Abstract
Introduction
Previously generated serum and plasma proteomic profiles were examined among adults with Down syndrome (DS) to determine whether these profiles could discriminate those with mild cognitive impairment (MCI‐DS) and Alzheimer's disease (DS‐AD) from those cognitively stable (CS).
Methods
Data were analyzed on n = 305 (n = 225 CS; n = 44 MCI‐DS; n = 36 DS‐AD) enrolled in the Alzheimer's Biomarker Consortium–Down Syndrome (ABC–DS).
Results
Distinguishing MCI‐DS from CS, the serum profile produced an area under the curve (AUC) = 0.95 (sensitivity [SN] = 0.91; specificity [SP] = 0.99) and an AUC = 0.98 (SN = 0.96; SP = 0.97) for plasma when using an optimized cut‐off score. Distinguishing DS‐AD from CS, the serum profile produced an AUC = 0.93 (SN = 0.81; SP = 0.99) and an AUC = 0.95 (SN = 0.86; SP = 1.0) for plasma when using an optimized cut‐off score. AUC remained unchanged to slightly improved when age and sex were included. Eotaxin3, interleukin (IL)‐10, C‐reactive protein, IL‐18, serum amyloid A , and FABP3 correlated fractions at r2 > = 0.90.
Discussion
Proteomic profiles showed excellent detection accuracy for MCI‐DS and DS‐AD.
1. INTRODUCTION
Alzheimer's disease (AD) is the most common neurodegenerative disease impacting nearly 5.8 million Americans (https://www.alz.org/alzheimers-dementia/facts-figures). Although the risk for AD increases with age, some populations face a disproportionate burden when it comes to development of AD neuropathology. One of the highest at‐risk groups is adults with Down syndrome (DS) due, at least in part, to the overexpression of the amyloid precursor protein (APP) located on chromosome 21. 1 Although the age at onset of AD varies widely, from under 40 years to over 70, in this population, recent estimates show that >70% of adults with DS over the age of 60 meet diagnostic criteria for AD. 2
Diagnosing a neurodegenerative cognitive disorder in adults with DS poses considerable challenges due to a wide range of premorbid intellectual and functional abilities. 3 Therefore, use of alternative measures such as biomarkers derived from neuroimaging, cerebrospinal fluid (CSF), or blood have all been increasingly explored in the population with DS as well as in the broader AD population. The use of blood‐based biomarkers as both individual and combined indicators of disease presence have gained considerable support for application in adults with DS due to the need for less invasive and burdensome (ie, time, cost) screening tools that can be used both in clinical practice and clinical trials.
Because adults with DS exhibit increased amounts of amyloid beta (Aβ) associated with the overexpression of APP, the majority of studies examining risk of AD have focused on Aβ. In plasma, an initial increase in Aβ 1‐42 was found to be associated with increased risk for dementia among adults with DS 4 ; follow‐up work indicated that when compared to the lowest quartile, those with middle to highest quartiles were more than twice as likely to develop AD, with the highest quartile also linked to increased mortality. 5 More recent work that evaluated Aβ as both individual peptides (Aβ1‐42 and Aβ1‐40) and in combination (Aβ1‐42/Aβ1‐40) identified that an increase in Aβ1‐40 along with a decrease in Aβ1‐42 and the Aβ1‐42/Aβ1‐40 ratio was associated with increased risk of development of AD in adults with DS. 6 Fortea et al. similarly examined Aβ peptides in both CSF and plasma as well as additional biomarkers associated with neurodegeneration including neurofilament light (NfL). 7 Their findings revealed that plasma NfL was the only blood‐based biomarker associated with prodromal AD and AD dementia in adults with DS whereas plasma Aβ peptides were not found to be associated with clinical status. 7
Among adults with DS and AD, additional individual plasma biomarkers have been evaluated including a number of inflammatory cytokines. Iulita et al. 8 identified elevations in tumor necrosis factor alpha (TNF‐α), interferon gamma (IFN‐y), interleukin 6 (IL‐6), and IL‐10 along with several additional proteins in adults with DS and AD when compared with healthy controls without DS. 8 In the same study, IL‐8 was also found to be significantly elevated among adults with DS and AD compared to those with DS but without evidence of dementia. 8 Recent work from our group identified an increase in several of the same plasma inflammatory proteins (ie, IL‐6, C‐reactive protein [CRP], IL‐10, IL‐18, thymus and activation regulated chemokine [TARC], TNF‐α, and thrombopoietin [TPO]) in a proteomic profile for prevalent AD in adults with DS with 89% accuracy. 9 Similar inflammatory plasma biomarkers were also found to drive the proteomic profile for prevalent mild cognitive impairment (MCI) in adults with DS (MCI‐DS) with 92% accuracy. 9
Despite a number of studies examining the link between plasma blood‐based biomarkers of AD among adults with DS, fewer studies have been conducted in serum. This is in contrast to the general AD population, in which a serum proteomic profile has been successfully validated across multiple cohorts, 10 assays, species, and tissue. 11 This same serum derived proteomic profile has also been successful in discriminating AD from other neurodegenerative conditions. 12 , 13 One study that examined neuronal exomes in adults with DS found no significant difference between serum and plasma Aβ1‐42, T‐T181‐tau, and P‐S396‐tau levels. 14 However, the authors found significant elevations in each of these markers among adults with DS when compared to neurotypical age‐matched controls. 14 This study is among the first to show the utility of serum blood‐based biomarkers among adults with DS.
Research in Context
Systematic review: Literature was identified and reviewed through PubMed. A growing body of literature supports the use of blood‐based biomarkers as a means of detecting mild cognitive impairment (MCI) and Alzheimer's disease (AD) in the neurotypical population. Most of the research examining blood biomarkers among adults with Down syndrome (DS) in the context of neurodegenerative cognitive disorders has been conducted in plasma. Only one study examined the utility of blood biomarkers in serum. No work to date has applied previously generated blood‐based proteomic profiles for detecting MCI and AD in the neurotypical population to adults with DS to examine the application of such profiles. Additionally, no work has examined the correlation of the same biomarker proteins across serum and plasma among adults with DS.
Interpretation: Our findings revealed that previously established proteomic profiles produced excellent detection accuracy when tasked with distinguishing MCI‐DS and DS‐AD from those with DS who are cognitively stable (CS). Detection accuracy based on an optimized cut‐off score reached an area under the curve (AUC) ranging from 95% to 98% for serum and plasma proteomic profiles for MCI‐DS when distinguishing from those with CS. The same serum and plasma proteomic profiles exhibited similar AUCs ranging from 93% to 95% in distinguishing DS‐AD from CS, again when an optimized cut‐off score was applied. Inclusion of sex and age improved almost all non‐optimized proteomic predictive models. Although most biomarkers were highly correlated across fraction, several correlated r2 > 90 and included Eotaxin3, interleukin (IL)‐10, C‐reactive protein, IL‐18, serum amyloid A, and fatty acid binding protein 3.
Future directions: This work was able to validate a previously generated serum and plasma proteomic profile from the neurotypical AD population among adults with DS. Findings from this study revealed the utility of serum blood‐based biomarkers, which have only been looked at in a limited capacity among adults with DS. Future work should expand on the current findings to evaluate cross‐over between serum and plasma biomarkers that, if combined, could potentially increase diagnostic accuracy.
In our prior work that examined proteomic biomarkers in a sample of older adults with and without AD in the general population, we found that several biomarkers were poorly associated across blood fractions. 10 To our knowledge, no study to date has examined the correlation of the same biomarkers spanning serum and plasma among adults with DS. Here we aimed to: (1) apply our previously generated serum and plasma proteomic profiles for AD and MCI in the general population to adults with DS and (2) examine the correlation of the same biomarker proteins across serum and plasma.
2. METHODS
2.1. Subjects
The study sample comprises n = 305 (n = 225 cognitively stable [CS]; n = 44 MCI‐DS; n = 36 DS‐AD) adult participants with DS enrolled in the Alzheimer's Biomarker Consortium–Down Syndrome (ABC–DS; https://www.nia.nih.gov/research/abc-ds). The ABC–DS is a prospective clinical cohort study of biomarkers associated with AD among adults with DS. ABC–DS sites include University of Pittsburgh, University of Wisconsin Madison, University of Cambridge, Washington University in St. Louis, Columbia University Irving Medical Center, Johns Hopkins University Bloomberg School of Public Health, University of California, Harvard/Massachusetts General Hospital, Hackensack University Medical Center, The New York State Institute for Basic Research in Developmental Disabilities, Georgetown University, and University of North Texas Health Science Center. The overall goals of ABC–DS are to: (1) identify sensitive neuropsychological measures of cognitive decline, imaging, blood‐based, and genetic biomarkers associated with transition from normal aging to MCI and AD among adults with DS; (2) identify critical factors that link cerebral amyloid deposition to neurodegeneration and ultimately dementia; (3) understand the relationships between biomarkers and pathways implicated in AD pathogenesis in preparation for clinical trials for AD in the DS population; and (4) provide rapid public access to all data, without embargo, and access to the biological samples by qualified scientific investigators. Study visits include a baseline visit, followed by subsequent assessments at 16 and 32 months. Data from this study come from blood collected at the ABC–DS baseline visit. Demographic characteristics of the cohort are presented in Table 1. The cohort comprises cognitively stable adults with DS and adults with and without prevalent MCI‐DS and AD. All ABC–DS sites operate under institutional review board approved protocols and informed consent and or assent was obtained for all participants.
TABLE 1.
Demographic characteristics of participants
| Characteristic | CS | MCI‐DS | DS‐AD |
|---|---|---|---|
| N | 225 | 44 | 36 |
| Age | 42.4 ± 9.1 | 52.9 ± 6.9 * | 54.3 ± 6.2 * |
| Sex N (%) | |||
| Male | 127 (52.5) | 35 (68.6) | 21(48.8) |
| Female | 115 (47.5) | 16 (31.4) * | 22 (51.2) |
| Level of function N (%) | |||
| Mild/moderate | 228 (94.2) | 37 (88.1) | 37 (88.1) |
| Severe/profound | 9 (3.7) | 5 (11.9) | 5 (11.6) |
| Ethnicity N (%) | |||
| White | 234 (97.1) | 47 (92.2) * | 40 (93.0) |
| Non‐white | 7 (2.9) | 4 (7.8) | 3 (7.0) |
| APOE ɛ4 allele N (%) | 45 (21.1) | 15 (34.1) | 14 (35.0) |
Note: Significance P‐value <.05*, <.001**
Abbreviations: AD, Alzheimer's disease; APOE, apolipoprotein E; CS, cognitively stable; DS, Down syndrome; MCI, mild cognitive impairment
2.2. Clinical assessment
Assessments included evaluations of cognition and functional abilities, behavioral and/or psychiatric conditions, and health status. Cognitive function was evaluated with a test battery designed for use with individuals with DS varying widely in their pre‐morbid levels of intellectual functioning. Structured interviews were conducted with caregivers to collect information on changes in cognition, day‐to‐day functioning, adaptive behavior, and medical status.
2.3. Classification of dementia
The classification of dementia status, dementia subtype, and age at onset was determined during clinical consensus conferences at which information from available sources including medical, clinical, and cognitive testing were reviewed. Participants were classified into three groups, generally consistent with the recommendations of the AAMR‐IASSID Working Group for the Establishment of Criteria for the Diagnosis of Dementia in Individuals with Developmental Disability. 15 , 16 Participants were classified as CS if they were without cognitive or functional decline. Participants were classified as having MCI‐DS if they demonstrated some cognitive and/or functional decline over and above what would be expected with aging per se based on performance on neuropsychological assessment as well as documented by informant report, but not severe enough to indicate the presence of dementia. Participants were categorized as having dementia (DS‐AD) if there was evidence of substantial progressive declines in cognitive functioning and daily living skills. An unable to determine category was used to indicate that declines were observed but that symptoms could be caused by life circumstance (eg, staff changes) or conditions unrelated to AD (eg, severe sensory loss, poorly resolved hip fracture, psychiatric diagnosis).
2.4. Apolipoprotein E (APOE)
DNA samples were genotyped for two APOE single‐nucleotide polymorphisms (SNPs) (rs429358 and rs7412) with the KASP genotyping system by LGC Genomics. Genotype data for these two SNPs were used to define APOE ε2, ε3, and ε4 alleles. For analysis, we classified individuals with at least one copy of APOE ε4 allele to be APOE ε4 carriers. APOE carrier status was included in the description of the sample but was otherwise omitted from follow‐up analyses conducted in this study.
2.5. Assays
Plasma and serum samples were analyzed at the Institute for Translational Research (ITR) Biomarker Core. Automation of the proteomic assay preparation was conducted using a customized Hamilton Robotics StarPlus system. This automated liquid handling workstation substantially improves reliability of assay preparation, which reduces error and coefficients of variation (CVs) and provides increased quality assurance/quality control (QA/QC) monitoring. Any re‐aliquot needs were conducted via the Hamilton easyBlood robotic system. Commercially available proteomic assays were obtained from Meso Scale Discovery (MSD; http://www.mesoscale.com) and assayed using electrochemiluminescence (ECL) per our previously published methods. 11 , 17 The ECL platform has been used extensively to assay biomarkers associated with a range of human diseases including AD. 18 , 19 , 20 , 21 ECL technology uses labels that emit light when electronically stimulated, which improves the sensitivity of detection of many analytes at very low concentrations. ECL measures have well‐established properties of being more sensitive and requiring less volume than conventional enzyme‐linked immunosorbent assays (ELISAs), 19 the gold standard for most assays. Although a number of platforms are available for assays including those considered to be more sensitive (ie, SIMOA), 22 prior work conducted in the neurotypical AD population has demonstrated how successful ECL technology can be when applied to this targeted proteomic panel with CVs < 10%. 17 , 23 Our lab maintains a database of more than n > 2000 samples conducted in duplicate on the same MSD plates and equipment referenced in this study. Our average CVs remain < 10% for all assays with > 60% having CVs less than or equal to 6%. Regarding quality control of the samples, five pooled plasma and five serum controls were included on each plate layout, which contained a similar distribution of age for DS samples and sibling controls. All determinations were conducted in singlicate. A total of 500 μL of plasma and serum were used to assay the following markers: fatty acid binding protein 3 (FABP3), beta 2 microglobulin (B2M), pancreatic polypeptide (PPY), CRP, TPO, α2 macroglobulin (A2M), exotaxin 3, TNF‐α, tenascin C, IL‐5, IL‐6, IL‐7, IL‐10, IL‐18, I‐309, factor VII, soluble intercellular adhesion molecule‐1 (sICAM‐1), circulating vascular cell adhesion molecule‐1 (sVCAM‐1), TARC, and serum amyloid A (SAA). Table S1 in supporting information provides the CVs, lower limit of detection (LLOD). and highest level of detection (HLOD) for each assay separated by fraction. CV was determined by finding the standard deviation/mean × 100 for both fractions as well as for each biomarker. LLOD for each analyte is determined at the concentration 2.5 standard deviations above the background measurement. HLOD is functionally determined from the performance of each assay. HLOD is usually set where immuno‐detection is still linear and has not reached a plateau (ie, top standard concentration).
2.6. Statistical analyses
Statistical analyses were conducted using the R (V 3.3.3) statistical software (R Development Core Team, 2009). Differences in demographic characteristics between diagnostic groups were determined by Fisher's exact test and Mann Whitney U test for categorical variables (sex) and continuous variables (age and level of premorbid intellectual functioning, as indicated by participants’ baseline cognitive assessment or clinical records). Support vector machine (SVM) analysis was used with blood‐biomarker prediction models. SVM is a method that performs classification tasks by constructing hyperplanes in a multidimensional space to separate cases of different class labels. The samples from the different groups were randomly distributed over the analytical runs. Because one biomarker (TPO) was shown to have a relatively high CV for plasma compared to all other proteins, SVM analyses were run both with and without this protein. Diagnostic accuracy was calculated using blood‐based biomarkers alone and in combination with demographic characteristics (ie, age and sex) using receiver operating characteristic (ROC) curves. SVM analyses were run with a five‐fold cross‐validation. The optimized cut‐off score is defined as a threshold to maximize the sum of sensitivity and specificity subject to reaching clinically acceptable levels. Correlations between plasma and serum proteomic markers were conducted using Pearson correlation coefficients.
3. RESULTS
When compared to CS participants, those with MCI‐DS were significantly older (P = .002), of white ethnicity (P = .030), and male (P = .035) but did not differ in the distribution of level of intellectual functioning (Table 1). Similarly, participants with DS‐AD were significantly older (P = .001) compared to those participants who were CS (Table 1). Although there was a trend toward significance, APOE ɛ4 status was comparable in CS and MCI‐DS and in CS and DS‐AD participants.
When distinguishing participants with MCI‐DS from those who were CS, the serum biomarkers alone produced an area under the curve (AUC) of 95% (sensitivity [SN] = 0.023; specificity [SP] = 1.00). The range of accuracy for the five cross‐validations is 79.6 to 87.0 with the average accuracy of 83.6. The inclusion of age and sex along with the serum biomarkers improved the AUC to 99% (SN = 0.45; SP = 1.00). The range of accuracy of the five cross‐validations is 79.6 to 88.7 with the average accuracy of 83.6. Use of an optimized cut‐off score of –0.977 for the serum biomarkers alone increased sensitivity to 0.91 while AUC and specificity remained relatively unchanged (AUC = 95%; SP = 0.99). Applying the same model but using plasma biomarkers alone produced an AUC of 98% (SN = 0.07; SP = 1.00). The range of accuracy for the five cross‐validations is 79.2 to 90.7 with the average accuracy of 83.6. The addition of age and sex did not significantly impact the model as AUC, sensitivity, and specificity remained relatively unchanged (AUC = 0.97; SN = 0.05; SP = 1.00). The range of accuracy for the five cross‐validations is 72.2 to 90.7 with an average accuracy of 83.3. Again, use of an optimized cut‐off score of –0.98 for the plasma biomarkers alone increased sensitivity to 0.96 while AUC and specificity remained stable (AUC = 98%; SP = 0.98; Figure 1).
FIGURE 1.
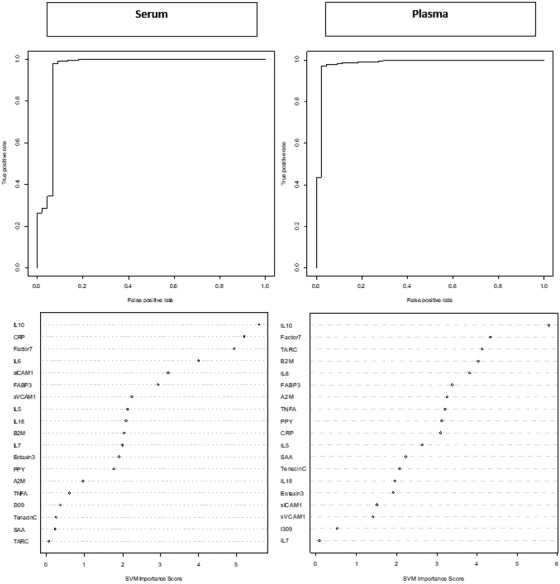
Receiver operating characteristic (ROC) curves and variable importance plots for serum and plasma proteomic profile for detecting mild cognitive impairment‐Down syndrome (MCI‐DS). Note: MCI‐DS, fatty acid binding protein (FABP3), beta 2 microglobulin (B2M), pancreatic polypeptide (PPY), C‐reactive protein (CRP), soluble intercellular adhesion molecule‐1 (sICAM‐1), circulating vascular cell adhesion molecule‐1 (sVCAM‐1), tumor necrosis factor‐alpha (TNF‐α), interleukin (IL)‐5, IL‐6, interleukin IL‐7, IL‐10, IL‐18, factor VII (Factor7), thymus and activation regulated chemokine (TARC), and serum amyloid A (SAA)
When distinguishing among participants with DS‐AD from those who were CS, the serum biomarkers alone produced an AUC of 93% (SN = 0.08; SP = 1.00). The range of accuracy for the five cross‐validations is 82.7 to 92.3 with an average accuracy of 86.2. The addition of age and sex increased the AUC to 98% (SN = 0.17; SP = 1.00). The range of accuracy for the five cross‐validations is 75.5 to 96.2 with an average accuracy of 86.2. Use of an optimized cut‐off score of –0.905 for serum biomarkers alone increased sensitivity to 0.81 while AUC and specificity remained relatively unchanged (AUC = 93%; SP = 0.99). The plasma proteomic profile alone produced an AUC of 95% (SN = 0.08; SP = 1.00). The range of accuracy for the five cross‐validations is 82.7 to 90.4 with an average accuracy of 86.2. The addition of age and sex increased the AUC to 98% (SN = 0.19; SP = 1.00). The range of accuracy for the five cross‐validations is 80.8 to 906 with an average accuracy of 85.8. Use of an optimized cut‐off score of –0.89 for plasma biomarkers alone increased sensitivity to 0.86 while AUC and specificity remained stable (AUC = 0.95; SP = 1.00; Figure 2).
FIGURE 2.
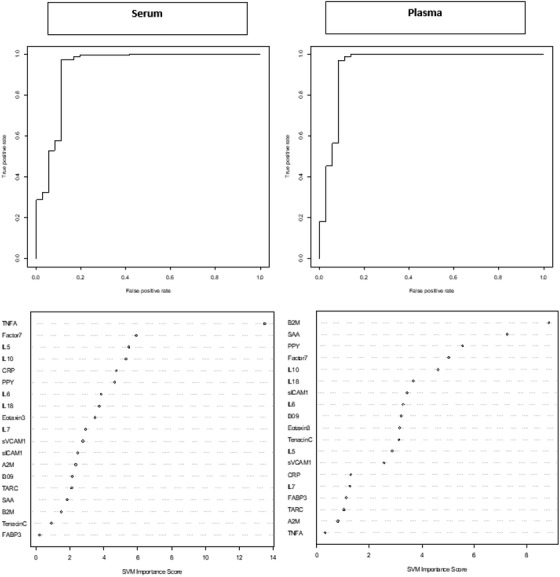
Receiver operating characteristic (ROC) curves and variable importance plots for serum and plasma proteomic profile for detecting Down syndrome‐Alzheimer's disease (DS‐AD). Note: fatty acid binding protein (FABP3), beta 2 microglobulin (B2M), pancreatic polypeptide (PPY), C‐reactive protein (CRP), soluble intercellular adhesion molecule‐1 (sICAM‐1), circulating vascular cell adhesion molecule‐1 (sVCAM‐1), tumor necrosis factor‐alpha (TNF‐α), interleukin (IL)‐5, IL‐6, IL‐7, IL‐10, IL‐18, factor VII (Factor7), thymus and activation regulated chemokine (TARC), and serum amyloid A (SAA)
When examining the SVM variable importance plots for predicting MCI‐DS, six of the top ten biomarkers were elevated across blood fractions and included IL‐10, CRP, Factor7, IL‐6, FABP3, and B2M. For predicting DS‐AD, six of the top ten biomarkers were elevated across blood fractions and included IL‐10, PPY, Factor7, IL‐18, IL‐6, and Eotaxin3 (Figures 1 and 2).
Of note, TPO was excluded from initial SVM models due to the unusually high assay performance parameters for plasma (CV > 40%; Table S1). Because assay performance parameters are derived from pooled DS sibling control cases, the SVM models were re‐run with the inclusion of TPO for both MCI‐DS and DS‐AD to see how this protein would (if at all) impact the proteomic profiles. Results revealed that when TPO was included, it was the top biomarker (as measured by the variable importance plot) for both the serum and plasma proteomic profiles for MCI‐DS. TPO was also among the top two biomarkers for the DS‐AD proteomic profiles again spanning across fractions. For MCI‐DS, there was no change to the testing set for serum biomarkers once TPO was removed, while for plasma biomarkers, when TPO was removed, the specificity for the testing set decreased; however, this change was not statistically significant (P‐value = .176). For DS‐AD, the sensitivity decreased for the testing set while the specificity for the testing set increased for the serum based proteomic profile; however, this change was again not statistically significant (P‐value = .859). For the plasma based proteomic profile for DS‐AD, the testing set slightly decreased after removing TPO; however, the change was not statistically significant (P‐value = .119).
Because the majority of work examining biomarkers related to disease status among adults with DS has been conducted in plasma, it was important to explore the correlation between biomarkers across fraction (serum and plasma). Those proteins with a high correlation reflect biomarkers that are able to be well detected within a disease status (MCI‐DS, DS‐AD) despite differences between fractions including clotting factors and so on. Findings revealed that the correlations of all proteins were statistically significant between serum and plasma (P < .001; Tables 2 and 3) with the majority of markers correlated at a level of 0.7 or higher. However, when considering the biological meaningfulness of the correlations, only Eotaxin3, IL‐6, IL‐10, CRP, IL‐18, SAA, and FABP3 correlated at a level to suggest at least an 80% of shared variance or greater (ie, r2 > = 0.90). Interestingly, many of the markers that correlated most highly across both blood fractions were also ranked very highly in the proteomic profiles for detecting DS‐MCI and DS‐AD across both blood fractions.
TABLE 2.
Correlation between serum and plasma biomarkers in the total sample
| R2 | P‐value | |
|---|---|---|
| Eotaxin3 | 1.00 | <.001 |
| IL‐6 | 1.00 | <.001 |
| IL‐10 | 0.99 | <.001 |
| CRP | 0.97 | <.001 |
| IL‐18 | 0.97 | <.001 |
| SAA | 0.97 | <.001 |
| FABP3 | 0.95 | <.001 |
| IL‐5 | 0.88 | <.001 |
| I309 | 0.84 | <.001 |
| TPO | 0.84 | <.001 |
| Factor7 | 0.80 | <.001 |
| B2M | 0.78 | <.001 |
| Tenascin C | 0.76 | <.001 |
| A2M | 0.73 | <.001 |
| PPY | 0.60 | <.001 |
| TARC | 0.58 | <.001 |
| sVCAM‐1 | 0.56 | <.001 |
| TNF‐α | 0.50 | <.001 |
| sICAM‐1 | 0.43 | .001 |
| IL‐7 | 0.34 | <.001 |
Abbreviations: A2M, alpha 2 macroglobulin; B2M, beta 2 microglobulin; CRP, c‐reactive protein; Factor7, factor VII; FABP3, fatty acid binding protein; IL‐10, interleukin‐10; IL‐18, interleukin‐18; IL‐5, interleukin‐5; IL‐6, interleukin‐6; IL‐7, interleukin‐7; PPY, pancreatic polypeptide; SAA, serum amyloid A; sICAM‐1, soluble intercellular adhesion molecule‐1; sVCAM‐1, circulating vascular cell adhesion molecule‐1; TARC, thymus and activation regulated chemokine; TNF‐α, tumor necrosis factor‐alpha; TPO, thrombopoietin
TABLE 3.
Correlation between serum and plasma biomarkers split by diagnostic group
| CS | MCI‐DS | DS‐AD | ||||
|---|---|---|---|---|---|---|
| R2 | P‐value | R2 | P‐value | R2 | P‐value | |
| A2M | 0.74 | <.001 | 0.81 | <.001 | 0.49 | .002 |
| B2M | 0.79 | <.001 | 0.71 | <.001 | 0.79 | <.001 |
| CRP | 0.97 | <.001 | 0.95 | <.001 | 0.96 | <.001 |
| Eotaxin3 | 1.00 | <.001 | 1.00 | <.001 | 0.61 | <.001 |
| FABP3 | 0.95 | <.001 | 0.93 | <.001 | 0.96 | <.001 |
| Factor7 | 0.79 | <.001 | 0.91 | <.001 | 0.75 | <.001 |
| I309 | 0.84 | <.001 | 0.85 | <.001 | 0.86 | <.001 |
| IL‐10 | 0.99 | <.001 | 0.87 | <.001 | 0.96 | <.001 |
| IL‐18 | 0.97 | <.001 | 0.97 | <.001 | 0.98 | <.001 |
| IL‐5 | 0.91 | <.001 | 0.82 | <.001 | 0.70 | <.001 |
| IL‐6 | 1.00 | <.001 | 0.46 | .002 | 0.97 | <.001 |
| IL‐7 | 0.30 | <.001 | 0.52 | <.001 | 0.34 | .04 |
| PPY | 0.57 | <.001 | 0.76 | .001 | 0.56 | .004 |
| SAA | 0.98 | <.001 | 0.93 | <.001 | 0.96 | <.001 |
| sICAM1 | 0.45 | <.001 | 0.29 | .063 | 0.45 | .005 |
| sVCAM1 | 0.52 | <.001 | 0.57 | <.001 | 0.65 | <.001 |
| TARC | 0.47 | <.001 | 0.58 | <.001 | 0.88 | <.001 |
| Tenascin C | 0.73 | <.001 | 0.84 | <.001 | 0.78 | <.001 |
| TNF‐α | 0.64 | <.001 | 0.74 | <.001 | 0.43 | .009 |
| TPO | 0.71 | <.001 | 1.00 | <.001 | 0.94 | <.001 |
Abbreviations: A2M, alpha 2 macroglobulin; AD, Alzheimer's disease; B2M, beta 2 microglobulin; CRP, c‐reactive protein; CS, cognitively stable; DS, Down syndrome; Factor7, factor VII; FABP3, fatty acid binding protein; IL‐10, interleukin‐10; IL‐18, interleukin‐18; IL‐5, interleukin‐5; IL‐6, interleukin‐6; IL‐7, interleukin‐7; MCI, mild cognitive impairment; PPY, pancreatic polypeptide; SAA, serum amyloid A; sICAM‐1, soluble intercellular adhesion molecule‐1; sVCAM‐1, circulating vascular cell adhesion molecule‐1; TARC, thymus and activation regulated chemokine; TNF‐α, tumor necrosis factor‐alpha; TPO, thrombopoietin
4. DISCUSSION
This study was the first to apply a specific panel of proteomic markers previously selected and validated in the neurotypical AD population, in adults with DS. The results reveal that proteomic profiles derived from both serum and plasma produce similar levels of accuracy across disease state (MCI‐DS, DS‐AD). The addition of age and sex increased accuracy for all proteomic profiles with the exception of the plasma proteomic profile for MCI‐DS, which remained elevated with an AUC of 98%. Use of an optimized cut‐off score improved most predictive models by increasing sensitivity. The detection accuracy from the derived proteomic profiles for both MCI‐DS and DS‐AD based on an optimized cut‐off score mirrored that observed in the neurotypical AD population showing its utility for application in the DS population. Our findings reveal that although many of the same biomarkers significantly impact the proteomic profiles across blood fractions, for most cases, the variable importance plots were distinct with only a few exceptions.
One example includes the MCI‐DS serum and plasma proteomic profiles, which comprised the same top biomarker (IL‐10) on the variable importance plot. Of note, the remaining combination of biomarkers comprising ranks 3 to 10 differed by fraction for MCI‐DS. When TPO was included in the model, it was found to play a significant role in both the MCI‐DS and DS‐AD proteomic profiles spanning fractions. TPO is a known glycoprotein hormone that aids in the production of platelets 24 , 25 and in DS, TPO has been almost exclusively studied among pediatric cases due to high level of thrombocytopenia. 26 , 27 , 28 Although TPO exhibited an unexpectedly high CV for plasma (and was initially excluded from derived protoemic profiles), the inclusion of TPO did not significantly change the detection accuracy of the proteomic profiles despite its prominence within the models. This suggests that TPO may play an important role among individuals with DS who also experience MCI or AD and should be explored further.
When looking more broadly at the proteomic profile for MCI‐DS, the serum proteomic profile was found to be driven by inflammatory based proteins such as IL‐10, CRP, and IL‐6. While the plasma proteomic profile for MCI‐DS showed similar elevations in inflammatory based proteins (IL‐10, B2M, TARC, IL‐6), the profile also included other biomarkers such as those related to endocrine function (PPY). The same inflammatory driven proteomic profile was found for DS‐AD (B2M, TNF‐α, IL‐5, IL‐10, CRP, IL‐18, IL‐6) again spanning fractions similar to MCI‐DS. This finding reflects that observed among non‐Hispanic whites in the neurotypical AD population who also present with a similar inflammatory based proteomic profile for AD. 10 Among individuals with DS, a number of the same inflammatory biomarkers have been shown to be elevated across the lifespan suggesting chronic neuroinflammation, 29 which corresponds to the increased predisposition for a number of autoimmune conditions 30 , 31 , 32 , 33 observed in this population. A meta‐analysis conducted primarily among children with DS revealed elevations in TNF‐α, IL‐1β, and IFN‐y, 34 while similar elevations in inflammatory cytokines and chemokines (IL‐6, IL‐22, and TNF‐α) were identified in a different study conducted among adults with DS compared to neurotypical healthy control adults. 35 Despite evidence to support chronic inflammation in this population, distinct elevations in inflammatory proteins between diagnostic categories (MCI‐DS and DS‐AD) suggests that specific inflammatory processes may uniquely contribute to the progression of cognitive decline among adults with DS. Understanding both the overall role inflammation plays in disease progression for adults with DS will continue to be important as well as the role specific proteins have across disease status.
One example of why it will be important to examine individual proteins more closely is due to the unique composition of each (serum and plasma) proteomic profile observed in this study for MCI‐DS and DS‐AD. Aside from a few select cases, most proteins held a distinct position on the variable importance plots, which differed by fraction and disease status for MCI‐DS and DS‐AD. One example of this was CRP, which is a well‐known biomarker of inflammation. 36 In the MCI‐DS serum proteomic profile, CRP was ranked as the third most important protein in the diagnostic model; however, in the MCI‐DS plasma proteomic profile, this same protein was ranked tenth on the variable importance plot. CRP, which has been previously been linked to AD in the neurotypical population 37 was only among the top 10 biomarkers for the serum protoemic profile for DS‐AD and was not found to be among the top plasma biomarkers.
It will be important for future work to expand on the current findings to evaluate potential cross‐over between serum and plasma biomarkers that, if combined, could possibly increase diagnostic accuracy. Further work, for example, is needed to evaluate how additional biomarkers linked with neurodegeneration such as NfL, tau, and Aβ 40 and 42 might impact the proteomic profiles included in this study or if the proteomic profiles are able to be refined down to only a smaller number of proteins. One limitation of the study is the relatively small sample size for those with DS‐AD as additional cases could impact the detection accuracy and variable importance plots. Another limitation to the study is use of DS sibling control cases as a control group, which produced several instances of high CVs across the proteomic assays. High CVs likely reflects individual variability among the DS sibling control cases and may not reflect poor performance of the assays within the DS sample itself. To determine this and help understand performance of the selected assays among this specific population, future work will include standardized control samples compared to DS sibling control samples. The ABC–DS is an ongoing study with continued recruitment, which will allow for future work to both validate the same proteomic profiles in a larger sample and to detect phenoconversion from CS to MCI‐DS and DS‐AD. This effort will also help to address inherent limitations with the diagnostic category of MCI‐DS given that adults with DS are at increased risk for AD due to higher rates of amyloid deposition. This work has implications not only for understanding DS‐AD but may provide important insights relevant to the neurotypical AD population.
CONFLICTS OF INTEREST
SEO has patents and patents pending regarding blood‐biomarkers for precision medicine in neurodegenerative diseases and served on an Advisory Board to Roche Diagnostics. No other authors have conflicts of interest to disclose.
Supporting information
Supplemental Table 1. CV, LLOD, and HHOD for each assay split by serum and plasma
ACKNOWLEDGMENTS
The Alzheimer's Biomarkers Consortium–Down Syndrome (ABC–DS) project is a longitudinal study of cognition and blood‐based, genetic, and imaging biomarkers of Alzheimer's disease. This publication was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number UL1TR001873. This work was also supported by grant IIRG‐08‐90655 from the Alzheimer's Association (Schupf, O'Bryant) and by additional grants P01HD035897 (Silverman) from National Institute for Child Health and Human Development (NICHD), and R01AG014673 (Schupf), R01AG058537 (O'Bryant), R01AG058252 (O'Bryant) and R01AG051848 (O'Bryant) from National Institute on Aging (NIA), and by NYS through its office for People with Developmental Disabilities. We thank the ABC–DS study participants and the ABC–DS research and support staff for their contributions to this study.
Samples from the National Centralized Repository for Alzheimer's Disease and Related Dementias (NCRAD), which receives government support under a cooperative agreement grant (U24 AG21886) awarded by the NIA, were used in this study. We thank contributors who collected samples used in this study, as well as patients and their families, whose help and participation made this work possible.
This manuscript has been reviewed by ABC–DS investigators for scientific content and consistency of data interpretation with previous ABC–DS study publications. We acknowledge the ABC–DS study participants and the ABC–DS research and support staff for their contributions to this study. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Petersen ME, Zhang F, Schupf N, et al. Proteomic profiles for Alzheimer's disease and mild cognitive impairment among adults with Down syndrome spanning serum and plasma: An Alzheimer's Biomarker Consortium–Down Syndrome (ABC–DS) study. Alzheimer's Dement. 2020;12:e12039 10.1002/dad2.12039
Alzheimer's Biomarker Consortium–Down Syndrome (ABC–DS) Investigators: Howard J. Aizenstein, MD, PhD; Beau M. Ances, MD, PhD; Howard F. Andrews, PhD; Karen Bell, MD; Rasmus M. Birn, PhD; Adam M. Brickman, PhD; Peter Bulova, MD; Amrita Cheema, PhD; Kewei Chen, PhD; Bradley T. Christian, PhD; Isabel Clare, PhD; Lorraine Clark, PhD; Ann D. Cohen, PhD; John N. Constantino, MD; Eric W. Doran, MS; Anne Fagan, PhD; Eleanor Feingold, PhD; Tatiana M. Foroud, PhD; Benjamin L. Handen, PhD; Sigan L. Hartley, PhD; Elizabeth Head, PhD; Rachel Henson, PhD; Christy Hom, PhD; Lawrence Honig, MD; Milos D. Ikonomovic, MD; Sterling C. Johnson, PhD; Courtney Jordan, RN; M. Ilyas Kamboh, PhD; David Keator, PhD; William E. Klunk, MD PhD; Julia K. Kofler, MD; William Charles Kreisl, MD; Sharon J. Krinsky‐McHale, PhD; Florence Lai, MD; Patrick Lao, PhD; Charles Laymon, PhD; Joseph Hyungwoo Lee, PhD; Ira T. Lott, MD; Victoria Lupson, PhD; Mark Mapstone, PhD; Chester A. Mathis, PhD; Davneet Singh Minhas, PhD; Neelesh Nadkarni, MD; Sid O'Bryant, PhD; Deborah Pang, MPH; Melissa Petersen, PhD; Julie C. Price, PhD; Margaret Pulsifer, PhD; Eric Reiman, MD; Batool Rizvi, MS; Herminia Diana Rosas, MD; Marwan N. Sabbagh, MD; Nicole Schupf, PhD; Wayne P. Silverman, PhD; Dana L. Tudorascu, PhD; Rameshwari Tumuluru, MD; Benjamin Tycko, MD, PhD; Badri Varadarajan, PhD; Desiree A. White, PhD; Michael A. Yassa, PhD; Shahid Zaman, MD, PhD; Fan Zhang, PhD
REFERENCES
- 1. Rumble B, Retallack R, Hilbich C, et al. Amyloid A4 protein and its precursor in Down's syndrome and Alzheimer's disease. N Engl J Med. 1989;320:1446‐1452. [DOI] [PubMed] [Google Scholar]
- 2. Lott IT, Head E. Dementia in Down syndrome: unique insights for Alzheimer disease research. Nat Rev Neurol. 2019;15:135‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sabbagh M, Edgin J. Clinical assessment of cognitive decline in adults with Down syndrome. Curr Alzheimer Res. 2015;13:30‐34. [DOI] [PubMed] [Google Scholar]
- 4. Schupf N, Patel B, Silverman W, et al. Elevated plasma amyloid β‐peptide 1‐42 and onset of dementia in adults with Down syndrome. Neurosci Lett. 2001;301:199‐203. [DOI] [PubMed] [Google Scholar]
- 5. Schupf N, Patel B, Pang D, et al. Elevated plasma β‐amyloid peptide Aβ42 levels, incident dementia, and mortality in Down syndrome. Arch Neurol. 2007;64:1007‐1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schupf N, Zigman WB, Tang MX, et al. Change in plasma Aβ peptides and onset of dementia in adults with Down syndrome. Neurology. 2010;75:1639‐1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fortea J, Carmona‐Iragui M, Benejam B, et al. Plasma and CSF biomarkers for the diagnosis of Alzheimer's disease in adults with Down syndrome: a cross‐sectional study. Lancet Neurol. 2018;17:860‐869. [DOI] [PubMed] [Google Scholar]
- 8. Iulita MF, Ower A, Barone C, et al. An inflammatory and trophic disconnect biomarker profile revealed in Down syndrome plasma: relation to cognitive decline and longitudinal evaluation. Alzheimer's Dement. 2016;12:1132‐1148. [DOI] [PubMed] [Google Scholar]
- 9. Petersen Melissa, Zhang Fan, Krinsky‐McHale Sharon J., Silverman Wayne, Lee Joseph H., Pang Deborah, Hall James, Schupf Nicole, O'Bryant Sid E. Proteomic profiles of prevalent mild cognitive impairment and Alzheimer's disease among adults with Down syndrome. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring. 2020;12:1: 10.1002/dad2.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. O'Bryant SE, Xiao G, Barber R, et al. A blood‐based screening tool for Alzheimer's disease that spans serum and plasma: findings from TARC and ADNI. PLoS One. 2011;6:e28092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O'Bryant SE, Xiao G, Zhang F, et al. Validation of a serum screen for Alzheimer's disease across assay platforms, species, and tissues. J Alzheimer's Dis. 2014;42:1325‐1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O'Bryant SE, Ferman TJ, Zhang F, et al. A proteomic signature for dementia with Lewy bodies. Alzheimer's Dement Diagnosis. Assess Dis Monit. 2019;11:270‐276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. O'Bryant SE, Edwards M, Zhang F, et al. Potential two‐step proteomic signature for parkinson's disease: pilot analysis in the Harvard biomarkers study. Alzheimer's dement diagnosis. Assess Dis Monit. 2019;11:374‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hamlett ED, Goetzl EJ, Ledreux A, et al. Neuronal exosomes reveal Alzheimer's disease biomarkers in Down syndrome. Alzheimer's Dement. 2017;13:541‐549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aylward EH, Burt DB, Thorpe LU, Lai F, Dalton A. Diagnosis of dementia in individuals with intellectual disability. J Intellect Disabil Res. 1997;41:152‐164. [DOI] [PubMed] [Google Scholar]
- 16. Burt DB, Aylward EH. Test battery for the diagnosis of dementia in individuals with intellectual disability. J Intellect Disabil Res. 2000;44:175‐180. [DOI] [PubMed] [Google Scholar]
- 17. O'Bryant SE, Edwards M, Johnson L, et al. A blood screening test for Alzheimer's disease. Alzheimer's dement diagnosis. Assess Dis Monit. 2016;3:83‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alves G, Brønnick K, Aarsland D, et al. CSF amyloid‐β and tau proteins, and cognitive performance, in early and untreated Parkinson's disease: the Norwegian Parkwest study. J Neurol Neurosurg Psychiatry. 2010;81:1080‐1086. [DOI] [PubMed] [Google Scholar]
- 19. Kuhle J, Regeniter A, Leppert D, et al. A highly sensitive electrochemiluminescence immunoassay for the neurofilament heavy chain protein. J Neuroimmunol. 2010;220:114‐119. [DOI] [PubMed] [Google Scholar]
- 20. Oh ES, Mielke MM, Rosenberg PB, et al. Comparison of conventional ELISA with electrochemiluminescence technology for detection of amyloid‐β in plasma. J Alzheimer's Dis. 2010;21:769‐773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bjerke M, Portelius E, Minthon L, et al. Confounding factors influencing amyloid beta concentration in cerebrospinal fluid. Int J Alzheimers Dis. 2010;2010:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kuhle J, Barro C, Andreasson U, et al. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin Chem Lab Med. 2016;54:1655‐1661. [DOI] [PubMed] [Google Scholar]
- 23. O'Bryant SE, Lista S, Rissman RA, et al. Comparing biological markers of Alzheimer's disease across blood fraction and platforms: comparing apples to oranges. Alzheimer's dement diagnosis. Assess Dis Monit. 2016;3:27‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wolber EM, Jelkmann W. Thrombopoietin: the novel hepatic hormone. News Physiol Sci. 2002;17:6‐10. [DOI] [PubMed] [Google Scholar]
- 25. Jelkmann W. The role of the liver in the production of thrombopoietin compared with erythropoietin. Eur J Gastroenterol Hepatol. 2001;13:791‐801. [DOI] [PubMed] [Google Scholar]
- 26. Matsubara K, Nigami H, Yura K, Inoue T, Isome K, Fukaya T. Serum thrombopoietin level and thrombocytopenia during the neonatal period in infants with Down's syndrome. J Perinatol. 2010;30:98‐102. [DOI] [PubMed] [Google Scholar]
- 27. Wiedmeier SE, Henry E, Christensen RD. Hematological abnormalities during the first week of life among neonates with trisomy 18 and trisomy 13: data from a multi‐hospital healthcare system. Am J Med Genet Part A. 2008;146A:312‐320. [DOI] [PubMed] [Google Scholar]
- 28. Henry E, Walker D, Wiedmeier SE, Christensen RD. Hematological abnormalities during the first week of life among neonates with Down syndrome: data from a multihospital healthcare system. Am J Med Genet Part A. 2007;143A:42‐50. [DOI] [PubMed] [Google Scholar]
- 29. Wilcock DM, Griffin WS. Down's syndrome, neuroinflammation, and Alzheimer neuropathogenesis. J Neuroinflammation. 2013;10:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Padmakumar B. Is arthritis more common in children with Down syndrome. Rheumatology. 2002;41:1191‐1193. [DOI] [PubMed] [Google Scholar]
- 31. Kinik ST, Özçay F, Varan B. Type I diabetes mellitus, Hashimoto's thyroiditis and celiac disease in an adolescent with Down syndrome. Pediatr Int. 2006;48:433‐435. [DOI] [PubMed] [Google Scholar]
- 32. Mårild K, Stephansson O, Grahnquist L, Cnattingius S, Söderman G, Ludvigsson JF. Down syndrome is associated with elevated risk of celiac disease: a nationwide case‐control study. J Pediatr. 2013;163:237‐242. [DOI] [PubMed] [Google Scholar]
- 33. Anwar AJ, Walker JD, Frier BM. Type 1 diabetes mellitus and Down's syndrome: prevalence, management and diabetic complications. Diabet Med. 1998;15:160‐163. [DOI] [PubMed] [Google Scholar]
- 34. Zhang Y, Che M, Yuan J, et al. Aberrations in circulating inflammatory cytokine levels in patients with Down syndrome: a meta‐analysis. Oncotarget. 2017;8:84489‐84496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sullivan KD, Evans D, Pandey A, et al. Trisomy 21 causes changes in the circulating proteome indicative of chronic autoinflammation. Sci Rep. 2017;7:14818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Luan YY, Yao YM. The clinical significance and potential role of C‐reactive protein in chronic inflammatory and neurodegenerative diseases. Front Immunol. 2018;9:1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. O'Bryant SE, Waring SC, Hobson V, et al. Decreased C‐reactive protein levels in Alzheimer disease. J Geriatr Psychiatry Neurol. 2010;23:49‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. CV, LLOD, and HHOD for each assay split by serum and plasma


