Abstract
Purpose
Only recently Tintelnotia was described as a new genus in the Phaeosphaeriaceae family of fungi containing two species, T. opuntiae and T. destructans. Until now, T. destructans keratitis was associated with contact lens wear and ocular trauma. We present the first case of T. destructans keratomycosis presenting as a superinfection in herpetic keratitis.
Observations
We present a case of a 53-year-old woman who presented with a unilateral keratitis since 3 weeks without history of trauma or contact lens wear, not responding to topical ofloxacin. Polymerase Chain Reaction (PCR) of the corneal ulcer was positive for Herpes Simplex Virus type 1 (HSV-1). Signs and symptoms progressively improved after starting topical and systemic antiviral therapy. Six weeks later however, our patient presented with a new white infiltrate in the previous herpetic epithelial defect. In vivo confocal microscopy showed fungal hyphae and culture from corneal scrapings identified a hyphomycete. Intensive antimycotic therapy could not prevent a corneal perforation 1 week later. Penetrating keratoplasty was performed with intracameral injection of amphotericin B. Culture of the corneal button and PCR and sequence analysis on the fungal isolate confirmed the diagnosis of T. destructans keratomycosis. Six months after penetrating keratoplasty, biomicroscopy showed a clear graft without recurrence of fungal activity.
Conclusions and importance
T. destructans is an emerging opportunistic pathogen causing severe keratomycosis. Despite intensive antimycotic therapy, rapid progression to corneal perforation can be seen. Early diagnosis using confocal microscopy, fungal culture and PCR can allow prompt initiation of treatment, which should be guided by in vitro susceptibility testing.
Keywords: Fungal keratitis, Herpetic keratitis, Phaeosphaeriaceae, Tintelnotia destructans, Voriconazole, Terbinafine
Abbreviations: .DNA, Deoxyribonucleic Acid; EUCAST, European Committee on Antimicrobial Susceptibility Testing; HSV, Herpes Simplex Virus; IVCM, In Vivo Confocal Microscopy; MIC, Minimal Inhibitory Concentration; PCR, Polymerase Chain Reaction; RNA, Ribonucleic Acid
1. Introduction
Only recently Tintelnotia was described as a new genus in the Phaeosphaeriaceae family of fungi containing two species, T. opuntiae and T. destructans.1 The Phaeosphaeriaceae constitute a large family within the Pleosporales characterized by coelomycetous anamorphs.2 Human infections by coelomycetous fungi typically present as superficial opportunistic infections such as keratitis and onychomycosis.3 Until now, only three cases of keratomycosis due to T. destructans have been described, of which 2 were associated with rigid gas-permeable contact lenses1,4 and 1 with ocular trauma.5 We describe the first case of a T. destructans superinfection in a previous herpetic keratitis, without other predisposing factors.
2. Case report
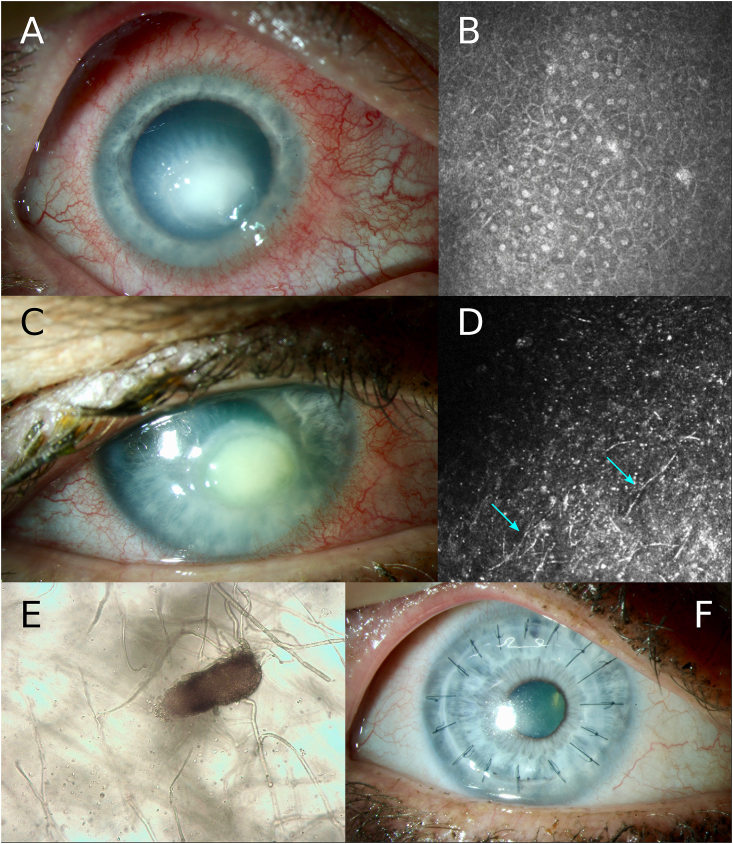
We present a case of a 53-year-old woman who was referred to our ophthalmology department for a keratitis in the right eye since 3 weeks, not responding to topical ofloxacin and indomethacin. Uncorrected visual acuity was limited to hand movements. Biomicroscopy showed a white corneal infiltrate with stromal edema and relatively calm anterior chamber (Fig. 1A). There was no history of trauma, contact lens wear or other predisposing factors. Fortified topical tobramycin 14mg/ml and cefazolin 50mg/ml were initiated hourly day and night. Corneal swab and scrapings were cultured for bacteria, fungi and Acanthamoeba but remained negative. Because of lack of improvement a second scraping was performed at day 5, which showed only inflammatory cells on direct examination while culture remained negative. In vivo confocal microscopy showed absence of hyphae or cysts (Fig. 1B). Herpetic keratitis was suspected and confirmed by PCR (Argene, bioMérieux) which was positive for HSV-1. Signs and symptoms improved after starting topical ganciclovir ointment, dexamethasone and moxifloxacin drops combined with oral valaciclovir 1500mg/day. A progressive decline in density of the stromal infiltrate and surface area of the overlying epithelial defect was noticed, however the epithelium did not heal completely. Uncorrected visual acuity improved to 20/63.
Fig. 1.
Slit lamp photography and microscopy images of T. destructans keratitis. Notice dense white corneal infiltrate with overlying epithelial defect at presentation (1A). Confocal microscopy showed absence of fungal hyphae (1B). Six weeks after presentation a new elevated corneal infiltrate was noticed (1C) and confocal microscopy confirmed presence of hyper-reflective branching fungal hyphae (1D, blue arrows). Culture from corneal scrapings identified a hyphomycete which produced phoma-like pycnidia (1E). Six months after penetrating keratoplasty, biomicroscopy showed a clear graft without recurrence of fungal activity (1F). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Six weeks later however, our patient presented with a new white infiltrate in the previous herpetic epithelial defect associated with stromal melting (Fig. 1C). Uncorrected visual acuity declined to counting fingers. To our surprise, in vivo confocal microscopy (IVCM) clearly showed fungal hyphae (Fig. 1D). Culture from corneal scrapings identified a hyphomycete which produced phoma-like pycnidia (Fig. 1E), without further identification at that time. In vitro susceptibility testing using European Committee on Antimicrobial Susceptibility Testing (EUCAST) methodology showed the lowest minimal inhibitory concentration (MIC) value for itraconazole (0.03μg/ml), the MIC value for voriconazole was 0.5 μg/ml and 0.125μg/ml for amphotericin B. Empirical treatment was started awaiting results from in vitro susceptibility testing.
Intensive therapy with topical voriconazole 10mg/ml hourly day and night and oral itraconazole 400mg/day and doxycycline 200mg/day could not prevent a corneal perforation 1 week later. Penetrating keratoplasty was performed combined with intracameral injection of amphotericin B 10μg/0.1ml. Postoperatively, topical dexamethasone and ofloxacin were added to voriconazole, combined with oral itraconazole and valaciclovir. Culture of the corneal button and sequence analysis on the isolate confirmed the diagnosis of T. destructans keratomycosis. Topical voriconazole was tapered and stopped six weeks after surgery. Both topical dexamethasone and oral valaciclovir were slowly tapered. Six months after penetrating keratoplasty, biomicroscopy showed a clear graft without recurrence of fungal activity (Fig. 1F). Best-corrected visual acuity improved to 20/63 again.
3. Discussion and conclusions
The incidence of mycotic keratitis and the specific pathogens vary greatly by geography. In India, one third of corneal ulcers are estimated to be of fungal source.6 In Europe, incidence of filamentous fungal keratitis is suggested to be increasing and half to two thirds of patients are reported to be contact lens wearers.7,8 Incidence varies between 0.6 and 1.5 cases per million per year.8,9 Predisposing factors such as contact lens wear, trauma, ocular surgery and topical steroids have been identified.10,11 Until now, T. destructans keratitis was associated with contact lens wear1,4 and ocular trauma.5 Coelomycetous fungi present as opportunistic superficial infections and are commonly acquired by traumatic implantation.1
Infectious keratitis is a major global cause of visual impairment and blindness and proper diagnosis of the causative organism is critical.12 However, diagnosing both herpetic and fungal keratitis can be challenging. The diagnosis of HSV keratitis is based on history and clinical presentation, complemented by laboratory confirmation, e.g. viral culture or PCR. Viral culture is labor-intensive and rather slow, as growth of HSV usually requires two days. PCR, in contrast, is more sensitive as it detects very small amounts of Deoxyribonucleic Acid (DNA) and Ribonucleic Acid (RNA) of both living and dead viral particles. In addition, PCR is easier to perform, providing results within a few hours.13 In our patient, empirical treatment with fortified antibiotics was initiated at presentation based on biomicroscopic findings. Suspicion for herpetic keratitis was raised after direct microscopy, culture and confocal microscopy all came back negative. Corneal swab for PCR confirmed the diagnosis of herpetic keratitis and signs and symptoms progressively improved after starting antiviral therapy. A superinfection was suspected after appearance of a new corneal infiltrate six weeks later. Confocal microscopy findings at that time allowed prompt initiation of antifungal therapy. Non-invasive techniques such as IVCM are being increasingly used for in vivo diagnosis of infectious keratitis.14,15 This can be of particular interest, knowing that approximately one-fourth of fungal cultures become positive only after 2 weeks.16 The reported specificity of IVCM for detecting fungal hyphae varies from 78% to 90%, while the sensitivity varies from 71% to 94%.17,18 IVCM does have an important intra- and interobserver variability, dependent on the level of the observer's experience and training.14,18 In our patient, identification of the fungus by means of culture and microscopy was possible up to Phaeosphaeriaceae family level, while PCR and sequence analysis was necessary to confirm the T. destructans genus and species. PCR directly on the clinical sample is a promising tool for diagnosis of fungal keratitis.19 Zhao et al.20 used a direct PCR assay without template DNA extraction for the diagnosis of infectious keratitis. In patients with high suspicion of fungal keratitis, the positive detection rate of direct PCR was 84.8%. This rate increased to 91.2% when repeated scrapings were excluded, and was significantly higher than the rates obtained with culture (35.3%) and smear (64.7%), and was also higher than the rate obtained with confocal microscopy (74.1%). Recently, the added value of multiplex PCR in diagnosing superinfection keratitis was highlighted,21 underlining the importance of viral PCR testing in any severe keratitis. Bacterial and fungal superinfection in herpetic keratitis has been described previously, however delay in diagnosis is common due to its atypical manifestation.21
The management of mycotic keratitis is difficult and remains a challenge for the ophthalmologist and the patient. If hyphae are seen by microscopy, direct or confocal, topical natamycin (5%) is the drug of choice, especially in Fusarium species.22 Based on in vitro susceptibility testing and due to unavailability of natamycin, combined therapy with topical voriconazole and oral itraconazole was started in our patient. Topical dexamethasone drops were stopped immediately, as corticosteroid use is a known predisposing factor in fungal keratitis.11 Interestingly, the two T. destructans keratitis cases treated with topical and systemic terbinafine resulted in slow but effective improvement without additional surgical intervention.1,4 Case 3 resulted in penetrating keratoplasty after corneal perforation despite topical, systemic and intracameral voriconazole administration,5 as was seen in our patient. Terbinafine is an allylamine with antifungal activity, which is however rarely used in ophthalmology and not routinely included in vitro antifungal susceptibility testing. Additionally, the intravenous solution of terbinafine, used for preparation of eyedrops, is not universally available. In a retrospective study of 90 filamentous mycotic keratitis cases, Liang et al.23 compared topical natamycin with terbinafine. A favorable response to terbinafine was reported in 89% of patients (n = 40/45), which was comparable to natamycin (93%, n = 42/45). However, the mean treatment duration was significantly longer in the terbinafine-group. In vitro susceptibility testing of T. destructans showed the lowest rate of minimal inhibitory concentration for terbinafine (MIC 0.12μg/ml),4 which was lower than the MIC value for voriconazole for the isolate of our patient (0.5 μg/ml).
In conclusion, we describe the first case of severe T. destructans keratomycosis presenting as a superinfection in herpetic keratitis. This case illustrates the importance of T. destructans as an emerging opportunistic pathogen. Despite intensive antimycotic therapy, rapid progression to corneal perforation can be seen. Early diagnosis using confocal microscopy, fungal culture and PCR can allow prompt initiation of treatment, which should be guided by in vitro susceptibility testing if available. Cases of severe keratitis should be followed closely, even with positive viral PCR. Further studies are needed to confirm that early administration of topical and systemic terbinafine in T. destructans keratitis can indeed prevent corneal perforation and the need for penetrating keratoplasty.
4. Patient consent
Written informed consents were obtained from the patient for publication of this Case Report and accompanying images. A copy of the written consent is available for review by the editors of this journal.
Funding
No funding or grant support.
Authorship
DR treated the patient and performed the corneal surgery. DR wrote the article and LC and KL revised it critically for important intellectual content. LC performed fungal cultures and KL performed fungal PCR. All authors approved the final manuscript. All authors attest that they meet the current ICMJE criteria for Authorship.
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Declaration of competing interest
The authors declare that they have no conflicts of interest.
Acknowledgements
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajoc.2020.100791.
Contributor Information
Dimitri Roels, Email: dimitri.roels@uzgent.bes.
Liselotte Coorevits, Email: liselotte.coorevits@UGent.be.
Katrien Lagrou, Email: katrien.lagrou@uzleuven.be.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Ahmed S.A., Hofmuller W., Seibold M. Tintelnotia, a new genus in Phaeosphaeriaceae harbouring agents of cornea and nail infections in humans. Mycoses. 2017;60(4):244–253. doi: 10.1111/myc.12588. [DOI] [PubMed] [Google Scholar]
- 2.Camara M.P., Palm M.E., van Berkum P., O'Neill N.R. Molecular phylogeny of leptosphaeria and phaeosphaeria. Mycologia. 2002;94(4):630–640. doi: 10.1080/15572536.2003.11833191. [DOI] [PubMed] [Google Scholar]
- 3.Sutton D.A. Coelomycetous fungi in human disease. A review: clinical entities, pathogenesis, identification and therapy. Rev Iberoam De Micol. 1999;16(4):171–179. [PubMed] [Google Scholar]
- 4.Behrens-Baumann W.J., Hofmuller W., Tammer I., Tintelnot K. Keratomycosis due to Tintelnotia destructans refractory to common therapy treated successfully with systemic and local terbinafine in combination with polyhexamethylene biguanide. Int Ophthalmol. 2018;Apr 28 doi: 10.1007/s10792-018-0930-2. [DOI] [PubMed] [Google Scholar]
- 5.Habbe K.J., Frings A., Schrader S. [Tintelnotia destructans: new enemy at the gates] Ophthalmologe. 2017;115(11):948–950. doi: 10.1007/s00347-017-0641-5. [DOI] [PubMed] [Google Scholar]
- 6.Lalitha P., Prajna N.V., Manoharan G. Trends in bacterial and fungal keratitis in South India, 2002-2012. Br J Ophthalmol. 2015;99(2):192–194. doi: 10.1136/bjophthalmol-2014-305000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ong H.S., Fung S.S.M., Macleod D., Dart J.K.G., Tuft S.J., Burton M.J. Altered patterns of fungal keratitis at a london ophthalmic referral hospital: an eight-year retrospective observational study. Am J Ophthalmol. 2016;168:227–236. doi: 10.1016/j.ajo.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farrell S., McElnea E., Moran S., Knowles S., Murphy C.C. Fungal keratitis in the republic of Ireland. Eye. 2017;31(10):1427–1434. doi: 10.1038/eye.2017.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nielsen S.E., Nielsen E., Julian H.O. Incidence and clinical characteristics of fungal keratitis in a Danish population from 2000 to 2013. Acta Ophthalmol. 2015;93(1):54–58. doi: 10.1111/aos.12440. [DOI] [PubMed] [Google Scholar]
- 10.Stapleton F., Edwards K., Keay L. Risk factors for moderate and severe microbial keratitis in daily wear contact lens users. Ophthalmology. 2012;119(8):1516–1521. doi: 10.1016/j.ophtha.2012.01.052. [DOI] [PubMed] [Google Scholar]
- 11.FlorCruz N.V., Evans J.R. Medical interventions for fungal keratitis. Cochrane Database Syst Rev. 2015;4 doi: 10.1002/14651858.CD004241.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Austin A., Lietman T., Rose-Nussbaumer J. Update on the management of infectious keratitis. Ophthalmology. 2017;124(11):1678–1689. doi: 10.1016/j.ophtha.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Remeijer L., Osterhaus A., Verjans G. Human herpes simplex virus keratitis: the pathogenesis revisited. Ocul Immunol Inflamm. 2004;12(4):255–285. doi: 10.1080/092739490500363. [DOI] [PubMed] [Google Scholar]
- 14.Hau S.C., Dart J.K., Vesaluoma M. Diagnostic accuracy of microbial keratitis with in vivo scanning laser confocal microscopy. Br J Ophthalmol. 2010;94(8):982–987. doi: 10.1136/bjo.2009.175083. [DOI] [PubMed] [Google Scholar]
- 15.Brasnu E., Bourcier T., Dupas B. In vivo confocal microscopy in fungal keratitis. Br J Ophthalmol. 2007;91(5):588–591. doi: 10.1136/bjo.2006.107243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Day D.M., Akrabawi P.L., Head W.S., Ratner H.B. Laboratory isolation techniques in human and experimental fungal infections. Am J Ophthalmol. 1979;87(5):688–693. doi: 10.1016/0002-9394(79)90305-2. [DOI] [PubMed] [Google Scholar]
- 17.Kanavi M.R., Javadi M., Yazdani S., Mirdehghanm S. Sensitivity and specificity of confocal scan in the diagnosis of infectious keratitis. Cornea. 2007;26(7):782–786. doi: 10.1097/ICO.0b013e318064582d. [DOI] [PubMed] [Google Scholar]
- 18.Kheirkhah A., Syed Z.A., Satitpitakul V. Sensitivity and specificity of laser-scanning in vivo confocal microscopy for filamentous fungal keratitis: role of observer experience. Am J Ophthalmol. 2017;179:81–89. doi: 10.1016/j.ajo.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 19.Vengayil S., Panda A., Satpathy G. Polymerase chain reaction-guided diagnosis of mycotic keratitis: a prospective evaluation of its efficacy and limitations. Invest Ophthalmol Vis Sci. 2009;50(1):152–156. doi: 10.1167/iovs.07-1283. [DOI] [PubMed] [Google Scholar]
- 20.Zhao G., Zhai H., Yuan Q., Sun S., Liu T., Xie L. Rapid and sensitive diagnosis of fungal keratitis with direct PCR without template DNA extraction. Clin Microbiol Infect. 2014;20(10):O776–O782. doi: 10.1111/1469-0691.12571. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida M., Hariya T., Yokokura S. Diagnosing superinfection keratitis with multiplex polymerase chain reaction. J Infect Chemother. 2018;24(12):1004–1008. doi: 10.1016/j.jiac.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 22.Prajna N.V., Krishnan T., Mascarenhas J. The mycotic ulcer treatment trial: a randomized trial comparing natamycin vs voriconazole. JAMA Ophthalmol. 2013;131(4):422–429. doi: 10.1001/jamaophthalmol.2013.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang Q.F., Jin X.Y., Wang X.L., Sun X.G. Effect of topical application of terbinafine on fungal keratitis. Chin Med J (Engl) 2009;122(16):1884–1888. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.