Keywords: acid secretion, growth factors, mucosal homeostasis, parietal cell, stomach
Abstract
Parietal cells are responsible for gastric acid secretion, which aids in the digestion of food, absorption of minerals, and control of harmful bacteria. However, a fine balance of activators and inhibitors of parietal cell-mediated acid secretion is required to ensure proper digestion of food, while preventing damage to the gastric and duodenal mucosa. As a result, parietal cell secretion is highly regulated through numerous mechanisms including the vagus nerve, gastrin, histamine, ghrelin, somatostatin, glucagon-like peptide 1, and other agonists and antagonists. The tight regulation of parietal cells ensures the proper secretion of HCl. The H+-K+-ATPase enzyme expressed in parietal cells regulates the exchange of cytoplasmic H+ for extracellular K+. The H+ secreted into the gastric lumen by the H+-K+-ATPase combines with luminal Cl− to form gastric acid, HCl. Inhibition of the H+-K+-ATPase is the most efficacious method of preventing harmful gastric acid secretion. Proton pump inhibitors and potassium competitive acid blockers are widely used therapeutically to inhibit acid secretion. Stimulated delivery of the H+-K+-ATPase to the parietal cell apical surface requires the fusion of intracellular tubulovesicles with the overlying secretory canaliculus, a process that represents the most prominent example of apical membrane recycling. In addition to their unique ability to secrete gastric acid, parietal cells also play an important role in gastric mucosal homeostasis through the secretion of multiple growth factor molecules. The gastric parietal cell therefore plays multiple roles in gastric secretion and protection as well as coordination of physiological repair.
This review summarizes the complex literature related to the physiology and cell biology of gastric parietal cell acid secretion and the impact of directed pharmacology in the therapeutic manipulation of acid secretion. In addition, the article addresses the role of gastric parietal cells as sources of growth factors and regulators of gastric mucosal homeostasis.
I. HISTORICAL PERSPECTIVE ON ACID SECRETORY PHYSIOLOGY AND PATHOPHYSIOLOGY
The pursuit of an understanding of gastric acidity has been a central focus of gastrointestinal medicine and physiology through the ages. Acid-peptic disease has been a consistent cause of morbidity and mortality throughout human history. Early physicians such as Galen and Vesalius recognized the caustic nature of gastric secretions in many animals including humans (402). Nevertheless, it was not until the 18th century that physiologists systematically sought to determine the chemical nature of gastric secretion. The insights into the composition of gastric juice began with the studies involving dubious ethics by William Beaumont who studied the effluent from a gastric-cutaneous fistula in a soldier wounded in the French and Indian War (31). These studies allowed Beaumont to determine a number of meal-related stimuli to the flow of gastric juice. While many believed that lactic acid accounted for the acidity in the stomach, in 1823, Prout determined definitively that the highly caustic nature of the gastric juice was due to HCl (258). This recognition of HCl secretion led to investigation of how neurons and humoral regulators control the secretion of acid from parietal cells. Similarly, the drive to understand acid secretion as a cause of ulcer disease led to extensive physiological studies beginning in the late 19th century focusing on identifying ways to moderate acid secretion and ameliorate acid-peptic disease. Latrajet (220) was the first to detail the innervation of the stomach, and Pavlov (289) expanded these insights to define neuronal regulation of acid secretion. The studies of Latrajet and Pavlov led to the development of acid suppressive surgery by Dragstedt, first through vagotomy and later through vagotomy and antrectomy (80). These operations, based on concepts of regulation of physiology, dominated the treatment of duodenal ulcer disease through most of the 20th century until the introduction of H2-histamine receptor blockers in the 1970s and the recognition in the 1980s of Helicobacter pylori infection as the predominant cause of duodenal ulcers.
II. THE CELLULAR ANATOMY OF THE STOMACH
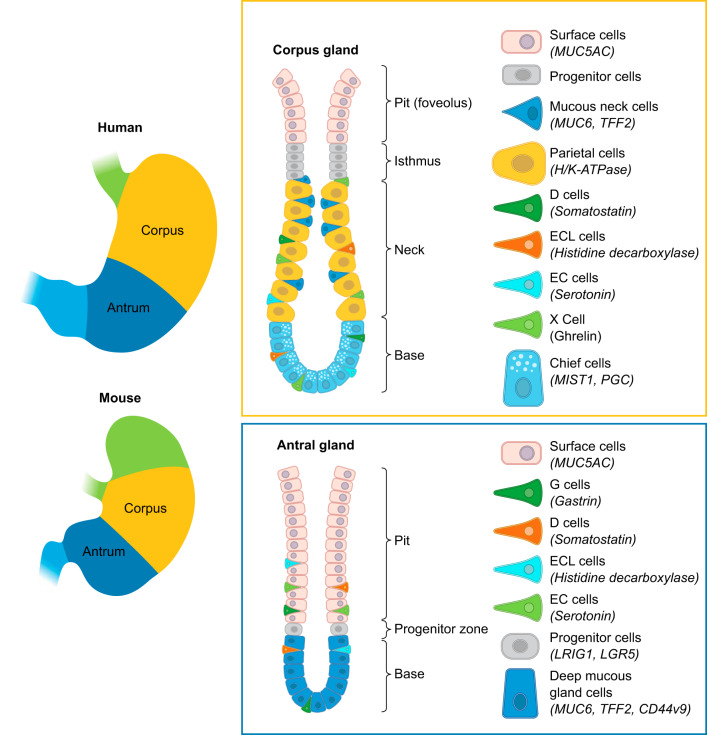
The human stomach is separated into three anatomical regions: the cardia, the corpus, and the antrum. The corpus represents the largest portion of the stomach and is populated by oxyntic glands. The oxyntic glands contain large numbers of acid-secreting parietal cells and an isthmal progenitor zone near the top quarter of the glands. Foveolar surface Muc5AC-expressing mucous cells migrate towards the lumen from the isthmus, while parietal cells migrate towards the base (188, 189). The oxyntic glands also show Muc6-expressing mucous neck cells that migrate towards the base and subsequently redifferentiate into pepsinogen-secreting chief cells (190). The position of the progenitor zone near the lumen is the result of differing lifetimes of corpus gland lineages. Thus surface mucous cells, which migrate towards the lumen, live 5–7 days (189). In contrast, the parietal and chief cell lineages that migrate towards the base live 90–120 days (187, 190, 417). The oxyntic glands are also defined specifically by the presence of ghrelin-secreting enteroendocrine cells and harbor histamine-secreting enterochromaffin-like (ECL) cells, somatostatin-secreting D cells, and a few serotonin-secreting enterochromaffin (EC) cells (77, 239) (FIGURE 1).
FIGURE 1.
Cellular anatomy of the stomach. The human stomach is composed of three distinct regions: the cardia, the corpus, and the antrum. The gastric cardia resides in the most proximal portion of the human stomach. The corpus contains the oxyntic glands that harbor an isthmal progenitor region and contains the majority of acid-secreting parietal cells and pepsinogen-secreting chief cells. Corpus glands uniquely contain ghrelin-secreting X cells. The antral glands are predominantly mucus secreting glands and uniquely harbor the gastrin expressing G cells. It is important to note that, in the human stomach, the antrum contains a mix of oxyntic and antral glands; however, the oxyntic-type glands in the antrum have significantly fewer chief cells and parietal cells compared with corpus glands (77).
In contrast, the antral or pyloric glands contain foveolar surface mucous cells and Muc6-expressing deep mucous cells. The presence of gastrin-expressing G cells defines the antrum, and these glands also show D cells and some EC cells (77). It is important to note that while the discrete separation of corpus oxyntic glands from mucus-secreting antral glands is very sharply demarcated in rodent and rabbit stomach, the human antrum usually contains a mixture of oxyntic- and antral-type glands. The oxyntic-type glands in the antrum do contain parietal cells and chief cells, but at significantly reduced numbers compared with corpus glands (77, 385). It is not clear whether the presence of parietal cells in the human antrum has consequences on the prevalence of duodenal ulcer disease.
The cardia region in humans as well as rabbits resides adjacent to the gastroesophageal junction and has variable size ranging from a few glands to 20–30 glands. Cardia glands are characterized by an absence of parietal cells and chief cells and have overall characteristics more similar to antral glands. All mammals studied possess a unique first gland directly after the squamo-columnar junction that has unique characteristics including Lgr5-positive stem cells, a general absence of endocrine cells or parietal cells, and an abundance of sensory tuft cells (182, 277). It remains controversial whether larger numbers of cardia glands in humans represents an expansion of the gland populations from the first gland. It should be noted that rodents do not have a real cardia. Rather rodents possess a large squamous epithelia-lined forestomach. Nevertheless, they still show a characteristic first gland at the squamo-columnar junction (277).
III. REGULATION OF GASTRIC ACID SECRETION
A. Neurohumoral Regulation of Parietal Cell Secretion
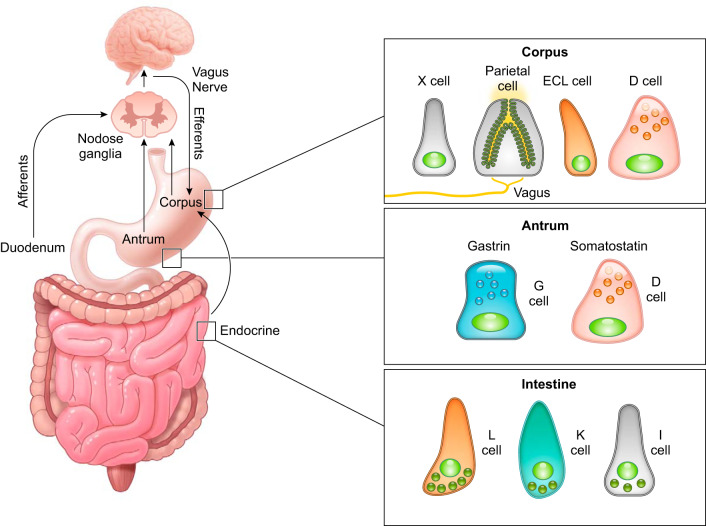
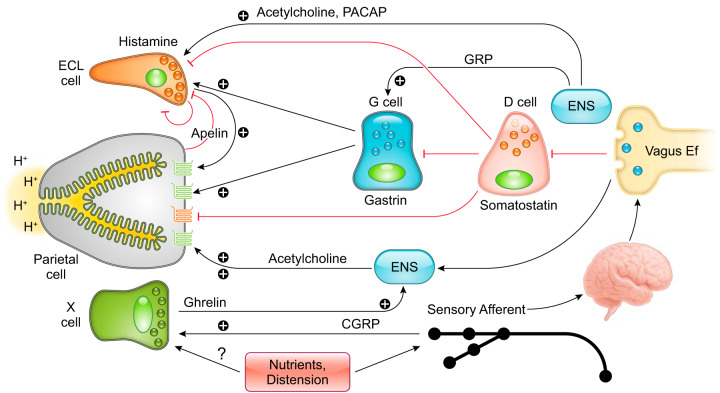
Hydrochloric acid secreted from gastric parietal cells generates the strongly acidic environment of the gastric lumen (pH <2) (305), which kills food-derived bacteria, facilitates food digestion, and promotes absorption of minerals including phosphate, calcium, and iron. High levels of acid secretion also represent a potentially harmful substance to the integrity of the gastric mucosa. Thus the gastric mucosa must maintain a balance between acid secretion and mechanisms for mucosal protection. The extrinsic and intrinsic neuroendocrine system of the stomach balances the influences of agonist and antagonist to maintain a safe range of acid secretion. Below we highlight the present knowledge of how the physiological balance between stimulatory and inhibitory pathways is integrated within the gastric mucosa (FIGURES 2 AND 3).
FIGURE 2.
Neurohumoral regulation of gastric acid secretion. Multiple pathways are involved in the regulation of gastric acid secretion, including the neuronal and endocrine pathways mediated by the enteric nervous system and enteroendocrine cells in the gastrointestinal mucosa. Histamine-producing enterochromaffin-like (ECL) cells and ghrelin-producing X cells are found in the corpus, while somatostatin-producing D cells are distributed throughout the stomach. Gastrin-producing G cells are specifically localized in the antrum. Small intestinal enteroendocrine cells have some overlapping expression of gastric peptides including ghrelin and somatostatin (93, 185).
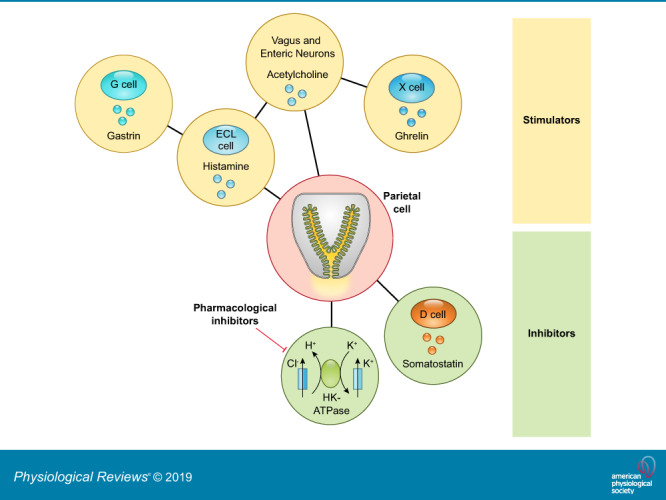
FIGURE 3.
Cellular components that control gastric acid secretion. Numerous cell types regulate gastric acid secretion. Enterochromaffin-like (ECL) cells through histamine and X cells that secrete ghrelin activate parietal cells via paracrine and neural pathways, respectively. Gastrin secreted from G cells binds directly on parietal cells or stimulates acid secretion mediated by histamine release from ECL cells. Vagal efferent mediated by the enteric nervous system (ENS) stimulates G cells through gastrin-releasing peptide (GRP) and acetylcholine (ACh) and stimulates ECL cells through pituitary adenylate cyclase-activating peptide (PACAP). The cholinergic signal can also inhibit somatostatin release from D cells, accelerating acid secretion. Additionally, calcitonin gene-related peptide (CGRP) released from vagal afferent terminals activates X cells to enhance the acid secretory pathway. Somatostatin directly inhibits gastric acid secretion from parietal cells as well as indirectly through its action on ECL cells and G cells. Histamine suppresses ECL cell activity as a negative feedback system. Apelin, produced by parietal cells, exerts inhibitory or stimulatory effects on ECL cells.
B. Stimulatory Mediators
1. Vagus nerve/acetylcholine
Extrinsic nerves densely innervate the upper gastrointestinal mucosa and regulate gastric acid secretion through afferent and efferent signals (FIGURE 2). The importance of the vagus nerve in stimulating acid secretion was first elaborated by Pavlov (289). Since vagotomy decreases basal and distension-induced acid secretion (136), this surgery was a mainstay of peptic ulcer treatment for decades (80). Afferent nerves processes from neural bodies in nodose ganglia consist of ~80% of vagus nerve fibers, implicating the importance of sensory function in the gastrointestinal mucosa. Sensory function for vagal components may be critical for luminal sensing and coordination of acid secretion and other functions including cell lineage homeostasis. Powley et al. (302) demonstrated the afferent terminals running close to epithelial cells of antral glands and duodenal villi and glands. However, they did not directly contact the lumen, suggesting that afferent nerves indirectly monitor luminal signals. Afferent nerve terminals and varicosities contain the neuropeptide calcitonin gene-related peptide (CGRP), released by local activation to stimulate ghrelin and somatostatin secretion from gastric X and antral D cells, respectively (98, 241, 446). Mechanical and chemical stimuli in the stomach activate a subset of vagal afferent neurons expressing glucagon-like peptide 1 receptor (GLP-1R) shown by in vivo ganglion imaging in reporter protein transgenic mice (422). The same study demonstrated that intraduodenal chemicals activated another subset of nodose neurons, which express GPR65, a proton sensing receptor (346, 422). Neuronal tracer studies in rats precisely mapped the afferent innervation and showed that right and left nodose afferents line antral mucosa, while left nodose predominantly innervates the duodenum (411). Unilateral vagotomy may alter the gastrointestinal response to intraduodenal foods. Afferent signals through nodose neurons are transduced to the nucleus tractus solitarius (NTS) in the medulla, which is influenced by postprandial circulating hormones and nutrients by the blood-brain barrier, which is relatively leaky to these molecules. NTS neurons contact dorsal motor nucleus (DMN), where the efferent nerves of the vagus originate. Electrical activation of DMN increases gastric acid secretion in cats (429). Vagal efferent nerves connect to gastric myenteric ganglia in the enteric nervous system (ENS), and the final vagal neurotransmitter is acetylcholine (ACh). Gastric mucosal nerves originating from intrinsic (submucosal and myenteric) ganglia predominantly contain ACh (15). Gene deletion of the neurturin receptor GFRα2 in mice causes a loss of mucosal cholinergic nerves, although basal and histamine-stimulated acid secretion as well as plasma gastrin level is similar to levels detected in wild-type mice (211). Unstimulated gastric acid output in GFRα2 knockout (KO) mice is reduced by the muscarinic antagonist atropine to the same extent as in wild-type mice (211), suggesting that a non-neural ACh source in gastric mucosa may exist to maintain the basal constitutive activity of muscarinic receptors.
Electrical stimulation of the cervical vagus nerve increases gastric acid secretion (41, 361). Vagal activation-induced acid secretion is reduced by atropine by 70% and abolished by the combination of atropine with ganglionic ACh receptor antagonist hexamethonium in anesthetized rats (347), suggesting the predominant contribution of the muscarinic pathway. The ACh analogue carbachol increases intracellular Ca2+ ([Ca2+]i) in isolated parietal cells, some ECL cells, and G cells to activate secretion (268, 439, 449). Distinct G protein-coupled muscarinic ACh receptors (M1R-M5R) differentially regulate gastric acid secretion. Selective antagonists for M3R increases inositol phosphate and [Ca2+]i in isolated parietal cells from rats and rabbits (296, 421). The deletion of each muscarinic receptor subtype in transgenic mice demonstrated that M3R mediates the greatest influence on stimulating acid secretion, with an additional contribution of M5R, but not M1R (8, 9). In addition, M4R activation suppresses somatostatin release from D cells, further enhancing gastric acid secretion (377).
2. Gastrin/G cell/CCK2 receptor
A gastric acid-stimulating hormone, gastrin, produced in the antrum was proposed in 1906 (92). Classic physiological experiments using isolated antrum with Heidenhain pouch in dogs demonstrated the presence of this humoral factor (139, 426, 427). Gregory and Tracy (135) extracted gastrinlike peptides from pig antral mucosa and determined its amino acid sequence. Subsequently, human gastrin was isolated as a heptadecapeptide with only one amino acid difference from pig gastrin (37). The gastrin gene was cloned from pig antrum in 1982 (440) and human in 1983 (50, 192). The gastrin-producing enteroendocrine cell (G cell), which has an apical brush border with direct access to the lumen, was first identified by Solcia et al. (364) in guinea pig antrum. Yalow and Berson (434) developed a radioimmunoassay with highly specific human gastrin antisera and showed that intragastric HCl decreased plasma gastrin level within a few minutes in pernicious anemia (autoimmune gastritis) patients, who have hypergastrinemia. The G cell was the first physiologically and histologically identified enteroendocrine cell and inspired further characterization of other enteroendocrine cell populations as gut nutrient sensor cells (112). Bioactive amidated gastrin has two major forms, G34 (big gastrin) and G17 (little gastrin), which share COOH-terminal polypeptides and are released into circulation after a meal (215). Most stored gastrin in human antrum is G17 (181, 310). G34 has a longer half-life and causes long-lasting acid stimulation (410), suggesting that the processing mechanism can regulate gastrin efficacy. Several Gq-coupled receptors for digested protein (peptone and amino acids) are found in G cells, such as CaSR, GPCR6A, and LPAR5 (102, 144, 309, 311). These chemical sensors are likely a mechanism of amino acid-induced gastrin secretion, subsequently stimulating acid secretion from parietal cells (100). A recent study, using CaSR agonists and the synthetic antagonist NPS2143, showed that CaSR activation stimulates gastrin release from dissected pig antral glands and that aromatic amino acids together with extracellular Ca2+ trigger this pathway (430).
The gastrin receptor, also designated as CCK2R by the International Union of Basic and Clinical Pharmacology (IUPHAR)/British Pharmacological Society (BPS) ‟Guide to Pharmacologyˮ (gene name: CCKBR; IC50 = 1 nM), was cloned in several animals including humans as an identical receptor in the stomach and brain (205, 298, 415). In human stomach, CCK2R immunoreactivity is localized on the basolateral membrane in the majority of parietal cells and some chromogranin-A containing ECL cells (210, 341). Histamine H2-receptor antagonists inhibit CCK2R-activated acid secretion in vivo (1, 46) and in parietal cells (71, 122), suggesting that gastrin indirectly stimulates acid secretion through histamine release from ECL cells. Although CCK2R is present on parietal cells, direct effect of gastrin on acid secretion in humans is likely minor. Synthetic G17 infusion increases acid secretion in fasted men, and this response is reduced >70% by atropine and abolished by famotidine (451). The primary pathway of gastrin-induced acid secretion in humans is likely paracrine release from ECL cells of histamine, which directly activates parietal cells. Additionally, gastrin-CCK2R signaling potentiates cholinergic input on parietal cells (117). CCK2R is also essential for parietal cell differentiation and maturation. Germline CCK2R-deficient mice demonstrate gastric mucosal atrophy and decreased parietal cell and ECL cell numbers, resulting in increased basal gastric pH and plasma gastrin level (216, 264). Likewise, gastrin-deficient mice have few parietal cells and low basal and stimulated acid secretion in vivo (110). However, isolated parietal cells from gastrin KO mice show higher Ca2+ responses than wild-type cells (163), suggesting that gastrin influences parietal cell characteristics and balances the receptor expression levels. Furthermore, gastrin is required for tonic expression of the gastric trefoil factors and parietal cell maturation (199, 285). The gastrin-secreting G cells regulate parietal cell function as a feedback system from the distal part of the stomach.
The CCK1 receptor (CCK1R; gene name: CCKAR) has 1,000-fold higher affinity to cholecystokinin (CCK)-8 than gastrin, while CCK2R has similar affinity to both peptides. CCK is produced and secreted by a subpopulation of the enteroendocrine cells of the duodenum in response to luminal fatty acids and digested protein (154, 227). CCK1R activation inhibits acid secretion in anesthetized rats and healthy humans (230, 338). CCK1R immunoreactivity is found in both chief cells and D cells, which are CCK2R negative (341, 343), suggesting that CCK contributes to postprandial acid inhibition likely through somatostatin release.
3. Histamine
Histamine is a bioactive amine and strong acid secretagogue, generated by histidine decarboxylase (HDC) in the ECL cells and mast cells in the corpus gastric glands (145). ECL cells, which are frequently found next to parietal cells, have no direct contact with the gastric lumen (closed type enteroendocrine cells). These ECL cells show long basal processes that are thought to come in apposition with multiple parietal cells (66, 99). Histamine release is directly activated by circulating gastrin via CCK2R (162) and neuronal pituitary adenylate cyclase-activating peptide (PACAP) via the PAC1R (447) (FIGURE 4). Histamine release is suppressed by somatostatin via SST2 and galanin via Gal1 (448) receptors on ECL cells. Diamine oxidase (DAO) and histamine-N-methyltransferase deactivate histamine. The infusion of exogenous DAO made from pig kidney inhibited histamine-induced gastric acid secretion in dogs (138). In a human study, heparin-induced gastric acid inhibition was mediated by endogenous DAO (151).
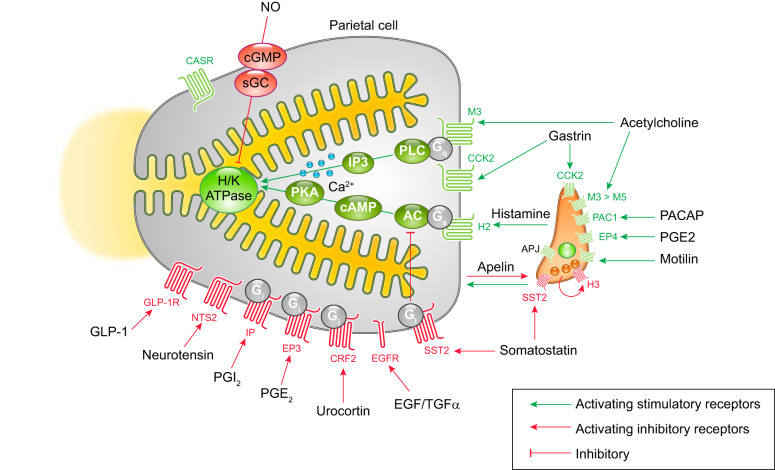
FIGURE 4.
Membrane receptors on parietal and enterochromaffin-like (ECL) cells and intracellular signaling pathways that regulate gastric acid secretion. Stimulatory pathways are indicated in green, and inhibitory pathways are indicated with red lines. The parietal cell expresses G protein-coupled receptors (GPCRs) for acid secretagogues, including the muscarinic receptor (M3) for acetylcholine, the gastrin receptor (CCK2), and the histamine receptor (H2). M3 and CCK2 receptors are coupled to G protein Gq that activates phospholipase C (PLC), which leads to intracellular Ca2+ release to potentiate acid secretion. Binding of histamine to the H2 receptor activates adenylate cyclase (AC) to generate cAMP through Gs signal, which potently stimulates H+-K+-ATPase (H/K) activity via cAMP-dependent protein kinase (PKA). Histamine release from ECL cells is stimulated by a variety of receptors, including CCK2, PAC1, EP4, and the motilin receptors. Several receptors for inhibitors of gastric acid secretion are present on parietal cells including GLP-1R, NTS2, IP, EP3, CRF2, EGFR, and SST2 receptors. The inhibitory G protein (Gi) suppresses AC activities to inhibit cAMP-mediated secretory pathways. Additionally, nitric oxide (NO) inhibits acid secretion through the cGMP pathway. Angiotensin-like peptide receptor (APJ), activated by parietal cell-derived apelin, exerts both inhibitory and stimulatory effects. The H3 histamine receptor, expressed on ECL cells, contributes to negative feedback for histamine release.
The H2 histamine receptor (H2R) was defined pharmacologically as a mediator of gastric acid secretion through the use of its antagonist, burimamide, in anesthetized rats (46). Acid secretion is insensitive to H1R antagonists, such as mepyramine. H2R antagonists inhibit cholinergic- and gastrin-induced acid secretion in vivo and in isolated gastric glands (40, 137). Synthetic H2R antagonists were the first targeted therapeutics for peptic ulcers and erosive esophagitis (46, 260) before the development of proton pump inhibitors. Parietal cells express Gs-coupled H2 receptors, which increase intracellular cAMP to stimulate acid secretion directly and potentiate Ca2+-induced acid secretion (40). Histamine induces accumulation of cAMP (238) and activates cAMP-dependent protein kinase in isolated parietal cells (69). H2R-activated cAMP production is the crucial and rate-limiting trigger of parietal acid secretion. HDC-deficient mice barely respond to carbachol and/or gastrin, but gastric acid secretion is stimulated by the combination of forskolin and carbachol (115). Similarly, H2R-deficient mice lack the response to gastrin, but basal acid secretion is maintained by M3R and CCK2R signaling (114). Transgenic mice with H+-K+-ATPase promotor-derived cholera toxin show consistently high intracellular cAMP concentration in parietal cells. These mice have high basal acid secretion and low plasma gastrin, and subsequently develop metaplasia with parietal cell loss by 15 mo of age (231).
Four subtypes of G protein-coupled histamine receptors (H1-H4R) have been cloned and identified in different types of cells in the gastrointestinal tract (326). Inhibitory H3 receptors on ECL cells likely mediate a direct autocrine feedback mechanism in rats and rabbits (166, 304); however, the functional expression of H3R in humans is still controversial.
4. Ghrelin
Ghrelin, which is an octanoylated 28-amino acid peptide released from the oxyntic mucosa of stomach, was identified by Kojima et al. (202) as a growth hormone secretagogue receptor (GHSR) ligand. Circulating ghrelin levels have a circadian rhythm and are increased by fasting and decreased by feeding in rodents (388) and humans (82). Ghrelin-producing X cells are closed-type enteroendocrine cells (83), which have no direct contact with the lumen and are distributed in corpus oxyntic glands reciprocally with gastrin in the antrum (77). A variety of metabolite and neurohumoral receptors are expressed on X cells. Bitter taste receptor (T2R)-coupling G proteins, such as gustducin and transducin, are colocalized with ghrelin in murine stomach. Bitter compound ingestion increases plasma ghrelin level within 40 min (177). FACS and qPCR techniques revealed that X cell release of ghrelin is inhibited by the activation of FFA2 (short-chain fatty acids), FFA4 (long-chain fatty acids), HCA1 (lactate), CaSR (extra cellular calcium, amino acids), and SST1–3 (somatostatin) receptors, explaining the decreased plasma ghrelin levels after a meal. Release of ghrelin from X cells is activated by β1-adrenergic, CGRP, gastric inhibitory polypeptide (GIP), and secretin (SCT) receptors, consistent with in vivo observations that postprandial nutrients and hormones regulate ghrelin release (98). Peripheral (248) and intracerebroventricular (84) administration of ghrelin stimulates gastric acid secretion through the vagus nerve in rats. Furthermore, in rats, coadministration of ghrelin with gastrin synergistically increases acid secretion stimulated through the vagus nerve (113, 433). Although most of human ghrelin cells are located close to parietal cells (99), model animal studies so far show that ghrelin action on acid secretion is predominantly mediated by GHSR on afferent nerves and a vagal reflex (FIGURE 3).
5. Apelin
Apelin was isolated from bovine stomach as an endogenous ligand of an orphan human G protein-coupled receptor (GPCR), angiotensin-like peptide receptor (APJ), with higher affinity for short COOH-terminal fragments (apelin-13 or -17) than long fragments (apelin-35) (381). Rat parietal cells express apelin mRNA, while ECL cells express APJ mRNA in purified rat gastric epithelial cells (214), suggesting a feedback pathway from the parietal cell to the ECL cell. In isolated rabbit gastric glands, 100 nM apelin inhibited [Ca2+]i responses in ECL cells and gastrin-induced parietal cell activation (214). In contrast, in vivo gastric perfusion experiments in rats showed that 100 µg/kg intravenous apelin-12 enhanced acid secretion through histamine release, independent from muscarinic cholinergic pathways (280). Different lengths of apelin fragments interact differently with APJ and its downstream Gi/o, inhibiting cAMP production in CHO cell expression systems (142), suggesting that different effects of endogenous apelin are influenced by truncation. Apelin immunoreactivities are broadly found in central and peripheral tissues, including in human mesenteric adipocytes with Crohn’s disease (118) and Kupffer cells in rat liver in addition to gastric parietal cells (382). In a stress-induced gastric lesion model in rats, apelin protein expression was increased, and APJ receptor antagonist delayed the mucosal healing, indicating an important role of apelin-APJ signaling in mucosal protection (43). Those observations suggest that apelin can locally and systemically modulate gastric acid secretion through APJ-histamine-H2R signaling under physiological and pathological conditions. Further examination of apelin functions in human stomach is needed.
6. Motilin
Motilin, which is in the same peptide family as ghrelin, was identified in pig intestine as a gastric motility activating peptide (52). Duodenal Mo cells release motilin during the interdigestive period by unknown stimuli. High doses (10 µg/kg) of motilin or a coadministration of a low dose (1 µg/kg) of motilin and ghrelin synergistically stimulate gastric acid secretion in anesthetized shrews (131). This peptide-induced acid secretion is mediated by histamine release and is independent from cholinergic pathways. Both motilin and ghrelin are released when the stomach is empty, suggesting that this combination is responsible for endocrine regulation of interdigestive motility-related gastric acid secretion.
7. Glucocorticoids
Glucocorticoids are essential steroid hormones for systemic homeostasis, primarily secreted by the adrenal cortex in a circadian manner and in response to stress. Other organs, including the intestine, also synthesize glucocorticoids, and local functions in immune regulation have been proposed (79). Glucocorticoids stimulate gastric acid secretion in dogs (81) and mice through serum- and glucocorticoid-inducible kinase (SGK1), which stimulates K+ channels, such as KCNQ1 (327). Adrenalectomy induces oxyntic atrophy and gastric inflammation through the spontaneous activation of CXCR2+ monocytes in mice, suggesting that glucocorticoids are essential for gastric homeostasis (56). In human studies, glucocorticoids induce hypergastrinemia (308), but do not affect acid secretion (164). Glucocorticoids bind ligand-dependent transcription factors, namely, mineralocorticoid (MR or NR3C2) and glucocorticoid (GR or NR3C1) receptors, that are broadly expressed in gastric cells, including parietal cells (56, 278). The activity of glucocorticoids is regulated by the balance of two subtypes of hydroxysteroid dehydrogenases (11β-HSD1 and 11β-HSD2), which either catalyze production of active glucocorticoids or inactivate them, respectively (63). Because human parietal cells highly express mineralocorticoid receptor and 11β-HSD2, mineralocorticoid-specific effects on parietal cells are suggested, rather than glucocorticoid (193). Yet the function of MR in regulating parietal cell mediated acid secretion remains unknown.
C. Inhibitory Mediators
1. Somatostatin
Somatostatin was identified as a growth-hormone release-inhibiting hormone in the hypothalamus and is found in enteroendocrine D cells in human gastric mucosa (219, 299). In anesthetized dogs, intragastric glucose, fat, and casein hydrolysate stimulates somatostatin release from the corpus to a greater degree than from the antrum (345). Conversely, intraduodenal and intragastric HCl infusion potently stimulates antral somatostatin secretion, but not from the corpus (345), suggesting that D cells throughout the gastric mucosa possess several nutrient receptors expressed at different levels and antral D cells are more sensitive to luminal acid. As oligopeptide and amino acid receptors in the human and pig antrum, immunoreactivities of CaSR, LPA5, and GPRC6A are detected in D cells (144), indicating that antral D cells are stimulated by luminal or circulating nutrients. From the circulation, gastrin and CCK regulate somatostatin secretion in the corpus and antral mucosa to different extents (443). More GPCRs were identified in isolated D cells from reporter mouse gastric tissue and primary cultured gastric epithelial cells using transcriptome techniques (6, 94). Trace amine-associated receptor 1, GLP-1R, GIPR, CGRP receptor subunits, vasoactive intestinal peptide R, adrenomodulin R, melanocortin MC1, muscarinic M3, CCK2, and adrenergic receptors are all stimulators of somatostatin release, whereas long-chain fatty acid receptor FFA4, SST1, and SST2 receptors are suppressers of somatostatin secretion (6, 94).
Intravenous somatostatin potently reduces feeding- or gastrin-stimulated acid secretion in conscious cats, dogs, and humans (27, 128, 204, 297). The direct effect of somatostatin on isolated gastric glands and parietal cells from rabbit stomach suggests that histamine secretion from ECL cells and acid production in parietal cells are both inhibited by somatostatin (68).
All of the five subtypes of somatostatin receptors (SST1-SST5R) are coupled with inhibitory G protein (Gi/o) and uniquely distributed in the gastric mucosa (11, 209). A splice variant of SST2R, SST2aR, is predominantly expressed in rat ECL cells and human G cells, while another variant, SST2bR, was identified in rat parietal cells (140, 336, 370). SST2R-deficient mice demonstrated higher basal acid secretion (247), confirming that SST2R activation suppresses cAMP generation and decreases gastric acid secretion.
2. GLP-1 and PYY/‟enterogastroneˮ effect
A substance from intestinal mucosa, which is released in response to intraluminal fat and inhibition of gastric acid secretion, was first described in 1930 as “enterogastrone” (206). Deficiency of apolipoprotein A-IV in transgenic mice abolishes the inhibitory effect of duodenal lipid on meal-stimulated gastric acid secretion (419), suggesting that absorption is essential for gastric acid inhibition by dietary lipid. Currently, an intestine-specific proglucagon product, glucagon-like peptide 1 (GLP-1), and peptide tyrosine-tyrosine (PYY), another inhibitory gut peptide causing the “ileal brake,” a negative feedback mechanism that slows food transit through the gastrointestinal tract, are considered as the molecular components accounting for enterogastrone. Intravenous GLP-1 or PYY inhibits stimulated gastric acid secretion in humans (141, 337). Plasma levels of GLP-1 and PYY are increased by nutrient perfusion, including lipid and carbohydrate in human ileum, correlating with the inhibition of gastric acid secretion (221, 418). PYY inhibition of histamine release from isolated rat ECL cells is mediated by the Gi/o-coupled Y1 receptor (450). PYY expression is detected in isolated D cells from murine stomach and only a small number of human stomach enteroendocrine cells (6, 94, 99), implicating direct PYY effects on gastric cells in a paracrine fashion. On the other hand, the GLP-1 receptor is expressed in rat parietal cells, and its agonists stimulate cAMP and acid production (339, 340). Since human parietal cells express GLP-1 receptors (51), elucidating the direct function of GLP-1 on human gastric glands would be important for understanding the side effects of incretin hormone analogues used in diabetic treatments.
3. Gaseous mediators: nitric oxide and hydrogen sulfide
Nitric oxide (NO) and hydrogen sulfide (H2S) are known to inhibit gastric acid secretion and enhance mucosal restitution. NO is produced from l-arginine by NO synthase (NOS) in the intramural neurons and gastric epithelial cells (53). Immunoreactivity for endothelial NOS was identified in surface epithelial cells and enteroendocrine cells in human gastric mucosa (38), and neuronal NOS was found in isolated rat parietal cells (303). NO activates soluble guanylate cyclase (sGC) to increase intracellular cGMP (FIGURE 4). The exogenous NO donor nitroprusside reduces histamine-induced acid secretion, and the NOS inhibitor NG-nitro-l-arginine methyl ester (l-NAME) prevents the inhibition of acid secretion induced by mucosal injury in anesthetized rats (378). Acid production in isolated oxyntic glands from rabbits and healthy human biopsies is inhibited by NO via elevations in cGMP, indicating that NO acts as a paracrine regulator (39, 200).
H2S is generated from l-cysteine by cystathionine β-synthase and cystathionine γ-lyase, which are expressed in the gastric mucosa as well as in parietal cells (245). The exogenous H2S donor NaHS increases luminal NO release in anesthetized rats and inhibits gastric acid secretion in response to gastric distension (242). Generation of H2S in gastric mucosa is increased in ulcerated mucosa and enhances mucosal healing independent from NO synthesis, suggesting that H2S maintains the gastric mucosa through several targets (407).
4. Neurotensin/xenin
Neurotensin and its related peptide, xenin, are neuropeptides that are produced in the central nervous system as well as from enteroendocrine cells in the distal and proximal intestine, respectively. Plasma concentrations of these peptides are elevated after a meal and influence gastrointestinal functions via high-affinity NTS1, low-affinity NTS2, and NTS3 receptors. Intravenous neurotensin or xenin (50 ng·kg−1·min−1) potently inhibits gastric acid secretion stimulated by pentagastrin, but not by histamine in dogs (13, 103). Vagotomy abolishes the antisecretory effect of (Gln4)-neurotensin, which has a glutamine residue at position 4 instead of glutamic acid in the natural form (14), and neurotensin-binding neurons were identified in rat nodose ganglia (198), suggesting the remarkable contribution of a vagal reflex. NTS1R, which is equally activated by neurotensin and xenin, is distributed in sensory neurons (300), and NTS2R is localized on basolateral membranes of parietal cells in human gastric mucosa (342). Both neuronal pathways and direct effects on parietal cells likely mediate postprandial antisecretory effects of neurotensin-related peptides.
5. Corticosterone releasing factor
Corticosterone releasing factor (CRF), a neuropeptide identified in the hypothalamus, and the related peptides urocortin (Ucn)1, Ucn2, and Ucn3 (400) are involved in stress-induced gastrointestinal dysfunctions through the G protein-coupled receptors CRF1R and CRF2R. CRF1R has high affinity for CRF and Ucn1, but not for Ucn2 or Ucn3. CRF2R prefers Ucn1, Ucn2, and Ucn3, but interacts poorly with CRF (156). Intracisternally or intravenously injected CRF inhibits gastrin-induced gastric acid secretion through vagal pathways, indicating the localization of CRF1R within the nervous system (374, 375). Immunoreactivity for CRF2R, but not CRF1R, was found in parietal cells and enteroendocrine cells in the oxyntic glands (65), and all urocortins are present in the gastric mucosa, including in gastric parietal cells (64, 208). These observations suggest local gastric acid regulation by urocortins-CRF2R signaling, but further studies are needed to clarify when urocortins are released and whether CRF2R contributes to physiological acid secretory function.
6. Prostaglandins
Prostaglandins are arachidonic acid metabolites, generated by cyclooxygenase (COX)-1 (Gene name: PTGS1) and COX-2 (PTGS2) in many types of cells in the normal gastric mucosa, including parietal cells, macrophages, and myofibroblasts (176). Major prostaglandins in human gastric mucosa are PGE2 and PGI2. In experimental animals, exogenous PGE2 and PGI2 suppress gastric acid secretion (237, 314, 420). However, the results in humans are controversial (32, 36), probably due to the different level of endogenous prostaglandins in different experimental paradigms. COX inhibition by nonsteroidal anti-inflammatory drugs (NSAIDs) augments acid secretion (224) and induces gastric mucosal lesions under fasted conditions. In isolated human parietal cells, PGE2 potently inhibits histamine-induced acid production, as assessed by aminopyrine uptake (178). Cultured gastric mucosa as well as isolated parietal cells generate PGE2 (283, 363), indicating that PGE2 functions as an autocrine feedback regulator suppressing gastric acid secretion and promoting mucosal protection. PGE2 activates four subtypes of membrane GPCRs (EP1–4R) with an affinity order of EP3 > EP4 > EP2 > EP1, coupling with distinct secondary messengers (4). Histamine- or forskolin-induced cAMP accumulation is inhibited by PGE2 (<1 µM) in isolated canine or rat parietal cells, indicating a Gi-coupled pathway (89, 365). In vivo gastric perfusion in anesthetized rats revealed that EP3R agonist inhibits pentagastrin- or histamine-stimulated acid secretion through the inhibition of parietal and ECL cells, whereas high concentrations of PGE2 (>100 µM) or Gs-coupled EP4R agonist enhances histamine release from ECL cells (194). High concentrations of PGE2 also stimulate bicarbonate secretion via EP1R to counteract histamine-upregulated acid secretion (379), suggesting that gastric pH is balanced by PGE2 production. EP3R is distributed on the basolateral membrane of gastric epithelial cells, and EP4R expression is weak in the epithelium but abundant in lamina propria mononuclear cells in human stomach, as shown by immunohistochemistry (376). Although expression of both isoforms of COX enzymes is increased in inflamed gastric mucosa, COX-1 predominantly mediates gastric acid inhibition through EP3R as well as IPR, a PGI2 receptor in isolated parietal cells (26, 176, 267, 272). Constitutive production of PGE2 and PGI2 at physiological concentration protects the gastric mucosa.
7. Adenosine
Extracellular adenosine inhibits histamine-induced acid secretion in isolated parietal cells from dogs and guinea pigs, likely through A1 receptors (123, 161). Rabbit parietal cells express A2B receptors, which stimulate adenylate cyclase activity and acid production (17, 18), suggesting species difference in direct adenosine function. A1 receptors are found in G cells as an inhibitory mechanism of acid secretion (438). Somatostatin release is enhanced by high concentration of adenosine via A2A receptor activation, while somatostatin release is inhibited by low concentrations of adenosine through an A1 receptor in mouse stomach (435). Thus the adenosine concentration in the microenvironment is likely important for acid secretory regulation through both direct and paracrine pathways.
8. Impact of Helicobacter pylori
Association between gastric colonization by Helicobacter pylori and gastric ulcer disease was discovered by Marshall and Warren (244). Research over the past three decades has demonstrated that chronic infection with different strains of H. pylori can lead to either hypersecretion or hyposecretion and their attendant pathological sequelae (95). Hyposecretion and corpus-predominant gastritis are related to the risk of gastric cancer, whereas hypersecretion and antrum-predominant gastritis are associated with duodenal ulcer (95, 243). Well-studied strain-specific virulence factors include the cytotoxin-associated gene pathogenicity island (cagPAI) gene, which encodes ~30 proteins of the type IV secretory system (T4SS) forming pili of bacterial outer membrane, and a variety of genotypes of vacuolating cytotoxin (vacA) gene (269, 276). Hypochlorhydria and mucosal inflammation are consistently observed with H. pylori colonization for a few days to few weeks after acute infection. H. pylori inhibits acid secretion by repressing H+-K+-ATPase α-subunit transcription and by augmented somatostatin release, enhancing microbial adhesion (130, 186, 261). Once attached to the epithelial cells, H. pylori can induce damage through secretion of the pore-forming protein VacA, resulting in apoptosis, disrupted tight junctions, and gastric inflammation. Infection of T4SS-expressing H. pylori strains increases the transcription factor nuclear factor (NF)-κB, binding the promoter region of the gastric H+-K+-ATPase to repress its transcription (149). A secreted oncoprotein CagA is the 3′-terminal product of cagPAI and is transferred into host cells to induce epithelial hyperproliferation and parietal cell apoptosis, resulting in a high risk of gastric cancer (270, 290, 324). Histamine- or carbachol-induced acid production in human parietal cell culture is acutely inhibited by incubation with a sonicated suspension of H. pylori (175). In addition, accumulation of H. pylori metabolites, such as fatty acids, suppresses parietal cell activities (35). Short-term (20 min) exposure of Ussing chambered rat gastric mucosa to H. pylori or its culture supernatant inhibits histamine release and stimulates somatostatin secretion via CGRP release from mucosal sensory nerves (442). Nevertheless, hyposecretion of acid and atrophic gastritis induced by chronic H. pylori infection may also be influenced by host characteristics including interleukin (IL)-1β polymorphisms (96).
Despite a number of mechanisms that can lead to hyposecretion and eventually atrophic gastritis associated with certain strains of H. pylori, it is likely that other strains are responsible for >95% of duodenal ulcers. Distinct virulence factors have been identified in different strains that are isolated from patients with gastric ulcer or duodenal ulcer (173, 282, 319). Yet the mechanisms of antrum- versus corpus-predominant inflammation have not been fully characterized. Children with duodenal ulcer and infected with H. pylori demonstrated elevated acid secretion and elevated basal and meal-stimulated serum gastrin levels (195). Eradication of H. pylori in duodenal ulcer patients causes reduced acid output (155). Since there is no evidence of acute stimulation of acid secretion by H. pylori, the hypersecretion in duodenal ulcer patients is likely caused by the alteration of inhibitory factors by chronic infection rather than direct effect on parietal cells. Indeed, the combination of increased gastrin and acid hypersecretion appears related to decreases in the secretion of somatostatin in the antrum (95, 133, 254, 261), although the precise mechanism for this deficit in somatostatin remains unclear.
9. Growth factors and cytokines
NSAID- or H. pylori-induced gastritis involves the production of proinflammatory cytokines, such as IL-1β, IL-6, and IL-8 in human gastric antrum, indicating that those cytokines are upstream of COX activation (148). Intravenous or intraperitoneal injection of IL-1β inhibits acid secretion through the central nervous system and PGE2 production in rat stomach (315, 328, 406). Consistent with those in vivo observations, IL-1β stimulates PGE2 generation in isolated rat ECL cells (228), consequently inhibiting acid secretion. In isolated mouse gastric glands, IL-2 and interferon-γ, but not IL-1β, suppress acid secretion, suggesting that a Th1 type immune response predominantly mediates direct acid inhibition (284). The IL-1 receptor type 1 (IL-1R1) was identified in rat and mouse parietal cells (335), and IL-1β suppresses sonic hedgehog expression in murine parietal cells in an IL-1R1-dependent manner (405). On the other hand, IL-1β as well as tumor necrosis factor (TNF)-α decrease basal and secretagogue-stimulated acid production in cultured rabbit parietal cells (29). Cytokine-mediated regulation of gastric acid secretion may vary among animal species, and the direct antisecretory effect of IL-1R1 in human parietal cells remains to be evaluated.
Transforming growth factor-α (TGF-α) is expressed throughout the gastrointestinal tract and is abundant in parietal cells (30). Epidermal growth factor (EGF) is secreted into salivary fluid (207), although whether breaches in the lumen can lead to access to basolateral EGF receptors in physiological conditions remains unclear. TGF-α and EGF share amino acid homology and a common receptor, namely, ErbB (EGFR), which is predominantly expressed on the basolateral membranes of parietal cells and is weakly detected in mucous neck cells and chief cells in healthy humans (3). EGF and TGF-α inhibit histamine-induced acid production in isolated rabbit, pig, and rat parietal cells (183, 203, 225, 348, 362, 412). ErbB ligands may regulate the physiological activity of parietal cells as autocrine and paracrine mediators. Other EGF family members, such as amphiregulin and heparin-binding EGF-like growth factor (HB-EGF), but not Cripto are produced by human gastric parietal cells (3, 263). The EGF level in the gastric lumen is lower in patients with H. pylori infection or Sjögren’s disease compared with healthy subjects, suggesting that loss of luminal EGF signal is linked to chronic gastritis (10, 233). In an experimental gastric injury model in rats, luminal or serosal EGF enhances mucosal restitution as measured by mucosal potential difference (255). In vivo gastric ulcer studies in rats also showed the therapeutic effect of oral EGF combined with an anti-ulcer drug, sucralfate (174). Since low levels of luminal EGF and chronic gastritis are correlated, luminal EGF may reach basolateral receptors and enhance restitution when the mucosa is damaged. Collectively, these findings suggest that EGFR ligands may play a dynamic role in regulating acid secretion and mucosal homeostasis in the gastric mucosa.
D. Luminal Sensing and the Regulation of Acid Secretion
Luminal contents also regulate acid secretion. Dietary proteins stimulate acid secretion, while dietary lipids suppress acid secretion (313). Nevertheless, little is known concerning the mechanisms that regulate luminal sensing in the gastric mucosa. Following the identification of gustatory signal transduction molecules, some of the taste sensor molecules were identified in gastric enteroendocrine cells (143, 401, 428) and parietal cells (67). Further characterization of orphan GPCRs revealed the molecular basis of chemical sensors in afferent nerves and enteroendocrine cells, which recognize food ingredients and regulate gastric physiological functions. Acid-sensing mechanisms have been proposed in the antrum and duodenum (129). However, exact pH sensing molecules and sensor cells are still unknown in the oxyntic mucosa. Since extracellular calcium receptor CaSR is activated by acidic extracellular pH (306) and is identified in parietal cells (67, 90), this GPCR-mediated signal is implicated as an acidity sensor in oxyntic mucosa. Rapid and local regulatory mechanisms of parietal cell activation by sensing microenvironmental pH might be important to maintain the basal tone of acid secretion independently from the central nervous system or systemic humoral control.
IV. CHARACTERIZATION OF THE H+-K+-ATPase
A. Gastric H+-K+-ATPase
Secretion of gastric acid by parietal cells is achieved through hydronium ion transport via the H+-K+-ATPase pump. This remarkable enzyme extrudes cytoplasmic protons against a steep concentration gradient while transporting extracellular potassium into parietal cells, resulting in electroneutral ion exchange (321). The H+-K+-ATPase is a P2-type ATPase similar to the Na+-K+-ATPase (78, 301, 373). Gastric H+-K+-ATPase is primarily found in gastric parietal cells and to a lesser degree is expressed in the renal medulla (7, 423). The dynamic membrane trafficking in gastric parietal cells regulates the activity of acid secretion into the lumen through H+-K+-ATPase. In resting parietal cells, gastric H+-K+-ATPase is present in tubulovesicles (106, 331). Upon stimulation, H+-K+-ATPase containing membranes fuse with the apical secretory canaliculi to form microvilli-like structures with a greatly expanded secretory surface (106, 331). The presence of the H+-K+-ATPase in the apical canalicular membrane enables the secretion of hydronium, provided by the H+-K+-ATPase enzyme. Debate continues as to the primary K+ and Cl− channels that supply the K+ necessary for the exchange of H+ and Cl− in gastric HCl. KCNQ1-KCNE2 likely provides the potassium necessary for exchange by the H+-K+-ATPase (213, 316, 399), while evidence suggests that the chloride in gastric HCl may be provided by the putative Cl− channel Clic6 or parchorin (257, 273, 323).
B. Initial Isolation and Cloning
In 1973, the presence of a K+-stimulated ATPase in bullfrog gastric mucosa provided evidence for a gastric proton pump (116). Subsequently, in 1976, gastric acid secretion was demonstrated to result from electroneutral ATP-dependent exchange of hydrogen for potassium (321). In this seminal report, Sachs et al. (321) reported that H+/K+ exchange was electroneutral in isolated gastric membrane fractions from hogs. This study established a model for acid secretion that required functional H+-K+-ATPase in the presence of luminal K+ to exchange intracellular H+. Fractionation and electrophoresis of hog gastric mucosal homogenates yielded evidence for the presence of H+-K+-ATPase in gastric parietal cells by antibody immunostaining (320). Of the two membrane fractions, one was identified as transporting H+ and K+ and originating from the secretory canalicular structure of the parietal cell (320). Isolation of gastric vesicles from stimulated and resting gastric mucosae resulted in cell membrane fractions with profound differences in gastric microsome size, density, and K+ transport activity (424, 425). Wolosin and Forte (424, 425) postulated that during stimulation there is a transformation from small microsomal vesicles to larger, denser structures that correspond to H+-K+-ATPase-rich apical membranes. These studies provided evidence for the theory of morphological transformation of gastric oxyntic cells during stimulation in which an expanded apical membrane is generated by fusion of tubulovesicular membranes (107, 403, 424, 425). Furthermore, they extended our understanding of the localization of the gastric H+-K+-ATPase providing important information for future studies targeted at inhibiting gastric acid secretion.
In 1986, Shull and Lingrel (359) were the first to deduce the primary amino acid sequence of the α-subunits of the gastric H+-K+-ATPase from the cDNA sequence of rat proton pump. This was soon followed by characterization of the sequence of H+-K+-ATPase α-subunits in hog (235), rabbit (23), dog (366), and human (236). The α-subunits of the H+-K+-ATPase contain the catalytic sites and are comprised of 1,033–1,034 amino acid sequences with significant homology between species (98%) (24, 235, 236, 359, 366). The H+-K+-ATPase α-subunit H+-K+-ATPase is also comprised of 8–10 transmembrane spanning segments (23, 25, 253, 262). Originally, only the α-subunit was identified; however, studies demonstrated the presence of a β-subunit in the Na+-K+-ATPase, indicating a high likelihood that the H+-K+-ATPase similarly had another subunit (235, 246, 358, 359). In 1990, several laboratories confirmed the presence of the β-subunit of the H+-K+-ATPase (61, 281, 312, 357, 384). The β-subunit contains 291 amino acids with 6 or 7 N-linked glycosylation sites (312, 355, 357, 384). The α- and β-subunits of the gastric H+-K+-ATPase are assembled in the endoplasmic reticulum (ER). Experimental results in Xenopus oocytes demonstrated that the proton pump is only properly trafficked and functionally active when the α-subunit is assembled with the β-subunit (19, 33, 34, 121). Expression of the α-subunit of the gastric H+-K+-ATPase alone leads to retention in the ER and degradation (34, 201). The β-subunit of the H+-K+-ATPase stabilizes the H+-K+-ATPase and is required for proper targeting of the enzyme from the ER to the Golgi and the apical membrane, as well as proper maturation of the α-subunit for proton pump function (5, 120, 168, 179). The assembled enzyme, consisting of an α/β-heterodimer, is sorted from the trans-Golgi network and trafficked to the plasma membrane as a heterodimeric oligomer (2, 147, 353, 354, 392, 396, 397). In both nonpolarized and polarized cells, sole expression of the β-subunit does not affect its ability to mature and traffic to the plasma membrane (132, 318, 393).
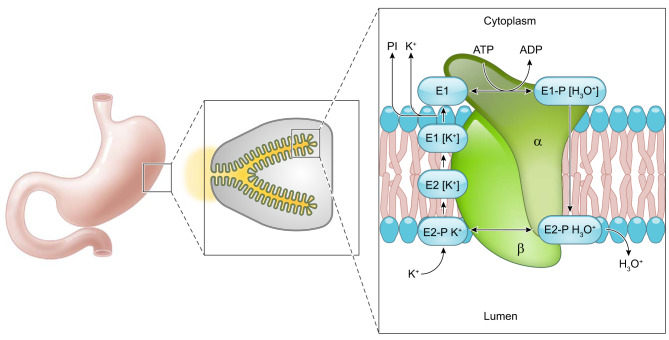
C. Functional Analysis
Parietal cells secrete acid through activation of the H+-K+-ATPase. This requires morphological changes in the cell to form the expanded canaliculi resulting from the fusion of tubulovesicles containing H+-K+-ATPase with the apical membrane and insertion of the pump into the canaliculi. Hydrolysis of ATP results in conformational changes in the gastric H+-K+-ATPase allowing for ion transport. Exchange of H+ and K+ results from conformational changes during the cycle of phosphorylation and dephosphorylation, which alters the orientation of the ion binding sites. The H+-K+-ATPase enzyme functions as an out of phase oligomeric heterodimer; therefore, if one heterodimer is in the E1 form, the other is necessarily in the E2 form (353) (FIGURE 5). The E1 conformation binds hydronium from the cytoplasmic side at high affinity (ion site in), while the E2 form (ion site out) has low H+ affinity and high affinity for K+ in the lumen. In the E1 conformation, a hydronium ion (H3O+) binds the cytoplasmic region of the H+-K+-ATPase enzyme. MgATP phosphorylates the catalytic subunit of the H+-K+-ATPase initiating a conformational change. The ion-binding site, which was previously oriented towards the cytoplasm, alters so that the hydronium ion is exposed to the extracytoplasmic region (the E2P form). In the E2P form, the H3O+ is released into the gastric lumen. Concomitantly, extracytoplasmic K+ binds the ion-binding site leading to dephosphorylation of the catalytic subunit to form the E2K conformation. The E2K form is converted to E1K with the potassium site now facing the cytoplasm. K+ is released from the ion-binding site following MgATP binding (47, 307, 371, 409) (FIGURE 5). The catalytic cycle of the H+-K+-ATPase allows for the pumping of hydronium ions out of parietal cells, while facilitating the uptake of K+ ions.
FIGURE 5.
Conformational changes of the H+-K+-ATPase. Phosphorylation and dephosphorylation of the gastric H+-K+-ATPase results in conformational changes facilitating the transport of H3O+ out of parietal cells concurrently with influx of K+. Initially, a hydronium ion binds the cytoplasmic surface of the H+-K+-ATPase and MgATP phosphorylates the protein to form the E1 conformation. In the E1 form, the ion-binding site faces the parietal cell cytoplasm. Next, the E1 form undergoes a conformational change to the E2 form where the ion binding site faces the gastric lumen. In this position the H3O+ is released into the gastric lumen. In this E2 conformation, K+ binds the ion site where H3O+ was previously bound. The enzyme is dephosphorylated, and a conformational change back to the E1 form results in the ion binding site facing the parietal cell cytoplasm where K+ is displaced by ATP binding (386, 387). Based on work on the Na+-K+-ATPase under physiological conditions, the E2 to E1 conformational transition of the unphosphorylated enzyme is postulated to be the rate-limiting step (232).
D. Proton Pump Inhibitors
Proton pump inhibitors (PPIs) are a class of compounds that inhibit gastric acid secretion through covalent binding of the H+-K+-ATPase. Timoprazole was the first PPI synthesized in 1975; however, omeprazole (Prilosec) was the first PPI used clinically in 1989. Generally, PPIs have a core structure that consists of substituted pyridylmethylsulfinyl benzimidazoles (101). The PPI timoprazole inhibited gastric acid secretion in vivo regardless of the stimulation pathway (dibutyryl cAMP, histamine, or ACh). However, timoprazole was ineffective in the absence of acid transport by the H+-K+-ATPase, although it could inhibit acid secretion in the presence of acid transport. The findings from these early experiments demonstrated that the initial PPIs were acid activated, an important step in determining the interaction of PPIs with the H+-K+-ATPase (408). PPIs were an improvement over H2-receptor antagonists, since the irreversible covalent binding of the H+-K+-ATPase pump results in a longer half-life of inhibition of gastric acid than for H2 receptor blockers (101, 323, 352, 356). PPIs accumulate in the secretory canaliculus of parietal cells after pyridine protonation. After a second protonation on the surface of the H+-K+-ATPase, PPIs are activated and form disulfide bonds with one or more accessible cysteines (351). All PPIs react with cysteine 813 on the α-subunit of the gastric H+-K+-ATPase. The reaction of PPIs with cysteine 813 arrests the H+-K+-ATPase enzyme in the E2 configuration. Different PPIs bind additional sites on the extracytoplasmic surface of the gastric H+-K+-ATPase α-subunit such as cysteine 892 for omeprazole and cysteine 822 for pantoprazole (42, 322, 350). The luminal exposure of cysteine 813 and 892 of the H+-K+-ATPase likely contributes to the reversibility of omeprazole (212). However, the covalent binding of PPIs with cysteine 822 located in the transport domain of the pump near ion binding sites results in inaccessibility to reducing agents due to the intramembranous location resulting in irreversibility of certain PPIs (42, 322, 350, 356, 394).
PPIs are weak bases. The weak base pKa of PPIs facilitates accumulation in the high acidity space in the secretory canaliculi of stimulated parietal cells or on the external surface of the H+-K+-ATPase pump. This attribute of PPIs is one of the reasons that they are so effective. The concentration of PPIs in the secretory canaliculus, where binding to the H+-K+-ATPase occurs, far exceeds the amount present in the blood (356). The initial protonation of pyridines in PPIs is followed by protonation of the benzimidazole moiety. This requirement for a second protonation of PPIs increases their therapeutic value because it allows for the conversion of the pro-drug to the active form in close proximity to the H+-K+-ATPase instead of in the lumen of the stomach. The protonation that regulates activation of PPIs results in irreversible binding of PPIs to accessible cysteines of the H+-K+-ATPase through disulfide bonds.
E. Potassium Competitive Acid Blockers
While the advent of PPIs was a significant advancement over the use of H2 receptor blockers, PPIs fail to meet the needs of several acid-related disorders including nonerosive reflux disease, severe erosive esophagitis, extra-esophageal reflux disease, NSAID ulcer, and nonvariceal upper gastrointestinal bleeding (87, 171, 196, 252, 389). Potassium competitive acid blockers (P-CAB) or acid pump antagonists (APAs) represent another type of proton pump inhibitor that may prove a more effective therapeutic for certain gastric acid-related disorders. This class of acid blockers results in a fast, effective, and reversible inhibition of gastric acid secretion (334, 398). P-CABs inhibit acid secretion by binding ionically to the H+-K+-ATPase following protonation. The large size of P-CABs likely prevents the access of K+ cations to their binding site, thus blocking activation of the H+-K+-ATPase by K+ (20, 21, 119, 392, 394, 395). Similar to other PPIs, P-CABs concentrate in the parietal cell canaliculi (119, 150). The accumulation of P-CABs in this highly acidic environment results in instantaneous protonation which facilitates ionic binding to the gastric H+-K+-ATPase and inhibition of acid secretion (20, 21, 119, 392, 394, 395). Administration of P-CABs results in a more rapid increase in intragastric pH and inhibits gastric acid secretion to a similar degree as PPIs (119, 441). However, unlike PPIs, P-CAB duration of inhibition of acid secretion is dependent on the level in the blood due to the reversible K+ competitive nature of P-CABs. While PPIs take repeat doses to reach full effect, P-CABs are fully effective after the first dose (16, 58, 167, 180, 184, 437). Thus P-CABs may represent another generation of gastric acid blockers to add to the compendium of therapeutic PPIs (12).
V. PARIETAL CELL LUMINAL POTASSIUM CHANNELS
Gastric acid secretion is dependent on K+. Lee et al. (223) were the first to develop an in vivo model that provided evidence for the dependence of acid secretion on the secretion of K+ into the gastric lumen. KCNQ1 knockout mice exhibit gastric mucosa hyperplasia, hypochlorhydria, and elevated levels of gastrin compared with controls. Moreover, KCNQ1 knockout mice harbored nonfunctional parietal cells, indicating that KCNQ1 was likely required for acid secretion. It was speculated that KCNQ1 maintains low levels of intracellular K+ through a K+ efflux channel to allow for exchange of H+ and K+ and thus acid secretion (223). Consistent with these findings, inhibition of the KCNQ1 channel in isolated rabbit gastric glands blocked acid secretion to a similar degree as histamine receptor antagonists and H+-K+-ATPase inhibitors (213). Thus KCNQ1 likely plays a critical role for K+ efflux during gastric acid secretion. Functionally, KCNQ1 and its regulatory subunit KCNE2 are proposed to form a luminal K+ channel (85, 134, 159). The interaction of KCNE2 with KCNQ1 results in a drastic change of KCNQ1 gating properties and current amplitude (383). KCNQ1 and KCNE2 are both highly expressed in parietal cells on the luminal membrane (85, 213). KCNQ1/KCNE2 K+ channels are stimulated by cAMP, and low extracellular pH was found to increase KCNQ1/KCNE2 current (158, 159). The KCNQ1/KCNE2 channel complex allows for the transformation of the voltage-dependent KCNQ1 current to a voltage-independent current (383). Without its regulatory subunit KCNE2, KCNQ1 is inhibited by a low extracellular pH (109, 159, 293). However, when KCNQ1 is in complex with KCNE2, K+ conductance increases in an acidic environment (109, 134, 159, 293). The importance of KCNE2 in acid secretion was demonstrated using KCNE2-deficient animals. Similar to KCNQ1 knockout mice, KCNE2 knockout mice showed dramatically decreased parietal cell proton secretion, altered parietal cell morphology, hyperplasia, and hypergastrinemia (316).
The exact localization of KCNE2/KCNQ1 remains controversial. Human KCNE2 and KCNQ1 are recovered on anti-H+-K+-ATPase-immunoisolated tubulovesicles (217). In contrast, Nguyen et al. (271) described the distinct localization of KCNQ1 in separate membrane compartments from H+-K+-ATPase in unstimulated parietal cells in wild-type mice and mice lacking the tubulovesicular membrane compartment in parietal cells (Atp4b-Y20A). In Atp4b-Y20A mice, the H+-K+-ATPase is anchored in the secretory canaliculi, and thus there are no H+-K+-ATPase-rich tubulovesicles in these mice. The Atp4b-20A mice demonstrated that the presence of H+-K+-ATPase at the secretory canaliculi is not sufficient to regulate acid secretion. Inhibition of KCNQ1 decreased acid secretion in wild-type as well as Atp4b-Y20A mice. These data suggested that trafficking of KCNQ1 to parietal cell canaliculi following stimulation might independently regulate gastric acid secretion. All of these studies and others provide convincing evidence that KCNQ1/KCNE2 form a luminal K+ channel that provides the extracellular K+ necessary for proper acid secretion (286, 368).
However, there are data to support a role for another K+ channel, the Kir family of inwardly rectifying K+ channels, in the regulation of gastric acid secretion and secretory membrane recycling. Fujita et al. (111) demonstrated that the Kir4.1, an inwardly rectifying K+ channel, was expressed on the apical membrane of parietal cells in rat gastric mucosa, suggesting a potential role for Kir4.1 in K+ recycling for proper H+-K+-ATPase activity. Other reports confirmed Fujita’s findings and demonstrated expression of Kir2.1 in parietal cells as well (197, 213, 240). Kir4.1 was also demonstrated to coprecipitate with H+-K+-ATPase from immunopurified tubulovesicles and stimulated secretory membranes (197). Work in Kir4.1-deficient mice demonstrated augmented acid secretion and suggested a role for Kir4.1 in secretory membrane recycling (367). Song et al. (367) suggested a role for parietal cell Kir4.1 channels in balancing rapid K+ loss via KCNQ1 and K+ absorption through the slower H+-K+-ATPase. Debate still exists as to the degree to which each individual K+ channel, KCNQ1/KCNE2 and Kir4.1, is responsible for apical K+ transport, but it appears clear that without functioning of either channel there is a reduction in gastric acid secretion (104, 160). Thus both channels likely play a role in the regulation of gastric acid secretion through distinct mechanisms.
VI. PARIETAL CELL MORPHOLOGICAL TRANSITION: THE MODEL FOR PLASMA MEMBRANE RECYCLING
The unusual cell morphology in the parietal cell was originally documented by Camillo Golgi, who found unusual wandering intracellular membranes, later designated as Muller-Golgi tubules, in silver stains of stomach mucosa (251). Electron microscopy documented the presence of an intracellular canaliculus in parietal cells that ramified within the parietal cell cytoplasm, but was contiguous with the lumen (45, 107). The parietal cell cytoplasm was also noted to have numerous tubulovesicular elements often in apposition to canalicular membranes. More impressive in electron microscopy studies was the morphological transformation that parietal cells underwent following stimulation, with marked expansion and dilation of the secretory canaliculi and the elongation of microvillar-like structures within canaliculi (44, 45, 106). Following acid secretory stimulation, in concert with the expansion of the canalicular network, there was a marked loss of tubulovesicular membranes within activated parietal cells. This morphological alteration is one of the largest reversible membrane alterations in mammalian cells.
The basis for the morphological transformation of parietal cells was contentious for a number of years. Some groups had suggested that the tubulovesicular network in parietal cells was connected to the intracellular canaliculus and that stimulation led to expansion of these elements through activation of the proton pump. In 1977, Forte et al. (105) first suggested a membrane recycling hypothesis to explain the morphological transitions in parietal cells (FIGURE 6). This hypothesis proposed that, in the resting parietal cell, tubulovesicles and the secretory canaliculus were distinct compartments. Forte et al. proposed that stimulation with histamine caused fusion of tubulovesicles with the canaliculus leading to the formation of elongated microvilli-like structures. Upon cessation of the stimulation, tubulovesicular membranes would be retrieved by endocytosis. This hypothesis was the first to suggest the existence of membrane recycling as a physiological process in cells. Rabbit gastric tubulovesicles could be isolated to high purity from resting gastric mucosa and were highly enriched for the α- and β-subunits of the H+-K+-ATPase, now designated as ATP4a and ATP4b, respectively (165, 222). One could also prepare rather enriched populations of stimulus-associated (SA) vesicles from stimulated parietal cells that contained the subunits of the H+-K+-ATPase along with putative canalicular F-actin (165). Further studies identified canalicular association of the F-actin binding phosphoprotein ezrin (152, 436), which was also enriched in SA vesicles. These latter studies suggested that ezrin defined the canalicular membranes, distinct from tubulovesicles.
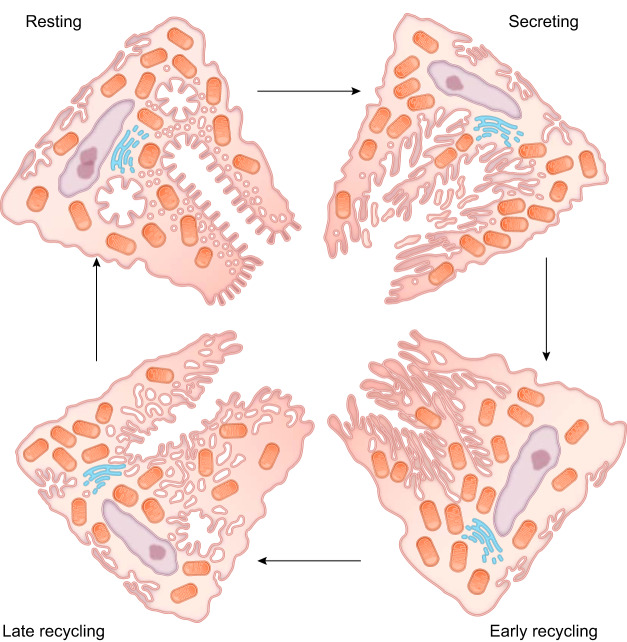
FIGURE 6.
Morphological changes in parietal cells that accompany gastric acid secretion. This graphic derived from the hand-drawn figure from John Forte depicts the morphological stages in the course of parietal cell stimulation (105). In the resting state, parietal cells contain many mitochondria, numerous membrane tubulovesicles, and collapsed intracellular canaliculi. Upon stimulation, exocytosis results in fusion of the H+-K+-ATPase rich tubulovesicles with the intracellular canaliculus, resulting in an expanded secretory canalicular membrane and the elongation of apical microvilli-like structures. Following stimulation, endocytosis occurs to retrieve the H+-K+-ATPase and recycle the membrane to tubulovesicles in the resting state and to prepare for the next stimulus and return.
The controversy over the role of tubulovesicle fusion in parietal cell morphological transformation was resolved with the identification of vesicle trafficking proteins associated with the canalicular membrane. First, early studies demonstrated the association of Rab small GTPases with rabbit parietal cell tubulovesicle membranes (28, 380). Then application of 3′-rapid amplification of cDNA ends (RACE) to parietal cells revealed a number of unrecognized Rab proteins including Rab11a, Rab25, and Rab14 (126). In particular, further studies on Rab11a revealed that it was highly enriched in parietal cells, and colocalized on tubulovesicles with H+-K+-ATPase (59, 127). Rab11a redistributed to the apical canaliculus in concert with the H+-K+-ATPase during stimulation (60). Immunoisolation of rabbit tubulovesicles with antibodies against the H+-K+-ATPase led to further insights into the association of trafficking proteins, first with the identification of SCAMPs as well as the vesicle SNARE protein VAMP-2 and target SNARE protein syntaxin3 on tubulovesicles and the observation of syntaxin1A on the membranes of the secretory canaliculus (54, 59, 60, 292). Immunoisolation of human tubulovesicles with H+-K+-ATPase antibodies confirmed these findings in rabbit membranes and also identified the presence of other vesicle trafficking proteins including VAMP8 and syntaxin7 in association with the proton-pump rich membranes (217). Together, these studies suggested that the tubulovesicular membranes and the secretory canaliculus did indeed represent the model system for large-scale regulated membrane recycling as proposed by Forte et al. Indeed, expression of a dominant negative Rab11a [Rab11a(N141I)] in parietal cells led to inhibition of acid secretion (91). In followup studies, alterations in VAMP2 and syntaxin3 also inhibited the process of H+-K+-ATPase translocation to the canalicular membranes (191, 229). It is also possible that other Rab proteins, in addition to Rab11a, are involved in H+-K+-ATPase recycling. For example, expression of dominant-negative Rab27b(N133I) can also inhibit parietal cell activation (372).
The recognition of Rab11a and v-SNAREs and t-SNAREs in association with the parietal cell secretory apparatus all supported the paradigm of regulated apical recycling in gastric parietal cells. Indeed, investigations utilizing parietal cell yeast-two hybrid libraries led to the identification of two critical families of Rab11-interacting proteins, the myosin V motors (MYO5A and MYO5B) as well as the Rab11-family interacting protein (Rab11-FIP) family (146, 218). Both Rab11-FIPs and MYO5B are highly expressed in parietal cells, but they were subsequently confirmed as critical regulators of apical recycling in polarized epithelial cells and generalized plasma membrane recycling in nonpolarized cells (22, 62, 146, 218). These findings therefore support the status of the parietal cell as the largest manifestation of apical plasma membrane recycling.
VII. PHYSIOLOGICAL ROLE OF PARIETAL CELLS IN GASTRIC MUCOSAL HOMEOSTASIS
Detailed investigations by Karam and Leblond (187–190) determined the patterns of cell differentiation from isthmal progenitor cells, with short-lived surface mucous cells differentiating towards the lumen and longer-lived parietal cells and mucous neck cells migrating towards the base. Mucous neck cells further redifferentiate into zymogen-secreting chief cells as they reach the gland base (190). While traditionally the focus on parietal cells has related to acid secretion, work over the past decade has brought increased recognition of the function of parietal cells in general mucosal homeostasis. Loss of parietal cells, or oxyntic atrophy, is the pathological finding most associated with gastric cancer (97). Multiple studies have now connected the loss of parietal cells with the development of metaplasia in the corpus mucosa. In part, based on this association, a number of investigators have evaluated roles for parietal cells other than acid secretion.
Loss of parietal cells occurs during infection of the stomach with Helicobacter species. The cause of parietal cell loss in the face of Helicobacter infection remains unclear. Loss of parietal cells can be replicated in rodent models with chronic infection with either Helicobacter pylori or Helicobacter felis (108, 291, 349, 413, 414). The loss of parietal cells appears to require the action of T cells, since T cell-deficient mice do not display oxyntic atrophy in response to Helicobacter felis infection (317). More recent studies have suggested that cytokines individually or together may lead to parietal cell death (57, 169). IL-17A induces loss of parietal cells in mouse models (48).
Experimental loss of parietal cells can be induced acutely with parietal cell-toxic drugs (124, 170, 265, 275). In these acute models, the loss of parietal cells is associated with prominent changes in gastric mucosal lineages. Foveolar hyperplasia (the expansion of surface mucous cells) develops rapidly after parietal cell loss, driven to a great extent by elevations in gastrin (275, 291). At the same time, spasmolytic polypeptide-expressing metaplasia (SPEM) develops through transdifferentiation of chief cells into mucous cell metaplasia (170, 265). The exact signals that coordinate these lineage changes remain unclear, and a recent publication indicates that parietal cell loss alone is not sufficient to induce SPEM (55). Interactions of parietal cells with intrinsic immune cells may lead to release of intermediate cytokine regulators (157, 294, 295) that are also required for induction of SPEM.
A. EGF Receptor Ligands
As noted above, EGF receptor ligands, including EGF and TGF-α, are inhibitors of acid secretion (225, 226). Other studies suggested that parietal cells were actually the major source of TGF-α in the gastric mucosa (30). In addition, other studies have demonstrated that parietal cells are also a source for amphiregulin (3) and HB-EGF (263), both potent ligands for the EGF receptor. These findings suggested that EGF receptor ligands were released from parietal cells in part as autocrine inhibitors of gastric acid secretion. Nevertheless, other investigations demonstrated that TGF-α could promote the expansion of surface cell lineages at the expense of glandular lineages such as parietal cells. The greatest impact of these growth factors on lineage derivation is observed in patients with Ménétrier’s disease, who show marked overproduction of TGF-α in the gastric mucosa, resulting in massive foveolar hyperplasia as well as loss of parietal cells and other glandular cells (86). Similar results have been reported in metallothionein (MT)-TGF-α transgenic mice following administration of oral zinc to induce TGF-α overexpression (49, 86, 125, 274). More recent investigations have suggested that expression of active Kras in isthmal progenitor cells also induces expansion of foveolar cells at the expense of glandular (parietal cell and chief cell) lineage differentiation (75, 249).
B. Sonic Hedgehog
Members of the sonic hedgehog (Shh) family regulate lineage development in many organs (234). Treatment of isolated dog parietal cells with EGF led to upregulated expression of Shh in parietal cells, and Shh promoted histamine-stimulated acid secretion (369). Later studies suggested that Shh was released from parietal cells in concert with the fusion of tubulovesicles with the apical canaliculus (444), and Shh is processed into an active form by pepsinogen (445). Shh release has been implicated in regulation of stomach cell lineages through feedback regulation of acid sensing (256). Loss of expression of Shh in gastric parietal cells leads to elevations of gastrin and foveolar hyperplasia (432). Similarly, inhibition of acid secretion by proton pump inhibitors or IL-1β decreases the expression of Shh (250, 405) and can be associated with oxyntic atrophy associated with H. pylori infection (344). Indeed, Shh appears to play a critical role in regulating mucosal homeostasis in the stomach (88), although the context of these effects appears variable. In mice with deletion of Shh in parietal cells, H. pylori infection does not lead to oxyntic atrophy and gastritis (344). Nevertheless, loss of Shh in parietal cells inhibits the resolution of acute acetic acid ulcers in the stomach (431). Overall, expression of Shh and perhaps other hedgehog family members appears critical for mucosal restitution and mucosal protection.
VIII. REMAINING QUESTIONS FOR ACID SECRETORY PHYSIOLOGY
Despite a number of prominent investigations during the past 30 yr, the exact cellular mechanisms that trigger second messenger-regulated acid secretion remain unknown. Although phosphoproteins associated with histamine and cholinergic stimulation have been identified (70, 72–74, 153, 259, 279, 287, 288, 390, 391, 416), the connection of these phosphorylation events to the induction of tubulovesicle fusion with the secretory canaliculus remains solely correlative. Thus alterations in the expression of ezrin or Lasp-1, the LIM and SH3 domain protein, can affect the fusion of tubulovesicles with the canaliculus in parietal cells, but the mechanisms of how these proteins act at a molecular level to promote regulated membrane fusion remain undetermined. No mouse models targeting phosphorylation sites have been characterized, so functional data remain sparse. Of interest, a recent study has demonstrated that a calcium channel resident in tubulovesicles, TRPML1 (ML1), is involved in cAMP-dependent stimulation of tubulovesicle fusion with the canaliculus (325). This finding suggests that discrete targets mediating the process of membrane fusion remain to be definitively identified.
Another question relates to the sensing of luminal pH in the stomach and how this regulates acid secretion. It has previously been assumed that G cells in the antrum or even ECL cells throughout the corpus might be able to sense the gastric lumen and respond to changes in acidity. Thus loss of acid secretion, which leads to increases in gastrin levels, would be triggered through G cells sensing of the lumen pH. Nevertheless, recent identification of classes of tuft cells, which present a highly specialized sensory apparatus towards the lumen (172, 330), has raised questions of whether tuft cells are a major sensor of luminal contents, including acid. Tuft cells are normally associated with the neck region of the corpus gastric glands and near the progenitor zone in the antrum (329). Importantly, following loss of parietal cells in gastritis models of mice, there is an increase in the number of tuft cells in the gastric mucosa (76, 266, 329). This increase in tuft cell numbers appears to be dependent on gastrin levels (76). Tuft cells can have direct connections with sensory nerves in multiple organs (332, 333), although these direct connections have not been validated in the stomach. Such intrinsic nerve connections could be involved in regulation of gastrin and histamine release from enteroendocrine cells. Tuft cells are known to release IL-25, which may stimulate the release of IL-13 and amphiregulin from intrinsic mucosal immune ILC2 cells (404). IL-13 and amphiregulin may have prominent effects on both lineage derivation and acid secretion in the gastric mucosa (294). Further studies are required to define the role of tuft cell chemosensing to acid secretory physiology.
Finally, the full compendium of growth factors that can be released from parietal cells remains unclear. Because of the long-lived status of parietal cells, it seems likely that this lineage is well placed to perform broad duties in the coordination of lineage maturation in the corpus of the stomach.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants R01 DK48370, RO1 DK101332, and R01 DK70856 and Department of Veterans Affairs Merit Review 1I01BX000930 (to J. R. Goldenring) and a gift from the Christine Volpe Fund. A. C. Engevik was supported by NIH Grants F32 DK111101 and T32 CA106183.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
This review is in honor of John G. Forte.
We thank Annie Meyer for assistance with figure graphics.
A. C. Engevik and I. Kaji contributed equally to this work.
Address for reprint requests and other correspondence: J. R. Goldenring, Epithelial Biology Center, Vanderbilt University Medical Center, 10435 Medical Research Building IV, 2213 Garland Ave., Nashville, TN 37232 (e-mail: jim.goldenring@vanderbilt.edu).
REFERENCES
- 1.Aadland E, Berstad A, Semb LS. Effect of cimetidine on pentagastrin-stimulated gastric secretion in healthy man. Scand J Gastroenterol 12: 501–506, 1977. doi: 10.3109/00365527709181695. [DOI] [PubMed] [Google Scholar]
- 2.Abe K, Kaya S, Taniguchi K, Hayashi Y, Imagawa T, Kikumoto M, Oiwa K, Sakaguchi K. Evidence for a relationship between activity and the tetraprotomeric assembly of solubilized pig gastric H/K-ATPase. J Biochem 138: 293–301, 2005. doi: 10.1093/jb/mvi127. [DOI] [PubMed] [Google Scholar]
- 3.Abe S, Sasano H, Katoh K, Ohara S, Arikawa T, Noguchi T, Asaki S, Yasui W, Tahara E, Nagura H, Toyota T. Immunohistochemical studies on EGF family growth factors in normal and ulcerated human gastric mucosa. Dig Dis Sci 42: 1199–1209, 1997. doi: 10.1023/A:1018897922644. [DOI] [PubMed] [Google Scholar]
- 4.Abramovitz M, Adam M, Boie Y, Carrière M, Denis D, Godbout C, Lamontagne S, Rochette C, Sawyer N, Tremblay NM, Belley M, Gallant M, Dufresne C, Gareau Y, Ruel R, Juteau H, Labelle M, Ouimet N, Metters KM. The utilization of recombinant prostanoid receptors to determine the affinities and selectivities of prostaglandins and related analogs. Biochim Biophys Acta 1483: 285–293, 2000. doi: 10.1016/S1388-1981(99)00164-X. [DOI] [PubMed] [Google Scholar]
- 5.Ackermann U, Geering K. Mutual dependence of Na,K-ATPase alpha- and beta-subunits for correct posttranslational processing and intracellular transport. FEBS Lett 269: 105–108, 1990. doi: 10.1016/0014-5793(90)81130-G. [DOI] [PubMed] [Google Scholar]
- 6.Adriaenssens A, Lam BY, Billing L, Skeffington K, Sewing S, Reimann F, Gribble F. A Transcriptome-Led Exploration of Molecular Mechanisms Regulating Somatostatin-Producing D-Cells in the Gastric Epithelium. Endocrinology 156: 3924–3936, 2015. doi: 10.1210/en.2015-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahn KY, Kone BC. Expression and cellular localization of mRNA encoding the “gastric” isoform of H+-K+-ATPase alpha-subunit in rat kidney. Am J Physiol Renal Physiol 268: F99–F109, 1995. doi: 10.1152/ajprenal.1995.268.1.F99. [DOI] [PubMed] [Google Scholar]
- 8.Aihara T, Fujishita T, Kanatani K, Furutani K, Nakamura E, Taketo MM, Matsui M, Chen D, Okabe S. Impaired gastric secretion and lack of trophic responses to hypergastrinemia in M3 muscarinic receptor knockout mice. Gastroenterology 125: 1774–1784, 2003. doi: 10.1053/j.gastro.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 9.Aihara T, Nakamura Y, Taketo MM, Matsui M, Okabe S. Cholinergically stimulated gastric acid secretion is mediated by M3 and M5 but not M1 muscarinic acetylcholine receptors in mice. Am J Physiol Gastrointest Liver Physiol 288: G1199–G1207, 2005. doi: 10.1152/ajpgi.00514.2004. [DOI] [PubMed] [Google Scholar]
- 10.Allen A, Hutton A, Leonard AJ, Pearson JP, Sellers LA. The role of mucus in the protection of the gastroduodenal mucosa. Scand J Gastroenterol 21, Suppl 125: 71–78, 1986. doi: 10.3109/00365528609093820. [DOI] [PubMed] [Google Scholar]
- 11.Allen JP, Canty AJ, Schulz S, Humphrey PP, Emson PC, Young HM. Identification of cells expressing somatostatin receptor 2 in the gastrointestinal tract of Sstr2 knockout/lacZ knockin mice. J Comp Neurol 454: 329–340, 2002. doi: 10.1002/cne.10466. [DOI] [PubMed] [Google Scholar]
- 12.Andersson K, Carlsson E. Potassium-competitive acid blockade: a new therapeutic strategy in acid-related diseases. Pharmacol Ther 108: 294–307, 2005. doi: 10.1016/j.pharmthera.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Andersson S, Chang D, Folkers K, Rosell S. Inhibition of gastric acid secretion in dogs by neurotensin. Life Sci 19: 367–370, 1976. doi: 10.1016/0024-3205(76)90040-0. [DOI] [PubMed] [Google Scholar]
- 14.Andersson S, Rosell S, Sjödin L, Folkers K. Inhibition of acid secretion from vagally innervated and denervated gastric pouches by (Gln4)-neurotensin. Scand J Gastroenterol 15: 253–256, 1980. doi: 10.3109/00365528009181465. [DOI] [PubMed] [Google Scholar]
- 15.Anlauf M, Schäfer MK, Eiden L, Weihe E. Chemical coding of the human gastrointestinal nervous system: cholinergic, VIPergic, and catecholaminergic phenotypes. J Comp Neurol 459: 90–111, 2003. doi: 10.1002/cne.10599. [DOI] [PubMed] [Google Scholar]
- 16.Arikawa Y, Nishida H, Kurasawa O, Hasuoka A, Hirase K, Inatomi N, Hori Y, Matsukawa J, Imanishi A, Kondo M, Tarui N, Hamada T, Takagi T, Takeuchi T, Kajino M. Discovery of a novel pyrrole derivative 1-[5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine fumarate (TAK-438) as a potassium-competitive acid blocker (P-CAB). J Med Chem 55: 4446–4456, 2012. doi: 10.1021/jm300318t. [DOI] [PubMed] [Google Scholar]
- 17.Arin RM, Vallejo AI, Rueda Y, Fresnedo O, Ochoa B. The A2B adenosine receptor colocalizes with adenosine deaminase in resting parietal cells from gastric mucosa. Biochemistry (Mosc) 80: 120–125, 2015. doi: 10.1134/S0006297915010149. [DOI] [PubMed] [Google Scholar]
- 18.Arin RM, Vallejo AI, Rueda Y, Fresnedo O, Ochoa B. Stimulation of gastric acid secretion by rabbit parietal cell A(2B) adenosine receptor activation. Am J Physiol Cell Physiol 309: C823–C834, 2015. doi: 10.1152/ajpcell.00224.2015. [DOI] [PubMed] [Google Scholar]
- 19.Asano S, Kawada K, Kimura T, Grishin AV, Caplan MJ, Takeguchi N. The roles of carbohydrate chains of the beta-subunit on the functional expression of gastric H+,K+-ATPase. J Biol Chem 275: 8324–8330, 2000. doi: 10.1074/jbc.275.12.8324. [DOI] [PubMed] [Google Scholar]
- 20.Asano S, Matsuda S, Hoshina S, Sakamoto S, Takeguchi N. A chimeric gastric H+,K+-ATPase inhibitable with both ouabain and SCH 28080. J Biol Chem 274: 6848–6854, 1999. doi: 10.1074/jbc.274.11.6848. [DOI] [PubMed] [Google Scholar]
- 21.Asano S, Yoshida A, Yashiro H, Kobayashi Y, Morisato A, Ogawa H, Takeguchi N, Morii M. The cavity structure for docking the K+-competitive inhibitors in the gastric proton pump. J Biol Chem 279: 13968–13975, 2004. doi: 10.1074/jbc.M308934200. [DOI] [PubMed] [Google Scholar]
- 22.Baetz NW, Goldenring JR. Rab11-family interacting proteins define spatially and temporally distinct regions within the dynamic Rab11a-dependent recycling system. Mol Biol Cell 24: 643–658, 2013. doi: 10.1091/mbc.e12-09-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bamberg K, Mercier F, Reuben MA, Kobayashi Y, Munson KB, Sachs G. cDNA cloning and membrane topology of the rabbit gastric H+/K+-ATPase alpha-subunit. Biochim Biophys Acta 1131: 69–77, 1992. doi: 10.1016/0167-4781(92)90100-E. [DOI] [PubMed] [Google Scholar]
- 24.Bamberg K, Nylander S, Helander KG, Lundberg LG, Sachs G, Helander HF. In situ hybridization of mRNA for the gastric H+,K+-ATPase in rat oxyntic mucosa. Biochim Biophys Acta 1190: 355–359, 1994. doi: 10.1016/0005-2736(94)90094-9. [DOI] [PubMed] [Google Scholar]
- 25.Bamberg K, Sachs G. Topological analysis of H+,K+-ATPase using in vitro translation. J Biol Chem 269: 16909–16919, 1994. [PubMed] [Google Scholar]
- 26.Barnett K, Bell CJ, McKnight W, Dicay M, Sharkey KA, Wallace JL. Role of cyclooxygenase-2 in modulating gastric acid secretion in the normal and inflamed rat stomach. Am J Physiol Gastrointest Liver Physiol 279: G1292–G1297, 2000. doi: 10.1152/ajpgi.2000.279.6.G1292. [DOI] [PubMed] [Google Scholar]
- 27.Barros D’Sa AA, Bloom SR, Baron JH. Direct inhibition of gastric acid by growth-hormone release-inhibiting hormone in dogs. Lancet 305: 886–887, 1975. doi: 10.1016/S0140-6736(75)91685-2. [DOI] [PubMed] [Google Scholar]
- 28.Basson MD, Goldenring JR, Tang LH, Lewis JJ, Padfield P, Jamieson JD, Modlin IM. Redistribution of 23 kDa tubulovesicle-associated GTP-binding proteins during parietal cell stimulation. Biochem J 279: 43–48, 1991. doi: 10.1042/bj2790043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beales IL, Calam J. Interleukin 1 beta and tumour necrosis factor alpha inhibit acid secretion in cultured rabbit parietal cells by multiple pathways. Gut 42: 227–234, 1998. doi: 10.1136/gut.42.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beauchamp RD, Barnard JA, McCutchen CM, Cherner JA, Coffey RJ Jr. Localization of transforming growth factor alpha and its receptor in gastric mucosal cells. Implications for a regulatory role in acid secretion and mucosal renewal. J Clin Invest 84: 1017–1023, 1989. doi: 10.1172/JCI114223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beaumont W. Further experiments on the case of Alexis St. Martin, who was wounded in the stomach by a load of buckshot, detailed in the Recorder for 1825. Med Recorder 9: 94–97, 1826. [Google Scholar]
- 32.Befrits R, Johansson C. Oral PGE2 inhibits gastric acid secretion in man. Prostaglandins 29: 143–152, 1985. doi: 10.1016/0090-6980(85)90159-5. [DOI] [PubMed] [Google Scholar]
- 33.Beggah AT, Béguin P, Bamberg K, Sachs G, Geering K. Beta-subunit assembly is essential for the correct packing and the stable membrane insertion of the H,K-ATPase alpha-subunit. J Biol Chem 274: 8217–8223, 1999. doi: 10.1074/jbc.274.12.8217. [DOI] [PubMed] [Google Scholar]
- 34.Béguin P, Hasler U, Staub O, Geering K. Endoplasmic reticulum quality control of oligomeric membrane proteins: topogenic determinants involved in the degradation of the unassembled Na,K-ATPase alpha subunit and in its stabilization by beta subunit assembly. Mol Biol Cell 11: 1657–1672, 2000. doi: 10.1091/mbc.11.5.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beil W, Birkholz C, Wagner S, Sewing K-F. Interaction of Helicobacter pylori and its fatty acids with parietal cells and gastric H+/K+-ATPase. Gut 35: 1176–1180, 1994. doi: 10.1136/gut.35.9.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bennett A, Stamford IF, Unger WG. Prostaglandin E2 and gastric acid secretion in man. J Physiol 229: 349–360, 1973. doi: 10.1113/jphysiol.1973.sp010142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bentley PH, Kenner GW, Sheppard RC. Structures of human gastrins I and II. Nature 209: 583–585, 1966. doi: 10.1038/209583b0. [DOI] [PubMed] [Google Scholar]
- 38.Berg A, Kechagias S, Sjöstrand SE, Ericson AC, Berg A, Kechagias S, Sjöstr SE. Morphological support for paracrine inhibition of gastric acid secretion by nitric oxide in humans. Scand J Gastroenterol 36: 1016–1021, 2001. doi: 10.1080/003655201750422594. [DOI] [PubMed] [Google Scholar]
- 39.Berg A, Redéen S, Grenegård M, Ericson AC, Sjöstrand SE. Nitric oxide inhibits gastric acid secretion by increasing intraparietal cell levels of cGMP in isolated human gastric glands. Am J Physiol Gastrointest Liver Physiol 289: G1061–G1066, 2005. doi: 10.1152/ajpgi.00230.2005. [DOI] [PubMed] [Google Scholar]
- 40.Berglindh T. Effects of common inhibitors of gastric acid secretion on secretagogue-induced respiration and aminopyrine accumulation in isolated gastric glands. Biochim Biophys Acta 464: 217–233, 1977. doi: 10.1016/0005-2736(77)90383-2. [DOI] [PubMed] [Google Scholar]
- 41.Berthoud HR, Laughton WB, Powley TL. Vagal stimulation-induced gastric acid secretion in the anesthetized rat. J Auton Nerv Syst 16: 193–204, 1986. doi: 10.1016/0165-1838(86)90025-1. [DOI] [PubMed] [Google Scholar]
- 42.Besancon M, Shin JM, Mercier F, Munson K, Miller M, Hersey S, Sachs G. Membrane topology and omeprazole labeling of the gastric hydrogen ion-potassium-adenosinetriphosphatase. Biochemistry 32: 2345–2355, 1993. doi: 10.1021/bi00060a028. [DOI] [PubMed] [Google Scholar]
- 43.Birsen İ, Gemici B, Acar N, Üstünel İ, İzgüt-Uysal VN. The role of apelin in the healing of water-immersion and restraint stress-induced gastric damage. J Physiol Sci 67: 373–385, 2017. doi: 10.1007/s12576-016-0469-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Black JA, Forte TM, Forte JG. The effects of microfilament disrupting agents on HCl secretion and ultrastructure of piglet gastric oxyntic cells. Gastroenterology 83: 595–604, 1982. [PubMed] [Google Scholar]
- 45.Black JA, Forte TM, Forte JG. Structure of oxyntic cell membranes during conditions of rest and secretion of HCl as revealed by freeze-fracture. Anat Rec 196: 163–172, 1980. doi: 10.1002/ar.1091960206. [DOI] [PubMed] [Google Scholar]
- 46.Black JW, Duncan WA, Durant CJ, Ganellin CR, Parsons EM. Definition and antagonism of histamine H2-receptors. Nature 236: 385–390, 1972. doi: 10.1038/236385a0. [DOI] [PubMed] [Google Scholar]
- 47.Blostein R, Dunbar L, Mense M, Scanzano R, Wilczynska A, Caplan MJ. Cation selectivity of gastric H,K-ATPase and Na,K-ATPase chimeras. J Biol Chem 274: 18374–18381, 1999. doi: 10.1074/jbc.274.26.18374. [DOI] [PubMed] [Google Scholar]
- 48.Bockerstett KA, Osaki LH, Petersen CP, Cai CW, Wong CF, Nguyen TM, Ford EL, Hoft DF, Mills JC, Goldenring JR, DiPaolo RJ. Interleukin-17A Promotes Parietal Cell Atrophy by Inducing Apoptosis. Cell Mol Gastroenterol Hepatol 5: 678–690.e1, 2018. doi: 10.1016/j.jcmgh.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bockman DE, Sharp R, Merlino G. Regulation of terminal differentiation of zymogenic cells by transforming growth factor alpha in transgenic mice. Gastroenterology 108: 447–454, 1995. doi: 10.1016/0016-5085(95)90073-X. [DOI] [PubMed] [Google Scholar]
- 50.Boel E, Vuust J, Norris F, Norris K, Wind A, Rehfeld JF, Marcker KA. Molecular cloning of human gastrin cDNA: evidence for evolution of gastrin by gene duplication. Proc Natl Acad Sci USA 80: 2866–2869, 1983. doi: 10.1073/pnas.80.10.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Broide E, Bloch O, Ben-Yehudah G, Cantrell D, Shirin H, Rapoport MJ. GLP-1 receptor is expressed in human stomach mucosa: analysis of its cellular association and distribution within gastric glands. J Histochem Cytochem 61: 649–658, 2013. doi: 10.1369/0022155413497586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brown JC, Cook MA, Dryburgh JR. Motilin, a gastric motor activity stimulating polypeptide: the complete amino acid sequence. Can J Biochem 51: 533–537, 1973. doi: 10.1139/o73-066. [DOI] [PubMed] [Google Scholar]
- 53.Brown JF, Hanson PJ, Whittle BJ. Nitric oxide donors increase mucus gel thickness in rat stomach. Eur J Pharmacol 223: 103–104, 1992. doi: 10.1016/0014-2999(92)90824-N. [DOI] [PubMed] [Google Scholar]
- 54.Brown MR, Chew CS. Carbachol-induced protein phosphorylation in parietal cells: regulation by [Ca2+]i. Am J Physiol Gastrointest Liver Physiol 257: G99–G110, 1989. doi: 10.1152/ajpgi.1989.257.1.G99. [DOI] [PubMed] [Google Scholar]
- 55.Burclaff J, Osaki LH, Liu D, Goldenring JR, Mills JC. Targeted Apoptosis of Parietal Cells Is Insufficient to Induce Metaplasia in Stomach. Gastroenterology 152: 762–766.e7, 2017. doi: 10.1053/j.gastro.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Busada JT, Ramamoorthy S, Cain DW, Xu X, Cook DN, Cidlowski JA. Endogenous glucocorticoids prevent gastric metaplasia by suppressing spontaneous inflammation. J Clin Invest 129: 1345–1358, 2019. doi: 10.1172/JCI123233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Buzzelli JN, Chalinor HV, Pavlic DI, Sutton P, Menheniott TR, Giraud AS, Judd LM. IL33 Is a Stomach Alarmin That Initiates a Skewed Th2 Response to Injury and Infection. Cell Mol Gastroenterol Hepatol 1: 203–221.e3, 2015. doi: 10.1016/j.jcmgh.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bytzer P, Morocutti A, Kennerly P, Ravic M, Miller N; ROSE Trial Investigators . Effect of rabeprazole and omeprazole on the onset of gastro-oesophageal reflux disease symptom relief during the first seven days of treatment. Scand J Gastroenterol 41: 1132–1140, 2006. doi: 10.1080/00365520600615781. [DOI] [PubMed] [Google Scholar]
- 59.Calhoun BC, Goldenring JR. Two Rab proteins, vesicle-associated membrane protein 2 (VAMP-2) and secretory carrier membrane proteins (SCAMPs), are present on immunoisolated parietal cell tubulovesicles. Biochem J 325: 559–564, 1997. doi: 10.1042/bj3250559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Calhoun BC, Lapierre LA, Chew CS, Goldenring JR. Rab11a redistributes to apical secretory canaliculus during stimulation of gastric parietal cells. Am J Physiol Cell Physiol 275: C163–C170, 1998. doi: 10.1152/ajpcell.1998.275.1.C163. [DOI] [PubMed] [Google Scholar]
- 61.Canfield VA, Okamoto CT, Chow D, Dorfman J, Gros P, Forte JG, Levenson R. Cloning of the H,K-ATPase beta subunit. Tissue-specific expression, chromosomal assignment, and relationship to Na,K-ATPase beta subunits. J Biol Chem 265: 19878–19884, 1990. [PubMed] [Google Scholar]
- 62.Casanova JE, Wang X, Kumar R, Bhartur SG, Navarre J, Woodrum JE, Altschuler Y, Ray GS, Goldenring JR. Association of Rab25 and Rab11a with the apical recycling system of polarized Madin-Darby canine kidney cells. Mol Biol Cell 10: 47–61, 1999. doi: 10.1091/mbc.10.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chapman K, Holmes M, Seckl J. 11β-Hydroxysteroid dehydrogenases: intracellular gate-keepers of tissue glucocorticoid action. Physiol Rev 93: 1139–1206, 2013. doi: 10.1152/physrev.00020.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chatzaki E, Charalampopoulos I, Leontidis C, Mouzas IA, Tzardi M, Tsatsanis C, Margioris AN, Gravanis A. Urocortin in human gastric mucosa: relationship to inflammatory activity. J Clin Endocrinol Metab 88: 478–483, 2003. doi: 10.1210/jc.2002-020853. [DOI] [PubMed] [Google Scholar]
- 65.Chatzaki E, Murphy BJ, Wang L, Million M, Ohning GV, Crowe PD, Petroski R, Taché Y, Grigoriadis DE. Differential profile of CRF receptor distribution in the rat stomach and duodenum assessed by newly developed CRF receptor antibodies. J Neurochem 88: 1–11, 2004. doi: 10.1046/j.1471-4159.2003.02078.x. [DOI] [PubMed] [Google Scholar]
- 66.Chen D, Zhao CM, Andersson K, Meister B, Panula P, Håkanson R. ECL cell morphology. Yale J Biol Med 71: 217–231, 1998. [PMC free article] [PubMed] [Google Scholar]
- 67.Cheng I, Qureshi I, Chattopadhyay N, Qureshi A, Butters RR, Hall AE, Cima RR, Rogers KV, Hebert SC, Geibel JP, Brown EM, Soybel DI. Expression of an extracellular calcium-sensing receptor in rat stomach. Gastroenterology 116: 118–126, 1999. doi: 10.1016/S0016-5085(99)70235-0. [DOI] [PubMed] [Google Scholar]
- 68.Chew CS. Inhibitory action of somatostatin on isolated gastric glands and parietal cells. Am J Physiol Gastrointest Liver Physiol 245: G221–G229, 1983. doi: 10.1152/ajpgi.1983.245.2.G221. [DOI] [PubMed] [Google Scholar]
- 69.Chew CS. Parietal cell protein kinases. Selective activation of type I cAMP-dependent protein kinase by histamine. J Biol Chem 260: 7540–7550, 1985. [PubMed] [Google Scholar]
- 70.Chew CS, Chen X, Bollag RJ, Isales C, Ding KH, Zhang H. Targeted disruption of the Lasp-1 gene is linked to increases in histamine-stimulated gastric HCl secretion. Am J Physiol Gastrointest Liver Physiol 295: G37–G44, 2008. doi: 10.1152/ajpgi.90247.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chew CS, Hersey SJ. Gastrin stimulation of isolated gastric glands. Am J Physiol Gastrointest Liver Physiol 242: G504–G512, 1982. [DOI] [PubMed] [Google Scholar]
- 72.Chew CS, Parente JA Jr, Zhou C, Chen X. PKC activation increases ezrin phosphorylation in gastric parietal cells. FASEB J 11: 1717, 1997. [Google Scholar]
- 73.Chew CS, Parente JA Jr, Zhou C, Baranco E, Chen X. Lasp-1 is a regulated phosphoprotein within the cAMP signaling pathway in the gastric parietal cell. Am J Physiol Cell Physiol 275: C56–C67, 1998. doi: 10.1152/ajpcell.1998.275.1.C56. [DOI] [PubMed] [Google Scholar]
- 74.Chew CS, Parente JA Jr, Chen X, Chaponnier C, Cameron RS. The LIM and SH3 domain-containing protein, lasp-1, may link the cAMP signaling pathway with dynamic membrane restructuring activities in ion transporting epithelia. J Cell Sci 113: 2035–2045, 2000. [DOI] [PubMed] [Google Scholar]
- 75.Choi E, Means AL, Coffey RJ, Goldenring JR. Active Kras Expression in Gastric Isthmal Progenitor Cells Induces Foveolar Hyperplasia but Not Metaplasia. Cell Mol Gastroenterol Hepatol 7: 251–253.e1, 2019. doi: 10.1016/j.jcmgh.2018.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Choi E, Petersen CP, Lapierre LA, Williams JA, Weis VG, Goldenring JR, Nam KT. Dynamic expansion of gastric mucosal doublecortin-like kinase 1-expressing cells in response to parietal cell loss is regulated by gastrin. Am J Pathol 185: 2219–2231, 2015. doi: 10.1016/j.ajpath.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Choi E, Roland JT, Barlow BJ, O’Neal R, Rich AE, Nam KT, Shi C, Goldenring JR. Cell lineage distribution atlas of the human stomach reveals heterogeneous gland populations in the gastric antrum. Gut 63: 1711–1720, 2014. doi: 10.1136/gutjnl-2013-305964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chow DC, Forte JG. Functional significance of the beta-subunit for heterodimeric P-type ATPases. J Exp Biol 198: 1–17, 1995. [DOI] [PubMed] [Google Scholar]
- 79.Cima I, Corazza N, Dick B, Fuhrer A, Herren S, Jakob S, Ayuni E, Mueller C, Brunner T. Intestinal epithelial cells synthesize glucocorticoids and regulate T cell activation. J Exp Med 200: 1635–1646, 2004. doi: 10.1084/jem.20031958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Clarke JS, Storer EH, Dragstedt LR. The effects of vagotomy on the physiology of the stomach in patients with peptic ulcer. J Clin Invest 26: 784–795, 1947. doi: 10.1172/JCI101861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cooke AR, Preshaw RM, Grossman ML. Effect of adrenalectomy and glucocorticoids on the secretion and absorption of hydrogen ion. Gastroenterology 50: 761–767, 1966. doi: 10.1016/S0016-5085(66)80004-5. [DOI] [PubMed] [Google Scholar]
- 82.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 50: 1714–1719, 2001. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 83.Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 141: 4255–4261, 2000. doi: 10.1210/endo.141.11.7757. [DOI] [PubMed] [Google Scholar]
- 84.Date Y, Nakazato M, Murakami N, Kojima M, Kangawa K, Matsukura S. Ghrelin acts in the central nervous system to stimulate gastric acid secretion. Biochem Biophys Res Commun 280: 904–907, 2001. doi: 10.1006/bbrc.2000.4212. [DOI] [PubMed] [Google Scholar]
- 85.Dedek K, Waldegger S. Colocalization of KCNQ1/KCNE channel subunits in the mouse gastrointestinal tract. Pflugers Arch 442: 896–902, 2001. doi: 10.1007/s004240100609. [DOI] [PubMed] [Google Scholar]
- 86.Dempsey PJ, Goldenring JR, Soroka CJ, Modlin IM, McClure RW, Lind CD, Ahlquist DA, Pittlekow MR, Lee DC, Sandgren EP, Page DL, Coffey RJ. Possible role of transforming growth factor α in the pathogenesis of Menetrier’s Disease: supportive evidence from humans and transgenic mice. Gastroenterology 103: 1950–1963, 1992. doi: 10.1016/0016-5085(92)91455-D. [DOI] [PubMed] [Google Scholar]
- 87.Dickman R, Maradey-Romero C, Gingold-Belfer R, Fass R. Unmet Needs in the Treatment of Gastroesophageal Reflux Disease. [Correction in J Neurogastroenterol Motil 25: 173, 2019.] J Neurogastroenterol Motil 21: 309–319, 2015. doi: 10.5056/jnm15105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ding L, Hayes MM, Photenhauer A, Eaton KA, Li Q, Ocadiz-Ruiz R, Merchant JL. Schlafen 4-expressing myeloid-derived suppressor cells are induced during murine gastric metaplasia. J Clin Invest 126: 2867–2880, 2016. doi: 10.1172/JCI82529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ding M, Kinoshita Y, Kishi K, Nakata H, Hassan S, Kawanami C, Sugimoto Y, Katsuyama M, Negishi M, Narumiya S, Ichikawa A, Chiba T. Distribution of prostaglandin E receptors in the rat gastrointestinal tract. Prostaglandins 53: 199–216, 1997. doi: 10.1016/S0090-6980(97)00015-4. [DOI] [PubMed] [Google Scholar]
- 90.Dufner MM, Kirchhoff P, Remy C, Hafner P, Müller MK, Cheng SX, Tang LQ, Hebert SC, Geibel JP, Wagner CA. The calcium-sensing receptor acts as a modulator of gastric acid secretion in freshly isolated human gastric glands. Am J Physiol Gastrointest Liver Physiol 289: G1084–G1090, 2005. doi: 10.1152/ajpgi.00571.2004. [DOI] [PubMed] [Google Scholar]
- 91.Duman JG, Tyagarajan K, Kolsi MS, Moore HP, Forte JG. Expression of rab11a N124I in gastric parietal cells inhibits stimulatory recruitment of the H+-K+-ATPase. Am J Physiol Cell Physiol 277: C361–C372, 1999. doi: 10.1152/ajpcell.1999.277.3.C361. [DOI] [PubMed] [Google Scholar]
- 92.Edkins JS. The chemical mechanism of gastric secretion. J Physiol 34: 133–144, 1906. doi: 10.1113/jphysiol.1906.sp001146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Egerod KL, Engelstoft MS, Grunddal KV, Nøhr MK, Secher A, Sakata I, Pedersen J, Windeløv JA, Füchtbauer EM, Olsen J, Sundler F, Christensen JP, Wierup N, Olsen JV, Holst JJ, Zigman JM, Poulsen SS, Schwartz TW. A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin. Endocrinology 153: 5782–5795, 2012. doi: 10.1210/en.2012-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Egerod KL, Engelstoft MS, Lund ML, Grunddal KV, Zhao M, Barir-Jensen D, Nygaard EB, Petersen N, Holst JJ, Schwartz TW. Transcriptional and Functional Characterization of the G Protein-Coupled Receptor Repertoire of Gastric Somatostatin Cells. Endocrinology 156: 3909–3923, 2015. doi: 10.1210/EN.2015-1388. [DOI] [PubMed] [Google Scholar]
- 95.El-Omar EM, Oien K, El-Nujumi A, Gillen D, Wirz A, Dahill S, Williams C, Ardill JE, McColl KE. Helicobacter pylori infection and chronic gastric acid hyposecretion. Gastroenterology 113: 15–24, 1997. doi: 10.1016/S0016-5085(97)70075-1. [DOI] [PubMed] [Google Scholar]
- 96.El-Omar EM, Rabkin CS, Gammon MD, Vaughan TL, Risch HA, Schoenberg JB, Stanford JL, Mayne ST, Goedert J, Blot WJ, Fraumeni JF Jr, Chow WH. Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology 124: 1193–1201, 2003. doi: 10.1016/S0016-5085(03)00157-4. [DOI] [PubMed] [Google Scholar]
- 97.El-Zimaity HMT, Ota H, Graham DY, Akamatsu T, Katsuyama T. Patterns of gastric atrophy in intestinal type gastric carcinoma. Cancer 94: 1428–1436, 2002. doi: 10.1002/cncr.10375. [DOI] [PubMed] [Google Scholar]
- 98.Engelstoft MS, Park WM, Sakata I, Kristensen LV, Husted AS, Osborne-Lawrence S, Piper PK, Walker AK, Pedersen MH, Nøhr MK, Pan J, Sinz CJ, Carrington PE, Akiyama TE, Jones RM, Tang C, Ahmed K, Offermanns S, Egerod KL, Zigman JM, Schwartz TW. Seven transmembrane G protein-coupled receptor repertoire of gastric ghrelin cells. Mol Metab 2: 376–392, 2013. doi: 10.1016/j.molmet.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fakhry J, Stebbing MJ, Hunne B, Bayguinov Y, Ward SM, Sasse KC, Callaghan B, McQuade RM, Furness JB. Relationships of endocrine cells to each other and to other cell types in the human gastric fundus and corpus. Cell Tissue Res 376: 37–49, 2019. doi: 10.1007/s00441-018-2957-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Feldman EJ, Grossman MI. Liver extract and its free amino acids equally stimulate gastric acid secretion. Am J Physiol Gastrointest Liver Physiol 239: G493–G496, 1980. doi: 10.1152/ajpgi.1980.239.6.G493. [DOI] [PubMed] [Google Scholar]
- 101.Fellenius E, Berglindh T, Sachs G, Olbe L, Elander B, Sjöstrand SE, Wallmark B. Substituted benzimidazoles inhibit gastric acid secretion by blocking (H+ + K+)ATPase. Nature 290: 159–161, 1981. doi: 10.1038/290159a0. [DOI] [PubMed] [Google Scholar]
- 102.Feng J, Petersen CD, Coy DH, Jiang JK, Thomas CJ, Pollak MR, Wank SA. Calcium-sensing receptor is a physiologic multimodal chemosensor regulating gastric G-cell growth and gastrin secretion. Proc Natl Acad Sci USA 107: 17791–17796, 2010. doi: 10.1073/pnas.1009078107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Feurle GE. XeninY–a review. Peptides 19: 609–615, 1998. doi: 10.1016/S0196-9781(97)00378-1. [DOI] [PubMed] [Google Scholar]
- 104.Forte JG. K+ channels in the secretory membrane of the parietal cell focus on “Gastric parietal cell secretory membrane contains PKA- and acid-activated Kir2.1 K+ channels”. Am J Physiol Cell Physiol 286: C478–C479, 2004. doi: 10.1152/ajpcell.00531.2003. [DOI] [PubMed] [Google Scholar]
- 105.Forte JG, Black JA, Forte TM, Machen TE, Wolosin JM. Ultrastructural changes related to functional activity in gastric oxyntic cells. Am J Physiol Gastrointest Liver Physiol 241: G349–G358, 1981. doi: 10.1152/ajpgi.1981.241.5.G349. [DOI] [PubMed] [Google Scholar]
- 106.Forte JG, Forte TM, Black JA, Okamoto C, Wolosin JM. Correlation of parietal cell structure and function. J Clin Gastroenterol 5, Suppl 1: 17–28, 1983. doi: 10.1097/00004836-198312001-00003. [DOI] [PubMed] [Google Scholar]
- 107.Forte TM, Machen TE, Forte JG. Ultrastructural changes in oxyntic cells associated with secretory function: a membrane-recycling hypothesis. Gastroenterology 73: 941–955, 1977. doi: 10.1016/S0016-5085(19)31740-8. [DOI] [PubMed] [Google Scholar]
- 108.Fox JG, Li X, Cahill RJ, Andrutis K, Rustgi AK, Odze R, Wang TC. Hypertrophic gastropathy in Helicobacter felis-infected wild-type C57BL/6 mice and p53 hemizygous transgenic mice. Gastroenterology 110: 155–166, 1996. doi: 10.1053/gast.1996.v110.pm8536852. [DOI] [PubMed] [Google Scholar]
- 109.Freeman LC, Lippold JJ, Mitchell KE. Glycosylation influences gating and pH sensitivity of I(sK). J Membr Biol 177: 65–79, 2000. doi: 10.1007/s002320001100. [DOI] [PubMed] [Google Scholar]
- 110.Friis-Hansen L, Sundler F, Li Y, Gillespie PJ, Saunders TL, Greenson JK, Owyang C, Rehfeld JF, Samuelson LC. Impaired gastric acid secretion in gastrin-deficient mice. Am J Physiol Gastrointest Liver Physiol 274: G561–G568, 1998. doi: 10.1152/ajpgi.1998.274.3.G561. [DOI] [PubMed] [Google Scholar]
- 111.Fujita A, Horio Y, Higashi K, Mouri T, Hata F, Takeguchi N, Kurachi Y. Specific localization of an inwardly rectifying K+ channel, Kir4.1, at the apical membrane of rat gastric parietal cells; its possible involvement in K+ recycling for the H+-K+-pump. J Physiol 540: 85–92, 2002. doi: 10.1113/jphysiol.2001.013439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fujita T. Concept of paraneurons. Arch Histol Jpn 40, Suppl: 1–12, 1977. doi: 10.1679/aohc1950.40.Supplement_1. [DOI] [PubMed] [Google Scholar]
- 113.Fukumoto K, Nakahara K, Katayama T, Miyazatao M, Kangawa K, Murakami N. Synergistic action of gastrin and ghrelin on gastric acid secretion in rats. Biochem Biophys Res Commun 374: 60–63, 2008. doi: 10.1016/j.bbrc.2008.06.114. [DOI] [PubMed] [Google Scholar]
- 114.Fukushima Y, Shindo T, Anai M, Saitoh T, Wang Y, Fujishiro M, Ohashi Y, Ogihara T, Inukai K, Ono H, Sakoda H, Kurihara Y, Honda M, Shojima N, Fukushima H, Haraikawa-Onishi Y, Katagiri H, Shimizu Y, Ichinose M, Ishikawa T, Omata M, Nagai R, Kurihara H, Asano T. Structural and functional characterization of gastric mucosa and central nervous system in histamine H2 receptor-null mice. Eur J Pharmacol 468: 47–58, 2003. doi: 10.1016/S0014-2999(03)01668-6. [DOI] [PubMed] [Google Scholar]
- 115.Furutani K, Aihara T, Nakamura E, Tanaka S, Ichikawa A, Ohtsu H, Okabe S. Crucial role of histamine for regulation of gastric acid secretion ascertained by histidine decarboxylase-knockout mice. J Pharmacol Exp Ther 307: 331–338, 2003. doi: 10.1124/jpet.103.052019. [DOI] [PubMed] [Google Scholar]
- 116.Ganser AL, Forte JG. K+-stimulated ATPase in purified microsomes of bullfrog oxyntic cells. Biochim Biophys Acta 307: 169–180, 1973. doi: 10.1016/0005-2736(73)90035-7. [DOI] [PubMed] [Google Scholar]
- 117.Gardner JD, Jackson MJ, Batzri S, Jensen RT. Potential mechanisms of interaction among secretagogues. Gastroenterology 74: 348–354, 1978. doi: 10.1016/0016-5085(78)90759-X. [DOI] [PubMed] [Google Scholar]
- 118.Ge Y, Li Y, Chen Q, Zhu W, Zuo L, Guo Z, Gong J, Cao L, Gu L, Li J. Adipokine apelin ameliorates chronic colitis in Il-10−/− mice by promoting intestinal lymphatic functions. Biochem Pharmacol 148: 202–212, 2018. doi: 10.1016/j.bcp.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 119.Gedda K, Briving C, Svensson K, Maxvall I, Andersson K. Mechanism of action of AZD0865, a K+-competitive inhibitor of gastric H+,K+-ATPase. Biochem Pharmacol 73: 198–205, 2007. doi: 10.1016/j.bcp.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 120.Geering K. The functional role of the beta-subunit in the maturation and intracellular transport of Na,K-ATPase. FEBS Lett 285: 189–193, 1991. doi: 10.1016/0014-5793(91)80801-9. [DOI] [PubMed] [Google Scholar]
- 121.Geering K, Beggah A, Good P, Girardet S, Roy S, Schaer D, Jaunin P. Oligomerization and maturation of Na,K-ATPase: functional interaction of the cytoplasmic NH2 terminus of the beta subunit with the alpha subunit. J Cell Biol 133: 1193–1204, 1996. doi: 10.1083/jcb.133.6.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Geibel J, Abraham R, Modlin I, Sachs G. Gastrin-stimulated changes in Ca2+ concentration in parietal cells depends on adenosine 3′,5′-cyclic monophosphate levels. Gastroenterology 109: 1060–1067, 1995. doi: 10.1016/0016-5085(95)90563-4. [DOI] [PubMed] [Google Scholar]
- 123.Gerber JG, Nies AS, Payne NA. Adenosine receptors on canine parietal cells modulate gastric acid secretion to histamine. J Pharmacol Exp Ther 233: 623–627, 1985. [PubMed] [Google Scholar]
- 124.Goldenring JR, Ray GS, Coffey RJ Jr, Meunier PC, Haley PJ, Barnes TB, Car BD. Reversible drug-induced oxyntic atrophy in rats. Gastroenterology 118: 1080–1093, 2000. doi: 10.1016/S0016-5085(00)70361-1. [DOI] [PubMed] [Google Scholar]
- 125.Goldenring JR, Ray GS, Soroka CJ, Smith J, Modlin IM, Meise KS, Coffey RJ Jr. Overexpression of transforming growth factor-alpha alters differentiation of gastric cell lineages. Dig Dis Sci 41: 773–784, 1996. doi: 10.1007/BF02213134. [DOI] [PubMed] [Google Scholar]
- 126.Goldenring JR, Shen KR, Vaughan HD, Modlin IM. Identification of a small GTP-binding protein, Rab25, expressed in the gastrointestinal mucosa, kidney, and lung. J Biol Chem 268: 18419–18422, 1993. [PubMed] [Google Scholar]
- 127.Goldenring JR, Soroka CJ, Shen KR, Tang LH, Rodriguez W, Vaughan HD, Stoch SA, Modlin IM. Enrichment of rab11, a small GTP-binding protein, in gastric parietal cells. Am J Physiol Gastrointest Liver Physiol 267: G187–G194, 1994. doi: 10.1152/ajpgi.1994.267.2.G187. [DOI] [PubMed] [Google Scholar]
- 128.Gomez-Pan A, Albinus M, Reed JD, Shaw B, Hall R, Besser GM, Coy DH, Kastin AJ, Schally AV. Direct inhibition of gastric acid and pepsin secretion by growth-hormone release-inhibiting hormone in cats. Lancet 305: 888–890, 1975. doi: 10.1016/S0140-6736(75)91686-4. [DOI] [PubMed] [Google Scholar]
- 129.Goo T, Akiba Y, Kaunitz JD. Mechanisms of intragastric pH sensing. Curr Gastroenterol Rep 12: 465–470, 2010. doi: 10.1007/s11894-010-0147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Göõz M, Hammond CE, Larsen K, Mukhin YV, Smolka AJ. Inhibition of human gastric H+-K+-ATPase alpha-subunit gene expression by Helicobacter pylori. Am J Physiol Gastrointest Liver Physiol 278: G981–G991, 2000. doi: 10.1152/ajpgi.2000.278.6.G981. [DOI] [PubMed] [Google Scholar]
- 131.Goswami C, Shimada Y, Yoshimura M, Mondal A, Oda S, Tanaka T, Sakai T, Sakata I. Motilin Stimulates Gastric Acid Secretion in Coordination with Ghrelin in Suncus murinus. PLoS One 10: e0131554, 2015. doi: 10.1371/journal.pone.0131554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gottardi CJ, Caplan MJ. An ion-transporting ATPase encodes multiple apical localization signals. J Cell Biol 121: 283–293, 1993. doi: 10.1083/jcb.121.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Graham DY, Lew GM, Lechago J. Antral G-cell and D-cell numbers in Helicobacter pylori infection: effect of H. pylori eradication. Gastroenterology 104: 1655–1660, 1993. doi: 10.1016/0016-5085(93)90642-P. [DOI] [PubMed] [Google Scholar]
- 134.Grahammer F, Wittekindt OH, Nitschke R, Herling AW, Lang HJ, Bleich M, Schmitt-Gräff A, Barhanin J, Warth R. The cardiac K+ channel KCNQ1 is essential for gastric acid secretion. Gastroenterology 120: 1363–1371, 2001. doi: 10.1053/gast.2001.24053. [DOI] [PubMed] [Google Scholar]
- 135.Gregory RA, Tracy HJ. The Constitution and Properties of Two Gastrins Extracted from Hog Antral Mucosa. Part I: The isolation of two gastrins from hog antral mucosa. Gut 5: 103–114, 1964. doi: 10.1136/gut.5.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Grossman MI. Secretion of acid and pepsin in response to distention of vagally innervated fundic gland area in dogs. Gastroenterology 42: 718–721, 1962. doi: 10.1016/S0016-5085(62)80123-1. [DOI] [PubMed] [Google Scholar]
- 137.Grossman MI, Konturek SJ. Inhibition of acid secretion in dog by metiamide, a histamine antagonist acting on H2 receptors. Gastroenterology 66: 517–521, 1974. doi: 10.1016/S0016-5085(74)80038-7. [DOI] [PubMed] [Google Scholar]
- 138.Grossman MI, Robertson CR. Inhibition by histaminase of gastric secretion in dogs. Am J Physiol 153: 447–453, 1948. doi: 10.1152/ajplegacy.1948.153.3.447. [DOI] [PubMed] [Google Scholar]
- 139.Grossman MI, Robertson CR, Ivy AC. Proof of a hormonal mechanism for gastric secretion: the humoral transmission of the distention stimulus. Am J Physiol 153: 1–9, 1948. doi: 10.1152/ajplegacy.1948.153.1.1. [DOI] [PubMed] [Google Scholar]
- 140.Gugger M, Waser B, Kappeler A, Schonbrunn A, Reubi JC. Cellular detection of sst2A receptors in human gastrointestinal tissue. Gut 53: 1431–1436, 2004. doi: 10.1136/gut.2004.042002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Guo YS, Singh P, Gomez G, Greeley GH Jr, Thompson JC. Effect of peptide YY on cephalic, gastric, and intestinal phases of gastric acid secretion and on the release of gastrointestinal hormones. Gastroenterology 92: 1202–1208, 1987. doi: 10.1016/S0016-5085(87)91078-X. [DOI] [PubMed] [Google Scholar]
- 142.Habata Y, Fujii R, Hosoya M, Fukusumi S, Kawamata Y, Hinuma S, Kitada C, Nishizawa N, Murosaki S, Kurokawa T, Onda H, Tatemoto K, Fujino M. Apelin, the natural ligand of the orphan receptor APJ, is abundantly secreted in the colostrum. Biochim Biophys Acta 1452: 25–35, 1999. doi: 10.1016/S0167-4889(99)00114-7. [DOI] [PubMed] [Google Scholar]
- 143.Haid D, Widmayer P, Breer H. Nutrient sensing receptors in gastric endocrine cells. J Mol Histol 42: 355–364, 2011. doi: 10.1007/s10735-011-9339-1. [DOI] [PubMed] [Google Scholar]
- 144.Haid DC, Jordan-Biegger C, Widmayer P, Breer H. Receptors responsive to protein breakdown products in g-cells and d-cells of mouse, swine and human. Front Physiol 3: 65, 2012. doi: 10.3389/fphys.2012.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Håkanson R, Sundler F. Histamine-producing cells in the stomach and their role in the regulation of acid secretion. Scand J Gastroenterol Suppl 26, sup180: 88–94, 1991. doi: 10.3109/00365529109093183. [DOI] [PubMed] [Google Scholar]
- 146.Hales CM, Griner R, Hobdy-Henderson KC, Dorn MC, Hardy D, Kumar R, Navarre J, Chan EK, Lapierre LA, Goldenring JR. Identification and characterization of a family of Rab11-interacting proteins. J Biol Chem 276: 39067–39075, 2001. doi: 10.1074/jbc.M104831200. [DOI] [PubMed] [Google Scholar]
- 147.Hall K, Perez G, Sachs G, Rabon E. Identification of H+/K+-ATPase alpha,beta-heterodimers. Biochim Biophys Acta 1077: 173–179, 1991. doi: 10.1016/0167-4838(91)90055-5. [DOI] [PubMed] [Google Scholar]
- 148.Hamlet A, Lindholm C, Nilsson O, Olbe L. Aspirin-induced gastritis, like Helicobacter pylori-induced gastritis disinhibits acid secretion in humans: relation to cytokine expression. Scand J Gastroenterol 33: 346–356, 1998. doi: 10.1080/00365529850170964. [DOI] [PubMed] [Google Scholar]
- 149.Hammond CE, Beeson C, Suarez G, Peek RM Jr, Backert S, Smolka AJ. Helicobacter pylori virulence factors affecting gastric proton pump expression and acid secretion. Am J Physiol Gastrointest Liver Physiol 309: G193–G201, 2015. doi: 10.1152/ajpgi.00099.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Han KS, Kim YG, Yoo JK, Lee JW, Lee MG. Pharmacokinetics of a new reversible proton pump inhibitor, YH1885, after intravenous and oral administrations to rats and dogs: hepatic first-pass effect in rats. Biopharm Drug Dispos 19: 493–500, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 151.Hansson R, Sundström G. Diamine oxidase (histaminase) and heparin inhibition of gastric secretion in man. Scand J Clin Lab Invest 26: 263–269, 1970. doi: 10.3109/00365517009046232. [DOI] [PubMed] [Google Scholar]
- 152.Hanzel D, Reggio H, Bretscher A, Forte JG, Mangeat P. The secretion-stimulated 80K phosphoprotein of parietal cells is ezrin, and has properties of a membrane cytoskeletal linker in the induced apical microvilli. EMBO J 10: 2363–2373, 1991. doi: 10.1002/j.1460-2075.1991.tb07775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hanzel DK, Urushidani T, Usinger WR, Smolka A, Forte JG. Immunological localization of an 80-kDa phosphoprotein to the apical membrane of gastric parietal cells. Am J Physiol Gastrointest Liver Physiol 256: G1082–G1089, 1989. doi: 10.1152/ajpgi.1989.256.6.G1082. [DOI] [PubMed] [Google Scholar]
- 154.Harden CJ, Jones AN, Maya-Jimenez T, Barker ME, Hepburn NJ, Garaiova I, Plummer SF, Corfe BM. Effect of different long-chain fatty acids on cholecystokinin release in vitro and energy intake in free-living healthy males. Br J Nutr 108: 755–758, 2012. doi: 10.1017/S0007114511006003. [DOI] [PubMed] [Google Scholar]
- 155.Harris AW, Gummett PA, Misiewicz JJ, Baron JH. Eradication of Helicobacter pylori in patients with duodenal ulcer lowers basal and peak acid outputs to gastrin releasing peptide and pentagastrin. Gut 38: 663–667, 1996. doi: 10.1136/gut.38.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hauger RL, Grigoriadis DE, Dallman MF, Plotsky PM, Vale WW, Dautzenberg FM. International Union of Pharmacology. XXXVI. Current status of the nomenclature for receptors for corticotropin-releasing factor and their ligands. Pharmacol Rev 55: 21–26, 2003. doi: 10.1124/pr.55.1.3. [DOI] [PubMed] [Google Scholar]
- 157.Hayakawa Y, Ariyama H, Stancikova J, Sakitani K, Asfaha S, Renz BW, Dubeykovskaya ZA, Shibata W, Wang H, Westphalen CB, Chen X, Takemoto Y, Kim W, Khurana SS, Tailor Y, Nagar K, Tomita H, Hara A, Sepulveda AR, Setlik W, Gershon MD, Saha S, Ding L, Shen Z, Fox JG, Friedman RA, Konieczny SF, Worthley DL, Korinek V, Wang TC. Mist1 Expressing Gastric Stem Cells Maintain the Normal and Neoplastic Gastric Epithelium and Are Supported by a Perivascular Stem Cell Niche. Cancer Cell 28: 800–814, 2015. doi: 10.1016/j.ccell.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Heitzmann D, Grahammer F, von Hahn T, Schmitt-Gräff A, Romeo E, Nitschke R, Gerlach U, Lang HJ, Verrey F, Barhanin J, Warth R. Heteromeric KCNE2/KCNQ1 potassium channels in the luminal membrane of gastric parietal cells. J Physiol 561: 547–557, 2004. doi: 10.1113/jphysiol.2004.075168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Heitzmann D, Koren V, Wagner M, Sterner C, Reichold M, Tegtmeier I, Volk T, Warth R. KCNE beta subunits determine pH sensitivity of KCNQ1 potassium channels. Cell Physiol Biochem 19: 21–32, 2007. doi: 10.1159/000099189. [DOI] [PubMed] [Google Scholar]
- 160.Heitzmann D, Warth R. No potassium, no acid: K+ channels and gastric acid secretion. Physiology (Bethesda) 22: 335–341, 2007. doi: 10.1152/physiol.00016.2007. [DOI] [PubMed] [Google Scholar]
- 161.Heldsinger AA, Vinik AI, Fox IH. Inhibition of guinea-pig oxyntic cell function by adenosine and prostaglandins. J Pharmacol Exp Ther 237: 351–356, 1986. [PubMed] [Google Scholar]
- 162.Hills DM, Gerskowitch VP, Roberts SP, Welsh NJ, Shankley NP, Black JW. Pharmacological analysis of the CCKB/gastrin receptors mediating pentagastrin-stimulated gastric acid secretion in the isolated stomach of the immature rat. Br J Pharmacol 119: 1401–1410, 1996. doi: 10.1111/j.1476-5381.1996.tb16052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hinkle KL, Bane GC, Jazayeri A, Samuelson LC. Enhanced calcium signaling and acid secretion in parietal cells isolated from gastrin-deficient mice. Am J Physiol Gastrointest Liver Physiol 284: G145–G153, 2003. doi: 10.1152/ajpgi.00283.2002. [DOI] [PubMed] [Google Scholar]
- 164.Hirschowitz BI, Streeten DH, London JA, Pollard HM. Effects of eight-hour intravenous infusions of ACTH and the adrenocortical steroids in normal man. I. Basal gastric secretion, and plasma and urinary pepsinogen. J Clin Invest 36: 1171–1182, 1957. doi: 10.1172/JCI103513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hirst BH, Forte JG. Redistribution and characterization of (H+ + K+)-ATPase membranes from resting and stimulated gastric parietal cells. Biochem J 231: 641–649, 1985. doi: 10.1042/bj2310641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hollande F, Bali JP, Magous R. Autoregulation of histamine synthesis through H3 receptors in isolated fundic mucosal cells. Am J Physiol Gastrointest Liver Physiol 265: G1039–G1044, 1993. doi: 10.1152/ajpgi.1993.265.6.G1039. [DOI] [PubMed] [Google Scholar]
- 167.Hori Y, Matsukawa J, Takeuchi T, Nishida H, Kajino M, Inatomi N. A study comparing the antisecretory effect of TAK-438, a novel potassium-competitive acid blocker, with lansoprazole in animals. J Pharmacol Exp Ther 337: 797–804, 2011. doi: 10.1124/jpet.111.179556. [DOI] [PubMed] [Google Scholar]
- 168.Horisberger JD, Jaunin P, Reuben MA, Lasater LS, Chow DC, Forte JG, Sachs G, Rossier BC, Geering K. The H,K-ATPase beta-subunit can act as a surrogate for the beta-subunit of Na,K-pumps. J Biol Chem 266: 19131–19134, 1991. [PubMed] [Google Scholar]
- 169.Howlett M, Chalinor HV, Buzzelli JN, Nguyen N, van Driel IR, Bell KM, Fox JG, Dimitriadis E, Menheniott TR, Giraud AS, Judd LM. IL-11 is a parietal cell cytokine that induces atrophic gastritis. Gut 61: 1398–1409, 2012. doi: 10.1136/gutjnl-2011-300539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Huh WJ, Khurana SS, Geahlen JH, Kohli K, Waller RA, Mills JC. Tamoxifen induces rapid, reversible atrophy, and metaplasia in mouse stomach. Gastroenterology 142: 21–24.e7, 2012. doi: 10.1053/j.gastro.2011.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hunt RH. Review article: the unmet needs in delayed-release proton-pump inhibitor therapy in 2005. Aliment Pharmacol Ther 22, Suppl 3: 10–19, 2005. doi: 10.1111/j.1365-2036.2005.02715.x. [DOI] [PubMed] [Google Scholar]
- 172.Isomäki AM. A new cell type (tuft cell) in the gastrointestinal mucosa of the rat. A transmission and scanning electron microscopic study. Acta Pathol Microbiol Scand A 240: 1–35, 1973. [PubMed] [Google Scholar]
- 173.Israel DA, Salama N, Arnold CN, Moss SF, Ando T, Wirth HP, Tham KT, Camorlinga M, Blaser MJ, Falkow S, Peek RM Jr. Helicobacter pylori strain-specific differences in genetic content, identified by microarray, influence host inflammatory responses. J Clin Invest 107: 611–620, 2001. doi: 10.1172/JCI11450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Itoh M, Matsuo Y. Gastric ulcer treatment with intravenous human epidermal growth factor: a double-blind controlled clinical study. J Gastroenterol Hepatol 9, Suppl 1: S78–S83, 1994. doi: 10.1111/j.1440-1746.1994.tb01307.x. [DOI] [PubMed] [Google Scholar]
- 175.Jablonowski H, Hengels KJ, Krämer N, Geis G, Opferkuch W, Strohmeyer G. Effect of Helicobacter pylori on dbc-AMP stimulated acid secretion by human parietal cells. Hepatogastroenterology 41: 546–548, 1994. [PubMed] [Google Scholar]
- 176.Jackson LM, Wu KC, Mahida YR, Jenkins D, Hawkey CJ. Cyclooxygenase (COX) 1 and 2 in normal, inflamed, and ulcerated human gastric mucosa. Gut 47: 762–770, 2000. doi: 10.1136/gut.47.6.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Janssen S, Laermans J, Verhulst PJ, Thijs T, Tack J, Depoortere I. Bitter taste receptors and α-gustducin regulate the secretion of ghrelin with functional effects on food intake and gastric emptying. Proc Natl Acad Sci USA 108: 2094–2099, 2011. doi: 10.1073/pnas.1011508108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Jaramillo E, Mårdh S, Gréen K, Persson B, Rubio C, Aly A. The effect of arachidonic acid and its metabolites on acid production in isolated human parietal cells. Scand J Gastroenterol 24: 1231–1237, 1989. doi: 10.3109/00365528909090792. [DOI] [PubMed] [Google Scholar]
- 179.Jaunin P, Horisberger JD, Richter K, Good PJ, Rossier BC, Geering K. Processing, intracellular transport, and functional expression of endogenous and exogenous alpha-beta 3 Na,K-ATPase complexes in Xenopus oocytes. J Biol Chem 267: 577–585, 1992. [PubMed] [Google Scholar]
- 180.Jenkins H, Sakurai Y, Nishimura A, Okamoto H, Hibberd M, Jenkins R, Yoneyama T, Ashida K, Ogama Y, Warrington S. Randomised clinical trial: safety, tolerability, pharmacokinetics and pharmacodynamics of repeated doses of TAK-438 (vonoprazan), a novel potassium-competitive acid blocker, in healthy male subjects. Aliment Pharmacol Ther 41: 636–648, 2015. doi: 10.1111/apt.13121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Jensen S, Borch K, Hilsted L, Rehfeld JF. Progastrin processing during antral G-cell hypersecretion in humans. Gastroenterology 96: 1063–1070, 1989. doi: 10.1016/0016-5085(89)91624-7. [DOI] [PubMed] [Google Scholar]
- 182.Jiang M, Li H, Zhang Y, Yang Y, Lu R, Liu K, Lin S, Lan X, Wang H, Wu H, Zhu J, Zhou Z, Xu J, Lee DK, Zhang L, Lee YC, Yuan J, Abrams JA, Wang TC, Sepulveda AR, Wu Q, Chen H, Sun X, She J, Chen X, Que J. Transitional basal cells at the squamous-columnar junction generate Barrett’s oesophagus. Nature 550: 529–533, 2017. doi: 10.1038/nature24269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Joshi V, Ray GS, Goldenring JR. Inhibition of parietal cell acid secretion is mediated by the classical epidermal growth factor receptor. Dig Dis Sci 42: 1194–1198, 1997. doi: 10.1023/A:1018845805806. [DOI] [PubMed] [Google Scholar]
- 184.Kagami T, Sahara S, Ichikawa H, Uotani T, Yamade M, Sugimoto M, Hamaya Y, Iwaizumi M, Osawa S, Sugimoto K, Miyajima H, Furuta T. Potent acid inhibition by vonoprazan in comparison with esomeprazole, with reference to CYP2C19 genotype. Aliment Pharmacol Ther 43: 1048–1059, 2016. doi: 10.1111/apt.13588. [DOI] [PubMed] [Google Scholar]
- 185.Kaji I, Akiba Y, Kato I, Maruta K, Kuwahara A, Kaunitz JD. Xenin augments duodenal anion secretion via activation of afferent neural pathways. J Pharmacol Exp Ther 361: 151–161, 2017. doi: 10.1124/jpet.116.238485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kaneko H, Nakada K, Mitsuma T, Uchida K, Furusawa A, Maeda Y, Morise K. Helicobacter pylori infection induces a decrease in immunoreactive-somatostatin concentrations of human stomach. Dig Dis Sci 37: 409–416, 1992. doi: 10.1007/BF01307736. [DOI] [PubMed] [Google Scholar]
- 187.Karam SM. Dynamics of epithelial cells in the corpus of the mouse stomach. IV. Bidirectional migration of parietal cells ending in their gradual degeneration and loss. Anat Rec 236: 314–332, 1993. doi: 10.1002/ar.1092360205. [DOI] [PubMed] [Google Scholar]
- 188.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. I. Identification of proliferative cell types and pinpointing of the stem cell. Anat Rec 236: 259–279, 1993. doi: 10.1002/ar.1092360202. [DOI] [PubMed] [Google Scholar]
- 189.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. II. Outward migration of pit cells. Anat Rec 236: 280–296, 1993. doi: 10.1002/ar.1092360203. [DOI] [PubMed] [Google Scholar]
- 190.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. III. Inward migration of neck cells followed by progressive transformation into zymogenic cells. Anat Rec 236: 297–313, 1993. doi: 10.1002/ar.1092360204. [DOI] [PubMed] [Google Scholar]
- 191.Karvar S, Zhu L, Crothers J Jr, Wong W, Turkoz M, Forte JG. Cellular localization and stimulation-associated distribution dynamics of syntaxin-1 and syntaxin-3 in gastric parietal cells. Traffic 6: 654–666, 2005. doi: 10.1111/j.1600-0854.2005.00306.x. [DOI] [PubMed] [Google Scholar]
- 192.Kato K, Hayashizaki Y, Takahashi Y, Himeno S, Matsubara K. Molecular cloning of the human gastrin gene. Nucleic Acids Res 11: 8197–8203, 1983. doi: 10.1093/nar/11.23.8197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kato K, Sasano H, Ohara S, Sekine H, Mochizuki S, Mune T, Yasuda K, Nagura H, Shimosegawa T, Toyota T, Krozowski Z. Coexpression of mineralocorticoid receptors and 11beta-hydroxysteroid dehydrogenase 2 in human gastric mucosa. J Clin Endocrinol Metab 84: 2568–2573, 1999. [DOI] [PubMed] [Google Scholar]
- 194.Kato S, Aihara E, Yoshii K, Takeuchi K. Dual action of prostaglandin E2 on gastric acid secretion through different EP-receptor subtypes in the rat. Am J Physiol Gastrointest Liver Physiol 289: G64–G69, 2005. doi: 10.1152/ajpgi.00397.2004. [DOI] [PubMed] [Google Scholar]
- 195.Kato S, Ozawa K, Koike T, Sekine H, Ohara S, Minoura T, Iinuma K. Effect of Helicobacter pylori infection on gastric acid secretion and meal-stimulated serum gastrin in children. Helicobacter 9: 100–105, 2004. doi: 10.1111/j.1083-4389.2004.00205.x. [DOI] [PubMed] [Google Scholar]
- 196.Katz PO, Scheiman JM, Barkun AN. Review article: acid-related disease–what are the unmet clinical needs? Aliment Pharmacol Ther 23, Suppl 2: 9–22, 2006. doi: 10.1111/j.1365-2036.2006.02944.x. [DOI] [PubMed] [Google Scholar]
- 197.Kaufhold MA, Krabbenhöft A, Song P, Engelhardt R, Riederer B, Fährmann M, Klöcker N, Beil W, Manns M, Hagen SJ, Seidler U. Localization, trafficking, and significance for acid secretion of parietal cell Kir4.1 and KCNQ1 K+ channels. Gastroenterology 134: 1058–1069, 2008. doi: 10.1053/j.gastro.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 198.Kessler JP, Beaudet A. Association of neurotensin binding sites with sensory and visceromotor components of the vagus nerve. J Neurosci 9: 466–472, 1989. doi: 10.1523/JNEUROSCI.09-02-00466.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Khan ZE, Wang TC, Cui G, Chi AL, Dimaline R. Transcriptional regulation of the human trefoil factor, TFF1, by gastrin. Gastroenterology 125: 510–521, 2003. doi: 10.1016/S0016-5085(03)00908-9. [DOI] [PubMed] [Google Scholar]
- 200.Kim H, Kim KH. Effects of a nitric oxide donor and nitric oxide synthase inhibitors on acid secretion of isolated rabbit gastric glands. Pharmacology 53: 331–339, 1996. doi: 10.1159/000139448. [DOI] [PubMed] [Google Scholar]
- 201.Kimura T, Tabuchi Y, Takeguchi N, Asano S. Mutational study on the roles of disulfide bonds in the beta-subunit of gastric H+,K+-ATPase. J Biol Chem 277: 20671–20677, 2002. doi: 10.1074/jbc.M200523200. [DOI] [PubMed] [Google Scholar]
- 202.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402: 656–660, 1999. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 203.Konturek SJ, Cieszkowski M, Jaworek J, Konturek J, Brzozowski T, Gregory H. Effects of epidermal growth factor on gastrointestinal secretions. Am J Physiol Gastrointest Liver Physiol 246: G580–G586, 1984. doi: 10.1152/ajpgi.1984.246.5.G580. [DOI] [PubMed] [Google Scholar]
- 204.Konturek SJ, Tasler J, Cieszkowski M, Coy DH, Schally AV. Effect of growth hormone release-inhibiting hormone on gastric secretion, mucosal blood flow, and serum gastrin. Gastroenterology 70: 737–741, 1976. doi: 10.1016/S0016-5085(76)80265-X. [DOI] [PubMed] [Google Scholar]
- 205.Kopin AS, Lee YM, McBride EW, Miller LJ, Lu M, Lin HY, Kolakowski LFJ Jr, Beinborn M. Expression cloning and characterization of the canine parietal cell gastrin receptor. Proc Natl Acad Sci USA 89: 3605–3609, 1992. doi: 10.1073/pnas.89.8.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kosaka TL, Lim RKS. Demonstration of the Humoral Agent in Fat Inhibition of Gastric Secretion. Proc Soc Exp Biol Med 27: 890–891, 1930. doi: 10.3181/00379727-27-5024. [DOI] [Google Scholar]
- 207.Koskenpato K, Ainola M, Przybyla B, Kouri VP, Virkki L, Koskenpato J, Ristimäki A, Konttinen YT. Diminished salivary epidermal growth factor secretion: a link between Sjögren’s syndrome and autoimmune gastritis? Scand J Rheumatol 45: 118–121, 2016. doi: 10.3109/03009742.2015.1072243. [DOI] [PubMed] [Google Scholar]
- 208.Kozicz T, Arimura A. Distribution of urocortin in the rat’s gastrointestinal tract and its colocalization with tyrosine hydroxylase. Peptides 23: 515–521, 2002. doi: 10.1016/S0196-9781(01)00639-8. [DOI] [PubMed] [Google Scholar]
- 209.Krempels K, Hunyady B, O’Carroll AM, Mezey E. Distribution of somatostatin receptor messenger RNAs in the rat gastrointestinal tract. Gastroenterology 112: 1948–1960, 1997. doi: 10.1053/gast.1997.v112.pm9178687. [DOI] [PubMed] [Google Scholar]
- 210.Kulaksiz H, Arnold R, Göke B, Maronde E, Meyer M, Fahrenholz F, Forssmann WG, Eissele R. Expression and cell-specific localization of the cholecystokinin B/gastrin receptor in the human stomach. Cell Tissue Res 299: 289–298, 2000. doi: 10.1007/s004410050027. [DOI] [PubMed] [Google Scholar]
- 211.Kupari J, Rossi J, Herzig KH, Airaksinen MS. Lack of cholinergic innervation in gastric mucosa does not affect gastrin secretion or basal acid output in neurturin receptor GFRα2 deficient mice. J Physiol 591: 2175–2188, 2013. doi: 10.1113/jphysiol.2012.246801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lambrecht N, Munson K, Vagin O, Sachs G. Comparison of covalent with reversible inhibitor binding sites of the gastric H,K-ATPase by site-directed mutagenesis. J Biol Chem 275: 4041–4048, 2000. doi: 10.1074/jbc.275.6.4041. [DOI] [PubMed] [Google Scholar]
- 213.Lambrecht NW, Yakubov I, Scott D, Sachs G. Identification of the K efflux channel coupled to the gastric H-K-ATPase during acid secretion. Physiol Genomics 21: 81–91, 2005. doi: 10.1152/physiolgenomics.00212.2004. [DOI] [PubMed] [Google Scholar]
- 214.Lambrecht NW, Yakubov I, Zer C, Sachs G. Transcriptomes of purified gastric ECL and parietal cells: identification of a novel pathway regulating acid secretion. Physiol Genomics 25: 153–165, 2006. doi: 10.1152/physiolgenomics.00271.2005. [DOI] [PubMed] [Google Scholar]
- 215.Lamers CB, Walsh JH, Jansen JB, Harrison AR, Ippoliti AF, van Tongere JH. Evidence that gastrin 34 is preferentially released from the human duodenum. Gastroenterology 83: 233–239, 1982. [PubMed] [Google Scholar]
- 216.Langhans N, Rindi G, Chiu M, Rehfeld JF, Ardman B, Beinborn M, Kopin AS. Abnormal gastric histology and decreased acid production in cholecystokinin-B/gastrin receptor-deficient mice. Gastroenterology 112: 280–286, 1997. doi: 10.1016/S0016-5085(97)90000-7. [DOI] [PubMed] [Google Scholar]
- 217.Lapierre LA, Avant KM, Caldwell CM, Ham AJ, Hill S, Williams JA, Smolka AJ, Goldenring JR. Characterization of immunoisolated human gastric parietal cells tubulovesicles: identification of regulators of apical recycling. Am J Physiol Gastrointest Liver Physiol 292: G1249–G1262, 2007. doi: 10.1152/ajpgi.00505.2006. [DOI] [PubMed] [Google Scholar]
- 218.Lapierre LA, Kumar R, Hales CM, Navarre J, Bhartur SG, Burnette JO, Provance DW Jr, Mercer JA, Bähler M, Goldenring JR. Myosin vb is associated with plasma membrane recycling systems. Mol Biol Cell 12: 1843–1857, 2001. doi: 10.1091/mbc.12.6.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Larsson LI, Goltermann N, de Magistris L, Rehfeld JF, Schwartz TW. Somatostatin cell processes as pathways for paracrine secretion. Science 205: 1393–1395, 1979. doi: 10.1126/science.382360. [DOI] [PubMed] [Google Scholar]
- 220.Latrajet A. Preliminaire sur l’innervation et l’enervation de l’estomach. Lyon Med 130: 160–166, 1921. [Google Scholar]
- 221.Layer P, Holst JJ, Grandt D, Goebell H. Ileal release of glucagon-like peptide-1 (GLP-1). Association with inhibition of gastric acid secretion in humans. Dig Dis Sci 40: 1074–1082, 1995. doi: 10.1007/BF02064202. [DOI] [PubMed] [Google Scholar]
- 222.Lee HC, Forte JG. A study of H+ transport in gastric microsomal vesicles using fluorescent probes. Biochim Biophys Acta 508: 339–356, 1978. doi: 10.1016/0005-2736(78)90336-X. [DOI] [PubMed] [Google Scholar]
- 223.Lee MP, Ravenel JD, Hu RJ, Lustig LR, Tomaselli G, Berger RD, Brandenburg SA, Litzi TJ, Bunton TE, Limb C, Francis H, Gorelikow M, Gu H, Washington K, Argani P, Goldenring JR, Coffey RJ, Feinberg AP. Targeted disruption of the Kvlqt1 gene causes deafness and gastric hyperplasia in mice. J Clin Invest 106: 1447–1455, 2000. doi: 10.1172/JCI10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Levine RA, Schwartzel EH. Effect of indomethacin on basal and histamine stimulated human gastric acid secretion. Gut 25: 718–722, 1984. doi: 10.1136/gut.25.7.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lewis JJ, Goldenring JR, Asher VA, Modlin IM. Effects of epidermal growth factor on signal transduction in rabbit parietal cells. Am J Physiol Gastrointest Liver Physiol 258: G476–G483, 1990. doi: 10.1152/ajpgi.1990.258.3.G476. [DOI] [PubMed] [Google Scholar]
- 226.Lewis JJ, Goldenring JR, Modlin IM, Coffey RJ. Inhibition of parietal cell H+ secretion by transforming growth factor alpha: a possible autocrine regulatory mechanism. Surgery 108: 220–226, 1990. [PubMed] [Google Scholar]
- 227.Liddle RA. Regulation of cholecystokinin secretion in humans. J Gastroenterol 35: 181–187, 2000. doi: 10.1007/s005350050328. [DOI] [PubMed] [Google Scholar]
- 228.Lindström E, Lerner UH, Håkanson R. Isolated rat stomach ECL cells generate prostaglandin E(2) in response to interleukin-1 beta, tumor necrosis factor-alpha and bradykinin. Eur J Pharmacol 416: 255–263, 2001. doi: 10.1016/S0014-2999(01)00881-0. [DOI] [PubMed] [Google Scholar]
- 229.Liu Y, Ding X, Wang D, Deng H, Feng M, Wang M, Yu X, Jiang K, Ward T, Aikhionbare F, Guo Z, Forte JG, Yao X. A mechanism of Munc18b-syntaxin 3-SNAP25 complex assembly in regulated epithelial secretion. FEBS Lett 581: 4318–4324, 2007. doi: 10.1016/j.febslet.2007.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lloyd KC, Raybould HE, Walsh JH. Cholecystokinin inhibits gastric acid secretion through type “A” cholecystokinin receptors and somatostatin in rats. Am J Physiol Gastrointest Liver Physiol 263: G287–G292, 1992. doi: 10.1152/ajpgi.1992.263.3.G287. [DOI] [PubMed] [Google Scholar]
- 231.Lopez-Diaz L, Hinkle KL, Jain RN, Zavros Y, Brunkan CS, Keeley T, Eaton KA, Merchant JL, Chew CS, Samuelson LC. Parietal cell hyperstimulation and autoimmune gastritis in cholera toxin transgenic mice. Am J Physiol Gastrointest Liver Physiol 290: G970–G979, 2006. doi: 10.1152/ajpgi.00461.2005. [DOI] [PubMed] [Google Scholar]
- 232.Lüpfert C, Grell E, Pintschovius V, Apell HJ, Cornelius F, Clarke RJ. Rate limitation of the Na(+),K(+)-ATPase pump cycle. Biophys J 81: 2069–2081, 2001. doi: 10.1016/S0006-3495(01)75856-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Lynch DA, Mapstone NP, Lewis F, Pentith J, Axon AT, Dixon MF, Quirke P. Serum and gastric luminal epidermal growth factor in Helicobacter pylori-associated gastritis and peptic ulcer disease. Helicobacter 1: 219–226, 1996. doi: 10.1111/j.1523-5378.1996.tb00042.x. [DOI] [PubMed] [Google Scholar]
- 234.Madison BB, Braunstein K, Kuizon E, Portman K, Qiao XT, Gumucio DL. Epithelial hedgehog signals pattern the intestinal crypt-villus axis. Development 132: 279–289, 2005. doi: 10.1242/dev.01576. [DOI] [PubMed] [Google Scholar]
- 235.Maeda M, Ishizaki J, Futai M. cDNA cloning and sequence determination of pig gastric (H+ + K+)-ATPase. Biochem Biophys Res Commun 157: 203–209, 1988. doi: 10.1016/S0006-291X(88)80033-0. [DOI] [PubMed] [Google Scholar]
- 236.Maeda M, Oshiman K, Tamura S, Futai M. Human gastric (H+ + K+)-ATPase gene. Similarity to (Na+ + K+)-ATPase genes in exon/intron organization but difference in control region. J Biol Chem 265: 9027–9032, 1990. [PubMed] [Google Scholar]
- 237.Main IH, Whittle BJ. The effects E and A prostaglandins on gastric mucosal blood flow and acid secretion in the rat. Br J Pharmacol 49: 428–436, 1973. doi: 10.1111/j.1476-5381.1973.tb17253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Major JS, Scholes P. The localization of a histamine H2-receptor adenylate cyclase system in canine parietal cells and its inhibition by prostaglandins. Agents Actions 8: 324–331, 1978. doi: 10.1007/BF01968611. [DOI] [PubMed] [Google Scholar]
- 239.Malfertheiner P, Megraud F, O’Morain CA, Gisbert JP, Kuipers EJ, Axon AT, Bazzoli F, Gasbarrini A, Atherton J, Graham DY, Hunt R, Moayyedi P, Rokkas T, Rugge M, Selgrad M, Suerbaum S, Sugano K, El-Omar EM; European Helicobacter and Microbiota Study Group and Consensus panel . Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 66: 6–30, 2017. doi: 10.1136/gutjnl-2016-312288. [DOI] [PubMed] [Google Scholar]
- 240.Malinowska DH, Sherry AM, Tewari KP, Cuppoletti J. Gastric parietal cell secretory membrane contains PKA- and acid-activated Kir2.1 K+ channels. Am J Physiol Cell Physiol 286: C495–C506, 2004. doi: 10.1152/ajpcell.00386.2003. [DOI] [PubMed] [Google Scholar]
- 241.Manela FD, Ren J, Gao J, McGuigan JE, Harty RF. Calcitonin gene-related peptide modulates acid-mediated regulation of somatostatin and gastrin release from rat antrum. Gastroenterology 109: 701–706, 1995. doi: 10.1016/0016-5085(95)90376-3. [DOI] [PubMed] [Google Scholar]
- 242.Mard SA, Askari H, Neisi N, Veisi A. Antisecretory effect of hydrogen sulfide on gastric acid secretion and the involvement of nitric oxide. BioMed Res Int 2014: 480921, 2014. doi: 10.1155/2014/480921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Marshall B. Helicobacter pylori: 20 years on. Clin Med (Lond) 2: 147–152, 2002. doi: 10.7861/clinmedicine.2-2-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 323: 1311–1315, 1984. doi: 10.1016/S0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 245.Martin GR, McKnight GW, Dicay MS, Coffin CS, Ferraz JG, Wallace JL. Hydrogen sulphide synthesis in the rat and mouse gastrointestinal tract. Dig Liver Dis 42: 103–109, 2010. doi: 10.1016/j.dld.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 246.Martin-Vasallo P, Dackowski W, Emanuel JR, Levenson R. Identification of a putative isoform of the Na,K-ATPase beta subunit. Primary structure and tissue-specific expression. J Biol Chem 264: 4613–4618, 1989. [PubMed] [Google Scholar]
- 247.Martinez V, Curi AP, Torkian B, Schaeffer JM, Wilkinson HA, Walsh JH, Taché Y. High basal gastric acid secretion in somatostatin receptor subtype 2 knockout mice. Gastroenterology 114: 1125–1132, 1998. doi: 10.1016/S0016-5085(98)70417-2. [DOI] [PubMed] [Google Scholar]
- 248.Masuda Y, Tanaka T, Inomata N, Ohnuma N, Tanaka S, Itoh Z, Hosoda H, Kojima M, Kangawa K. Ghrelin stimulates gastric acid secretion and motility in rats. Biochem Biophys Res Commun 276: 905–908, 2000. doi: 10.1006/bbrc.2000.3568. [DOI] [PubMed] [Google Scholar]
- 249.Matsuo J, Kimura S, Yamamura A, Koh CP, Hossain MZ, Heng DL, Kohu K, Voon DC, Hiai H, Unno M, So JBY, Zhu F, Srivastava S, Teh M, Yeoh KG, Osato M, Ito Y. Identification of Stem Cells in the Epithelium of the Stomach Corpus and Antrum of Mice. Gastroenterology 152: 218–231.e14, 2017. doi: 10.1053/j.gastro.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 250.Matsuzaki J, Suzuki H, Minegishi Y, Sugai E, Tsugawa H, Yasui M, Hibi T. Acid suppression by proton pump inhibitors enhances aquaporin-4 and KCNQ1 expression in gastric fundic parietal cells in mouse. Dig Dis Sci 55: 3339–3348, 2010. doi: 10.1007/s10620-010-1167-8. [DOI] [PubMed] [Google Scholar]
- 251.Mazzarello P. Camillo Golgi’s scientific biography. J Hist Neurosci 8: 121–131, 1999. doi: 10.1076/jhin.8.2.121.1836. [DOI] [PubMed] [Google Scholar]
- 252.McColl KE. Helicobacter pylori-negative nonsteroidal anti-inflammatory drug-negative ulcer. Gastroenterol Clin North Am 38: 353–361, 2009. doi: 10.1016/j.gtc.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 253.Melle-Milovanovic D, Milovanovic M, Nagpal S, Sachs G, Shin JM. Regions of association between the alpha and the beta subunit of the gastric H,K-ATPase. J Biol Chem 273: 11075–11081, 1998. doi: 10.1074/jbc.273.18.11075. [DOI] [PubMed] [Google Scholar]
- 254.Mihaljevic S, Katicic M, Karner I, Vuksic-Mihaljevic Z, Dmitrovic B, Ivandic A. The influence of Helicobacter pylori infection on gastrin and somatostatin values present in serum. Hepatogastroenterology 47: 1482–1484, 2000. [PubMed] [Google Scholar]
- 255.Miller CA, Debas HT. Epidermal growth factor stimulates the restitution of rat gastric mucosa in vitro. Exp Physiol 80: 1009–1018, 1995. doi: 10.1113/expphysiol.1995.sp003898. [DOI] [PubMed] [Google Scholar]
- 256.Minegishi Y, Suzuki H, Arakawa M, Fukushima Y, Masaoka T, Ishikawa T, Wright NA, Hibi T. Reduced Shh expression in TFF2-overexpressing lesions of the gastric fundus under hypochlorhydric conditions. J Pathol 213: 161–169, 2007. doi: 10.1002/path.2221. [DOI] [PubMed] [Google Scholar]
- 257.Mizukawa Y, Nishizawa T, Nagao T, Kitamura K, Urushidani T. Cellular distribution of parchorin, a chloride intracellular channel-related protein, in various tissues. Am J Physiol Cell Physiol 282: C786–C795, 2002. doi: 10.1152/ajpcell.00239.2001. [DOI] [PubMed] [Google Scholar]
- 258.Modlin IM. From Prout to the proton pump–a history of the science of gastric acid secretion and the surgery of peptic ulcer. Surg Gynecol Obstet 170: 81–96, 1990. [PubMed] [Google Scholar]
- 259.Modlin IM, Oddsdottir M, Adrian TE, Zdon MJ, Zucker KA, Goldenring JR. A specific histamine-stimulated phosphoprotein in isolated parietal cells. J Surg Res 42: 348–353, 1987. doi: 10.1016/0022-4804(87)90168-5. [DOI] [PubMed] [Google Scholar]
- 260.Morton DM. Pharmacology and toxicology of nizatidine. Scand J Gastroenterol Suppl 22, sup136: 1–8, 1987. doi: 10.3109/00365528709094479. [DOI] [PubMed] [Google Scholar]
- 261.Moss SF, Calam J, Legon S, Bishop AE, Polak JM. Effect of Helicobacter pylori on gastric somatostatin in duodenal ulcer disease. Lancet 340: 930–932, 1992. doi: 10.1016/0140-6736(92)92816-X. [DOI] [PubMed] [Google Scholar]
- 262.Munson K, Lambrecht N, Shin JM, Sachs G. Analysis of the membrane domain of the gastric H(+)/K(+)-ATPase. J Exp Biol 203: 161–170, 2000. [DOI] [PubMed] [Google Scholar]
- 263.Murayama Y, Miyagawa J, Higashiyama S, Kondo S, Yabu M, Isozaki K, Kayanoki Y, Kanayama S, Shinomura Y, Taniguchi N, Matsuzawa Y. Localization of heparin-binding epidermal growth factor-like growth factor in human gastric mucosa. Gastroenterology 109: 1051–1059, 1995. doi: 10.1016/0016-5085(95)90562-6. [DOI] [PubMed] [Google Scholar]
- 264.Nagata A, Ito M, Iwata N, Kuno J, Takano H, Minowa O, Chihara K, Matsui T, Noda T. G protein-coupled cholecystokinin-B/gastrin receptors are responsible for physiological cell growth of the stomach mucosa in vivo. Proc Natl Acad Sci USA 93: 11825–11830, 1996. doi: 10.1073/pnas.93.21.11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Nam KT, Lee H-J, Sousa JF, Weis VG, O’Neal RL, Finke PE, Romero-Gallo J, Shi G, Mills JC, Peek RM Jr, Konieczny SF, Goldenring JR. Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology 139: 2028–2037.e9, 2010. doi: 10.1053/j.gastro.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Nam KT, O’Neal R, Lee YS, Lee YC, Coffey RJ, Goldenring JR. Gastric tumor development in Smad3-deficient mice initiates from forestomach/glandular transition zone along the lesser curvature. Lab Invest 92: 883–895, 2012. doi: 10.1038/labinvest.2012.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Nandi J, Das PK, Zinkievich JM, Baltodano JD, Levine RA. Cyclo-oxygenase-1 inhibition increases acid secretion by modulating H+,K+-ATPase expression and activation in rabbit parietal cells. Clin Exp Pharmacol Physiol 36: 127–134, 2009. doi: 10.1111/j.1440-1681.2008.05032.x. [DOI] [PubMed] [Google Scholar]
- 268.Negulescu PA, Machen TE. Intracellular Ca regulation during secretagogue stimulation of the parietal cell. Am J Physiol Cell Physiol 254: C130–C140, 1988. doi: 10.1152/ajpcell.1988.254.1.C130. [DOI] [PubMed] [Google Scholar]
- 269.Nejati S, Karkhah A, Darvish H, Validi M, Ebrahimpour S, Nouri HR. Influence of Helicobacter pylori virulence factors CagA and VacA on pathogenesis of gastrointestinal disorders. Microb Pathog 117: 43–48, 2018. doi: 10.1016/j.micpath.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 270.Neu B, Randlkofer P, Neuhofer M, Voland P, Mayerhofer A, Gerhard M, Schepp W, Prinz C. Helicobacter pylori induces apoptosis of rat gastric parietal cells. Am J Physiol Gastrointest Liver Physiol 283: G309–G318, 2002. doi: 10.1152/ajpgi.00546.2001. [DOI] [PubMed] [Google Scholar]
- 271.Nguyen N, Kozer-Gorevich N, Gliddon BL, Smolka AJ, Clayton AH, Gleeson PA, van Driel IR. Independent trafficking of the KCNQ1 K+ channel and H+-K+-ATPase in gastric parietal cells from mice. Am J Physiol Gastrointest Liver Physiol 304: G157–G166, 2013. doi: 10.1152/ajpgi.00346.2012. [DOI] [PubMed] [Google Scholar]
- 272.Nishio H, Terashima S, Nakashima M, Aihara E, Takeuchi K. Involvement of prostaglandin E receptor EP3 subtype and prostacyclin IP receptor in decreased acid response in damaged stomach. J Physiol Pharmacol 58: 407–421, 2007. [PubMed] [Google Scholar]
- 273.Nishizawa T, Nagao T, Iwatsubo T, Forte JG, Urushidani T. Molecular cloning and characterization of a novel chloride intracellular channel-related protein, parchorin, expressed in water-secreting cells. J Biol Chem 275: 11164–11173, 2000. doi: 10.1074/jbc.275.15.11164. [DOI] [PubMed] [Google Scholar]
- 274.Nomura S, Settle SH, Leys CM, Means AL, Peek RM Jr, Leach SD, Wright CV, Coffey RJ, Goldenring JR. Evidence for repatterning of the gastric fundic epithelium associated with Ménétrier’s disease and TGFalpha overexpression. Gastroenterology 128: 1292–1305, 2005. doi: 10.1053/j.gastro.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 275.Nomura S, Yamaguchi H, Ogawa M, Wang TC, Lee JR, Goldenring JR. Alterations in gastric mucosal lineages induced by acute oxyntic atrophy in wild-type and gastrin-deficient mice. Am J Physiol Gastrointest Liver Physiol 288: G362–G375, 2005. doi: 10.1152/ajpgi.00160.2004. [DOI] [PubMed] [Google Scholar]
- 276.Noto JM, Peek RM Jr. The Helicobacter pylori cag Pathogenicity Island. Methods Mol Biol 921: 41–50, 2012. doi: 10.1007/978-1-62703-005-2_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.O’Neil A, Petersen CP, Choi E, Engevik AC, Goldenring JR. Unique Cellular Lineage Composition of the First Gland of the Mouse Gastric Corpus. J Histochem Cytochem 65: 47–58, 2017. doi: 10.1369/0022155416678182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Oakley RH, Cidlowski JA. The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease. J Allergy Clin Immunol 132: 1033–1044, 2013. doi: 10.1016/j.jaci.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Oddsdottir M, Goldenring JR, Adrian TE, Zdon MJ, Zucker KA, Modlin IM. Identification and characterization of a cytosolic 30 kDa histamine stimulated phosphoprotein in parietal cell cytosol. Biochem Biophys Res Commun 154: 489–496, 1988. doi: 10.1016/0006-291X(88)90166-0. [DOI] [PubMed] [Google Scholar]
- 280.Ohno S, Yakabi K, Ro S, Ochiai M, Onouchi T, Sakurada T, Takabayashi H, Ishida S, Takayama K. Apelin-12 stimulates acid secretion through an increase of histamine release in rat stomachs. Regul Pept 174: 71–78, 2012. doi: 10.1016/j.regpep.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 281.Okamoto CT, Karpilow JM, Smolka A, Forte JG. Isolation and characterization of gastric microsomal glycoproteins. Evidence for a glycosylated beta-subunit of the H+/K+-ATPase. Biochim Biophys Acta 1037: 360–372, 1990. doi: 10.1016/0167-4838(90)90038-H. [DOI] [PubMed] [Google Scholar]
- 282.Oleastro M, Monteiro L, Lehours P, Mégraud F, Ménard A. Identification of markers for Helicobacter pylori strains isolated from children with peptic ulcer disease by suppressive subtractive hybridization. Infect Immun 74: 4064–4074, 2006. doi: 10.1128/IAI.00123-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Ota S, Razandi M, Krause W, Terano A, Hiraishi H, Ivey KJ. Prostaglandin E2 output is greater by isolated rat gastric parietal cells than non-parietal cells. Prostaglandins 36: 585–587, 1988. doi: 10.1016/0090-6980(88)90004-4. [DOI] [PubMed] [Google Scholar]
- 284.Padol IT, Hunt RH. Effect of Th1 cytokines on acid secretion in pharmacologically characterised mouse gastric glands. Gut 53: 1075–1081, 2004. doi: 10.1136/gut.2003.026435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Pagliocca A, Hegyi P, Venglovecz V, Rackstraw SA, Khan Z, Burdyga G, Wang TC, Dimaline R, Varro A, Dockray GJ. Identification of ezrin as a target of gastrin in immature mouse gastric parietal cells. Exp Physiol 93: 1174–1189, 2008. doi: 10.1113/expphysiol.2008.042648. [DOI] [PubMed] [Google Scholar]
- 286.Pan Q, Ma J, Zhou Q, Li J, Tang Y, Liu Y, Yang Y, Xiao J, Peng L, Li P, Liang D, Zhang H, Chen YH. KCNQ1 loss-of-function mutation impairs gastric acid secretion in mice. Mol Biol Rep 37: 1329–1333, 2010. doi: 10.1007/s11033-009-9511-9. [DOI] [PubMed] [Google Scholar]
- 287.Parente JA Jr, Chen X, Zhou C, Petropoulos AC, Chew CS. Isolation, cloning, and characterization of a new mammalian coronin family member, coroninse, which is regulated within the protein kinase C signaling pathway. J Biol Chem 274: 3017–3025, 1999. doi: 10.1074/jbc.274.5.3017. [DOI] [PubMed] [Google Scholar]
- 288.Parente JA Jr, Goldenring JR, Petropoulos AC, Hellman U, Chew CS. Purification, cloning, and expression of a novel, endogenous, calcium-sensitive, 28-kDa phosphoprotein. J Biol Chem 271: 20096–20101, 1996. doi: 10.1074/jbc.271.33.20096. [DOI] [PubMed] [Google Scholar]
- 289.Pavlov I. The Work of the DIgestive Glands. London: Griffin, 1902. [Google Scholar]
- 290.Peek RM Jr, Moss SF, Wang S, Holt PR, Tham KT, Blaser MJ, Pérez-Pérez GI, Miller GG, Atherton JC. Helicobacter pylori cagA+ strains and dissociation of gastric epithelial cell proliferation from apoptosis. J Natl Cancer Inst 89: 863–868, 1997. doi: 10.1093/jnci/89.12.863. [DOI] [PubMed] [Google Scholar]
- 291.Peek RM Jr, Wirth HP, Moss SF, Yang M, Abdalla AM, Tham KT, Zhang T, Tang LH, Modlin IM, Blaser MJ. Helicobacter pylori alters gastric epithelial cell cycle events and gastrin secretion in Mongolian gerbils. Gastroenterology 118: 48–59, 2000. doi: 10.1016/S0016-5085(00)70413-6. [DOI] [PubMed] [Google Scholar]
- 292.Peng XR, Yao X, Chow D-C, Forte JG, Bennett MK. Association of syntaxin 3 and vesicle-associated membrane protein (VAMP) with H+/K+-ATPase-containing tubulovesicles in gastric parietal cells. Mol Biol Cell 8: 399–407, 1997. doi: 10.1091/mbc.8.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Peretz A, Schottelndreier H, Aharon-Shamgar LB, Attali B. Modulation of homomeric and heteromeric KCNQ1 channels by external acidification. J Physiol 545: 751–766, 2002. doi: 10.1113/jphysiol.2002.028381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Petersen CP, Meyer AR, De Salvo C, Choi E, Schlegel C, Petersen A, Engevik AC, Prasad N, Levy SE, Peebles RS, Pizarro TT, Goldenring JR. A signalling cascade of IL-33 to IL-13 regulates metaplasia in the mouse stomach. Gut 67: 805–817, 2018. doi: 10.1136/gutjnl-2016-312779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Petersen CP, Weis VG, Nam KT, Sousa JF, Fingleton B, Goldenring JR. Macrophages promote progression of spasmolytic polypeptide-expressing metaplasia after acute loss of parietal cells. Gastroenterology 146: 1727–38.e8, 2014. doi: 10.1053/j.gastro.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Pfeiffer A, Rochlitz H, Noelke B, Tacke R, Moser U, Mutschler E, Lambrecht G. Muscarinic receptors mediating acid secretion in isolated rat gastric parietal cells are of the glandular M3-type. Gastroenterology 98: 218–222, 1990. doi: 10.1016/0016-5085(90)91314-v. [DOI] [PubMed] [Google Scholar]
- 297.Phillip J, Domschke S, Domschke W, Urbach HJ, Reiss M, Demling L. Inhibition by somatostatin of gastrin release and gastric acid responses to meals and to pentagastrin in man. Scand J Gastroenterol 12: 261–265, 1977. doi: 10.3109/00365527709180926. [DOI] [PubMed] [Google Scholar]
- 298.Pisegna JR, de Weerth A, Huppi K, Wank SA. Molecular cloning of the human brain and gastric cholecystokinin receptor: structure, functional expression and chromosomal localization. Biochem Biophys Res Commun 189: 296–303, 1992. doi: 10.1016/0006-291X(92)91557-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Polak JM, Grimelius L, Pearse AG, Bloom SR, Arimura A. Growth-hormone release-inhibiting hormone in gastrointestinal and pancreatic D cells. Lancet 305: 1220–1222, 1975. doi: 10.1016/S0140-6736(75)92198-4. [DOI] [PubMed] [Google Scholar]
- 300.Porzionato A, Macchi V, Amagliani A, Castagliuolo I, Parenti A, De Caro R. Neurotensin receptor 1 immunoreactivity in the peripheral ganglia and carotid body. Eur J Histochem 53: 135–142, 2009. doi: 10.4081/ejh.2009.e16. [DOI] [PubMed] [Google Scholar]
- 301.Post RL, Hegyvary C, Kume S. Activation by adenosine triphosphate in the phosphorylation kinetics of sodium and potassium ion transport adenosine triphosphatase. J Biol Chem 247: 6530–6540, 1972. [PubMed] [Google Scholar]
- 302.Powley TL, Spaulding RA, Haglof SA. Vagal afferent innervation of the proximal gastrointestinal tract mucosa: chemoreceptor and mechanoreceptor architecture. J Comp Neurol 519: 644–660, 2011. doi: 10.1002/cne.22541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Premaratne S, Xue C, McCarty JM, Zaki M, McCuen RW, Johns RA, Schepp W, Neu B, Lippman R, Melone PD, Schubert ML. Neuronal nitric oxide synthase: expression in rat parietal cells. Am J Physiol Gastrointest Liver Physiol 280: G308–G313, 2001. doi: 10.1152/ajpgi.2001.280.2.G308. [DOI] [PubMed] [Google Scholar]
- 304.Prinz C, Kajimura M, Scott DR, Mercier F, Helander HF, Sachs G. Histamine secretion from rat enterochromaffinlike cells. Gastroenterology 105: 449–461, 1993. doi: 10.1016/0016-5085(93)90719-S. [DOI] [PubMed] [Google Scholar]
- 305.Quigley EM, Turnberg LA. pH of the microclimate lining human gastric and duodenal mucosa in vivo. Studies in control subjects and in duodenal ulcer patients. Gastroenterology 92: 1876–1884, 1987. doi: 10.1016/0016-5085(87)90619-6. [DOI] [PubMed] [Google Scholar]
- 306.Quinn SJ, Bai M, Brown EM. pH sensing by the calcium-sensing receptor. J Biol Chem 279: 37241–37249, 2004. doi: 10.1074/jbc.M404520200. [DOI] [PubMed] [Google Scholar]
- 307.Rabon E, Sachs G, Bassilian S, Leach C, Keeling D. A K+-competitive fluorescent inhibitor of the H,K-ATPase. J Biol Chem 266: 12395–12401, 1991. [PubMed] [Google Scholar]
- 308.Raptis S, von Berger L, Dollinger HC, Fazekas AA, Pfeiffer EF. Hypergastrinemia induced by glucocorticoid and corticotropin treatment in man. Am J Dig Dis 21: 376–380, 1976. doi: 10.1007/BF01072659. [DOI] [PubMed] [Google Scholar]
- 309.Ray JM, Squires PE, Curtis SB, Meloche MR, Buchan AM. Expression of the calcium-sensing receptor on human antral gastrin cells in culture. J Clin Invest 99: 2328–2333, 1997. doi: 10.1172/JCI119413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Rehfeld JF, Johnsen AH. Identification of gastrin component I as gastrin-71. The largest possible bioactive progastrin product. Eur J Biochem 223: 765–773, 1994. doi: 10.1111/j.1432-1033.1994.tb19051.x. [DOI] [PubMed] [Google Scholar]
- 311.Rettenberger AT, Schulze W, Breer H, Haid D. Analysis of the protein related receptor GPR92 in G-cells. Front Physiol 6: 261, 2015. doi: 10.3389/fphys.2015.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Reuben MA, Lasater LS, Sachs G. Characterization of a beta subunit of the gastric H+/K+-transporting ATPase. Proc Natl Acad Sci USA 87: 6767–6771, 1990. doi: 10.1073/pnas.87.17.6767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Richardson CT, Walsh JH, Hicks MI, Fordtran JS. Studies on the mechanisms of food-stimulated gastric acid secretion in normal human subjects. J Clin Invest 58: 623–631, 1976. doi: 10.1172/JCI108509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Robert A, Nezamis JE, Phillips JP. Inhibition of gastric secretion by prostaglandins. Am J Dig Dis 12: 1073–1076, 1967. doi: 10.1007/BF02233268. [DOI] [PubMed] [Google Scholar]
- 315.Robert A, Olafsson AS, Lancaster C, Zhang WR. Interleukin-1 is cytoprotective, antisecretory, stimulates PGE2 synthesis by the stomach, and retards gastric emptying. Life Sci 48: 123–134, 1991. doi: 10.1016/0024-3205(91)90405-Z. [DOI] [PubMed] [Google Scholar]
- 316.Roepke TK, Anantharam A, Kirchhoff P, Busque SM, Young JB, Geibel JP, Lerner DJ, Abbott GW. The KCNE2 potassium channel ancillary subunit is essential for gastric acid secretion. J Biol Chem 281: 23740–23747, 2006. doi: 10.1074/jbc.M604155200. [DOI] [PubMed] [Google Scholar]
- 317.Roth KA, Kapadia SB, Martin SM, Lorenz RG. Cellular immune responses are essential for the development of Helicobacter felis-associated gastric pathology. J Immunol 163: 1490–1497, 1999. [PubMed] [Google Scholar]
- 318.Roush DL, Gottardi CJ, Naim HY, Roth MG, Caplan MJ. Tyrosine-based membrane protein sorting signals are differentially interpreted by polarized Madin-Darby canine kidney and LLC-PK1 epithelial cells. J Biol Chem 273: 26862–26869, 1998. doi: 10.1074/jbc.273.41.26862. [DOI] [PubMed] [Google Scholar]
- 319.Saberi S, Schmidt A, Eybpoosh S, Esmaili M, Talebkhan Y, Mohajerani N, Oghalaie A, Eshagh Hosseini M, Mohagheghi MA, Bugaytova J, Borén T, Mohammadi M. Helicobacter pylori Strains from Duodenal Ulcer Patients Exhibit Mixed babA/B Genotypes with Low Levels of BabA Adhesin and Lewis b Binding. Dig Dis Sci 61: 2868–2877, 2016. doi: 10.1007/s10620-016-4217-z. [DOI] [PubMed] [Google Scholar]
- 320.Saccomani G, Helander HF, Crago S, Chang HH, Dailey DW, Sachs G. Characterization of gastric mucosal membranes. X. Immunological studies of gastric (H+ + K+)-ATPase. J Cell Biol 83: 271–283, 1979. doi: 10.1083/jcb.83.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Sachs G, Chang HH, Rabon E, Schackman R, Lewin M, Saccomani G. A nonelectrogenic H+ pump in plasma membranes of hog stomach. J Biol Chem 251: 7690–7698, 1976. [PubMed] [Google Scholar]
- 322.Sachs G, Shin JM, Besancon M, Prinz C. The continuing development of gastric acid pump inhibitors. Aliment Pharmacol Ther 7, Suppl 1: 4–12, 1993. doi: 10.1111/j.1365-2036.1993.tb00582.x. [DOI] [PubMed] [Google Scholar]
- 323.Sachs G, Shin JM, Vagin O, Lambrecht N, Yakubov I, Munson K. The gastric H,K ATPase as a drug target: past, present, and future. J Clin Gastroenterol 41, Suppl 2: S226–S242, 2007. doi: 10.1097/MCG.0b013e31803233b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Saha A, Hammond CE, Beeson C, Peek RM Jr, Smolka AJ. Helicobacter pylori represses proton pump expression and inhibits acid secretion in human gastric mucosa. Gut 59: 874–881, 2010. doi: 10.1136/gut.2009.194795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Sahoo N, Gu M, Zhang X, Raval N, Yang J, Bekier M, Calvo R, Patnaik S, Wang W, King G, Samie M, Gao Q, Sahoo S, Sundaresan S, Keeley TM, Wang Y, Marugan J, Ferrer M, Samuelson LC, Merchant JL, Xu H. Gastric Acid Secretion from Parietal Cells Is Mediated by a Ca2+ Efflux Channel in the Tubulovesicle. Dev Cell 41: 262–273.e6, 2017. doi: 10.1016/j.devcel.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Sander LE, Lorentz A, Sellge G, Coëffier M, Neipp M, Veres T, Frieling T, Meier PN, Manns MP, Bischoff SC. Selective expression of histamine receptors H1R, H2R, and H4R, but not H3R, in the human intestinal tract. Gut 55: 498–504, 2006. doi: 10.1136/gut.2004.061762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Sandu C, Artunc F, Grahammer F, Rotte A, Boini KM, Friedrich B, Sandulache D, Metzger M, Just L, Mack A, Skutella T, Rexhepaj R, Risler T, Wulff P, Kuhl D, Lang F. Role of the serum and glucocorticoid inducible kinase SGK1 in glucocorticoid stimulation of gastric acid secretion. Pflugers Arch 455: 493–503, 2007. doi: 10.1007/s00424-007-0305-4. [DOI] [PubMed] [Google Scholar]
- 328.Saperas E, Cominelli F, Taché Y. Potent inhibition of gastric acid secretion by intravenous interleukin-1 beta and -1 alpha in rats. Peptides 13: 221–226, 1992. doi: 10.1016/0196-9781(92)90100-H. [DOI] [PubMed] [Google Scholar]
- 329.Saqui-Salces M, Keeley TM, Grosse AS, Qiao XT, El-Zaatari M, Gumucio DL, Samuelson LC, Merchant JL. Gastric tuft cells express DCLK1 and are expanded in hyperplasia. Histochem Cell Biol 136: 191–204, 2011. doi: 10.1007/s00418-011-0831-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Sato A. Tuft cells. Anat Sci Int 82: 187–199, 2007. doi: 10.1111/j.1447-073X.2007.00188.x. [DOI] [PubMed] [Google Scholar]
- 331.Sawaguchi A, Aoyama F, Ide S, Suganuma T. The cryofixation of isolated rat gastric mucosa provides new insights into the functional transformation of gastric parietal cells: an in vitro experimental model study. Arch Histol Cytol 68: 151–160, 2005. doi: 10.1679/aohc.68.151. [DOI] [PubMed] [Google Scholar]
- 332.Sbarbati A, Osculati F. A new fate for old cells: brush cells and related elements. J Anat 206: 349–358, 2005. doi: 10.1111/j.1469-7580.2005.00403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Sbarbati A, Osculati F. The taste cell-related diffuse chemosensory system. Prog Neurobiol 75: 295–307, 2005. doi: 10.1016/j.pneurobio.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 334.Scarpignato C, Hunt RH. Proton pump inhibitors: the beginning of the end or the end of the beginning? Curr Opin Pharmacol 8: 677–684, 2008. doi: 10.1016/j.coph.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 335.Schepp W, Dehne K, Herrmuth H, Pfeffer K, Prinz C. Identification and functional importance of IL-1 receptors on rat parietal cells. Am J Physiol Gastrointest Liver Physiol 275: G1094–G1105, 1998. doi: 10.1152/ajpgi.1998.275.5.G1094. [DOI] [PubMed] [Google Scholar]
- 336.Schindler M, Humphrey PP. Differential distribution of somatostatin sst2 receptor splice variants in rat gastric mucosa. Cell Tissue Res 297: 163–168, 1999. doi: 10.1007/s004410051344. [DOI] [PubMed] [Google Scholar]
- 337.Schjoldager BT, Mortensen PE, Christiansen J, Orskov C, Holst JJ. GLP-1 (glucagon-like peptide 1) and truncated GLP-1, fragments of human proglucagon, inhibit gastric acid secretion in humans. Dig Dis Sci 34: 703–708, 1989. doi: 10.1007/BF01540341. [DOI] [PubMed] [Google Scholar]
- 338.Schmidt WE, Schenk S, Nustede R, Holst JJ, Fölsch UR, Creutzfeldt W. Cholecystokinin is a negative regulator of gastric acid secretion and postprandial release of gastrin in humans. Gastroenterology 107: 1610–1620, 1994. doi: 10.1016/0016-5085(94)90799-4. [DOI] [PubMed] [Google Scholar]
- 339.Schmidtler J, Dehne K, Allescher HD, Schusdziarra V, Classen M, Holst JJ, Polack A, Schepp W. Rat parietal cell receptors for GLP-1-(7-36) amide: northern blot, cross-linking, and radioligand binding. Am J Physiol Gastrointest Liver Physiol 267: G423–G432, 1994. doi: 10.1152/ajpgi.1994.267.3.G423. [DOI] [PubMed] [Google Scholar]
- 340.Schmidtler J, Schepp W, Janczewska I, Weigert N, Fürlinger C, Schusdziarra V, Classen M. GLP-1-(7-36) amide, -(1-37), and -(1-36) amide: potent cAMP-dependent stimuli of rat parietal cell function. Am J Physiol Gastrointest Liver Physiol 260: G940–G950, 1991. doi: 10.1152/ajpgi.1991.260.6.G940. [DOI] [PubMed] [Google Scholar]
- 341.Schmitz F, Göke MN, Otte JM, Schrader H, Reimann B, Kruse ML, Siegel EG, Peters J, Herzig KH, Fölsch UR, Schmidt WE. Cellular expression of CCK-A and CCK-B/gastrin receptors in human gastric mucosa. Regul Pept 102: 101–110, 2001. doi: 10.1016/S0167-0115(01)00307-X. [DOI] [PubMed] [Google Scholar]
- 342.Schulz S, Röcken C, Ebert MP, Schulz S. Immunocytochemical identification of low-affinity NTS2 neurotensin receptors in parietal cells of human gastric mucosa. J Endocrinol 191: 121–128, 2006. doi: 10.1677/joe.1.06903. [DOI] [PubMed] [Google Scholar]
- 343.Schulz S, Röcken C, Mawrin C, Schulz S. Immunohistochemical localization of CCK1 cholecystokinin receptors in normal and neoplastic human tissues. J Clin Endocrinol Metab 90: 6149–6155, 2005. doi: 10.1210/jc.2005-0172. [DOI] [PubMed] [Google Scholar]
- 344.Schumacher MA, Donnelly JM, Engevik AC, Xiao C, Yang L, Kenny S, Varro A, Hollande F, Samuelson LC, Zavros Y. Gastric Sonic Hedgehog acts as a macrophage chemoattractant during the immune response to Helicobacter pylori. Gastroenterology 142: 1150–1159.e6, 2012. doi: 10.1053/j.gastro.2012.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Schusdziarra V, Harris V, Conlon JM, Arimura A, Unger R. Pancreatic and gastric somatostatin release in response to intragastric and intraduodenal nutrients and HCl in the dog. J Clin Invest 62: 509–518, 1978. doi: 10.1172/JCI109154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Seuwen K, Ludwig MG, Wolf RM. Receptors for protons or lipid messengers or both? J Recept Signal Transduct Res 26: 599–610, 2006. doi: 10.1080/10799890600932220. [DOI] [PubMed] [Google Scholar]
- 347.Sharkey KA, Oland LD, Kirk DR, Davison JS. Capsaicin-sensitive vagal stimulation-induced gastric acid secretion in the rat: evidence for cholinergic vagal afferents. Br J Pharmacol 103: 1997–2003, 1991. doi: 10.1111/j.1476-5381.1991.tb12366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Shaw GP, Hatt JF, Anderson NG, Hanson PJ. Action of epidermal growth factor on acid secretion by rat isolated parietal cells. Biochem J 244: 699–704, 1987. doi: 10.1042/bj2440699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Shimizu N, Ikehara Y, Inada K, Nakanishi H, Tsukamoto T, Nozaki K, Kaminishi M, Kuramoto S, Sugiyama A, Katsuyama T, Tatematsu M. Eradication diminishes enhancing effects of Helicobacter pylori infection on glandular stomach carcinogenesis in Mongolian gerbils. Cancer Res 60: 1512–1514, 2000. [PubMed] [Google Scholar]
- 350.Shin JM, Besancon M, Simon A, Sachs G. The site of action of pantoprazole in the gastric H+/K+-ATPase. Biochim Biophys Acta 1148: 223–233, 1993. doi: 10.1016/0005-2736(93)90133-K. [DOI] [PubMed] [Google Scholar]
- 351.Shin JM, Cho YM, Sachs G. Chemistry of covalent inhibition of the gastric (H+, K+)-ATPase by proton pump inhibitors. J Am Chem Soc 126: 7800–7811, 2004. doi: 10.1021/ja049607w. [DOI] [PubMed] [Google Scholar]
- 352.Shin JM, Grundler G, Senn-Bilfinger J, Simon WA, Sachs G. Functional consequences of the oligomeric form of the membrane-bound gastric H,K-ATPase. Biochemistry 44: 16321–16332, 2005. doi: 10.1021/bi051342q. [DOI] [PubMed] [Google Scholar]
- 353.Shin JM, Sachs G. Differences in binding properties of two proton pump inhibitors on the gastric H+,K+-ATPase in vivo. Biochem Pharmacol 68: 2117–2127, 2004. doi: 10.1016/j.bcp.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 354.Shin JM, Sachs G. Dimerization of the gastric H+,K+-ATPase. J Biol Chem 271: 1904–1908, 1996. doi: 10.1074/jbc.271.4.1904. [DOI] [PubMed] [Google Scholar]
- 355.Shin JM, Sachs G. Identification of a region of the H,K-ATPase alpha subunit associated with the beta subunit. J Biol Chem 269: 8642–8646, 1994. [PubMed] [Google Scholar]
- 356.Shin JM, Sachs G. Restoration of acid secretion following treatment with proton pump inhibitors. Gastroenterology 123: 1588–1597, 2002. doi: 10.1053/gast.2002.36593. [DOI] [PubMed] [Google Scholar]
- 357.Shull GE. cDNA cloning of the beta-subunit of the rat gastric H,K-ATPase. J Biol Chem 265: 12123–12126, 1990. [PubMed] [Google Scholar]
- 358.Shull GE, Lane LK, Lingrel JB. Amino-acid sequence of the beta-subunit of the (Na+ + K+)ATPase deduced from a cDNA. Nature 321: 429–431, 1986. doi: 10.1038/321429a0. [DOI] [PubMed] [Google Scholar]
- 359.Shull GE, Lingrel JB. Molecular cloning of the rat stomach (H+ + K+)-ATPase. J Biol Chem 261: 16788–16791, 1986. [PubMed] [Google Scholar]
- 360.Silverstein FE, Graham DY, Senior JR, Davies HW, Struthers BJ, Bittman RM, Geis S. Misoprostil reduces serious gastrointestinal complications in patients with rheumatois arthritis receiving nonsteroidal anti-inflammatory drugs. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 123: 241–249, 1995. doi: 10.7326/0003-4819-123-4-199508150-00001. [DOI] [PubMed] [Google Scholar]
- 361.Sjödin L. Gastric acid responses to graded vagal stimulation in the anaesthetized cat. Digestion 12: 17–24, 1975. doi: 10.1159/000197649. [DOI] [PubMed] [Google Scholar]
- 362.Sjödin L, Dahlén HG, Viitanen E. Binding of epidermal growth factor to receptors in preparations of enriched porcine parietal cells and inhibition of aminopyrine uptake. Scand J Gastroenterol 27: 495–500, 1992. doi: 10.3109/00365529209000111. [DOI] [PubMed] [Google Scholar]
- 363.Skoglund ML, Gerber JG, Murphy RC, Nies AS. Prostaglandin production by intact isolated gastric parietal cells. Eur J Pharmacol 66: 145–148, 1980. doi: 10.1016/0014-2999(80)90309-X. [DOI] [PubMed] [Google Scholar]
- 364.Solcia E, Vassallo G, Sampietro R. Endocrine cells in the antro-pyloric mucosa of the stomach. Z Zellforsch Mikrosk Anat 81: 474–486, 1967. doi: 10.1007/BF00541009. [DOI] [PubMed] [Google Scholar]
- 365.Soll AH. Specific inhibition by prostaglandins E2 and I2 of histamine-stimulated [14C]aminopyrine accumulation and cyclic adenosine monophosphate generation by isolated canine parietal cells. J Clin Invest 65: 1222–1229, 1980. doi: 10.1172/JCI109777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Song I, Mortell MP, Gantz I, Brown DR, Yamada T. Molecular cloning and structural analysis of canine gastric H+,K+-ATPase. Biochem Biophys Res Commun 196: 1240–1247, 1993. doi: 10.1006/bbrc.1993.2385. [DOI] [PubMed] [Google Scholar]
- 367.Song P, Groos S, Riederer B, Feng Z, Krabbenhöft A, Manns MP, Smolka A, Hagen SJ, Neusch C, Seidler U. Kir4.1 channel expression is essential for parietal cell control of acid secretion. J Biol Chem 286: 14120–14128, 2011. doi: 10.1074/jbc.M110.151191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Song P, Groos S, Riederer B, Feng Z, Krabbenhöft A, Smolka A, Seidler U. KCNQ1 is the luminal K+ recycling channel during stimulation of gastric acid secretion. J Physiol 587: 3955–3965, 2009. doi: 10.1113/jphysiol.2009.173302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Stepan V, Ramamoorthy S, Nitsche H, Zavros Y, Merchant JL, Todisco A. Regulation and function of the sonic hedgehog signal transduction pathway in isolated gastric parietal cells. J Biol Chem 280: 15700–15708, 2005. doi: 10.1074/jbc.M413037200. [DOI] [PubMed] [Google Scholar]
- 370.Sternini C, Wong H, Wu SV, de Georgio R, Yang M, Reeve J Jr, Brecha NC, Walsh JH. Somatostatin 2A receptor is expressed by enteric neurons, and by interstitial cells of Cajal and enterochromaffin-like cells of the gastrointestinal tract. J Comp Neurol 386: 396–408, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- 371.Stewart B, Wallmark B, Sachs G. The interaction of H+ and K+ with the partial reactions of gastric (H+ + K+)-ATPase. J Biol Chem 256: 2682–2690, 1981. [PubMed] [Google Scholar]
- 372.Suda J, Zhu L, Okamoto CT, Karvar S. Rab27b localizes to the tubulovesicle membranes of gastric parietal cells and regulates acid secretion. Gastroenterology 140: 868–878.e2, 2011. doi: 10.1053/j.gastro.2010.09.044. [DOI] [PubMed] [Google Scholar]
- 373.Sweadner KJ, Donnet C. Structural similarities of Na,K-ATPase and SERCA, the Ca2+-ATPase of the sarcoplasmic reticulum. Biochem J 356: 685–704, 2001. doi: 10.1042/bj3560685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Taché Y, Goto Y, Gunion M, Rivier J, Debas H. Inhibition of gastric acid secretion in rats and in dogs by corticotropin-releasing factor. Gastroenterology 86: 281–286, 1984. doi: 10.1016/0016-5085(84)90412-8. [DOI] [PubMed] [Google Scholar]
- 375.Taché Y, Goto Y, Gunion MW, Vale W, River J, Brown M. Inhibition of gastric acid secretion in rats by intracerebral injection of corticotropin-releasing factor. Science 222: 935–937, 1983. doi: 10.1126/science.6415815. [DOI] [PubMed] [Google Scholar]
- 376.Takafuji VA, Evans A, Lynch KR, Roche JK. PGE2 receptors and synthesis in human gastric mucosa: perturbation in cancer. Prostaglandins Leukot Essent Fatty Acids 66: 71–81, 2002. doi: 10.1054/plef.2001.0299. [DOI] [PubMed] [Google Scholar]
- 377.Takeuchi K, Endoh T, Hayashi S, Aihara T. Activation of Muscarinic Acetylcholine Receptor Subtype 4 Is Essential for Cholinergic Stimulation of Gastric Acid Secretion: Relation to D Cell/Somatostatin. Front Pharmacol 7: 278, 2016. doi: 10.3389/fphar.2016.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Takeuchi K, Takehara K, Kaneko T, Okabe S. Nitric oxide and prostaglandins in regulation of acid secretory response in rat stomach following injury. J Pharmacol Exp Ther 272: 357–363, 1995. [PubMed] [Google Scholar]
- 379.Takeuchi K, Yagi K, Kato S, Ukawa H. Roles of prostaglandin E-receptor subtypes in gastric and duodenal bicarbonate secretion in rats. Gastroenterology 113: 1553–1559, 1997. doi: 10.1053/gast.1997.v113.pm9352857. [DOI] [PubMed] [Google Scholar]
- 380.Tang LH, Stoch SA, Modlin IM, Goldenring JR. Identification of rab2 as a tubulovesicle-membrane-associated protein in rabbit gastric parietal cells. Biochem J 285: 715–719, 1992. doi: 10.1042/bj2850715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou MX, Kawamata Y, Fukusumi S, Hinuma S, Kitada C, Kurokawa T, Onda H, Fujino M. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun 251: 471–476, 1998. doi: 10.1006/bbrc.1998.9489. [DOI] [PubMed] [Google Scholar]
- 382.Tatemoto K, Takayama K, Zou MX, Kumaki I, Zhang W, Kumano K, Fujimiya M. The novel peptide apelin lowers blood pressure via a nitric oxide-dependent mechanism. Regul Pept 99: 87–92, 2001. doi: 10.1016/S0167-0115(01)00236-1. [DOI] [PubMed] [Google Scholar]
- 383.Tinel N, Diochot S, Borsotto M, Lazdunski M, Barhanin J. KCNE2 confers background current characteristics to the cardiac KCNQ1 potassium channel. EMBO J 19: 6326–6330, 2000. doi: 10.1093/emboj/19.23.6326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Toh BH, Gleeson PA, Simpson RJ, Moritz RL, Callaghan JM, Goldkorn I, Jones CM, Martinelli TM, Mu FT, Humphris DC. The 60- to 90-kDa parietal cell autoantigen associated with autoimmune gastritis is a beta subunit of the gastric H+/K+-ATPase (proton pump). Proc Natl Acad Sci USA 87: 6418–6422, 1990. doi: 10.1073/pnas.87.16.6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Tominaga K. Distribution of parietal cells in the antral mucosa of human stomachs. Gastroenterology 69: 1201–1207, 1975. doi: 10.1016/S0016-5085(19)32319-4. [DOI] [PubMed] [Google Scholar]
- 386.Toyoshima C, Nakasako M, Nomura H, Ogawa H. Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 A resolution. Nature 405: 647–655, 2000. doi: 10.1038/35015017. [DOI] [PubMed] [Google Scholar]
- 387.Toyoshima C, Nomura H, Sugita Y. Crystal structures of Ca2+-ATPase in various physiological states. Ann N Y Acad Sci 986: 1–8, 2003. doi: 10.1111/j.1749-6632.2003.tb07131.x. [DOI] [PubMed] [Google Scholar]
- 388.Tschöp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature 407: 908–913, 2000. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 389.Tytgat GN. Are there unmet needs in acid suppression? Best Pract Res Clin Gastroenterol 18, Suppl: 67–72, 2004. doi: 10.1016/j.bpg.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 390.Urushidani T, Hanzel DK, Forte JG. Characterization of an 80-kDa phosphoprotein involved in parietal cell stimulation. Am J Physiol Gastrointest Liver Physiol 256: G1070–G1081, 1989. doi: 10.1152/ajpgi.1989.256.6.G1070. [DOI] [PubMed] [Google Scholar]
- 391.Urushidani T, Hanzel DK, Forte JG. Protein phosphorylation associated with stimulation of rabbit gastric glands. Biochim Biophys Acta 930: 209–219, 1987. doi: 10.1016/0167-4889(87)90033-4. [DOI] [PubMed] [Google Scholar]
- 392.Vagin O, Denevich S, Munson K, Sachs G. SCH28080, a K+-competitive inhibitor of the gastric H,K-ATPase, binds near the M5-6 luminal loop, preventing K+ access to the ion binding domain. Biochemistry 41: 12755–12762, 2002. doi: 10.1021/bi025921w. [DOI] [PubMed] [Google Scholar]
- 393.Vagin O, Denevich S, Sachs G. Plasma membrane delivery of the gastric H,K-ATPase: the role of beta-subunit glycosylation. Am J Physiol Cell Physiol 285: C968–C976, 2003. doi: 10.1152/ajpcell.00068.2003. [DOI] [PubMed] [Google Scholar]
- 394.Vagin O, Munson K, Denevich S, Sachs G. Inhibition kinetics of the gastric H,K-ATPase by K-competitive inhibitor SCH28080 as a tool for investigating the luminal ion pathway. Ann N Y Acad Sci 986: 111–115, 2003. doi: 10.1111/j.1749-6632.2003.tb07147.x. [DOI] [PubMed] [Google Scholar]
- 395.Vagin O, Munson K, Lambrecht N, Karlish SJ, Sachs G. Mutational analysis of the K+-competitive inhibitor site of gastric H,K-ATPase. Biochemistry 40: 7480–7490, 2001. doi: 10.1021/bi0105328. [DOI] [PubMed] [Google Scholar]
- 396.Vagin O, Turdikulova S, Sachs G. The H,K-ATPase beta subunit as a model to study the role of N-glycosylation in membrane trafficking and apical sorting. J Biol Chem 279: 39026–39034, 2004. doi: 10.1074/jbc.M405453200. [DOI] [PubMed] [Google Scholar]
- 397.Vagin O, Turdikulova S, Yakubov I, Sachs G. Use of the H,K-ATPase beta subunit to identify multiple sorting pathways for plasma membrane delivery in polarized cells. J Biol Chem 280: 14741–14754, 2005. doi: 10.1074/jbc.M412657200. [DOI] [PubMed] [Google Scholar]
- 398.Vakily M, Zhang W, Wu J, Atkinson SN, Mulford D. Pharmacokinetics and pharmacodynamics of a known active PPI with a novel Dual Delayed Release technology, dexlansoprazole MR: a combined analysis of randomized controlled clinical trials. Curr Med Res Opin 25: 627–638, 2009. doi: 10.1185/03007990802693883. [DOI] [PubMed] [Google Scholar]
- 399.Vallon V, Grahammer F, Volkl H, Sandu CD, Richter K, Rexhepaj R, Gerlach U, Rong Q, Pfeifer K, Lang F. KCNQ1-dependent transport in renal and gastrointestinal epithelia. Proc Natl Acad Sci USA 102: 17864–17869, 2005. doi: 10.1073/pnas.0505860102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, Chan R, Turnbull AV, Lovejoy D, Rivier C, Rivier J, Sawchenko PE, Vale W. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature 378: 287–292, 1995. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- 401.Vegezzi G, Anselmi L, Huynh J, Barocelli E, Rozengurt E, Raybould H, Sternini C. Diet-induced regulation of bitter taste receptor subtypes in the mouse gastrointestinal tract. PLoS One 9: e107732, 2014. doi: 10.1371/journal.pone.0107732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Vesalius A. De Homani Corporis Fabrica. Padua: School of Medicine, 1543. [Google Scholar]
- 403.Vial JD, Garrido J. Actin-like filaments amd membrane rearrangement in oxyntic cells. Proc Natl Acad Sci USA 73: 4032–4036, 1976. doi: 10.1073/pnas.73.11.4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Von Moltke J, Ji M, Liang HE, Locksley RM. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 529: 221–225, 2016. doi: 10.1038/nature16161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Waghray M, Zavros Y, Saqui-Salces M, El-Zaatari M, Alamelumangapuram CB, Todisco A, Eaton KA, Merchant JL. Interleukin-1beta promotes gastric atrophy through suppression of Sonic Hedgehog. Gastroenterology 138: 562–572.e2, 2010. doi: 10.1053/j.gastro.2009.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Wallace JL, Cucala M, Mugridge K, Parente L. Secretagogue-specific effects of interleukin-1 on gastric acid secretion. Am J Physiol Gastrointest Liver Physiol 261: G559–G564, 1991. doi: 10.1152/ajpgi.1991.261.4.G559. [DOI] [PubMed] [Google Scholar]
- 407.Wallace JL, Dicay M, McKnight W, Martin GR. Hydrogen sulfide enhances ulcer healing in rats. FASEB J 21: 4070–4076, 2007. doi: 10.1096/fj.07-8669com. [DOI] [PubMed] [Google Scholar]
- 408.Wallmark B, Sachs G, Mardh S, Fellenius E. Inhibition of gastric (H+ + K+)-ATPase by the substituted benzimidazole, picoprazole. Biochim Biophys Acta 728: 31–38, 1983. doi: 10.1016/0005-2736(83)90433-9. [DOI] [PubMed] [Google Scholar]
- 409.Wallmark B, Stewart HB, Rabon E, Saccomani G, Sachs G. The catalytic cycle of gastric (H+ + K+)-ATPase. J Biol Chem 255: 5313–5319, 1980. [PubMed] [Google Scholar]
- 410.Walsh JH, Isenberg JI, Ansfield J, Maxwell V. Clearance and acid-stimulating action of human big and little gastrins in duodenal ulcer subjects. J Clin Invest 57: 1125–1131, 1976. doi: 10.1172/JCI108379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Wang FB, Young YK, Kao CK. Abdominal vagal afferent pathways and their distributions of intraganglionic laminar endings in the rat duodenum. J Comp Neurol 520: 1098–1113, 2012. doi: 10.1002/cne.22812. [DOI] [PubMed] [Google Scholar]
- 412.Wang L, Lucey MR, Fras AM, Wilson EJ, Del Valle J. Epidermal growth factor and transforming growth factor-alpha directly inhibit parietal cell function through a similar mechanism. J Pharmacol Exp Ther 265: 308–313, 1993. [PubMed] [Google Scholar]
- 413.Wang TC, Dangler CA, Chen D, Goldenring JR, Koh T, Raychowdhury R, Coffey RJ, Ito S, Varro A, Dockray GJ, Fox JG. Synergistic interaction between hypergastrinemia and Helicobacter infection in a mouse model of gastric cancer. Gastroenterology 118: 36–47, 2000. doi: 10.1016/S0016-5085(00)70412-4. [DOI] [PubMed] [Google Scholar]
- 414.Wang TC, Goldenring JR, Dangler C, Ito S, Mueller A, Jeon WK, Koh TJ, Fox JG. Mice lacking secretory phospholipase A2 show altered apoptosis and differentiation with Helicobacter felis infection. Gastroenterology 114: 675–689, 1998. doi: 10.1016/S0016-5085(98)70581-5. [DOI] [PubMed] [Google Scholar]
- 415.Wank SA, Pisegna JR, de Weerth A. Brain and gastrointestinal cholecystokinin receptor family: structure and functional expression. Proc Natl Acad Sci USA 89: 8691–8695, 1992. doi: 10.1073/pnas.89.18.8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Watson C, Zho, Forte JG, Yao X. . Ezrin is required for stimulation-mediated remodeling of apical membrane in gastric parietal cells. Mol Biol Cell 173a, 2000. [Google Scholar]
- 417.Weis VG, Petersen CP, Weis JA, Meyer AR, Choi E, Mills JC, Goldenring JR. Maturity and age influence chief cell ability to transdifferentiate into metaplasia. Am J Physiol Gastrointest Liver Physiol 312: G67–G76, 2017. doi: 10.1152/ajpgi.00326.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Wettergren A, Maina P, Boesby S, Holst JJ. Glucagon-like peptide-1 7-36 amide and peptide YY have additive inhibitory effect on gastric acid secretion in man. Scand J Gastroenterol 32: 552–555, 1997. doi: 10.3109/00365529709025098. [DOI] [PubMed] [Google Scholar]
- 419.Whited KL, Lu D, Tso P, Kent Lloyd KC, Raybould HE. Apolipoprotein A-IV is involved in detection of lipid in the rat intestine. J Physiol 569: 949–958, 2005. doi: 10.1113/jphysiol.2005.097634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Whittle BJ, Moncada S, Vane JR. Comparison of the effects of prostacyclin (PGI2), prostaglandin E1 and D2 on platelet aggregation in different species. Prostaglandins 16: 373–388, 1978. doi: 10.1016/0090-6980(78)90216-2. [DOI] [PubMed] [Google Scholar]
- 421.Wilkes JM, Kajimura M, Scott DR, Hersey SJ, Sachs G. Muscarinic responses of gastric parietal cells. J Membr Biol 122: 97–110, 1991. doi: 10.1007/BF01872634. [DOI] [PubMed] [Google Scholar]
- 422.Williams EK, Chang RB, Strochlic DE, Umans BD, Lowell BB, Liberles SD. Sensory Neurons that Detect Stretch and Nutrients in the Digestive System. Cell 166: 209–221, 2016. doi: 10.1016/j.cell.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Wingo CS. Active proton secretion and potassium absorption in the rabbit outer medullary collecting duct. Functional evidence for proton-potassium-activated adenosine triphosphatase. J Clin Invest 84: 361–365, 1989. doi: 10.1172/JCI114165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Wolosin JM, Forte JG. Changes in the membrane environment of the (K+ + H+)-ATPase following stimulation of the gastric oxyntic cell. J Biol Chem 256: 3149–3152, 1981. [PubMed] [Google Scholar]
- 425.Wolosin JM, Forte JG. Functional differences between K+-ATPase rich membranes isolated from resting or stimulated rabbit fundic mucosa. FEBS Lett 125: 208–212, 1981. doi: 10.1016/0014-5793(81)80720-X. [DOI] [PubMed] [Google Scholar]
- 426.Woodward ER, Lyon ES, Landor J, Dragstedt LR. The physiology of the gastric antrum: experimental studies on isolated antrum pouches in dogs. Gastroenterology 27: 766–785, 1954. doi: 10.1016/S0016-5085(19)36076-7. [DOI] [PubMed] [Google Scholar]
- 427.Woodward ER, Robertson C, Fried W, Schapiro H. Further studies on the isolated gastric antrum. Gastroenterology 32: 868–877, 1957. doi: 10.1016/S0016-5085(57)80033-X. [DOI] [PubMed] [Google Scholar]
- 428.Wu SV, Rozengurt N, Yang M, Young SH, Sinnett-Smith J, Rozengurt E. Expression of bitter taste receptors of the T2R family in the gastrointestinal tract and enteroendocrine STC-1 cells. Proc Natl Acad Sci USA 99: 2392–2397, 2002. doi: 10.1073/pnas.042617699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Wyrwicka W, Garcia R. Effect of electrical stimulation of the dorsal nucleus of the vagus nerve on gastric acid secretion in cats. Exp Neurol 65: 315–325, 1979. doi: 10.1016/0014-4886(79)90101-8. [DOI] [PubMed] [Google Scholar]
- 430.Xian Y, Zhao X, Wang C, Kang C, Ding L, Zhu W, Hang S. Phenylalanine and tryptophan stimulate gastrin and somatostatin secretion and H+-K+-ATPase activity in pigs through calcium-sensing receptor. Gen Comp Endocrinol 267: 1–8, 2018. doi: 10.1016/j.ygcen.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 431.Xiao C, Feng R, Engevik AC, Martin JR, Tritschler JA, Schumacher M, Koncar R, Roland J, Nam KT, Goldenring JR, Zavros Y. Sonic Hedgehog contributes to gastric mucosal restitution after injury. [Correction in Lab Invest 93: 264, 2013.] Lab Invest 93: 96–111, 2013. doi: 10.1038/labinvest.2012.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Xiao C, Ogle SA, Schumacher MA, Orr-Asman MA, Miller ML, Lertkowit N, Varro A, Hollande F, Zavros Y. Loss of parietal cell expression of Sonic hedgehog induces hypergastrinemia and hyperproliferation of surface mucous cells. Gastroenterology 138: 550–561.e8, 2010. doi: 10.1053/j.gastro.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Yakabi K, Kawashima J, Kato S. Ghrelin and gastric acid secretion. World J Gastroenterol 14: 6334–6338, 2008. doi: 10.3748/wjg.14.6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Yalow RS, Berson SA. Radioimmunoassay of gastrin. Gastroenterology 58: 1–14, 1970. doi: 10.1016/S0016-5085(70)80086-5. [DOI] [PubMed] [Google Scholar]
- 435.Yang GK, Chen JF, Kieffer TJ, Kwok YN. Regulation of somatostatin release by adenosine in the mouse stomach. J Pharmacol Exp Ther 329: 729–737, 2009. doi: 10.1124/jpet.108.146050. [DOI] [PubMed] [Google Scholar]
- 436.Yao X, Thibodeau A, Forte JG. Ezrin-calpain I interactions in gastric parietal cells. Am J Physiol Cell Physiol 265: C36–C46, 1993. doi: 10.1152/ajpcell.1993.265.1.C36. [DOI] [PubMed] [Google Scholar]
- 437.Yi S, Lee H, Jang SB, Byun HM, Yoon SH, Cho JY, Jang IJ, Yu KS. A novel K+ competitive acid blocker, YH4808, sustains inhibition of gastric acid secretion with a faster onset than esomeprazole: randomised clinical study in healthy volunteers. Aliment Pharmacol Ther 46: 337–346, 2017. doi: 10.1111/apt.14148. [DOI] [PubMed] [Google Scholar]
- 438.Yip L, Leung HC, Kwok YN. Role of adenosine A1 receptor in the regulation of gastrin release. J Pharmacol Exp Ther 310: 477–487, 2004. doi: 10.1124/jpet.104.066654. [DOI] [PubMed] [Google Scholar]
- 439.Yokotani K, DelValle J, Park J, Yamada T. Muscarinic M3 receptor-mediated release of gastrin from canine antral G cells in primary culture. Digestion 56: 31–34, 1995. doi: 10.1159/000201218. [DOI] [PubMed] [Google Scholar]
- 440.Yoo OJ, Powell CT, Agarwal KL. Molecular cloning and nucleotide sequence of full-length of cDNA coding for porcine gastrin. Proc Natl Acad Sci USA 79: 1049–1053, 1982. doi: 10.1073/pnas.79.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Yu KS, Bae KS, Shon JH, Cho JY, Yi SY, Chung JY, Lim HS, Jang IJ, Shin SG, Song KS, Moon BS. Pharmacokinetic and pharmacodynamic evaluation of a novel proton pump inhibitor, YH1885, in healthy volunteers. J Clin Pharmacol 44: 73–82, 2004. doi: 10.1177/0091270003261321. [DOI] [PubMed] [Google Scholar]
- 442.Zaki M, Coudron PE, McCuen RW, Harrington L, Chu S, Schubert ML. H. pylori acutely inhibits gastric secretion by activating CGRP sensory neurons coupled to stimulation of somatostatin and inhibition of histamine secretion. Am J Physiol Gastrointest Liver Physiol 304: G715–G722, 2013. doi: 10.1152/ajpgi.00187.2012. [DOI] [PubMed] [Google Scholar]
- 443.Zavros Y, Fleming WR, Hardy KJ, Shulkes A. Regulation of fundic and antral somatostatin secretion by CCK and gastrin. Am J Physiol Gastrointest Liver Physiol 274: G742–G750, 1998. doi: 10.1152/ajpgi.1998.274.4.G742. [DOI] [PubMed] [Google Scholar]
- 444.Zavros Y, Orr MA, Xiao C, Malinowska DH. Sonic hedgehog is associated with H+-K+-ATPase-containing membranes in gastric parietal cells and secreted with histamine stimulation. Am J Physiol Gastrointest Liver Physiol 295: G99–G111, 2008. doi: 10.1152/ajpgi.00389.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Zavros Y, Waghray M, Tessier A, Bai L, Todisco A, L Gumucio D, Samuelson LC, Dlugosz A, Merchant JL. Reduced pepsin A processing of sonic hedgehog in parietal cells precedes gastric atrophy and transformation. J Biol Chem 282: 33265–33274, 2007. doi: 10.1074/jbc.M707090200. [DOI] [PubMed] [Google Scholar]
- 446.Zdon MJ, Adrian TE, Modlin IM. Gastric somatostatin release: evidence for direct mediation by calcitonin gene-related peptide and vasoactive intestinal peptide. J Surg Res 44: 680–686, 1988. doi: 10.1016/0022-4804(88)90100-X. [DOI] [PubMed] [Google Scholar]
- 447.Zeng N, Athmann C, Kang T, Lyu RM, Walsh JH, Ohning GV, Sachs G, Pisegna JR. PACAP type I receptor activation regulates ECL cells and gastric acid secretion. J Clin Invest 104: 1383–1391, 1999. doi: 10.1172/JCI7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Zeng N, Kang T, Wen Y, Wong H, Walsh J, Sachs G. Galanin inhibition of enterochromaffin-like cell function. Gastroenterology 115: 330–339, 1998. doi: 10.1016/S0016-5085(98)70199-4. [DOI] [PubMed] [Google Scholar]
- 449.Zeng N, Walsh JH, Kang T, Helander KG, Helander HF, Sachs G. Selective ligand-induced intracellular calcium changes in a population of rat isolated gastric endocrine cells. Gastroenterology 110: 1835–1846, 1996. doi: 10.1053/gast.1996.v110.pm8964409. [DOI] [PubMed] [Google Scholar]
- 450.Zeng N, Walsh JH, Kang T, Wu SV, Sachs G. Peptide YY inhibition of rat gastric enterochromaffin-like cell function. Gastroenterology 112: 127–135, 1997. doi: 10.1016/S0016-5085(97)70227-0. [DOI] [PubMed] [Google Scholar]
- 451.Zimmerhackl B, Wünsch E, Classen M, Schusdziarra V, Schepp W. In man histamine and muscarinergic mechanisms are essential mediators of acid secretion in response to synthetic human gastrin (1-17). Regul Pept 46: 583–592, 1993. doi: 10.1016/0167-0115(93)90260-F. [DOI] [PubMed] [Google Scholar]