Abstract
Anisodamine hydrobromide (AniHBr) is a Chinese medicine used to treat septic shock. However, whether AniHBr could ameliorate septic acute kidney injury and the underlying mechanism were not investigated. In the present study, 18 male Sprague-Dawley rats (200–250 g) were randomly divided into control, lipopolysaccharide (LPS) and LPS+AniHBr groups. Rats were intravenously administrated with LPS or normal saline (for control). After 4 h, the rats were intravenously administrated with AniHBr (LPS+AniHBr) or normal saline at 4 h intervals. Hemodynamic parameters including blood pressure and heart rate were measured. The histopathologic evaluation of kidney tissues was performed. Lactate, creatine kinase, inflammatory cytokines and oxidative stress indicators were determined. Using Seahorse analysis, the metabolic analysis of mitochondrial stress and glycolytic stress in human renal proximal tubular epithelial cells treated with TNF-α in the presence of AniHBr was performed. AniHBr administration significantly reduced serum creatine kinase and lactate following LPS treatment. AniHBr significantly improved hemodynamics in sepsis rats including increase in the mean atrial pressure and reduction in the heart rate. AniHBr significantly attenuated LPS-induced TNF-α, IL-6 and IL-1β in serum, and LPS-induced TNF-α and IL-1β in renal tissues. The LPS-reduced SOD activity and LPS-increased MDA content were reversed by AniHBr. In vitro, TNF-α increased mitochondrial oxygen consumption and glycolysis, but inhibited the ATP generation, which was reversed by AniHBr. Thus, AniHBr protects against the LPS-induced inflammatory cytokines, mitochondrial dysfunction and oxidative stress, and thus attenuates the LPS-induced acute kidney injury, showing AniHBr is a promising therapeutic drug for septic kidney injury.
Keywords: metabolic analysis, oxidative stress, renal injury, sepsis
Introduction
Sepsis or septic shock is the major cause of death from infection. Depending of the extent of the inflammatory response, the patients may suffer multiple organ failure, including the heart, liver, lungs and acute kidney injury (AKI). Sepsis is the greatest risk for septic AKI among hospitalized patients, which was account for approximately 30–70% of AKI patients. The incidence of sepsis is 29% in overall intensive care units and the in-hospital mortality rate is approximately 33% [1]. Therefore, it is important to develop diagnostic and treatment strategies to protect the patients from sepsis. In fact, there is still no effective drug available in the clinical setting.
Sepsis-induced AKI is closely modulated by inflammatory cytokines such as tumor necrosis factor α (TNF-α), which could induce most of septic symptoms and signs including AKI. It was demonstrated that suppression of TNF-α signaling by anti-TNF-α antibody could result in sepsis resistance [2]. The bacterial endotoxin lipopolysaccharide (LPS) is extremely strong stimulators of inflammatory reactions, which was administered to construct the sepsis animal model that is mostly studied. The LPS-induced AKI could be suppressed by inactivation of TNF-α signaling.
As adenosine triphosphate (ATP) generation was suppressed in septic patients, the failure of oxygen usage in mitochondria will damage the organic cell and tissues, as well as cause the metabolic abnormalities. High level of reactive oxygen species (ROS) is required for pathogen clearance and intracellular signaling. It was proposed the pathogenesis of septic-induced AKI based on an assumption that the adaptive responses to injury such as interplay among inflammation and dysfunctions of vascular microcirculatory and oxidative stress are driven by mitochondria [3].
Anisodamine hydrobromide (C17H23NO4·HBr, AniHBr) is an active ingredient extracted from the root of a Chinese specialty plant Scopolia tangutica maxim in 1956. The anisodamine has been synthesized and used clinically in China for improving the blood circulation in patients with organphosphorus poisoning or septic shock since 1956 [4] and was a proved powerful inhibitor of platelet aggregation possibly through inhibition of cyclo-oxygenase or thromboxane synthetase at 1982 [5]. It was implicated that anisodamine is an antioxidant and antagonist of muscarinic acetylcholine receptor to prevent free radical and thus protects against cellular damage [6]. Anisodamine could reduce the damage of myocardial mitochondria and thus inhibiting the cell apoptosis [7–9]. In recent, it was reported that anisodamine could significantly inhibit oxidative stress and attenuate the TNF-α in myocardial ischemia/reperfusion injured rats [10,11]. In mice with Shiga toxin-producing Escherichia coli infection, anisodamine administration suppressed TNF-α mRNA expression [12]. In glycerol-induced AKI, anisodamine administration reduced ROS-induced oxidative stress and thus protected against AKI [13]. Therefore, we hypothesized that the natural extract AniHBr prevent kidney from septic injury by suppressing the mitochondrial metabolism and subsequently mitochondrial dysfunction via suppression of oxidative stress and release of inflammatory cytokines.
In the present study, we aimed to investigate the role of AniHBr in renal injury in septic rats, and explore the effects of AniHBr on inflammation and inflammation-damaged mitochondrial function by in vitro experiments.
Materials and methods
Animals and experimental protocols
The present study is undertaken according to the recommendations in the Guide for the Care and Use of Laboratory Animals with approval by the Animal Research Ethics Committee of Laboratory Animals Center of Sichuan University (No.2019-5-15), Chengdu, China. Male Sprague-Dawley rats (200–250 g) were provided by the Experimental Animal Center of the Sichuan University (Chengdu, China). The rats were housed in solid-bottom cage at constant temperature (25 ± 2°C) and humidity (55 ± 10%) in a temperature-controlled facility in a standard breeding environment, with 12:12 h light/dark cycles in Laboratory Animals Center of Sichuan University. All rats were allowed access to food and water ad libitum, but they were fasted for 1 h before the experiments.
All rats were randomly divided into three groups as follows: control group (n=6), LPS group (n=6) and LPS+AniHBr group (n=6). The rats were kept grouped in cages (6 rats per cage). LPS from Escherichia coli O55:B5 (L2880; Sigma, Mo, U.S.A.) was administrated intravenously by tail vein injection with 5 mg/kg LPS in conscious rats. Rats were administrated intravenously by tail vein injection with AniHBr (the purity of C17H23NO4·HBr > 98.5%, Lot. 190501, Chengdu NO.1 Pharmaceutical Co., Ltd, China) with 3.6 mg/kg or an equal volume of vehicle (normal saline, for control group) at 4 h intervals. The first injection of AniHBr is at 4 h after LPS administration, and the last dose was given at 20 h after LPS administration. The mean arterial pressures and heart rates were measured by Medlab non-invasive pressure monitoring system (KEW, Nanjing, China). The blood specimens were collected from the orbital venous plexus at 24 h after LPS injection, and serum was isolated by centrifugation at 1500 g for 15 min at 4°C within 1 h after blood collection. After 24 h, all rats were euthanasia using carbon dioxide [14] and kidneys were isolated for further analysis. For histopathologic evaluation, part of each kidney was fixed in 4% paraformaldehyde solution, and then the remaining tissue was frozen immediately in liquid nitrogen and stored at −80°C.
Histopathologic evaluation
The kidney tissues were dehydrated and embedded in paraffin, and then were sliced into 3 μm sections and stained with hematoxylin and eosin for histopathologic evaluation. Images were acquired with an Olympus DP70 microscopy with a digital camera (Olympus Optical Co, Ltd., Tokyo, Japan). Using ImageJ software v.1.52s (National Institutes of Health, U.S.A.), the swelling, vacuolar degeneration, necrosis and desquamation of tubular epithelial cells were graded using a semi-quantitative scoring method as previously described [13]: (I) <25% of cortical tubules; (II) 25–50% of cortical tubules; (III) 50–75% of cortical tubules; (IV) >75% of cortical tubules. The extent of LPS-induced renal injury and recovery by administration of AniHBr was performed. All histological evaluation was performed in a double blinded manner.
Assessment of renal function, inflammation and oxidative stress
For renal function assessment, lactate (BC2235, Solarbio, Beijing, China) and creatine kinase (BC1145, Solarbio, Beijing, China) were measured using assay kits according to the manufacturer’s instructions. For inflammatory cytokines, serum TNF-α (Neobioscience, Shenzhen, China), IL-1β (Solarbio, Beijing, China) and IL-6 (Neobioscience, Shenzhen, China) were determined using commercial enzyme-linked immunosorbent assay (ELISA) kits. For ELISA, data were detected by absorbance at 450 nm using a Labserv K3 Touch microplate reader (ThermoFisher, U.S.A.).
Kidney tissues was gently homogenized in homogenization solution (10 mM Tris-HCl, 1 Mm EDTA, pH 7.4). After centrifuged at 3500 g for 10 min at 4°C, the supernatant was used for the measurement of malondialdehyde (MDA) concentration (A003-1, Nanjing Jiancheng Bioengineering Institute, Jiangsu, China) and superoxide dismutase (SOD) activity (A001-1, Nanjing Jiancheng Bioengineering Institute, Jiangsu, China) using assay kits according to the manufacturer’s instructions, and IL-6 level (Neobioscience, Shenzhen, China) was determined using the ELISA kit.
In vitro cell culture, mito stress test and glycolytic stress test
Human renal proximal tubular epithelial cell line HK-2 (ATCC, Rockville, Md, U.S.A.) were cultured in Dulbecco Modified Eagle medium (DMEM, Hyclone, U.S.A.) supplemented with 10% fetal bovine serum (Tianhang, Zhejiang, China) and 1% penicillin/streptomycin (Invitrogen, ThermoFisher, U.S.A.) at 37°C with 5% CO2.
Cells were treated with or without a final concentration of 10 μg/ml AniHBr (Lot.190303, dissolved in ddH2O; Chengdu NO.1 Pharmaceutical Co., Ltd, China) in the presence of a final concentration of 10 ng/ml TNF-α (T6674, Sigma, U.S.A.) for 24 h. Then, the mito stress test was performed with the XF96 Extracellular Flux Analyzer (Seahorse Bioscience, MA, U.S.A.) according to the supper’s instructions [15]. After calibration (baseline), with an addition of 1 μM oligomycin, 2 μM carbonylcyanide p-(trifluoromethoxy) phenylhydrazone (FCCP) and 0.5 μM antimycin A, the oxygen consumption rate (OCR) was measured and then the basal OCR, maximal OCR and ATP-linked OCR (ATP production) were analyzed using Seahorse Wave software v.2.6.1 (Agilent, U.S.A.). With an addition of 10 mM glucose, 1 μM oligomycin and 0.5 mM 2-deoxyglucose (2-DG), the extracellular acidification rate (ECAR; glycolysis) was measured and analyzed. Graphs were performed using Garphpad Prism 7.0 software (Graphpad software, Inc., U.S.A.).
Statistical analysis
Data are expressed as means ± SD from at least three independent experiments. All statistical analyses were performed using SPSS 25 (IBM, U.S.A.) with one-way analysis of variance (ANOVA) with least significant difference (LSD) post hoc test. Values of P<0.05 were considered statistical significant.
Results
AniHBr reduces serum creatine kinase and lactate concentrations in sepsis rats
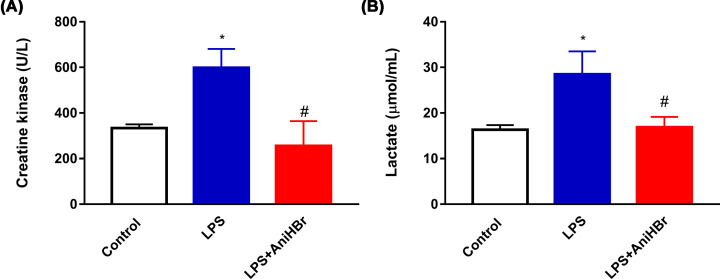
LPS rats showed higher serum creatine kinase compared with control rats after 24 h. Administration of AniHBr significantly suppressed the serum creatine kinase (Control group, 333.3 ± 17.2 U/l; LPS group, 598.0 ± 83.2 U/l; LPS+AniHBr, 255.7 ± 108.9 U/l) (Figure 1A).
Figure 1. Serum creatine kinase (A) and lactate (B) concentrations at 24 h after the administration of LPS or vehicle (normal saline, control).
The rats were treated with LPS, and then administrated with vechicle, or AniHBr. Mean ± SD, *P<0.05 vs. control; #P<0.05 vs. LPS.
Compared with control rats, LPS significantly increased the lactate concentrations at 24 h, but AniHBr administration significantly prevented this increase (Control group, 16.34 ± 1.0 μmol/ml; LPS group, 28.5 ± 5.0 μmol/ml; LPS+AniHBr, 16.9 ± 2.3 μmol/ml) (Figure 1B).
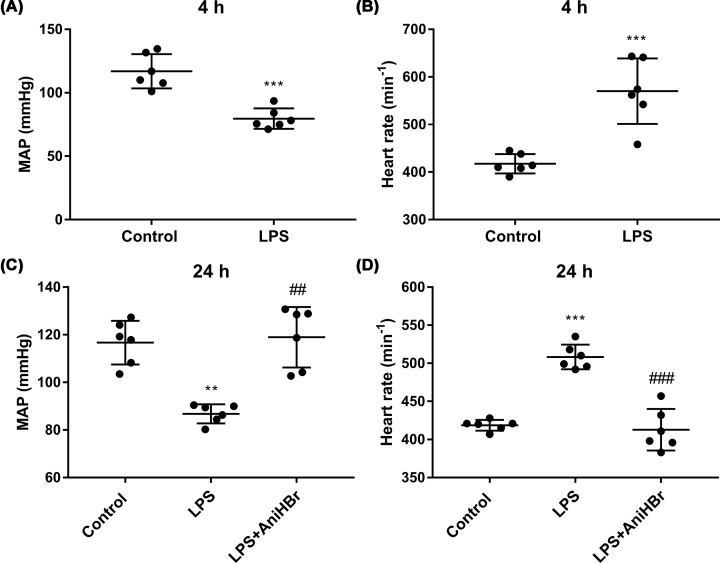
AniHBr improve hemodynamics in sepsis rats
The hemodynamic parameters including mean arterial pressure (Figure 2A,C) and heart rate (Figure 2B,D) were measured. LPS significantly increased the heart rate (Control group, 116.9 ± 13.5 s−1; LPS group, 79.6 ± 8.1 s−1; Figure 2B) and reduced the mean atrial pressure (Control group, 417.5 ± 20.5 mmHg; LPS group, 570.0 ± 69.0 mmHg; Figure 2A) at 4 h compared with control group. After 24 h, the heart rate of rats in LPS group was slightly reduced, compared with the heart rate of rats in LPS group at 4 h (P>0.05). AniHBr significantly increased the mean atrial pressure (Control group, 116.7 ± 9.2 mmHg; LPS group, 86.8 ± 4.0 mmHg; LPS+AniHBr, 118.9 ± 12.7 mmHg; Figure 2C) and reduced the heart rate (Control group, 418.7 ± 7.1 s−1; LPS group, 508.3 ± 16.2 s−1; LPS+AniHBr, 412.8 ± 27.3 s−1; Figure 2D) at 24 h, compared with the LPS group. Overall, AniHBr improved hemodynamics in sepsis rats.
Figure 2. Hemodynamic parameters.
The mean atrial pressure (MAP; A and C) and heart rate (B and D) were measured at 4 h and 24 h after the administration of LPS or vehicle (normal saline, control). The rats were treated with LPS, and then administrated with vechicle, or AniHBr. Mean ± SD, **P<0.01, ***P<0.05 vs. control; ##P<0.01, ###P<0.001 vs. LPS.
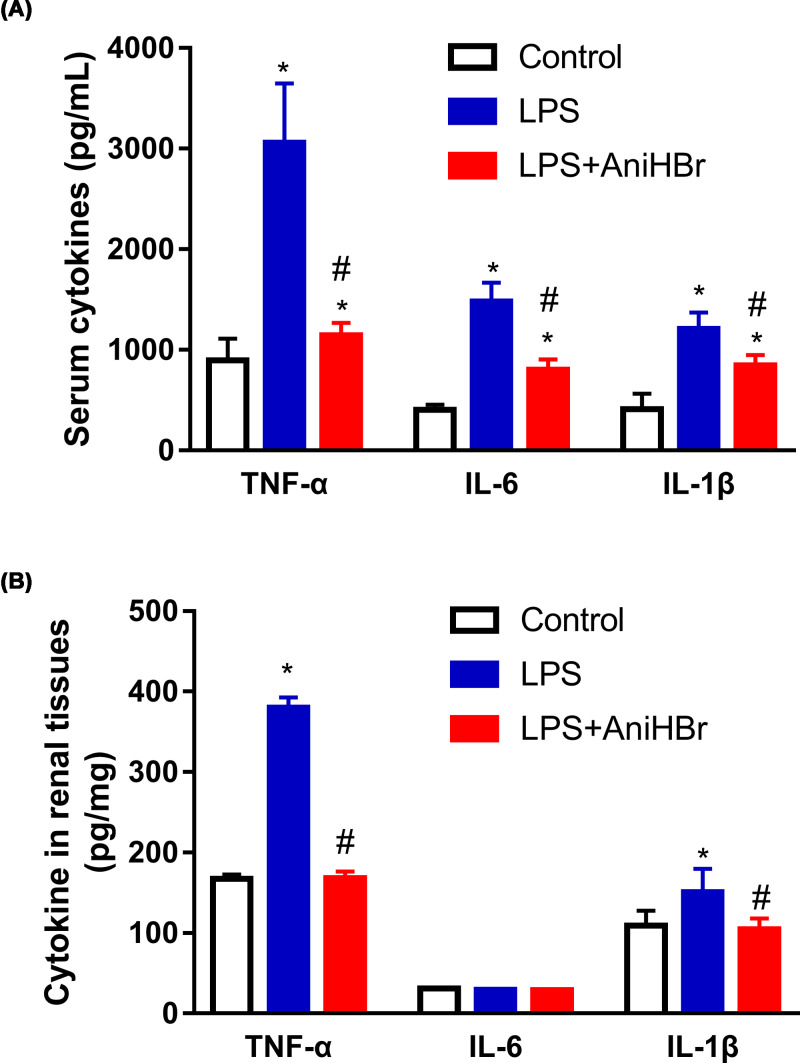
AniHBr inhibits inflammation in sepsis rats
Inflammatory cytokines including TNF-α, IL-6 and IL-1β in serum and renal tissues specimens isolated from rats after LPS and AniHBr administration were detected (Figure 3). Compared with control rats, TNF-α, IL-6 and IL-1β in serum were significantly increased in the rats after LPS administration with or without AniHBr (Figure 3A). Notably, AniHBr administration significantly attenuated the LPS-induced secretion of serum TNF-α, IL-6 and IL-1β (Figure 3A). In renal tissues, TNF-α and IL-1β were demonstrated to significantly produce after the administration of LPS compared with the control group (Figure 3B). AniHBr adminstration significantly abolished the LPS-induced increases of TNF-α and IL-1β in renal tissues (Figure 3B). However, it was not observed significant differences in IL-6 levels among the control group, LPS group and LPS+AniHBr group.
Figure 3. Serum and renal TNF-α, IL-1β and IL-6 levels at 24 h.
The rats were treated with LPS, and then administrated with vechicle or AniHBr. (A) The cytokine in serum. (B) The renal cytokine concentration is presented in mg of tissue wet. Mean ± SD, *P<0.05 vs. control; #P<0.05 vs. LPS.
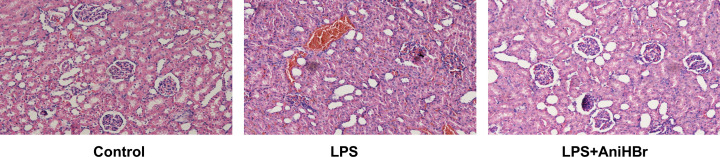
AniHBr alleviates LPS-induced renal damage
Renal histology was performed after AniHBr administration (Figure 4). There were no pathological changes in the control group. Aggravated cortical tubular damages including tubular dilation, tubular necrosis, brush border loss, tubule degeneration and tubular cast formation were observed in kidney cortex and medulla after 24 h of LPS administration (Grade III). With AniHBr treatment, those histopathological changes were ameliorated (Grade I).
Figure 4. Effect of AniHBr in LPS-induced renal injury.
The rats were treated with LPS, and then administrated with vechicle, or AniHBr. Renal histology was performed after AniHBr administration.
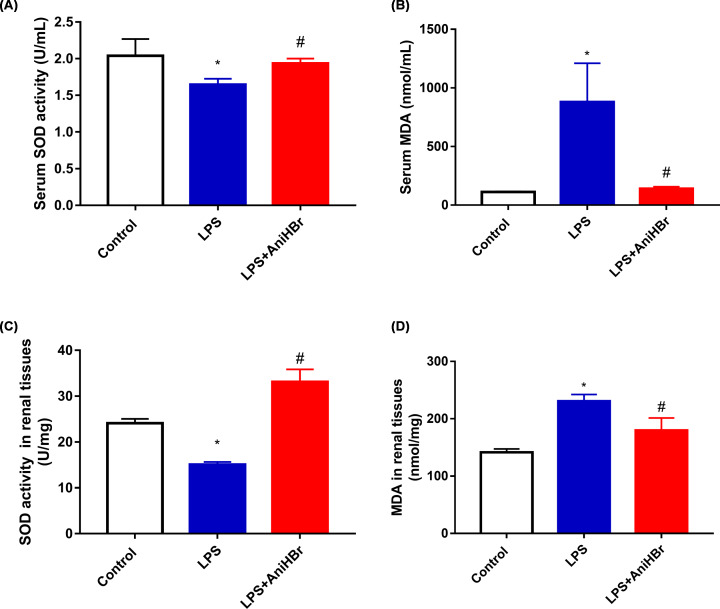
AniHBr reduces MDA levels and increased SOD activity after LPS-induced renal injury
The antioxidative effect of AniHBr in LPS rats was investigated using ELISA (Figure 5). Compared with the control group, in both serum and renal tissues, the SOD activity was significantly reduced (Figure 5A,C) and MDA was significantly increased (Figure 5B,D) in LPS group. However, AniHBr administration present an opposite effect on SOD and MDA in both serum and renal tissues. The serum and renal tissues levels of SOD and MDA were significantly reversed by AniHBr administration in the LPS rats.
Figure 5. Serum and renal SOD, and MDA levels at 24 h.
The rats were treated with LPS, and then administrated with vechicle, or AniHBr. (A) Serum SOD activity. (B) Serum MDA. (C) SOD activity in renal tissues. (D) MDA in renal tissues. Mean ± SD, *P<0.05 vs. control; #P<0.05 vs. LPS.
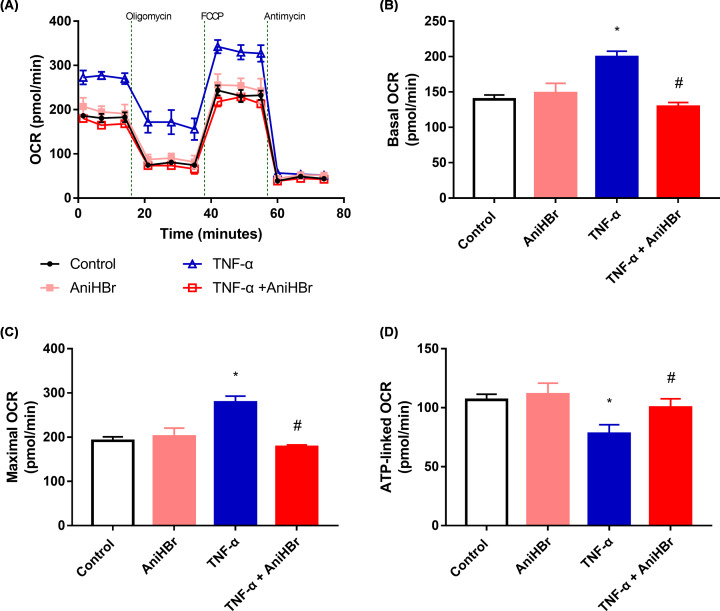
AniHBr alleviated TNF-α inhibited oxidative phosphorylation
Mito stress tests (Figure 6A) showed that the basal OCR (Figure 6B) and maximal OCR (Figure 6C) in the 10 ng/ml TNF-α induction group were significantly increased, and ATP-linked OCR (Figure 6D) was significantly reduced, compared with in control group. In the presence of AniHBr, basal OCR, maximal OCR and ATP-linked OCR were significantly reversed compared with the TNF-α induction group. The basal OCR, maximal OCR and ATP-linked OCR were not significantly changed in rats with the addition of AniHBr, compared with the control rats.
Figure 6. AniHBr rescued TNF-α inhibited metabolic switch.
Cells were treated with or without a final concentration of 10 μg/ml AniHBr in the presence of a final concentration of 10 ng/ml TNF-α for 24 h. Then, the mito stress test was performed. (A) Oxygen consumption rate (OCR) curves. (B) Basal OCR. (C) Maximal OCR. (D) ATP-linked OCR. n=3, Mean ± SD, *P<0.05 vs. control; #P<0.05 vs. TNF-α.
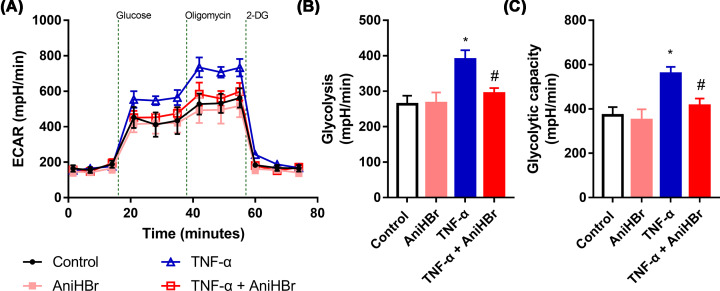
Glycolysis stress tests (Figure 7A) showed that the glycolysis (Figure 7B) and glycolytic capacity (Figure 7C) in the presence of 10 ng/ml TNF-α was significantly increased, while it was not observed significant changes with the addition of AniHBr compared with the control group, suggesting the increase of glycolysis and glycolytic capacity by TNF-α was attenuated by the addition of AniHBr.
Figure 7. AniHBr rescued TNF-α inhibited glycolysis and glycolytic capacity.
Cells were treated with or without a final concentration of 10 μg/ml AniHBr in the presence of a final concentration of 10 ng/ml TNF-α for 24 h. Then, the glycolysis was measured and analyzed. (A) Extracellular acidification rate (ECAR). (B) Glycolysis. (C) Glycolytic capacity. n=3, Mean ± SD, *P<0.05 vs. control; #P<0.05 vs. TNF-α.
Discussion
Tissue hypoxia and mitochondrial dysfunctions are thought to be important mediators of sepsis-induced multiple organ dysfunction [16]. In recent, it was contradicted renal ischemia contributed to AKI [17]. Renal blood flow is even increased in septic AKI, suggesting intrarenal vasodilation together with microcirculatory changes lead to renal functional changes [17]. Nevertheless, inflammation and mitochondrial dysfunction was closely associated with AKI [18,19]. Nowadays, there was no available specific pharmacological therapy for renal protection [17]. In the present study, we reported that AniHBr inhibits inflammatory cytokines and thus protects against AKI by attenuating mitochondrial dysfunction.
Anisodamine administration could improve the hemodynamics, maintain the histology by inhibiting the consecutive tubular necrosis and protect against functional impairment. Raised lactate concentration is an important prognostic marker for sepsis, and the reduction of lactate content is associated with improved outcomes [20]. AniHBr significantly suppressed the serum creatine kinase and lactate concentrations in rats with administration of intravenous LPS, showing a protective effect in LPS-induced rat septic AKI. The LD50 (median lethal dose) of AniHBr i.v. administration is 742 mg/kg. Thus, the multiply injection of AniHBr is safe.
During sepsis, inflammation mediators will not only activate the immune systems but also mediate host cellular injury. TNF-α elicited by LPS might be responsible for the septic shock [21]. Depletion of M2 macrophage could induce the secretion of TNF-α during sepsis-induced AKI, and significantly suppress the proliferation of tubular cells [22], suggesting TNF-α is associated with the tubular cell necrosis. It was demonstrated that mitochondrial dysfunction could decrease ATP generation, enhance mitochondrial oxidative stress, cell necrosis and apoptosis, and thus contributes to AKI [19]. Amelioration of mitochondrial dysfunction could inhibit the tubule epithelial cell apoptosis and protect LPS-induced AKI [18]. Oxidative phosphorylation takes place in the inner mitochondrial membrane where electron transport chain lies. The transfer of electrons leads to ATP production. Molecular oxygen is the final receptor of the electrons. Thus, oxygen consumption rate was used to assess the mitochondrial function [23]. It was demonstrated that proinflammatory TNF-α induced oxygen consumption in vitro HEK293 cells [24]. AniHBr inhibited the TNF-α induced oxygen consumption.
Mitochondria is a main source of intracellular ROS. In contrast-induced AKI, there was a protective effect against AKI by attenuating mitochondrial dysfunction and oxidative stress [25]. AniHBr significantly increased SOD activity and reduced the MDA concentration in the serum and renal tissues in septic rats. In the presence of TNF-α, oxygen consumption rate was significantly increased, and ATP-linked oxygen consumption rate (ATP production) was significantly reduced. In consistent to the change in mito stress, glycolysis and glycolysis capacity was significantly increased by TNF-α, suggesting proinflammatory cytokine TNF-α induced mitochondrial dysfunction, enhanced mitochondrial oxidative stress and glycolysis. AniHBr significantly inhibited those changes induced by TNF-α. Thus, AniHBr is an efficient anti-inflammatory and antioxidant agent. The molecular mechanism of AniHBr in protection of tubular cell necrosis should be investigated in the future. Moreover, the molecular mechanism by which AniHBr can significantly attenuate LPS-induced TNF-α, IL-6 and IL-1β in serum and attenuate oxidative stress will be investigated in the future.
The anti-inflammatory effect of anisodamine had been reported in LPS-induced pancreatic acinar cells [26]. Anisodamine might play a significant role in LPS-induced microvascular endothelial cells [27]. Endothelial cells interact with other cells via exosome [28]. Anisodamine acts as a cholinergic antagonist to improve smooth muscle function [29]. In recent, it was demonstrated that antagonist of M2 subtype muscarinic cholinergic receptors (AF-DX116) might accelerate LPS-induced lung and hepatic injuries, and antagonist of M1 subtype muscarinic cholinergic receptors (pirenzepine) and non-selective competitive antagonist atropine relieved those injuries [30]. The interplay of microvascular dysfunction and inflammation, and oxidative stress might lead to mitochondrial dysfunction via muscarinic cholinergic receptors. Future biomarkers for prevention and therapy of AKI may represent a better protection of the microvasculature and maintain the function of the endothelium.
Conclusion
In the present study, we concluded that inflammation, mitochondrial dysfunction and oxidative stress are involved in LPS-induced AKI, and AniHBr protects against the LPS-induced inflammatory cytokines, mitochondrial dysfunction and oxidative stress, and thus attenuates the LPS-induced AKI. AniHBr might improve the balance in energy utilization via modulation of inflammation, which is a promising therapeutic drug for the treatment of LPS-induced acute kidney injury.
Abbreviations
- 2-DG
2-deoxyglucose
- AKI
acute kidney injury
- AniHBr
anisodamine hydrobromide
- ANOVA
analysis of variance
- ATP
adenosine triphosphate
- ECAR
extracellular acidification rate
- FCCP
carbonylcyanide p-(trifluoromethoxy) phenylhydrazone
- LPS
lipopolysaccharide
- LSD
least significant difference
- MDA
malondialdehyde
- OCR
oxygen consumption rate
- ROS
reactive oxygen species
- SOD
superoxidedismutase
- TNF-α
tumor necrosis factor α
Data Availability
All data generated or analyzed during this study are included in this published article.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
Supported by funding from Chengdu No.1 Pharmaceutical Co., Ltd. Chengdu, China [grant number 19H0383].
Author Contribution
F.W., X.Q.D., H.L., X.L.H. and Y.Z. performed the experiments, analyzed and interpreted the data. X.Q.D., X.L.H. and Y.Z. performed the histological examination of the kidney. All authors read and approved the final manuscript.
Ethics Approval
This study is undertaken according to the recommendations in the Guide for the Care and Use of Laboratory Animals with approval by the Animal Research Ethics Committee of Sichuan University, Chengdu, China.
References
- 1.Zhou J., Qian C., Zhao M., Yu X., Kang Y., Ma X. et al. (2014) Epidemiology and outcome of severe sepsis and septic shock in intensive care units in mainland China. PLoS ONE 9, e107181 10.1371/journal.pone.0107181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qu W., Han C., Li M., Zhang J. and Jiang Z. (2018) Anti-TNF-α antibody alleviates insulin resistance in rats with sepsis-induced stress hyperglycemia. J. Endocrinol. Invest. 41, 455–463 10.1007/s40618-017-0742-7 [DOI] [PubMed] [Google Scholar]
- 3.Gomez H., Ince C., De Backer D., Pickkers P., Payen D., Hotchkiss J. et al. (2014) A unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock (Augusta, Ga) 41, 3–11 10.1097/SHK.0000000000000052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang S.T. and Kuo N.L. (1978) Experience in emergency treatment of shock due to infection. Chin. Med. J. 4, 497–500 [PubMed] [Google Scholar]
- 5.Xiu R.J., Hammerschmidt D.E., Coppo P.A. and Jacob H.S. (1982) Anisodamine inhibits thromboxane synthesis, granulocyte aggregation, and platelet aggregation. A possible mechanism for its efficacy in bacteremic shock. JAMA 247, 1458–1460 10.1001/jama.247.10.1458 [DOI] [PubMed] [Google Scholar]
- 6.Poupko J.M., Baskin S.I. and Moore E. (2007) The pharmacological properties of anisodamine. J. Appl. Toxicol. 27, 116–121 10.1002/jat.1154 [DOI] [PubMed] [Google Scholar]
- 7.Liu Y.H., Zhou M.H., Dai Z., Zhang J., Zhu H.Y., Li Y.H. et al. (2013) Protective effect of anisodamine against myocardial cell apoptosis through mitochondria impairment in cardiac arrest in pigs. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 25, 88–91 [DOI] [PubMed] [Google Scholar]
- 8.Xing K., Fu X., Jiang L., Wang Y., Li W., Gu X. et al. (2015) Cardioprotective Effect of Anisodamine Against Myocardial Ischemia Injury and its Influence on Cardiomyocytes Apoptosis. Cell Biochem. Biophys. 73, 707–716 10.1007/s12013-015-0642-4 [DOI] [PubMed] [Google Scholar]
- 9.Yuan X., Zheng Y., Chen C. and Wang C. (2017) Anisodamine inhibits endoplasmic reticulum stress-associated TXNIP/NLRP3 inflammasome activation in rhabdomyolysis-induced acute kidney injury. Apoptosis: An Int. J. Programmed Cell Death 22, 1524–1531 10.1007/s10495-017-1414-y [DOI] [PubMed] [Google Scholar]
- 10.Yin X.L., Shen H., Zhang W. and Yang Y. (2011) Inhibition of endoplasm reticulum stress by anisodamine protects against myocardial injury after cardiac arrest and resuscitation in rats. Am. J. Chin. Med. 39, 853–866 10.1142/S0192415X11009251 [DOI] [PubMed] [Google Scholar]
- 11.Yao B.J., He X.Q., Lin Y.H. and Dai W.J. (2018) Cardioprotective effects of anisodamine against myocardial ischemia/reperfusion injury through the inhibition of oxidative stress, inflammation and apoptosis. Mol. Med. Rep. 17, 1253–1260 [DOI] [PubMed] [Google Scholar]
- 12.Zhang H.M., Ou Z.L. and Yamamoto T. (2001) Anisodamine inhibits shiga toxin type 2-mediated tumor necrosis factor-alpha production in vitro and in vivo. Exp. Biol. Med. (Maywood, N.J.) 226, 597–604 10.1177/153537020122600614 [DOI] [PubMed] [Google Scholar]
- 13.Li Y.F., Xu B.Y., An R., Du X.F., Yu K., Sun J.H. et al. (2019) Protective effect of anisodamine in rats with glycerol-induced acute kidney injury. BMC Nephrol. 20, 223 10.1186/s12882-019-1394-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shomer N.H., Allen-Worthington K.H., Hickman D.L., Jonnalagadda M., Newsome J.T., Slate A.R. et al. (2020) Review of Rodent Euthanasia Methods. J. Am. Assoc. Laboratory Animal Sci.: JAALAS 59, 242–253 10.30802/AALAS-JAALAS-19-000084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leung D.T.H. and Chu S. (2018) Measurement of Oxidative Stress: Mitochondrial Function Using the Seahorse System. Methods Mol. Biol. 1710, 285–293 10.1007/978-1-4939-7498-6_22 [DOI] [PubMed] [Google Scholar]
- 16.Singer M. (2014) The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence 5, 66–72 10.4161/viru.26907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.John S. (2020) Lessons learned from kidney dysfunction: Preventing organ failure. Medizinische Klinik, Intensivmedizin und Notfallmedizin 10.1007/s00063-020-00659-2 [DOI] [PubMed] [Google Scholar]
- 18.Ding Y., Zheng Y., Huang J., Peng W., Chen X., Kang X. et al. (2019) UCP2 ameliorates mitochondrial dysfunction, inflammation, and oxidative stress in lipopolysaccharide-induced acute kidney injury. Int. Immunopharmacol. 71, 336–349 10.1016/j.intimp.2019.03.043 [DOI] [PubMed] [Google Scholar]
- 19.Zuk A. and Bonventre J.V. (2016) Acute Kidney Injury. Annu. Rev. Med. 67, 293–307 10.1146/annurev-med-050214-013407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cecconi M., Evans L., Levy M. and Rhodes A. (2018) Sepsis and septic shock. Lancet (London, England) 392, 75–87 10.1016/S0140-6736(18)30696-2 [DOI] [PubMed] [Google Scholar]
- 21.Mul Fedele M.L., Aiello I., Caldart C.S., Golombek D.A., Marpegan L. and Paladino N. (2020) Differential Thermoregulatory and Inflammatory Patterns in the Circadian Response to LPS-Induced Septic Shock. Front. Cell. Infection Microbiol. 10, 100 10.3389/fcimb.2020.00100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X., Mu G., Song C., Zhou L., He L., Jin Q. et al. (2018) Role of M2 Macrophages in Sepsis-Induced Acute Kidney Injury. Shock (Augusta, Ga) 50, 233–239 10.1097/SHK.0000000000001006 [DOI] [PubMed] [Google Scholar]
- 23.Quan Y., Xin Y., Tian G., Zhou J. and Liu X. (2020) Mitochondrial ROS-Modulated mtDNA: A Potential Target for Cardiac Aging. Oxidative Med. Cell. Longev. 2020, 9423593 10.1155/2020/9423593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiyonaga N., Moriyama T. and Kanmura Y. (2019) Effects of Landiolol in Lipopolysaccharide-Induced Acute Kidney Injury in Rats and In Vitro. Shock (Augusta, Ga) 52, e117–e123 10.1097/SHK.0000000000001306 [DOI] [PubMed] [Google Scholar]
- 25.Yang X., Yan X., Yang D., Zhou J., Song J. and Yang D. (2018) Rapamycin attenuates mitochondrial injury and renal tubular cell apoptosis in experimental contrast-induced acute kidney injury in rats. Biosci. Rep. 38, BSR20180876 10.1042/BSR20180876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Z., Xu C., Tao Y., Liang Y., Liang Q., Li J. et al. (2020) Anisodamine alleviates lipopolysaccharide-induced pancreatic acinar cell injury through NLRP3 inflammasome and NF-κB signaling pathway. J. Recept. Signal Transduct. Res. 40, 58–66 10.1080/10799893.2020.1713808 [DOI] [PubMed] [Google Scholar]
- 27.You Q.H., Zhang D., Niu C.C., Zhu Z.M., Wang N., Yue Y. et al. (2014) Expression of IL-17A and IL-17F in lipopolysaccharide-induced acute lung injury and the counteraction of anisodamine or methylprednisolone. Cytokine 66, 78–86 10.1016/j.cyto.2013.12.019 [DOI] [PubMed] [Google Scholar]
- 28.Zeng Y., Yao X., Liu X., He X., Li L., Liu X. et al. (2019) Anti-angiogenesis triggers exosomes release from endothelial cells to promote tumor vasculogenesis. J. Extracell. Vesicles 8, 1629865 10.1080/20013078.2019.1629865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu G.N., Yang K., Xu Z.P., Zhu L., Hou L.N., Qi H. et al. (2012) Protective effects of anisodamine on cigarette smoke extract-induced airway smooth muscle cell proliferation and tracheal contractility. Toxicol. Appl. Pharmacol. 262, 70–79 10.1016/j.taap.2012.04.020 [DOI] [PubMed] [Google Scholar]
- 30.Wang Z., Li M., Liu L. and Geng B. (2019) Muscarinic M1 and M2 receptor subtypes play opposite roles in LPS-induced septic shock. Pharmacol. Rep.: PR 71, 1108–1114 10.1016/j.pharep.2019.06.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.