Abstract
Sepsis is conceptually defined as life-threatening organ dysfunction that is caused by a dysregulated host response to infection. Although there has been significant advancement in recent decades in defining and understanding sepsis pathology, clinical management of sepsis is challenging due to difficulties in diagnosis, a lack of reliable prognostic biomarkers, and treatment options that are largely limited to antibiotic therapy and fundamental supportive measures. The lack of reliable diagnostic and prognostic tests makes it difficult to triage patients who are in need of more urgent care. Furthermore, while the acute inpatient treatment of sepsis warrants ongoing attention and investigation, efforts must also be directed toward longer term survival and outcomes. Sepsis survivors experience incomplete recovery, with long-term health impairments that may require both cognitive and physical treatment and rehabilitation. This review summarizes recent advances in sepsis prognosis research and discusses progress made in elucidating the underlying causes of prolonged health deficits experienced by patients surviving the early phases of sepsis.
Keywords: Sepsis, Inflammation, Innate immunity
INTRODUCTION
Sepsis is a life-threatening condition that is triggered by microbial infection and characterized by an uncontrolled and detrimental host inflammatory immune response. Over 1.7 million people develop sepsis in the U.S. each year, which accounts for 25%–30% of hospital deaths and over 270,000 deaths annually (1). Worldwide, there are nearly 50 million cases of sepsis per year and sepsis accounts for nearly 20% of all deaths (2). In recent years it appears the incidence and mortality of sepsis remain unchanged, with approximately 20% of patients dying in the hospital or being discharged to hospice care (1). Further improvement in clinical outcomes will likely require improvements in diagnostic methods that allow rapid recognition and the discovery of novel treatments that influence clinically relevant aspects of sepsis pathology. The former will likely require new diagnostic biomarkers and the latter will require discovery of novel prognostic biomarkers that may define the most clinically important pathophysiological pathways to target for therapeutic manipulation.
DEFINITION
For nearly 25 years, sepsis has been defined as systemic inflammatory response syndrome caused by infection (3). This concept was revised in 2016 and the current definition of sepsis is life-threatening organ dysfunction caused by a dysregulated host response to infection (4). However, defining the presence, source, and specific microbiological pathogen of infection is often challenging in the clinical environment. Bacteria are responsible for most cases of sepsis, but viral, fungal, or parasitic infections can also trigger the syndrome. A large national cohort study indicates the most common sites of infection were the lungs, genitourinary tract, abdomen, and direct intravascular devices (5). However, in many cases an infectious source cannot be defined, especially early in the course of illness. It is often even more challenging to identify the pathogenic organism causing the infection. Blood cultures are only positive in 30%–40% of all septic patients (1), and the likelihood of positive cultures varies depending on the source of infection. Culturing techniques often take days to turn positive, thus levels of uncertainty are high early in the course of illness when prompt intervention is important, especially in the presence of hypotension. In recent years, a number of technologies have emerged in an effort to reliably and rapidly diagnose sepsis, but none have yet to be widely incorporated into clinical practice (6,7). Due to frequent diagnostic uncertainty (8), it is not particularly surprising that available time-sensitive sepsis treatments are often delayed (9). In an effort to achieve more rapid management, state and federal governments have instituted mandated reporting of sepsis care “bundles” (10), which focus on the first 6 h of management and consist of basic diagnostic tests, supportive measures, and prompt antibiotic therapy (11). While early evidence suggests bundle management may be effective, it could be advantageous to incorporate complementary strategies focusing on manipulation of downstream pathophysiological events that occur once sepsis diagnosis is confirmed and the clinical trajectory is defined (12).
Sepsis mortality occurs due to vital organ dysfunction, driven in part by microvascular dysfunction characterized by impaired vascular barrier function, infiltration of hyperinflammatory immune cells, excessive coagulation, impaired vasoregulation, and ischemia, ultimately resulting in multiorgan failure and death (4). Although treatment often involves broad-spectrum antibiotics and fluid resuscitation, these basic efforts may be insufficient for recovery. Despite elimination of the microbial source of sepsis, the maladaptive immune response can persist, leading to ongoing multi-organ injury. The challenge ahead is to define elements of this complex, uncontrolled immune response that are amenable to therapeutic intervention.
CLINICAL DIAGNOSIS AND MANAGEMENT
The diagnosis of unstable patients with sepsis cannot wait the hours or days often required to confirm infection. Therefore, clinical criteria are utilized for identification of patients with probable sepsis. The onset of sepsis is characterized by various clinical surveillance definitions. Clinical scores classically include measurements of body temperature, blood pressure, white blood cell (WBC) counts, respiratory function, and cognitive ability. The criteria are WBC counts of >12,000/mm3 or <4,000/mm3, hyperthermia (>38°C) or hypothermia (<36°C), a heart rate of >90 beats/min, and respiratory rates of >20 breaths/min (3). The presence of 2 or more of these criteria and a suspected or confirmed source of infection have defined sepsis syndrome for over 2 decades. More recently, new classification criteria have been proposed that define sepsis as suspected or confirmed infection associated with new or worsening vital organ dysfunction, defined by a change in the sequential sepsis-related organ failure assessment (SOFA) score of ≥2 (4).
The standard early treatment of critically ill septic patients includes intravenous fluid resuscitation and broad-spectrum antibiotics (11). Importantly, early administration of antibiotic treatment may be one of the most powerful ways to decrease mortality (9,13,14). Therefore, early detection and immediate treatment are imperative to save patients, as the risk of mortality can increase by 10% with every hour of delayed intervention (14).
Outcome prediction for sepsis patients is imperative for treatment decisions and resource allocation and is the driving influence behind the new sepsis 3 definitions. Studies of outcome prediction using clinical parameters have been conducted using large datasets that may include thousands of patients (15). While these studies are useful for clinical identification of subjects at risk of experiencing poorer outcome, they do not shed light on to pathophysiological pathways that could generate new treatment targets. Therefore, they do not provide insights that allow clinicians to tailor treatment according to the patient's biological response to infection, and this type of personalized medicine approach is probably necessary for substantive progress in reducing sepsis morbidity and mortality (12).
Biomarkers
There is ongoing intense interest in identifying biomarkers both for the diagnosis and prognostication in sepsis patients. Identifying new prognostic markers could be an important step in developing novel treatment strategies. Conventional sepsis biomarkers include circulating factors released from specific organ systems, surface molecules expressed on overactivated cells, or metabolites secreted from damaged tissues (16).
C-reactive protein (CRP) and procalcitonin (PCT) are 2 of the most widely studied biomarkers in the diagnosis of severe sepsis (17). CRP is synthesized in the liver during very acute phases of systemic inflammation, and it plays a role in complement activation, typically through the signaling of classical cascade initiator molecules. CRP concentrations in serum can be measured very quickly, and levels can fluctuate in very short periods of time, contributing to its sensitivity and usefulness as a biomarker (18,19). However, CRP levels vary highly among septic individuals, and for accurate and predictive measurements, CRP must be compared to the individual's own baseline. Often, this is difficult or even impossible, as patients may be infected and septic long before ICU admission. PCT may be even less useful, as its levels do not correlate well with SOFA scores, lengths of stay in the hospital, or even patient mortality (20). PCT may still be useful in combination with other tools and sepsis scoring methods, but as a single factor for diagnosis or prognosis, it falls short. CRP and PCT have been held as gold standards for predicting sepsis outcomes, but the requirements for sepsis diagnosis must already be in place (21).
In comparison to circulating factors as biomarkers, other methods use the detection of upregulated proteins on the surfaces of activated immune cells. Chemokine receptors, including C-C chemokine receptor type 2 (CCR2) and CX3CR1, are useful biomarkers that are expressed on monocytes during sepsis (22). CCR2 is highly upregulated on “inflammatory monocytes” and coincides with the rapid release of these cells from sites of maturation, including the bone marrow and spleen. CX3CR1 is more likely to be found on “patrolling monocytes”, which are a less hyperinflammatory version of the same cell type. Both molecules serve specialized functions that are crucial in the context of sepsis and are thus considered useful biomarkers of persistent inflammation (23,24,25).
In addition to chemokines and their receptors, integrins are a family of molecules expressed on the surfaces of circulating immune cells that facilitate their binding and extravasation into inflamed tissues. Recently, it has been shown that integrin α3β1 or very late antigen-3 (VLA-3) is highly expressed on a subset of neutrophils, causing them to exhibit hyperinflammatory behaviors that include excessive extravasation into tissues (26). Other important molecules present on cells during the immune response to infection include Fc receptors. These specialized molecules exist in many subclasses and bind to the constant portions of antibodies, which may be bound to foreign antigens such as bacteria to mark them for clearance. One such molecule is Fc-gamma (γ)- receptor-1 (CD64), which binds to monomeric IgG antibodies with very high affinity (27,28). Neutrophil CD64 expression is an excellent biomarker that indicates active infection, as its expression is only increased during sepsis and is negligible on resting neutrophils (29). Combining clinical variables with immune cell activation markers such as VLA-3 and CD64 expression to create a sepsis risk prediction score is may provide greater diagnostic and prognostic accuracy than any of these parameters alone (Table 1).
Table 1. Sepsis biomarkers.
| Marker | Function | Clinical relevance | Ref. | |
|---|---|---|---|---|
| Soluble | ||||
| Lactate | Byproduct of glucose metabolism, produced from pyruvate during anaerobic metabolism | • Hyperlactatemia indicative of cell hypoxia states when aerobic is converted to anaerobic metabolism | (30,31) | |
| • Also elevated with reduced lactate clearance from sepsis-induced liver dysfunction | ||||
| • Also elevated with excess glycolysis, thiamine deficiency, and other conditions, therefore non-specific | ||||
| Blood pH | Indicative of metabolic acidosis, a result of increased anion production | • Acidified blood correlates with a decrease in tissue perfusion and contributes to reduced cardiac contractility, ATP generation, and negatively impacts the immune response | (32,33) | |
| • Can be a result of other disease states, and not specific to sepsis | ||||
| CRP | Binds to pathogens and dying cells to facilitate enhanced phagocytosis and clearance | • Produced by the liver at early phases of sepsis in response to bacterial infection, but also as a response to many other inflammatory stimuli | (34,35,36) | |
| PCT | Prohormone of calcitonin secreted in response to bacterial stimulation | • PCT concentrations above 0.1 ng/mL indicative of bacterial infection | (36,37) | |
| • Half-life is relatively short, and concentrations can normalize quickly | ||||
| HMGB-1 | HMGB-1 protein binds to DNA and creates a scaffold for chromatin formation | • Alarmin released from cells under stress | (38,39) | |
| • During sepsis, can bind inflammatory mediators such as RAGE and TLRs | ||||
| • Higher levels in the blood indicative of inflammation, however not specific to sepsis | ||||
| Cell surface | ||||
| CD64 | High affinity Fc-g-receptor, highly expressed on macrophages and eosinophils, binds to immunoglobulins and mediates clearance of antibody coated cells | • Expressed on neutrophils only during sepsis, specifically during bacterial infections | (36,40) | |
| VLA-3 (a3b1) | Member of the integrin family, mediates adhesion of immune cells to fibronectin and collagen in extracellular matrices during cell migration | • Upregulated on hyperinflammatory neutrophils exclusively during sepsis, distinguishing from sterile inflammation or SIRS | (26,41,42) | |
| CCR2 | Chemokine receptor 2, expressed on monocytes and some macrophages to facilitate chemotaxis and regulation of tissue specific immune cell homing | • Higher expression levels during sepsis indicative of pro-inflammatory monocyte egress from bone marrow and subsequent infiltration into inflamed tissues | (23,43,44,45) | |
| CX3CR1 | Fractalkine receptor, highly expressed on tissue-resident macrophages, facilitates leukocyte adhesion and migration during steady state | • Expressed on monocytes during immunosuppressive phases of sepsis | (22,25,46,47,48) | |
SIRS, systemic inflammatory response syndrome; HMGB-1, high mobility group protein B1; RAGE, receptor for advanced glycation end products.
INFLAMMATORY MEDIATORS OF SEPSIS
Even when sepsis is promptly diagnosed at the time of hospital admission, the diagnosis is changed about a third of the time when additional clinical information becomes available later during the hospital stay. Inaccuracies in diagnosis and prognosis stem from the marked heterogeneity of patients with severe sepsis. This heterogeneity originates largely from variation in the immune responses of patients with regard to the phase of the sepsis inflammatory cascade. Following sections will describe the diversity and heterogeneity of the sepsis-associated inflammatory pathways.
Recognition of molecular patterns
The host response to pathogen invasion is initiated by the pattern recognition receptors (PRRs) of the innate immune system, which recognize pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs). PAMPs are unique motifs found on microbes that are recognized by PRRs and allow the innate immune system to distinguish self from non-self, while DAMPs are a sign that there is damage to the host. Both PAMPs and DAMPs function as “molecular warnings” that activate circulating and tissue-resident immune cells. The broad nature of the innate immune system's response to infection and damage is largely facilitated by well-conserved subclasses of PRRs (49). PRRs are made up of several distinct subfamilies of receptors, including TLRs, Nod-like receptors (NLRs), RIG-I-like receptors, AIM2-like receptors, and C-type lectin receptors (50).
During sepsis, PRRs play a critical role in initiating the response to infection. Perhaps the most well studied PRRs are TLRs and NLRs, which bind a multitude of both PAMPs and DAMPs that are displayed during sepsis. TLRs exist in both the cell membrane and endosomal compartments and distinguish extracellular and intracellular pathogens, respectively. Transmembrane TLRs mainly recognize microbial membrane components such as LPS by TLR4, lipoproteins, peptidoglycans, lipoteichoic acids, zymosan, and mannan by TLRs 1, 2, and 6, and flagellin by TLR5. Intracellular TLRs detect nucleic acids from both bacterial and viral sources that include dsDNA (TLR3), ssRNA (TLR7), and CpG-DNA motifs (TLR9), in addition to profilin, a component of the parasite Toxoplasma gondii (TLR11) (51,52). TLRs also respond to host products such as heme or high mobility group protein B1 through TLRs 4 and 2, respectively (53).
NLRs recognize various ligands from microbial pathogens and host cells. NLRs sense viral ssRNA (NOD2), bacterial flagellin (NLRB), and cytosolic products of host stress, such as ATP. Activation of NLRs leads to distinct functional mechanisms, including the formation of the inflammasome, transcriptional activation of proinflammatory cytokines, and autophagy (54).
Other PRRs include P2X and P2Y receptors, which respond to host nucleotide products such as ATP, ADP, UTP, and UDP (55). Heat shock proteins and uric acid are other examples of host products that innate immune cells can sense as a sign of cellular damage (56). All PRRs exert a multitude of functions that ultimately lead to cell secretion of antimicrobial products or signals to other cells.
During sepsis, sustained immune activation is achieved by initial infection and recognition of foreign material through PAMPs, followed by the release of host components during tissue damage (DAMPs or alarmins), leading to a vicious cycle of amplified inflammation. The innate immune system response is absolutely necessary as the first line of defense towards pathogen invasion, yet the pathophysiology of sepsis occurs when these same immune cells become overactivated and dysregulated. In this regard, PRRs have been established as therapeutic targets during sepsis. This field of research is very dynamic and numerous clinical trials are in place that test the efficacy of various TLR antagonists, with the majority of studies centered around TLR4. Many small molecule drugs are in the earlier stages of clinical trials but appear to be well tolerated by healthy subjects (57,58,59). Unfortunately, at present, treatments targeting specific elements of the dysregulated immune response of sepsis remain elusive.
Proinflammatory cytokine responses
Many signal transduction pathways stemming from activation of PRRs culminate in the activation of transcription factors (TFs), including interferon-regulatory factors and the master regulator NF-κB (60). These TFs result in the expression and secretion of proinflammatory cytokines such as IL-6 and IL-12 and IFNs, which are required for host defense against pathogens and long-term adaptive immunity (61). Another well-characterized example of PRR downstream signaling is inflammasome-mediated induction of caspase-1, an enzyme that cleaves the pro-forms of IL-1β and IL-18 to mediate their release (62). The -proinflammatory cytokines IL-1β, IL-18, IL-6, or TNF-α may be double-edged swords, as these cytokines have essential functions in signaling to other immune cells but ultimately exacerbate inflammation and contribute to many harmful symptoms of sepsis. IL-6 activates prostaglandin E2 in thermoregulatory neurons within the hypothalamus, where downstream signaling results in hyperthermia or fever (63). TNF-α is an especially important multifunctional molecule that is produced during sepsis. Among other effects, it causes a hypercoagulable state promoting intravascular clotting and disrupting microvascular blood flow, a hallmark of sepsis pathology (64).
Targeting TNF-α and IL-1β is a novel pharmacological modulation strategy for treating sepsis. Although blocking these proinflammatory cytokines proved efficacious in mouse models of disease (65), clinical trials in humans were unsuccessful (66). Antagonists of IFN-γ similarly did not improve mortality rates when given intravenously to severely septic patients (67). The bulk of these randomized trials occurred decades ago. To date, there are still no cytokine modulators on the market for sepsis treatment. However, other soluble factors are therapeutic targets, and some play even more extensive roles during severe sepsis and the outcome. One such example is the activation of humoral immune components known as complement.
Complement
Complement activation occurs via 3 different routes: classical, alternative, and mannose binding lectin pathways (68). All 3 have multiple unique factors, but all converge on the C3 component and culminate in the formation of the membrane attack complex (MAC) (69). The MAC creates a transmembrane pore in a target cell, facilitating lysis and death. Targets include bacteria or infected host cells, making regulating of the complement pathway essential during sepsis. The antimicrobial properties of complement include direct lysis of microorganisms, opsonization of cells as markers for phagocytosis, and signaling molecules that coordinate further responses to infection (70). Complement release and activation occurs rapidly after pathogen invasion, and each pathway's components are tightly regulated by a systematic cascade of events when the system is functioning normally. Complement components are made primarily by endothelial cells in the liver, but during inflammation, they can also be synthesized and released by immune cells.
The production and circulation of complement factors are highly upregulated during sepsis. While the protective functions of complement are an essential facet of innate immunity, complement factor concentrations are strongly and positively associated with disease prognosis (70). Complement is comprised of multiple fluid-phase and membrane-associated proteins, which must be tightly regulated to distinguish self from non-self (71). Multiple factors exist to control the activation and assembly of the complement cascade, including endogenous inhibitors that sequester complement components in the absence of infection. Sepsis is frequently marked by a loss of complement regulation, which can include both overactivation and deficiency. Exploitation of the complement cascade has been a focus of research for the past 20 years. A therapeutic strategy targeting complement has been tested in autoimmune diseases, transplant-related complications, and neurodegeneration (72).
Coagulation factors
During acute inflammation, the coagulation system is diffusively and systemically activated, resulting in disseminated intravascular coagulation (73). Different degrees of hemostatic dysregulation occur at different timepoints throughout sepsis, resulting in both bleeding abnormalities and tissue ischemia. This makes therapeutic manipulation of the coagulation cascade extremely challenging. Furthermore, clot formation and disruption both have noncanonical roles outside of hemodynamic processes. These include host defense against pathogens and clearance of infection. This interconnection between coagulation and immune pathways is called immunothrombosis (74). Small amounts of controlled clot formation can trap circulating pathogens and impede dissemination of these pathogens through circulation and into tissues (75). Despite this, the majority of therapeutic treatments targeting the coagulation cascade are inhibitory. The administration of DNase was designed to directly prevent the nets that are formed during immunothrombotic bacterial sequestration (76). The majority of anticoagulation therapies are targeted towards deactivating specific components of the coagulation cascade. For example, several antithrombin therapies have been tested in multiple clinical trials worldwide, with mixed results (77). Some antithrombin-based anticoagulant therapies have resulted in a modest change in septic outcomes, decreasing 28-day mortality by up to 9% (77). The benefits and risks of antithrombin treatment are still under active investigation, as antithrombin therapy increases the likelihood of bleeding events in up to 7% of severely septic patients without a significant reduction in the 28-day all-cause mortality (78,79).
Leukocyte recruitment
The most essential mediators of sepsis pathology are the cells of the innate immune system. Neutrophils and monocytes are the 2 cell types that are primarily responsible for preventing the progression of microbial infection. In the context of sepsis, however, abundance and widespread activation of these cell types facilitates much of the damage seen during the acute phases of the disease.
Neutrophils
Neutrophils display various cell surface molecules that recruit them from their site of maturation in the bone marrow into circulation and peripheral tissues. Once exited from the bone marrow, neutrophils follow endogenous chemokine gradients that localize them to sites of infection, where they are subsequently recruited by bacterial products and complement factors (80). Under infection and host stress conditions, neutrophils release their granules, which exist in primary, secondary, and tertiary forms. Some of the main antimicrobial components secreted by neutrophils include myeloperoxidase (MPO), ROS, azurocidin (primary), cathelicidin (secondary), peptidoglycan recognition proteins (tertiary), and their own DNA and histones via the generation of neutrophil extracellular traps (81). The antimicrobial properties of neutrophils are critical for the disruption of bacterial cell membranes, resulting in pathogen clearance (81).
Granules released from neutrophils also mediate their extravasation from circulation into tissues. Matrix metalloproteases (MMPs), in particular MMP9, are found in the brain following acute ischemic injury (82). MMP9 was first described in neutrophils and is released from primary granules following neutrophil activation. Release of MMP9 facilitates the breakdown of tight junction proteins, including claudin-5, occludin, and zonula occludens-1, which are all found between endothelial cells, including those of the blood brain barrier (BBB) (82,83). Therefore, hyperinflammatory neutrophils releasing MMP9 are capable of breaching the endothelial layer that separated circulating leukocytes from tissues, including the brain, thus allowing more immune cells to infiltrate organs and promote damage.
Although neutrophils become hyperactivated and induce much of the tissue damage observed during sepsis, they are also essential for the clearance of pathogens. Therefore, extensive research has been devoted to further understanding and exploiting the mechanisms behind neutrophil dysfunction during inflammation. Exploration of neutrophil heterogeneity within different diseases has uncovered the potential for perturbing the distribution of neutrophil subpopulations. During tumorigenesis, neutrophils have been reported to exist in a state between a pro- and anti-inflammatory phenotype (84), similar to what has been described in M1 vs M2 macrophages (85). Here, N1 vs N2 neutrophils may have similar traits to their macrophage counterparts, and in the context of sepsis, controlling the infiltration of one type over the other may prove advantageous from a therapeutic standpoint. As previously stated, integrin VLA-3 is upregulated on a subpopulation of hyperinflammatory neutrophils during sepsis (26). VLA-3high neutrophils exhibit elevated MPO and proinflammatory cytokine production, lending to their antimicrobial properties but also to their infliction of tissue damage (26). How to best control the neutrophil response is still under active investigation.
Monocytes
Monocytes are another crucial cell that migrates to inflamed tissue sites during the first few hours of infection. During infection, monocytes further differentiate into macrophages. Monocytes and macrophages specialize in phagocytosis and the removal of pathogens, antigen presentation, and the production of cytokines and chemokines to facilitate communication with the adaptive immune system (86). Like neutrophils, monocyte recruitment and migration depend on chemotactic cues and adhesion to the vascular endothelium. Additionally, there are numerous subpopulations of monocytes that are broadly categorized as “classical” and “nonclassical” subtypes. The chemokine and fractalkine receptors CCR2 and CX3CR1, respectively, are commonly used to identify “inflammatory” (classical) vs “patrolling” (nonclassical) populations. Inflammatory monocytes are typically CCR2high Ly6C+ CX3CR1Low and are rapidly recruited to damaged and inflamed tissues, whereas patrolling monocytes are CCR2Low Ly6C+ CX3CR1High, allowing these cells to circulate and clear damaged cells to facilitate repair (87). During sepsis, CCR2high monocytes extravasate into tissues, where they secrete proinflammatory cytokines and growth factors and transport antigens to draining lymph nodes to activate the adaptive immune response (88). During infection, CX3CR1+ monocytes are found in circulation and localized to the marginal zone of the spleen, where their purpose is to phagocytose bacteria and recruit additional inflammatory monocytes (89).
To identify the function of monocytic chemokine receptors during sepsis, several studies have used cecal ligation and puncture (CLP) to investigate the role of monocyte trafficking. Andonegui et al. (44) revealed that targeting CCR2+ inflammatory monocytes during septic peritonitis led to significantly fewer infiltrating cells in the central nervous system (CNS) by 24 h post-infection. This finding was critical, as decreased monocyte recruitment to the brain correlated with fewer behavioral abnormalities in these animals at 9 weeks after infection (44). Furthermore, in the absence of nonclassical CX3CR1+ monocytes during CLP, mortality rates increased from 33% in wild-type to 75% in CX3CR1 knockout mice within 7 days of polymicrobial peritonitis when these patrolling monocytes were not present to clear bacteria (90). Thus, both classical and nonclassical monocyte recruitment is important for bacterial clearance and sepsis recovery. Furthermore, chemokine receptors such as CCR2 and CX3CR1 are only partially responsible for the process that facilitates migration into inflamed tissues.
Tissue-resident macrophages
An indispensable role of monocyte trafficking during sepsis is to deliver tissue-resident macrophages, which exist in a temporary or permanent state after differentiation. Macrophages received their name over 100 years ago, when Élie Metchnikoff coined the term “phagocytosis” (91). It is often thought that the main purpose of macrophages is to clear debris and dying cells from tissues, both during homeostasis and disease. Tissue-resident macrophages are critical innate immune sentinels that recognize pathogens via PRRs and signal to other cells. As a first line of defense, resident macrophages are considered “gate-keepers” of the tissues, where they rapidly respond to infection and initiate recruitment of circulating/inflammatory innate cells. Our knowledge of their diverse functions has since expanded, including their ability to crosstalk with other cells, secrete both pro- and anti-inflammatory cytokines, and even present antigens during pathogen invasion (92). During acute inflammation, macrophages serve as acute sensors of their environment, which is critical during the initiation of sepsis.
More recently, research devoted to exploring the ontogeny of tissue-resident macrophages has accelerated our understanding of their regulation and roles in peripheral tissues. Resident macrophages make up a diverse population with unique developmental origins. The majority of tissue-resident macrophages are derived from embryonic precursors and can be self-maintained throughout the lifetime of the host (93). The homeostatic support provided by these cells is achieved through tissue repair and crosstalk with other cells. Although tissue-resident macrophages are typically seeded in early development during embryogenesis, they may be replenished by bone marrow-derived monocytes during infection and inflammation (94). The contribution of adult myeloid progenitors to tissue-resident macrophages is context- and organ-dependent. In many different diseases, such as cancer, colitis, and sepsis, tissue-resident macrophages and their replenishment are required for host immunity (95,96,97,98). During disease progression, tissue-resident macrophages exhibit increased plasticity and adapt rapidly to changing microenvironments. Tissue specification determines macrophage function, highlighting the need for a focus on individual organ systems. Every organ contains its own form of sentinel macrophage, with considerable research concentrating on microglia in the brain, Kupffer cells in the liver, and macrophages in the peritoneal cavity.
Kupffer cells are the largest population of tissue-resident macrophages in the body, perhaps explaining the extensive research surrounding their homeostatic functions during inflammation (99). Kupffer cell activity changes based on the disease scenario, promoting tissue repair during drug-induced injury (100) but also contributing to chronic damage in the context of fatty-liver diseases (101). As is the case with most immune sentinels, Kupffer cells have both beneficial and harmful effects during sepsis. Some research shows deleterious consequences of Kupffer cells on the liver sinusoidal endothelial vasculature (102), while other studies show that these cells protect the endothelium (103). As is frequently the case during sepsis-related organ dysfunction, damage versus protection appears to be a fine balance that depends on timing and location.
Microglia are tissue-resident macrophages in the CNS and have a unique morphology and purpose throughout the lifetime of the host. Their role during brain development includes phagocytosing dead cells and debris while also promoting synapse formation and reorganization (104,105,106). While the brain is historically considered an immune-privileged area, microglia also serve as the interface between the nervous and peripheral immune systems. During sepsis, the BBB breaks down, and immune privilege is lost. At this time, microglia continue to provide support and feedback to local neurons but also become activated in the context of massive systemic inflammation. Overactivation of microglia exerts detrimental effects on neuronal structure, but much of this damage may be the direct result of peripheral immune cell infiltration. Microglial function during sepsis is a surprisingly understudied field, but new advances in understanding the robust response to infection in the brain are quickly growing (45,107).
SEPSIS RESOLUTION AND TISSUE REPAIR
Patient survival is highly dependent on the ability to overcome the dampened immune response and regain control and balance of both hyper- and anti-inflammatory modulation. The onset of tissue repair must start with the resolution of infection and clearance of microbial products (108). The resolution of inflammation is very complicated and involves more than just the termination of proinflammatory responses. Although pathogen invasion may be under control, the body must also limit the release of DAMPs from damaged cells (109). There are several complex subcellular processes that eliminate damage signals and promote wound healing and regeneration.
Clearance of molecular patterns that can further activate cells is essential in promoting inflammatory resolution. Eradication of PAMPs and DAMPs is facilitated by autophagy. Autophagy is an elegant and precise way for the cell to recycle and dispose of its own damaged organelles, residual pathogenic material, and cellular proteins (108,110). By decreasing the likelihood of further immune cell stimulation, autophagy is used globally throughout the body and relies heavily on subcellular checkpoints that decide whether a cell lives or dies. Recently, there has been evidence to support a link between autophagy and apoptosis. During sepsis, rapid influx and turnover of effector cells in tissues require both self-consumption and cell death. While the turnover and destruction of organelles within cells and cells within organisms are functionally distinct mechanisms (111), the theory that autophagy and apoptosis pathways share molecular signals is becoming widely accepted (112).
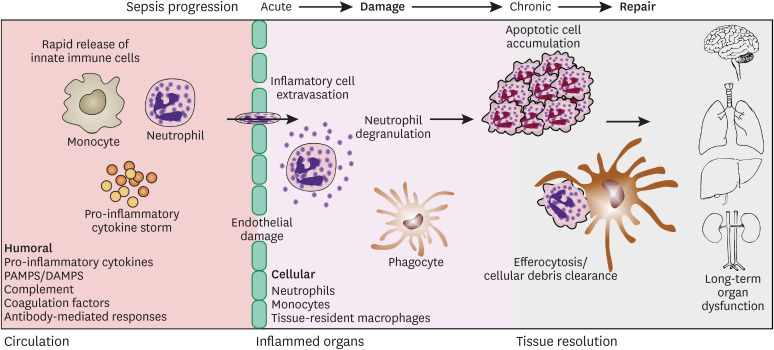
Apoptosis is a programmed cell death process that is executed in both endogenous and paracrine manners. During sepsis, effector cells are constantly turning over and require continuous clearance and repopulation in infected tissues. Apoptosis precedes many downstream effector functions, including direct signaling to phagocytes to commence efferocytosis. Efferocytosis is defined as phagocytosis of dying cells and is crucial during inflammatory resolution. The ability of phagocytic cells to engulf billions of cellular corpses during and after systemic inflammation is one of the hallmark features of successful recovery (Fig. 1) (113). Macrophages that engulf apoptotic cells contain metabolite-sensing equipment in the form of nuclear receptors that are directly involved in the regulation of inflammation (114). Several reports have demonstrated the anti-inflammatory properties of efferocytosis, including its most obvious role in sequestering and limiting the spread of DAMPs by dying cells, which would perpetuate inflammation (114). Furthermore, efferocytosis induces the production of anti-inflammatory cytokines such as IL-10 and TGF-β, which act on local microenvironments or within the phagocyte itself (115). The unique ability of efferocytosis to promote a resolution phenotype makes it one of the most crucial features of sepsis recovery.
Figure 1. Cartoon of working model of host immune responses from early sepsis progression to the chronic phase after resolution of inflammation.
CHALLENGES IN RECOVERY
Although acute management of sepsis accounts for a significant clinical and financial burden to society, recovery after sepsis is delayed and often incomplete (Fig. 1). These patients often experience post-intensive care syndrome (PICS), defined as “new or worsening problems in physical, cognitive, or mental health status arising after a critical illness and persisting beyond acute care hospitalization” (116). An increasing number of clinics have been established to evaluate and treat these patients (117). A number of landmark epidemiologic studies have clearly documented deficits in physical, mental, and cognitive health in survivors of critically ill patients with sepsis (118,119,120), but the underlying pathophysiology of PICS is not well understood.
Prolonged organ dysfunction
Long-term organ dysfunction after sepsis is poorly understood, but the acute inflammatory process likely plays a role. The potential link between acute illness/inflammation and the occurrence of persistent vital organ dysfunction is exemplified by the cognitive deficits of PICS. During acute illness, 3 quarters of critically ill patients experience sepsis-associated delirium, a symptom that develops during systemic inflammation and ranges from confusion to coma (120). In turn, the duration of delirium during acute illness is correlated with the severity of persistent cognitive dysfunction as measured a full year after hospital discharge (120). In a large cohort investigation performed within the Health and Retirement Study, functional disability and cognitive impairment were examined in patients before and after a sepsis hospitalization. This study was able to compare patients to their own baselines before sepsis and indicated marked cognitive decline that remained even 3 years after recovery from acute illness (119). The study found that many survivors had difficulties in everyday tasks such as grocery shopping, managing money, or using the telephone.
Although there is extensive research devoted to understanding the mechanisms and prevention of brain injury during sepsis, very little is currently known. One theory involves brain microglial cells. Microglia are immune sentinels that are unique in their ability to self-renew and/or survive throughout the lifetime of the host. With sepsis leading to permanent defects in multiple organ systems such as the brain, a role for long-lived tissue-resident macrophages in prolonged immunity and inflammation is a hypothesized target in research on long-term cognitive impairment. To date, multiple theories regarding the role of microglia during sepsis-induced neuroinflammation have been identified. Infiltration of peripheral immune cells undoubtedly influences brain-resident macrophages, both through direct and indirect contacts (45,121,122). Current research exploring the relationships between infiltrating and sentinel immune cells during inflammation is rapidly growing.
Tolerized immunity
Sepsis survivors have an increased incidence of rehospitalization after discharge, and these readmissions are frequently infection-related (123,124,125). The concept of cellular reprogramming is new in the immunology field but is quickly being accepted as an important component of immune system rechallenge. Patients who are discharged from hospitals after sepsis often experience persistent low-grade inflammation and increased immunosuppression, in part due to elevated levels of the anti-inflammatory cytokines IL-10, PD-L1, and IL-7, which are found up to one year after recovery (126). Evidence of protracted immune disorders begs the question of the possibility of tolerized immunity.
Several reports have examined the potential for innate immune cells to retain memory (127,128,129). While it is a widely accepted aspect of the adaptive immune system, memory in the innate immune system is more controversial. Understanding cellular reprogramming has opened up the field considerably, with current literature highlighting the remodeling of epigenetic landscapes as a way for cells to incur permanent genetic changes (130). A popular example of this phenomenon arises in endotoxin tolerance, which can be achieved by TLR-induced chromatin modification (131). LPS-induced tolerance has also been observed in microglia, where primary exposure to TLR4 stimulation directly influences future responses to endotoxin (132). Enhancement of microglial proinflammatory reactions to secondary exposure has also been linked to numerous neurodegenerative diseases, including Alzheimer's disease, stroke, and Parkinson's disease (132). The concept of innate immune cell memory could have significant impact on our ability to treat sepsis, particularly its longer-term adverse health consequences.
DISCUSSION
There have been large investments devoted to clinical investigations manipulating both the hyperinflammatory and immunosuppressive states of sepsis. Unfortunately, most therapeutic treatments have failed in clinical trials, a harsh reality reflected in the number of sepsis-related deaths each year. Sepsis remains one of the most fatal diseases worldwide.
The ever-expanding networks and connections between both proinflammatory and anti-inflammatory mediators in sepsis provide an unparalleled level of complexity. The interplay between the innate immune system, tissue-resident macrophages, adaptive immunosuppression, and abundant circulating humoral factors leave myriad possibilities for investigation. The acute phase of sepsis requires further understanding to effectively dampen early and excessive host responses. However, the later stages of sepsis deserve special focus, as cellular and metabolic reprogramming probably exert important roles in the longer survival and health of acute sepsis survivors. The search for new and effective therapeutic innovation is complex and successes will require coordinated multidisciplinary efforts by a diverse array of biomedical researchers. We are hopeful that collaborative research and technological advances will aid in defining new diagnostic and prognostic biomarkers and yield effective therapeutic advances.
ACKNOWLEDGEMENTS
This research is funded by grants from the NIH (R01HL147525, M.K.).
Abbreviations
- BBB
blood brain barrier
- CCR2
C-C chemokine receptor type 2
- CLP
cecal ligation and puncture
- CNS
central nervous system
- CRP
C-reactive protein
- DAMP
damage-associated molecular pattern
- MAC
membrane attack complex
- MMP
matrix metalloprotease
- MPO
myeloperoxidase
- NLR
Nod-like receptor
- PAMP
pathogen-associated molecular pattern
- PCT
procalcitonin
- PICS
post-intensive care syndrome
- PRR
pattern recognition receptor
- SOFA
sequential sepsis-related organ failure assessment
- TF
transcription factor
- VLA-3
very late antigen-3
- WBC
white blood cell
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
- Conceptualization: Trzeciak A, Pietropaoli AP, Kim M.
- Funding acquisition: Kim M.
- Project administration: Kim M.
- Supervision: Kim M.
- Writing - original draft: Trzeciak A, Pietropaoli AP, Kim M.
- Writing - review & editing: Trzeciak A, Pietropaoli AP, Kim M.
References
- 1.Rhee C, Dantes R, Epstein L, Murphy DJ, Seymour CW, Iwashyna TJ, Kadri SS, Angus DC, Danner RL, Fiore AE, et al. Incidence and trends of sepsis in us hospitals using clinical vs claims data, 2009–2014. JAMA. 2017;318:1241–1249. doi: 10.1001/jama.2017.13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer S, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395:200–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 4.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeganathan N, Yau S, Ahuja N, Otu D, Stein B, Fogg L, Balk R. The characteristics and impact of source of infection on sepsis-related ICU outcomes. J Crit Care. 2017;41:170–176. doi: 10.1016/j.jcrc.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 6.Sinha M, Jupe J, Mack H, Coleman TP, Lawrence SM, Fraley SI. Emerging technologies for molecular diagnosis of sepsis. Clin Microbiol Rev. 2018;31:e00089-17. doi: 10.1128/CMR.00089-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller RR, 3rd, Lopansri BK, Burke JP, Levy M, Opal S, Rothman RE, D'Alessio FR, Sidhaye VK, Aggarwal NR, Balk R, et al. Validation of a host response assay, septicyte lab, for discriminating sepsis from systemic inflammatory response syndrome in the ICU. Am J Respir Crit Care Med. 2018;198:903–913. doi: 10.1164/rccm.201712-2472OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rhee C, Kadri SS, Danner RL, Suffredini AF, Massaro AF, Kitch BT, Lee G, Klompas M. Diagnosing sepsis is subjective and highly variable: a survey of intensivists using case vignettes. Crit Care. 2016;20:89. doi: 10.1186/s13054-016-1266-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seymour CW, Gesten F, Prescott HC, Friedrich ME, Iwashyna TJ, Phillips GS, Lemeshow S, Osborn T, Terry KM, Levy MM. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med. 2017;376:2235–2244. doi: 10.1056/NEJMoa1703058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kahn JM, Davis BS, Yabes JG, Chang CH, Chong DH, Hershey TB, Martsolf GR, Angus DC. Association between state-mandated protocolized sepsis care and in-hospital mortality among adults with sepsis. JAMA. 2019;322:240–250. doi: 10.1001/jama.2019.9021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med. 2017;45:486–552. doi: 10.1097/CCM.0000000000002255. [DOI] [PubMed] [Google Scholar]
- 12.Angus DC. The search for effective therapy for sepsis: back to the drawing board? JAMA. 2011;306:2614–2615. doi: 10.1001/jama.2011.1853. [DOI] [PubMed] [Google Scholar]
- 13.Liu VX, Fielding-Singh V, Greene JD, Baker JM, Iwashyna TJ, Bhattacharya J, Escobar GJ. The timing of early antibiotics and hospital mortality in sepsis. Am J Respir Crit Care Med. 2017;196:856–863. doi: 10.1164/rccm.201609-1848OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 15.Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A, Rubenfeld G, Kahn JM, Shankar-Hari M, Singer M, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315:762–774. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho SY, Choi JH. Biomarkers of sepsis. Infect Chemother. 2014;46:1–12. doi: 10.3947/ic.2014.46.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henriquez-Camacho C, Losa J. Biomarkers for sepsis. BioMed Res Int. 2014;2014:547818. doi: 10.1155/2014/547818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anush MM, Ashok VK, Sarma RI, Pillai SK. Role of C-reactive protein as an indicator for determining the outcome of sepsis. Indian J Crit Care Med. 2019;23:11–14. doi: 10.5005/jp-journals-10071-23105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mustafić S, Brkić S, Prnjavorac B, Sinanović A, Porobić Jahić H, Salkić S. Diagnostic and prognostic value of procalcitonin in patients with sepsis. Med Glas (Zenica) 2018;15:93–100. doi: 10.17392/963-18. [DOI] [PubMed] [Google Scholar]
- 20.Yunus I, Fasih A, Wang Y. The use of procalcitonin in the determination of severity of sepsis, patient outcomes and infection characteristics. PLoS One. 2018;13:e0206527. doi: 10.1371/journal.pone.0206527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szederjesi J, Almasy E, Lazar A, Huțanu A, Badea I, Georgescu A. An evaluation of serum procalcitonin and c-reactive protein levels as diagnostic and prognostic biomarkers of severe sepsis. J Crit Care Med (Targu Mures) 2015;1:147–153. doi: 10.1515/jccm-2015-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chousterman BG, Boissonnas A, Poupel L, Baudesson de Chanville C, Adam J, Tabibzadeh N, Licata F, Lukaszewicz AC, Lombès A, Deterre P, et al. Ly6chigh monocytes protect against kidney damage during sepsis via a CX3CR1-dependent adhesion mechanism. J Am Soc Nephrol. 2016;27:792–803. doi: 10.1681/ASN.2015010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiu F, Stanojcic M, Wang V, Qi P, Jeschke MG. C-C chemokine receptor type 2 expression on monocytes before sepsis onset is higher than that of postsepsis in septic burned patients: a new predictor for sepsis in burned injury. Ann Surg. 2016;264:392–398. doi: 10.1097/SLA.0000000000001531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nemeth ZH, Bilaniuk JW, Di Fazio LT, Paglinco SR, Rolandelli RH. Chemokine receptor CCR2-expressing inflammatory monocytes contribute to the exacerbated inflammatory response associated with sepsis. J Am Coll Surg. 2015;221:S40–S41. [Google Scholar]
- 25.Pachot A, Cazalis MA, Venet F, Turrel F, Faudot C, Voirin N, Diasparra J, Bourgoin N, Poitevin F, Mougin B, et al. Decreased expression of the fractalkine receptor CX3CR1 on circulating monocytes as new feature of sepsis-induced immunosuppression. J Immunol. 2008;180:6421–6429. doi: 10.4049/jimmunol.180.9.6421. [DOI] [PubMed] [Google Scholar]
- 26.Lerman YV, Lim K, Hyun YM, Falkner KL, Yang H, Pietropaoli AP, Sonnenberg A, Sarangi PP, Kim M. Sepsis lethality via exacerbated tissue infiltration and TLR-induced cytokine production by neutrophils is integrin α3β1-dependent. Blood. 2014;124:3515–3523. doi: 10.1182/blood-2014-01-552943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jämsä J, Ala-Kokko T, Huotari V, Ohtonen P, Savolainen ER, Syrjälä H. Neutrophil CD64, C-reactive protein, and procalcitonin in the identification of sepsis in the ICU - Post-test probabilities. J Crit Care. 2018;43:139–142. doi: 10.1016/j.jcrc.2017.08.038. [DOI] [PubMed] [Google Scholar]
- 28.Mahmoodpoor A, Paknezhad S, Shadvar K, Hamishehkar H, Movassaghpour AA, Sanaie S, Ghamari AA, Soleimanpour H. Flow cytometry of CD64, HLA-DR, CD25, and TLRs for diagnosis and prognosis of sepsis in critically ill patients admitted to the intensive care unit: a review article. Anesth Pain Med. 2018;8:e83128. doi: 10.5812/aapm.83128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faix JD. Biomarkers of sepsis. Crit Rev Clin Lab Sci. 2013;50:23–36. doi: 10.3109/10408363.2013.764490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moran JL, Santamaria J. Reconsidering lactate as a sepsis risk biomarker. PLoS One. 2017;12:e0185320. doi: 10.1371/journal.pone.0185320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang HE, Park DW. Lactate as a biomarker for sepsis prognosis? Infect Chemother. 2016;48:252–253. doi: 10.3947/ic.2016.48.3.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ganesh K, Sharma RN, Varghese J, Pillai MG. A profile of metabolic acidosis in patients with sepsis in an Intensive Care Unit setting. Int J Crit Illn Inj Sci. 2016;6:178–181. doi: 10.4103/2229-5151.195417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Z, Zhu C, Mo L, Hong Y. Effectiveness of sodium bicarbonate infusion on mortality in septic patients with metabolic acidosis. Intensive Care Med. 2018;44:1888–1895. doi: 10.1007/s00134-018-5379-2. [DOI] [PubMed] [Google Scholar]
- 34.Póvoa P, Almeida E, Moreira P, Fernandes A, Mealha R, Aragão A, Sabino H. C-reactive protein as an indicator of sepsis. Intensive Care Med. 1998;24:1052–1056. doi: 10.1007/s001340050715. [DOI] [PubMed] [Google Scholar]
- 35.Wang HE, Shapiro NI, Safford MM, Griffin R, Judd S, Rodgers JB, Warnock DG, Cushman M, Howard G. High-sensitivity C-reactive protein and risk of sepsis. PLoS One. 2013;8:e69232. doi: 10.1371/journal.pone.0069232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yeh CF, Wu CC, Liu SH, Chen KF. Comparison of the accuracy of neutrophil CD64, procalcitonin, and C-reactive protein for sepsis identification: a systematic review and meta-analysis. Ann Intensive Care. 2019;9:5. doi: 10.1186/s13613-018-0479-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nargis W, Ibrahim M, Ahamed BU. Procalcitonin versus C-reactive protein: usefulness as biomarker of sepsis in ICU patient. Int J Crit Illn Inj Sci. 2014;4:195–199. doi: 10.4103/2229-5151.141356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang H, Ward MF, Sama AE. Targeting HMGB1 in the treatment of sepsis. Expert Opin Ther Targets. 2014;18:257–268. doi: 10.1517/14728222.2014.863876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stevens NE, Chapman MJ, Fraser CK, Kuchel TR, Hayball JD, Diener KR. Therapeutic targeting of HMGB1 during experimental sepsis modulates the inflammatory cytokine profile to one associated with improved clinical outcomes. Sci Rep. 2017;7:5850. doi: 10.1038/s41598-017-06205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X, Li ZY, Zeng L, Zhang AQ, Pan W, Gu W, Jiang JX. Neutrophil CD64 expression as a diagnostic marker for sepsis in adult patients: a meta-analysis. Crit Care. 2015;19:245. doi: 10.1186/s13054-015-0972-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lerman YV, Kim M. Neutrophil migration under normal and sepsis conditions. Cardiovasc Hematol Disord Drug Targets. 2015;15:19–28. doi: 10.2174/1871529x15666150108113236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sarangi PP, Hyun YM, Lerman YV, Pietropaoli AP, Kim M. Role of β1 integrin in tissue homing of neutrophils during sepsis. Shock. 2012;38:281–287. doi: 10.1097/SHK.0b013e31826136f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ngo LY, Kasahara S, Kumasaka DK, Knoblaugh SE, Jhingran A, Hohl TM. Inflammatory monocytes mediate early and organ-specific innate defense during systemic candidiasis. J Infect Dis. 2014;209:109–119. doi: 10.1093/infdis/jit413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andonegui G, Zelinski EL, Schubert CL, Knight D, Craig LA, Winston BW, Spanswick SC, Petri B, Jenne CN, Sutherland JC, et al. Targeting inflammatory monocytes in sepsis-associated encephalopathy and long-term cognitive impairment. JCI Insight. 2018;3:e99364. doi: 10.1172/jci.insight.99364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trzeciak A, Lerman YV, Kim TH, Kim MR, Mai N, Halterman MW, Kim M. Long-term microgliosis driven by acute systemic inflammation. J Immunol. 2019;203:2979–2989. doi: 10.4049/jimmunol.1900317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee M, Lee Y, Song J, Lee J, Chang SY. Tissue-specific role of CX3CR1 expressing immune cells and their relationships with human disease. Immune Netw. 2018;18:e5. doi: 10.4110/in.2018.18.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Friggeri A, Cazalis MA, Pachot A, Cour M, Argaud L, Allaouchiche B, Floccard B, Schmitt Z, Martin O, Rimmelé T, et al. MIP Rea Study Group. Decreased CX3CR1 messenger RNA expression is an independent molecular biomarker of early and late mortality in critically ill patients. Crit Care. 2016;20:204. doi: 10.1186/s13054-016-1362-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ancuta P, Rao R, Moses A, Mehle A, Shaw SK, Luscinskas FW, Gabuzda D. Fractalkine preferentially mediates arrest and migration of CD16+ monocytes. J Exp Med. 2003;197:1701–1707. doi: 10.1084/jem.20022156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tan RS, Ho B, Leung BP, Ding JL. TLR cross-talk confers specificity to innate immunity. Int Rev Immunol. 2014;33:443–453. doi: 10.3109/08830185.2014.921164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kawasaki T, Kawai T. Toll-like receptor signaling pathways. Front Immunol. 2014;5:461. doi: 10.3389/fimmu.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kanamaru T, Kamimura N, Yokota T, Nishimaki K, Iuchi K, Lee H, Takami S, Akashiba H, Shitaka Y, Ueda M, et al. Intravenous transplantation of bone marrow-derived mononuclear cells prevents memory impairment in transgenic mouse models of Alzheimer's disease. Brain Res. 2015;1605:49–58. doi: 10.1016/j.brainres.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 52.Yarovinsky F, Zhang D, Andersen JF, Bannenberg GL, Serhan CN, Hayden MS, Hieny S, Sutterwala FS, Flavell RA, Ghosh S, et al. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science. 2005;308:1626–1629. doi: 10.1126/science.1109893. [DOI] [PubMed] [Google Scholar]
- 53.Matta BM, Reichenbach DK, Blazar BR, Turnquist HR. Alarmins and their receptors as modulators and indicators of alloimmune responses. Am J Transplant. 2017;17:320–327. doi: 10.1111/ajt.13887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim YK, Shin JS, Nahm MH. Nod-like receptors in infection, immunity, and diseases. Yonsei Med J. 2016;57:5–14. doi: 10.3349/ymj.2016.57.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.von Kügelgen I, Hoffmann K. Pharmacology and structure of P2Y receptors. Neuropharmacology. 2016;104:50–61. doi: 10.1016/j.neuropharm.2015.10.030. [DOI] [PubMed] [Google Scholar]
- 56.Roh JS, Sohn DH. Damage-associated molecular patterns in inflammatory diseases. Immune Netw. 2018;18:e27. doi: 10.4110/in.2018.18.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tidswell M, Tillis W, Larosa SP, Lynn M, Wittek AE, Kao R, Wheeler J, Gogate J, Opal SM Eritoran Sepsis Study Group. Phase 2 trial of eritoran tetrasodium (E5564), a Toll-like receptor 4 antagonist, in patients with severe sepsis. Crit Care Med. 2010;38:72–83. doi: 10.1097/CCM.0b013e3181b07b78. [DOI] [PubMed] [Google Scholar]
- 58.Rice TW, Wheeler AP, Bernard GR, Vincent JL, Angus DC, Aikawa N, Demeyer I, Sainati S, Amlot N, Cao C, et al. A randomized, double-blind, placebo-controlled trial of TAK-242 for the treatment of severe sepsis. Crit Care Med. 2010;38:1685–1694. doi: 10.1097/CCM.0b013e3181e7c5c9. [DOI] [PubMed] [Google Scholar]
- 59.Savva A, Roger T. Targeting Toll-like receptors: promising therapeutic strategies for the management of sepsis-associated pathology and infectious diseases. Front Immunol. 2013;4:387. doi: 10.3389/fimmu.2013.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. 2009;22:240–273. doi: 10.1128/CMR.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yanai H, Negishi H, Taniguchi T. The IRF family of transcription factors: Inception, impact and implications in oncogenesis. OncoImmunology. 2012;1:1376–1386. doi: 10.4161/onci.22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nagar A, DeMarco RA, Harton JA. Inflammasome and caspase-1 activity characterization and evaluation: an imaging flow cytometer-based detection and assessment of inflammasome specks and caspase-1 activation. J Immunol. 2019;202:1003–1015. doi: 10.4049/jimmunol.1800973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nilsberth C, Elander L, Hamzic N, Norell M, Lönn J, Engström L, Blomqvist A. The role of interleukin-6 in lipopolysaccharide-induced fever by mechanisms independent of prostaglandin E2. Endocrinology. 2009;150:1850–1860. doi: 10.1210/en.2008-0806. [DOI] [PubMed] [Google Scholar]
- 64.Aderka D. Role of tumor necrosis factor in the pathogenesis of intravascular coagulopathy of sepsis: potential new therapeutic implications. Isr J Med Sci. 1991;27:52–60. [PubMed] [Google Scholar]
- 65.Zeni F, Freeman B, Natanson C. Anti-inflammatory therapies to treat sepsis and septic shock: a reassessment. Crit Care Med. 1997;25:1095–1100. doi: 10.1097/00003246-199707000-00001. [DOI] [PubMed] [Google Scholar]
- 66.Cohen J, Carlet J. INTERSEPT: an international, multicenter, placebo-controlled trial of monoclonal antibody to human tumor necrosis factor-alpha in patients with sepsis. Crit Care Med. 1996;24:1431–1440. doi: 10.1097/00003246-199609000-00002. [DOI] [PubMed] [Google Scholar]
- 67.Polk HC, Jr, Cheadle WG, Livingston DH, Rodriguez JL, Starko KM, Izu AE, Jaffe HS, Sonnenfeld G. A randomized prospective clinical trial to determine the efficacy of interferon-gamma in severely injured patients. Am J Surg. 1992;163:191–196. doi: 10.1016/0002-9610(92)90099-d. [DOI] [PubMed] [Google Scholar]
- 68.Mastellos DC, Ricklin D, Lambris JD. Clinical promise of next-generation complement therapeutics. Nat Rev Drug Discov. 2019;18:707–729. doi: 10.1038/s41573-019-0031-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ricklin D, Reis ES, Lambris JD. Complement in disease: a defence system turning offensive. Nat Rev Nephrol. 2016;12:383–401. doi: 10.1038/nrneph.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Markiewski MM, DeAngelis RA, Lambris JD. Complexity of complement activation in sepsis. J Cell Mol Med. 2008;12:2245–2254. doi: 10.1111/j.1582-4934.2008.00504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ram S, Lewis LA, Rice PA. Infections of people with complement deficiencies and patients who have undergone splenectomy. Clin Microbiol Rev. 2010;23:740–780. doi: 10.1128/CMR.00048-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ricklin D, Lambris JD. New milestones ahead in complement-targeted therapy. Semin Immunol. 2016;28:208–222. doi: 10.1016/j.smim.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Simmons J, Pittet JF. The coagulopathy of acute sepsis. Curr Opin Anaesthesiol. 2015;28:227–236. doi: 10.1097/ACO.0000000000000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. 2013;13:34–45. doi: 10.1038/nri3345. [DOI] [PubMed] [Google Scholar]
- 75.Fiusa MM, Carvalho-Filho MA, Annichino-Bizzacchi JM, De Paula EV. Causes and consequences of coagulation activation in sepsis: an evolutionary medicine perspective. BMC Med. 2015;13:105. doi: 10.1186/s12916-015-0327-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Camicia G, Pozner R, de Larrañaga G. Neutrophil extracellular traps in sepsis. Shock. 2014;42:286–294. doi: 10.1097/SHK.0000000000000221. [DOI] [PubMed] [Google Scholar]
- 77.Iba T, Nagaoka I, Boulat M. The anticoagulant therapy for sepsis-associated disseminated intravascular coagulation. Thromb Res. 2013;131:383–389. doi: 10.1016/j.thromres.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 78.Iba T, Saito D, Wada H, Asakura H. Efficacy and bleeding risk of antithrombin supplementation in septic disseminated intravascular coagulation: a prospective multicenter survey. Thromb Res. 2012;130:e129–e133. doi: 10.1016/j.thromres.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 79.Vincent JL, Francois B, Zabolotskikh I, Daga MK, Lascarrou JB, Kirov MY, Pettilä V, Wittebole X, Meziani F, Mercier E, et al. Effect of a recombinant human soluble thrombomodulin on mortality in patients with sepsis-associated coagulopathy: the scarlet randomized clinical trial. JAMA. 2019;321:1993–2002. doi: 10.1001/jama.2019.5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 81.Soehnlein O. Direct and alternative antimicrobial mechanisms of neutrophil-derived granule proteins. J Mol Med (Berl) 2009;87:1157–1164. doi: 10.1007/s00109-009-0508-6. [DOI] [PubMed] [Google Scholar]
- 82.Lakhan SE, Kirchgessner A, Tepper D, Leonard A. Matrix metalloproteinases and blood-brain barrier disruption in acute ischemic stroke. Front Neurol. 2013;4:32. doi: 10.3389/fneur.2013.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chou SH, Feske SK, Simmons SL, Konigsberg RG, Orzell SC, Marckmann A, Bourget G, Bauer DJ, De Jager PL, Du R, et al. Elevated peripheral neutrophils and matrix metalloproteinase 9 as biomarkers of functional outcome following subarachnoid hemorrhage. Transl Stroke Res. 2011;2:600–607. doi: 10.1007/s12975-011-0117-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shaul ME, Levy L, Sun J, Mishalian I, Singhal S, Kapoor V, Horng W, Fridlender G, Albelda SM, Fridlender ZG. Tumor-associated neutrophils display a distinct N1 profile following TGFβ modulation: a transcriptomics analysis of pro- vs. antitumor TANs. OncoImmunology. 2016;5:e1232221. doi: 10.1080/2162402X.2016.1232221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ferreira da Mota NV, Brunialti MK, Santos SS, Machado FR, Assuncao M, Azevedo LC, Salomao R. Immunophenotyping of monocytes during human sepsis shows impairment in antigen presentation: a shift toward nonclassical differentiation and upregulation of FCγRi-receptor. Shock. 2018;50:293–300. doi: 10.1097/SHK.0000000000001078. [DOI] [PubMed] [Google Scholar]
- 87.Gasco S, Zaragoza P, García-Redondo A, Calvo AC, Osta R. Inflammatory and non-inflammatory monocytes as novel prognostic biomarkers of survival in SOD1G93A mouse model of Amyotrophic Lateral Sclerosis. PLoS One. 2017;12:e0184626. doi: 10.1371/journal.pone.0184626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Burgess M, Wicks K, Gardasevic M, Mace KA. CX3CR1 expression identifies distinct macrophage populations that contribute differentially to inflammation and repair. Immunohorizons. 2019;3:262–273. doi: 10.4049/immunohorizons.1900038. [DOI] [PubMed] [Google Scholar]
- 90.Ishida Y, Hayashi T, Goto T, Kimura A, Akimoto S, Mukaida N, Kondo T. Essential involvement of CX3CR1-mediated signals in the bactericidal host defense during septic peritonitis. J Immunol. 2008;181:4208–4218. doi: 10.4049/jimmunol.181.6.4208. [DOI] [PubMed] [Google Scholar]
- 91.Nathan C. Metchnikoff's legacy in 2008. Nat Immunol. 2008;9:695–698. doi: 10.1038/ni0708-695. [DOI] [PubMed] [Google Scholar]
- 92.Kierdorf K, Prinz M, Geissmann F, Gomez Perdiguero E. Development and function of tissue resident macrophages in mice. Semin Immunol. 2015;27:369–378. doi: 10.1016/j.smim.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lavin Y, Mortha A, Rahman A, Merad M. Regulation of macrophage development and function in peripheral tissues. Nat Rev Immunol. 2015;15:731–744. doi: 10.1038/nri3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity. 2014;41:21–35. doi: 10.1016/j.immuni.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shaw TN, Houston SA, Wemyss K, Bridgeman HM, Barbera TA, Zangerle-Murray T, Strangward P, Ridley AJ, Wang P, Tamoutounour S, et al. Tissue-resident macrophages in the intestine are long lived and defined by TIM-4 and CD4 expression. J Exp Med. 2018;215:1507–1518. doi: 10.1084/jem.20180019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhao Y, Zou W, Du J, Zhao Y. The origins and homeostasis of monocytes and tissue-resident macrophages in physiological situation. J Cell Physiol. 2018;233:6425–6439. doi: 10.1002/jcp.26461. [DOI] [PubMed] [Google Scholar]
- 97.Loyher PL, Hamon P, Laviron M, Meghraoui-Kheddar A, Goncalves E, Deng Z, Torstensson S, Bercovici N, Baudesson de Chanville C, Combadière B, et al. Macrophages of distinct origins contribute to tumor development in the lung. J Exp Med. 2018;215:2536–2553. doi: 10.1084/jem.20180534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Poh AR, Ernst M. Targeting macrophages in cancer: from bench to bedside. Front Oncol. 2018;8:49. doi: 10.3389/fonc.2018.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dixon LJ, Barnes M, Tang H, Pritchard MT, Nagy LE. Kupffer cells in the liver. Compr Physiol. 2013;3:785–797. doi: 10.1002/cphy.c120026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ju C, Reilly TP, Bourdi M, Radonovich MF, Brady JN, George JW, Pohl LR. Protective role of Kupffer cells in acetaminophen-induced hepatic injury in mice. Chem Res Toxicol. 2002;15:1504–1513. doi: 10.1021/tx0255976. [DOI] [PubMed] [Google Scholar]
- 101.Nagy LE. Recent insights into the role of the innate immune system in the development of alcoholic liver disease. Exp Biol Med (Maywood) 2003;228:882–890. doi: 10.1177/153537020322800803. [DOI] [PubMed] [Google Scholar]
- 102.Hutchins NA, Wang F, Wang Y, Chung CS, Ayala A. Kupffer cells potentiate liver sinusoidal endothelial cell injury in sepsis by ligating programmed cell death ligand-1. J Leukoc Biol. 2013;94:963–970. doi: 10.1189/jlb.0113051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hutchins NA, Chung CS, Borgerding JN, Ayala CA, Ayala A. Kupffer cells protect liver sinusoidal endothelial cells from Fas-dependent apoptosis in sepsis by down-regulating gp130. Am J Pathol. 2013;182:742–754. doi: 10.1016/j.ajpath.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Weinhard L, di Bartolomei G, Bolasco G, Machado P, Schieber NL, Neniskyte U, Exiga M, Vadisiute A, Raggioli A, Schertel A, et al. Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction. Nat Commun. 2018;9:1228. doi: 10.1038/s41467-018-03566-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Miyamoto A, Wake H, Ishikawa AW, Eto K, Shibata K, Murakoshi H, Koizumi S, Moorhouse AJ, Yoshimura Y, Nabekura J. Microglia contact induces synapse formation in developing somatosensory cortex. Nat Commun. 2016;7:12540. doi: 10.1038/ncomms12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Thion MS, Ginhoux F, Garel S. Microglia and early brain development: an intimate journey. Science. 2018;362:185–189. doi: 10.1126/science.aat0474. [DOI] [PubMed] [Google Scholar]
- 107.Russo MV, McGavern DB. Immune surveillance of the CNS following infection and injury. Trends Immunol. 2015;36:637–650. doi: 10.1016/j.it.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hotchkiss RS, Coopersmith CM, McDunn JE, Ferguson TA. The sepsis seesaw: tilting toward immunosuppression. Nat Med. 2009;15:496–497. doi: 10.1038/nm0509-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Delano MJ, Ward PA. The immune system's role in sepsis progression, resolution, and long-term outcome. Immunol Rev. 2016;274:330–353. doi: 10.1111/imr.12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fullerton JN, Gilroy DW. Resolution of inflammation: a new therapeutic frontier. Nat Rev Drug Discov. 2016;15:551–567. doi: 10.1038/nrd.2016.39. [DOI] [PubMed] [Google Scholar]
- 111.Mariño G, Niso-Santano M, Baehrecke EH, Kroemer G. Self-consumption: the interplay of autophagy and apoptosis. Nat Rev Mol Cell Biol. 2014;15:81–94. doi: 10.1038/nrm3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gump JM, Thorburn A. Autophagy and apoptosis: what is the connection? Trends Cell Biol. 2011;21:387–392. doi: 10.1016/j.tcb.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Green DR, Oguin TH, Martinez J. The clearance of dying cells: table for two. Cell Death Differ. 2016;23:915–926. doi: 10.1038/cdd.2015.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Elliott MR, Koster KM, Murphy PS. Efferocytosis signaling in the regulation of macrophage inflammatory responses. J Immunol. 2017;198:1387–1394. doi: 10.4049/jimmunol.1601520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Korns D, Frasch SC, Fernandez-Boyanapalli R, Henson PM, Bratton DL. Modulation of macrophage efferocytosis in inflammation. Front Immunol. 2011;2:57. doi: 10.3389/fimmu.2011.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Needham DM, Davidson J, Cohen H, Hopkins RO, Weinert C, Wunsch H, Zawistowski C, Bemis-Dougherty A, Berney SC, Bienvenu OJ, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders' conference. Crit Care Med. 2012;40:502–509. doi: 10.1097/CCM.0b013e318232da75. [DOI] [PubMed] [Google Scholar]
- 117.Mehlhorn J, Freytag A, Schmidt K, Brunkhorst FM, Graf J, Troitzsch U, Schlattmann P, Wensing M, Gensichen J. Rehabilitation interventions for postintensive care syndrome: a systematic review. Crit Care Med. 2014;42:1263–1271. doi: 10.1097/CCM.0000000000000148. [DOI] [PubMed] [Google Scholar]
- 118.Huang M, Parker AM, Bienvenu OJ, Dinglas VD, Colantuoni E, Hopkins RO, Needham DM, National Institutes of Health, National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Network Psychiatric symptoms in acute respiratory distress syndrome survivors: a 1-year national multicenter study. Crit Care Med. 2016;44:954–965. doi: 10.1097/CCM.0000000000001621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, Brummel NE, Hughes CG, Vasilevskis EE, Shintani AK, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369:1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Greenhalgh AD, Zarruk JG, Healy LM, Baskar Jesudasan SJ, Jhelum P, Salmon CK, Formanek A, Russo MV, Antel JP, McGavern DB, et al. Peripherally derived macrophages modulate microglial function to reduce inflammation after CNS injury. PLoS Biol. 2018;16:e2005264. doi: 10.1371/journal.pbio.2005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Getts DR, Terry RL, Getts MT, Müller M, Rana S, Shrestha B, Radford J, Van Rooijen N, Campbell IL, King NJ. Ly6c+ “inflammatory monocytes” are microglial precursors recruited in a pathogenic manner in West Nile virus encephalitis. J Exp Med. 2008;205:2319–2337. doi: 10.1084/jem.20080421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shankar-Hari M, Rubenfeld GD. Understanding long-term outcomes following sepsis: implications and challenges. Curr Infect Dis Rep. 2016;18:37. doi: 10.1007/s11908-016-0544-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sun A, Netzer G, Small DS, Hanish A, Fuchs BD, Gaieski DF, Mikkelsen ME. Association between index hospitalization and hospital readmission in sepsis survivors. Crit Care Med. 2016;44:478–487. doi: 10.1097/CCM.0000000000001464. [DOI] [PubMed] [Google Scholar]
- 125.Jones TK, Fuchs BD, Small DS, Halpern SD, Hanish A, Umscheid CA, Baillie CA, Kerlin MP, Gaieski DF, Mikkelsen ME. Post-acute care use and hospital readmission after sepsis. Ann Am Thorac Soc. 2015;12:904–913. doi: 10.1513/AnnalsATS.201411-504OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Riché F, Chousterman BG, Valleur P, Mebazaa A, Launay JM, Gayat E. Protracted immune disorders at one year after ICU discharge in patients with septic shock. Crit Care. 2018;22:42. doi: 10.1186/s13054-017-1934-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chan LC, Chaili S, Filler SG, Miller LS, Solis NV, Wang H, Johnson CW, Lee HK, Diaz LF, Yeaman MR. Innate immune memory contributes to host defense against recurrent skin and skin structure infections caused by methicillin-resistant staphylococcus aureus. Infect Immun. 2017;85:e00876-16. doi: 10.1128/IAI.00876-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chang YH, Kumar R, Ng TH, Wang HC. What vaccination studies tell us about immunological memory within the innate immune system of cultured shrimp and crayfish. Dev Comp Immunol. 2018;80:53–66. doi: 10.1016/j.dci.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 129.Gourbal B, Pinaud S, Beckers GJ, Van Der Meer JW, Conrath U, Netea MG. Innate immune memory: an evolutionary perspective. Immunol Rev. 2018;283:21–40. doi: 10.1111/imr.12647. [DOI] [PubMed] [Google Scholar]
- 130.Boraschi D, Italiani P. Innate immune memory: time for adopting a correct terminology. Front Immunol. 2018;9:799. doi: 10.3389/fimmu.2018.00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature. 2007;447:972–978. doi: 10.1038/nature05836. [DOI] [PubMed] [Google Scholar]
- 132.Wendeln AC, Degenhardt K, Kaurani L, Gertig M, Ulas T, Jain G, Wagner J, Häsler LM, Wild K, Skodras A, et al. Innate immune memory in the brain shapes neurological disease hallmarks. Nature. 2018;556:332–338. doi: 10.1038/s41586-018-0023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]