Abstract
Direct oral anticoagulants (DOACs) have had a positive impact in preventing cardioembolic stroke in patients with atrial fibrillation (AF) who were associated with lower bleeding complications; however, data on subjects with concomitant advanced liver diseases (ALDs) are poor. This meta‐analysis evaluates bleeding and thromboembolic complications in patients with coexisting AF and ALD who were treated with DOACs or vitamin K antagonists (VKAs). We performed a meta‐analysis of randomized controlled trials and observational studies identified by the PubMed and Embase databases using a combination of the following keywords: “direct oral anticoagulants,” “advanced liver disease,” “cirrhosis,” “bleeds,” “stroke.” No time restriction was applied to the research. Two physicians reviewed data on outcome measures and assessed the quality rating. The main outcome was major bleeding, and the secondary outcomes were bleedings (all, intracranial, and gastrointestinal) and ischemic strokes. A total of four studies (one prospective, three retrospective) were identified involving 3,483 subjects with AF and ALD; of these, 1,547 were on VKAs and 1,936 on DOACs. Advanced liver disease was defined as liver cirrhosis or fibrosis‐4 score >3.25. Compared to VKA use, DOAC use was associated with reduced risk for major bleedings (hazard ratio [HR], 0.58; 95% confidence interval [CI], 0.44‐0.77; P < 0.001), total bleedings (HR, 0.45; 95% CI, 0.36‐0.55; P < 0.05), intracranial hemorrhage (HR, 0.51; 95% CI, 0.32‐0.80; P < 0.004), and gastrointestinal bleedings (HR, 0.61; 95% CI, 0.42‐0.88; P < 0.008). Efficacy analysis showed no significant difference between VKA‐ and DOAC‐treated patients (HR, 0.83; 95% CI, 0.58‐1.15; P = 0.31). Conclusion: In patients with AF and ALD, the safety and efficacy profile of DOACs did not appear to differ from those with AF without ALD.
Abbreviations
- AF
atrial fibrillation
- ALD
advanced liver disease
- CI
confidence interval
- DOAC
direct oral anticoagulant
- HR
hazard ratio
- LD
liver disease
- RCT
randomized clinical trial
- VKA
vitamin K antagonist
Atrial fibrillation (AF) is the most common cardiac arrhythmia. It occurs in roughly 1% of the general population and is associated with an enhanced risk of cardioembolic stroke.( 1 ) The use of oral vitamin K antagonists (VKAs) and, more recently, direct oral anticoagulants (DOACs) has had a positive impact in terms of reduction of cardioembolic stroke and bleeding complications.( 2 ) An important caveat of the trials with DOACs has been the exclusion of patients with liver disease (LD), likely due to the assumption that a coexistent coagulopathy or changes in pharmacokinetics/pharmacodynamics of DOACs could theoretically favor bleedings. Therefore, it is still unclear if patients with AF may benefit from DOACs in cases of coexistence with LD.
Data regarding the impact of DOACs on bleeding complications in patients with coexisting AF and LD essentially stem from observational studies, which provided inconclusive results. Thus, two meta‐analyses with a relatively small number of patients showed no difference in terms of bleeding complications between DOAC‐ and VKA‐treated patients or lower bleeding complications in DOAC‐treated patients.( 3 , 4 ) Furthermore, comparative analysis of the two types of treatment regarding ischemic stroke has not been performed. Here, we report the results of a larger meta‐analysis focusing on bleeding and thromboembolic complications in patients with coexisting AF and advanced liver disease (ALD).
Materials and Methods
Eligibility Criteria
We included all original clinical research articles in the English language with full text available. In particular, observational clinical studies that reported data on bleedings and ischemic events in patients with AF with advanced ALD defined as liver cirrhosis (according to Child‐Pugh classification) or the validated( 5 ) fibrosis‐4 score (>3.25) were collected. We did not include case reports, editorials/comments, letters, cross‐sectional studies, reviews, and meta‐analysis; subgroup analysis from the same clinical trial; or studies reporting data on randomized controlled trials (RCTs) aimed at drug registration.
Information Sources and Search Strategy
Studies were identified by the PubMed and Embase databases using a combination of the following terms: “advanced liver disease,” “cirrhosis,” “bleeds,” “strokes,” “direct oral anticoagulants.”
We limited our search to human studies. The research strategy included only English language journal articles with full papers available with no time restrictions. The search ended on October 1, 2019, and was performed according to Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines.( 6 )
Study Selection and Quality Assessment
The study selection was performed in multiple phases. In the first phase, potentially relevant studies were obtained by combined searches of electronic databases using the selected above‐mentioned keywords. Then, studies not in the English language, not involving humans, involving fewer than 100 patients, or not addressing study questions were excluded. In the second phase, studies were reviewed and selected according to the inclusion/exclusion criteria. The third phase consisted of a detailed analysis of full‐text articles to assess if they provided the necessary data to be included in the meta‐analysis (Fig. 1). Quality assessment of studies included in the meta‐analysis was performed using the Newcastle‐Ottawa Scale (NOS) to assess the quality of nonrandomized cohort studies.( 7 ) Studies with a score ≥7 were considered good quality.
Fig. 1.
Flow diagram of the search strategy.
Data Collection Process and Data Items
Two physicians (D.P. and P.P.) independently screened the titles and abstracts of manuscripts identified through the database searches to identify studies potentially eligible for further assessment. Controversies were resolved by a third investigator (F.V.). For each study, we collected the following information: authors, year of publication, study type, number of participants included, percentage of male individuals, age, follow‐up (years), number (n/%), and rate of bleedings and strokes (%/year).
Statistical Analysis
Outcomes of interest for this meta‐analysis were major bleeding events, all bleedings, intracranial hemorrhages, gastrointestinal bleedings, and ischemic stroke. Hazard ratios (HRs) were used as summary statistics. When available, HRs were recorded from each study. When HRs were not reported, they were estimated from summary statistics with the methods described by Tierney et al.( 8 )
Because the number of studies to be included in the meta‐analyses was small, we decided to use a hierarchical Bayesian meta‐analysis approach with heterogeneity and informative priors, according to the guidelines proposed by Higgins et al.( 9 ) All statistical analyses for the meta‐analysis were performed using the open‐source software R (version 3.5.1).
Results
Study Selection
By combining the keywords “direct oral anticoagulants,” “advanced liver disease,” “cirrhosis,” “bleeds,” and “stroke,” we found 637 studies merging the searches performed in PubMed and Embase. Of these studies, seven studies were excluded as duplicates; 323 reports were excluded (102 not in English, 84 not involving humans, and 137 because they did not include patients treated with DOACs). In addition, 307 articles were selected for detailed analysis; of these, 258 were excluded (42 reviews; six meta‐analyses; 63 case reports; 147 with no AF and ALD). The 49 remaining studies were analyzed for eligibility; of these, 45 were excluded because they were not addressing the specific study question. Four studies were finally included in the analysis (Fig. 1). Quality assessment by NOS found that the four selected studies were of good quality (score ≥7) (Table 1).
Table 1.
Newcastle‐Ottawa Quality Assessment Scale for Cohort Studies
| Pastori et al.( 5 ) | Goriacko et al.( 10 ) | Lee HF et al.( 11 ) | Lee SR et al.( 12 ) | ||
|---|---|---|---|---|---|
| Selection | |||||
| Representativeness of the exposed cohort | |||||
| A Truly representative of the average in the community* | A | B | B | A | |
| B Somewhat representative of the average in the community* | |||||
| C Selected group of users (e.g., nurses, volunteers) | |||||
| D No description of the derivation of the cohort | |||||
| Selection of the nonexposed cohort | |||||
| A Drawn from the same community as the exposed cohort* | A | A | A | A | |
| B Drawn from a different source | |||||
| C No description of the derivation of the nonexposed cohort | |||||
| Ascertainment of exposure | |||||
| A Secure record (e.g., surgical records)* | A | A | A | A | |
| B Structured interview* | |||||
| C Written self‐report | |||||
| D No description | |||||
| Demonstration that outcome of interest was not present at start of study | |||||
| A Yes* | A | A | A | A | |
| B No | |||||
| Comparability | |||||
| Comparability of cohorts on the basis of the design or analysis | |||||
| A Study controls for age and/or sex*, † | B | B | B | B | |
| B Study controls for any additional factor*, † | |||||
| Outcome | |||||
| Assessment of outcome | |||||
| A Independent blind assessment* | A | B | B | B | |
| B Record linkage* | |||||
| C Self‐report | |||||
| D No description | |||||
| Was follow‐up long enough for outcomes to occur? | |||||
| A Yes (≥12 months)* | A | B | A | A | |
| B No | |||||
| Adequacy of follow‐up of cohorts | |||||
| A Complete follow‐up (all subjects accounted for)* | D | D | D | D | |
| B Subjects lost to follow‐up unlikely to introduce bias (small number lost) >80% in follow‐up or description provided of those lost* | |||||
| C Follow‐up rate <80% and no description of those lost | |||||
| D No statement | |||||
| Total score † | 8 | 7 | 8 | 8 | |
Point assigned only to these criteria.
1 point if studies adjusted for age and/or sex; 2 points if studies adjusted for age and sex and/or any other cardiovascular risk factor.
Overall Study Analysis
Of the studies, four studies (one prospective, three retrospective)( 5 , 10 , 11 , 12 ) were identified involving a total of 3,483 subjects with AF with ALD. Of these, 1,547 were on VKA treatment and 1,936 on DOAC treatment. Mean age at baseline was 70.04 and 71.97 years for VKAs and DOACs, respectively. Male subjects were 59.3% and 58.3% of the VKA‐ and DOAC‐treated subjects, respectively. The mean follow‐up was 27.73 months for VKA users and 15.12 months for DOAC users. Data from the four studies are summarized in Table 2.
Table 2.
Clinical Characteristics
| Study 1 | Study 2 | Study 3 | Study 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Authors (year) | Pastori et al.( 5 ) | Goriacko et al.( 10 ) | Lee HF et al.( 11 ) | Lee SR et al.( 12 ) | ||||
| Study design | Prospective | Retrospective | Retrospective | Retrospective | ||||
| Anticoagulant | NOAC | VKA | NOAC | VKA | NOAC | VKA | NOAC | VKA |
| Follow‐up (months) | N/A | N/A | N/A | N/A | 13.56 | 15.6 | N/A | N/A |
| Sex (men, %) | 60.5 | 57.3 | 59.5 | 63.65 | 63.70 | 68.6 | 72.2 | |
| Mean CHA2DS2 VASc | 3.4 | N/A | N/A | 3.72 | 3.66 | 3.4 | 3.0 | |
| Mean age (years) | 78.9 | 66 | 65 | 72.81 | 72.41 | 69.5 | 66.9 | |
| NOACs/VKAs (n) | 52 | 77 | 75 | 158 | 1,438 (after PSSWs 1,397) | 990 (after PSSWs 946) | 446 | 322 |
| Dabigatran | N/A | 35 | 535 | 132 | ||||
| Apixaban | N/A | 11 | 171 | 94 | ||||
| Rivaroxaban | N/A | 29 | 732 | 190 | ||||
| Edoxaban | N/A | ‐* | ‐ | 30 | ||||
| Definition of LD | Advanced liver fibrosis (FIB‐4 >3.25) | Liver cirrhosis (Child‐Pugh A‐B‐C) | Liver cirrhosis (ICD‐9‐CM) | Liver cirrhosis (ICD‐10‐CM) | ||||
*‐ indicates none.
Abbreviations: CHA2DS2 VASc, congestive heart failure (1 point), hypertension (1 point), age (2 points if above 75), diabetes (1 point), previous stroke (2 points), vascular disease (1 point), age (1 point if between 65 and 74 years), sex (1 point if female); FIB‐4, fibrosis‐4; ICD‐9/10‐CM, International Classification of Diseases, Ninth/Tenth Revision, Clinical Modification; N/A, not applicable; NOAC, nonvitamin K anticoagulant; PSSW, propensity score‐based stabilized weight.
Compared to VKA, the use of DOACs was associated with a reduced risk for total bleedings (HR, 0.45; 95% confidence interval [CI], 0.36‐0.55; P < 0.05), major bleedings (HR, 0.58; 95% CI, 0.44‐0.77; P < 0.001), intracranial hemorrhage (HR 0.51; 95% CI, 0.32‐0.80; P < 0.004), and gastrointestinal bleedings (HR 0.61; 95% CI, 0.42‐0.88; P < 0.008) (Fig. 2). Similar efficacy was found in patients treated or not with DOACs (HR, 0.83; 95% CI, 0.58‐1.15; P = 0.31) (Fig. 3).
Fig. 2.
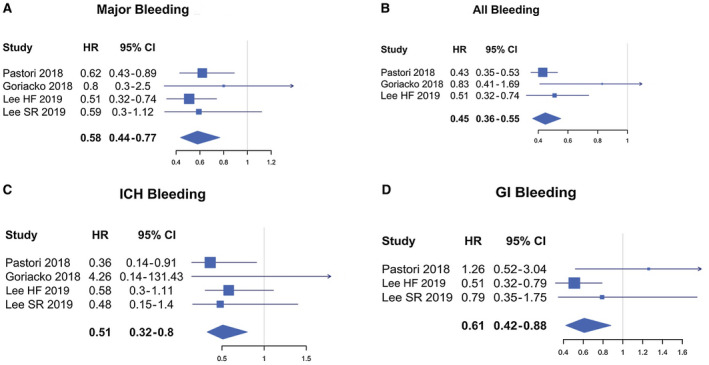
Forest plots for different outcomes comparing VKAs and DOACs. (A) Major bleedings VKA. (B) All bleedings VKA. (C) Intracranic hemorrhage. (D) Gastrointestinal bleedings VKA. Abbreviations: GI, gastrointestinal; ICH, intracranic hemorrhage.
Fig. 3.
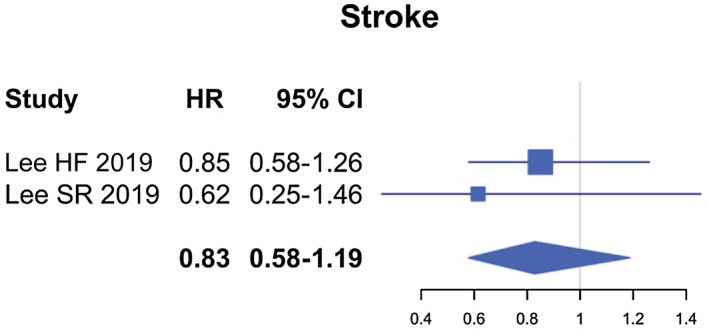
Forest plots for outcomes of ischemic stroke comparing VKA to DOACs users in the entire population studied.
Discussion
Our study shows that in patients with AF with ALD, the use of DOACs reduces the risk of bleedings compared to warfarin. This is consistent with the safety profile of this drug category in patients with AF without LD.
The clinical impact of DOACs in patients with coexisting AF and LD has not been fully investigated due to the perception of an increased bleeding risk as a consequence of the potentially impaired drug pharmacokinetics and/or intrinsic bleeding risk of patients with ALD. Although studies performed in patients with mild LD seem to support the safety profile of DOACs with a tendency to lower bleeding risk compared to warfarin, data regarding ALD are scarce.( 3 , 13 , 14 ) In the present study, we meta‐analyzed four observational studies that included 3,483 patients with AF and ALD. The analysis showed that in this setting, the safety profile is similar to that detected in AF without ALD because a lower risk of bleeding was found in DOAC‐ versus VKA‐treated patients. This difference was observed for major bleeding, all bleedings, and cerebral hemorrhage, which are all significantly lower in DOAC‐treated patients. Previous RCT studies showed a tendency to enhanced risk of gastrointestinal bleeding in DOAC‐ compared to VKA‐treated patients.( 2 ) In the context of LD, this point is of particular relevance because patients with ALD have an increased risk of gastrointestinal bleeding, which is essentially dependent on portal hypertension and/or portal vein thrombosis.( 15 ) Hence, administration of anticoagulants in patients with ALD may be of concern because the positive safety profile detected in patients with AF alone may be outweighed by an increased risk of gastrointestinal bleeding in patients with coexisting AF and ALD. The present analysis support the safety profile of DOACs also in the context of gastrointestinal bleeding, which, in fact, is significantly reduced in DOAC‐treated patients compared to warfarin‐treated patients. Although this favorable effect is difficult to explain, a possible interpretation may rely on the positive effect played by DOACs on portal circulation where they could lower the risk of portal vein thrombosis and eventually gastrointestinal bleeding.( 16 ) Finally, in accordance with RCTs in AF alone, we found that DOACs display a similar efficacy compared to VKAs in preventing ischemic stroke.
This study has implications and limitations. An implication of this report is that in patients with AF and ALD, DOACs display similar efficacy but a better safety profile compared to VKAs. However, our meta‐analysis is limited by observational and prevalently retrospective studies and a relatively small sample size and therefore needs to be confirmed by RCTs. Moreover, these results cannot be applied to subjects with severe LD, such as those in Child‐Pugh C, because these data are scarce. Finally, due to the small sample size, we could not examine the impact of each DOAC on the safety and efficacy profile. The results of this meta‐analysis, therefore, suggest that the safety and efficacy profile of DOACs in patients with AF with ALD parallels that of the AF population without liver disease.
Potential conflict of interest: Nothing to report.
References
Author names in bold designate shared co‐first authorship.
- 1. Stewart S, Hart CL, Hole DJ, McMurray JJ. A population‐based study of the long‐term risks associated with atrial fibrillation: 20‐year follow‐up of the Renfrew/Paisley study. Am J Med 2002;113:359‐364. [DOI] [PubMed] [Google Scholar]
- 2. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomised trials. Lancet 2014;383:955‐962. [DOI] [PubMed] [Google Scholar]
- 3. Chokesuwattanaskul R, Thongprayoon C, Bathini T, Torres‐Ortiz A, O'Corragain OA, Watthanasuntorn K, et al. Efficacy and safety of anticoagulation for atrial fibrillation in patients with cirrhosis: a systematic review and meta‐analysis. Dig Liver Dis 2019;51:489‐495. [DOI] [PubMed] [Google Scholar]
- 4. Fu Y, Zhu W, Zhou Y, Chen H, Yan L, He W. Non‐vitamin K antagonist oral anticoagulants versus warfarin in patients with atrial fibrillation and liver disease: a meta‐analysis and systematic review. Am J Cardiovasc Drugs 2020;20:139‐147. [DOI] [PubMed] [Google Scholar]
- 5. Pastori D, Lip GYH, Farcomeni A, Del Sole F, Sciacqua A, Perticone F, et al.; ATHERO‐AF study group. Incidence of bleeding in patients with atrial fibrillation and advanced liver fibrosis on treatment with vitamin K or non‐vitamin K antagonist oral anticoagulants. Int J Cardiol 2018;264:58‐63. [DOI] [PubMed] [Google Scholar]
- 6. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle‐Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta‐Analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Published 2014. Accessed January 2020.
- 8. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Higgins JP, Thompson SG, Spiegelhalter DJ. A re‐evaluation of random‐effects meta‐analysis. J R Stat Soc Ser A Stat Soc 2009;172:137‐159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goriacko P, Veltri KT. Safety of direct oral anticoagulants vs warfarin in patients with chronic liver disease and atrial fibrillation. Eur J Haematol 2018;100:488‐493. [DOI] [PubMed] [Google Scholar]
- 11. Lee HF, Chan YH, Chang SH, Tu HT, Chen SW, Yeh YH, et al. Effectiveness and safety of non‐vitamin K antagonist oral anticoagulant and warfarin in cirrhotic patients with nonvalvular atrial fibrillation. J Am Heart Assoc 2019;8:e011112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee SR, Lee HJ, Choi EK, Han KD, Jung JH, Cha MJ, et al. Direct oral anticoagulants in patients with atrial fibrillation and liver disease. J Am Coll Cardiol 2019;73:3295‐3308. [DOI] [PubMed] [Google Scholar]
- 13. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al.; ENGAGE AF‐TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093‐2104. [DOI] [PubMed] [Google Scholar]
- 14. Qamar A, Antman EM, Ruff CT, Nordio F, Murphy SA, Grip LT, et al. Edoxaban versus warfarin in patients with atrial fibrillation and history of liver disease. J Am Coll Cardiol 2019;74:179‐189. [DOI] [PubMed] [Google Scholar]
- 15. Loffredo L, Pastori D, Farcomeni A, Violi F. Effects of anticoagulants in patients with cirrhosis and portal vein thrombosis: a systematic review and meta‐analysis. Gastroenterology 2017;153:480‐487.e1. [DOI] [PubMed] [Google Scholar]
- 16. Villa E, Camma C, Marietta M, Luongo M, Critelli R, Colopi S, et al. Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis. Gastroenterology 2012;143:1253‐1260.e4. [DOI] [PubMed] [Google Scholar]


