Abstract
In the progression of atherosclerosis, macrophages are the key immune cells for foam cell formation. During hyperlipidemic condition, phagocytic cells such as monocytes and macrophages uptake oxidized low-density lipoproteins (oxLDLs) accumulated in subintimal space, and lipid droplets are accumulated in their cytosols. In this review, we discussed the characteristics and phenotypic changes of macrophages in atherosclerosis and the effect of cytosolic lipid accumulation on macrophage phenotype. Due to macrophage plasticity, the inflammatory phenotypes triggered by oxLDL can be re-programmed by cytosolic lipid accumulation, showing downregulation of NF-κB activation followed by activation of anti-inflammatory genes, leading to tissue repair and homeostasis. We also discuss about various in vivo and in vitro models for atherosclerosis research and next generation sequencing technologies for foam cell gene expression profiling. Analysis of the phenotypic changes of macrophages during the progression of atherosclerosis with adequate approach may lead to exact understandings of the cellular mechanisms and hint therapeutic targets for the treatment of atherosclerosis.
Keywords: Atherosclerosis, Macrophage, Lipid uptake, In vivo model, In vitro model
BASIC FUNCTIONS OF MACROPHAGES AS ESSENTIAL IMMUNE CELLS
Macrophages play a fundamental role in the immune system, providing immediate defense against pathogens by clearing pathogenic invasions through phagocytosis (1). Macrophages are specialized immune cells that degrade engulfed cargo and may also present antigens, but are not capable of migrating to lymph node tissues to stimulate T cells as dendritic cells do (2). Macrophages respond to the surrounding microenvironment, showing various phenotypes and biological functions (3). Pro-inflammatory cytokines may be induced through either exogenous or endogenous sources. Exogenous inflammation inducers from microorganisms are known as pathogen-associated molecular patterns (PAMPs) and are recognized by pattern-recognition receptors (PRRs) (4). Endogenous inflammation inducers are produced by damaged cells, release of ATP, K+ ions, and the high-mobility group box 1 (HMGB1) proteins, which in cooperation with TLRs induce inflammatory responses. Macrophages sense the inflammatory signals and get recruited to the site of tissue injury, which is vital for elimination of the inflammation triggers and contributes to tissue repair (5).
Macrophages originate from either yolk sac progenitors before birth or bone marrow-derived monocytes after birth (6). Each organ retains different combinations of embryonic and adult-derived macrophage subsets, which are maintained by local proliferation and influx of circulating blood monocytes (7). A significant proportion of tissue-resident macrophages is seeded into the tissues before birth and self-replenish independently of hematopoiesis (8). Macrophages from the yolk sac progenitors or fetal liver are tissue-resident and prenatally establish the majority of cardiac macrophages, as demonstrated through fate mapping studies using the macrophage marker CX3CR1, in vivo cell tracking, parabiosis, and bone marrow transplants (9). Bone-marrow-derived hematopoietic stem cells and progenitor cells (HSPCs) develop into circulating Ly6Chi monocytes upon the action of M-CSF and differentiate into macrophages (10). Under certain circumstances, bone-marrow-derived HSPCs populate in the spleen and undergo extramedullary hematopoiesis (11). In the heart, Ly6Chi monocytes reside in the cardiac tissue and are the dominant tissue macrophage population upon local inflammation (7). These monocyte-derived macrophages are recruited through the C-C chemokine receptor 2 (CCR2) and are crucial in the inflammatory environment (12). CCR2 expression is typically associated with infiltrating Ly6Chi monocytes and is used to distinguish between infiltrating and tissue-resident macrophages (13). Bajpai et al also demonstrated that tissue-resident CCR2+ macrophages within the heart are responsible for monocyte recruitment through the myeloid differentiation primary response 88 (MYD88) pathway, leading to the release of the MCP and contribute to heart failure pathogenesis. Unlike monocyte-derived macrophages, tissue-resident macrophages contribute to the initiation of inflammation and tissue homeostasis via apoptotic cell clearance (14).
CLASSIFICATION OF MACROPHAGE PHENOTYPES: INFLAMMATORY AND ANTI-INFLAMMATORY
Among various immune cells, macrophages are remarkably plastic in their ability to respond to microenvironmental changes or immunological challenges, also known as macrophage polarization, eliciting appropriate responses to the cues. Although macrophages are heterogeneous cells, they are broadly classified in two groups: classically activated and alternatively activated macrophages. Classically activated macrophages are associated with host defense and produce pro-inflammatory cytokines such as TNF and IL-1β, the latter resulting from the nucleotide oligomerization domain (NOD)-, leucine-rich repeat (LRR)-, and the NOD-, LRR- and pyrin domain-containing 3 (NLRP3) inflammasome (15). Upon recognition of PAMPs through PRRs such as TLRs and NOD receptors, macrophages are activated (16). Alternatively-activated or anti-inflammatory macrophages are associated with tissue repair, wound healing, and metabolic processes, and maintain homeostasis through the production of arginase and specialized pro-resolving mediators such as TGF-β. With the aid of pro-resolving mediators such as resolvins and protectins, alternatively-activated macrophages limit local inflammation and lead to inflammation resolution (17). Alternatively-activated macrophages are polarized through TH2 cytokines IL-4, IL-13, or IL-10, and show activation of the interferon regulatory factor/STAT via STAT6 (18). Activation of PPAR-γ in adipose tissue showed downregulation of classically activated macrophage markers such as IL-18, whereas alternatively activated macrophage markers such as arginase 1 and IL-10 were upregulated, suggesting PPAR-γ and alternatively activated macrophages leas to tissue remodeling (19). The IL-33 amplifies IL-13-induced macrophage polarization, leading to upregulation of arginase-1 (Arg-1), CCL17, and CCL24 (20). In addition, alternatively-activated macrophages produce CCL13, CCL8, or CCL26 derived from the host defense against extracellular pathogens (21). These anti-inflammatory signals are necessary to suppress inflammation, leading to tissue remodeling and retaining homeostasis (22).
Although infiltrating monocyte-derived macrophages predominantly express inflammatory markers, they develop an anti-inflammatory phenotype after they perform phagocytosis (23). Macrophages recruited to the mice kidney performed tissue repair in the late stages of inflammation, through the expression of pro-wound healing factors such as the CCL17, insulin-like growth factor 1, and platelet-derived growth factor subunit B (24). Lavin et al. (25) profiled four histone modifications on various tissue-resident macrophage populations and revealed that the local microenvironment reconstructs the tissue-resident macrophage enhancer landscape, giving macrophages a combination of tissue- and lineage-specific transcription factors, and allowing them to be reprogrammed upon encountering with new microenvironments. The ability of macrophages to show resilient characteristics corresponding to surrounding cues enables them to polarize and initiate an inflammatory response or maintain homeostasis through transcriptional regulation and reprogramming in response to exogenous or endogenous stimuli (26).
INITIATION AND PROGRESSION OF ATHEROSCLEROSIS UNDER HYPERLIPIDEMIC CONDITION
Atherosclerosis is a chronic inflammatory disease due to the narrowing of arteries, which could lead to cardiac arrest, ischemic stroke, myocardial infarction, renal impairment, and aneurysms with hypertension (27). Diagnosis of atherosclerosis is made through various tests; however, as atherosclerosis is asymptomatic until plaque rupture or side effects caused by the narrowing of arteries, it is considered one of the most problematic causes of death in western societies. Atherosclerosis results from interactions among modified lipoproteins, immune cells such as macrophages and T cells, and non-immune vascular cells such as smooth muscle and endothelial cells (28).
Progression of atherosclerosis is initiated with increased lipid accumulation in the subintimal space and oxidative modification of lipids. Gerrity et al. (29) demonstrated that blood monocytes penetrate the intima adhere to aortic endothelium at early progression stages of atherosclerosis. Circulating monocytes adhere to endothelium via P-selectin glycoprotein ligand-1 (PSGL-1) interacting with P-selectin and E-selectin, and also endothelium interact with vascular cell adhesion molecule 1 (VCAM1) or intercellular adhesion molecule 1 (ICAM1) (30,31). CCL2, CCR2, CCR5 and CX3CR1 also contribute to monocyte migration to arterial walls (32,33), and IFNβ accelerates lesion formation by enhancing macrophage adhesion to endothelial cells and promoting leukocyte infiltration (34).
The blood mononuclear cells are the major foam cell precursor and subsequently undergo transformation into macrophages (35). Phagocytic cells such as monocytes or macrophages uptake and deposit oxidized low-density lipoproteins (oxLDL) via diverse mechanisms such as action of scavenger receptors, cholesterol hydrolysis, or phagocytosis, during which membrane-bound lipid droplets are accumulated and lead to foam cell formation (36). Macrophages participate in all developmental phases of atherosclerosis and affect the status of a lesion through cellular responses, such as cytokine secretion, apoptosis, and necrosis.
Apart from mononuclear phagocytes, numerous adaptive immune cells are involved in atherosclerosis progression. In the arterial adventitia, large numbers of naïve T cells, CD4+ and CD8+ T cells, regulatory T cells (Tregs), and memory B cells are recruited (37). These adaptive immune cells play perplexing roles in atherosclerosis, inducing proatherogenic effects through the CD4+ Th1 effector cells to release proinflammatory cytokines, IFN-γ, and TNF, or inhibiting inflammation through the secretion of anti-inflammatory cytokines, TGF-β, and IL-10 through activated Tregs (38). During atherosclerosis progression, FoxP3 expressing Tregs are overwhelmed by effector T cells, replacing athero-protective immunity with proatherogenic functions (39). T lymphocytes and macrophages are dominant in ruptured plaques, and combinatorially generate plaque rupture or superficial erosion conditions (40). The immediate site of plaque rupture or erosion is characterized with an inflammatory process, with abundant expression of HLA-DR antigens on inflammatory cells. B cells develop into innate-like B1 cells or adaptive-functioning B2 cells, the former recognize low-density lipoprotein (LDL) epitopes and release low-affinity IgM antibodies, and the latter differentiate into plasma cells and express high-affinity IgG antibodies against atherogenic antigens and show athero-protective responses (39).
MACROPHAGES IN PROGRESSION AND REGRESSION OF ATHEROSCLEROSIS
The progression and exacerbation of atherosclerosis is regulated between recruitment and emigration of macrophages within the plaque. At early plaque stages, luminal movement of Ly6Chi monocyte-derived macrophages show initial response and emigrate into the artery, and increase up to 20-fold within mouse aorta by proliferation (41,42). However, as macrophages become foam cells, emigration capacity is diminished and less emigration is detected in progressive plaques (43). Plaque macrophages promote lesional progression into rupture-prone plaques with impeded inflammation resolution, which drives macrophage apoptosis, inhibition of efficient efferocytosis and apoptotic cell accumulation leading to necrotic core formation in atherosclerotic plaque (42).
Several heterogenous macrophage subsets are classified in murine atherosclerotic cardiovascular disease. Hemoglobin associated macrophages (Mhem) phagocytize erythrocyte remnants via CD163 and provoke secretion of anti-inflammatory cytokines such as IL-10 and cardio-protective responses (44). Mhem macrophages show high expression of ABCA1, ABCG1, and liver X receptor (LXR), therefore are resistant to foam cell formation (45). Mox macrophages, which are induced by oxidized phospholipids, account for 30% of all CD11b+/CD11c+ macrophages in advanced atherosclerotic lesions of low density lipoprotein receptor deficient (Ldlr−/−) mice (46). M4 macrophages are polarized by CXCL4, and show different transcriptome clusters compared with M1 and M2 macrophages (47). M4 macrophages are specifically identified from other macrophage subsets by combination of CD68, matrix metalloproteinase7 and S100A8 (48). M4 macrophages do not show phagocytosis ability, and show lower cholesterol efflux transporters, resulting in lower LDL content. Also, as CXCL4 has proinflammatory effects in atherosclerosis, M4 macrophages showing downregulation of CD163 mRNA could be defined as atherogenic (49).
Lesional macrophages are regulated by entry of circulating monocytes, local macrophage proliferation and apoptosis. Infiltrating intima macrophages egress into the bloodstream, lymphatic vessels or undergo apoptosis and efferocytotic clearance. In early lesional stage, macrophage egression associated with atherosclerosis regression is observed, followed by plaque progression and declined macrophage emigration rate (50). The retention and emigration signals within the plaque determine the content of intraplaque macrophages (50). Several cell signals are involved in the retention and emigration of macrophage foam cells, such as CD146, LXR and neuroimmune guidance cues. Semaphorin 3A and Netrin-1, members of the neuroimmune guidance cue family, are responsible for macrophage retention, as they inhibit chemokine-directed monocyte migration, whereas EphrinB2 functions as a chemoattractant and promotes leukocyte migration (51). Netrin-1 acts as negative regulator of leukocyte migration, and inhibits CCL2 and CCL19 directed macrophage migration via its receptor UNC5b, which may role in chronic inflammation persistence (52). Semaphorin 3E regulates macrophage retention with its immunomodulatory function, inhibits macrophage motility and promotes macrophage accumulation in plaques (53). CD146 shows high correlation with plaque vulnerability and atherosclerosis inflammation, as the expression was mainly found in infiltrated macrophages and intraplaque blood vessels in human atherosclerotic plaques (54). In response to oxLDL uptake, CD146 drives CD36 internalization, and macrophages show reduced migratory capacities to CCL19 and CCL21, indicating CD146 may play a pivotal role in atherosclerosis progression (55). Other factors such as adhesion molecule αDβ2 integrin and Junctional Adhesion Molecule C also contribute to macrophage retention by inhibiting cell movement (56).
Unlike retention phase, macrophage regression is associated with increased cell migratory capacity, associated with suppressed macrophages retention factors such as sempahorin 3E, netrin-1 and adhesion molecule expression (56). Atherosclerosis regression is promoted via CCR7-dependent emigration pathway, in which sterol regulatory element-binding proteins (SREBPs) increase CCR7 expression and promote CD68+ cell emigration from plaques (57). LXRs, especially phosphorylation to LXRα serine 198 (S198) also modulates CCR7 expression. In the regression environment, low level of S198 phosphorylation was observed with high level of CCR7 expression, and nonphosphorylated LXRα in RAW 264.7 cells showed induction of anti-inflammatory genes and repression of proinflammatory genes (58). Remarkably, genetic disruption of LXRα phosphorylation increased phagocytic molecules expression and apoptotic cell removal by macrophages, leading to reduced necrotic cores (59). Comparing regressing plaque CD68+ cells with progressing cells identified high expression of genes associated with cellular movement such as actin and myosin, whereas cell adhesion-related genes such as cadherins and vinculin were downregulated (60). Collectively, macrophages in regression stage preferentially express genes to reduce cellular adhesion, promote cellular motility and inhibit inflammation. These findings demonstrate that several plaque development-associated factors undergo transcription changes leading to both quantitative and phenotypic changes of macrophages in atherosclerotic plaque.
LIPID-LADEN FOAM CELL FORMATION IN ATHEROSCLEROSIS
A prominent feature of an atherosclerotic lesion is the accumulation of foam cells within the lesion. Although vascular smooth muscle cells can transdifferentiate into macrophage-like cells and form lipid-laden foam cells, macrophages are the most prominent phagocytic cells which contribute to the foam cell formation in atherosclerotic lesion (61,62,63). Accumulation of LDL particles in macrophages is mediated by scavenger receptors (64). Scavenger receptors SR-A, MARCO, CD36, SR-B1, lectin-like oxidized LDL rceptor-1 (LOX1) and CXCL16 bind to oxLDL and promote foam cell formation, SR-A and CD36 mediating 75%–90% of LDL degradation in vitro (65). SR-A and CD36 have prominent roles in systemic and cellular metabolism of cholesterol (66). SR-A mediates monocyte-derived macrophages to invade the lesion area through interaction with Scavenger receptor expressed by endothelial cells (SREC) and lectin-like oxidized LDL rceptor-1 (LOX-1) expressed by endothelial cells, and also activates plaque macrophages and enhances macrophages to uptake modified LDL particles (67). CD36 also mediates cholesterol influx by binding and scavenging oxLDL on monocytes and macrophages (68). Although CD36 has less binding affinity to oxLDL compared to SR-A, it has wider extended cellular distribution to monocytes, macrophages, erythroid precursors, endothelium, and platelets (69). Scavenger receptor class BI (SR-BI) is highly homologous and has a similar ligand repertoire to CD36; It may bind to high-density lipoprotein (HDL), LDL, very low-density lipoprotein (VLDL), and modified forms of LDL such as acetylated LDL, oxLDL, and maleylated-bovine serum albumin (70). However, SR-BI shows distinct functions in lipoprotein metabolism since it facilitates selective cholesterol uptake and reverse cholesterol transport (71).
oxLDL endocytosed by scavenger receptors is delivered to lysosomes, and oxLDL-derived cholesterol and 7-ketocholesterol are esterified into oxidized fatty acids (FAs) (72). In the lysosomal compartment, cholesterol esters are hydrolyzed into free cholesterol through the lysosomal acid lipase (LIPA) (73). LIPA enhances cholesterol efflux through the production of 25- and 27-hydroxycholesterol and liver X receptor activation (74). The influx of cholesterol released from the lysosomal compartment into the endoplasmic reticulum (ER) via the NPC factor lead to significant activation of acyl-coenzyme A:cholesterol acyltransferase (ACAT) (75). At the ER, upon reaching certain cholesterol concentration, ACAT re-esterifies free cholesterol and forms cholesterol ester, which accumulates as cytoplasmic lipid droplets (75). Cytosolic lipid droplets in macrophages leads to foamy macrophage formation (66), and the lipids could be effluxed via lipolysis or lipophagy (65).
PHENOTYPIC CHANGES OF MACROPHAGES BY EXOGENOUS LIPID ACCUMULATION
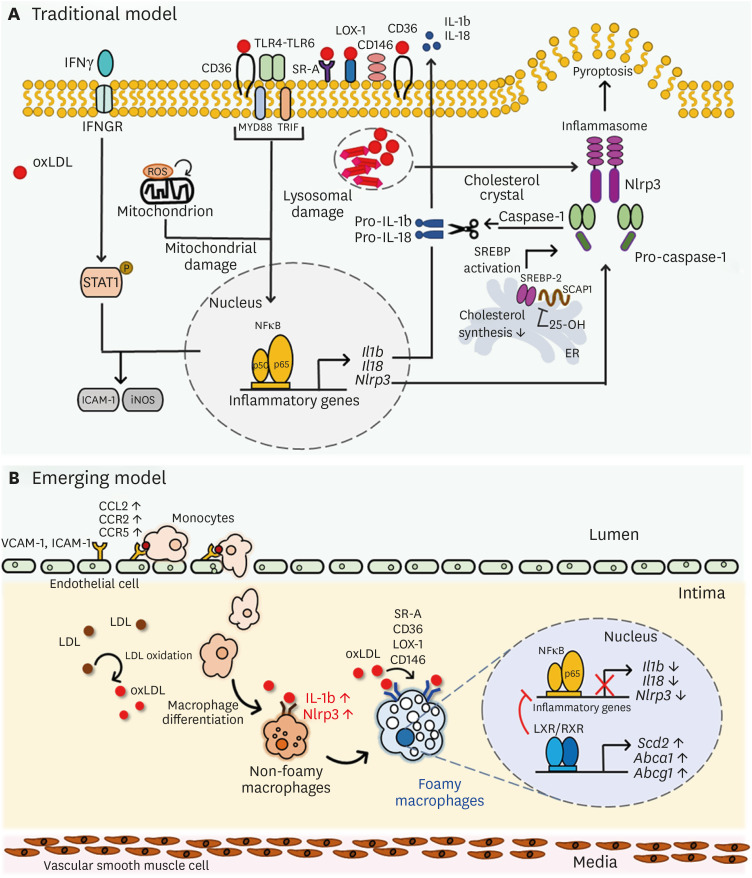
Since macrophages play a fundamental role in foam cell formation in all stages of atherogenesis, it is important to understand the molecular mechanisms and phenotypes involved in oxLDL-induced foam cells (76). It was previously demonstrated that uptake of exogenous oxLDL induces inflammatory responses in macrophages leading to the initiation, development, and progression of atherosclerosis (77). The cholesterol accumulation induces macrophages to undergo inflammatory responses through an increase in TLR, inflammasome activation, and monocyte production from the bone marrow and spleen, which can ultimately lead to development of complex lesions (78). Modified LDL such as oxLDL functions as a ligand for a macrophage pattern recognition receptor, such as CD36, which cooperates with the TLR4-TLR6 heterodimer and induces inflammatory response (79). CD36-mediated uptake of oxLDL as a danger-associated molecular pattern generates NLRP3-activators, triggers the TLR signaling pathway, and activates MYD88 and NF-κB to produce proinflammatory cytokines and chemokines such as IL-1β and IL-18 (80). Uptake or formation of intracellular cholesterol crystals by macrophages leads to lysosomal damage and dysfunction, resulting in NLRP3 inflammasome activation in atherosclerotic lesions (81). The NLRP3-inflammasome complex cleaves caspase-1, which catalyzes cytokine proproteins such as pro-IL-1β and pro-IL-18, promoting active cytokines as well as IL-1α (82). Furthermore, ERK signaling interacting with STAT1 signaling drives macrophage activation and atherosclerosis, and phospho-STAT1 and NF-κB associatively regulates ICAM-1 and iNOS expression (83). Early stages of plaque development are highly contributed by IFNγ and TLR4 signals, leading to increased leukocyte attraction (84). Macrophage inflammatory responses are also triggered by mitochondrial metabolic switch, in which long-chain FA uptake, mitochondrial import and decreased FA oxidation collectively lead to mitochondrial structure and function alteration, superoxide production and NF-κB activation (85). Collectively, these findings suggest that hypercholesterolemia leads to macrophage-foam cell formation and exerts inflammatory effects through increasing inflammatory mediators (Fig. 1A). Moreover, it has been reported that FA synthesis is immediately reduced in response to TLR4 activation in RAW264.7 macrophages, leading to increases in eicosanoid synthesis and delayed sphingolipid and sterol biosynthesis, indicating that inflammatory mediators activate the innate immune system and alters mammalian lipid metabolism (86).
Figure 1. Phenotypic changes of macrophages by lipid uptake in atherosclerotic lesion. (A) Traditional model of oxLDL-mediated inflammatory processes in macrophages. oxLDL activates TLR pathway, leading to NF-κB activation and consecutive pro-inflammatory cytokine secretion such as Il1b, Il18 and Nlrp3. Intracellular ROS accompanied by CD36 signaling leads to mitochondrial metabolic reprogramming and pro-atherogenic signaling, and cholesterol crystals activate NLRP3 and inflammasome formation. (B) An overall diagram describing emerging concept of foam cell formation during hyperlipidemic conditions. Non-foamy macrophages which are newly infiltrated into the intima have low level of cytosolic lipids and show high level of inflammatory genes such as IL-1b and Nlrp3. Foamy macrophages, retained in subintimal space for relatively prolonged time, have high level of lipids in their cytosol and show high level of genes involved in tissue repair and homeostasis, whereas inflammatory genes are relatively downregulated. Uptake of oxidized LDL via scavenger receptors SR-A, CD36, LOX-1, and CD146 induce inflammatory processes in macrophages, leading to production of pro-inflammatory cytokine production. However, after inflammatory responses in early phase, excess cholesterol inhibits Dhcr24, leading to accumulation of desmosterol and LXR/RXR signaling pathway activation. Excess desmosterol also inhibit NF-κB, thereby leading to resolution of acute inflammation. LXR activation promotes resolution of inflammation and cholesterol homeostasis in resolution phages of hyperlipidemic conditions. Unsaturated fatty acids synthesized by LXR pathway inhibit NF-κB -induced pro-inflammation and promote sterol efflux.
LOX-1, lectin-like oxidized LDL rceptor-1; SR, scavenger receptor.
Interestingly, an overall reduction in inflammatory gene expression in peritoneal macrophages isolated from western diet-fed Ldlr−/− mice had been observed. The reduction of inflammatory gene expression was mediated through the accumulation of desmosterol, an agonist of the LXR (87). Macrophages synthesize anti-inflammatory FAs in response to LXRs, which exert anti-inflammatory functions through binding to G protein-coupled receptor 120, thereby repressing macrophage-induced tissue inflammation (88). Moreover, oxLDL treatment induced anti-inflammatory phenotype in macrophages, showing high level of TGF-β IL-10, mannose receptors, PPARγ and arginase-1, whereas IL-12 and iNOS showed low levels (89). Mox macrophage, noticeably different from conventional classically activated macrophage and alternatively-activated macrophage phenotypes (46). Mox macrophages show decreased phagocytotic and chemotactic capacity, and induce unique gene expression patterns, which is largely mediated by Nrf2 and consequent redox-regulating transcriptions. Surprisingly, although Mox macrophages are ineffective at clearing apoptotic cells due to low efferocytosis and weak phagocytic activity, theses macrophages are expected to exert anti-inflammatory roles (90). Mox macrophages express IL-10 and vascular endothelial growth factor, which suppress T cell and macrophage activation and aid endothelial cell proliferation and survival. Anti-oxidization related enzymes such as heme oxygenase, sulfiredoxin-1 and thioredoxin reductase 1 further protect endothelial cells and smooth muscle cells from oxidative stress.
Thus, cholesterol and FA homeostasis are highly involved in the regulation of macrophage activity, and are regulated by several important transcription factors such as LXR and SREBP1 and 2 (91). SREBPs have related but distinct roles in embryogenesis, FA, and cholesterol syntheses (92). SREBP-2 preferentially induces genes involved in cellular cholesterol synthesis and import (92). SREBP-1c preferentially activate genes involved in FA, triglyceride metabolism, and phospholipids via activating the transcription of the acetyl CoA carboxylase, FA synthase, stearoyl CoA desaturase-1 (SCD1), and glycerol-3-phosphate acyltransferase (93,94). Oleate, the end product of de novo FA synthesis and a result of steric acid desaturation, specifically increases SREBP-1 nuclear accumulation and affect both the precursor and mature forms of SREBP-1 expression (95). As SREBP-1 isoforms activate FA synthesis and induce FA elongation and SCD1, FA biosynthesis and cholesterol biosynthesis are regulated transcriptionally by SREBPs (96). SREBP-1a promotes an acute inflammatory response through activating Caspase-1 and secreting IL-1β and their downstream regulators. SREBP-1a also activates FA biosynthesis-related genes, indicating that its pivotal role in linking lipid metabolism and the innate immune response (97). Moreover, Oishi et al reported that SREBP1 not only promoted IL-1β production, but also contributed to the resolution of TLR4-induced gene activation through increase of anti-inflammatory FA biosynthesis mRNAs such as Scd2, Fads, Elov, and inhibition of NFκB activation and corresponding decrease of Nos2, Cxcl10 and IL-6 (98).
Along with insulin and glucagon, LXR selectively regulates transcription of SREBP-1c, by inducing FA biosynthesis- associated gene expression and raising plasma triglyceride concentrations (99). Previous studies have proposed that oxLDL loading into macrophages negatively regulates transcription of TLR-induced proinflammatory gene expression at late stages (100). It has been reported that LXRs and ABCA1 repress inflammatory gene expression through disrupting MyD88 and TRAF6 recruitment, which lead to the inhibition of TLR2, 4, 9 and the downstream effectors NF-κB and MAPK (101). Upon hypercholesterolemia, deactivation of LXR-independent inflammatory gene expression leads to suppressed Nrf2 pathway, which regulates pentose phosphate pathway and the inhibition of macrophage inflammatory responses (102). These studies show that overloading lipids in cytosol of macrophages deactivate inflammatory responses, both through LXR- dependent and independent pathways.
IN VIVO MICE MODELS TO STUDY LIPID-LADEN FOAM CELLS IN ATHEROSCLEROTIC LESION
Mouse and rabbit models are widely used in atherosclerosis research, followed by pig and non-human primate models (103). Among these animal models, mouse model is most favored for its rapid reproduction, genetic manipulation ability, and reasonable time frame for atherogenesis formation and observation. However, mice have different lipid profile compared to humans, as most of the cholesterol is transported via HDL and retain low concentration of LDL and VLDL (104). Therefore, genetic modification of mice to achieve manipulation of their lipid metabolism and study atherosclerosis is required (105).
Apolipoprotein E-deficient (ApoE−/−) and Ldlr−/− mice are the most widely used along with the ApoE/LDLr double-knockout, ApoE3-Leiden, and proprotein convertase subtilisin/kexin type 9 (Pcsk9) via adeno-associated virus (PCSk9-AAV) mice. Apolipoprotein E (ApoE) is a glycoprotein synthesized in the liver and the brain, and is present in all lipoproteins except LDL (105). It functions as a ligand for receptors that clear chylomicrons and VLDL remnants; hence, its deficiency leads to increased cholesterol in plasma, especially in chylomicron fractions and VLDL-sized particles (106). ApoE−/− mice retain significant hypercholesterolemia, and therefore develop atherosclerosis on a normal diet and rapidly develop plaques with more advanced lesion upon a high-cholesterol diet (107). However, as ApoE affects inflammation thereby influencing plaque development, the lipid profile is dissimilar to that of humans (103).
Ldlr−/− mice are deficient of the LDL receptor, which is a membrane receptor that mediates LDL endocytosis, thus maintaining the LDL plasma concentration. Mice without LDL receptors show modestly elevated plasma cholesterol concentrations on a normal diet, with increased intermediate-density lipoprotein- and LDL-sized particles, whereas HDL and triglycerides are unaffected (108). With high fat and cholesterol-based western diets, Ldlr−/− mice show dramatic changes in the lipoprotein profile, with atherosclerotic lesion development showing lipid profiles similar to that of humans (103).
Pcsk9 is a subtilisin serine protease that internalizes LDL receptors into lysosomes and leads to increase in total plasma cholesterol. Pcsk9 expressed via adenovirus results in an LDL receptor knockout phenotype confirmed by decreased LDLr protein without change in LDLr mRNA level, a mechanism that is more rapid than SREBP-mediated transcription of LDLr (109). Pcsk9 regulates LDLr protein inhibitor in liver; therefore, mice lacking Pcsk9 show increased LDLr protein, causing clearance of circulating lipoproteins and reduced plasma cholesterol concentration (110). The overexpression of functional Pcsk9-AAV can induce hyperlipidemia and atherosclerosis in normal mouse (111), (112). Although PCSK9 expression shows gender variation due to different tissue distribution of AAVs and induces hyperlipidemia and atherosclerosis more effectively in male mice (113), injection of pcsk9-AAV appears to be a very useful method to induce hyperlipidemia and foam cell formation in mouse aorta without cross-breeding to induce hyperlipidemia.
LESSON FROM THE PHENOTYPE ANALYSIS OF LESIONAL MACROPHAGES
Distinguishing the cellular components of a lesion is complex, as characterization of macrophages requires analysis of numerous cellular biomarkers many of which are shared with other cell types, such as dendritic cells (114). Researchers have developed various methods to investigate lipid-enriched foam cells within atherosclerotic lesions. Roberts and Thompson quantitated the degree of atherosclerosis through staining lipids in lesions with Oil red O (114). The use of Oil red O demonstrates the presence of triglycerides, lipids, and lipoproteins in atherosclerotic lesions, allowing researchers to quantitatively evaluate lesion formation. Macrophages within atherosclerotic plaques of ApoE−/− mice are identified using nanospheres conjugated with an anti-CD68 antibody using dual-modal US imaging and magnetic resonance imaging (115). CD68 is one of the macrophage membrane proteins that bind to oxLDL; therefore, this model allows researchers to observe oxLDL-enriched macrophages (116).
Feig and Fisher (117) demonstrated that foam cell-specific RNA in plaques is isolated by laser capture microdissection of plaques. The use of laser capture microdissection facilitates the isolation of foam cells and RNA extraction, which leads to observation of significantly decreased expression of inflammatory genes MCP-1 and VCAM-1, and upregulation of cholesterol efflux genes LXRα, ABCA1, and SR-BI in foam cells under atherosclerotic regression conditions. This technology also shows that CCR7, a migratory factor that is functionally required for depletion of foam cells during regression, is upregulated. A surgical sponge containing Matrigel may be implanted to harvest macrophages (118). Feeding high-fat diet post subcutaneous insertion of surgical sponges led to the observation that lipid droplets are only found in macrophages and that foam cell formation induces pro-fibrotic transcriptions related to plaque stability rather than macrophage polarization towards an inflammatory or an anti-inflammatory state (118). Development of next-generation sequencing technologies and single-cell RNA sequencing allows deep sequencing and analyses of transcriptomes of diverse cells on single cell basis. The characterization of cells based on cell surface markers is widely used; however, as only few markers are known to specify a cell type, more studies are needed to identify markers such as the cluster of differentiation in immune cells and denotation of specific gene expression in heterogeneous cells. Recently, Kim et al. (62) developed a new method to analyze and isolate lipid-laden foam cells from atherosclerotic aorta. Using boron-dipyrromethene 493/503 (BODIPY493/503), lipid-laden foamy and non-foamy macrophages within atherosclerotic lesions were easily sorted out using flow cytometry and underwent the gene expression analysis of foamy and non-foamy macrophages using bulk RNA sequencing. Interestingly, the gene expression profiles of intimal foamy and non-foamy macrophages were strikingly different, the foamy macrophages expressed less inflammatory genes compared to non-foamy counterpart. Further characterization of aortic leukocytes in single cell level confirmed that foamy macrophages expressed lipid-processing genes with less expression of inflammatory genes, whereas non-foamy macrophages expressed IL-1β and other inflammatory genes (62). In human aorta, as gene and protein expression analysis of human plaques evidenced that pro-inflammatory macrophage markers are highly expressed in symptomatic plaques, whereas alternatively-activated macrophages markers, mannose receptor, CD163 and Th2 cytokines are highly related with disease progression (119). Collectively, the macrophages just recruited into subintimal space show inflammatory phenotype and contribute to lesion formation, after which become foamy cells with less inflammatory phenotype and high expression of lipid-processing genes (Fig. 1B). However, further studies analyzing lesional macrophages in single-cell resolution with fate-mapping approach are needed to understand the detailed phenotype changes of macrophages at different time points of the disease.
IN VITRO CELL MODELS TO UNDERSTAND PHENOTYPIC CHANGES OF MACROPHAGES BY LIPID UPTAKE
Since the macrophages residing in human and mouse atherosclerotic lesion have been affected by local micro-environmental factors for long period of time, the results obtained from in vitro macrophage culture may be different from in vivo data, and for this reason, the interpretation of results from in vitro cell experiments should be carefully done. Nevertheless, in vitro experiments are useful for monitoring explicit pathways or correlation between genes and rapidly obtaining and comprehending preliminary results. The treatment of oxLDL has been widely used to generate foamy macrophages and understand the molecular mechanism involved in phenotypic changes of macrophages during atherogenesis (120). The macrophages may be obtained from two main sources, animal primary cells or cell lines. Animal primary cells are isolated from living tissue and cultured in cell culture plates. Cell lines are immortalized through induction of mutations to inhibit cellular senescence (121), and therefore have the advantage that cells may be easily grown in vitro for prolonged periods of time. Cell lines are often used in research as they provide a pure population of cells, guaranteeing consistent material and reproducible results (122). One of the most commonly used macrophage cell lines is RAW264.7, which is a murine leukemia cell line (123). There are four immortalized human monocyte–macrophage cell lines: THP-1, U937, ML-2, and Mono Mac 6 cells (124). The THP-1 cell line shows a round single-cell morphology and expresses distinct monocytic markers (125). Upon treatment with phorbol-12-myristate-13-acetate or 1,25-dihydroxyvitamin D3 (1,25[OH]2D3), THP-1 cells differentiate into flat and amoeboid macrophage phenotypes. However, as cell lines have undergone significant genetic mutations to become immortal, their cellular biology and phenotypes may differ from actual cells from living organisms; therefore, this limitation should be considered when using cell lines.
Macrophages are widely distributed throughout the body; therefore, primary macrophages may be obtained from various sources such as the bone marrow, spleen, and peritoneal cavity (126). Bone marrow derived macrophages (BMDMs) and peritoneal macrophages are the most frequently used cells in atherosclerotic experiments because of the easiness to obtain large number of cells. BMDMs may be obtained by isolating bone marrow cells and culturing them with M-CSF or L929 cell-produced GM-CSF (127). It has been reported that BMDMs show high CD169 expression, moderate CD115 and MHCII expression, and low CD11b expression, whereas peritoneal macrophages show high CD115 and CD11b expression and a MHCII lower expression (128).The peritoneum cavity is a specialized compartment in which various immune cells localize, half of which are macrophages (129). Approximately 60% of the peritoneal cavity cells express CD11b and F4/80, hence characterized as macrophages (130), and most macrophages from peritoneum are maintained by embryonic precursors as tissue resident macrophages (131). Peritoneal cavity macrophages are classified into two subsets based on size differences, large peritoneal macrophages (LPMs) or small peritoneal macrophages (SPMs), which retain unique developmental characteristics and corresponding functions and phenotypes (130). LPMs predominate within the peritoneal macrophage population and represent approximately 90% of the population in unstimulated mice. LPMs are tissue-resident, therefore they self-maintain their population with minimal contribution from circulating monocytes (132). Transcriptional factor GATA-6 is involved in different lineages from the mesoderm during embryo development, and is restricted to the precardiac mesoderm, the embryonic heart tube, and the primitive gut (133). Also, Gautier et al revealed that GATA6 acts as a regulator of peritoneal macrophages (2). GATA-6 is highly and specifically expressed in LPMs, whereas SPMs and other tissue macrophages do not show such expression level (133). The intraperitoneal injection of thioglycollate medium has been widely used to elicit and obtain a large number of peritoneal macrophages (134). Thioglycollate medium-elicited macrophages are treated with oxLDL to generate foamy macrophages (87). However, along with the Bacillus Calmette-Guérin vaccine, LPS, or zymosan, thioglycollate medium acts as a sterile irritant and promotes the rapid migration of macrophages to the omentum (135). The use of thioglycollate medium or LPS stimulation leads to inflammatory stimuli within the peritoneal cavity, leading to irretrievable LPMs to disappear rapidly in the lavage and promoting the macrophage disappearance reaction (136). Surprisingly, blood monocyte-derived SPMs, which are less abundant and only occupy 10% of normal peritoneal macrophages, migrate to the peritoneal cavity in response to inflammation stimulation and becomes the predominant population. Although both subsets orchestrate the immune response and maintain tissue homeostasis, these 2 different peritoneal macrophage subsets show heterogeneous cell markers (137). These 2 populations are expressed differently on surface molecules such as granulocytic marker (Gr-1), MHC II, CD11b and CD11c. LPMs express higher levels of CD11b, F4/80, CD40, CD80, CD86, CD11c, and TLR4 than SPMs, and only LPMS are known to express Gr-1 and AA4.1. In contrast, only SPMs express a high level of the MHC II marker, CD62L and Dectin-1, and express low level of F4/80, CD11b, GR-1 and CD86 (137). Thus thioglycollate-elicited peritoneal macrophages may have different cellular responses to oxLDL uptake compared to the peritoneal macrophages in resting status. Recently, we analyzed the cellular responses of thioglycollate-elicited and resting peritoneal macrophages upon oxLDL treatment and compared their gene expressions with the gene expression pattern of lesional foamy macrophages shown in our previous report (62). Interestingly, oxLDL stimulation to resident peritoneal macrophages showed gene expression changes similar to those of intimal foamy macrophages, whereas thioglycollate-elicited peritoneal macrophages, BMDM, RAW264.7 cells did not (unpublished/private observation). These collectively suggest resident peritoneal macrophages may be the optimized in vitro cell system for studying foam cells in vitro. And further studies need to be done with resident peritoneal macrophages to dissect the molecular mechanism of phenotypic changes upon oxLDL treatment.
CONCLUDING REMARKS
Recent technical advances allow us to analyze the gene expression, chromatin accessibility, and localization of gene expression based on single cell level. Two independent groups firstly adopted single cell RNA sequencing to analyze the cellular heterogeneity of atherosclerotic aorta (138,139). Kim et al. (62) analyzed the gene expression patterns of intimal foam and nonfoamy macrophages using bulk- and single cell RNA sequencing. Cochain et al. (139) showed three aortic macrophage populations including resident-like macrophages, inflammatory macrophages, and Trem2hi macrophages. Interestingly, we also found similar gene expression patterns in previous bulk and single cell RNA sequencing (62) and concluded that the “Trem2hi macrophages” are intimal foamy macrophages and other two macrophages are intimal non-foamy (“inflammatory”) and adventitial macrophages (“resident-like”) (140). The consistency of single cell RNA sequencing data obtained by independent research groups indicate that single cell RNA sequencing is a highly useful technique to understand the phenotypic changes of macrophages during the progression of atherosclerosis. It seems that the single cell gene expression map in atherosclerosis will be available in near future. In addition to gene expression profiling, the analysis of proteomics/metabolomics and chromatic accessibility on single cell level will provide us new understanding on pathogenesis of atherosclerosis and molecular or cellular target to treat atherosclerosis. As next approach, we will then validate the new therapeutic targets and molecular mechanisms using in vitro cell system most mimicking in vivo cellular phenotypes.
ACKNOWLEDGEMENTS
This research was supported by the research grants (NRF-2016M3A9D5A01952413, 2018R1A2B6003393 and 2015M3A9B6029138) supported by the National Research Foundation of Korea.
Abbreviations
- AAV
adeno-associated virus
- ACAT
acyl-coenzyme A:cholesterol acyltransferase
- ApoE
apolipoprotein E
- ApoE−/−
apolipoprotein E-deficient
- BMDM
bone marrow derived macrophage
- CCR2
C-C chemokine receptor 2
- ER
endoplasmic reticulum
- FA
fatty acid
- HDL
high-density lipoprotein
- HMGB1
high-mobility group box 1
- HSPC
hematopoietic stem cells and progenitor cell
- LDL
low-density lipoprotein
- LDLr
low-density lipoprotein-receptor
- Ldlr−/−
LDL receptor deficient
- LIPA
lysosomal acid lipase
- LOX-1
lectin-like oxidized LDL rceptor-1
- LPM
large peritoneal macrophage
- LRR
leucine-rich repeat
- LXR
liver X receptor
- Mhem
hemoglobin associated macrophage
- MYD88
myeloid differentiation primary response 88
- NLRP3
NOD-, LRR- and pyrin domain-containing 3
- NOD
nucleotide oligomerization domain
- oxLDL
oxidized low-density lipoprotein
- PAMP
pathogen-associated molecular pattern
- Pcsk9
proprotein convertase subtilisin/kexin type 9
- PRR
pattern-recognition receptor
- SCD1
stearoyl CoA desaturase-1
- SPM
small peritoneal macrophage
- SR-BI
scavenger receptor class BI
- SREBP
sterol regulatory element-binding protein
- VLDL
very low-density lipoprotein
Footnotes
Conflicts of Interest: The authors declare no potential conflicts of interest.
- Conceptualization: Lee J, Choi JH.
- Investigation: Lee J, Choi JH.
- Supervision: Choi JH.
- Writing - original draft: Lee J, Choi JH.
- Writing - review & editing: Lee J, Choi JH.
References
- 1.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 2.Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, Helft J, Chow A, Elpek KG, Gordonov S, et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. 2012;13:1118–1128. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, Mohammadi A, Afshari JT, Sahebkar A. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233:6425–6440. doi: 10.1002/jcp.26429. [DOI] [PubMed] [Google Scholar]
- 4.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 5.Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44:450–462. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nourshargh S, Alon R. Leukocyte migration into inflamed tissues. Immunity. 2014;41:694–707. doi: 10.1016/j.immuni.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity. 2014;41:21–35. doi: 10.1016/j.immuni.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies LC, Taylor PR. Tissue-resident macrophages: then and now. Immunology. 2015;144:541–548. doi: 10.1111/imm.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, Brija T, Gautier EL, Ivanov S, Satpathy AT, et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014;40:91–104. doi: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones CV, Ricardo SD. Macrophages and CSF-1: implications for development and beyond. Organogenesis. 2013;9:249–260. doi: 10.4161/org.25676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tabas I, Lichtman AH. Monocyte-macrophages and t cells in atherosclerosis. Immunity. 2017;47:621–634. doi: 10.1016/j.immuni.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davies LC, Rosas M, Jenkins SJ, Liao CT, Scurr MJ, Brombacher F, Fraser DJ, Allen JE, Jones SA, Taylor PR. Distinct bone marrow-derived and tissue-resident macrophage lineages proliferate at key stages during inflammation. Nat Commun. 2013;4:1886. doi: 10.1038/ncomms2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bajpai G, Bredemeyer A, Li W, Zaitsev K, Koenig AL, Lokshina I, Mohan J, Ivey B, Hsiao HM, Weinheimer C, et al. Tissue resident ccr2- and ccr2+ cardiac macrophages differentially orchestrate monocyte recruitment and fate specification following myocardial injury. Circ Res. 2019;124:263–278. doi: 10.1161/CIRCRESAHA.118.314028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liddiard K, Rosas M, Davies LC, Jones SA, Taylor PR. Macrophage heterogeneity and acute inflammation. Eur J Immunol. 2011;41:2503–2508. doi: 10.1002/eji.201141743. [DOI] [PubMed] [Google Scholar]
- 15.Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol. 2011;11:738–749. doi: 10.1038/nri3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 17.Serhan CN. Novel lipid mediators and resolution mechanisms in acute inflammation: to resolve or not? Am J Pathol. 2010;177:1576–1591. doi: 10.2353/ajpath.2010.100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang N, Liang H, Zen K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front Immunol. 2014;5:614. doi: 10.3389/fimmu.2014.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stienstra R, Duval C, Keshtkar S, van der Laak J, Kersten S, Müller M. Peroxisome proliferator-activated receptor gamma activation promotes infiltration of alternatively activated macrophages into adipose tissue. J Biol Chem. 2008;283:22620–22627. doi: 10.1074/jbc.M710314200. [DOI] [PubMed] [Google Scholar]
- 20.Kurowska-Stolarska M, Stolarski B, Kewin P, Murphy G, Corrigan CJ, Ying S, Pitman N, Mirchandani A, Rana B, van Rooijen N, et al. IL-33 amplifies the polarization of alternatively activated macrophages that contribute to airway inflammation. J Immunol. 2009;183:6469–6477. doi: 10.4049/jimmunol.0901575. [DOI] [PubMed] [Google Scholar]
- 21.Lech M, Anders HJ. Macrophages and fibrosis: How resident and infiltrating mononuclear phagocytes orchestrate all phases of tissue injury and repair. Biochim Biophys Acta. 2013;1832:989–997. doi: 10.1016/j.bbadis.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Patel U, Rajasingh S, Samanta S, Cao T, Dawn B, Rajasingh J. Macrophage polarization in response to epigenetic modifiers during infection and inflammation. Drug Discov Today. 2017;22:186–193. doi: 10.1016/j.drudis.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujiu K, Wang J, Nagai R. Cardioprotective function of cardiac macrophages. Cardiovasc Res. 2014;102:232–239. doi: 10.1093/cvr/cvu059. [DOI] [PubMed] [Google Scholar]
- 24.Lin SL, Castaño AP, Nowlin BT, Lupher ML, Jr, Duffield JS. Bone marrow Ly6Chigh monocytes are selectively recruited to injured kidney and differentiate into functionally distinct populations. J Immunol. 2009;183:6733–6743. doi: 10.4049/jimmunol.0901473. [DOI] [PubMed] [Google Scholar]
- 25.Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, Jung S, Amit I. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 2014;159:1312–1326. doi: 10.1016/j.cell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atri C, Guerfali FZ, Laouini D. Role of human macrophage polarization in inflammation during infectious diseases. Int J Mol Sci. 2018;19:19. doi: 10.3390/ijms19061801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 28.Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014;114:1852–1866. doi: 10.1161/CIRCRESAHA.114.302721. [DOI] [PubMed] [Google Scholar]
- 29.Gerrity RG, Naito HK, Richardson M, Schwartz CJ. Dietary induced atherogenesis in swine. Morphology of the intima in prelesion stages. Am J Pathol. 1979;95:775–792. [PMC free article] [PubMed] [Google Scholar]
- 30.Xu H, Jiang J, Chen W, Li W, Chen Z. Vascular macrophages in atherosclerosis. J Immunol Res. 2019;2019:4354786. doi: 10.1155/2019/4354786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mestas J, Ley K. Monocyte-endothelial cell interactions in the development of atherosclerosis. Trends Cardiovasc Med. 2008;18:228–232. doi: 10.1016/j.tcm.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Combadière C, Potteaux S, Rodero M, Simon T, Pezard A, Esposito B, Merval R, Proudfoot A, Tedgui A, Mallat Z. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117:1649–1657. doi: 10.1161/CIRCULATIONAHA.107.745091. [DOI] [PubMed] [Google Scholar]
- 33.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goossens P, Gijbels MJ, Zernecke A, Eijgelaar W, Vergouwe MN, van der Made I, Vanderlocht J, Beckers L, Buurman WA, Daemen MJ, et al. Myeloid type I interferon signaling promotes atherosclerosis by stimulating macrophage recruitment to lesions. Cell Metab. 2010;12:142–153. doi: 10.1016/j.cmet.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 35.Gerrity RG. The role of the monocyte in atherogenesis: I. Transition of blood-borne monocytes into foam cells in fatty lesions. Am J Pathol. 1981;103:181–190. [PMC free article] [PubMed] [Google Scholar]
- 36.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ketelhuth DF, Hansson GK. Modulation of autoimmunity and atherosclerosis - common targets and promising translational approaches against disease. Circ J. 2015;79:924–933. doi: 10.1253/circj.CJ-15-0167. [DOI] [PubMed] [Google Scholar]
- 38.Ketelhuth DF, Hansson GK. Cellular immunity, low-density lipoprotein and atherosclerosis: break of tolerance in the artery wall. Thromb Haemost. 2011;106:779–786. doi: 10.1160/TH11-05-0321. [DOI] [PubMed] [Google Scholar]
- 39.Wolf D, Ley K. Immunity and inflammation in atherosclerosis. Circ Res. 2019;124:315–327. doi: 10.1161/CIRCRESAHA.118.313591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van der Wal AC, Becker AE, van der Loos CM, Das PK. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation. 1994;89:36–44. doi: 10.1161/01.cir.89.1.36. [DOI] [PubMed] [Google Scholar]
- 41.Gerrity RG, Naito HK. Lipid clearance from fatty streak lesions by foam cell migration. Artery. 1980;8:215–219. [PubMed] [Google Scholar]
- 42.Barrett TJ. Macrophages in atherosclerosis regression. Arterioscler Thromb Vasc Biol. 2020;40:20–33. doi: 10.1161/ATVBAHA.119.312802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Llodrá J, Angeli V, Liu J, Trogan E, Fisher EA, Randolph GJ. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc Natl Acad Sci U S A. 2004;101:11779–11784. doi: 10.1073/pnas.0403259101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boyle JJ, Harrington HA, Piper E, Elderfield K, Stark J, Landis RC, Haskard DO. Coronary intraplaque hemorrhage evokes a novel atheroprotective macrophage phenotype. Am J Pathol. 2009;174:1097–1108. doi: 10.2353/ajpath.2009.080431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boyle JJ. Heme and haemoglobin direct macrophage Mhem phenotype and counter foam cell formation in areas of intraplaque haemorrhage. Curr Opin Lipidol. 2012;23:453–461. doi: 10.1097/MOL.0b013e328356b145. [DOI] [PubMed] [Google Scholar]
- 46.Kadl A, Meher AK, Sharma PR, Lee MY, Doran AC, Johnstone SR, Elliott MR, Gruber F, Han J, Chen W, et al. Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circ Res. 2010;107:737–746. doi: 10.1161/CIRCRESAHA.109.215715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gleissner CA, Shaked I, Little KM, Ley K. CXC chemokine ligand 4 induces a unique transcriptome in monocyte-derived macrophages. J Immunol. 2010;184:4810–4818. doi: 10.4049/jimmunol.0901368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Erbel C, Tyka M, Helmes CM, Akhavanpoor M, Rupp G, Domschke G, Linden F, Wolf A, Doesch A, Lasitschka F, et al. CXCL4-induced plaque macrophages can be specifically identified by co-expression of MMP7+S100A8+ in vitro and in vivo. Innate Immun. 2015;21:255–265. doi: 10.1177/1753425914526461. [DOI] [PubMed] [Google Scholar]
- 49.Gleissner CA, Shaked I, Erbel C, Böckler D, Katus HA, Ley K. CXCL4 downregulates the atheroprotective hemoglobin receptor CD163 in human macrophages. Circ Res. 2010;106:203–211. doi: 10.1161/CIRCRESAHA.109.199505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chistiakov DA, Grechko AV, Myasoedova VA, Melnichenko AA, Orekhov AN. The role of monocytosis and neutrophilia in atherosclerosis. J Cell Mol Med. 2018;22:1366–1382. doi: 10.1111/jcmm.13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Gils JM, Ramkhelawon B, Fernandes L, Stewart MC, Guo L, Seibert T, Menezes GB, Cara DC, Chow C, Kinane TB, et al. Endothelial expression of guidance cues in vessel wall homeostasis dysregulation under proatherosclerotic conditions. Arterioscler Thromb Vasc Biol. 2013;33:911–919. doi: 10.1161/ATVBAHA.112.301155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Gils JM, Derby MC, Fernandes LR, Ramkhelawon B, Ray TD, Rayner KJ, Parathath S, Distel E, Feig JL, Alvarez-Leite JI, et al. The neuroimmune guidance cue netrin-1 promotes atherosclerosis by inhibiting the emigration of macrophages from plaques. Nat Immunol. 2012;13:136–143. doi: 10.1038/ni.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wanschel A, Seibert T, Hewing B, Ramkhelawon B, Ray TD, van Gils JM, Rayner KJ, Feig JE, O'Brien ER, Fisher EA, et al. Neuroimmune guidance cue Semaphorin 3E is expressed in atherosclerotic plaques and regulates macrophage retention. Arterioscler Thromb Vasc Biol. 2013;33:886–893. doi: 10.1161/ATVBAHA.112.300941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qian YN, Luo YT, Duan HX, Feng LQ, Bi Q, Wang YJ, Yan XY. Adhesion molecule CD146 and its soluble form correlate well with carotid atherosclerosis and plaque instability. CNS Neurosci Ther. 2014;20:438–445. doi: 10.1111/cns.12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luo Y, Duan H, Qian Y, Feng L, Wu Z, Wang F, Feng J, Yang D, Qin Z, Yan X. Macrophagic CD146 promotes foam cell formation and retention during atherosclerosis. Cell Res. 2017;27:352–372. doi: 10.1038/cr.2017.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moore KJ, Koplev S, Fisher EA, Tabas I, Björkegren JL, Doran AC, Kovacic JC. Macrophage trafficking, inflammatory resolution, and genomics in atherosclerosis: JACC macrophage in CVD series (part 2) J Am Coll Cardiol. 2018;72:2181–2197. doi: 10.1016/j.jacc.2018.08.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feig JE, Shang Y, Rotllan N, Vengrenyuk Y, Wu C, Shamir R, Torra IP, Fernandez-Hernando C, Fisher EA, Garabedian MJ. Statins promote the regression of atherosclerosis via activation of the CCR7-dependent emigration pathway in macrophages. PLoS One. 2011;6:e28534. doi: 10.1371/journal.pone.0028534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu C, Hussein MA, Shrestha E, Leone S, Aiyegbo MS, Lambert WM, Pourcet B, Cardozo T, Gustafson JA, Fisher EA, et al. Modulation of macrophage gene expression via liver x receptor alpha serine 198 phosphorylation. Mol Cell Biol. 2015;35:2024–2034. doi: 10.1128/MCB.00985-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gage MC, Bécares N, Louie R, Waddington KE, Zhang Y, Tittanegro TH, Rodríguez-Lorenzo S, Jathanna A, Pourcet B, Pello OM, et al. Disrupting LXRα phosphorylation promotes FoxM1 expression and modulates atherosclerosis by inducing macrophage proliferation. Proc Natl Acad Sci U S A. 2018;115:E6556–E6565. doi: 10.1073/pnas.1721245115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feig JE, Vengrenyuk Y, Reiser V, Wu C, Statnikov A, Aliferis CF, Garabedian MJ, Fisher EA, Puig O. Regression of atherosclerosis is characterized by broad changes in the plaque macrophage transcriptome. PLoS One. 2012;7:e39790. doi: 10.1371/journal.pone.0039790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Y, Dubland JA, Allahverdian S, Asonye E, Sahin B, Jaw JE, Sin DD, Seidman MA, Leeper NJ, Francis GA. Smooth muscle cells contribute the majority of foam cells in apoe (apolipoprotein e)-deficient mouse atherosclerosis. Arterioscler Thromb Vasc Biol. 2019;39:876–887. doi: 10.1161/ATVBAHA.119.312434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim K, Shim D, Lee JS, Zaitsev K, Williams JW, Kim KW, Jang MY, Seok Jang H, Yun TJ, Lee SH, et al. Transcriptome analysis reveals nonfoamy rather than foamy plaque macrophages are proinflammatory in atherosclerotic murine models. Circ Res. 2018;123:1127–1142. doi: 10.1161/CIRCRESAHA.118.312804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cochain C, Zernecke A. Macrophages in vascular inflammation and atherosclerosis. Pflugers Arch. 2017;469:485–499. doi: 10.1007/s00424-017-1941-y. [DOI] [PubMed] [Google Scholar]
- 64.Suzuki H, Kurihara Y, Takeya M, Kamada N, Kataoka M, Jishage K, Ueda O, Sakaguchi H, Higashi T, Suzuki T, et al. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature. 1997;386:292–296. doi: 10.1038/386292a0. [DOI] [PubMed] [Google Scholar]
- 65.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13:709–721. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- 67.de Winther MP, van Dijk KW, Havekes LM, Hofker MH. Macrophage scavenger receptor class A: a multifunctional receptor in atherosclerosis. Arterioscler Thromb Vasc Biol. 2000;20:290–297. doi: 10.1161/01.atv.20.2.290. [DOI] [PubMed] [Google Scholar]
- 68.Endemann G, Stanton LW, Madden KS, Bryant CM, White RT, Protter AA. CD36 is a receptor for oxidized low density lipoprotein. J Biol Chem. 1993;268:11811–11816. [PubMed] [Google Scholar]
- 69.Murphy JE, Tedbury PR, Homer-Vanniasinkam S, Walker JH, Ponnambalam S. Biochemistry and cell biology of mammalian scavenger receptors. Atherosclerosis. 2005;182:1–15. doi: 10.1016/j.atherosclerosis.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 70.Acton SL, Scherer PE, Lodish HF, Krieger M. Expression cloning of SR-BI, a CD36-related class B scavenger receptor. J Biol Chem. 1994;269:21003–21009. [PubMed] [Google Scholar]
- 71.Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271:518–520. doi: 10.1126/science.271.5248.518. [DOI] [PubMed] [Google Scholar]
- 72.Brown AJ, Mander EL, Gelissen IC, Kritharides L, Dean RT, Jessup W. Cholesterol and oxysterol metabolism and subcellular distribution in macrophage foam cells. Accumulation of oxidized esters in lysosomes. J Lipid Res. 2000;41:226–237. [PubMed] [Google Scholar]
- 73.Ouimet M, Franklin V, Mak E, Liao X, Tabas I, Marcel YL. Autophagy regulates cholesterol efflux from macrophage foam cells via lysosomal acid lipase. Cell Metab. 2011;13:655–667. doi: 10.1016/j.cmet.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Viaud M, Ivanov S, Vujic N, Duta-Mare M, Aira LE, Barouillet T, Garcia E, Orange F, Dugail I, Hainault I, et al. Lysosomal cholesterol hydrolysis couples efferocytosis to anti-inflammatory oxysterol production. Circ Res. 2018;122:1369–1384. doi: 10.1161/CIRCRESAHA.117.312333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chang TY, Chang CC, Cheng D. Acyl-coenzyme A:cholesterol acyltransferase. Annu Rev Biochem. 1997;66:613–638. doi: 10.1146/annurev.biochem.66.1.613. [DOI] [PubMed] [Google Scholar]
- 76.de Winther MP, Hofker MH. Scavenging new insights into atherogenesis. J Clin Invest. 2000;105:1039–1041. doi: 10.1172/JCI9919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 78.Tall AR, Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat Rev Immunol. 2015;15:104–116. doi: 10.1038/nri3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sheedy FJ, Grebe A, Rayner KJ, Kalantari P, Ramkhelawon B, Carpenter SB, Becker CE, Ediriweera HN, Mullick AE, Golenbock DT, et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat Immunol. 2013;14:812–820. doi: 10.1038/ni.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nuñez G, Schnurr M, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kuida K, Lippke JA, Ku G, Harding MW, Livingston DJ, Su MS, Flavell RA. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 83.Randolph GJ. Mechanisms that regulate macrophage burden in atherosclerosis. Circ Res. 2014;114:1757–1771. doi: 10.1161/CIRCRESAHA.114.301174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sikorski K, Chmielewski S, Olejnik A, Wesoly JZ, Heemann U, Baumann M, Bluyssen H. STAT1 as a central mediator of IFNγ and TLR4 signal integration in vascular dysfunction. JAK-STAT. 2012;1:241–249. doi: 10.4161/jkst.22469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen Y, Yang M, Huang W, Chen W, Zhao Y, Schulte ML, Volberding P, Gerbec Z, Zimmermann MT, Zeighami A, et al. Mitochondrial metabolic reprogramming by cd36 signaling drives macrophage inflammatory responses. Circ Res. 2019;125:1087–1102. doi: 10.1161/CIRCRESAHA.119.315833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dennis EA, Deems RA, Harkewicz R, Quehenberger O, Brown HA, Milne SB, Myers DS, Glass CK, Hardiman G, Reichart D, et al. A mouse macrophage lipidome. J Biol Chem. 2010;285:39976–39985. doi: 10.1074/jbc.M110.182915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Spann NJ, Garmire LX, McDonald JG, Myers DS, Milne SB, Shibata N, Reichart D, Fox JN, Shaked I, Heudobler D, et al. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell. 2012;151:138–152. doi: 10.1016/j.cell.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rios FJ, Koga MM, Pecenin M, Ferracini M, Gidlund M, Jancar S. Oxidized LDL induces alternative macrophage phenotype through activation of CD36 and PAFR. Mediators Inflamm. 2013;2013:198193. doi: 10.1155/2013/198193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Butcher MJ, Galkina EV. Phenotypic and functional heterogeneity of macrophages and dendritic cell subsets in the healthy and atherosclerosis-prone aorta. Front Physiol. 2012;3:44. doi: 10.3389/fphys.2012.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Goldstein JL, DeBose-Boyd RA, Brown MS. Protein sensors for membrane sterols. Cell. 2006;124:35–46. doi: 10.1016/j.cell.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 92.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bennett MK, Lopez JM, Sanchez HB, Osborne TF. Sterol regulation of fatty acid synthase promoter. Coordinate feedback regulation of two major lipid pathways. J Biol Chem. 1995;270:25578–25583. doi: 10.1074/jbc.270.43.25578. [DOI] [PubMed] [Google Scholar]
- 94.Ericsson J, Jackson SM, Kim JB, Spiegelman BM, Edwards PA. Identification of glycerol-3-phosphate acyltransferase as an adipocyte determination and differentiation factor 1- and sterol regulatory element-binding protein-responsive gene. J Biol Chem. 1997;272:7298–7305. doi: 10.1074/jbc.272.11.7298. [DOI] [PubMed] [Google Scholar]
- 95.Lounis MA, Bergeron KF, Burhans MS, Ntambi JM, Mounier C. Oleate activates SREBP-1 signaling activity in SCD1-deficient hepatocytes. Am J Physiol Endocrinol Metab. 2017;313:E710–E720. doi: 10.1152/ajpendo.00151.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Moon YA, Shah NA, Mohapatra S, Warrington JA, Horton JD. Identification of a mammalian long chain fatty acyl elongase regulated by sterol regulatory element-binding proteins. J Biol Chem. 2001;276:45358–45366. doi: 10.1074/jbc.M108413200. [DOI] [PubMed] [Google Scholar]
- 97.Im SS, Yousef L, Blaschitz C, Liu JZ, Edwards RA, Young SG, Raffatellu M, Osborne TF. Linking lipid metabolism to the innate immune response in macrophages through sterol regulatory element binding protein-1a. Cell Metab. 2011;13:540–549. doi: 10.1016/j.cmet.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Oishi Y, Spann NJ, Link VM, Muse ED, Strid T, Edillor C, Kolar MJ, Matsuzaka T, Hayakawa S, Tao J, et al. Srebp1 contributes to resolution of pro-inflammatory tlr4 signaling by reprogramming fatty acid metabolism. Cell Metab. 2017;25:412–427. doi: 10.1016/j.cmet.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schultz JR, Tu H, Luk A, Repa JJ, Medina JC, Li L, Schwendner S, Wang S, Thoolen M, Mangelsdorf DJ, et al. Role of LXRs in control of lipogenesis. Genes Dev. 2000;14:2831–2838. doi: 10.1101/gad.850400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jongstra-Bilen J, Zhang CX, Wisnicki T, Li MK, White-Alfred S, Ilaalagan R, Ferri DM, Deonarain A, Wan MH, Hyduk SJ, et al. Oxidized low-density lipoprotein loading of macrophages downregulates tlr-induced proinflammatory responses in a gene-specific and temporal manner through transcriptional control. J Immunol. 2017;199:2149–2157. doi: 10.4049/jimmunol.1601363. [DOI] [PubMed] [Google Scholar]
- 101.Ito A, Hong C, Rong X, Zhu X, Tarling EJ, Hedde PN, Gratton E, Parks J, Tontonoz P. LXRs link metabolism to inflammation through Abca1-dependent regulation of membrane composition and TLR signaling. eLife. 2015;4:e08009. doi: 10.7554/eLife.08009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Baardman J, Verberk SGS, Prange KHM, van Weeghel M, van der Velden S, Ryan DG, Wust RCI, Neele AE, Speijer D, Denis SW, et al. A defective pentose phosphate pathway reduces inflammatory macrophage responses during hypercholesterolemia. Cell Rep. 2018;25:2044–2052.e2045. doi: 10.1016/j.celrep.2018.10.092. [DOI] [PubMed] [Google Scholar]
- 103.Emini Veseli B, Perrotta P, De Meyer GR, Roth L, Van der Donckt C, Martinet W, De Meyer GR. Animal models of atherosclerosis. Eur J Pharmacol. 2017;816:3–13. doi: 10.1016/j.ejphar.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 104.Getz GS, Reardon CA. Animal models of atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:1104–1115. doi: 10.1161/ATVBAHA.111.237693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Meir KS, Leitersdorf E. Atherosclerosis in the apolipoprotein-E-deficient mouse: a decade of progress. Arterioscler Thromb Vasc Biol. 2004;24:1006–1014. doi: 10.1161/01.ATV.0000128849.12617.f4. [DOI] [PubMed] [Google Scholar]
- 106.Piedrahita JA, Zhang SH, Hagaman JR, Oliver PM, Maeda N. Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embryonic stem cells. Proc Natl Acad Sci U S A. 1992;89:4471–4475. doi: 10.1073/pnas.89.10.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Silvestre-Roig C, de Winther MP, Weber C, Daemen MJ, Lutgens E, Soehnlein O. Atherosclerotic plaque destabilization: mechanisms, models, and therapeutic strategies. Circ Res. 2014;114:214–226. doi: 10.1161/CIRCRESAHA.114.302355. [DOI] [PubMed] [Google Scholar]
- 108.Ishibashi S, Goldstein JL, Brown MS, Herz J, Burns DK. Massive xanthomatosis and atherosclerosis in cholesterol-fed low density lipoprotein receptor-negative mice. J Clin Invest. 1994;93:1885–1893. doi: 10.1172/JCI117179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Maxwell KN, Breslow JL. Adenoviral-mediated expression of Pcsk9 in mice results in a low-density lipoprotein receptor knockout phenotype. Proc Natl Acad Sci U S A. 2004;101:7100–7105. doi: 10.1073/pnas.0402133101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rashid S, Curtis DE, Garuti R, Anderson NN, Bashmakov Y, Ho YK, Hammer RE, Moon YA, Horton JD. Decreased plasma cholesterol and hypersensitivity to statins in mice lacking Pcsk9. Proc Natl Acad Sci U S A. 2005;102:5374–5379. doi: 10.1073/pnas.0501652102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Roche-Molina M, Sanz-Rosa D, Cruz FM, García-Prieto J, López S, Abia R, Muriana FJ, Fuster V, Ibáñez B, Bernal JA. Induction of sustained hypercholesterolemia by single adeno-associated virus-mediated gene transfer of mutant hPCSK9. Arterioscler Thromb Vasc Biol. 2015;35:50–59. doi: 10.1161/ATVBAHA.114.303617. [DOI] [PubMed] [Google Scholar]
- 112.Goettsch C, Hutcheson JD, Hagita S, Rogers MA, Creager MD, Pham T, Choi J, Mlynarchik AK, Pieper B, Kjolby M, et al. A single injection of gain-of-function mutant PCSK9 adeno-associated virus vector induces cardiovascular calcification in mice with no genetic modification. Atherosclerosis. 2016;251:109–118. doi: 10.1016/j.atherosclerosis.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vozenilek AE, Blackburn CM, Schilke RM, Chandran S, Castore R, Klein RL, Woolard MD. AAV8-mediated overexpression of mPCSK9 in liver differs between male and female mice. Atherosclerosis. 2018;278:66–72. doi: 10.1016/j.atherosclerosis.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Paigen B, Morrow A, Holmes PA, Mitchell D, Williams RA. Quantitative assessment of atherosclerotic lesions in mice. Atherosclerosis. 1987;68:231–240. doi: 10.1016/0021-9150(87)90202-4. [DOI] [PubMed] [Google Scholar]
- 115.Ji R, Li X, Zhou C, Tian Q, Li C, Xia S, Wang R, Feng Y, Zhan W. Identifying macrophage enrichment in atherosclerotic plaques by targeting dual-modal US imaging/MRI based on biodegradable Fe-doped hollow silica nanospheres conjugated with anti-CD68 antibody. Nanoscale. 2018;10:20246–20255. doi: 10.1039/c8nr04703k. [DOI] [PubMed] [Google Scholar]
- 116.Lougheed M, Lum CM, Ling W, Suzuki H, Kodama T, Steinbrecher U. High affinity saturable uptake of oxidized low density lipoprotein by macrophages from mice lacking the scavenger receptor class A type I/II. J Biol Chem. 1997;272:12938–12944. doi: 10.1074/jbc.272.20.12938. [DOI] [PubMed] [Google Scholar]
- 117.Feig JE, Fisher EA. Laser capture microdissection for analysis of macrophage gene expression from atherosclerotic lesions. Methods Mol Biol. 2013;1027:123–135. doi: 10.1007/978-1-60327-369-5_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Thomas AC, Eijgelaar WJ, Daemen MJ, Newby AC. Foam cell formation in vivo converts macrophages to a pro-fibrotic phenotype. PLoS One. 2015;10:e0128163. doi: 10.1371/journal.pone.0128163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.de Gaetano M, Crean D, Barry M, Belton O. M1- and m2-type macrophage responses are predictive of adverse outcomes in human atherosclerosis. Front Immunol. 2016;7:275. doi: 10.3389/fimmu.2016.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jin X, Kruth HS. Culture of macrophage colony-stimulating factor differentiated human monocyte-derived macrophages. J Vis Exp. 2016:54244. doi: 10.3791/54244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.MacDonald C. Development of new cell lines for animal cell biotechnology. Crit Rev Biotechnol. 1990;10:155–178. doi: 10.3109/07388559009068265. [DOI] [PubMed] [Google Scholar]
- 122.Kaur G, Dufour JM. Cell lines: valuable tools or useless artifacts. Spermatogenesis. 2012;2:1–5. doi: 10.4161/spmg.19885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mäkinen PI, Lappalainen JP, Heinonen SE, Leppänen P, Lähteenvuo MT, Aarnio JV, Heikkilä J, Turunen MP, Ylä-Herttuala S. Silencing of either SR-A or CD36 reduces atherosclerosis in hyperlipidaemic mice and reveals reciprocal upregulation of these receptors. Cardiovasc Res. 2010;88:530–538. doi: 10.1093/cvr/cvq235. [DOI] [PubMed] [Google Scholar]
- 124.Qin Z. The use of THP-1 cells as a model for mimicking the function and regulation of monocytes and macrophages in the vasculature. Atherosclerosis. 2012;221:2–11. doi: 10.1016/j.atherosclerosis.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 125.Tsuchiya S, Yamabe M, Yamaguchi Y, Kobayashi Y, Konno T, Tada K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1) Int J Cancer. 1980;26:171–176. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- 126.Zhao YL, Tian PX, Han F, Zheng J, Xia XX, Xue WJ, Ding XM, Ding CG. Comparison of the characteristics of macrophages derived from murine spleen, peritoneal cavity, and bone marrow. J Zhejiang Univ Sci B. 2017;18:1055–1063. doi: 10.1631/jzus.B1700003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Trouplin V, Boucherit N, Gorvel L, Conti F, Mottola G, Ghigo E. Bone marrow-derived macrophage production. J Vis Exp. 2013:e50966. doi: 10.3791/50966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lavin Y, Merad M. Macrophages: gatekeepers of tissue integrity. Cancer Immunol Res. 2013;1:201–209. doi: 10.1158/2326-6066.CIR-13-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sohn M, Na HY, Ryu SH, Choi W, In H, Shin HS, Park JS, Shim D, Shin SJ, Park CG. Two distinct subsets are identified from the peritoneal myeloid mononuclear cells expressing both CD11c and CD115. Immune Netw. 2019;19:e15. doi: 10.4110/in.2019.19.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ghosn EE, Cassado AA, Govoni GR, Fukuhara T, Yang Y, Monack DM, Bortoluci KR, Almeida SR, Herzenberg LA, Herzenberg LA. Two physically, functionally, and developmentally distinct peritoneal macrophage subsets. Proc Natl Acad Sci U S A. 2010;107:2568–2573. doi: 10.1073/pnas.0915000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, Becker CD, See P, Price J, Lucas D, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38:792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Morrisey EE, Ip HS, Lu MM, Parmacek MS. GATA-6: a zinc finger transcription factor that is expressed in multiple cell lineages derived from lateral mesoderm. Dev Biol. 1996;177:309–322. doi: 10.1006/dbio.1996.0165. [DOI] [PubMed] [Google Scholar]
- 134.Leijh PC, van Zwet TL, ter Kuile MN, van Furth R. Effect of thioglycolate on phagocytic and microbicidal activities of peritoneal macrophages. Infect Immun. 1984;46:448–452. doi: 10.1128/iai.46.2.448-452.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Meza-Perez S, Randall TD. Immunological functions of the omentum. Trends Immunol. 2017;38:526–536. doi: 10.1016/j.it.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang N, Czepielewski RS, Jarjour NN, Erlich EC, Esaulova E, Saunders BT, Grover SP, Cleuren AC, Broze GJ, Edelson BT, et al. Expression of factor V by resident macrophages boosts host defense in the peritoneal cavity. J Exp Med. 2019;216:1291–1300. doi: 10.1084/jem.20182024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cassado AA, D’Império Lima MR, Bortoluci KR. Revisiting mouse peritoneal macrophages: heterogeneity, development, and function. Front Immunol. 2015;6:225. doi: 10.3389/fimmu.2015.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Winkels H, Ehinger E, Vassallo M, Buscher K, Dinh HQ, Kobiyama K, Hamers AA, Cochain C, Vafadarnejad E, Saliba AE, et al. Atlas of the immune cell repertoire in mouse atherosclerosis defined by single-cell RNA-sequencing and mass cytometry. Circ Res. 2018;122:1675–1688. doi: 10.1161/CIRCRESAHA.117.312513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cochain C, Vafadarnejad E, Arampatzi P, Pelisek J, Winkels H, Ley K, Wolf D, Saliba AE, Zernecke A. Single-cell RNA-seq reveals the transcriptional landscape and heterogeneity of aortic macrophages in murine atherosclerosis. Circ Res. 2018;122:1661–1674. doi: 10.1161/CIRCRESAHA.117.312509. [DOI] [PubMed] [Google Scholar]
- 140.Kim K, Choi JH. Response by Kim and Choi to letter regarding article, “Transcriptome analysis reveals nonfoamy rather than foamy plaque macrophages are proinflammatory in atherosclerotic murine models”. Circ Res. 2018;123:e50. doi: 10.1161/CIRCRESAHA.118.314163. [DOI] [PubMed] [Google Scholar]