Abstract
Despite different developmental and pathological processes affecting lung vascular remodeling in both patient populations, differences in 4D MRI findings between children and adults with PAH have not been studied. The purpose of this study was to compare flow hemodynamic state, including flow-mediated shear forces, between pediatric and adult patients with PAH matched by severity of pulmonary vascular resistance index (PVRi). Adults (n = 10) and children (n = 10) with PAH matched by pulmonary vascular resistance index (PVRi) and healthy adult (n = 10) and pediatric (n = 10) subjects underwent comprehensive 4D-flow MRI to assess peak systolic wall shear stress (WSSmax) measured in the main (MPA), right (RPA), and left pulmonary arteries (LPA), viscous energy loss (EL) along the MPA-RPA and MPA-LPA tract, and qualitative analysis of secondary flow hemodynamics. WSSmax was decreased in all pulmonary vessels in children with PAH when compared with the same age group (all P < 0.05). Similarly, WSSmax was decreased in all pulmonary vessels in adult PAH patients when compared with healthy adult subjects (all P < 0.01). Average EL was increased in adult patients with PAH when compared with the same age group along both MPA-RPA (P = 0.020) and MPA-LPA (P = 0.025) tracts. There were no differences in EL indices between adults and pediatric patients. Children and adult patients with PAH have decreased shear hemodynamic forces. However, pathological flow hemodynamic formations appear to be more consistent in adult patients, whereas flow hemodynamic abnormalities appear to be more variable in children with PAH for comparable severity of PVRi.
NEW & NOTEWORTHY Both children and adult patients with PAH have decreased shear hemodynamic forces inside the pulmonary arteries associated with the degree of vessel dilation and stiffness. These differences also exist between healthy normotensive children and adults. However, pathological flow hemodynamic formations appear to more uniform in adult patients, whereas in children with PAH flow, hemodynamic abnormalities appear to be more variable. Pathological flow formations appear not to have a major effect on viscous energy loss associated with the flow conduction through proximal pulmonary arteries.
Keywords: cardiac magnetic resonance, flow, magnetic resonance imaging, pediatric and adult, pulmonary arterial hypertension
INTRODUCTION
Pulmonary arterial hypertension (PAH) is associated with a diverse spectrum of diseases, coupled with cardiac, pulmonary, and systemic comorbidities, and causes significant morbidity and mortality in both adult and pediatric populations (1, 19, 23). Although the definition of PAH and hemodynamic profiles of children and adults with PAH are generally similar, the natural history of pediatric PAH is typically linked with altered cardiopulmonary development, including compromised lung growth and function. Furthermore, right ventricular (RV) adaptation and pulmonary vascular responsiveness to the flow-mediated injury appear to be age dependent, with the timing or nature of vascular injury playing an important role in the pediatric setting (4, 15, 16, 41). The close link between development and disease makes pediatric PAH distinct from adult manifestations of PAH. Nevertheless, diagnostic and therapeutic strategies for children with PAH are derived predominantly from the results of randomized control trials conducted in adults (4, 18).
Comparative studies between children and adults with PAH are rarely performed due to intrinsic demographic differences and a large spectrum of heterogenous comorbidities affecting the respective populations. From a hemodynamic point of view, PAH has been characterized extensively using standard catheterization based hemodynamic parameters in both pediatric and adult populations (5, 7, 17, 43). Presently, noninvasively derived hemodynamics from MRI and echocardiography have been shown to have a strong potential to predict clinical outcomes (13, 20, 25, 36). However, the vast majority of imaging techniques focus on the tissue biomechanical analysis to better understand the RV adaptation throughout the course of the disease, whereas the flow hemodynamic mapping and investigations considering flow-mediated pathology are scarce (12). Consequently, the physical properties of flow in the pulmonary circulation in the context of PAH have not been well defined.
Hemodynamic mapping using 4D-flow MRI is a novel imaging modality for qualitative characterization of the nature and impact of flow dynamics through the proximal pulmonary arteries (10). More specifically, 4D-flow MRI enables noninvasive quantitative analysis of flow-mediated shear forces acting on the endothelial surface of pulmonary arteries that are capable of modulating vessel wall function, growth, and remodeling (9, 40). Past studies from 4D-flow MRI studies of adults with PAH have described abnormal wall shear stress (WSS) measurements in proximal pulmonary arteries that were associated with measures of proximal pulmonary arterial stiffness (3, 33). Given the recognized strong, independent predictive ability of proximal pulmonary arterial stiffness toward clinical outcomes, further insights into the fluid-tissue interactions in both pediatric and adult patients are necessary. 4D-flow MRI can additionally quantify the presence of vortical and helical flow previously described in PAH patients in terms of viscous energy loss (EL) and relate these findings to RV mechanical work. However, similar studies applying 4D-flow MRI in pulmonary arteries with comprehensive flow hemodynamic assessment have not been performed in children.
The purpose of this study was to compare flow hemodynamic state between pediatric and adult patients with PAH who were matched by baseline pulmonary vascular resistance index (PVRi), as measured by cardiac catheterization. We further sought to evaluate the efficiency of pulmonary blood flow conduction determined by qualitative assessment of secondary flow structures and computation of viscous energy loss (EL) measured in proximal pulmonary arteries. We hypothesized that 1) shear forces and overall pulmonary flow formations will differ between pediatric and adult PAH patients and their respective age-matched healthy controls and 2) similar differences will exist between pediatric and adult subjects with PAH. Improved understanding of flow hemodynamic characteristics assessed via noninvasive imaging might identify novel pathophysiological phenomena affecting proximal pulmonary arteries and more specific therapeutic targets in both adult and pediatric patient populations.
METHODS
This study was approved by the Colorado Multi-Institutional Review Board and by an institutional review board at National Jewish Health, with all subjects providing informed consent. We performed a prospective 4D-flow MRI study to evaluate patients with previously diagnosed PAH conducted at National Jewish Health Center and Children’s Hospital Colorado. PAH was defined as main pulmonary arterial pressure (mPAP) ≥25 mmHg and pulmonary arterial wedge pressure <15 mmHg by right heart catheterization (1, 14). As a part of a prospective study involving comprehensive flow hemodynamic mapping, adult patients with PAH (n = 10) and age-matched healthy controls (n = 10) underwent comprehensive 4D-flow MRI evaluation along with standard functional and volumetric analyses. Adult PAH patients also underwent cardiac catheterization. Similarly, pediatric patients (n = 10) diagnosed with PAH underwent 4D-flow MRI that was performed within 24 h of right heart catheterization. Healthy pediatric subjects without a known history of cardiovascular or pulmonary disease were prospectively recruited and studied by 4D-flow MRI without a catheterization component. Pediatric and adult PAH patients were matched by baseline PVRi determined by catheterization for subsequent comparison of flow hemodynamic differences between both groups. Additional markers of PAH severity, trans-annular plane systolic excursion (TAPSE), and brain natriuretic peptide (BNP) were sampled in both pediatric and adult patient groups.
MRI Studies
Adult studies.
Standard steady-state free precession technique for ventricular volumetric and functional evaluation along with 4D-flow MRI technique was performed using a 1.5-T MRI Siemens system (MAGNETOM; Avanto, Erlangen, Germany) with an eight-channel phased array coil, using a previously described protocol (31). 4D-flow MRI gradient pulse sequence with prospective ECG gating and respiratory navigation using respiratory bellows were implemented using a standard cartesian k-space encoding in a sagittal direction with three-dimensional (3D) volume covering entire heart cavity and great vessels. Depending on patient size and field of view, typical scanning parameters were as follows: spatial resolution 2.4–2.6 × 2.4–2.6 × 2.4–3.0 mm3; flip angle = 14–15°; echo time/repetition time 2.85/48.56 ms, 14–18 cardiac phases resulting in temporal resolution between 42 and 48 ms. Velocity-encoding values were adjusted per maximum velocities encountered during phase contrast MRI scout sequences to avoid aliasing artifact (typical values ranged from 100 to 150 cm/s). A cine of steady-state free precession two-dimensional (2D) images were acquired uniformly from the base to the apex during brief end-expiratory breath holds using contiguous short-, long-, and four-chamber axis slices in 8-mm increments. Additional steady-state free precession images were acquired in the mid-main pulmonary artery (MPA) for the vascular geometric analysis.
Pediatric studies.
MRI evaluation was performed using a 3.0T system (Ingenia; Philips Medical Systems, Best, The Netherlands), using a 32-channel coil. 4D-flow MRI gradient pulse sequence with retrospective ECG gating with respiratory navigation using diaphragmatic tracking was applied in a sagittal direction covering the myocardium and pulmonary vasculature out to the third generation. The typical scanning parameters were as follows: echo time 2.4–2.6 ms, repetition times = 4.2–5.0 ms, flip angle = 10°, 14 cardiac phases resulting in temporal resolution between 38 and 48 ms, and field of view: 250–320 × 200–250 mm2, providing voxel size range =2.0 × 2.0 × 2.0–2.6 mm3. Velocity-encoding value was determined from previous 2D phase contrast MRI scout images positioned in main and branch pulmonary arteries (100–150 cm/s). The typical acquisition time was between 5 and 10 min, depending on heart rate and respiratory gating efficiency. The ventricular and functional analysis was performed from standardly prescribed 2D cine steady-state free precession short-, long-, and four-chamber axis images along with MPA cross-sectional images acquired during end-expiratory breath holds.
MRI Postprocessing
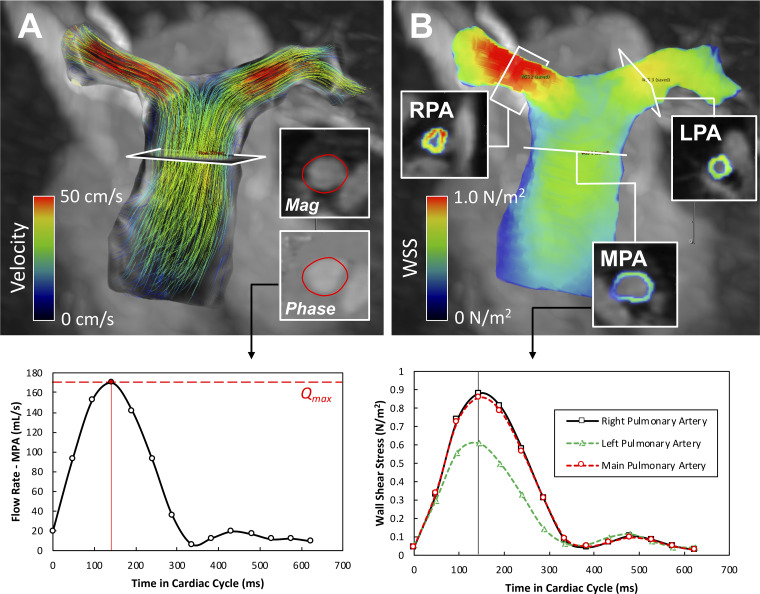
Analysis for ventricular volumetric and functional assessment as well as 4D-flow MRI postprocessing was performed on a CVI42 platform (version 5.9.1; Circle Cardiovascular Imaging, Calgary, AB, Canada). Measured volumetric and functional indices were indexed for body surface area. Collected velocity data sets were corrected for background offset errors and velocity-aliasing artifacts in each encoding direction per common consensus recommendation (10). The postprocessing algorithm is depicted in Fig. 1. A 3D volume rendering projection derived from the 4D-flow data set was created for detailed segmentation of the right ventricular outflow tract, MPA, and primary branch arteries. Shear hemodynamic indices, including maximum systolic WSS (WSSmax) and time-averaged WSS (WSSTA), were measured in the mid-MPA, mid-right pulmonary artery (RPA), and mid-left pulmonary artery (LPA). Additionally, standard flow hemodynamic analysis was performed in the mid-MPA including the collection of maximum flow rate (Qmax) and peak velocity (Vmax). Luminal size of the main pulmonary artery (MPA) and respective relative area change (RAC), calculated as [(Amax − Amin)/Amax × 100%, were measured from cross-sectional MPA cine images.
Fig. 1.
The postprocessing pipeline for the calculation of hemodynamic wall shear stress (WSS) using 4D-flow MRI. A: after segmentation of the right ventricular (RV) outflow tract and proximal pulmonary arteries, flow analysis was achieved using interactive path line visualization. Principle flow hemodynamic curve was generated by positioning the plane of analysis into the mid-main pulmonary artery (MPA). B: visualization of calculated WSS map through the same segmented region of interest. WSS was measured in the standardized fashion in the mid-MPA and 3 cm from the center of the bifurcation in each branch pulmonary artery. LPA; left pulmonary artery; RPA, right pulmonary artery.
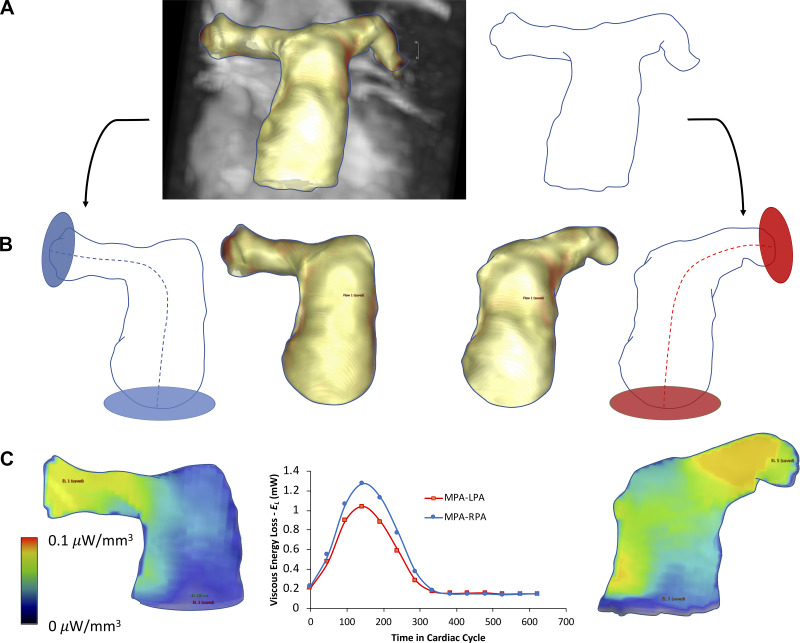
Viscous energy loss (EL) was calculated within the predefined segmented proximal pulmonary arterial tract. EL dissipation due to viscous energy loss has previously been theorized and described by Barker et al. (2), where viscous dissipation is calculated per individual voxel bases within a predefined region of interest by computation of the viscous component of the Navier-Stokes flow energy equation for incompressible fluid. In other words, this approach applies computation of viscous stress tensor per unit of volume defined by voxel size for individual cardiac phase under an assumption of uniform viscosity and boundary slip condition. Unlike the turbulent kinetic energy loss, EL calculates the energy loss due to secondary nonturbulent flow, and therefore, it is sensitive to the presence of large scale vortices and helices. The central line through pulmonary arteries was developed to help delineate EL loss in a predefined volumetric region (Fig. 2). EL was therefore quantified for 1) the MPA – RPA tract, starting at a plane defined by the pulmonary valve and ending at the plane that defined the ostia of the right middle and right lower lobar arteries and 2) the MPA – LPA tract starting at the identical plane and ending 3 cm distally from the main bifurcation. Peak systolic and average EL loss per cardiac cycle were sampled per considered tract. Finally, qualitative flow grading was performed to characterize large scale secondary flow characteristics typically present in PAH. We applied the grading scheme proposed by Bürk et al. (6), describing the strength of prominent secondary flow formations: helices and vortices as normal or mild (grade 0), with flow rotation <180°, moderate supraphysiological (grade 1) with flow rotation between 180 and 360°, and severe (grade 2) with flow rotation >360°.
Fig. 2.
Viscous energy loss (EL) analysis scheme in pulmonary vasculature. A: from the segmented 3-dimentional contour depicting the proximal pulmonary vasculature, EL was calculated along 2 standardized anatomic trajectories. B: the first trajectory, main pulmonary artery (MPA)-right pulmonary artery (RPA), was defined by the centerline placement from the pulmonary valve 5 cm from the center of the bifurcation. Identical approach was applied for the MPA-left pulmonary artery (LPA) trajectory. C: mean intensity projection of the EL field within each trajectory, with the EL waveform depicting the amount of the viscous energy loss at various time through cardiac cycle.
Statistical Analysis
Analyses were performed in Prism (version 7; GraphPad Software, La Jolla, CA). Variables were checked for the distributional assumption of normality using normal plots, in addition to Kolmogorov-Smirnov and Shapiro-Wilks tests. All normally distributed variables were reported as means ± SD or as median with corresponding interquantile ranges. Multi-group comparisons were performed using one-way ANOVA or Kruskal-Wallis, as dictated by the distribution of given variable. Demographic and clinical characteristics between groups were compared using Student’s t-test for normally distributed continuous variables, Mann-Whitney test was used for non-normally distributed variables, and χ2-test was used for categorical variables. The relationship between the shear hemodynamics and pulmonary arterial biomechanical indices and RV function were analyzed through a simple linear regression analysis using Pearson r values. Significance was based on P values < 0.05.
RESULTS
Comprehensive demographic and standard hemodynamic characteristics are reported in Table 1. There were no differences in age, size, or sex distribution within respective pediatric and adult groups. All pediatric PAH patients received the right heart catheterization and 4D-flow MRI studies within 24 h. In adult patients, there was a median time of 65 days between catheterization and 4D-flow MRI (range: 0–519 days). The mPAP in pediatric group was 45 ± 17 mmHg (range: 27–62), with median PVRi of 9.7 WU/m2 (range: 3.1–16.4). Adult PAH subjects had an average mPAP of 38 ± 11 mmHg (range: 26–59), with median PVRi of 8.4 WU (range: 3.5–15.3). There were no differences in catheterization-derived pulmonary hemodynamics between pediatric and adult PAH groups. Within the pediatric group, one patient had heritable PAH, seven patients had idiopathic PAH, and two patients had PAH associated with congenital heart disease (1 atrial and 1 ventricular septal defect, with both closed in infancy). All adult patients had idiopathic PAH.
Table 1.
Demographics and standard hemodynamics
| Pediatric |
Adult |
|||
|---|---|---|---|---|
| PAH | Control | PAH | Control | |
| Age, yr | 13.8 ± 3.2++ | 12.1 ± 4.3++ | 62.7 ± 8.8 | 57.7 ± 9.2 |
| BSA, m2 | 1.26 ± 0.34++ | 1.15 ± 0.21++ | 1.74 ± 0.31 | 1.87 ± 0.25 |
| Sex (%female) | 7 (70) | 6 (60) | 4 (40) | 5 (50) |
| mPAP, mmHg | 45 ± 17 | 38 ± 11 | ||
| PVRi, WU/m2 | 9.7 (6.1–11.3) | 8.4 (5.4–10.6) | ||
| TAPSE, cm | 2.4 ± 0.4 | 2.0 ± 0.6 | ||
| BNP, ng/l | 14 (14–17) | 23 (8–64) | ||
| RV EDVi, ml/m2 | 106 ± 19** | 79 ± 11 | 90 ± 23* | 70 ± 13 |
| RV ESVi, ml/m2 | 51 ± 15** | 33 ± 15 | 52 ± 17* | 37 ± 13 |
| RV SVi, ml/m2 | 55 ± 10*+ | 46 ± 10+ | 38 ± 13 | 35 ± 5 |
| RV EF, % | 52 ± 8*+ | 58 ± 4++ | 42 ± 12 | 49 ± 6 |
| RV CI, l·min−1·m2 | 4.1 ± 1.2+ | 3.6 ± 0.2++ | 2.9 ± 1.0* | 2.0 ± 0.3 |
Values are means ± SD; n = 10. BNP, brain natriuretic peptide; BSA, CI, cardiac index; EDVi, end-diastolic volume index; EF, ejection fraction; ESVi, end-systolic volume index; mPAP, mean pulmonary arterial pressure; PAH, pulmonary arterial hypertension; PVRi, pulmonary vascular resistance index; RV, right ventricle; SVi, stroke volume index; TAPSE, trans-annular plane systolic excursion.
P < 0.05 and
P < 0.01 from respective control;
P < 0.05 and
P < 0.01 from respective PAH or control group.
Not surprisingly, the pediatric patients had elevated average RV end-diastolic volume index (106 vs. 79 ml/m2, P = 0.001) and RV end-systolic volume index (51 vs. 33 ml/m2, P = 0.004) when compared with the control group. Additionally, pediatric patients had elevated RV stroke volume index (55 vs. 46 ml/m2, P = 0.045) and reduced RV ejection fraction (52 vs. 58%, P = 0.037) when compared with the pediatric control group. In the adult population, patients with PAH had similarly elevated RV end-diastolic volume index (90 vs. 70 ml/m2, P = 0.037), RV end-systolic volume index (52 vs. 37 ml/m2, P = 0.038), and RV cardiac index (2.9 vs. 2.0 l·min−1·m2, P = 0.023) when compared with healthy controls. There was no difference in RV ejection fraction among the adult groups (42 vs. 49%, P = 0.119). In comparison with adult PAH patients, pediatric PAH patients had increased RV stroke volume index (55 vs. 35 ml/min2, P = 0.029), RV ejection fraction (52 vs. 42%, P = 0.049), and RV cardiac index (4.1 vs. 2.9 ml·min−1·m2, P = 0.044). Finally, there were no differences between pediatric and adult patient groups in echocardiography derived TAPSE (2.4 ± 0.4 vs. 2.0 ± 0.6 cm, respectively, P = 0.105) and sampled BNP [median: 14 (14−17) vs. 23 (8–64) ng/l, P = 0.247].
Comprehensive 4D-flow hemodynamics and MPA vessel characteristics are reported in Table 2. Children with PAH had reduced RAC measured in the MPA compared with pediatric controls (29 vs. 38%, P = 0.037), and as in the pediatric group, adult PAH patients had significantly reduced RAC when compared with the adult control group (17 vs. 29%, P < 0.001). Children with PAH had better preserved RAC when compared with adults with PAH (29 vs. 17%, P = 0.011). Correspondingly, control children had increased RAC when compared with healthy adults (38 vs. 29%, P < 0.001). There were no differences in Vmax, Qmax, and diameter measured in the MPA between groups.
Table 2.
4D-flow MRI hemodynamics
| Pediatric |
Adult |
|||
|---|---|---|---|---|
| PAH | Control | PAH | Control | |
| MPA | ||||
| WSSmax, N/m2 | 0.17 ± 0.05** | 0.27 ± 0.06 | 0.18 ± 0.08** | 0.30 ± 0.09 |
| WSSTA, N/m2 | 0.08 ± 0.02 | 0.09 ± 0.02 | 0.08 ± 0.03 | 0.10 ± 0.03 |
| RAC, % | 29 ± 11*+ | 38 ± 3++ | 17 ± 4** | 29 ± 5 |
| Vmax, cm/s | 93 ± 36 | 82 ± 15 | 78 ± 30 | 89 ± 20 |
| Qmax, ml/s | 299 ± 100 | 230 ± 50 | 273 ± 70 | 248 ± 37 |
| Diameter, cm | 3.1 ± 0.3**++ | 2.1 ± 0.2 | 3.7 ± 0.3** | 2.3 ± 0.4 |
| RPA | ||||
| WSSmax, N/m2 | 0.31 ± 0.11**++ | 0.54 ± 0.15++ | 0.16 ± 0.04** | 0.37 ± 0.11 |
| WSSTA, N/m2 | 0.13 ± 0.04*++ | 0.19 ± 0.06++ | 0.08 ± 0.03** | 0.13 ± 0.04 |
| LPA | ||||
| WSSmax, N/m2 | 0.39 ± 0.15*++ | 0.55 ± 0.17++ | 0.16 ± 0.05** | 0.29 ± 0.09 |
| WSSTA, N/m2 | 0.16 ± 0.05++ | 0.19 ± 0.06++ | 0.08 ± 0.03* | 0.11 ± 0.03 |
| EL, mW | ||||
| MPA-RPA | ||||
| Maximum | 1.25 (0.56–1.56) | 0.51 (0.46–0.86) | 0.82 (0.53–1.44) | 0.57 (0.50–0.92) |
| Average | 0.47 (0.24–0.53) | 0.19 (0.15–0.39) | 0.49 (0.33–0.66)* | 0.25 (0.15–0.33) |
| MPA-LPA | ||||
| Maximum | 0.92 (0.44–1.26) | 0.43 (0.31–0.68) | 0.92 (0.48–1.25) | 0.56 (0.45–0.88) |
| Average | 0.34 (0.16–0.52) | 0.20 (0.09–0.34) | 0.45 (0.28–0.66)* | 0.23 (0.17–0.29) |
Values are means ± SD; n = 10. EL, viscous energy loss; KE, kinetic energy; LPA, left pulmonary artery; MPA, main pulmonary artery; Qmax, maximum flow rate; PAH, pulmonary arterial hypertension; RAC, relative area change; RPA, right pulmonary artery; WSSmax, maximum wall shear stress; WSSTA, time-averaged wall shear stress; Vmax, maximum velocity.
P < 0.05 and
P < 0.01 from respective control;
P < 0.05 and
P < 0.01 from respective PAH or control group.
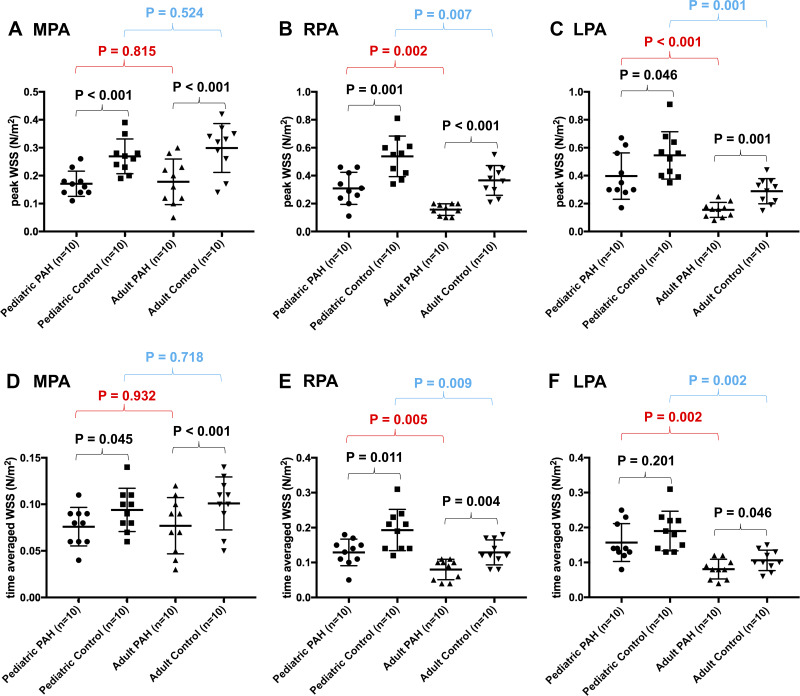
The measurements of shear hemodynamics as assessed in the MPA and branch pulmonary arteries are shown in Fig. 3. In comparison with the respective control groups, 4D-flow-derived WSSmax as measured in the MPA was decreased in children (0.17 vs. 0.27 N/m2, P < 0.001) and adult patients with PAH (0.18 vs. 0.30 N/m2, P < 0.001). In the RPA, WSSmax (0.31 vs. 0.54 N/m2, P = 0.001) and WSSTA (0.13 vs. 0.19 N/m2, P = 0.011) were reduced in children with PAH with respect to the pediatric control group. Similarly, in adults with PAH, both WSSmax (0.16 vs. 0.37 N/m2, P < 0.001) and WSSTA were decreased (0.08 vs. 0.13 N/m2, P = 0.004) when compared with the same age control group. With regard to the LPA, WSSmax was reduced in children with PAH (0.39 vs. 0.55 N/m2, P = 0.046), but there was no difference in WSSTA (0.16 vs. 0.19 N/m2, P = 0.201). In comparison with adult controls, adults with PAH had decreased WSSmax (0.16 vs. 0.29 N/m2, P = 0.001) and WSSTA (0.08 vs. 0.11 N/m2, P = 0.042).
Fig. 3.
Hemodynamic wall shear stress (WSS) measured in the proximal pulmonary arteries. A–C: plots depicting the variability in maximum systolic WSS (WSSmax) between all considered groups revealed significantly decreased WSSmax between pulmonary arterial hypertension (PAH) and control group in each age group. Additionally, there was a difference in time-averaged WSS (WSSTA) between age-specific groups in respective PAH and control groups when the branch pulmonary arteries were considered. D–F: identical plots for WSSTA.
In addition to the differences observed within the same age group, there were differences in shear hemodynamics between respective control and PAH populations (Fig. 3). When compared with adult PAH patients, children with PAH had increased WSSmax measured in both RPA (0.31 vs. 0.16 N/m2, P = 0.002) and LPA (0.39 vs. 0.16, P < 0.001). Similarly, WSSTA was increased in children with PAH when compared with adult patients in both RPA (0.13 vs. 0.08, P = 0.009) and LPA (0.19 vs. 0.11, P = 0.002). A similar comparison of shear metrics measured in the MPA did not reveal any differences.
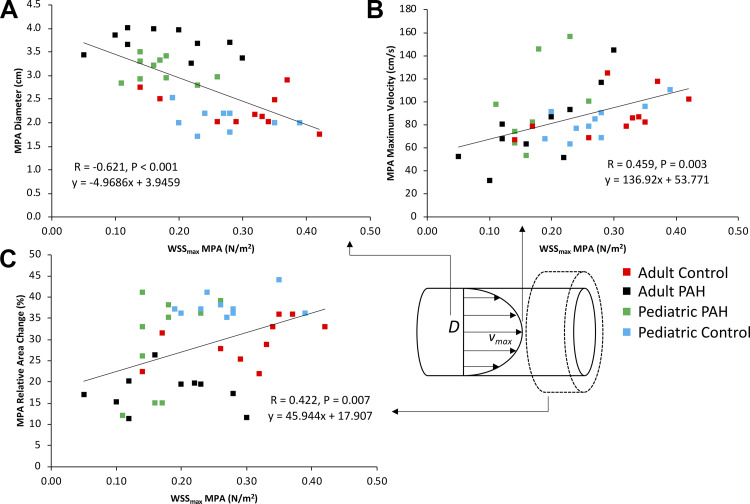
To better understand the nature of WSS in the MPA, we investigated the relationship between WSSmax as measured in the MPA across all considered groups with its theoretical determinants (diameter and flow velocity), as defined by the Haagen-Poisseuille equation (Fig. 4). As expected, we found a negative relationship between WSSmax and MPA diameter (r = −0.621, P < 0.001) and a positive relationship with Vmax (r = 0.459, P = 0.003). Additionally, we observed a positive correlation between WSSmax and RAC (r = 0.422, P = 0.007). There were no significant associations between WSSmax and traditional RV functional and volumetric indices.
Fig. 4.
Associations between peak (maximum) systolic wall shear stress (WSSmax) and the primary determinants of WSS, vessel diameter (A) and peak flow velocity (B), measured in the main pulmonary artery (MPA). Both correlations obeyed the relationship between WSS, vessel size, and flow velocity as dictated by steady-state Haagen-Poisseuille equation. C: positive correlation existed between the relative area change (RAC) and WSSmax. PAH, pulmonary arterial hypertension.
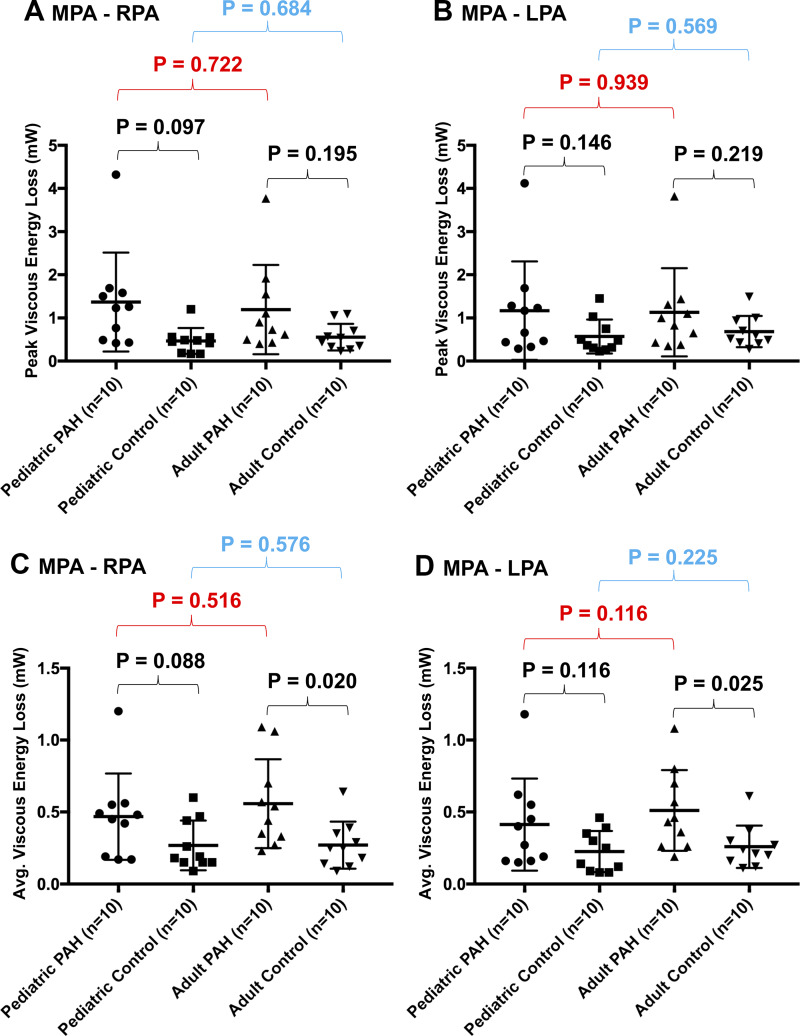
A comprehensive overview of EL measurements (high EL values represent higher rate of viscous energy dissipation) is summarized in Fig. 5. There were no differences between PAH groups regarding maximum systolic EL as assessed along both the MPA-RPA and MPA-LPA tracts. However, there was an increase in the average EL in adult patients with PAH when compared with adult controls, as measured along the MPA-RPA tract (0.49 vs. 0.25 mW, P = 0.020) and similarly along the MPA-LPA tract (0.45 vs. 0.23 mW, P = 0.025). There were no differences in measured average EL between pediatric groups.
Fig. 5.
Summary of viscous energy loss (EL) measures across all considered groups. There were no differences in maximum EL along either the main pulmonary artery (MPA)-right pulmonary artery (RPA) (A) or MPA-left pulmonary artery (LPA) (B) tracts. However, there was a higher average EL in adult pulmonary arterial hypertension (PAH) patients when compared with normotensive adults along both the MPA-RPA (C) and MPA-LPA (D) tracts.
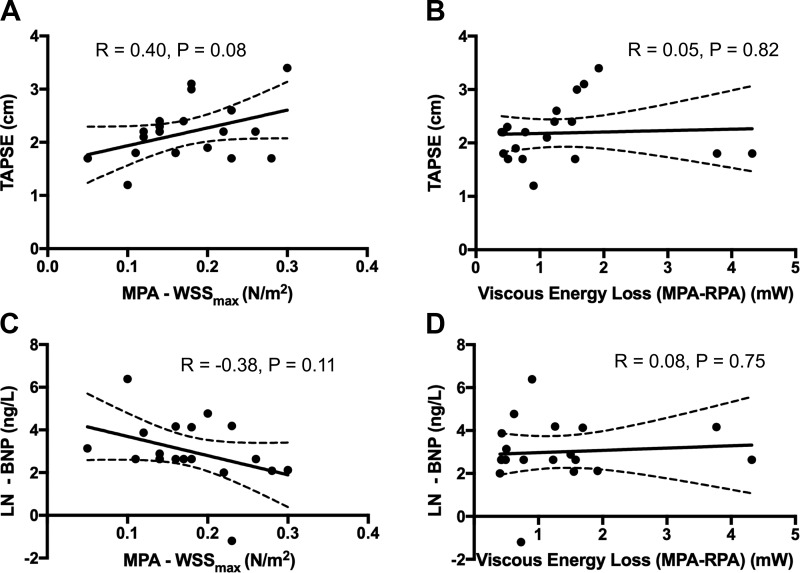
We correlated WSSmax sampled in MPA and maximum peak systolic EL measured along the MPA-RPA tract with conventional markers of PAH severity TAPSE and BNP (Fig. 6). There were no correlations between WSSmax and TAPSE (r = 0.40, P = 0.082) or between WSSmas and BNP levels (r = −0.38, P = 0.110). Similarly, there was no relationship between EL measured along the MPA-RPA tract and TAPSE (r = 0.05, P = 0.821) and between EL and BNP levels (r = 0.08, P = 0.752).
Fig. 6.
Summary of correlation analyses between conventional markers of pulmonary arterial hypertension severity [trans-annular plane systolic excursion (TAPSE) and brain natriuretic peptide (BNP)] and measured 4D-flow hemodynamic indices. TAPSE showed a mild but not significant trend with peak (maximum) systolic wall shear stress (WSSmax) measured in the main pulmonary artery (MPA) (A) and no relationship with viscous energy loss (EL) measured along the MPA-right pulmonary artery (RPA) tract (B). Similarly, we did not find any relationships between the BNP levels and WSSmax (C) or between BNP and EL (D). BNP values were natural log transformed.
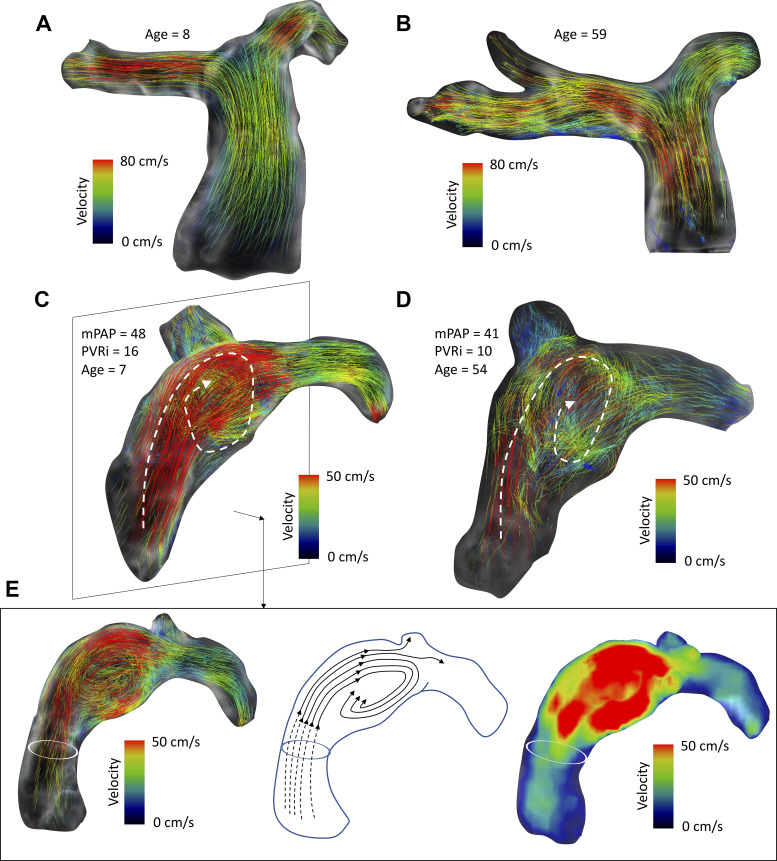
Qualitative grading of secondary flow characteristics, i.e., vortex and helix formation, was performed successfully in each subject. Control subjects from both adult and pediatric groups presented uniform flow propagation through proximal pulmonary arteries characterized by cohesive path line propagation through the majority of the cardiac cycle (Fig. 7). All adult patients with PAH revealed vortex formation within the MPA, with four patients (40%) presenting with mild grade 0 vortex, four patients (40%) with moderate strength grade 1 vortex, and two patients (20%) with large-scale grade 2 vortex.
Fig. 7.
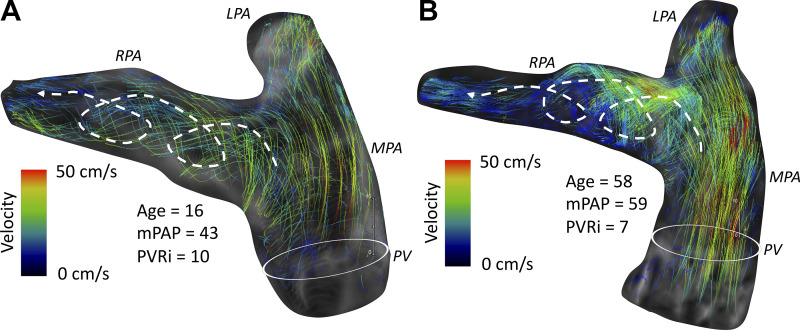
Summary of observed qualitative flow hemodynamic features. A and B: all healthy pediatric and adult subjects revealed normal flow through pulmonary arteries without any secondary flow features or disturbances as visualized by cohesive path lines in representative pediatric (A) and adult (B) subjects. C and D: prominent large-scale vortices were present in 50% of children with pulmonary arterial hypertension (C) and present in all adult patients (D). E: different representation of vortex structure from the sagittal view depicting path lines forming along the dilated main pulmonary artery (MPA) lumen (left), with artistic representation of streamlines depicting the flow trajectory from the right ventricular (RV) outflow tract to branch pulmonary arteries (middle) and velocity mean intensity projection revealing the flow acceleration as part of the vortex formation (right). mPAP, mean pulmonary arterial pressure; PVRi, pulmonary vascular resistance index.
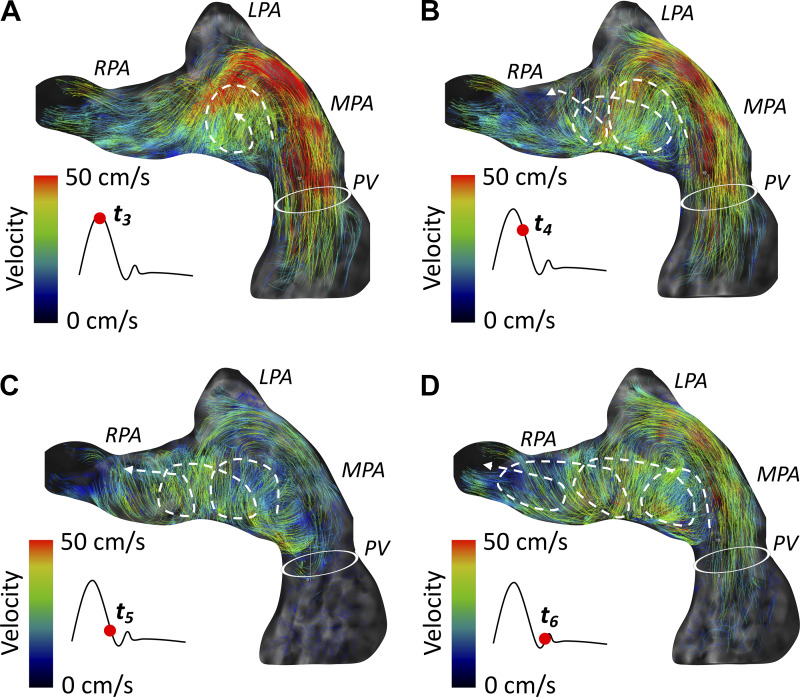
The next prominent finding was the formation of the helices within the RPA, which typically resulted from diffusion of the primary vortex formed in the MPA (Fig. 6). Large-scale grade 2 helices were formed within the RPA in three adult patients (30%), and two adult patients (20%) showed moderate grade 1 helices (Fig. 8). In comparison with adult patients, vortex formation inside the MPA was present in children with PAH only in five patients (50%). Specifically, four pediatric patients (40%) had present moderate grade 1 vortex inside the MPA, with one patient revealing large-scale grade 2 vortex. Two pediatric patients (20%) had normal flow conduction through the pulmonary arteries without any presence of secondary flow formations (Fig. 9). Interestingly, three pediatric patients (30%) showed isolated grade 1 helical formation inside the RPA without any prominent secondary flow formations inside the MPA.
Fig. 8.
Typical formation of the right pulmonary artery (RPA) helix in a representative adult patient. A–D: the origin of the clockwise RPA helix begins at the main pulmonary artery (MPA) vortex diffusing through the RPA ostium and spirals distally throughout systole.
Fig. 9.
Representative cases of children with moderate (A) and severe (B) pulmonary arterial hypertension (PAH) with normal flow pattern without any formation of vortices and helices in pulmonary arteries despite noticeable main pulmonary artery (MPA) luminal dilation. mPAP, mean pulmonary arterial pressure.
DISCUSSION
Comprehensive flow mapping of pulmonary arteries using 4D-flow MRI technology is a relatively novel method of investigating the hemodynamic pathologies associated with PAH, which could be applied in both pediatric and adult patient populations. We report that 1) flow through the proximal pulmonary arteries is abnormal in both pediatric and adult patients with PAH, 2) different abnormal flow hemodynamic formations exist between pediatric and adult patients, 3) decreased shear mediated forces in proximal pulmonary arteries are strongly associated with the presence of PAH regardless of age category, and 4) reduction in branch pulmonary arterial WSS is more profound in adult population regardless of the disease state. These findings are interesting, as comparative hemodynamic studies between adult and pediatric PAH patients are rare despite the recognized differences in pathological processes affecting the pulmonary vascular remodeling (4, 41, 42). Furthermore, there are very few clinical trials in pediatric PAH, which is due at least partly to the need for better study end points, including identification of novel biomarkers and noninvasive pathophysiological assessments, which are provided by approaches such as 4D-flow MRI studies.
Interestingly, we did not observe any correlations between 4D-flow-derived flow hemodynamic markers and typical measures of PAH severity TAPSE and BNP. This may be partially due to the fact that conventional clinical end points recognized in adult PAH studies are not well translated to the pediatric patient population (18). Traditional prognostic markers of clinical worsening in adult trials such as TAPSE, BNP, or the 6-min walk test typically do not have a comparable strong prognostic potential in pediatric studies (27, 28). Furthermore, TAPSE and BNP are more reflective of ventricular mechanical function and stress, whereas 4D-flow, MRI-derived hemodynamic indices are concerned with the flow abnormalities typically related to proximal pulmonary vascular remodeling.
Differences in Shear Hemodynamics Between Children and Adults
Stiffness measured in proximal pulmonary arteries is one of the strongest independent predictors of clinical outcomes in patients with PAH (36, 37). Importantly, proximal pulmonary arteries can undergo intrinsic remodeling toward stiffer character due to abnormal fluid-tissue interactions, which are typically assessed by hemodynamic WSS (13, 21, 35). Our previous work showed that alteration in hemodynamic shear forces throughout course of the disease parallels with a change in pulmonary artery stiffness (13). Studies evaluating flow-induced shear forces present in proximal pulmonary arteries have been conducted predominantly in adult PAH patients. In the context of PAH, microvascular endothelial cells are exposed to abnormally high WSS due to characteristic luminal narrowing, whereas the endothelial cells lining large proximal pulmonary arteries are exposed to reduced WSS (33, 38). Barker et al. (3) reported a nearly twofold decrease in WSSmax derived by 4D-flow MRI in the MPA and branch pulmonary arteries of adult patients with mixed PAH etiology, which was associated with the degree pulmonary artery dilation. These results were supported by Odagiri et al. (26), who observed the same degree of reduction in WSSmax in adult patients with idiopathic PAH and PAH associated with lupus erythematosus and congenital heart disease. Previous studies have suggested that reduced and oscillatory WSS causes the formation of permeable and adhesive endothelial cells, increased reactive oxygen species, increased inflammatory cell infiltration, enhanced matrix-metalloprotein proteases synthesis by macrophages, and smooth muscle cell proliferation and infiltration, which can then increase vascular stiffness (8, 22, 24). However, the exact role of altered shear forces on the endothelial surface of pulmonary arteries in the context of PAH is uncertain. In our previous study of adult PAH patients, we observed a strong correlation between 4D-flow-derived WSSmax measured in MPA and pulmonary arterial compliance assessed by cardiac catheterization (33). In this study, we observed nearly identical reductions in WSSmax in adult PAH patients, as described in the aforementioned studies.
Similar 4D-flow studies focused on pediatric population are missing. Shear hemodynamics have previously been investigated in children with PAH using 2D WSS derived from conventional phase contrast MRI (13, 32, 39). Initial work from our institution suggested that reduction in WSSmax as measured in RPA is driven primarily by vessel dilation similar to adult PAH patients (39). Our subsequent studies have shown that peak systolic WSSmax measured in the MPA is longitudinally associated with stiffness as measured by relative area change, suggesting the close relationship between vessel geometric remodeling and shear forces (13). Indeed, in this study, WSSmax was significantly associated with the theoretical determinants of WSS (vessel diameter and peak velocity) defined by the Haagen-Poisseuille relationship for steady-state flow conditions. However, 2D WSS considers only through-plane velocity component and, consequently, can underappreciate circumferential velocity, which can be considerable in the setting of PAH as signified by the presence of prominent secondary flow formations in pulmonary arteries. Interestingly, pediatric patients showed the same scale reduction in 4D-flow-derived WSS as the adult counterpart in each of the PA segments. Importantly, significant changes in shear hemodynamics existed between control and PAH subjects from the pediatric and adult populations. Our results suggest that adult patients experience significantly lower shear forces in branch pulmonary arteries than those measured in children despite similar levels of PVR. Furthermore, WSS metrics measured in normotensive healthy children were higher when compared with healthy adults. These observations support the relationship defined by the Haagen-Poisseuille equation, which states that WSS is inversely related to vessel radius. Therefore, age and disease-related PA dilation might be primarily responsible for these results. Future studies investigating the close relationship between age, vessel stiffness, and shear hemodynamic across a larger age spectrum of patients are required to better understand the role between flow-mediated forces and vessel remodeling with respect to the aging process.
Viscous Energy Loss in Pulmonary Arteries
Energy loss (EL) due to viscous frictional forces acting on any given blood particle represents a component of mechanically produced kinetic energy within a generated stroke volume that is irreversibly converted to thermal energy. This conversion represents immediate loss in a mechanical energy provided by the RV to a propelled stroke volume. Such an immediate loss in kinetic energy effectively introduces additional work on the RV and ultimately elevates the afterload. In our study, only adult patients with PAH had prominent elevation in average EL when compared with their same age controls. The calculation of EL considers the presence of arge-scale complex secondary flow formations that were present in both children and adult patients. Therefore, we speculate that size and nature of observed vortices/helices might be a reason behind an increased EL in adult patients and no observed differences in pediatric group. Furthermore, not all pediatric patients exhibited vortex or helix formations in pulmonary arteries.
However, it is important to note that EL considers only nonturbulent flow. 4D-flow MRI-derived velocity field cannot directly assess regions of turbulent flow, which currently can be analyzed only by means of turbulent kinetic energy calculation, using a voxel-based spatiotemporal velocity fluctuations in relation to obtained MRI signal, as proposed by Dyverfeldt et al. (11). Furthermore, as stated previously, EL is a rather sensitive metric highly dependent on vessel wall geometry and should be applied cautiously for analysis of anatomic regions associated with high gradients such a stenotic lesions or significant vascular tortuosity (2). Unfortunately, no prior studies using EL were applied in pulmonary circulation, and therefore, we lack a comparative reference frame for our observed values. Additional work considering the EL calculation in a larger cohort of patients with PAH should be performed with regard to the RV power to better understand the interplay between secondary flow formations, viscous energy dissipation, and RV afterload.
Different Pulmonary Flow Formations are Present in Children and Adults with PAH
The presence of vortex formation in large pulmonary arteries in adult patients with PAH is already a widely recognized phenomenon. The original work by Reiter et al. (29) described for the first time the pathophysiological presence of vortex formation inside the MPA using 4D-flow MRI. Ever since then, different quantitative flow hemodynamic indices have been applied to better understand the role and character of formed vortices in proximal pulmonary arteries (30, 31). To our knowledge, this is the first study investigating flow hemodynamic formations in children with PAH. Although secondary flow formations are present in large arteries of children with PAH, their character appears to be different from adult patients. All adult patients presented with “typical” MPA vortex formation, which is due largely to a disproportionate dilation of the MPA in relation to the branch arteries. Interestingly, vortices were present in only five (50%) pediatric patients, with two (20%) children with PAH revealing normal flow patterns through pulmonary arteries despite high mPAP and PVRi. Furthermore, isolated supraphysiological RPA helices existed in three pediatric PAH patients without the presence of major flow disturbance inside the MPA. We speculate that the uniform flow pathologies observed in adult patients might be related to similar PAH etiologies since all adult patients had idiopathic forms of PAH. On the other hand, pediatric PAH tends to be more heterogenous and is most frequently associated with congenital heart disease. The other possible explanation is related to a spectrum of pediatric ages, which directly influence geometry and PA size and which are primary determinants for flow hemodynamics. On the other hand, adults have typically more uniform sizes, and pathological flow may then have a similar influence on PA structure and dilation.
The exact nature behind formation of secondary flow in proximal pulmonary arteries is yet to be determined, but we speculate that it is due to complex interplay between heterogenous type of proximal pulmonary arterial dilation and overall geometry, degree of vessel wall stiffness, and the RV systolic function. A previous study by Wehrum et al. (44) investigating the natural progression of flow hemodynamic differences in pulmonary vasculature revealed the low incidence rate of pathological vortices present inside the MPA in 3% of 126 healthy individuals across a wide age spectrum. Additionally, pathological flow formations are typically absent in the LPA. Again, this might be due predominantly to bifurcation geometry, which allows for more continuous flow between MPA and LPA, and different branching pattern of pulmonary arteries on the left side. Larger studies assessing comprehensive geometry of pulmonary arteries and flow hemodynamic patterns across all age spectrums will be necessary to better understand the pathological role of flow hemodynamic formations and their use for stratification of patients with PAH.
Limitations
We would like to acknowledge limitations of this work. First, our study was limited by small sample sizes in each considered subgroup to assure a close hemodynamic match between pediatric and adult patients by PVRi. Second, there was a median 65-day difference between the right heart catheterization and 4D-flow MRI acquisition in adult patients, yet we believe that only minimal changes in invasive hemodynamics would occur between this time period. Third, pediatric and adult 4D-flow acquisitions were performed using two different MRI systems with different magnetic field strengths, which needs to be considered as a part of result interpretation. However, both MRI system and field strength variability have previously been shown to not play important roles when shear hemodynamic measures are assessed (3). We attempted to mitigate additional differences by applying the identical postprocessing for each subject. Additionally, 4D-flow MRI acquisitions are associated with high spatiotemporal resolution when compared with conventional 2D phase contrast imaging, which needs to be considered when calculating shear hemodynamic indices between children and adult populations. To receive the similar number of voxels across the vessel lumen for WSS calculations, we have reduced the actual voxel size in pediatric acquisitions. Finally, pediatric MRI evaluations in children younger than 8 yr of age require per institutional protocol general anesthesia, which limited the recruitment of younger children.
Conclusion
Both children and adult patients with PAH have decreased shear hemodynamic forces inside the pulmonary arteries associated with the degree of vessel dilation and stiffness. These differences also exist between healthy normotensive children and adults. However, pathological flow hemodynamic formations appear to be more uniform in adult patients, whereas in children with PAH flow hemodynamic abnormalities appear to be more variable. Pathological flow formations appear not to have a major effect on viscous energy loss associated with the flow conduction through proximal pulmonary arteries. Future studies are required to determine whether different flow hemodynamic patterns between pediatric and adult patients contribute to specific features of pulmonary arterial remodeling.
GRANTS
This study was supported by Jayden DeLuca and Rady Family Foundations.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.S., D.D.I., S.H.A., L.P.B., M.B.M., N.W., J.K.B., B.E.F., and U.T. conceived and designed research; M.S., L.P.B., A.J.B., A.S., M.K., N.N., B.F., M.D., B.E.F., and U.T. performed experiments; M.S., S.H.A., K.S., L.P.B., A.J.B., G.J.M., A.S., M.K., N.N., B.F., M.D., K.S.H., V.K., and B.E.F. analyzed data; M.S., D.D.I., S.H.A., K.S., A.J.B., M.B.M., G.J.M., N.W., A.S., M.K., J.K.B., K.S.H., V.K., B.E.F., and U.T. interpreted results of experiments; M.S., A.S., and N.N. prepared figures; M.S., S.H.A., N.W., B.E.F., and U.T. drafted manuscript; M.S., D.D.I., S.H.A., K.S., L.P.B., A.J.B., M.B.M., G.J.M., N.W., A.S., M.K., N.N., B.F., M.D., J.K.B., K.S.H., V.K., B.E.F., and U.T. edited and revised manuscript; M.S., D.D.I., S.H.A., K.S., L.P.B., A.J.B., M.B.M., G.J.M., N.W., A.S., M.K., N.N., B.F., M.D., J.K.B., K.S.H., V.K., B.E.F., and U.T. approved final version of manuscript.
REFERENCES
- 1.Abman SH, Hansmann G, Archer SL, Ivy DD, Adatia I, Chung WK, Hanna BD, Rosenzweig EB, Raj JU, Cornfield D, Stenmark KR, Steinhorn R, Thébaud B, Fineman JR, Kuehne T, Feinstein JA, Friedberg MK, Earing M, Barst RJ, Keller RL, Kinsella JP, Mullen M, Deterding R, Kulik T, Mallory G, Humpl T, Wessel DL; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Surgery and Anesthesia; and the American Thoracic Society . Pediatric pulmonary hypertension: Guidelines from the American Heart Association and American Thoracic Society. Circulation 132: 2037–2099, 2015. [Correction in Circulation 133: e368, 2016.]. doi: 10.1161/CIR.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 2.Barker AJ, van Ooij P, Bandi K, Garcia J, Albaghdadi M, McCarthy P, Bonow RO, Carr J, Collins J, Malaisrie SC, Markl M. Viscous energy loss in the presence of abnormal aortic flow. Magn Reson Med 72: 620–628, 2014. doi: 10.1002/mrm.24962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker AJ, Roldán-Alzate A, Entezari P, Shah SJ, Chesler NC, Wieben O, Markl M, François CJ. Four-dimensional flow assessment of pulmonary artery flow and wall shear stress in adult pulmonary arterial hypertension: results from two institutions. Magn Reson Med 73: 1904–1913, 2015. doi: 10.1002/mrm.25326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barst RJ, Ertel SI, Beghetti M, Ivy DD. Pulmonary arterial hypertension: a comparison between children and adults. Eur Respir J 37: 665–677, 2011. doi: 10.1183/09031936.00056110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berger RM, Beghetti M, Humpl T, Raskob GE, Ivy DD, Jing ZC, Bonnet D, Schulze-Neick I, Barst RJ. Clinical features of paediatric pulmonary hypertension: a registry study. Lancet 379: 537–546, 2012. doi: 10.1016/S0140-6736(11)61621-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bürk J, Blanke P, Stankovic Z, Barker A, Russe M, Geiger J, Frydrychowicz A, Langer M, Markl M. Evaluation of 3D blood flow patterns and wall shear stress in the normal and dilated thoracic aorta using flow-sensitive 4D CMR. J Cardiovasc Magn Reson 14: 84, 2012. doi: 10.1186/1532-429X-14-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Champion HC, Michelakis ED, Hassoun PM. Comprehensive invasive and noninvasive approach to the right ventricle-pulmonary circulation unit: state of the art and clinical and research implications. Circulation 120: 992–1007, 2009. doi: 10.1161/CIRCULATIONAHA.106.674028. [DOI] [PubMed] [Google Scholar]
- 8.Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev 91: 327–387, 2011. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med 6: 16–26, 2009. doi: 10.1038/ncpcardio1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dyverfeldt P, Bissell M, Barker AJ, Bolger AF, Carlhäll C-J, Ebbers T, Francios CJ, Frydrychowicz A, Geiger J, Giese D, Hope MD, Kilner PJ, Kozerke S, Myerson S, Neubauer S, Wieben O, Markl M. 4D flow cardiovascular magnetic resonance consensus statement. J Cardiovasc Magn Reson 17: 72, 2015. doi: 10.1186/s12968-015-0174-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dyverfeldt P, Hope MD, Tseng EE, Saloner D. Magnetic resonance measurement of turbulent kinetic energy for the estimation of irreversible pressure loss in aortic stenosis. JACC Cardiovasc Imaging 6: 64–71, 2013. doi: 10.1016/j.jcmg.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freed BH, Collins JD, François CJ, Barker AJ, Cuttica MJ, Chesler NC, Markl M, Shah SJ. MR and CT Imaging for the Evaluation of Pulmonary Hypertension. JACC Cardiovasc Imaging 9: 715–732, 2016. doi: 10.1016/j.jcmg.2015.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friesen RM, Scha M, Ivy DD, Abman SH, Stenmark K, Browne LP, Barker AJ, Hunter KS, Truong U. Proximal pulmonary vascular stiffness as a prognostic factor in children with pulmonary arterial hypertension. Eur Heart J Cardiovasc Imaging 20: 209–217, 2019. doi: 10.1093/ehjci/jey069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galiè N, Humbert M, Vachiéry J-L, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M; ESC Scientific Document Group . 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 37: 67–119, 2016. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 15.Haworth SG. Development of the normal and hypertensive pulmonary vasculature Exp Physiol 80: 843–853, 1995. doi: 10.1113/expphysiol.1995.sp003892. [DOI] [PubMed] [Google Scholar]
- 16.Haworth SG. Pulmonary hypertension in the young. Heart 88: 658–664, 2002. doi: 10.1136/heart.88.6.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoeper MM, Barberà JA, Channick RN, Hassoun PM, Lang IM, Manes A, Martinez FJ, Naeije R, Olschewski H, Pepke-Zaba J, Redfield MM, Robbins IM, Souza R, Torbicki A, McGoon M. Diagnosis, assessment, and treatment of non-pulmonary arterial hypertension pulmonary hypertension. J Am Coll Cardiol 54, Suppl: S85–S96, 2009. doi: 10.1016/j.jacc.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 18.Hopper RK, Abman SH, Ivy DD. Persistent Challenges in Pediatric Pulmonary Hypertension. Chest 150: 226–236, 2016. doi: 10.1016/j.chest.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ivy DD, Abman SH, Barst RJ, Berger RMF, Bonnet D, Fleming TR, Haworth SG, Raj JU, Rosenzweig EB, Schulze Neick I, Steinhorn RH, Beghetti M. Pediatric pulmonary hypertension. J Am Coll Cardiol 62, Suppl: D117–D126, 2013. doi: 10.1016/j.jacc.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 20.Jone P-N, Schäfer M, Li L, Craft M, Ivy DD, Kutty S. Right Atrial Deformation in Predicting Outcomes in Pediatric Pulmonary Hypertension. Circ Cardiovasc Imaging 10: e006250, 2017. doi: 10.1161/CIRCIMAGING.117.006250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lammers SR, Kao PH, Qi HJ, Hunter K, Lanning C, Albietz J, Hofmeister S, Mecham R, Stenmark KR, Shandas R. Changes in the structure-function relationship of elastin and its impact on the proximal pulmonary arterial mechanics of hypertensive calves. Am J Physiol Heart Circ Physiol 295: H1451–H1459, 2008. doi: 10.1152/ajpheart.00127.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA 282: 2035–2042, 1999. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- 23.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, Rubin LJ, Tapson VF, Varga J, Harrington RA, Anderson JL, Bates ER, Bridges CR, Eisenberg MJ, Ferrari VA, Grines CL, Hlatky MA, Jacobs AK, Kaul S, Lichtenberg RC, Lindner JR, Moliterno DJ, Mukherjee D, Pohost GM, Rosenson RS, Schofield RS, Shubrooks SJ, Stein JH, Tracy CM, Weitz HH, Wesley DJ; ACCF/AHA . ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation 119: 2250–2294, 2009. doi: 10.1161/CIRCULATIONAHA.109.192230. [DOI] [PubMed] [Google Scholar]
- 24.Meng H, Tutino VM, Xiang J, Siddiqui A. High WSS or low WSS? Complex interactions of hemodynamics with intracranial aneurysm initiation, growth, and rupture: toward a unifying hypothesis. AJNR Am J Neuroradiol 35: 1254–1262, 2014. doi: 10.3174/ajnr.A3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moledina S, Pandya B, Bartsota M, Mortensen KH, McMillan M, Quyam S, Taylor AM, Haworth SG, Schulze-Neick I, Muthurangu V. Prognostic significance of cardiac magnetic resonance imaging in children with pulmonary hypertension. Circ Cardiovasc Imaging 6: 407–414, 2013. doi: 10.1161/CIRCIMAGING.112.000082. [DOI] [PubMed] [Google Scholar]
- 26.Odagiri K, Inui N, Hakamata A, Inoue Y, Suda T, Takehara Y, Sakahara H, Sugiyama M, Alley MT, Wakayama T, Watanabe H. Non-invasive evaluation of pulmonary arterial blood flow and wall shear stress in pulmonary arterial hypertension with 3D phase contrast magnetic resonance imaging. Springerplus 5: 1071, 2016. doi: 10.1186/s40064-016-2755-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ploegstra MJ, Arjaans S, Zijlstra WM, Douwes JM, Vissia-Kazemier TR, Roofthooft MT, Hillege HL, Berger RM. Clinical Worsening as Composite Study End Point in Pediatric Pulmonary Arterial Hypertension. Chest 148: 655–666, 2015. doi: 10.1378/chest.14-3066. [DOI] [PubMed] [Google Scholar]
- 28.Ploegstra MJ, Zijlstra WM, Douwes JM, Hillege HL, Berger RM. Prognostic factors in pediatric pulmonary arterial hypertension: A systematic review and meta-analysis. Int J Cardiol 184: 198–207, 2015. doi: 10.1016/j.ijcard.2015.01.038. [DOI] [PubMed] [Google Scholar]
- 29.Reiter G, Reiter U, Kovacs G, Kainz B, Schmidt K, Maier R, Olschewski H, Rienmueller R. Magnetic resonance-derived 3-dimensional blood flow patterns in the main pulmonary artery as a marker of pulmonary hypertension and a measure of elevated mean pulmonary arterial pressure. Circ Cardiovasc Imaging 1: 23–30, 2008. doi: 10.1161/CIRCIMAGING.108.780247. [DOI] [PubMed] [Google Scholar]
- 30.Reiter U, Reiter G, Kovacs G, Stalder AF, Gulsun MA, Greiser A, Olschewski H, Fuchsjäger M. Evaluation of elevated mean pulmonary arterial pressure based on magnetic resonance 4D velocity mapping: comparison of visualization techniques. PLoS One 8: e82212, 2013. doi: 10.1371/journal.pone.0082212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schäfer M, Barker AJ, Kheyfets V, Stenmark KR, Crapo J, Yeager ME, Truong U, Buckner JK, Fenster BE, Hunter KS. Helicity and Vorticity of Pulmonary Arterial Flow in Patients With Pulmonary Hypertension: Quantitative Analysis of Flow Formations. J Am Heart Assoc 6: e007010, 2017. doi: 10.1161/JAHA.117.007010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schäfer M, Ivy DD, Barker AJ, Kheyfets V, Shandas R, Abman SH, Hunter KS, Truong U. Characterization of CMR-derived haemodynamic data in children with pulmonary arterial hypertension. Eur Heart J Cardiovasc Imaging 18: 424–431, 2017. doi: 10.1093/ehjci/jew152. [DOI] [PubMed] [Google Scholar]
- 33.Schäfer M, Kheyfets VO, Schroeder JD, Dunning J, Shandas R, Buckner JK, Browning J, Hertzberg J, Hunter KS, Fenster BE. Main pulmonary arterial wall shear stress correlates with invasive hemodynamics and stiffness in pulmonary hypertension. Pulm Circ 6: 37–45, 2016. doi: 10.1086/685024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schäfer M, Myers C, Brown RD, Frid MG, Tan W, Hunter K, Stenmark KR. Pulmonary Arterial Stiffness: Toward a New Paradigm in Pulmonary Arterial Hypertension Pathophysiology and Assessment. Curr Hypertens Rep 18: 4, 2016. doi: 10.1007/s11906-015-0609-2. [DOI] [PubMed] [Google Scholar]
- 36.Swift AJ, Capener D, Johns C, Hamilton N, Rothman A, Elliot C, Condliffe R, Charalampopoulos A, Rajaram S, Lawrie A, Campbell MJ, Wild JM, Kiely DG. Magnetic Resonance Imaging in the Prognostic Evaluation of Patients with Pulmonary Arterial Hypertension. Am J Respir Crit Care Med 196: 228–239, 2017. doi: 10.1164/rccm.201611-2365OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swift AJ, Rajaram S, Condliffe R, Capener D, Hurdman J, Elliot C, Kiely DG, Wild JM. Pulmonary artery relative area change detects mild elevations in pulmonary vascular resistance and predicts adverse outcome in pulmonary hypertension. Invest Radiol 47: 571–577, 2012. doi: 10.1097/RLI.0b013e31826c4341. [DOI] [PubMed] [Google Scholar]
- 38.Szulcek R, Happé CM, Rol N, Fontijn RD, Dickhoff C, Hartemink KJ, Grünberg K, Tu L, Timens W, Nossent GD, Paul MA, Leyen TA, Horrevoets AJ, de Man FS, Guignabert C, Yu PB, Vonk-Noordegraaf A, van Nieuw Amerongen GP, Bogaard HJ. Delayed Microvascular Shear-adaptation in Pulmonary Arterial Hypertension: Role of PECAM-1 Cleavage. Am J Respir Crit Care Med 33: 1–58, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Truong U, Fonseca B, Dunning J, Burgett S, Lanning C, Ivy DD, Shandas R, Hunter K, Barker AJ. Wall shear stress measured by phase contrast cardiovascular magnetic resonance in children and adolescents with pulmonary arterial hypertension. J Cardiovasc Magn Reson 15: 81, 2013. doi: 10.1186/1532-429X-15-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437: 426–431, 2005. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 41.Wagenvoort CA. The pathology of primary pulmonary hypertension. J Pathol 101: Pi–Pxxiii, 1970. doi: 10.1002/path.1711010408. [DOI] [PubMed] [Google Scholar]
- 42.Wagenvoort CA, Wagenvoort N. Primary pulmonary hypertension: a pathologic study of the lung vessels in 156 clinically diagnosed cases. Circulation 42: 1163–1184, 1970. doi: 10.1161/01.CIR.42.6.1163. [DOI] [Google Scholar]
- 43.Weatherald J, Boucly A, Chemla D, Savale L, Peng M, Jevnikar M, Jaïs X, Taniguchi Y, O’Connell C, Parent F, Sattler C, Hervé P, Simonneau G, Montani D, Humbert M, Adir Y, Sitbon O. The prognostic value of follow-up hemodynamic variables after initial management in pulmonary arterial hypertension. Circulation 137: 693–704, 2018. doi: 10.1161/CIRCULATIONAHA.117.029254. [DOI] [PubMed] [Google Scholar]
- 44.Wehrum T, Hagenlocher P, Lodemann T, Vach W, Dragonu I, Hennemuth A, von Zur Mühlen C, Stuplich J, Ngo BT, Harloff A. Age dependence of pulmonary artery blood flow measured by 4D flow cardiovascular magnetic resonance: results of a population-based study. J Cardiovasc Magn Reson 18: 31, 2016. doi: 10.1186/s12968-016-0252-3. [DOI] [PMC free article] [PubMed] [Google Scholar]