Abstract
Microelectrode recordings were performed during awake deep brain stimulation surgery for obsessive-compulsive disorder, revealing robust brain oscillations that were plainly visible throughout the ventral striatum. There was an elegant topological correspondence between each oscillation and the underlying brain anatomy, most prominently a ~35-Hz gamma-oscillation specific to the nucleus accumbens. Direct provocation of the patient’s contamination obsession modulated both firing rate and gamma-oscillation amplitude within the nucleus accumbens.
NEW & NOTEWORTHY Surgical implantation of deep brain stimulating electrodes (DBS) to treat obsessive-compulsive disorder (OCD) is an option for patients who have not fully responded to medical intervention or cognitive behavioral therapy. We measured the electrophysiology of a collection of deep brain structures during awake DBS surgery for an OCD patient with an obsession about cleanliness and contamination. The anatomic delineation of these deep brain structures was revealed by distinct brain rhythms, most notably a ~35 Hz oscillation specific to the nucleus accumbens. In the first ever measurement of a human obsessive thought, we found that this ~35-Hz biomarker, as well as the local neuronal action potential rate, were modulated by handing the patient a toothbrush to bring to his face and instructing him to “imagine brushing your teeth with this dirty toothbrush.”
Keywords: deep brain stimulation, electrophysiology, gamma oscillation, nucleus accumbens, obsessive-compulsive disorder
INTRODUCTION
Have you ever gone back into your house shortly after leaving to make sure the oven was turned off, despite remembering turning it off? Have you then had the urge to check it yet again? These transient motivations are a normal part of the human experience that reinforce patterned behavior, and most of us can suppress them when they contradict what we know to be reasonable. But this ability to suppress is dysfunctional in those with obsessive-compulsive disorder (OCD), a neuropsychiatric disease characterized by repetitive physical or mental acts (compulsions) directed toward unwanted persistent images, thoughts, or impulses (obsessions) (Westphal 1877). The execution of compulsions consumes the time and effort of individuals to the degree that they dramatically interrupt personal and professional activities (American Psychiatric Association 2013). Standard treatment is a combination of systematic exposure to the objects of obsession during cognitive behavioral therapy and medical intervention (Grant 2014).
Beginning 15 years ago, deep brain stimulation (DBS) emerged as a therapy for patients who fail the most aggressive standard treatment (Sturm et al. 2003). As a region of confluent cortical, striatal, and thalamic projections, the nucleus accumbens (NAc) was felt to be an ideal initial target for DBS. Long-term studies of therapeutic outcome have substantiated its efficacy in many patients (Alonso et al. 2015; Fayad et al. 2016; Sheth et al. 2013). However, NAc DBS does not help some patients (Mian et al. 2010), and this can likely be attributed to variability in electrode positioning and individuals’ functional anatomy. Variable-response DBS for other diseases is partially mitigated by performing electrode implantation awake, making microelectrode recordings to identify neuronal populations whose activity correlates with disease-related tasks (e.g., limb movement in Parkinson’s disease) (Romanelli et al. 2004). This strategy had remained unrealized for OCD until a patient of ours with a particular contamination obsession underwent awake NAc-DBS surgery. The provocable nature of his disease allowed for electrophysiological characterization of the fundamental processes that underlie obsessive thoughts.
METHODS
All recorded data, as well as analysis code in MATLAB format are available at https://searchworks.stanford.edu/view/xf387wq3868 (open “kjm_NAc_OCD_Read_Me.pdf” at this URL for a complete description). There is also a supplement with methodological illustration and some minor additional results at the same URL, in the file “kjm_NAc_OCD_Supplemental.pdf.”
Patient and surgical implantation.
A 64-yr-old male patient with intractable obsessive-compulsive disorder, refractory to all medications, presented for bilateral DBS electrode implantation of the ventral capsule/ventral striatum, a region that includes the NAc. His disease centered on cleanliness and bathroom-related activities, particularly brushing his teeth, causing marked impairment in his ability to carry out his normal activities of daily life. The patient consented to participate in a research protocol during the awake surgery for implantation of these leads. Stanford’s internal review board approved the study and the consent process (IRB no. 33146). Stereotactic targeting and alignment to the left NAc was performed with the NexFrame and Stealth S7 system (Medtronic, Minneapolis, MN). A cannula was stereotactically passed from the middle frontal gyrus to the ventromedial internal capsule aligned to NAc in-plane with the anterior commissure (Fig. 1A). From the tip of the cannula, a microelectrode (0.5–1 MΩ platinum–iridium; FHC, Bowdoin, ME) was advanced 20 mm to a target in the ventral NAc (Fig. 1A). The target location for the stereotactic placement in the anterior commissure-posterior commissure (AC-PC) coordinate system was at x = 6.0 mm, y = 15.1 mm, z = −6.6 mm, with a trajectory of 34.2° from the midsagittal plane and 60.3° from the axial plane. With prolonged clinical stimulation at the border of the accumbens/commissure (Medtronic 3391 electrode spanning 7–10 mm above target in Fig. 1), the patient achieved a 30% reduction in Yale-Brown obsessive-compulsive scale. The Medtronic 3391 lead cleared for humanitarian exemption contains 3-mm leads separated by 4 mm. Because of the large 3-mm size of these leads, they cannot delineate NAc subregions. Likely for this reason, widespread stimulation throughout the NAc does not always provide optimal therapy, and a more dorsal stimulation program is often employed (Alonso et al. 2015).
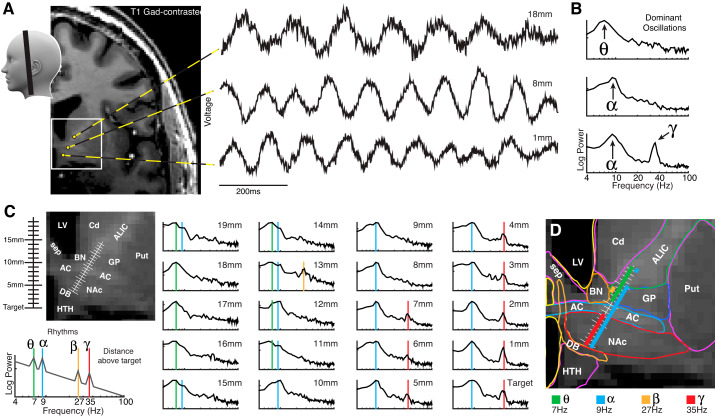
Fig. 1.
A robust map of brain oscillations in the ventral capsule/ventral striatal region of the human brain. A: oscillations of different frequency are plainly visible in the raw voltage trace at three exemplar sites (+1, +8, +18 mm above target). B: power spectral densities (PSDs) on log-log axes for the three sites in A show clear peaks in the theta (7 Hz), alpha (9 Hz), and gamma (35 Hz) frequencies above a 1/f background shape (Miller et al. 2009). C: PSDs were measured at 1-mm intervals from the cannula to target location. Colored background lines show significant oscillations (green-7 Hz/θ; blue-9 Hz/α; orange-25 Hz/β; red-35 Hz/γ). D: anatomical plotting of oscillations revealed a plain topological correspondence between each oscillation and the underlying brain anatomy. AC, anterior commissure; ALIC, anterior limb of internal capsule; BN, bed nucleus of the stria terminalis; Cd, caudate; DB, diagonal band of Broca; GP, globus pallidus; HTH, hypothalamus; LV, lateral ventricle; NAc, nucleus accumbens; Put, putamen; sep, septal nuclei/fornix.
Signal analysis.
Raw voltage, V0(t), was measured from the microelectrode, referenced to the cannula, and sampled at 50 kHz using a Guideline 3000 microelectrode recording system (Axon Instruments) (gain, 10,000; band-pass filtered from 1 Hz to 10 kHz), passed through a CyberAmp 380 amplifier/filter (Axon Instruments, Foster City, CA) (band-pass filtered from 1 Hz to 6 kHz), and sampled at 50,000 samples/s using a data-acquisition interface (Power1401) and Spike software (version 2.7, Cambridge Electronic Design, Cambridge, UK). Although previous studies were able to extract meaningful measurements of phase below 4 Hz (Wu et al. 2018), there was significant signal amplitude attenuation in this range, so we have limited our exploration in this study to frequencies above 4 Hz.
A number of steps were employed to isolate spikes from the raw voltage trace (illustrated in the online methodological supplement):
-
•
First, the raw voltage trace was high-pass filtered at 300 Hz, . A linear threshold was then visually fit to the filtered voltage trace at each location to capture characteristic action potential voltage deflections.
-
•
Seven-millisecond windows of data were obtained surrounding the sample of furthest excursion from baseline for each action potential deflection, τq, from 2 ms before to 5 ms after [e.g., , where −2 ms≤t′≤5 ms]. The average of these windows gives the characteristic action potential shape.
-
•
These data windows surrounding action potential times were then decomposed with a principal component approach. A singular value decomposition is used to determine the eigenvalues λk and eigenvectors of the correlation matrix: . Note that the baseline is, in effect, subtracted off each window as a by-product of the high-pass filtering. These eigenvectors, C, reveal characteristic shapes in the temporal shape of the action potential that vary orthogonally and are ordered by magnitude of corresponding eigenvalue: (where T number of time points in intervals of −2 to 5 ms). If we define the rotation matrix , then the projection, W(k,q, of each individual spike in the ensemble into the new eigenvector space is . The inverse rotation matrix A−1 (where ) allows us to remove the weighted spike components (the first 3 eigenvectors) surrounding spike at time τq from the raw voltage time series, leaving the local field potential (LFP): .
From this LFP, oscillations were characterized as follows:
-
•
Power spectral densities (PSDs) were calculated using Welch’s averaged periodogram method, with 1-s windows, using a Hann window, stepping through V(t) in 250-ms intervals (Fig. 1). Peaks in the PSDs were visually apparent above a 1/f background shape, centered at 7 Hz (theta), 9 Hz (alpha), 25 Hz (beta), and 36 Hz (gamma).
-
•
Rhythm amplitudes were calculated by band-passing the local field potential, V(t), using a 3rd order Butterworth filter for a specified frequency range, F, to obtain the “band-limited” potential, V(F,t). A complex analytic signal, , was constructed using the Hilbert transform, which can also be expressed in polar notation as . In this study, the alpha range is F→8–10 Hz, and the gamma range is F→31–39 Hz.
Anatomic localization.
As illustrated in Fig. 1A, microelectrode recording position was determined by fusion of the postsurgical CT to the presurgical MRI, using a normalized mutual information approach, and reslicing in-plane with the DBS shank while preserving midline symmetry. Then the intraoperative microelectrode recording positions were inferred from the corresponding postimplantation DBS electrode lead positions (where the terminal lead was at the target position). Gray matter-nulled MR, white matter-nulled MR, and postgadolinium contrasted T1 were overlaid, so underlying ventral striatal anatomic structures could be clearly delineated.
Compulsion provocation.
A simple provocation test was designed based on a self-reported fear known to trigger his compulsive full body cleaning. After a brief baseline period, a psychiatrist at the bedside (N. R. Williams) handed the patient a toothbrush, telling him first to bring it to his face, and then told to “imagine brushing your teeth with this dirty toothbrush.” The toothbrush was then taken back from the patient, and, as a control, the patient was then instructed to bring his hand back to his face without the toothbrush (Fig. 2, Supplemental video). This toothbrush provocation testing was performed twice, once each at 3 mm and 1 mm above target, where actively spiking neurons had been identified. Spike rate counts and average oscillation amplitudes of r(F,t) were calculated in 1-s blocks (block size chosen arbitrarily) to calculate statistical significance during the task (as shown in Fig. 2).
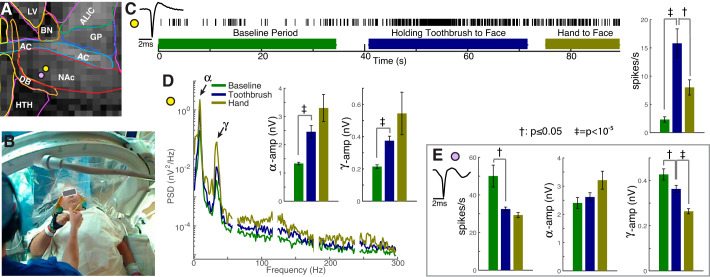
Fig. 2.
Physiological changes during provocation of an obsessive fear that drives compulsive cleaning behavior. A: provocation was performed at two nucleus accumbens (NAc) sites (yellow/purple dots 4/6 mm ventral to the dorsal NAc border). B: after resting baseline, the patient was handed a toothbrush to bring to his face and was told “imagine brushing your teeth with this dirty toothbrush,” followed by bringing his hand to his face without a toothbrush (see Supplemental video). C: action potential rate selectively increased during toothbrush provocation at the yellow site. (‡P = 1×10−6/t = 5.4; †P = 0.05/t = −2.0, by unpaired t-test. Error bars show SE of 1-s blocks). D: power spectral densities reveal progressive power increase across all frequencies during the task at the yellow site. Inset axes show isolated alpha-range (8–10 Hz, ‡P = 2×10−6/t = 5.2) and gamma-range (31–39 Hz, ‡P = 7×10−7/t = 5.5) amplitudes. E: conversely, a neuron captured 2 mm ventral (purple site) exhibited a significant decrease in spike rate (P = †8×10−3/t = −2.7) and gamma-range amplitude (†P = 0.04/t = −2.1; ‡P = 4×10−6/t = 5.0) with provocation.
RESULTS
A map of human brain oscillations in the ventral capsule/ventral striatal region.
Field potentials measured were measured at every millimeter from the opening of a stereotactic guidance cannula to the NAc target 2 cm below (Fig. 1). The raw potential traces showed visually apparent oscillations, plainly reflected by peaks in the PSDs. When these PSDs are viewed alongside one another, a clear topological relationship between oscillatory frequency and brain anatomy emerges:
-
•
A robust 35-Hz-centered gamma oscillation (hγ35) was found specifically in the NAc and nowhere else. Based on comparison with recent human segmentations using diffusion tractography (Baliki et al. 2013), our NAc recordings are most likely in the shell subregion.
-
•
7-Hz-centered theta oscillations extended throughout the recorded portion of the anterior limb of the internal capsule (ALIC), including where capsular fibers were colocalized with the globus pallidus (GP) and the bed nucleus of the stria terminalis (BNST).
-
•
9-Hz-centered alpha oscillations were present throughout all structures except for the ALIC.
-
•
A small focus of 27-Hz-centered beta oscillation was found at the confluence of ALIC, GP, and BNST, making it difficult to attribute to a single structure.
Physiological changes during provocation of an obsessive fear.
As the microelectrode tip neared the ventral NAc target, the patient was handed a toothbrush and told to bring it to his face, and then to imagine it dirty while also imagining brushing his teeth with it. This test was performed for clinical purposes: to attempt neuronal action potential modulation correlated with his contamination obsession and confirm regional involvement of his disease, much like sensorimotor testing is used in movement disorders (Benabid et al. 2000). Robustly firing neurons were studied at two sites within NAc, 2 mm from one another, and 4 and 6 mm ventral to the dorsal border (Fig. 2). In response to the provocation test, we made the following observations:
-
•
At the more dorsal NAc site, firing rate of the measured neuron increased specifically during provocation of the compulsion. The amplitude of both the alpha and gamma oscillations increased with provocation (Fig. 2, also illustrated in the supplemental videos at https://searchworks.stanford.edu/view/xf387wq3868). Unfortunately, the persistent postprovocation hand movement AP rate increase compared with resting seen during (though decreased compared with provocation) cannot be disentangled from ongoing obsessive feelings.
-
•
Conversely, at the more ventral NAc site, firing rate of an isolated neuron as well as gamma amplitude decreased with provocation of the compulsion. At this location, there was significant correlation between 1-s blocks of gamma oscillation amplitude and log10 spike rate during the preprovocation period (Pearson’s R = 0.56, P = 4×10−6), and not for the periods during or following provocation with the toothbrush. This effect was not seen at the more dorsal NAc site.
DISCUSSION
This NAc-specific hγ35 oscillation implies a common physiological element among NAc microcircuits, which are known to be composed of medium spiny neurons (MSNs) and a variety of different classes of interneurons. The observation that obsession provocation induces opposite hγ35-amplitude responses at different NAc sites implies that this common element is present across different NAc microcircuit types. In rats, a ~50-Hz gamma oscillation (rγ50) is present in NAc and not in the remainder of the striatum (Berke et al. 2004). Using pharmacological manipulation, it was shown that rγ50 is specifically attributable to subthreshold fluctuations in the membrane potential of parvalbumin-positive GABAergic fast-spiking interneurons (FSIs) (Bracci et al. 2003). Furthermore, emerging work shows that output from these FSIs specifically constrains impulsive action (Pisansky et al. 2019). The rat rγ50 may help us interpret the human hγ35 if, as we hypothesize, both emerge from genetically homologous microcircuits that slightly diverged during evolution. Such human versus rat homology is seen in 7- versus 10-Hz hippocampal oscillations (DeCoteau et al. 2007; Kahana et al. 1999). Computational modeling of ventral striatal networks shows that γ-oscillations emerge from the MSN-FSI interactions, and a small change in the time constant of GABAergic postsynaptic current (such as might happen evolutionarily) could induce a shift from 50 to 35 Hz in the emergent global oscillation of the microcircuit (Wu et al. 2017). Careful measurement showed that some NAc rγ50 are coherent with select sites in prefrontal cortex, piriform cortex, and the hippocampus (Berke 2009). Assuming hγ35-rγ50 homology, hγ35 coherence might reveal NAc interactions with these other brain areas in the human, which could be used as a tool for paired stimulation in neurosurgical intervention.
One might speculate that these oscillations actually facilitate information transfer between brain regions, beyond serving only as a signature of interaction, but that cannot be established from this case alone. Measurements from DBS macroelectrodes in the NAc found a similar ~10-Hz oscillation during task engagement, but nothing consistent with the hγ35 in the signal (Cohen et al. 2009a, 2009b). This discrepancy might be explained if the microcircuit motif that generates a 35-Hz oscillation isn’t coherent across a large enough volume to be picked up by the DBS macroelectrode, which fits with the observation that the hγ35 motif has conjugate changes in sites separated by just 2 mm (Fig. 2).
The finding that provocation of the patient’s contamination obsession induced physiological changes in NAc is an initial step forward to better understand how obsessions are processed in the human brain. In isolation, differential action potential firing activity at different sites within NAc could be attributed to the capture of different neuron types within a functionally isotropic region. However, local field potentials reflect a property of the local ensemble of neurons and were also opposite in the magnitude of their shift with provocation, suggesting that the different electrophysiological responses we observed within the NAc reflect distinct microcircuits with different functions. Likely, our two conjugate responses to compulsive fear are from different types of microcircuits that share the hγ35-FSI type and may be related to different MSN types (e.g., D1 versus D2 dopamine receptors) (Graziane et al. 2016). The ~0.3- to 0.5-mm scale of the arborization of these FSIs allows for the possibility of differential microcircuits across the 2-mm distance where are differential observations were made (Koós and Tepper 1999). There are distinct MSN-based microcircuits in the rat NAc shell, which are topologically organized differentially by positive and negative motivational valence (Reynolds and Berridge 2002). In light of this, differential NAc responses we observed may reveal conjugate motivational-valence microcircuits, with obsession triggered increased firing rate and hγ35-amplitude more dorsally, and the physiological converse 2 mm beneath. During intraoperative stimulation testing of a DBS electrode advanced to target, our patient began smiling with an outwardly euphoric affect (Haq et al. 2011), while verbally stating this distressed him. This effect was not seen with more dorsal stimulation, and the 3-mm-long electrode at target produced current density spread across both of the sites where the toothbrush task was performed. It may be that these contrasting effects were induced by simultaneous stimulation of multiple accumbens microcircuit types. Although NAc involvement in a brain circuit underlying OCD has been demonstrated with functional imaging (Figee et al. 2011) and inferred by clinical improvement with NAc-DBS (Fayad et al. 2016; Haq et al. 2011; Sturm et al. 2003), this case shows directly that provocation of an obsession is associated with changes in firing rate and LFP oscillatory power in human NAc.
GRANTS
This study was supported by the Van Wagenen Fellowship (K. J. Miller), funds from K12NS080223 (C. H. Halpern), the Brain & Behavior Research Foundation (N. R. Williams and C. H. Halpern), the Neurosurgery Research and Education Foundation (C. H. Halpern), the John A. Blume Foundation (C. H. Halpern), the William Randolph Hearst Foundation (C. H. Halpern), the Stanford Neuroscience Institute's Neurochoice Initiative (C. H. Halpern), and start-up funds from Stanford’s Department of Neurosurgery (C. H. Halpern).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors. Since this paper has been accepted, a provisional patent has been placed by Stanford University on the ~35-Hz oscillation, with serial number 62/854,892, filing date of May 30, 2019.
AUTHOR CONTRIBUTIONS
K.J.M., N.R.W., and C.H.H. conceived and designed research; K.J.M., T.P., N.R.W., and C.H.H. performed experiments; K.J.M. analyzed data; K.J.M., T.P., N.R.W., and C.H.H. interpreted results of experiments; K.J.M. prepared figures; K.J.M. drafted manuscript; K.J.M., T.P., N.R.W., and C.H.H. edited and revised manuscript; K.J.M., T.P., N.R.W., and C.H.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful for the intraoperative support of the Bronte-Stewart laboratory and the helpful discussion with Drs. Dora Hermes, Kelly Foote, Joshua Berke, and Robert Malenka while interpreting the case and preparing the manuscript.
REFERENCES
- Alonso P, Cuadras D, Gabriëls L, Denys D, Goodman W, Greenberg BD, Jimenez-Ponce F, Kuhn J, Lenartz D, Mallet L, Nuttin B, Real E, Segalas C, Schuurman R, Tezenas du Montcel S, Menchon JM. Deep brain stimulation for obsessive-compulsive disorder: a meta-analysis of treatment outcome and predictors of response. PLoS One 10: e0133591, 2015. doi: 10.1371/journal.pone.0133591 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders (DSM-5). Washington, DC: American Psychiatric Association Publishing, 2013. [Google Scholar]
- Baliki MN, Mansour A, Baria AT, Huang L, Berger SE, Fields HL, Apkarian AV. Parceling human accumbens into putative core and shell dissociates encoding of values for reward and pain. J Neurosci 33: 16383–16393, 2013. doi: 10.1523/JNEUROSCI.1731-13.2013 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benabid A-L, Krack PP, Benazzouz A, Limousin P, Koudsie A, Pollak P. Deep brain stimulation of the subthalamic nucleus for Parkinson’s disease: methodologic aspects and clinical criteria. Neurology 55, Suppl 6: S40–S44, 2000. [PubMed] [Google Scholar]
- Berke JD. Fast oscillations in cortical-striatal networks switch frequency following rewarding events and stimulant drugs. Eur J Neurosci 30: 848–859, 2009. doi: 10.1111/j.1460-9568.2009.06843.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke JD, Okatan M, Skurski J, Eichenbaum HB. Oscillatory entrainment of striatal neurons in freely moving rats. Neuron 43: 883–896, 2004. doi: 10.1016/j.neuron.2004.08.035 . [DOI] [PubMed] [Google Scholar]
- Bracci E, Centonze D, Bernardi G, Calabresi P. Voltage-dependent membrane potential oscillations of rat striatal fast-spiking interneurons. J Physiol 549: 121–130, 2003. doi: 10.1113/jphysiol.2003.040857 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Axmacher N, Lenartz D, Elger CE, Sturm V, Schlaepfer TE. Good vibrations: cross-frequency coupling in the human nucleus accumbens during reward processing. J Cogn Neurosci 21: 875–889, 2009a. doi: 10.1162/jocn.2009.21062 . [DOI] [PubMed] [Google Scholar]
- Cohen MX, Axmacher N, Lenartz D, Elger CE, Sturm V, Schlaepfer TE. Nuclei accumbens phase synchrony predicts decision-making reversals following negative feedback. J Neurosci 29: 7591–7598, 2009b. doi: 10.1523/JNEUROSCI.5335-08.2009 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoteau WE, Thorn C, Gibson DJ, Courtemanche R, Mitra P, Kubota Y, Graybiel AM. Learning-related coordination of striatal and hippocampal theta rhythms during acquisition of a procedural maze task. Proc Natl Acad Sci USA 104: 5644–5649, 2007. doi: 10.1073/pnas.0700818104 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayad SM, Guzick AG, Reid AM, Mason DM, Bertone A, Foote KD, Okun MS, Goodman WK, Ward HE. Six-nine year follow-up of deep brain stimulation for obsessive-compulsive disorder. PLoS One 11: e0167875, 2016. doi: 10.1371/journal.pone.0167875 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figee M, Vink M, de Geus F, Vulink N, Veltman DJ, Westenberg H, Denys D. Dysfunctional reward circuitry in obsessive-compulsive disorder. Biol Psychiatry 69: 867–874, 2011. doi: 10.1016/j.biopsych.2010.12.003 . [DOI] [PubMed] [Google Scholar]
- Grant JE. Obsessive-compulsive disorder. N Engl J Med 371: 646–653, 2014. doi: 10.1056/NEJMcp1402176 . [DOI] [PubMed] [Google Scholar]
- Graziane NM, Sun S, Wright WJ, Jang D, Liu Z, Huang YH, Nestler EJ, Wang YT, Schlüter OM, Dong Y. Opposing mechanisms mediate morphine- and cocaine-induced generation of silent synapses. Nat Neurosci 19: 915–925, 2016. doi: 10.1038/nn.4313 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haq IU, Foote KD, Goodman WG, Wu SS, Sudhyadhom A, Ricciuti N, Siddiqui MS, Bowers D, Jacobson CE, Ward H, Okun MS. Smile and laughter induction and intraoperative predictors of response to deep brain stimulation for obsessive-compulsive disorder. Neuroimage 54, Suppl 1: S247–S255, 2011. doi: 10.1016/j.neuroimage.2010.03.009 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahana MJ, Sekuler R, Caplan JB, Kirschen M, Madsen JR. Human theta oscillations exhibit task dependence during virtual maze navigation. Nature 399: 781–784, 1999. doi: 10.1038/21645 . [DOI] [PubMed] [Google Scholar]
- Koós T, Tepper JM. Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nat Neurosci 2: 467–472, 1999. doi: 10.1038/8138 . [DOI] [PubMed] [Google Scholar]
- Mian MK, Campos M, Sheth SA, Eskandar EN. Deep brain stimulation for obsessive-compulsive disorder: past, present, and future. Neurosurg Focus 29: E10, 2010. doi: 10.3171/2010.4.FOCUS10107 . [DOI] [PubMed] [Google Scholar]
- Miller KJ, Sorensen LB, Ojemann JG, den Nijs M. Power-law scaling in the brain surface electric potential. PLOS Comput Biol 5: e1000609, 2009. doi: 10.1371/journal.pcbi.1000609 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisansky MT, Lefevre EM, Retzlaff CL, Trieu BH, Rothwell PE. Nucleus accumbens fast-spiking interneurons constrain impulsive action (Preprint). bioRxiv: 516609, 2019. doi: 10.1101/516609. [DOI] [PMC free article] [PubMed]
- Reynolds SM, Berridge KC. Positive and negative motivation in nucleus accumbens shell: bivalent rostrocaudal gradients for GABA-elicited eating, taste “liking”/“disliking” reactions, place preference/avoidance, and fear. J Neurosci 22: 7308–7320, 2002. doi: 10.1523/JNEUROSCI.22-16-07308.2002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanelli P, Heit G, Hill BC, Kraus A, Hastie T, Brontë-Stewart HM. Microelectrode recording revealing a somatotopic body map in the subthalamic nucleus in humans with Parkinson disease. J Neurosurg 100: 611–618, 2004. doi: 10.3171/jns.2004.100.4.0611 . [DOI] [PubMed] [Google Scholar]
- Sheth SA, Neal J, Tangherlini F, Mian MK, Gentil A, Cosgrove GR, Eskandar EN, Dougherty DD. Limbic system surgery for treatment-refractory obsessive-compulsive disorder: a prospective long-term follow-up of 64 patients. J Neurosurg 118: 491–497, 2013. doi: 10.3171/2012.11.JNS12389 . [DOI] [PubMed] [Google Scholar]
- Sturm V, Lenartz D, Koulousakis A, Treuer H, Herholz K, Klein JC, Klosterkötter J. The nucleus accumbens: a target for deep brain stimulation in obsessive-compulsive- and anxiety-disorders. J Chem Neuroanat 26: 293–299, 2003. doi: 10.1016/j.jchemneu.2003.09.003 . [DOI] [PubMed] [Google Scholar]
- Westphal CFO. Ueber Zwangsvorstellungen. Berliner Klinische Wochenschrift 46: 669–672, 1877. [Google Scholar]
- Wu H, Miller KJ, Blumenfeld Z, Williams NR, Ravikumar VK, Lee KE, Kakusa B, Sacchet MD, Wintermark M, Christoffel DJ, Rutt BK, Bronte-Stewart H, Knutson B, Malenka RC, Halpern CH. Closing the loop on impulsivity via nucleus accumbens delta-band activity in mice and man. Proc Natl Acad Sci USA 115: 192–197, 2018. doi: 10.1073/pnas.1712214114 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Guo A, Fu X. Generation of low-gamma oscillations in a GABAergic network model of the striatum. Neural Netw 95: 72–90, 2017. doi: 10.1016/j.neunet.2017.08.004 . [DOI] [PubMed] [Google Scholar]