimize suffering, and the number of animals used was minimized by the experimental design. After one-week of acclimatization maintained on a normal chow diet, mice were randomly divided into three groups: the pair-fed group, ethanol-fed group, and ethanol-fed group pretreated with GPS (40 mg/kg, body weight). Each group had 6 mice. Because we reported in in previous study that GPS alone at 40 mg/kg for 10 days showed no toxicity in the liver including liver injury and steatosis (Li et al., 2018), we omitted investigation of the effect of GPS alone on liver steatosis in the current research. The ethanol group was fed a Lieber-DeCarli diet (TROPHIC Animal Feed High-tech Co., Ltd, Nantong, Jiangsu, China) with the concentration of ethanol gradually increased from 1% to 4% (vol/vol) every two days, followed by a continuous 5% (vol/vol) ethanol-containing Lieber-DeCarli diet for 28 days as described previously (Cai et al., 2016). The mice in the pair-fed group were fed Lieber-DeCarli liquid diets containing isocaloric maltose dextrin. Mice were gavaged with GPS (40 mg/kg) every morning for 28 days. Nine hours after the last feeding, all mice were anesthetized in a closed chamber flushed with isoflurane (1%) in mixed air for 2-5 min until immobile, blood was collected by cardiac puncture, and then, the liver was removed and rapidly frozen in liquid nitrogen. Whole blood was allowed to clot at room temperature for 30 min and the serum was separated by centrifugation at 1,800× g for 30 min. Mouse livers and serum samples were stored at –80°C for further analysis.
Serum aminotransferase and triglyceride measurement
Serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and triglyceride (TG) were measured using an Autodry Chemistry Analyzer (SPOTCHEM SP4410, Arkray, Kyoto, Japan).
ELISA
Blood were determined using murine IL-1α or IL-1β Standard ABTS ELISA Development Kit (PeproTech, Rock Hill, NJ, USA) according to the manufacturer’s instruction.
Cell culture
The human hepatic stellate cell line LX-2 was cultured in DMEM containing 10% fetal bovine serum (FBS), 100 U/mL penicillin and 100 mg/mL streptomycin at 37°C adding 5% CO2-95% air. Phosphate-buffered saline (PBS) was used to wash the cells. Cells were passaged by trypsinization every two or three days. AML12, an immortalized mouse hepatocyte cell line, was incubated in DMEM/F12 supplemented with 10% FBS, 100 U/mL penicillin, 100 mg/mL streptomycin, 10 μg/mL insulin, 5.5 μg/mL transferrin, 6.7 ng/mL selenium and 40 ng/mL dexamethasone at 37°C under 5% CO2.
Western blotting
Equal amounts of protein extracted from cells and mouse livers were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to PVDF membranes. The membranes were blocked in 5% skim milk in PBST (PBS containing 0.05% Tween 20) for 1 h at room temperature and probed with specific primary antibodies at 4°C overnight. The membranes were washed and incubated with appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies. Finally, the proteins were visualized using Clarity™ Western ECL Substrate (Bio-Rad, Hercules, CA, USA). The density of each band was analyzed by Bio-Rad Quantity One software (Bio-Rad).
RT-PCR analyses
Total RNA was extracted using the SV Total RNA Isolation System (Promega, Madison, WI, USA). The cDNA was prepared by using 500 ng of total RNA and RT-PCR was performed as described previously (Jiang et al., 2017b). The following primers were used for analyses of gene expression in LX-2 cells: hCOL1A1-forward, 5′-CAAGACGAAGACATCCCAC-3′; hCOL1A1-reverse, 5′-CGGTTGATTTCTCATCATAGC-3′; hACTA2-forward, 5′-TATGCCTCTGGACGCACAAC-3′; hACTA2-reverse, 5′-CACGCTCAGCAGTAGTAACG-3′; hGAPDH-forward, 5′-GGCTCTCCAGAACATCATC-3′; hGAPDH-reverse, 5′-CTCTTCCTCTTGTGCTCTTG-3′.
Liver histological analysis and immunohistochemistry
Liver tissues were fixed in 10% neutral buffered formalin and embedded in paraffin, cut into 5-μm-thick sections and stained with hematoxylin and eosin (H&E) or Masson-trichrome (Fuzhou Maixin Biotechnology Development Co. Ltd., Fuzhou, China). Five-micrometer-thick cryosections of liver were stained with oil red O, and then counterstained with hematoxylin as described previously (Li et al., 2018). Five-micrometer-thick paraffin sections were generated for immunocytochemistry staining using mouse anti-SREBP1 and anti-α-SMA antibodies as described previously (Zhang et al., 2018).
Nile Red staining and cytoimmunofluorescence staining
AML12 cells were grown on coverslips in 6-well plates and then treated appropriately. AML12 cells were fixed with 4% paraformaldehyde and permeabilized in 0.1% TritonX-100. AML12 cells were then stained with 1 μg/mL Nile red (Sigma-Aldrich, St. Louis, MO, USA), an excellent vital stain for the detection of intracellular lipid droplets, and incubated for 15 minutes at 37°C. Alternatively, fixed and permeabilized AML12 cells were blocked with 2% bovine serum albumin for 1 h at room temperature, followed by incubation with SREBP1 antibody as previously described in detail (Li et al., 2018). Images of immunostained slides or coverslips were acquired by microscopy (Nikon TI-E, Nikon, Tokyo, Japan).
Statistical analysis
Statistical analysis was performed using the GraphPad Prism program (GraphPad Software, Inc., San Diego, USA) and the experimental results were expressed as the mean ± SD. Comparison of results was performed using one-way ANOVA and Tukey’s multiple comparison tests. The level of statistical significance (p value) was less than 0.05.
RESULTS
GPS inhibited chronic alcohol intake-induced liver steatosis
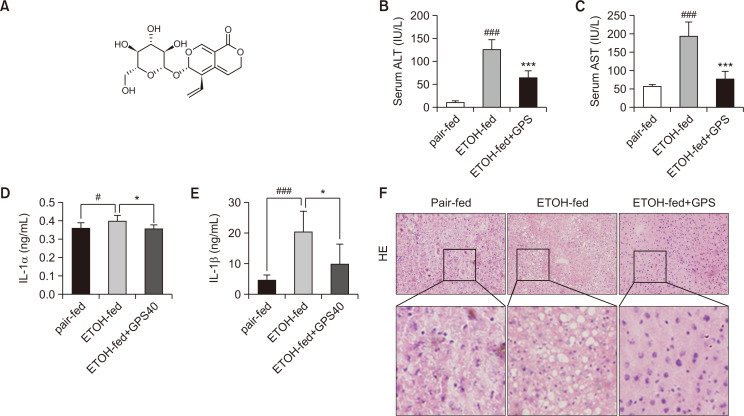
Chronic ethanol challenge for 4 weeks induced a significant increase in serum ALT and AST levels (Fig. 1B, 1C). Histological analysis showed that chronic ethanol feeding resulted in serious steatosis and inflammatory infiltration (Fig. 1F). The accumulation of hepatic lipid droplets was decreased in the liver tissues from GPS pretreatment mice compared with chronic ethanol-fed mice, indicating the protective effect of GPS on chronic alcoholic hepatic steatosis. In addition, IL-1α and IL-1β were increased in the ethanol-fed group, while GPS pretreatment suppressed the production of proinflammatory cytokines (Fig. 1D, 1F). These results indicated that GPS treatment could suppress liver steatosis caused by chronic alcohol intake and decrease inflammatory cytokine secretion.
Fig. 1.
GPS ameliorated liver steatosis in chronic alcohol-fed mice. (A) Chemical structure of GPS. (B, C) Serum ALT and AST levels. (D, E) IL-1α and IL-1β level in serum were analyzed by ELISA. (F) Hematoxylin and eosin (H&E) staining (×200 magnification). Data represent the mean ± SD of three independent experiments. #p<0.05, ###p<0.001, significantly different from the pair-fed group; *p<0.05, ***p<0.001 significantly different from the ethanol-fed group. Representative images are shown. All histograms represent the mean ± SD of at least three independent assays.
GPS regulated lipid metabolism in chronic alcohol intake-induced mouse steatotic liver and ethanol-treated hepatocytes
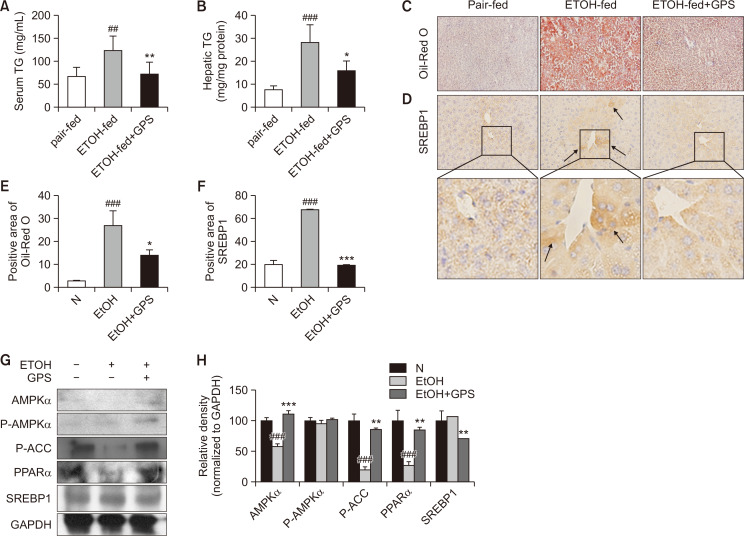
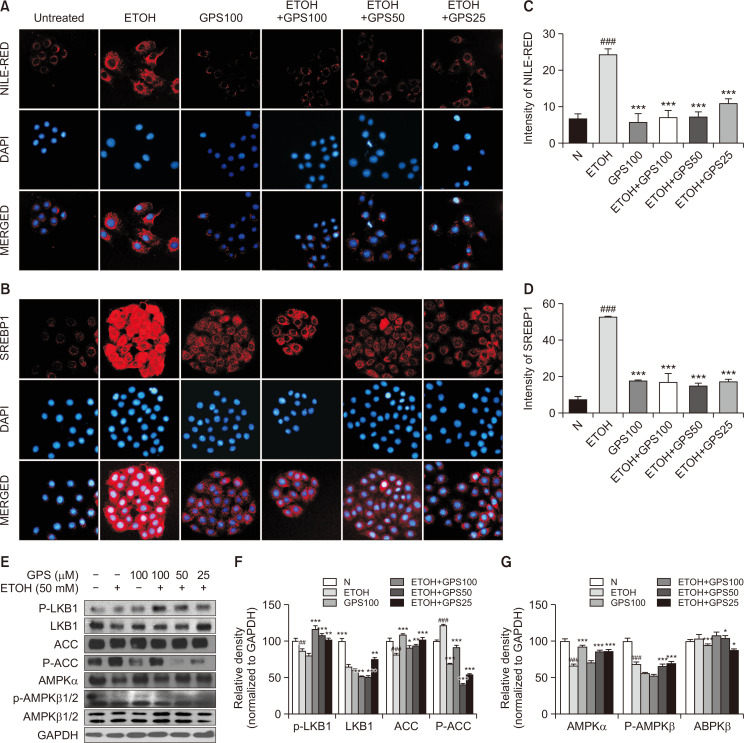
Chronic ethanol-fed mice exhibited increased hepatic and serum TG levels compared with those of the pair-fed group, while GPS administration reduced hepatic and serum TG levels after ethanol exposure (Fig. 2A, 2B). Lipid droplets in mouse liver exposed to ethanol were notably red-stained with oil red O, while positive oil red O staining was decreased with GPS pretreatment in mice suffering from alcoholic steatosis (Fig. 2C). Confirmed by immunohistochemical staining for SREBP1, chronic ethanol feeding significantly increased the expression of SREBP1 compared with that in the pair-fed group, while SREBP1 expression in mouse liver significantly declined with GPS pretreatment (Fig. 2D). Moreover, we determined proteins that were closely related to lipid anabolism, such as AMPK, PPARα, ACC and SREBP1 by western blot. The expression of phosphorylated-ACC and PPARα in mouse livers of the chronic ethanol-fed group was significantly decreased (Fig. 2G). After pretreatment with GPS, the expression of total- and phosphorylated-AMPK, PPARα, and phosphorylated-ACC was significantly increased compared with that in the chronic alcohol-fed group, while SREBP1 expression was downregulated with GPS pretreatment. As expected, GPS ameliorated these lipid metabolism-related factors to normal levels (Fig. 2G, 2H). As shown in Fig 3A-3G, we also observed that GPS reversed ethanol-induced lipid accumulation by inhibiting lipid synthesis and promoting lipid oxidation in AML12 mouse hepatocyte cell line via LKB1-AMPK, which is consistent with previous work performed in HepG2 cells and primary mouse hepatocytes (Li et al., 2018). Our in vitro data related to ethanol-treated steatotic AML12 cells was also in line with previously published literature (Gao et al., 2018). These data hinted that GPS protected against liver steatosis induced by chronic ethanol consumption.
Fig. 2.
GPS regulated lipid metabolism in the livers of mice exposed to chronic alcohol. Serum (A) and hepatic (B) TG levels. (C) Oil-red O staining. (D) Immunohistochemical staining of SREBP1. Representative images were captured with 200× magnification. The averaged percentages of immunostaining areas for oil red O (E) and SREBP1 (F) were analyzed with Image Pro-Plus 6.0 software (Media Cybernetics, Inc., Bethesda, MD, USA). (G) AMPKα, P-AMPKα, P-ACC, PPARα and SREBP1 proteins were detected by western blotting. GAPDH was used as a loading control. (H) Densitometric tracing analysis was done for each western blot band normalized to GAPDH. Data represent the mean ± SD of three independent experiment. ##p<0.01, ###p<0.001, significantly different from the pair-fed group; *p<0.05, **p<0.01, ***p<0.001 significantly different from the ethanol-fed group. Representative images are shown. All histograms represent the mean ± SD of at least three independent assays.
GPS reversed ECM deposition in chronic alcohol intake-induced liver fibrosis
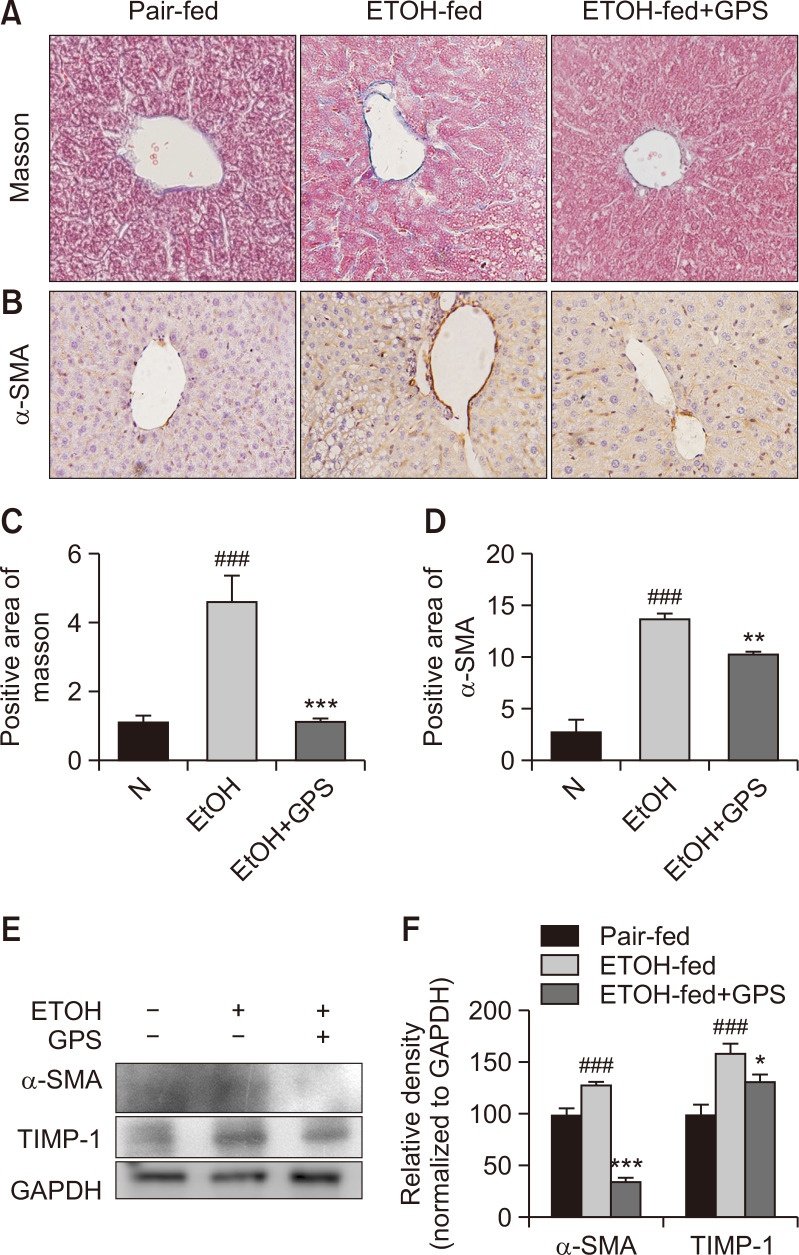
Long-term administration of excessive ethanol promoted hepatic steatosis developing into hepatic fibrosis. Although ad libitum oral feeding with a Lieber-DeCarli ethanol liquid diet could not induce obvious liver fibrosis (Bertola et al., 2013), some literature reported that in the mice fed a Lieber-DeCarli diet containing 5% (v/v) alcohol, proteins related to liver fibrosis were upregulated (Chang et al., 2017). Masson trichrome staining data of mouse livers showed that mice exposed to chronic and excessive alcohol exhibited increased collagen deposition, indicating that excessive alcohol consumption might induce a mild fibrosis in mouse livers (Fig. 4A, 4C). GPS administration reduced the positive staining of collagen deposition. Similar to previously reported research (Wu et al., 2016), after chronic alcohol intake, overexpression of α-SMA was observed in alcohol-exposed mouse livers as confirmed by immunohistochemistry staining and western blot analysis, but GPS pretreatment downregulated this protein expressions (Fig. 4B, 4D-4F). Additionally, GPS suppressed the protein expression of tissue inhibitor of metal protease 1 (TIMP-1), which was increased by alcohol exposure. From the α-SMA immunohistochemical staining results, the chronic alcohol group showed significant positive expression around the central vein. These results suggested GPS could decelerate the progression from hepatic steatosis to mild hepatic fibrosis in the early stage of liver fibrosis.
Fig. 4.
GPS reversed ECM deposition in chronic alcohol intake-induced liver fibrosis (A) Masson’s trichrome staining. (B) Immunohistochemical analysis of α-SMA. Representative images were captured with 200× magnification. The averaged percentages of positive staining areas for Masson (C) and α-SMA (D) were analyzed with Image Pro-Plus 6.0 software (Media Cybernetics, Inc.). (E) Western blot analysis of α-SMA, TIMP-1 and GAPDH (loading control). (F) Densitometric tracing analysis was done for each western blot band normalized to GAPDH. Data represent the mean ± SD of three independent experiment. ###p<0.001, significantly different from the pair-fed group; *p<0.05, **p<0.01, ***p<0.001, significantly different from the ethanol-fed group. Representative images are shown. All histograms represent the mean ± SD of at least three independent assays.
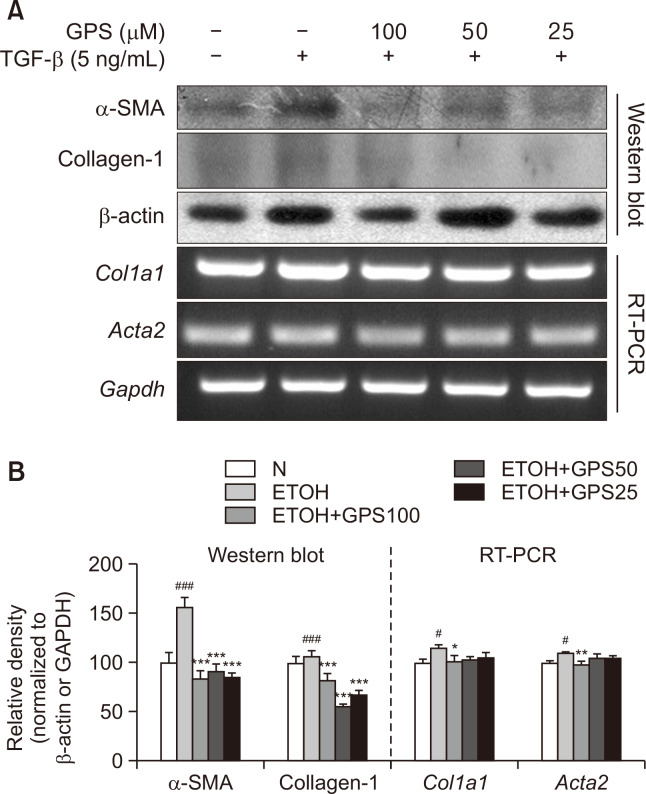
Activation of hepatic stellate cells is the cellular basis of hepatic fibrosis. A immortal human stellate cell line, LX-2, demonstrates a sensitive response to TGF-β, resembling a myofibroblast-like phenotype of activated HSCs (Herrmann et al., 2007). We reported previously upregulated protein and mRNA expression of α-SMA and collagen I in LX-2 cells (Yang et al., 2016). GPS pretreatment at concentrations of 25, 50 and 100 μM notably inhibited protein and mRNA expression of α-SMA and collagen I in TGF-β-activated LX-2 cells (Fig. 5).
Fig. 5.
GPS suppressed TGF-β-stimulated HSC activation. LX-2 cells were pretreated with GPS for 1h in the presence of TGF-β (5 ng/mL) for 24 h. (A) Protein expression of α-SMA and collagen I was analyzed by western blotting. mRNA expression of Acta2 (encoding α-SMA) or Col1a1 (encoding collagen I) was analyzed by RT-PCR. (B) Densitometric tracing analysis was done for each western blot band or RT-PCR band and normalized to β-actin or GAPDH. #p<0.05, ###p<0.001, significantly different from untreated cells; *p<0.05, **p<0.01, ***p<0.001 significantly different from TGF-β-stimulated cells. All histograms represent the mean ± SD of at least three independent assays.
DISCUSSION
GM is an herbal medicine for chronic hepatitis, which has been used from ancient times in China. GPS, a water-soluble ingredient in GM has anti-inflammatory and hepatoprotective effects (Mirzaee et al., 2017; Wu et al., 2017). Previously, we reported GPS could reverse ethanol-induced liver steatosis and drug-induced liver injury (Lian et al., 2010b; Wang et al., 2010). In addition, it was reported that GPS can ameliorate pulmonary fibrosis (Chen et al., 2018a). However, whether and how GPS could prevent the progression from liver steatosis to liver fibrosis during chronic ethanol consumption is still elusive. Our current research is distinguished from the above studies: Herein we elucidated that GPS could prevent chronic alcohol consumption-induced liver steatosis and effectively inhibited the development to a mild status of fibrosis.
Alcohol exposure directly or indirectly regulates lipid metabolism-related transcription factors and nuclear hormone receptors, such as SREBP1 and PPARα (Li et al., 2016; Carneiro et al., 2017). Chronic ethanol intake increased the expression of SREBP1 and reduced the expression of PPARα and phosphorylated-ACC in liver (Fig. 2D, 2G). Long term and excessive ethanol consumption can inhibit AMPK phosphorylation by affecting the upstream kinase LKB1 (Jiang et al., 2015). AMPK is linked to lipid hemostasis by regulating ACC activity through phosphorylation. AMPK stimulates fatty acid oxidation through suppressing ACC activity (Minokoshi et al., 2002). GPS administration can reverse chronic ethanol-induced fatty liver, characterized by the inhibition of hepatic lipid accumulation with decreased SREBP1 and increased AMPKα, PPARα and phosphorylated-ACC in mouse liver, as well as in hepatocytes. The inhibition capacity of GPS in lipid accumulation confirmed that GPS also reversed chronic alcoholic liver steatosis by promoting lipid oxidation and inhibiting lipid synthesis.
Chronic inflammation and continuous damage to hepatocytes caused by long-term and excessive alcohol intake initiates hepatic fibrosis, accompanied by the production of ECM over hepatocyte regeneration and recruitment of immune cells to sites of inflammation. Accumulated immune cells release a variety of pro-inflammatory cytokines upon encountering stimuli derived from damaged hepatocytes. This may be due to the reduction of hepatic steatosis leading to a decrease in cytokines. Fed with a Lieber-DeCarli ethanol liquid diet, pro-inflammatory cytokines in mouse serum including IL-1β and IL-1α, were remarkably increased (Fig. 1D, 1E). Along with the promoted inflammatory response, the levels of liver fibrosis markers, such as α-SMA, collagens and TIMP-1, were elevated in alcohol-fed mice. In addition, with increasing collagen deposition, our data indicated that mild liver fibrosis might have occurred in mice fed with a Lieber-DeCarli ethanol liquid diet for 4 weeks, which was similar to a previous report (Chang et al., 2017). With GPS administration, those liver fibrosis marker levels were restored to normal levels, suggesting GPS might possess the ability to restrain the progression from alcoholic hepatic steatosis to hepatic fibrosis. In addition, due the pivotal pathogenic role of HSCs in liver fibrosis, we examined whether GPS targeted the activation of HSCs to prohibit the transformation of those quiescent HSCs to a myofibroblast response to TGF-β. Activation of LX-2, an immortalized human hepatic stellate cell, with TGF-β is used to mimic myofibroblast-like HSCs (Xu et al., 2005). As expected, GPS pretreatment decreased collagen I and α-SMA was upregulated by TGF-β in LX-2 cells (Fig. 5A). This indicated that GPS could reverse the initiation of hepatic fibrosis during chronic alcoholic liver disease.
In summary, GPS could prevent the development of hepatic steatosis to fibrosis caused by chronic alcohol exposure, targeting HSC activation through AMPK, which suggested GPS might be a promising therapy for the treatment of alcoholic liver diseases. We hopefully provide the promising clinical application of GPS for treating the development of hepatic steatosis to fibrosis.
Fig. 3.
GPS inhibited lipid accumulation in ethanol-treated steatotic AML-12 cells. (A) Nile-red staining. Nile red (red, intracellular lipid droplets), DAPI (blue, nucleus). (B) Immunofluorescence staining of SREBP1 (SREBP1, red; DAPI, blue). Relative fluorescence intensity of Nile red (C) and SREBP1 (D) was analyzed with Image Pro-Plus 6.0 software (Media Cybernetics, Inc.). (E) P-LKB1, LKB1, ACC, P-ACC, AMPKα, AMPKβ and P-AMPKβ proteins levels were detected by western blotting. GAPDH was used as a loading control. (F, G) Densitometric tracing analysis was done for each western blot band normalized to GAPDH. ##p<0.01, ###p<0.001, significantly different from untreated cells; *p<0.05, **p<0.01, ***p<0.001 significantly different from ethanol-stimulated cells. Representative images are shown. All histograms represent the mean ± SD of at least three independent assays.
ACKNOWLEDGMENTS
This study was supported by a grant from the National Natural Science Foundation of China (81960677, 81660689, 81560597 and 81860751), and partially by Science and Technology Planning Projects from the Science and Technology Department of Jilin Province (20180414048GH, 20180201065YY and 20180519010JH) and Science and Technology Planning Project of the Jilin Provincial Education Department (JJKH20191155KJ).
Footnotes
CONFLICT OF INTEREST
The authors have declared no conflict of interest.
REFERENCES
- Bertola A., Mathews S., Ki S. H., Wang H., Gao B. Mouse model of chronic and binge ethanol feeding (the NIAAA model) Nat. Protoc. 2013;8:627–637. doi: 10.1038/nprot.2013.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruha R., Dvorak K., Petrtyl J. Alcoholic liver disease. World J. Hepatol. 2012;4:81–90. doi: 10.4254/wjh.v4.i3.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y., Jogasuria A., Yin H., Xu M. J., Hu X., Wang J., Kim C., Wu J., Lee K., Gao B., You M. The detrimental role played by lipocalin-2 in alcoholic fatty liver in mice. Am. J. Pathol. 2016;186:2417–2428. doi: 10.1016/j.ajpath.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro L., Asrih M., Repond C., Sempoux C., Stehle J. C., Leloup C., Jornayvaz F. R., Pellerin L. AMPK activation caused by reduced liver lactate metabolism protects against hepatic steatosis in MCT1 haploinsufficient mice. Mol. Metab. 2017;6:1625–1633. doi: 10.1016/j.molmet.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceni E., Mello T., Galli A. Pathogenesis of alcoholic liver disease: role of oxidative metabolism. World J. Gastroenterol. 2014;20:17756–17772. doi: 10.3748/wjg.v20.i47.17756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. Y., Liu Y. C., Kuo Y. H., Lin Y. L., Wu Y. S., Chen J. W., Chen Y. C. Effects of antrosterol from Antrodia camphorata submerged whole broth on lipid homeostasis, antioxidation, alcohol clearance, and anti-inflammation in livers of chronic-alcohol fed mice. J. Ethnopharmacol. 2017;202:200–207. doi: 10.1016/j.jep.2017.03.003. [DOI] [PubMed] [Google Scholar]
- Chen C., Wang Y. Y., Wang Y. X., Cheng M. Q., Yin J. B., Zhang X., Hong Z. P. Gentiopicroside ameliorates bleomycin-induced pulmonary fibrosis in mice via inhibiting inflammatory and fibrotic process. Biochem. Biophys. Res. Commun. 2018a;495:2396–2403. doi: 10.1016/j.bbrc.2017.12.112. [DOI] [PubMed] [Google Scholar]
- Chen F., Xie L., Kang R., Deng R., Xi Z., Sun D., Zhu J., Wang L. Gentiopicroside inhibits RANKL-induced osteoclastogenesis by regulating NF-kappaB and JNK signaling pathways. Biomed. Pharmacother. 2018b;100:142–146. doi: 10.1016/j.biopha.2018.02.014. [DOI] [PubMed] [Google Scholar]
- Ezhilarasan D., Evraerts J., Brice S., Buc-Calderon P., Karthikeyan S., Sokal E., Najimi M. Silibinin inhibits proliferation and migration of human hepatic stellate LX-2 cells. J. Clin. Exp. Hepatol. 2016;6:167–174. doi: 10.1016/j.jceh.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Chen S., Qiu Z., Fang L., Zhang L., Guo C., Chen T., Qiu L. Myricitrin ameliorates ethanol-induced steatosis in mouse AML12 liver cells by activating AMPK, and reducing oxidative stress and expression of inflammatory cytokines. Mol. Med. Rep. 2018;17:7381–7387. doi: 10.3892/mmr.2018.8740. [DOI] [PubMed] [Google Scholar]
- Herrmann J., Gressner A. M., Weiskirchen R. Immortal hepatic stellate cell lines: useful tools to study hepatic stellate cell biology and function? J. Cell. Mol. Med. 2007;11:704–722. doi: 10.1111/j.1582-4934.2007.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S., Li T., Yang Z., Yi W., Di S., Sun Y., Wang D., Yang Y. AMPK orchestrates an elaborate cascade protecting tissue from fibrosis and aging. Ageing Res. Rev. 2017a;38:18–27. doi: 10.1016/j.arr.2017.07.001. [DOI] [PubMed] [Google Scholar]
- Jiang S., Zhang Y., Zheng J. H., Li X., Yao Y. L., Wu Y. L., Song S. Z., Sun P., Nan J. X., Lian L. H. Potentiation of hepatic stellate cell activation by extracellular ATP is dependent on P2X7R-mediated NLRP3 inflammasome activation. Pharmacol. Res. 2017b;117:82–93. doi: 10.1016/j.phrs.2016.11.040. [DOI] [PubMed] [Google Scholar]
- Jiang Z., Zhou J., Zhou D., Zhu Z., Sun L., Nanji A. A. The adiponectin-SIRT1-AMPK pathway in alcoholic fatty liver disease in the rat. Alcohol. Clin. Exp. Res. 2015;39:424–433. doi: 10.1111/acer.12641. [DOI] [PubMed] [Google Scholar]
- Lee H. I., Yun K. W., Seo K. I., Kim M. J., Lee M. K. Scopoletin prevents alcohol-induced hepatic lipid accumulation by modulating the AMPK-SREBP pathway in diet-induced obese mice. Metabolism. 2014;63:593–601. doi: 10.1016/j.metabol.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Li X., Zhang Y., Jin Q., Xia K. L., Jiang M., Cui B. W., Wu Y. L., Song S. Z., Lian L. H., Nan J. X. Liver kinase B1/AMP-activated protein kinase-mediated regulation by gentiopicroside ameliorates P2X7 receptor-dependent alcoholic hepatosteatosis. Br. J. Pharmacol. 2018;175:1451–1470. doi: 10.1111/bph.14145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Zhao X., Feng X., Liu X., Deng C., Hu C. H. Berberine alleviates olanzapine-induced adipogenesis via the AMPKalpha-SREBP pathway in 3T3-L1 cells. Int. J. Mol. Sci. 2016;17:E1865. doi: 10.3390/ijms17111865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian L. H., Wu Y. L., Song S. Z., Wan Y., Xie W. X., Li X., Bai T., Ouyang B. Q., Nan J. X. Gentiana manshurica Kitagawa reverses acute alcohol-induced liver steatosis through blocking sterol regulatory element-binding protein-1 maturation. J. Agric. Food Chem. 2010a;58:13013–13019. doi: 10.1021/jf103976y. [DOI] [PubMed] [Google Scholar]
- Lian L. H., Wu Y. L., Wan Y., Li X., Xie W. X., Nan J. X. Anti-apoptotic activity of gentiopicroside in D-galactosamine/lipopolysaccharide-induced murine fulminant hepatic failure. Chem. Biol. Interact. 2010b;188:127–133. doi: 10.1016/j.cbi.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Liu N., Li Y. X., Gong S. S., Du J., Liu G., Jin S. J., Zhao C. J., Niu Y., Sun T., Yu J. Q. Antinociceptive effects of gentiopicroside on neuropathic pain induced by chronic constriction injury in mice: a behavioral and electrophysiological study. Can. J. Physiol. Pharmacol. 2016;94:769–778. doi: 10.1139/cjpp-2015-0462. [DOI] [PubMed] [Google Scholar]
- Mandal S., Mukhopadhyay S., Bandhopadhyay S., Sen G., Biswas T. 14-Deoxyandrographolide alleviates ethanol-induced hepatosteatosis through stimulation of AMP-activated protein kinase activity in rats. Alcohol. 2014;48:123–132. doi: 10.1016/j.alcohol.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Minokoshi Y., Kim Y. B., Peroni O. D., Fryer L. G., Muller C., Carling D., Kahn B. B. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- Mirzaee F., Hosseini A., Jouybari H. B., Davoodi A., Azadbakht M. Medicinal, biological and phytochemical properties of Gentiana species. J. Tradit. Complement. Med. 2017;7:400–408. doi: 10.1016/j.jtcme.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicoro A., Ramachandran P., Iredale J. P., Fallowfield J. A. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat. Rev. Immunol. 2014;14:181–194. doi: 10.1038/nri3623. [DOI] [PubMed] [Google Scholar]
- Torruellas C., French S. W., Medici V. Diagnosis of alcoholic liver disease. World J. Gastroenterol. 2014;20:11684–11699. doi: 10.3748/wjg.v20.i33.11684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. Y., Lian L. H., Jiang Y. Z., Wu Y. L., Nan J. X. Gentiana manshurica Kitagawa prevents acetaminophen-induced acute hepatic injury in mice via inhibiting JNK/ERK MAPK pathway. World J. Gastroenterol. 2010;16:384–391. doi: 10.3748/wjg.v16.i3.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Zern M. A. Hepatic stellate cells: a target for the treatment of liver fibrosis. J. Gastroenterol. 2000;35:665–672. doi: 10.1007/s005350070045. [DOI] [PubMed] [Google Scholar]
- Wu S., Ning Y., Zhao Y., Sun W., Thorimbert S., Dechoux L., Sollogoub M., Zhang Y. Research progress of natural product gentiopicroside - a secoiridoid compound. Mini Rev. Med. Chem. 2017;17:62–77. doi: 10.2174/1389557516666160624124127. [DOI] [PubMed] [Google Scholar]
- Wu Y. L., Zhang Y. J., Yao Y. L., Li Z. M., Han X., Lian L. H., Zhao Y. Q., Nan J. X. Cucurbitacin E ameliorates hepatic fibrosis in vivo and in vitro through activation of AMPK and blocking mTOR-dependent signaling pathway. Toxicol. Lett. 2016;258:147–158. doi: 10.1016/j.toxlet.2016.06.2102. [DOI] [PubMed] [Google Scholar]
- Xiao J., Liong E. C., Ching Y. P., Chang R. C., Fung M. L., Xu A. M., So K. F., Tipoe G. L. Lycium barbarum polysaccharides protect rat liver from non-alcoholic steatohepatitis-induced injury. Nutr. Diabetes. 2013;3:e81. doi: 10.1038/nutd.2013.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Hui A. Y., Albanis E., Arthur M. J., O'Byrne S. M., Blaner W. S., Mukherjee P., Friedman S. L., Eng F. J. Human hepatic stellate cell lines, LX-1 and LX-2: new tools for analysis of hepatic fibrosis. Gut. 2005;54:142–151. doi: 10.1136/gut.2004.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Bai T., Yao Y. L., Zhang D. Q., Wu Y. L., Lian L. H., Nan J. X. Upregulation of SIRT1-AMPK by thymoquinone in hepatic stellate cells ameliorates liver injury. Toxicol. Lett. 2016;262:80–91. doi: 10.3847/0004-637X/831/1/80. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Jin Q., Li X., Jiang M., Cui B. W., Xia K. L., Wu Y. L., Lian L. H., Nan J. X. Amelioration of alcoholic liver steatosis by dihydroquercetin through the modulation of AMPK-dependent lipogenesis mediated by P2X7R-NLRP3-inflammasome activation. J. Agric. Food Chem. 2018;66:4862–4871. doi: 10.1021/acs.jafc.8b00944. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Li B., Meng X., Yao S., Jin L., Yang J., Wang J., Zhang H., Zhang Z., Cai D., Zhang Y., Ning G. Berberine prevents progression from hepatic steatosis to steatohepatitis and fibrosis by reducing endoplasmic reticulum stress. Sci. Rep. 2016;6:20848. doi: 10.1038/srep20848. [DOI] [PMC free article] [PubMed] [Google Scholar]