Keywords: development, gene regulation, heart diseases, mRNA, post-transcriptional, RNA binding protein, RNA processing
Abstract
The central dogma of molecular biology illustrates the importance of mRNAs as critical mediators between genetic information encoded at the DNA level and proteomes/metabolomes that determine the diverse functional outcome at the cellular and organ levels. Although the total number of protein-producing (coding) genes in the mammalian genome is ~20,000, it is evident that the intricate processes of cardiac development and the highly regulated physiological regulation in the normal heart, as well as the complex manifestation of pathological remodeling in a diseased heart, would require a much higher degree of complexity at the transcriptome level and beyond. Indeed, in addition to an extensive regulatory scheme implemented at the level of transcription, the complexity of transcript processing following transcription is dramatically increased. RNA processing includes post-transcriptional modification, alternative splicing, editing and transportation, ribosomal loading, and degradation. While transcriptional control of cardiac genes has been a major focus of investigation in recent decades, a great deal of progress has recently been made in our understanding of how post-transcriptional regulation of mRNA contributes to transcriptome complexity. In this review, we highlight some of the key molecular processes and major players in RNA maturation and post-transcriptional regulation. In addition, we provide an update to the recent progress made in the discovery of RNA processing regulators implicated in cardiac development and disease. While post-transcriptional modulation is a complex and challenging problem to study, recent technological advancements are paving the way for a new era of exciting discoveries and potential clinical translation in the context of cardiac biology and heart disease.
In this review we describe and highlight the complex processes involved in the post-transcriptional regulation of mRNAs in shaping the functional outcomes of the cardiac transcriptome. We feature recent advancements in the field and discuss a paradigm-shifting role for mRNA metabolism in cardiac function that has led to a greater understanding of cardiac biology. We describe the key players in and the functional impact of post-transcriptional regulation. This includes a description of key RNA binding proteins involved in the modification, splicing, and turnover of mRNA within the context of cardiac development and disease. Finally, we discuss the current challenges and future directions in this exciting area of research, which has far-reaching implications for new insights in cardiac biology and for the development of novel therapies to treat heart disease.
I. INTRODUCTION
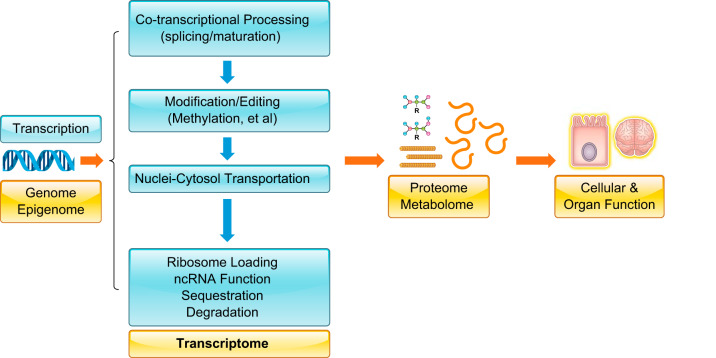
In sync with transcriptional synthesis by the RNA polymerase II complex, the nascent mRNA transcripts are immediately processed by several highly integrated RNA modification steps, including the classic 5′-capping, intron splicing, and 3′-polyadenylation to yield matured mRNA transcripts. The processed mRNAs are subsequently transported out of the nucleus into the cytoplasm where they will be either loaded onto ribosomes for polypeptide synthesis or shuttled to other discrete compartments, such as the stress granules or P-bodies, for sequestration and/or degradation (101, 153, 162). Although much of the core machinery involved in the modification, splicing, and degradation of RNA are highly conserved, each step of these post-transcriptional processes can be modulated by developmental cues, cellular signaling, or environmental conditions in a cell type- and tissue-specific manner, and this is accomplished by a large number of RNA binding proteins (22, 33, 42). Furthermore, post-transcriptional modifications and editing are emerging as additional reprogramming signals that can potentially affect the functionality of RNA molecules (15, 55, 63, 139, 210, 247). Consequently, a majority of protein coding genes in humans can produce multiple mRNA species encoding different protein products with potentially different regulatory properties that alter their intracellular localization, translational capacities, and turnover rates (FIGURE 1). Therefore, the finite number of protein coding genes in mammalian genome can contribute, through alternative splicing and distinct modifications, to many orders of magnitude greater complexity to a cell’s transcriptome that leads to diverse and specific cellular functions and to dynamic responses to specific external conditions. Thus it is not difficult to appreciate that the highly regulated processes of mRNA maturation and disposal (i.e., mRNA metabolism) should have critical importance in a wide array of physiological and disease settings.
FIGURE 1.
Transcriptome in linking genome with proteome/metabolome and cellular/organ function. It features the flow of genetic information from DNA through multiple layers of post-transcriptional regulation to generate a cellular transcriptome before the establishment of the proteome and metabolome.
Post-transcriptional modulation is an integral part of the transcriptome programming during cardiogenic processes in developing embryos. The emerging genetic and molecular evidence implicates post-transcriptional regulation of mRNA as an important underlying mechanism to physiological adaptation or pathological maladaptation in the heart (17, 72, 86, 110, 159). Matching the complexity of the post-transcriptional RNA modulatory processes, the complexity of the world of RNA-binding proteins (numbering ~1,500) encoded by the human genome is manifest by the large number of this class of protein (42, 136). They work in a highly coordinated and regulated manner to accomplish each of the metabolic activities during the life cycle of RNA transcripts. Despite such multilayered complexities, post-transcriptional regulation of mRNA is still very much understudied compared with the intense research efforts afforded to gene expression in the heart. The lack of progress is also due to the technical challenges uniquely associated with the experimental manipulation of RNA. However, recent advancement in RNA-focused molecular approaches has now provided new opportunities to extend our knowledge regarding the scope of RNA metabolic dynamics in cardiac development and disease. In this review and based on recent discoveries in model organisms, we provide an overview of the major players in the key processes of RNA metabolism, and will discuss their role in cotranscriptional processing and maturation to targeted translation and degradation. Then, we highlight some of the recent findings implicating specific RNA post-transcriptional regulators in cardiac development and diseases (FIGURE 1). Finally, we discuss the key gaps in our knowledge, and the main challenges in RNA studies, as well as potential opportunities for future progress offered by recent advancements in RNA-focused technologies. This review will remain confined to protein-coding RNAs since the topics of the noncoding RNAs, particularly the microRNAs and the long-non-coding RNAs, have been well covered in recent excellent reviews by Beermann et al. (16) and others (128, 191, 209).
A. mRNA Maturation Process
Like all eukaryotes, mammalian mRNAs are generally transcribed by the RNA polymerase II complex (PolII). The nascent mRNA molecules are capped at the 5′ end with a m7G structure to prevent degradation and facilitate nuclei export and ribosomal loading. The emerging intronic sequences in the pre-mRNAs are efficiently removed by the RNA spliceosome while the PolII continues RNA synthesis along the DNA template (184). At the end of transcription, RNA cleavage occurs, and the polyadenylated tail is then added to the 3′ end after its release from the PolII complex (FIGURE 2).
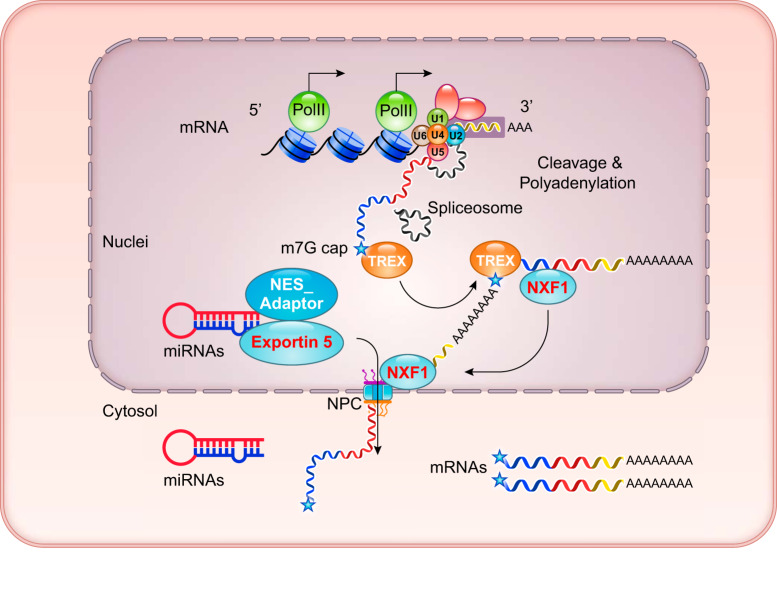
FIGURE 2.
Coupled RNA processing and transcription in the nucleus. This nuclei illustrates cotranscriptional RNA modification and splicing before nuclear to cytosol export. Spliceosome, including U1, U2, U4, and U6 core; TREX, transcription/export complex; NXF1, nuclear export factor 1; NPC, nuclear pore complex.
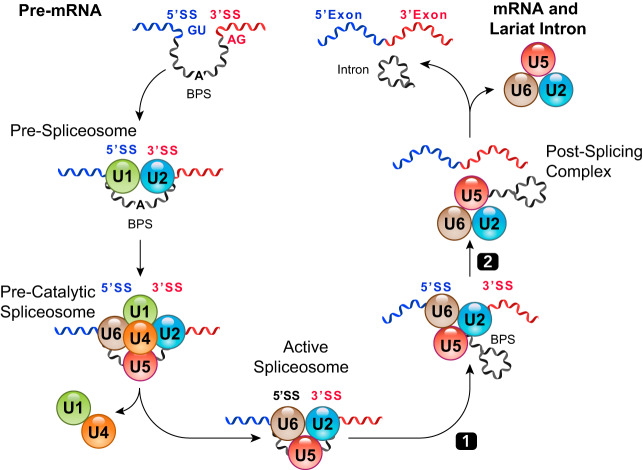
It remains enigmatic how sequential RNA splicing events are accurately and efficiently performed along the multi-exon RNA molecules, particularly when the exon/intron junctions appear to be interchangeable. One solution to resolve this issue is to couple RNA splicing with RNA polymerase-mediated RNA elongation in a highly synchronized manner (FIGURES 2 and 3) (12, 17, 27, 85, 86, 120, 178, 184). As soon as the nascent RNA molecule is synthesized and emerges from the PolII complex, the spliceosome begins to assemble on the mRNA template (85), starting with the recruitment of U1/U2 small-nuclear ribonucleoproteins (snRNPs) to demarcate the 5′ and the 3′ splicing site (SS) at the exon/intron junctions. The subsequent recruitment and maturation of the splicing machinery (by stepwise recruitment of U2, U5, and U6 snRNPs and other factors) lead to a two-step reaction that yields two spliced exons and a lariat intron RNA (FIGURE 4).
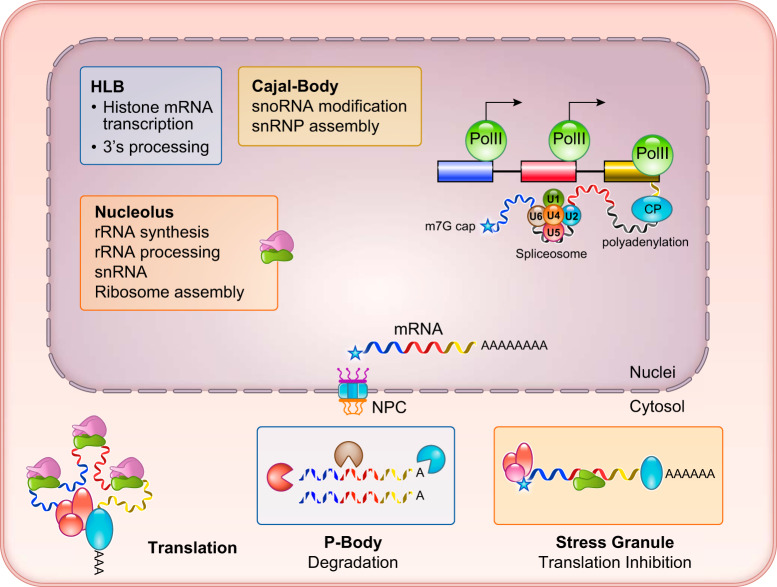
FIGURE 3.
Localized RNA biogenesis and post-transcriptional processing. Specialized RNA processing centers are illustrated, including the nucleolus, rRNA synthesis, snoRNA modification, and ribosome assembly. HLB, histone locus body; NPC, nuclear pore complex.
FIGURE 4.
Overview of RNA splicing process. A prototypic splicing event from pre-mRNA to spliced exons and lariat intron RNAs. BPS, branch point site.
Following proper cleavage and polyadenylation at the 3′ end, the fully processed mRNAs are recognized by a highly conserved multicomponent transcription/export complex (TREX) (50, 84), which interacts with the hyperphosphorylated PolII COOH-terminal domain (CTD) (77), the exon-junction complex (EJC) (69), the 5′-cap binding complex (176, 201, 238), and the polyA binding protein (PABP) (194). Through additional interaction with nuclear RNA export factor 1 (NXF1), the bulk of mRNAs are transported out of the nucleus into the cytosol through the nuclear pore complex (NPC) (95, 174). As illustrated in FIGURES 2 and 3, the entire maturation process of mRNA is highly integrated and synchronized to ensure efficient and accurate excision of intronic sequences for the ultimate and timely transport of mature mRNAs out of the nucleus. Miscapped or misspliced products are quickly and efficiently degraded by extensive mRNA quality control mechanisms (56, 106, 169, 213, 237, 238). As a consequence, under normal conditions the intron-containing pre-mRNAs are often detected at very low levels relative to the mature mRNAs.
Compared with mRNAs and perhaps a majority of the long-noncoding RNAs, the maturation processes for several other species of noncoding RNAs and histone mRNAs are different and highly specialized. For example, the small nucleolar RNAs (snoRNAs) guided modifications of spliceosomal small nuclear RNA (snRNAs) involve pseudouridylation and 2′-O-methylation, which are restricted to the Cajal body (8, 143). In contrast, histone mRNAs do not require splicing, but they need specialized 3′ modification involving stem-loop-dependent trimming and uridylation instead of polyadenylation. These specialized post-transcriptional modifications of histone mRNAs are carried out in histone locus bodies (HLB) (26, 143) where transcription from the histone locus and post-transcriptional processing are integrated in the same physical location. Finally, ribosomal RNA (rRNA) synthesis, processing (including snoRNA guided pseudouridylation and 2′-O-methylation), and ribosome assembly are carried out in the nucleolus (96, 202). In contrast, the maturation and export of primary microRNA (miRNA) requires a dedicated trimming machinery involving Drosha, with exportin 5 facilitating miRNA export (230) (FIGURE 3).
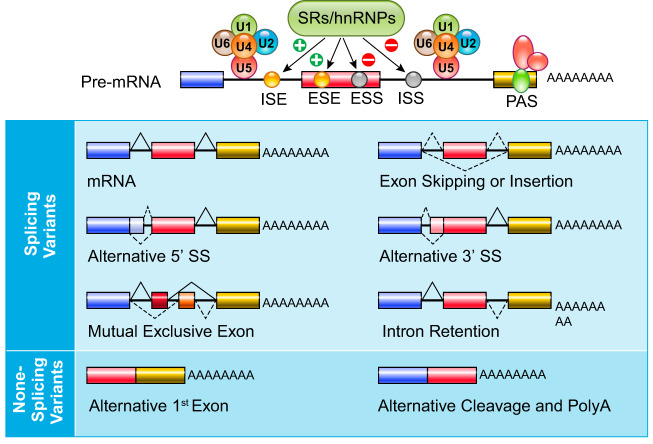
Among the mRNA maturation processes, alternative splicing, alternative initiation, and alternative polyadenylation contribute the most to transcriptomic variation (9, 12, 17, 156). In addition to canonic mRNA spliceosome core subunits (U1-U6 RNPs) (FIGURE 4), a large number of RNA binding proteins serve as RNA splicing regulators. They serve as trans-acting regulators interacting with cis-regulatory elements (RNA sequence motifs) to manipulate spliceosome activities on the targeted intron/exon junction (FIGURE 5). These alternative RNA splicing events can significantly enrich the overall diversity of the mRNA species generated from a single gene through a combination of intron retention, exon skipping/insertion, alternative 5′ or 3′ splicing site utilization, and mutually exclusive exon utilization (FIGURE 5). Among the more than 1,500 known RNA binding proteins (RBPs) in the human genome, only a very small number of them are identified as RNA splicing regulators and remain functionally uncharacterized. According to SpliceAid F, a database cataloguing human splicing factors and their binding sites (74), a total of 71 human trans-acting splicing regulatory proteins are known, including 13 serine/arginine-rich proteins, 27 heterogeneous nuclear ribonucleoprotein (hnRNP) proteins, and 31 additional proteins from other protein families (ELAV, KHDRBS, CUGBP, Nova, and RBFox). Considering that the majority of human mRNAs have multiple RNA species due to alternative splicing, it would be very surprising if the number of RNA splicing regulators remains at the current number. As the main mediators of RNA splicing regulation, these RBPs are subject to additional layers of regulation, which often gives rise to cell type specificity. These additional layers of RBP regulation include differential expression, post-translational modification, localization (nuclear retention vs. exclusion), and alternative splicing of RBP transcripts (120, 177, 204). Similar to transcription factors, the functional activity of the RBPs is quite diverse and often determined by the specific context of their interaction with pre-mRNA, which can be inhibitory or activating towards a specific splicing event (FIGURE 5). In addition, RNA splicing factors often have multifunctional roles in gene expression, as in the case of RBFox2, which serves as regulator of both RNA splicing and epigenetics (226). RNA splicing factors are also linked to RNA degradation (45) and miRNA biogenesis (178) that highlights the integrated nature of RNA splicing with other aspects of RNA metabolism. Finally, considering the highly synchronized coupling with transcription, RNA splicing is also influenced by PolII at the exon-intron junction (184). This appears to be the underlying mechanism linking epigenetics and alternative RNA splicing through targeted chromatin and DNA modifications (3, 85, 186). It has been reported that CCCTC-binding factor (CTCF), an important regulator of chromatin architecture, can promote PolII pausing at intron/exon junctions and modulates RNA splicing (3, 186).
FIGURE 5.
Alternative splicing and splicing variants of mRNAs. Alternative splicing regulated intron/exon is illustrated with potential products. Cis-regulatory elements are marked as ISE, intron splicing enhancer; ESE, exon splicing enhancer; ESS, exon splicing suppressor; or ISS, intro splicing suppressor. SRs, serine/arginine rich; hnRNP, heterogeneous ribonucleotide protein.
Since mRNA maturation has such a major impact on the functional output of our genome, it is not surprising that genetic defects or population variants resulting in aberrant RNA splicing can lead to many diseases including cancer and neural diseases (22, 27, 66). We present greater detail of mRNA splicing as a focal point of cardiac development and pathological remodeling later in this review.
B. mRNA Localization, Ribosomal Loading, and Translation
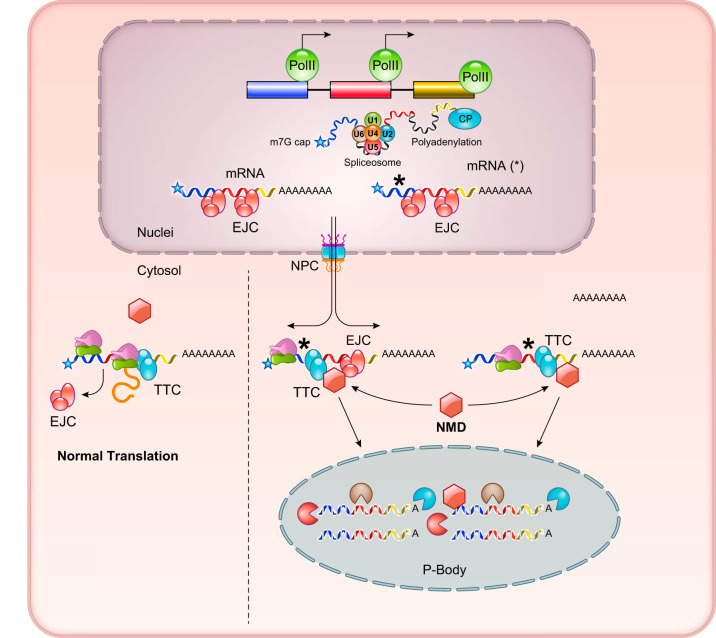
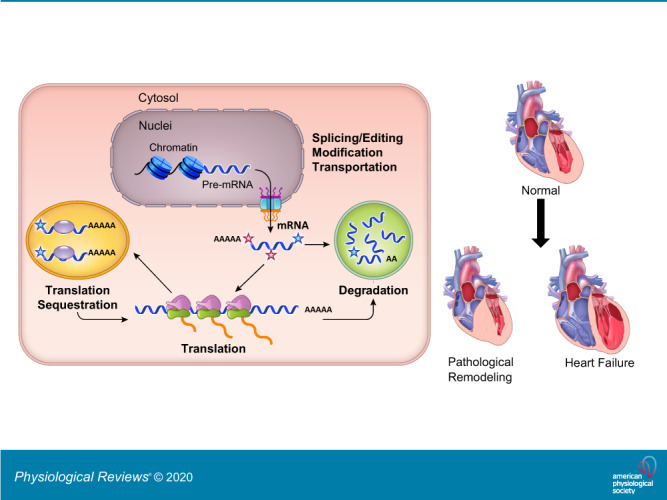
When the processed mRNAs are transported outside of the nucleus, they are generally destined for one of two fates (FIGURE 6). One is cap-dependent translation after the mRNA is loaded onto ribosomes, and the second is degradation. With respect to translation, certain mRNA transcripts are targeted to specific cellular compartments to generate cellular polarity and/or molecular gradients (6, 148, 203). This is particularly prevalent to maternal mRNA species in the developing embryos (89, 196). In addition, mRNAs encoding endoplasmic reticulum (ER) targeted proteins (for transmembrane or secreted proteins) will be anchored on ER membrane through specific receptor complexes interacting with the membrane targeting recognition signal in the nascent peptides (44, 183, 188). Similarly, targeted translation has been observed for mitochondrial-targeted proteins (75, 122). Under stress conditions, such as starvation, mRNAs with stalled translation are sequestered in a structure called stress granules where translation is suppressed, and where the mRNAs remain intact while being decorated with 40S ribosome subunit and other RNA processing proteins as protection against conditions that may adversely affect the integrity of the RNA molecules (5, 51, 81, 82, 193) (FIGURE 3). With respect to RNA degradation, the misprocessed or mistranscribed mRNAs are targeted delivered to P-bodies where the nonsense-mediated degradation (NMD) machinery triggers decapping and deadenylation before undergoing exosome-mediated degradation (166) (FIGURE 6). Beyond the degradation of mutant RNA molecules as a quality control mechanism, NMD is also involved in the targeted degradation of specific mRNA transcripts in a highly regulated and precise manner (56, 107, 135).
FIGURE 6.
Functional fates of mRNAs in cytosol. Normal and mutant mRNAs carrying premature termination signal (*) are directed to either ribosome for protein translation or P-bodies for degradation. EJC, exon junction complex; TTC, translation termination complex; NMD, nonsense mediated degradation.
The selectivity and efficiency of ribosomal loading and translational initiation are contributed by the interactions between eIF-4E (for m7GpppG cap recognition) and translation initiation complexes (eIF-4F) (7, 65). This process is modulated by sequence and structural features in the 5′- (65) or the 3′-untranslated regions (UTR) of mRNAs. Different from the housekeeping genes, which in theory have steady translational activities, a subset of stress-inducible genes have their mRNAs blocked from translation by interacting with translation-suppressing RBPs, such as HuR (31, 187, 218). Upon stimulation, the RBP interactions suppressing translation are removed and the abundance of these mRNAs can be increased as a consequence of higher transcriptional activity, or the abundance of the encoded protein can be increased as a consequence of increased ribosomal loading. The examples include p38 mitogen-activated protein kinase (MAPK)-mediated induction of inflammatory genes such as IL-1β and TNF-α (1, 138, 168). Another group of specialized mRNAs are ribosomal protein encoding mRNAs, which are highly abundant transcripts, but the translation of these transcripts is generally basally suppressed by LARP1 (60). These mRNAs have a shared 5′-terminal oligopyrimidine motif in their 5′-UTR, also referred as TOP mRNAs. Upon nutrient and growth signaling stimulation, mTORC1-mediated signaling leads to translational derepression by LARP1 on the TOP mRNAs (172). mTORC1-mediated phosphorylation of eIF4E-binding proteins (4E-BPs) and the ribosomal S6 kinases (S6Ks) leads to a robust induction of translational initiation efficiency (60). Thus stabilizing mRNAs through a 3′-UTR-mediated RNA-protein interaction or coordinated ribosomal loading can dictate the functional activities of specific mRNAs under different conditions.
C. mRNA Degradation and Stability
Misspliced mRNAs or mRNA transcripts carrying premature termination codon (PTC) mutations are efficiently removed by NMD (107) (FIGURES 3 and 6). This is an important step for translation-dependent mRNA quality control since aberrant production of truncated proteins can lead to disastrous outcomes. There are two highly conserved schemes that contribute to the efficiency and target specificity of NMD (93, 102, 106), both involving a RNA-dependent helicase and the ATPase UPF1. One is EJC dependent, involving the recognition by UPF1 of the abnormal (>50 nucleotides) distances between EJC and translation termination complex (TTC). Another is EJC independent, where UPF1 recognizes the extra-long distances (>1,000 nucleotides) between TTC and the polyA structure (FIGURE 6). These common PTC features allow UPF1 to efficiently direct them to RNA degradation machinery within the P-bodies (5, 134, 239).
Other than clearance of “rogue” mRNA species due to missplicing or premature nonsense mutations, normal turnover of mRNAs occurs in and outside the P-bodies. Different mRNAs exhibit very broad spectrum of turnover rates due to the differential regulation of their stabilities through a myriad of RBP interactions (51, 147, 166). However, RNA degradation often shares similar molecular machinery in 5′-decapping by the Dcp1/Dcp2, and 3′-deadenylation by the CCR4/POP2/NOT complex. The resulting unprotected mRNAs will be degraded quickly by exonucleases such as Xrn1 (134). NMD is also employed for targeted mRNA degradation via specific interactions of RBPs (121).
It is important to recognize that the translational activity of an mRNA molecule must be highly orchestrated with its stability. When premature termination is detected by NMD, ribosomes will be released from the affected mRNAs followed by 5′ decapping and 3′ deadenylation, and quick degradation by the RNA exosomes (157, 187). However, aberrant expression of mutant proteins due to NMD escape is associated with diseased conditions (56, 106).
In summary, mRNAs undergo an elaborate maturation process following PolII-mediated synthesis by 5′ and 3′ modification and alternative splicing. The mature mRNAs that are destined for translation or degradation are transported to specific compartments in the cytosol by dedicated molecular pathways. RNA processing is highly integrated and coupled with RBPs, which serve as the molecular links and entry points for external signaling modulations (FIGURES 3 and 6).
II. mRNA POST-TRANSCRIPTIONAL MODIFICATIONS
Other than the above discussed 5′ capping and 3′ polyadenylation, recent studies have revealed a pervasive level of post-transcriptional modification in the mRNA transcriptome. In fact, more than 100 chemical moieties have been identified on the RNA molecules, and this undoubtedly contributes to an explosive increase in transcriptome complexity (43, 63, 161, 170, 182, 200). This generally describes the area of research that aims to elucidate the role that transcriptional modifications play in transcript fate, and is now referred to as the field of epi-transcriptomics. As a field of research, epi-transcriptomics is in its infancy, with only a few types of RNA modifications having been studied for their functional significance.
A. Post-transcriptional Modification in mRNA Metabolism
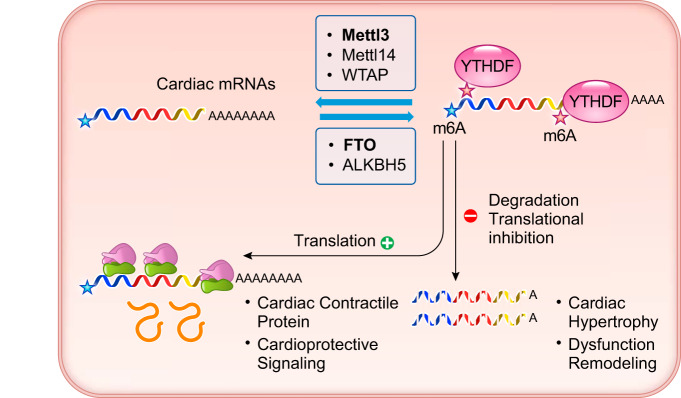
For mRNAs, two types of post-transcriptional modifications are relatively well studied. One is adenosine methylation at the nitrogen-6 position (m6A) and another is uridylation at the 3′-end. Although these modifications have long been observed, the biological function and molecular players have just begun to emerge from recent studies. With the use of mi-CLIP seq, a high-throughput method for identifying m6A modifications in eukaryotic RNA, the full scale of m6A modification was profiled for the human transcriptome, and distinct modification patterns emerged (126). For mRNA m6A modification, the homeostatic level is accomplished by the balance between the epigenetic writers (m6A methyltransferases), including METTL3 and METTL14 and WTAP, and the epigenetic erasers (N6A demethylase), including FTO and ALKBH5. The modified mRNAs are recognized by the epigenetic readers, consisting of YTHDF1–3 family members, which bind to the m6A modified mRNAs (FIGURE 7). The m6A modification sites are highly enriched at the 5′-translation start site (TSS) and the 3′-UTR. In general, m6A modification promotes mRNA translation and stability. Modification of these sites can influence many steps of mRNA metabolism, including CAP-independent translation, nuclear retention, alternative splicing, P-body targeting, translational modulation, and mRNA degradation (FIGURE 7) (2, 10, 25, 29, 43, 57, 63, 158, 161, 170, 182, 200). Despite a broad impact on mRNA metabolism, many of the molecular details involving m6A-dependent regulation of RNA remain unknown (29). On the other hand, uridylation at the 3′-tail of mRNA is another form of post-transcriptional modification that is carried out by a family of terminal uridylyl transferases (TUTases) (36, 119, 247). The consensus is that adding the uridylation to polyadenylated mRNAs promotes exonuclease digestion of stalled RNA molecules; thus this type of modification effectively leads to “gene silencing” by targeted degradation of the mRNA (36, 49, 143, 151, 247).
FIGURE 7.
Summary of mRNA m6A modification in cardiac physiology and pathogenesis. Cardiac mRNAs are modified for m6A by writer genes including Mettl3 (methyltransferase like 3), Mettl14 (methyltransferase like 14), and WTAP (Wilms' tumor 1-associating protein) and by eraser genes including FTO (fat mass and obesity-associated protein, also known as α-ketoglutarate-dependent dioxygenase 9 or ALKBH9) and ALKBH5 (α-ketoglutarate-dependent dioxygenase 5). m6A-modified mRNAs will be recognized by reader genes such as YTHDF (YTH domain-containing family) to affect translation and stability.
B. Functional Impact of mRNA Modification in Cardiac Hypertrophy and Dysfunction
There is increasing evidence that m6A mRNA modification is an important epitranscriptomic feature for normal gene regulation during development and disease, including cancer and neurological disorders (46, 63, 200). Early studies of m6A writers showed Mettl3 knockout in mice caused complete abrogation and m6A modification of RNA in developing embryos, and led to early lethality due to adverse changes in mRNA stability for key molecules involved in the regulation of pluripotency.
In a pair of recent studies, the functional impact of m6A modification is investigated in the context of heart failure. Both studies found that diseased hearts, resulting from different pathological etiologies, including pressure-overload and ischemic injury, are characterized by changes in m6A modification, and these changes can be classified as molecular hallmarks of heart disease (54, 140). In the study reported by Dorn et al. (54), genetic inactivation of the main m6A writer Mettl3 blocks the development of pathological hypertrophy while activation of Mettl3 expression is sufficient to induce hypertrophic remodeling in mouse hearts. On the other hand, Mathiyalagan et al. (140) observed a loss of the m6A eraser FTO is associated with the pathogenesis of heart failure, and genetic induction or silencing of FTO expression leads to either cardioprotection or deterioration of cardiac function, respectively. These two studies provide complementary evidence that m6A modification is dynamically regulated in diseased heart, and elevated m6A level is a pathological contributor to the onset of heart failure. At a mechanistic level, the authors of these two reports find that m6A modification affects the expression levels of the cardiac mRNAs encoding hypertrophy-related signaling molecules and contractile proteins, as a result of modulating the stabilities and translation capacities of these mRNAs. Thus targeted manipulations of m6A modification via its writer or eraser may have a major impact on the pathological outcome in the stressed hearts (FIGURE 7).
Despite these exciting new findings, the molecular basis of m6A-mediated modulation of cardiac mRNA turnover or translation is largely unknown. Nevertheless, other forms of mRNA modifications and their potential contribution to the complexity and function of cardiac transcriptome still need to be explored. Epitranscriptomics is proving to be a new and exciting area of research in cardiac biology and medicine and is opening new possibilities for understanding the molecular factors underpinning heart function and disease.
III. RNA SPLICING REGULATION
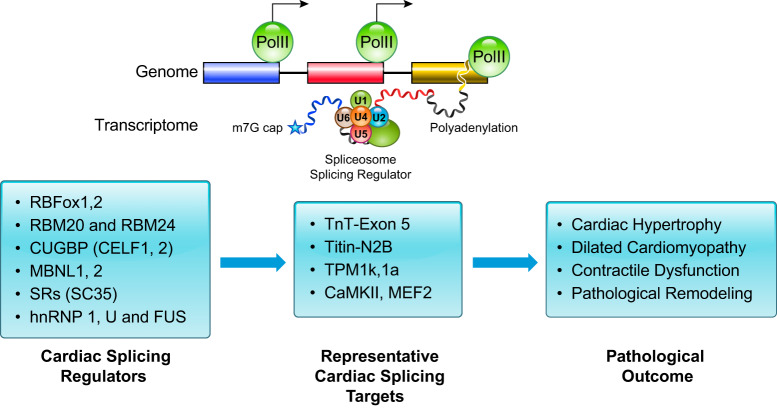
Compared with epitranscriptomics, the field of RNA splicing is well established. Constitutive splicing is performed by the core spliceosome, which includes the ubiquitous ribonucleoprotein complex (FIGURE 3). In contrast, alternative splicing is regulated by specific cis-regulatory elements located in the pre-mRNA and the trans-acting factors with dynamic and tissue-specific expression patterns. These alternative splicing events can generate a variety of splicing isoforms from a single gene. (FIGURE 5). To date, several cardiac specific splicing regulator families have been identified and characterized (FIGURE 8). However, the scope of targeted mRNA splicing by each splicing regulator in the heart is not fully established. Although the proteins of each family recognize unique cis-regulatory elements, each mRNA may be regulated by multiple splicing factors. While splicing is well described, the regulation of alternative splicing remains only superficially characterized, and what we do know is primarily based on the dynamic expression pattern of the specific splicing factors. Moreover, the molecular details of the regulation of alternative splicing in heart remains a very active area of research.
FIGURE 8.
Summary of cardiac mRNA splicing regulation with representative splicing regulators, known downstream mRNA targets, and functional outcomes. Cardiac mRNAs associated with sarcomere and signaling are regulated by alternative splicing, leading to different isoforms that contribute to hypertrophy, chamber dilation, contractile dysfunction, and remodeling.
A. RBFox Family
The RBFox family of proteins (RBFox1, RBFox2, and RBFox3) constitutes a collection of highly conserved, sequence-specific RNA binding proteins. They share a single high-affinity RNA recognition motif (RRM) domain that binds to the cis-regulatory element containing a consensus sequence motif of (U)GCAUG located in the pre-mRNA intronic, exonal, and 3′-UTR regions. Among the three family members, both RBFox1 and RBFox2 showed high expression in the heart but with distinct dynamic patterns (47, 155). The expression of RBFox1 is low in the embryonic heart but upregulated in the matured postnatal heart, while the RBFox2 expression is high in the embryonic heart but downregulated postnatally (67). RBFox1 is highly conserved and has been shown to play critical roles in multiple species. For example, mutation of RBFox1 in Caenorhabditis elegans affects RNA splicing patterns of the nematode’s body wall muscle (108), inactivation of RBFox1 in zebrafish causes developmental defects in the heart (61, 64), and RBFox1 cardiac specific conditional knockout mice show exacerbated heart failure upon stress (67). In addition to RBFox1, RBFox2 also plays important roles during cardiac pathogenesis. Both RBFox1 and RBFox2 showed a decreased expression in response to pressure overload in mouse heart (67, 225), while RBFox2 is elevated in hearts affected with diabetic cardiomyopathy (160). Ablation of RBFox2 in a cardiac specific manner led to a lethal cardiac phenotype in mice that is associated with a global shift in alternative splicing pattern, and is similar to the changes observed in the pressure-overloaded failing hearts (225). Interestingly, besides splicing regulation, RBFox2 also has a direct role in epigenetic regulation through the direct interaction with the polycomb repressing complex 2 (PRC2) (226). Indeed, Hu et al. (90) reported recently that RBFox2 regulates cardiac function through transcriptional suppression of miR-34a expression and its target Junctophilin 2. Therefore, many of the RNA splicing regulators may “moonlight” in other steps of gene regulation, providing more regulatory links with other transcriptional or post-transcriptional processes.
Although both RBFox1 and RBFox2 have been demonstrated to be critical in cardiac pathological remodeling in vivo, RBFox1 and RBFox2 appear to target a different set of alternative splicing events (67, 217, 225). This is likely the result of differential interacting partners for RBFox1 and RBFox2. However, the detailed molecular basis of their target specificity and the interacting partners responsible for the functional outcome in heart remain to be determined. More importantly, RBFox1 and RBFox2 undergo extensive alternative splicing themselves, yielding different isoforms which may affect their molecular function and target specificities. Therefore, loss of RBFox1 and RBFox2 expression and misregulation of these proteins may contribute significantly to the pathogenesis of heart failure.
B. RBM Family
Another class of well-characterized splicing regulators in the heart is the RNA Binding Motif (RBM) family of proteins. In particular, RBM20 features two RNA binding zinc finger (ZnF) domains: one is the RNA-recognition motif (RRM) and another is the arginine/serine-rich domain (224). Mutations in the human Rbm20 gene are linked to dilated cardiomyopathy (224, 228). RBM20 has been suggested to be the main splicing regulator for TTN mRNA, which contains 363 exons and encodes the TITIN protein. In the normal adult heart, TTN mRNA undergoes extensive exon skipping within the regions corresponding to the middle immunoglobulin (Ig) and the proline-glutamate-valine-lysine-abundant (PEVK) domains, but inactivation of RBM20 abolishes these alternative splicing events (125). RBM20 is enriched in nuclear speckles where TTN pre-mRNAs are processed. Although mutations in the Rbm20 gene leads to dilated cardiomyopathy, reducing the RBM20 activity improves cardiac diastolic dysfunction and cardiac atrophy, possibly due to the increasing lengths of the TITIN isoforms as well as other downstream splicing targets regulated by RBM20 (87).
RBM24, another key member of the RBM family of proteins, shows high expression in human and mouse hearts. Interestingly, the expression of RBM24 can also regulate cardiac gene expression, sarcomeric assembly, and cardiac contractility in zebrafish (173). RBM24 expression is induced during the differentiation of human embryonic stem cells into cardiomyocytes. With the use of an inducible RBM24 expression system, it was demonstrated that RBM24 can also regulate a large number of alternative splicing events during mouse embryonic stem cell differentiation (242). RBM38, a homolog of RBM24, can also function in mRNA splicing and stability, but is dispensable for pressure overload-induced cardiac remodeling (205). Finally, RBM10 mutations may be causal of the talipes equinovarus, atrial septal defect, Robin sequence, and persistent left superior vena cava (TARP) syndrome (77a). The expression of RBM10 is reduced during H9C2 myoblasts differentiation (131). At the mechanistic level, RBM10 seems to regulate alternative exon skipping as well as 3′-UTR-dependent processing during cardiac hypertrophic response (145, 219). Overall, the RBM family of genes plays an important role in the genetics of cardiomyopathy.
C. CUGBP Family
Members of the CUG binding protein (CUGBP) family of proteins are also highly expressed in heart, including CUGBP1, also known as CUGBP CUGElav-like family member 1 (CELF1), and CUGBP2, also known as CELF2. Both CUGBP1 and 2 are present in the nucleus and cytoplasm, with high expression in the embryonic heart but downregulated postnatally (111). With the use of CLIP-Seq analysis, it was found that the CUGBP1 binding motifs are highly conserved across different tissues, including heart, muscle, and myoblasts (214). CUGBP1 is also implicated in embryonic heart development (48, 98). In the Cugbp1 knockout mouse model, Giudice et al. (73) observed a significantly lower body weight, which was associated with compromised cardiac function. With the use of RNA-seq analysis, it was further shown that a total of 45 splicing events are affected after Cugbp1 depletion. Among them, 69% contain at least one Cugbp1-CLIP tag adjacent to the alternatively spliced exon, implicating them as direct targets of splicing modulation by Cugbp1 (73). In myotonic dystrophy (MD), accumulation of CUG triplet nucleotides due to mutational expansion leads to CUGBP1 sequestration, CUGBP1 induction, and aberrant RNA splicing (117, 197, 198, 215). CUGBP1 is induced in MD muscle via protein kinase C (PKC)-dependent phosphorylation and protein stabilization (109). Overexpression of CUGBP1 is sufficient to confer the MD phenotype to skeletal and cardiac muscle (105, 221). These findings highlight the importance of CUBGP1 in the phenotypic manifestation of MD. It is now important to determine if and how the CUGBP family of genes contributes to the overall transcriptome reprogramming in the common forms of heart failure.
D. MBNL Family
The human muscleblind-like splicing regulator (MBNL) genes include MBNL1, MBNL2, and MBNL3 and are homologous to the Drosophila gene muscleblind. Like CUBGP1, the recruitment of human MBNL proteins to the CUG expansions in mRNAs is also implicated in the pathogenesis of MD (52, 144). In the heart, the expression of the MBNL1 protein is upregulated during cardiac development (98). In addition to binding to the CUG expansion-containing mRNAs, MBNL1 can also regulate alternative splicing of cardiac sodium channels (such as SCN5A), which potentially contributes to cardiac conduction block and cardiac arrhythmia (62). Reducing MBNL1/CUGBP1-mediated CUG RNA foci via gene targeting or small molecule-based approaches represents a potential therapeutic approach to treat MD (103, 132).
E. SR Family
Members of the serine/arginine-rich (SR) gene family are important components of the generic splicing machinery and are characterized by their ability to interact simultaneously with RNA and other proteins (185). Some of the SR proteins are among the earliest proteins that were demonstrated to have an important role in cardiac-specific alternative splicing regulation. The ablation of the SR protein SC35 (or SRSF2) during early cardiogenesis has no detectable effect on embryonic cardiac development. However, SC35 knockout in adult mice developed significant cardiac hypertrophy associated with chamber dilation (53). Although it is unclear what the main downstream targets of SC35 are in the heart, evidence suggests that the expression of SC35 can alter the pre-mRNA splicing of atrial natriuretic factor (58). In contrast to SC35, ASF1/SF2 (or SRSF1) is critical during embryonic cardiac development as ablation of ASF1/SF2 in cardiomyocytes leads to a hypercontractile phenotype due to a defect in postnatal splicing switch for numerous cardiac genes, including cardiac troponin T and calmodulin kinase-IIδ (231).
F. hnRNP Family
The hnRNP family encompasses a large number of RNA binding proteins that contribute to multiple aspects of alternative splicing. The functions of hnRNPs are different based on their unique cellular localization. In fact, many hnRNPs can shuttle between the cytosol and the nucleus upon stimulation (71). Some of the hnRNPs were suggested to play important roles in cardiac development and function. For example, Liu et al. (129) reported that ablation of hnRNPA1 in mice causes muscle defects, which includes dilated cardiomyopathy with myofibril hypoplasia. Similarly, knocking down hnRNPA1 in zebrafish causes cardiac edema, which suggests that hnRNPA1 has a conserved function in cardiac function and development (129).
PTB (polypyrimidine tract binding protein; also known as hnRNPI) is an RNA binding protein known to regulate alternative splicing in both muscle and neurons. PTB is highly expressed in embryonic heart, and its expression is reduced during cardiac maturation. In the heart, PTB regulates alternative splicing of sarcomeric proteins, including β-tropomyosin, α-tropomyosin, α-actinin, and troponin-T (32, 150, 171). PTB also regulates the alternative splicing of apoptotic genes in the differentiating cardiomyocytes. Its overexpression in cardiomyocytes leads to elevated caspase-dependent apoptosis (240). PTB activity is post-translationally regulated by histone deacetylase (HDAC)5 in cardiomyocytes. Inhibition of HDAC5 in myocytes caused a reduced caspase-dependent cleavage of PTB (235). PTB intersects with cardiac signaling through multiple downstream targets and upstream regulators, and this ultimately impacts cardiac physiology.
Other hnRNPs, such as hnRNPU and the fused in sarcoma/translated in liposarcoma (FUS; also known as TLS or hnRNPP2) are also involved in cardiac development and disease. hnRNPU inactivation leads to dilated cardiomyopathy and premature death 2 wk after birth (234), while FUS is shown to be downregulated during both cardiac and skeletal muscle differentiation and maturation (92). However, the exact functions and downstream splicing targets of hnRNPU and FUS in the heart remain to be established.
IV. FUNCTIONAL IMPACT OF ALTERNATIVE mRNA SPLICING IN HEART
Alternative mRNA splicing generates multiple mRNA species from one gene. The changes in the exon composition of the final mRNAs can lead to either subtle or disparate changes in the functional properties of the encoded proteins (91). Some striking changes were demonstrated for the protein isoforms encoded by different splice variants of an ion channel gene, where localization to the cytoplasm or the mitochondrial membrane was dependent on the splice variant encoding the particular ion channel isoform (189, 233). In addition, some dramatic changes, even those resulting in a protein isoform with functional opposition to the primary protein, have been reported (e.g., Bcl-xS and Bcl-xL) (11, 21). Indeed, a majority of human mRNAs are subjected to alternative RNA splicing, and the splicing variants help to define the cellular identity and differentiation status in a significant way (12). It is conceivable that a majority of cardiac genes are subjected to alternative splicing, and this has a high likelihood of affecting cardiac physiology and pathology. While there is a myriad of genes regulated by alternative splicing, herein we will only discuss the well-characterized role of alternative splicing in isoform switching observed with the sarcomeric proteins, which are critical during cardiac development and disease.
A. Alternative mRNA Splicing in Cardiac Development
At a global level, using a large-scale splicing microarray combined with reverse transcriptase-polymerase chain reaction, Kalsotra et al. (98) were the first to identify a large number of altered alternative splicing events during cardiac development. These alternative splicing events are highly conserved between mouse and chicken. Among these alternative splicing events, a subset of them are coordinated at specific time points during mouse heart development, and this supports the notion that mRNA splicing during cardiac development is highly regulated. With the use of computational analysis, it was further observed that both the GCAUG (RBFox binding motif) and the GUGUG (CUGBP binding motif) elements are enriched in the intronic regions of the alternative spliced exons in the heart, suggesting these RNA binding proteins coordinately regulate at least a subset of alternative splicing events during mouse cardiac development (98). Global mRNA splicing shifts are also observed in human hearts during development, again supporting the conservation of RNA splicing regulation in heart (111, 204, 216).
One of the earliest documented alternative splicing events in the heart occurs with the cardiac troponin T (or TNNT2) gene (41). The embryonic isoform of cardiac troponin T contains exon 5, and the adult isoform lacks exon 5, which contains 10 highly acidic amino acids, and is the result of both cis-regulatory elements and trans-acting factors (39, 40). Another example of cardiac specific alternative splicing is the titin (or TTN) gene. At both transcript and protein levels, the N2BA isoform of titin (3.7 MDa) is the dominant isoform expressed in the perinatal heart. However, the N2BA isoform is rapidly replaced by a smaller N2B isoform (3.0 MDa) due to mRNA splicing (163, 223). This alternative splicing event is critical during postnatal heart development as force measurements show that titin isoform switching greatly increases myofibrillar passive tension (28).
B. Alternative mRNA Splicing in Cardiac Diseases
During cardiac pathological remodeling in both human patients and mouse heart failure models, alternative splicing of mRNAs is dramatically changed at a global scale (204). Using an exon array, Kong et al. (104) compared the left ventricle RNA from both control and ischemic cardiomyopathy patients and identified a significant decrease in RNA splicing efficiency in the ischemic heart. Among the differentially spliced genes, a large set of sarcomere genes are shown to be altered in the diseased human heart. Motif analysis further demonstrated that MBNL and hnRNP F binding motifs are enriched in the sequences flanking the differentially spliced exons. Similar to humans, in mice with pressure overload-induced failing hearts, Lee et al. (116) identified a large set of differentially spliced genes using whole transcriptome RNA-seq. In this study, a total of 1,087 genes were identified to be differentially spliced due to either alternative transcription start site utilization or alternative splicing. As the heart progresses into decompensated heart failure following long-term pressure overload, a larger number of alternative splicing events are identified, showing a correlation between alternative splicing and progression of heart failure (116). Among the differentially spliced genes, many have a known role in cardiac regulation and function, including Rgs12, Rabgap1, and Hdac7 (116). In addition to ischemic and dilated cardiomyopathy, diabetic cardiomyopathy also shows a global change of alternative splicing. Using a mouse type 1 diabetic cardiomyopathy model established with streptozotocin treatment, Verma et al. (206) identified a total of 967 alternative splicing events in diabetic mouse hearts. Many of these alternatively spliced genes are involved in cardiac development and RNA metabolism (206). Interestingly, the alternative splicing observed in diseased heart also appears to be part of the phenomenon of “fetal gene reprogramming,” which occurs in diseased hearts (e.g., dilated cardiomyopathy and diabetic cardiomyopathy) and is characterized by an alternative splicing pattern typically observed during embryonic development (67, 206).
Among the differentially spliced genes in heart failure, sarcomere genes are perhaps the best characterized group. Similar to cardiac development, titin is also subjected to extensive alternative splicing regulation in cardiomyopathy. The titin PEVK segment (named for its enriched Pro-Glu-Val-Lys amino acids) is regulated by RBM20-mediated splicing of exon 8. Mutations in RBM20 cause dilated cardiomyopathy and aberrant titin alternative splicing (80, 125). Interestingly, a mutation in the glutamate-rich region of RBM20 (E913K/+) caused massive inclusion of titin exons coding for the spring region, leading to a dramatic shift from the less compliant N2B towards the highly compliant N2BA isoforms (18). In addition to RBM20, RBM24 has also been shown to regulate alternative splicing of sarcomere genes, including titin. Using a reporter construct, Liu et al. (127) demonstrated that expression of RBM24 is sufficient to promote the exon 13 inclusion of titin. hnRNPU deletion also changes titin alternative splicing, although it is unclear whether hnRNPU directly regulates titin splicing in diseased hearts (234).
Tropomyosins are a family of actin-binding proteins that are expressed in both muscle and non-muscle cells. They are important regulators for actin filament function (78, 88) and can form rod-shaped homodimer or heterodimer structures arranged as a coiled coil. In humans, there are four conserved genes (TPM1, TPM2, TPM3, and TPM4) encoding four different tropomyosin protein isoforms, TPM1, TPM2, TPM3, and TPM4 (236). Each of these genes can also produce multiple transcript variants and protein isoforms through alternative promoter usage, alternative splicing, and alternative polyadenylation (220). For example, the TPM1 gene is subject to tissue-specific alternative splicing, generating TPM1k and TPM1a that are highly expressed in the heart. In human failing hearts, TPM1k appears to be upregulated (99, 175). To examine the impact of elevated TPM1k levels on heart function, Rajan et al. (175) overexpressed TPM1k in the mouse heart, and this led to systolic and diastolic dysfunction. Thus alternative splicing of tropomyosin could potentially contribute to cardiac disease progression. TPM1 splicing is regulated by RBM20 and PTB, although it is unclear whether they are directly responsible for the TPM1a and TPM1k isoform-switch during cardiac development and diseases (80, 235). Troponin T (TnT or TNNT2) links the troponin complex to tropomyosin and is also subject to alternative splicing in the heart. In humans, there are at least four isoforms of cardiac TnT (cTnT) that result from the alternative splicing of exon 4 and 5 (133). The splicing regulator responsible for the inclusion of exon 5 into cTnT is SRp55. Inactivation of SRp55 suppresses exon 5 inclusion into cTnT, while overexpression of SRp55 facilitates its inclusion as shown by minigene reporter analysis (133, 154). Similarly, Mbnl can also recognize its binding motif within intron 4 of the cTnT transcript, an interaction that mediates exon 5 splicing (222). In addition to exon 5, exon 7 of cTnT also plays an important role in cardiac function. This was demonstrated in transgenic mice overexpressing a mutant cTnT isoform lacking exon 7 that caused a significant decrease in cardiac function (59). Nevertheless, it is not clear which factors are the responsible trans-acting regulator(s) of exon 7 splicing in the heart.
During heart disease progression, apoptotic genes also undergo extensive alternative splicing. For example, the Bcl-x gene can generate two isoforms as a result of alternative splicing. The short protein Bcl-xS is pro-apoptotic, while the large protein Bcl-xL is anti-apoptotic (21).The alternative splicing of Bcl-x can be regulated by both protein arginine methyltransferase 2 (PRMT2) and polypyrimidine tract-binding protein 1 (PTBP1) (19, 208). In the failing heart, the expression of Bcl-xL mRNA is significantly increased after ventricular assist device implantation (14). This increase leads to an imbalance in the ratio of Bcl-xL to Bcl-xS that leads to mitochondrial stress and cell apoptosis, which may, in part, contribute to an increased risk of heart failure (181).
V. mRNA TRANSLATION AND DEGRADATION IN HEART
The proteome of a cell largely defines its unique functions, with different proteomic profiles specifying the myriad of cell types in the animal kingdom, and it is the maintenance of this proteome that characterizes the life of most cells in the human body. However, acute stress causes a transient cellular response, which is strongly dependent on the temporal and dynamic expression of key proteins (23). Long-term or chronic exposure to insults often leads to irreversible changes in the proteome that causes an altered and persistent dysfunctional phenotype in affected cells and tissues. In the heart, it is commonly recognized that the rate of protein synthesis in mature cardiomyocytes is relatively low, but is dramatically increased during cardiac hypertrophy (68, 124). While translation of mRNA into protein occurs at the translation machinery (i.e., ribosomes), RNA binding proteins, which bind to and shuttle the newly synthesized mRNAs to the ribosomes, present another level for the regulation of mRNA translation. In fact, a lot of the aforementioned RNA binding proteins are able to translocate between the nucleus and cytosol, or undergo alternative splicing to generate both nuclear and cytosolic isoforms. These properties suggest that RNA binding proteins can regulate both alternative splicing and other processes of RNA metabolism, including mRNA translation and stability regulation (FIGURE 9).
FIGURE 9.
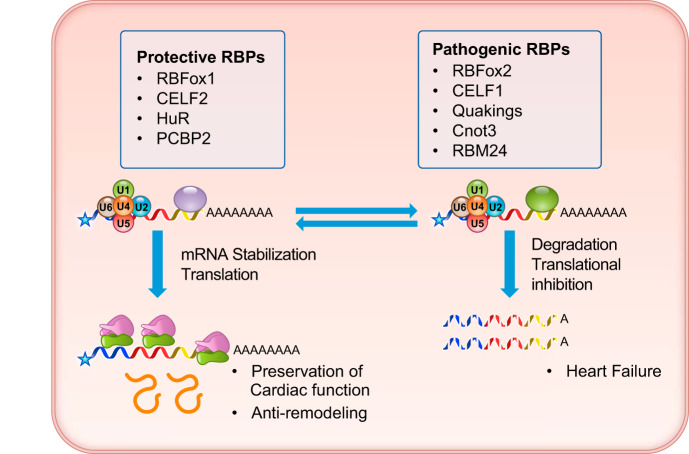
Summary of cardiac mRNA translation and degradation control with representative regulators. Translation and stability of cardiac mRNAs are regulated by protective RBPs (RNA binding proteins) versus pathogenic RBPs based on functional analysis.
A. RBPs in Cardiac Regulation of mRNA Translation and Decay
1. RBFox family
In addition to the canonical activity in mRNA splicing, RBFox1 also undergoes alternative splicing, which generates two splicing isoforms, each located either in the nucleus or the cytosol (114, 115). While the function of the nuclear RBFox1 in alternative splicing has been well characterized, emerging evidence also suggests that the cytosolic RBFox1 could play important roles in regulating mRNA stability. In a recent study, Ray et al. (180) demonstrated that the RBFox1 binding motif is present in the 3′-UTR of various RNA transcripts across different tissues. In the neuron, double inactivation of both RBFox1 and RBFox3 caused significant changes in mRNA abundance for a large number of mRNA transcripts, an effect that was rescued by reintroducing the cytosolic isoform of RBFox1, but not the nuclear isoform. With the use of the iCLIP-Seq method, cytosolic RBFox1 is found to directly interact with 3′-UTRs and has the effect of increasing mRNA stability. One of the possible mechanisms in the cytosolic RBFox1-mediated mRNA regulation may be due to competition with miRNA-mediated regulation of mRNA stability and translation. Conceptually, this competitive model is plausible given that there may be overlap between the binding motifs for each molecule within the 3′-UTR (114, 212). Similar to RBFox1, a recent study shows that RBFox2 is associated with cytoplasmic stress granules, a translational silencing machinery assembled in response to cellular stress (164) (see FIGURE 3). In a study performed by Park et al. (164), a subset of cell cycle-related genes including Rb1 are molecular targets of RBFox2 in stress granule-mediated translational suppression, leading to changes in cell proliferation. In the heart, Verma et al. (207) demonstrated a change in the subcellular localization of RBFox2 in individuals affected with hypoplastic left heart syndrome (HLHS), towards a more cytoplasmic distribution. A portion of the differentially expressed genes in the HLHS ventricles may be RBFox1/2 downstream targets, given that there is a significant enrichment in the differentially expressed gene list for transcripts harboring RBFox binding sites in the 3′-UTR (207). However, the detailed mechanism underlying RBFox2-mediated gene expression in the heart remains unclear. In addition to directly regulating mRNA stability and translation, RBFox2 could also have an indirect impact on downstream mRNA abundance through miRNA synthesis. For example, Chen et al. (34) suggest that RBFox proteins can regulate miRNA biogenesis by binding to their precursors and regulating the miRNA processer Dicer.
2. CUGBP protein family
CUGBP1 (also known as CUGBP Elav-like family member 1, CELF1) is also a multifunctional RNA-binding protein regulating pre-mRNA alternative splicing, translation, and mRNA stability (48). CUGBP1 appears to localize to both the nucleus and cytosol. In the cytosol, CUGBP1, but not CUGBP2, can specifically bind to the GU-rich element (GRE) in the 3′-UTR region and promote mRNA degradation (118, 179, 211). In addition to 3′-UTR, CUGBP1 can also interact with mRNA 5′-UTR and regulate mRNA translation for genes such as p21 (94, 199). In the heart, CUGBP1 binds to the 3′-UTR of Cx43, a major cardiac gap junction protein containing UG-rich motifs, and downregulates Cx43 mRNA by interacting with a 3′- to 5′-exoribonuclease, RRP6, to promote Cx43 mRNA degradation. Depletion of CUGBP1 CUGBP1in the infarcted heart preserves Cx43 mRNA levels and attenuates contractile dysfunction (30). Another example of CUGBP1-mediated mRNA degradation is the heme-oxygenase 1 (HO-1) gene. CUGBP1 downregulates HO-1 mRNA levels through interacting with the conserved GU-rich elements in the HO-1 3′-UTR, and this promotes HO-1 mRNA degradation in myoblasts (130). In addition, CUGBP1 expression is downregulated by carbon monoxide in H9C2 cells and highly induced in hypertrophic cardiomyopathy hearts. Therefore, it may serve as an important player in the positive regulatory circuit for carbon monoxide and heme clearance in the heart. Interestingly, in contrast to CUGBP1, the CUGBP2 gene seems to have the opposite effect on mRNA stability compared with CUGBP1. For example, CUGBP2 can bind to the A/U-rich sequence in the 3′-UTR of cyclooxygenase-2 (COX-2) and stabilizes the COX-2 mRNA while inhibiting its translation (149, 152). This appears to be an important mechanism in the paradox observed for COX-2 in cancer cells, where an abundance of COX-2 induced mRNA is contrasted with a relatively low abundance of COX-2 protein. Another downstream target of CUGBP2 is Mcl-1 mRNA, which encodes an anti-apoptotic protein. Expression of CUGBP2 reduces the expression of Mcl-1 protein by interacting with the Mcl-1 mRNA 3′-UTR and destabilizing it, effectively blocking the Mcl-1 mRNA from interacting with the translation machinery (195). In short, CUBGP1 and 2 are both able to regulate mRNA stability and protein translation, but their full spectrum of downstream targets in cardiac tissue is yet to be fully established.
3. Heterogeneous nuclear ribonucleoproteins
HuR (also known as ELAVL1) plays a key role in many aspects of RNA metabolism, including mRNA alternative splicing and mRNA decay. HuR recognizes and interacts with adenylate-uridylate-rich elements (ARE) within the 3′-UTR of the target mRNA (13, 20, 141). In cardiomyocytes, one established downstream target for the HuR is transcription factor Mef2c. HuR protein increases Mef2c mRNA expression by protecting its mRNA from degradation (244). A similar mechanism is also attributed to NADPH oxidase-catalytic subunit 1 (NOX-1) in vascular smooth muscle cells and cardiac sodium channel gene SCN5A in cardiomyocytes. HuR blockade reduces NOX-1 mRNA stability, and binding of HuR to SCN5A mRNA protects it from decay (4, 245). SCN5A is also a splicing target of MBNL in promoting cardiac arrhythmia associated with myotonic dystrophy (62). Therefore, in addition to splicing regulation, HuR can coordinate with other RBPs to regulate gene expression and cellular physiology in cardiac tissue by modulating target mRNA splicing and stability.
4. Other RBPs
a) quaking.
The quaking homolog KH domain RNA binding (QKI) family of RNA binding proteins is important in heart physiology (50). Studies by de Bruin et al. (50) demonstrated that QKI5 protects cardiomyocytes from apoptosis by reducing FoxO1 expression. This regulation plays an important role in obesity as a result of ER stress activation. Both mRNA and protein levels of the FoxO1 gene are markedly reduced by QKI5 in the ob/ob mouse hearts through QKI5-mediated mRNA degradation (79).
b) poly(C)-binding proteins.
Poly(C)-binding proteins (PCBPs) are a family of RNA binding proteins that bind to single-stranded poly C sequence. The PCBP family includes PCBP1–4 and are expressed across different tissues with multiple roles in mRNA metabolism (137). Among them, PCBP2 has been demonstrated to be downregulated in human failing hearts and mouse hypertrophic hearts and is an anti-hypertrophic factor that can protect cardiomyocytes from angiotensin II-induced hypertrophy (243). In cultured cardiomyocytes, one of the downstream targets of PCBP2 is GPR56. Inactivation of PCBP2 reduced the decay rate of GPR56 mRNA, which is induced during cardiac hypertrophy (243).
B. Functional Impact of mRNA Translation and Decay in Cardiac Development
During cardiac development and cardiomyocyte differentiation, Cnot3, a key regulator of the de-adenylation complex, is critical for cardiomyocyte proliferation in vitro, an effect that was demonstrated using human embryonic stem cell-derived cardiomyocytes (hiCM). Cnot3 is predominantly found in the cytoplasm. Under actinomycin D treatment, depletion of Cnot3 significantly reduces the degradation of cyclin D kinase inhibitor 1A (CDKI) mRNA, which encodes the cell cycle inhibitor p21. This observation provides a potential mechanism for the regulation of cardiomyocyte proliferation (246).
The tumor suppressor 53 (TP53 or p53) protein plays a critical role in cardiac development and is a master regulator of stress-induced gene expression. Post-transcriptional regulation of p53 is mediated by RBM24 via direct binding to the p53 3′-UTR (241). In vitro, RBM24 knock down significantly increases p53 protein levels. It is believed that this regulation is a consequence of the interaction between RBM24 and the 5′-cap-binding protein eIF4E, an interaction that may lead to dissociation of eIF4E from the p53 mRNA 5′-cap and p53 mRNA translation suppression. Deficiency of RBM24 in vivo causes endocardial cushion defect, and this is associated with elevated p53 levels, an effect that was rescued by p53 deletion (241).
C. Functional Impact of mRNA Translation and Decay in Heart Diseases
Using a genome-wide approach, Park et al. (165) compared mRNA isoforms between mouse hearts with transverse aortic constriction (TAC)-induced hypertrophy and “healthy” hearts (i.e., sham operated). The investigators identified a global shift towards an abundance of mRNA isoforms with shorter 3′-UTRs in 1 wk TAC hearts, compared with healthy hearts. Because mRNAs with short 3′-UTRs are generally considered to be more stable than mRNAs with longer 3′-UTRs, the finding in TAC hearts suggests that the heart’s response to TAC-induced hypertrophy leads to a stabilization of some/many mRNA species, a feature that may be characteristic of cardiac hypertrophy. In parallel, Park et al. (165) compared miRNA expression and did not find increased activity for any miRNA against their respective mRNA targets, suggesting that miRNA activity is generally inhibited in cardiac hypertrophy. Taken together, the authors concluded that hypertrophic hearts tend to have more stable mRNA transcripts compared with healthy hearts (165). However, because the observation was made at an early stage of hypertrophic remodeling, it remains to be demonstrated if such a shift in the profile of mRNA isoforms is functionally compensatory or detrimental.
In addition to a shift towards an abundance of stable mRNA transcripts during hypertrophy, the translation of mRNAs is also regulated in hypertrophic hearts. Poly(A) binding protein C1 (PABPC1) is a ubiquitously expressed mRNA binding protein that facilitates translation while also protecting the mRNA from exonucleases (38, 97, 123). In a recent study, Chorghade et al. (35) demonstrated that PABPC1 protein levels are regulated post-transcriptionally and found that shortening of the PABPC1 mRNA poly(A) tail leads to reduced polysome association and lower translation activity. During cardiac development, the PABPC1 is silenced. Interestingly, hypertrophic cardiomyocytes stimulated by both endurance exercise and heart disease can restore the PABPC1 poly(A) tail length and protein expression. This restoration of PABPC1 is important and necessary for global protein translation in cardiomyocytes and for their physiological growth (35).
In addition to extensive alternative splicing regulation, titin is also subject to translation regulation through the 5′-UTR region of its mRNA. The 5′-UTR of titin is highly conserved among human, mouse, and rat and contains a stem-loop structure and two upstream AUG codons (uAUGs) converging on a shared in-frame stop codon. Using a luciferase reporter construct containing the 5′-UTR sequence of titin, Cadar et al. (24) showed that the translation efficiency mediated by this 5′-UTR sequence was reduced in cardiomyocytes unless both uAUG sequences were mutated. Furthermore, treatment of cardiomyocytes with doxorubicin-induced stress reduced titin 5′-UTR-mediated translation suppression in cardiomyocytes (24). However, the physiological significance of such regulation remains to be demonstrated.
In addition to pathological stressors, genetic variants affecting mRNA stability also have an important role in cardiomyopathies. For example, long QT syndrome type 2 (LQT2) is caused by mutations in the human hERG gene, which encodes the Kv11.1 potassium channel in the heart. Interestingly, over 30% of the identified LQT2 mutations are nonsense or frameshift mutations, and the associated RNA transcripts are potentially subject to degradation through nonsense-mediated mRNA decay (76).
VI. CHALLENGES AND OPPORTUNITIES FOR mRNA METABOLISM STUDIES IN HEART
Despite the significant progress in the past decade, major gaps remain in our understanding of mRNA metabolism, as well as its relevance to cardiac biology and medicine. The enormous complexity found in the processes that encompass mRNA metabolism and the technical challenges of experimentally manipulating RNA molecules present significant barriers to elucidating the role of RNA metabolism in cardiac biology and disease. Several key questions remain to be addressed.
What are the molecular players in mRNA metabolic regulation in the heart? There are thousands of known RBPs in the human genome, but only a small fraction of them are fully characterized and validated (70). RBP and the current CLIP-seq and iCLIP-seq approaches suffer from specificity and signal-noise issues (126, 229). Most of the interactions can only be assessed by in vitro biochemical assays because of the technical difficulties of using in situ methods when the conditions are more physiologically relevant. Therefore, identifying the molecular basis for many of the mechanisms regulating mRNA metabolism remains just beyond our reach.
What is the functional status of mRNA in living cells? mRNA metabolic regulation involves a multitude of highly dynamic processes in mRNA processing, modification, transportation, and anchoring (142, 192). Most of our current analysis of mRNA function relies on global and bulk assessment of mRNA expression levels, stability, and ribosomal loading, with poor temporal and special resolution. A real-time approach to investigating the life cycle of mRNA molecules, during synthesis, modification, translation, and degradation, will offer tremendous insight into the underlying mechanisms involved in mRNA metabolic regulation in health and disease.
What are the emerging frontiers in mRNA regulation? Clearly, mRNA modification (63, 146), editing (190, 201, 232), and compartmented function will open new areas for exploration. Beyond m6A modification, other forms of post-transcriptional modifications and interactions with other noncoding RNAs in subcellular compartments and organelles will shed new light on the ever-expanding universe of mRNA metabolism.
Current technological advancements and the advent of large data-based systems approaches have offered many exciting opportunities for rapid progress in the field. Molecular imaging techniques have finally shined some light on the mRNA life cycle at the resolution of a single molecule (113, 167). Large-scale gene editing tools and whole transcriptome sequencing make it feasible to perform a comprehensive analysis for all RBP proteins in the human genome (37, 100). An unbiased systems approach may uncover key regulators in the RBP network and establish novel links (83). These powerful tools (142) will help us better understand the intricate regulatory network underlying mRNA metabolism that may lead to new discoveries critical for developing novel diagnostic and therapeutic approaches for the clinical treatment of heart disease.
GRANTS
The work is in part supported by a K99 Award from the National Institutes of Health (NIH) and a postdoctoral fellowship award and Career Development Award from American Heart Association (to C. Gao) and grants from NIH and the Department of Defense (to Y. Wang).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We are grateful to colleagues for their input and discussion and to Ricardo Frausto for proofreading.
Address for reprint requests and other correspondence: Y. Wang, 650 Charles E. Young St., Room CHS 37200J, Los Angeles, CA 90095 (e-mail: yibinwang@mednet.ucla.edu).
REFERENCES
- 1.Abdelmohsen K, Kuwano Y, Kim HH, Gorospe M. Posttranscriptional gene regulation by RNA-binding proteins during oxidative stress: implications for cellular senescence. Biol Chem 389: 243–255, 2008. doi: 10.1515/BC.2008.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adhikari S, Xiao W, Zhao YL, Yang YG. m6A: Signaling for mRNA splicing. RNA Biol 13: 756–759, 2016. doi: 10.1080/15476286.2016.1201628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agirre E, Bellora N, Alló M, Pagès A, Bertucci P, Kornblihtt AR, Eyras E. A chromatin code for alternative splicing involving a putative association between CTCF and HP1α proteins. BMC Biol 13: 31, 2015. doi: 10.1186/s12915-015-0141-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aguado A, Fischer T, Rodríguez C, Manea A, Martínez-González J, Touyz RM, Hernanz R, Alonso MJ, Dixon DA, Briones AM, Salaices M. Hu antigen R is required for NOX-1 but not NOX-4 regulation by inflammatory stimuli in vascular smooth muscle cells. J Hypertens 34: 253–265, 2016. doi: 10.1097/HJH.0000000000000801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson P, Kedersha N, Ivanov P. Stress granules, P-bodies and cancer. Biochim Biophys Acta 1849: 861–870, 2015. doi: 10.1016/j.bbagrm.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andreassi C, Crerar H, Riccio A. Post-transcriptional Processing of mRNA in Neurons: The Vestiges of the RNA World Drive Transcriptome Diversity. Front Mol Neurosci 11: 304, 2018. doi: 10.3389/fnmol.2018.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andreev DE, O’Connor PB, Loughran G, Dmitriev SE, Baranov PV, Shatsky IN. Insights into the mechanisms of eukaryotic translation gained with ribosome profiling. Nucleic Acids Res 45: 513–526, 2017. doi: 10.1093/nar/gkw1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arias Escayola D, Neugebauer KM. Dynamics and Function of Nuclear Bodies during Embryogenesis. Biochemistry 57: 2462–2469, 2018. doi: 10.1021/acs.biochem.7b01262. [DOI] [PubMed] [Google Scholar]
- 9.Auboeuf D. Alternative mRNA processing sites decrease genetic variability while increasing functional diversity. Transcription 9: 75–87, 2018. doi: 10.1080/21541264.2017.1373891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balacco DL, Soller M. The m6A writer: Rise of a machine for growing tasks. Biochemistry 58: 363–378, 2019. doi: 10.1021/acs.biochem.8b01166. [DOI] [PubMed] [Google Scholar]
- 11.Baralle D, Buratti E. RNA splicing in human disease and in the clinic. Clin Sci (Lond) 131: 355–368, 2017. doi: 10.1042/CS20160211. [DOI] [PubMed] [Google Scholar]
- 12.Baralle FE, Giudice J. Alternative splicing as a regulator of development and tissue identity. Nat Rev Mol Cell Biol 18: 437–451, 2017. doi: 10.1038/nrm.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barreau C, Paillard L, Osborne HB. AU-rich elements and associated factors: are there unifying principles? Nucleic Acids Res 33: 7138–7150, 2005. doi: 10.1093/nar/gki1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartling B, Milting H, Schumann H, Darmer D, Arusoglu L, Koerner MM, El-Banayosy A, Koerfer R, Holtz J, Zerkowski HR. Myocardial gene expression of regulators of myocyte apoptosis and myocyte calcium homeostasis during hemodynamic unloading by ventricular assist devices in patients with end-stage heart failure. Circulation 100, Suppl 2: II216–II223, 1999. doi: 10.1161/01.CIR.100.suppl_2.II-216. [DOI] [PubMed] [Google Scholar]
- 15.Batista PJ. The RNA Modification N6-methyladenosine and Its Implications in Human Disease. Genomics Proteomics Bioinformatics 15: 154–163, 2017. doi: 10.1016/j.gpb.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beermann J, Piccoli MT, Viereck J, Thum T. Non-coding RNAs in Development and Disease: Background, Mechanisms, and Therapeutic Approaches. Physiol Rev 96: 1297–1325, 2016. doi: 10.1152/physrev.00041.2015. [DOI] [PubMed] [Google Scholar]
- 17.Beqqali A. Alternative splicing in cardiomyopathy. Biophys Rev 10: 1061–1071, 2018. doi: 10.1007/s12551-018-0439-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beqqali A, Bollen IAE, Rasmussen TB, van den Hoogenhof MM, van Deutekom HWM, Schafer S, Haas J, Meder B, Sørensen KE, van Oort RJ, Mogensen J, Hubner N, Creemers EE, van der Velden J, Pinto YM. A mutation in the glutamate-rich region of RNA-binding motif protein 20 causes dilated cardiomyopathy through missplicing of titin and impaired Frank-Starling mechanism. Cardiovasc Res 112: 452–463, 2016. doi: 10.1093/cvr/cvw192. [DOI] [PubMed] [Google Scholar]
- 19.Bielli P, Bordi M, Di Biasio V, Sette C. Regulation of BCL-X splicing reveals a role for the polypyrimidine tract binding protein (PTBP1/hnRNP I) in alternative 5′ splice site selection. Nucleic Acids Res 42: 12070–12081, 2014. doi: 10.1093/nar/gku922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blackinton JG, Keene JD. Functional coordination and HuR-mediated regulation of mRNA stability during T cell activation. Nucleic Acids Res 44: 426–436, 2016. doi: 10.1093/nar/gkv1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boise LH, González-García M, Postema CE, Ding L, Lindsten T, Turka LA, Mao X, Nuñez G, Thompson CB. Bcl-x, a bcl-2 related gene that functions as a dominant regulator of apoptotic cell death. Cell 74: 597–608, 1993. doi: 10.1016/0092-8674(93)90508-N. [DOI] [PubMed] [Google Scholar]
- 22.Brinegar AE, Cooper TA. Roles for RNA-binding proteins in development and disease. Brain Res 1647: 1–8, 2016. doi: 10.1016/j.brainres.2016.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buszczak M, Signer RA, Morrison SJ. Cellular differences in protein synthesis regulate tissue homeostasis. Cell 159: 242–251, 2014. doi: 10.1016/j.cell.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cadar AG, Zhong L, Lin A, Valenzuela MO, Lim CC. Upstream open reading frame in 5′-untranslated region reduces titin mRNA translational efficiency. Biochem Biophys Res Commun 453: 185–191, 2014. doi: 10.1016/j.bbrc.2014.09.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao G, Li HB, Yin Z, Flavell RA. Recent advances in dynamic m6A RNA modification. Open Biol 6: 160003, 2016. doi: 10.1098/rsob.160003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao T, Rajasingh S, Samanta S, Dawn B, Bittel DC, Rajasingh J. Biology and clinical relevance of noncoding sno/scaRNAs. Trends Cardiovasc Med 28: 81–90, 2018. doi: 10.1016/j.tcm.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carey KT, Wickramasinghe VO. Regulatory Potential of the RNA Processing Machinery: Implications for Human Disease. Trends Genet 34: 279–290, 2018. doi: 10.1016/j.tig.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 28.Cazorla O, Freiburg A, Helmes M, Centner T, McNabb M, Wu Y, Trombitás K, Labeit S, Granzier H. Differential expression of cardiac titin isoforms and modulation of cellular stiffness. Circ Res 86: 59–67, 2000. doi: 10.1161/01.RES.86.1.59. [DOI] [PubMed] [Google Scholar]
- 29.Chandola U, Das R, Panda B. Role of the N6-methyladenosine RNA mark in gene regulation and its implications on development and disease. Brief Funct Genomics 14: 169–179, 2015. doi: 10.1093/bfgp/elu039. [DOI] [PubMed] [Google Scholar]
- 30.Chang K-T, Cheng C-F, King P-C, Liu S-Y, Wang G-S. CELF1 Mediates Connexin 43 mRNA Degradation in Dilated Cardiomyopathy. Circ Res 121: 1140–1152, 2017. doi: 10.1161/CIRCRESAHA.117.311281. [DOI] [PubMed] [Google Scholar]
- 31.Chang SH, Elemento O, Zhang J, Zhuang ZW, Simons M, Hla T. ELAVL1 regulates alternative splicing of eIF4E transporter to promote postnatal angiogenesis. Proc Natl Acad Sci USA 111: 18309–18314, 2014. doi: 10.1073/pnas.1412172111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Charlet-B N, Singh G, Cooper TA, Logan P. Dynamic antagonism between ETR-3 and PTB regulates cell type-specific alternative splicing. Mol Cell 9: 649–658, 2002. doi: 10.1016/S1097-2765(02)00479-3. [DOI] [PubMed] [Google Scholar]
- 33.Chen Q, Hu G. Post-transcriptional regulation of the pluripotent state. Curr Opin Genet Dev 46: 15–23, 2017. doi: 10.1016/j.gde.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Y, Zubovic L, Yang F, Godin K, Pavelitz T, Castellanos J, Macchi P, Varani G. Rbfox proteins regulate microRNA biogenesis by sequence-specific binding to their precursors and target downstream Dicer. Nucleic Acids Res 44: 4381–4395, 2016. doi: 10.1093/nar/gkw177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chorghade S, Seimetz J, Emmons R, Yang J, Bresson SM, Lisio M, Parise G, Conrad NK, Kalsotra A. Poly(A) tail length regulates PABPC1 expression to tune translation in the heart. eLife 6: e24139, 2017. doi: 10.7554/eLife.24139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chung CZ, Seidl LE, Mann MR, Heinemann IU. Tipping the balance of RNA stability by 3′ editing of the transcriptome. Biochim Biophys Acta, Gen Subj 1861, 11 Pt B: 2971–2979, 2017. doi: 10.1016/j.bbagen.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 37.Churko JM, Garg P, Treutlein B, Venkatasubramanian M, Wu H, Lee J, Wessells QN, Chen SY, Chen WY, Chetal K, Mantalas G, Neff N, Jabart E, Sharma A, Nolan GP, Salomonis N, Wu JC. Defining human cardiac transcription factor hierarchies using integrated single-cell heterogeneity analysis. Nat Commun 9: 4906, 2018. doi: 10.1038/s41467-018-07333-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coller JM, Gray NK, Wickens MP. mRNA stabilization by poly(A) binding protein is independent of poly(A) and requires translation. Genes Dev 12: 3226–3235, 1998. doi: 10.1101/gad.12.20.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cooper TA, Cardone MH, Ordahl CP. Cis requirements for alternative splicing of the cardiac troponin T pre-mRNA. Nucleic Acids Res 16: 8443–8465, 1988. doi: 10.1093/nar/16.17.8443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cooper TA, Ordahl CP. Nucleotide substitutions within the cardiac troponin T alternative exon disrupt pre-mRNA alternative splicing. Nucleic Acids Res 17: 7905–7921, 1989. doi: 10.1093/nar/17.19.7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cooper TA, Ordahl CP. A single cardiac troponin T gene generates embryonic and adult isoforms via developmentally regulated alternate splicing. J Biol Chem 260: 11140–11148, 1985. [PubMed] [Google Scholar]
- 42.Corbett AH. Post-transcriptional regulation of gene expression and human disease. Curr Opin Cell Biol 52: 96–104, 2018. doi: 10.1016/j.ceb.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Covelo-Molares H, Bartosovic M, Vanacova S. RNA methylation in nuclear pre-mRNA processing. Wiley Interdiscip Rev RNA 9: e1489, 2018. doi: 10.1002/wrna.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cui XA, Palazzo AF. Localization of mRNAs to the endoplasmic reticulum. Wiley Interdiscip Rev RNA 5: 481–492, 2014. doi: 10.1002/wrna.1225. [DOI] [PubMed] [Google Scholar]
- 45.Da Costa PJ, Menezes J, Romão L. The role of alternative splicing coupled to nonsense-mediated mRNA decay in human disease. Int J Biochem Cell Biol 91, Pt B: 168–175, 2017. doi: 10.1016/j.biocel.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 46.Dai D, Wang H, Zhu L, Jin H, Wang X. N6-methyladenosine links RNA metabolism to cancer progression. Cell Death Dis 9: 124, 2018. doi: 10.1038/s41419-017-0129-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Damianov A, Black DL. Autoregulation of Fox protein expression to produce dominant negative splicing factors. RNA 16: 405–416, 2010. doi: 10.1261/rna.1838210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dasgupta T, Ladd AN. The importance of CELF control: molecular and biological roles of the CUG-BP, Elav-like family of RNA-binding proteins. Wiley Interdiscip Rev RNA 3: 104–121, 2012. doi: 10.1002/wrna.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Almeida C, Scheer H, Zuber H, Gagliardi D. RNA uridylation: a key posttranscriptional modification shaping the coding and noncoding transcriptome. Wiley Interdiscip Rev RNA 9: e1440, 2018. doi: 10.1002/wrna.1440. [DOI] [PubMed] [Google Scholar]
- 50.De Bruin RG, Rabelink TJ, van Zonneveld AJ, van der Veer EP. Emerging roles for RNA-binding proteins as effectors and regulators of cardiovascular disease. Eur Heart J 38: 1380–1388, 2017. doi: 10.1093/eurheartj/ehw567. [DOI] [PubMed] [Google Scholar]
- 51.Decker CJ, Parker R. P-bodies and stress granules: possible roles in the control of translation and mRNA degradation. Cold Spring Harb Perspect Biol 4: a012286, 2012. doi: 10.1101/cshperspect.a012286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deniaud A, Karuppasamy M, Bock T, Masiulis S, Huard K, Garzoni F, Kerschgens K, Hentze MW, Kulozik AE, Beck M, Neu-Yilik G, Schaffitzel C. A network of SMG-8, SMG-9 and SMG-1 C-terminal insertion domain regulates UPF1 substrate recruitment and phosphorylation. Nucleic Acids Res 43: 7600–7611, 2015. doi: 10.1093/nar/gkv668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ding JH, Xu X, Yang D, Chu PH, Dalton ND, Ye Z, Yeakley JM, Cheng H, Xiao RP, Ross J Jr, Chen J, Fu XD. Dilated cardiomyopathy caused by tissue-specific ablation of SC35 in the heart. EMBO J 23: 885–896, 2004. doi: 10.1038/sj.emboj.7600054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dorn LE, Lasman L, Chen J, Xu X, Hund TJ, Medvedovic M, Hanna JH, van Berlo JH, Accornero F. The N6-Methyladenosine mRNA Methylase METTL3 Controls Cardiac Homeostasis and Hypertrophy. Circulation 139: 533–545, 2019. doi: 10.1161/CIRCULATIONAHA.118.036146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Duan HC, Wang Y, Jia G. Dynamic and reversible RNA N6-methyladenosine methylation. Wiley Interdiscip Rev RNA 10: e1507, 2019. doi: 10.1002/wrna.1507. [DOI] [PubMed] [Google Scholar]
- 56.Dyle MC, Kolakada D, Cortazar MA, Jagannathan S. How to get away with nonsense: mechanisms and consequences of escape from nonsense-mediated RNA decay. Wiley Interdiscip Rev RNA 11: e1560, 2020. doi: 10.1002/wrna.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Erson-Bensan AE, Begik O. m6A Modification and Implications for microRNAs. MicroRNA 6: 97–101, 2017. doi: 10.2174/2211536606666170511102219. [DOI] [PubMed] [Google Scholar]
- 58.Fan C, Chen Q, Wang QK. Functional role of transcriptional factor TBX5 in pre-mRNA splicing and Holt-Oram syndrome via association with SC35. J Biol Chem 284: 25653–25663, 2009. doi: 10.1074/jbc.M109.041368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Feng H-Z, Chen G, Nan C, Huang X, Jin J-P. Abnormal splicing in the N-terminal variable region of cardiac troponin T impairs systolic function of the heart with preserved Frank-Starling compensation. Physiol Rep 2: e12139, 2014. doi: 10.14814/phy2.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fonseca BD, Lahr RM, Damgaard CK, Alain T, Berman AJ. LARP1 on TOP of ribosome production. Wiley Interdiscip Rev RNA 9: e1480, 2018. doi: 10.1002/wrna.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Frese KS, Meder B, Keller A, Just S, Haas J, Vogel B, Fischer S, Backes C, Matzas M, Köhler D, Benes V, Katus HA, Rottbauer W. RNA splicing regulated by RBFOX1 is essential for cardiac function in zebrafish. J Cell Sci 128: 3030–3040, 2015. doi: 10.1242/jcs.166850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Freyermuth F, Rau F, Kokunai Y, Linke T, Sellier C, Nakamori M, Kino Y, Arandel L, Jollet A, Thibault C, Philipps M, Vicaire S, Jost B, Udd B, Day JW, Duboc D, Wahbi K, Matsumura T, Fujimura H, Mochizuki H, Deryckere F, Kimura T, Nukina N, Ishiura S, Lacroix V, Campan-Fournier A, Navratil V, Chautard E, Auboeuf D, Horie M, Imoto K, Lee K-Y, Swanson MS, de Munain AL, Inada S, Itoh H, Nakazawa K, Ashihara T, Wang E, Zimmer T, Furling D, Takahashi MP, Charlet-Berguerand N. Splicing misregulation of SCN5A contributes to cardiac-conduction delay and heart arrhythmia in myotonic dystrophy. Nat Commun 7: 11067, 2016. doi: 10.1038/ncomms11067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Frye M, Harada BT, Behm M, He C. RNA modifications modulate gene expression during development. Science 361: 1346–1349, 2018. doi: 10.1126/science.aau1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gallagher TL, Arribere JA, Geurts PA, Exner CRT, McDonald KL, Dill KK, Marr HL, Adkar SS, Garnett AT, Amacher SL, Conboy JG. Rbfox-regulated alternative splicing is critical for zebrafish cardiac and skeletal muscle functions. Dev Biol 359: 251–261, 2011. doi: 10.1016/j.ydbio.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Galloway A, Cowling VH. mRNA cap regulation in mammalian cell function and fate. Biochim Biophys Acta Gene Regul Mech 1862: 270–279, 2019. doi: 10.1016/j.bbagrm.2018.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gandal MJ, Zhang P, Hadjimichael E, Walker RL, Chen C, Liu S, Won H, van Bakel H, Varghese M, Wang Y, Shieh AW, Haney J, Parhami S, Belmont J, Kim M, Moran Losada P, Khan Z, Mleczko J, Xia Y, Dai R, Wang D, Yang YT, Xu M, Fish K, Hof PR, Warrell J, Fitzgerald D, White K, Jaffe AE, Peters MA, Gerstein M, Liu C, Iakoucheva LM, Pinto D, Geschwind DH; PsychENCODE Consortium . Transcriptome-wide isoform-level dysregulation in ASD, schizophrenia, and bipolar disorder. Science 362: eaat8127, 2018. doi: 10.1126/science.aat8127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gao C, Ren S, Lee J-H, Qiu J, Chapski DJ, Rau CD, Zhou Y, Abdellatif M, Nakano A, Vondriska TM, Xiao X, Fu X-D, Chen J-N, Wang Y. RBFox1-mediated RNA splicing regulates cardiac hypertrophy and heart failure. J Clin Invest 126: 195–206, 2015. doi: 10.1172/JCI84015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garlick PJ, McNurlan MA, Preedy VR. A rapid and convenient technique for measuring the rate of protein synthesis in tissues by injection of [3H]phenylalanine. Biochem J 192: 719–723, 1980. doi: 10.1042/bj1920719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gerbracht JV, Gehring NH. The exon junction complex: structural insights into a faithful companion of mammalian mRNPs. Biochem Soc Trans 46: 153–161, 2018. doi: 10.1042/BST20170059. [DOI] [PubMed] [Google Scholar]
- 70.Gerstberger S, Hafner M, Tuschl T. A census of human RNA-binding proteins. Nat Rev Genet 15: 829–845, 2014. doi: 10.1038/nrg3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Geuens T, Bouhy D, Timmerman V. The hnRNP family: insights into their role in health and disease. Hum Genet 135: 851–867, 2016. doi: 10.1007/s00439-016-1683-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Giudice J, Cooper TA. RNA-binding proteins in heart development. Adv Exp Med Biol 825: 389–429, 2014. doi: 10.1007/978-1-4939-1221-6_11. [DOI] [PubMed] [Google Scholar]
- 73.Giudice J, Xia Z, Li W, Cooper TA. Neonatal cardiac dysfunction and transcriptome changes caused by the absence of Celf1. Sci Rep 6: 35550, 2016. doi: 10.1038/srep35550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Giulietti M, Piva F, D’Antonio M, D’Onorio De Meo P, Paoletti D, Castrignanò T, D’Erchia AM, Picardi E, Zambelli F, Principato G, Pavesi G, Pesole G. SpliceAid-F: a database of human splicing factors and their RNA-binding sites. Nucleic Acids Res 41, D1: D125–D131, 2013. doi: 10.1093/nar/gks997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Golani-Armon A, Arava Y. Localization of Nuclear-Encoded mRNAs to Mitochondria Outer Surface. Biochemistry (Mosc) 81: 1038–1043, 2016. doi: 10.1134/S0006297916100023. [DOI] [PubMed] [Google Scholar]
- 76.Gong Q, Zhou Z. Nonsense-Mediated mRNA Decay of hERG Mutations in Long QT Syndrome. In: Potassium Channels: Methods and Protocols , edited by Shyng S-L, Valiyaveetil FI, Whorton M. New York: Springer, 2018, p. 37–49. [DOI] [PubMed] [Google Scholar]
- 77.Greenleaf AL. Human CDK12 and CDK13, multi-tasking CTD kinases for the new millennium. Transcription 10: 91–110, 2019. doi: 10.1080/21541264.2018.1535211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77a.Gripp KW, Hopkins E, Johnston JJ, Krause C, Dobyns WB, Biesecker LG. Long-term survival in TARP syndrome and confirmation of RBM10 as the disease-causing gene. Am J Med Genet A 155: 2516–2520, 2011. doi: 10.1002/ajmg.a.34190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gunning PW, Hardeman EC, Lappalainen P, Mulvihill DP. Tropomyosin–master regulator of actin filament function in the cytoskeleton. J Cell Sci 128: 2965–2974, 2015. doi: 10.1242/jcs.172502. [DOI] [PubMed] [Google Scholar]
- 79.Guo W, Jiang T, Lian C, Wang H, Zheng Q, Ma H. QKI deficiency promotes FoxO1 mediated nitrosative stress and endoplasmic reticulum stress contributing to increased vulnerability to ischemic injury in diabetic heart. J Mol Cell Cardiol 75: 131–140, 2014. doi: 10.1016/j.yjmcc.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 80.Guo W, Schafer S, Greaser ML, Radke MH, Liss M, Govindarajan T, Maatz H, Schulz H, Li S, Parrish AM, Dauksaite V, Vakeel P, Klaassen S, Gerull B, Thierfelder L, Regitz-Zagrosek V, Hacker TA, Saupe KW, Dec GW, Ellinor PT, MacRae CA, Spallek B, Fischer R, Perrot A, Özcelik C, Saar K, Hubner N, Gotthardt M. RBM20, a gene for hereditary cardiomyopathy, regulates titin splicing. Nat Med 18: 766–773, 2012. doi: 10.1038/nm.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guzikowski AR, Chen YS, Zid BM. Stress-induced mRNP granules: form and function of processing bodies and stress granules. Wiley Interdiscip Rev RNA 10: e1524, 2019. doi: 10.1002/wrna.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Harvey R, Dezi V, Pizzinga M, Willis AE. Post-transcriptional control of gene expression following stress: the role of RNA-binding proteins. Biochem Soc Trans 45: 1007–1014, 2017. doi: 10.1042/BST20160364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hasin-Brumshtein Y, Khan AH, Hormozdiari F, Pan C, Parks BW, Petyuk VA, Piehowski PD, Brümmer A, Pellegrini M, Xiao X, Eskin E, Smith RD, Lusis AJ, Smith DJ. Hypothalamic transcriptomes of 99 mouse strains reveal trans eQTL hotspots, splicing QTLs and novel non-coding genes. eLife 5: e15614, 2016. doi: 10.7554/eLife.15614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hautbergue GM. RNA Nuclear Export: From Neurological Disorders to Cancer. Adv Exp Med Biol 1007: 89–109, 2017. doi: 10.1007/978-3-319-60733-7_6. [DOI] [PubMed] [Google Scholar]
- 85.Herzel L, Ottoz DSM, Alpert T, Neugebauer KM. Splicing and transcription touch base: co-transcriptional spliceosome assembly and function. Nat Rev Mol Cell Biol 18: 637–650, 2017. doi: 10.1038/nrm.2017.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hinkle ER, Wiedner HJ, Black AJ, Giudice J. RNA processing in skeletal muscle biology and disease. Transcription 10: 1–20, 2019. doi: 10.1080/21541264.2018.1558677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hinze F, Dieterich C, Radke MH, Granzier H, Gotthardt M. Reducing RBM20 activity improves diastolic dysfunction and cardiac atrophy. J Mol Med (Berl) 94: 1349–1358, 2016. doi: 10.1007/s00109-016-1483-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hitchcock-DeGregori SE, Barua B. Tropomyosin Structure, Function, and Interactions: A Dynamic Regulator. Subcell Biochem 82: 253–284, 2017. doi: 10.1007/978-3-319-49674-0_9. [DOI] [PubMed] [Google Scholar]
- 89.Houston DW. Regulation of cell polarity and RNA localization in vertebrate oocytes. Int Rev Cell Mol Biol 306: 127–185, 2013. doi: 10.1016/B978-0-12-407694-5.00004-3. [DOI] [PubMed] [Google Scholar]
- 90.Hu J, Gao C, Wei C, Xue Y, Shao C, Hao Y, Gou LT, Zhou Y, Zhang J, Ren S, Chen J, Wang Y, Fu XD. RBFox2-miR-34a-Jph2 axis contributes to cardiac decompensation during heart failure. Proc Natl Acad Sci USA 116: 6172–6180, 2019. doi: 10.1073/pnas.1822176116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hu Z, Liang MC, Soong TW. Alternative Splicing of L-type CaV1.2 Calcium Channels: Implications in Cardiovascular Diseases. Genes (Basel) 8: 344, 2017. doi: 10.3390/genes8120344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huang C, Xia PY, Zhou H. Sustained expression of TDP-43 and FUS in motor neurons in rodent’s lifetime. Int J Biol Sci 6: 396–406, 2010. doi: 10.7150/ijbs.6.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hug N, Longman D, Cáceres JF. Mechanism and regulation of the nonsense-mediated decay pathway. Nucleic Acids Res 44: 1483–1495, 2016. doi: 10.1093/nar/gkw010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Iakova P, Wang GL, Timchenko L, Michalak M, Pereira-Smith OM, Smith JR, Timchenko NA. Competition of CUGBP1 and calreticulin for the regulation of p21 translation determines cell fate. EMBO J 23: 406–417, 2004. doi: 10.1038/sj.emboj.7600052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jansen RP, Niessing D, Baumann S, Feldbrügge M. mRNA transport meets membrane traffic. Trends Genet 30: 408–417, 2014. doi: 10.1016/j.tig.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 96.Jia W, Yao Z, Zhao J, Guan Q, Gao L. New perspectives of physiological and pathological functions of nucleolin (NCL). Life Sci 186: 1–10, 2017. doi: 10.1016/j.lfs.2017.07.025. [DOI] [PubMed] [Google Scholar]
- 97.Kahvejian A, Svitkin YV, Sukarieh R, M’Boutchou M-N, Sonenberg N. Mammalian poly(A)-binding protein is a eukaryotic translation initiation factor, which acts via multiple mechanisms. Genes Dev 19: 104–113, 2005. doi: 10.1101/gad.1262905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kalsotra A, Xiao X, Ward AJ, Castle JC, Johnson JM, Burge CB, Cooper TA. A postnatal switch of CELF and MBNL proteins reprograms alternative splicing in the developing heart. Proc Natl Acad Sci USA 105: 20333–20338, 2008. doi: 10.1073/pnas.0809045105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Karam CN, Warren CM, Rajan S, de Tombe PP, Wieczorek DF, Solaro RJ. Expression of tropomyosin-κ induces dilated cardiomyopathy and depresses cardiac myofilament tension by mechanisms involving cross-bridge dependent activation and altered tropomyosin phosphorylation. J Muscle Res Cell Motil 31: 315–322, 2011. doi: 10.1007/s10974-010-9237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kargapolova Y, Levin M, Lackner K, Danckwardt S. sCLIP–an integrated platform to study RNA-protein interactomes in biomedical research: identification of CSTF2tau in alternative processing of small nuclear RNAs. Nucleic Acids Res 45: 6074–6086, 2017. doi: 10.1093/nar/gkx152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kasprowicz-Maluśki A, Kwiatkowski W, Starosta A, Wojtaszek P. Journey from the Center of the Cell–the intra- and intercellular transport of mRNA. Acta Biochim Pol 63: 693–699, 2016. doi: 10.18388/abp.2016_1359. [DOI] [PubMed] [Google Scholar]
- 102.Kishor A, Fritz SE, Hogg JR. Nonsense-mediated mRNA decay: the challenge of telling right from wrong in a complex transcriptome. Wiley Interdiscip Rev RNA 10: e1548, 2019. doi: 10.1002/wrna.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Klein AF, Dastidar S, Furling D, Chuah MK. Therapeutic Approaches for Dominant Muscle Diseases: Highlight on Myotonic Dystrophy. Curr Gene Ther 15: 329–337, 2015. doi: 10.2174/1566523215666150630120537. [DOI] [PubMed] [Google Scholar]
- 104.Kong SW, Hu YW, Ho JWK, Ikeda S, Polster S, John R, Hall JL, Bisping E, Pieske B, dos Remedios CG, Pu WT. Heart failure-associated changes in RNA splicing of sarcomere genes. Circ Cardiovasc Genet 3: 138–146, 2010. doi: 10.1161/CIRCGENETICS.109.904698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Koshelev M, Sarma S, Price RE, Wehrens XH, Cooper TA. Heart-specific overexpression of CUGBP1 reproduces functional and molecular abnormalities of myotonic dystrophy type 1. Hum Mol Genet 19: 1066–1075, 2010. doi: 10.1093/hmg/ddp570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kurosaki T, Maquat LE. Nonsense-mediated mRNA decay in humans at a glance. J Cell Sci 129: 461–467, 2016. doi: 10.1242/jcs.181008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kurosaki T, Popp MW, Maquat LE. Quality and quantity control of gene expression by nonsense-mediated mRNA decay. [Correction in Nat Rev Mol Cell Biol 20: 384, 2019.] Nat Rev Mol Cell Biol 20: 406–420, 2019. doi: 10.1038/s41580-019-0126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kuroyanagi H, Kobayashi T, Mitani S, Hagiwara M. Transgenic alternative-splicing reporters reveal tissue-specific expression profiles and regulation mechanisms in vivo. Nat Methods 3: 909–915, 2006. doi: 10.1038/nmeth944. [DOI] [PubMed] [Google Scholar]
- 109.Kuyumcu-Martinez NM, Wang G-S, Cooper TA. Increased steady-state levels of CUGBP1 in myotonic dystrophy 1 are due to PKC-mediated hyperphosphorylation. Mol Cell 28: 68–78, 2007. doi: 10.1016/j.molcel.2007.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ladd AN. New Insights Into the Role of RNA-Binding Proteins in the Regulation of Heart Development. Int Rev Cell Mol Biol 324: 125–185, 2016. doi: 10.1016/bs.ircmb.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 111.Ladd AN, Stenberg MG, Swanson MS, Cooper TA. Dynamic balance between activation and repression regulates pre-mRNA alternative splicing during heart development. Dev Dyn 233: 783–793, 2005. doi: 10.1002/dvdy.20382. [DOI] [PubMed] [Google Scholar]
- 113.Lee BH, Bae SW, Shim JJ, Park SY, Park HY. Imaging Single-mRNA Localization and Translation in Live Neurons. Mol Cells 39: 841–846, 2016. doi: 10.14348/molcells.2016.0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lee J-A, Damianov A, Lin C-H, Fontes M, Parikshak NN, Anderson ES, Geschwind DH, Black DL, Martin KC. Cytoplasmic Rbfox1 Regulates the Expression of Synaptic and Autism-Related Genes. Neuron 89: 113–128, 2016. doi: 10.1016/j.neuron.2015.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lee J-A, Tang Z-Z, Black DL. An inducible change in Fox-1/A2BP1 splicing modulates the alternative splicing of downstream neuronal target exons. Genes Dev 23: 2284–2293, 2009. doi: 10.1101/gad.1837009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lee J-H, Gao C, Peng G, Greer C, Ren S, Wang Y, Xiao X. Analysis of transcriptome complexity through RNA sequencing in normal and failing murine hearts. Circ Res 109: 1332–1341, 2011. doi: 10.1161/CIRCRESAHA.111.249433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee JE, Cooper TA. Pathogenic mechanisms of myotonic dystrophy. Biochem Soc Trans 37: 1281–1286, 2009. doi: 10.1042/BST0371281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lee JE, Lee JY, Wilusz J, Tian B, Wilusz CJ. Systematic analysis of cis-elements in unstable mRNAs demonstrates that CUGBP1 is a key regulator of mRNA decay in muscle cells. PLoS One 5: e11201, 2010. doi: 10.1371/journal.pone.0011201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lee M, Kim B, Kim VN. Emerging roles of RNA modification: m6A and U-tail. Cell 158: 980–987, 2014. doi: 10.1016/j.cell.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 120.Lee Y, Rio DC. Mechanisms and Regulation of Alternative Pre-mRNA Splicing. Annu Rev Biochem 84: 291–323, 2015. doi: 10.1146/annurev-biochem-060614-034316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lejeune F. Nonsense-mediated mRNA decay at the crossroads of many cellular pathways. BMB Rep 50: 175–185, 2017. doi: 10.5483/BMBRep.2017.50.4.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lesnik C, Golani-Armon A, Arava Y. Localized translation near the mitochondrial outer membrane: an update. RNA Biol 12: 801–809, 2015. doi: 10.1080/15476286.2015.1058686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lewis CJT, Pan T, Kalsotra A. RNA modifications and structures cooperate to guide RNA-protein interactions. Nat Rev Mol Cell Biol 18: 202–210, 2017. doi: 10.1038/nrm.2016.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lewis SEM, Kelly FJ, Goldspink DF. Pre- and post-natal growth and protein turnover in smooth muscle, heart and slow- and fast-twitch skeletal muscles of the rat. Biochem J 217: 517–526, 1984. doi: 10.1042/bj2170517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li S, Guo W, Dewey CN, Greaser ML. Rbm20 regulates titin alternative splicing as a splicing repressor. Nucleic Acids Res 41: 2659–2672, 2013. doi: 10.1093/nar/gks1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Linder B, Grozhik AV, Olarerin-George AO, Meydan C, Mason CE, Jaffrey SR. Single-nucleotide-resolution mapping of m6A and m6Am throughout the transcriptome. Nat Methods 12: 767–772, 2015. doi: 10.1038/nmeth.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu J, Kong X, Zhang M, Yang X, Xu X. RNA binding protein 24 deletion disrupts global alternative splicing and causes dilated cardiomyopathy. Protein Cell 10: 405–416, 2019. doi: 10.1007/s13238-018-0578-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liu N, Olson EN. MicroRNA regulatory networks in cardiovascular development. Dev Cell 18: 510–525, 2010. doi: 10.1016/j.devcel.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu T-Y, Chen Y-C, Jong Y-J, Tsai H-J, Lee C-C, Chang Y-S, Chang J-G, Chang Y-F. Muscle developmental defects in heterogeneous nuclear Ribonucleoprotein A1 knockout mice. Open Biol 7: 160303, 2017. doi: 10.1098/rsob.160303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liu Y, Wang H, Wang J, Wei B, Zhang X, Zhang M, Cao D, Dai J, Wang Z, Nyirimigabo E, Ji G. A positive feedback regulation of Heme oxygenase 1 by CELF1 in cardiac myoblast cells. Biochim Biophys Acta 1862: 209–218, 2019. doi: 10.1016/j.bbagrm.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 131.Loiselle JJ, Sutherland LC. Differential downregulation of Rbm5 and Rbm10 during skeletal and cardiac differentiation. In Vitro Cell Dev Biol Anim 50: 331–339, 2014. doi: 10.1007/s11626-013-9708-z. [DOI] [PubMed] [Google Scholar]
- 132.López-Morató M, Brook JD, Wojciechowska M. Small Molecules Which Improve Pathogenesis of Myotonic Dystrophy Type 1. Front Neurol 9: 349, 2018. doi: 10.3389/fneur.2018.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lu S, Yin X. Overexpression of Dyrk1A regulates cardiac troponin T splicing in cells and mice. Biochem Biophys Res Commun 473: 993–998, 2016. doi: 10.1016/j.bbrc.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 134.Luo Y, Na Z, Slavoff SA. P-Bodies: Composition, Properties, and Functions. Biochemistry 57: 2424–2431, 2018. doi: 10.1021/acs.biochem.7b01162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lykke-Andersen S, Jensen TH. Nonsense-mediated mRNA decay: an intricate machinery that shapes transcriptomes. Nat Rev Mol Cell Biol 16: 665–677, 2015. doi: 10.1038/nrm4063. [DOI] [PubMed] [Google Scholar]
- 136.MacNicol MC, Cragle CE, Arumugam K, Fosso B, Pesole G, MacNicol AM. Functional Integration of mRNA Translational Control Programs. Biomolecules 5: 1580–1599, 2015. doi: 10.3390/biom5031580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Makeyev AV, Liebhaber SA. The poly(C)-binding proteins: a multiplicity of functions and a search for mechanisms. RNA 8: 265–278, 2002. doi: 10.1017/S1355838202024627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Man SM, Kanneganti TD. Regulation of inflammasome activation. Immunol Rev 265: 6–21, 2015. doi: 10.1111/imr.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Martinez NM, Gilbert WV. Pre-mRNA modifications and their role in nuclear processing. Quant Biol 6: 210–227, 2018. doi: 10.1007/s40484-018-0147-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mathiyalagan P, Adamiak M, Mayourian J, Sassi Y, Liang Y, Agarwal N, Jha D, Zhang S, Kohlbrenner E, Chepurko E, Chen J, Trivieri MG, Singh R, Bouchareb R, Fish K, Ishikawa K, Lebeche D, Hajjar RJ, Sahoo S. FTO-Dependent N6-Methyladenosine Regulates Cardiac Function During Remodeling and Repair. Circulation 139: 518–532, 2019. doi: 10.1161/CIRCULATIONAHA.118.033794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Matoulkova E, Michalova E, Vojtesek B, Hrstka R. The role of the 3′ untranslated region in post-transcriptional regulation of protein expression in mammalian cells. RNA Biol 9: 563–576, 2012. doi: 10.4161/rna.20231. [DOI] [PubMed] [Google Scholar]
- 142.Medioni C, Besse F. The Secret Life of RNA: Lessons from Emerging Methodologies. Methods Mol Biol 1649: 1–28, 2018. doi: 10.1007/978-1-4939-7213-5_1. [DOI] [PubMed] [Google Scholar]
- 143.Meier UT. RNA modification in Cajal bodies. RNA Biol 14: 693–700, 2017. doi: 10.1080/15476286.2016.1249091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Miller JW, Urbinati CR, Teng-Umnuay P, Stenberg MG, Byrne BJ, Thornton CA, Swanson MS. Recruitment of human muscleblind proteins to (CUG)n expansions associated with myotonic dystrophy. EMBO J 19: 4439–4448, 2000. doi: 10.1093/emboj/19.17.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mohan N, Kumar V, Kandala DT, Kartha CC, Laishram RS. A Splicing-Independent Function of RBM10 Controls Specific 3′ UTR Processing to Regulate Cardiac Hypertrophy. Cell Rep 24: 3539–3553, 2018. doi: 10.1016/j.celrep.2018.08.077. [DOI] [PubMed] [Google Scholar]
- 146.Mohanty BK, Kushner SR. Analysis of post-transcriptional RNA metabolism in prokaryotes. Methods 155: 124–130, 2019. doi: 10.1016/j.ymeth.2018.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Morton DJ, Kuiper EG, Jones SK, Leung SW, Corbett AH, Fasken MB. The RNA exosome and RNA exosome-linked disease. RNA 24: 127–142, 2018. doi: 10.1261/rna.064626.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Moujaber O, Stochaj U. Cytoplasmic RNA Granules in Somatic Maintenance. Gerontology 64: 485–494, 2018. doi: 10.1159/000488759. [DOI] [PubMed] [Google Scholar]
- 149.Mukhopadhyay D, Houchen CW, Kennedy S, Dieckgraefe BK, Anant S. Coupled mRNA stabilization and translational silencing of cyclooxygenase-2 by a novel RNA binding protein, CUGBP2. Mol Cell 11: 113–126, 2003. doi: 10.1016/S1097-2765(03)00012-1. [DOI] [PubMed] [Google Scholar]
- 150.Mulligan GJ, Guo W, Wormsley S, Helfman DM. Polypyrimidine tract binding protein interacts with sequences involved in alternative splicing of beta-tropomyosin pre-mRNA. J Biol Chem 267: 25480–25487, 1992. [PubMed] [Google Scholar]
- 151.Munoz-Tello P, Rajappa L, Coquille S, Thore S. Polyuridylation in Eukaryotes: A 3′-End Modification Regulating RNA Life. BioMed Res Int 2015: 968127, 2015. doi: 10.1155/2015/968127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Murmu N, Jung J, Mukhopadhyay D, Houchen CW, Riehl TE, Stenson WF, Morrison AR, Arumugam T, Dieckgraefe BK, Anant S. Dynamic antagonism between RNA-binding protein CUGBP2 and cyclooxygenase-2-mediated prostaglandin E2 in radiation damage. Proc Natl Acad Sci USA 101: 13873–13878, 2004. doi: 10.1073/pnas.0406066101. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 153.Nachtergaele S, He C. The emerging biology of RNA post-transcriptional modifications. [Correction in RNA Biol 14: 1269, 2017.] RNA Biol 14: 156–163, 2017. doi: 10.1080/15476286.2016.1267096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nagel RJ, Lancaster AM, Zahler AM. Specific binding of an exonic splicing enhancer by the pre-mRNA splicing factor SRp55. RNA 4: 11–23, 1998. [PMC free article] [PubMed] [Google Scholar]
- 155.Nakahata S, Kawamoto S. Tissue-dependent isoforms of mammalian Fox-1 homologs are associated with tissue-specific splicing activities. Nucleic Acids Res 33: 2078–2089, 2005. doi: 10.1093/nar/gki338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nakka K, Ghigna C, Gabellini D, Dilworth FJ. Diversification of the muscle proteome through alternative splicing. Skelet Muscle 8: 8, 2018. doi: 10.1186/s13395-018-0152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nasif S, Contu L, Mühlemann O. Beyond quality control: The role of nonsense-mediated mRNA decay (NMD) in regulating gene expression. Semin Cell Dev Biol 75: 78–87, 2018. doi: 10.1016/j.semcdb.2017.08.053. [DOI] [PubMed] [Google Scholar]
- 158.Nishida K, Kuwano Y, Nishikawa T, Masuda K, Rokutan K. RNA Binding Proteins and Genome Integrity. Int J Mol Sci 18: 1341, 2017. doi: 10.3390/ijms18071341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nishida K, Yamaguchi O, Otsu K. Degradation systems in heart failure. J Mol Cell Cardiol 84: 212–222, 2015. doi: 10.1016/j.yjmcc.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 160.Nutter CA, Jaworski EA, Verma SK, Deshmukh V, Wang Q, Botvinnik OB, Lozano MJ, Abass IJ, Ijaz T, Brasier AR, Garg NJ, Wehrens XHT, Yeo GW, Kuyumcu-Martinez MN. Dysregulation of RBFOX2 Is an Early Event in Cardiac Pathogenesis of Diabetes. Cell Rep 15: 2200–2213, 2016. doi: 10.1016/j.celrep.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Oerum S, Dégut C, Barraud P, Tisné C. m1A Post-Transcriptional Modification in tRNAs. Biomolecules 7: 20, 2017. doi: 10.3390/biom7010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ogorodnikov A, Kargapolova Y, Danckwardt S. Processing and transcriptome expansion at the mRNA 3′ end in health and disease: finding the right end. Pflugers Arch 468: 993–1012, 2016. doi: 10.1007/s00424-016-1828-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Opitz CA, Leake MC, Makarenko I, Benes V, Linke WA. Developmentally regulated switching of titin size alters myofibrillar stiffness in the perinatal heart. Circ Res 94: 967–975, 2004. doi: 10.1161/01.RES.0000124301.48193.E1. [DOI] [PubMed] [Google Scholar]
- 164.Park C, Choi S, Kim Y-E, Lee S, Park S-H, Adelstein RS, Kawamoto S, Kim KK. Stress Granules Contain Rbfox2 with Cell Cycle-related mRNAs. Sci Rep 7: 11211, 2017. doi: 10.1038/s41598-017-11651-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Park JY, Li W, Zheng D, Zhai P, Zhao Y, Matsuda T, Vatner SF, Sadoshima J, Tian B. Comparative analysis of mRNA isoform expression in cardiac hypertrophy and development reveals multiple post-transcriptional regulatory modules. PLoS One 6: e22391, 2011. doi: 10.1371/journal.pone.0022391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Parker R, Sheth U. P bodies and the control of mRNA translation and degradation. Mol Cell 25: 635–646, 2007. doi: 10.1016/j.molcel.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 167.Patange S, Girvan M, Larson DR. Single-cell systems biology: probing the basic unit of information flow. Curr Opin Syst Biol 8: 7–15, 2018. doi: 10.1016/j.coisb.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Peake JM, Della Gatta P, Suzuki K, Nieman DC. Cytokine expression and secretion by skeletal muscle cells: regulatory mechanisms and exercise effects. Exerc Immunol Rev 21: 8–25, 2015. [PubMed] [Google Scholar]
- 169.Peck SA, Hughes KD, Victorino JF, Mosley AL. Writing a wrong: coupled RNA polymerase II transcription and RNA quality control. Wiley Interdiscip Rev RNA 10: e1529, 2019. doi: 10.1002/wrna.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Peer E, Rechavi G, Dominissini D. Epitranscriptomics: regulation of mRNA metabolism through modifications. Curr Opin Chem Biol 41: 93–98, 2017. doi: 10.1016/j.cbpa.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 171.Pérez I, Lin CH, McAfee JG, Patton JG. Mutation of PTB binding sites causes misregulation of alternative 3′ splice site selection in vivo. RNA 3: 764–778, 1997. [PMC free article] [PubMed] [Google Scholar]
- 172.Philippe L, Vasseur JJ, Debart F, Thoreen CC. La-related protein 1 (LARP1) repression of TOP mRNA translation is mediated through its cap-binding domain and controlled by an adjacent regulatory region. Nucleic Acids Res 46: 1457–1469, 2018. doi: 10.1093/nar/gkx1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Poon KL, Tan KT, Wei YY, Ng CP, Colman A, Korzh V, Xu XQ. RNA-binding protein RBM24 is required for sarcomere assembly and heart contractility. Cardiovasc Res 94: 418–427, 2012. doi: 10.1093/cvr/cvs095. [DOI] [PubMed] [Google Scholar]
- 174.Pratt CA, Mowry KL. Taking a cellular road-trip: mRNA transport and anchoring. Curr Opin Cell Biol 25: 99–106, 2013. doi: 10.1016/j.ceb.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rajan S, Jagatheesan G, Karam CN, Alves ML, Bodi I, Schwartz A, Bulcao CF, D’Souza KM, Akhter SA, Boivin GP, Dube DK, Petrashevskaya N, Herr AB, Hullin R, Liggett SB, Wolska BM, Solaro RJ, Wieczorek DF. Molecular and functional characterization of a novel cardiac-specific human tropomyosin isoform. Circulation 121: 410–418, 2010. doi: 10.1161/CIRCULATIONAHA.109.889725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ramanathan A, Robb GB, Chan SH. mRNA capping: biological functions and applications. Nucleic Acids Res 44: 7511–7526, 2016. doi: 10.1093/nar/gkw551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ramanouskaya TV, Grinev VV. The determinants of alternative RNA splicing in human cells. Mol Genet Genomics 292: 1175–1195, 2017. doi: 10.1007/s00438-017-1350-0. [DOI] [PubMed] [Google Scholar]
- 178.Ratnadiwakara M, Mohenska M, Änkö ML. Splicing factors as regulators of miRNA biogenesis–links to human disease. Semin Cell Dev Biol 79: 113–122, 2018. doi: 10.1016/j.semcdb.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 179.Rattenbacher B, Beisang D, Wiesner DL, Jeschke JC, von Hohenberg M, St. Louis-Vlasova IA, Bohjanen PR. Analysis of CUGBP1 targets identifies GU-repeat sequences that mediate rapid mRNA decay. Mol Cell Biol 30: 3970–3980, 2010. doi: 10.1128/MCB.00624-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ray D, Kazan H, Cook KB, Weirauch MT, Najafabadi HS, Li X, Gueroussov S, Albu M, Zheng H, Yang A, Na H, Irimia M, Matzat LH, Dale RK, Smith SA, Yarosh CA, Kelly SM, Nabet B, Mecenas D, Li W, Laishram RS, Qiao M, Lipshitz HD, Piano F, Corbett AH, Carstens RP, Frey BJ, Anderson RA, Lynch KW, Penalva LOF, Lei EP, Fraser AG, Blencowe BJ, Morris QD, Hughes TR. A compendium of RNA-binding motifs for decoding gene regulation. Nature 499: 172–177, 2013. doi: 10.1038/nature12311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Rohrbach S, Muller-Werdan U, Werdan K, Koch S, Gellerich NF, Holtz J. Apoptosis-modulating interaction of the neuregulin/erbB pathway with anthracyclines in regulating Bcl-xS and Bcl-xL in cardiomyocytes. J Mol Cell Cardiol 38: 485–493, 2005. doi: 10.1016/j.yjmcc.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 182.Roundtree IA, Evans ME, Pan T, He C. Dynamic RNA Modifications in Gene Expression Regulation. Cell 169: 1187–1200, 2017. doi: 10.1016/j.cell.2017.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ryder PV, Lerit DA. RNA localization regulates diverse and dynamic cellular processes. Traffic 19: 496–502, 2018. doi: 10.1111/tra.12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Saldi T, Cortazar MA, Sheridan RM, Bentley DL. Coupling of RNA Polymerase II Transcription Elongation with Pre-mRNA Splicing. J Mol Biol 428: 2623–2635, 2016. doi: 10.1016/j.jmb.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Shepard PJ, Hertel KJ. The SR protein family. Genome Biol 10: 242, 2009. doi: 10.1186/gb-2009-10-10-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Shukla S, Kavak E, Gregory M, Imashimizu M, Shutinoski B, Kashlev M, Oberdoerffer P, Sandberg R, Oberdoerffer S. CTCF-promoted RNA polymerase II pausing links DNA methylation to splicing. Nature 479: 74–79, 2011. doi: 10.1038/nature10442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Simms CL, Thomas EN, Zaher HS. Ribosome-based quality control of mRNA and nascent peptides. Wiley Interdiscip Rev RNA 8: e1366, 2017. doi: 10.1002/wrna.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Singer-Krüger B, Jansen RP. Here, there, everywhere. mRNA localization in budding yeast. RNA Biol 11: 1031–1039, 2014. doi: 10.4161/rna.29945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Singh H, Lu R, Bopassa JC, Meredith AL, Stefani E, Toro L. MitoBK(Ca) is encoded by the Kcnma1 gene, and a splicing sequence defines its mitochondrial location. [Correction in Proc Natl Acad Sci USA 110: 18024, 2013.] Proc Natl Acad Sci USA 110: 10836–10841, 2013. doi: 10.1073/pnas.1302028110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sinigaglia K, Wiatrek D, Khan A, Michalik D, Sambrani N, Sedmik J, Vukic D, O’Connell MA, Keegan LP. ADAR RNA editing in innate immune response phasing, in circadian clocks and in sleep. Biochim Biophys Acta Gene Regul Mech 1862: 356–369, 2018. doi: 10.1016/j.bbagrm.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 191.Small EM, Frost RJ, Olson EN. MicroRNAs add a new dimension to cardiovascular disease. Circulation 121: 1022–1032, 2010. doi: 10.1161/CIRCULATIONAHA.109.889048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.St Johnston D. The intracellular localization of messenger RNAs. Cell 81: 161–170, 1995. doi: 10.1016/0092-8674(95)90324-0. [DOI] [PubMed] [Google Scholar]
- 193.Stoecklin G, Kedersha N. Relationship of GW/P-bodies with stress granules. Adv Exp Med Biol 768: 197–211, 2013. doi: 10.1007/978-1-4614-5107-5_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sträßer K, Masuda S, Mason P, Pfannstiel J, Oppizzi M, Rodriguez-Navarro S, Rondón AG, Aguilera A, Struhl K, Reed R, Hurt E. TREX is a conserved complex coupling transcription with messenger RNA export. Nature 417: 304–308, 2002. doi: 10.1038/nature746. [DOI] [PubMed] [Google Scholar]
- 195.Subramaniam D, Natarajan G, Ramalingam S, Ramachandran I, May R, Queimado L, Houchen CW, Anant S. Translation inhibition during cell cycle arrest and apoptosis: Mcl-1 is a novel target for RNA binding protein CUGBP2. Am J Physiol Gastrointest Liver Physiol 294: G1025–G1032, 2008. doi: 10.1152/ajpgi.00602.2007. [DOI] [PubMed] [Google Scholar]
- 196.Susor A, Kubelka M. Translational Regulation in the Mammalian Oocyte. Results Probl Cell Differ 63: 257–295, 2017. doi: 10.1007/978-3-319-60855-6_12. [DOI] [PubMed] [Google Scholar]
- 197.Tang ZZ, Yarotskyy V, Wei L, Sobczak K, Nakamori M, Eichinger K, Moxley RT, Dirksen RT, Thornton CA. Muscle weakness in myotonic dystrophy associated with misregulated splicing and altered gating of Ca(V)1.1 calcium channel. Hum Mol Genet 21: 1312–1324, 2012. doi: 10.1093/hmg/ddr568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Timchenko NA, Cai ZJ, Welm AL, Reddy S, Ashizawa T, Timchenko LT. RNA CUG repeats sequester CUGBP1 and alter protein levels and activity of CUGBP1. J Biol Chem 276: 7820–7826, 2001. doi: 10.1074/jbc.M005960200. [DOI] [PubMed] [Google Scholar]
- 199.Timchenko NA, Iakova P, Cai Z-J, Smith JR, Timchenko LT. Molecular basis for impaired muscle differentiation in myotonic dystrophy. Mol Cell Biol 21: 6927–6938, 2001. doi: 10.1128/MCB.21.20.6927-6938.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Tong J, Flavell RA, Li HB. RNA m6A modification and its function in diseases. Front Med 12: 481–489, 2018. doi: 10.1007/s11684-018-0654-8. [DOI] [PubMed] [Google Scholar]
- 201.Trotman JB, Schoenberg DR. A recap of RNA recapping. Wiley Interdiscip Rev RNA 10: e1504, 2019. doi: 10.1002/wrna.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Tsekrekou M, Stratigi K, Chatzinikolaou G. The Nucleolus: In Genome Maintenance and Repair. Int J Mol Sci 18: 1411, 2017. doi: 10.3390/ijms18071411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ulianov SV, Gavrilov AA, Razin SV. Nuclear compartments, genome folding, and enhancer-promoter communication. Int Rev Cell Mol Biol 315: 183–244, 2015. doi: 10.1016/bs.ircmb.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 204.Van den Hoogenhof MM, Pinto YM, Creemers EE. RNA Splicing: Regulation and Dysregulation in the Heart. Circ Res 118: 454–468, 2016. doi: 10.1161/CIRCRESAHA.115.307872. [DOI] [PubMed] [Google Scholar]
- 205.Van den Hoogenhof MMG, van der Made I, Beqqali A, de Groot NE, Damanafshan A, van Oort RJ, Pinto YM, Creemers EE. The RNA-binding protein Rbm38 is dispensable during pressure overload-induced cardiac remodeling in mice. PLoS One 12: e0184093, 2017. doi: 10.1371/journal.pone.0184093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Verma SK, Deshmukh V, Liu P, Nutter CA, Espejo R, Hung M-L, Wang G-S, Yeo GW, Kuyumcu-Martinez MN. Reactivation of fetal splicing programs in diabetic hearts is mediated by protein kinase C signaling. J Biol Chem 288: 35372–35386, 2013. doi: 10.1074/jbc.M113.507426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Verma SK, Deshmukh V, Nutter CA, Jaworski E, Jin W, Wadhwa L, Abata J, Ricci M, Lincoln J, Martin JF, Yeo GW, Kuyumcu-Martinez MN. Rbfox2 function in RNA metabolism is impaired in hypoplastic left heart syndrome patient hearts. Sci Rep 6: 30896, 2016. doi: 10.1038/srep30896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Vhuiyan MI, Pak ML, Park MA, Thomas D, Lakowski TM, Chalfant CE, Frankel A. PRMT2 interacts with splicing factors and regulates the alternative splicing of BCL-X. J Biochem 162: 17–25, 2017. doi: 10.1093/jb/mvw102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Viereck J, Thum T. Long Noncoding RNAs in Pathological Cardiac Remodeling. Circ Res 120: 262–264, 2017. doi: 10.1161/CIRCRESAHA.116.310174. [DOI] [PubMed] [Google Scholar]
- 210.Visvanathan A, Somasundaram K. mRNA Traffic Control Reviewed: N6-Methyladenosine (m6A) Takes the Driver’s Seat. BioEssays 40: 1700093, 2018. doi: 10.1002/bies.201700093. [DOI] [PubMed] [Google Scholar]
- 211.Vlasova IA, Bohjanen PR. Posttranscriptional regulation of gene networks by GU-rich elements and CELF proteins. RNA Biol 5: 201–207, 2008. doi: 10.4161/rna.7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Vuong CK, Wei W, Lee J-A, Lin C-H, Damianov A, de la Torre-Ubieta L, Halabi R, Otis KO, Martin KC, O’Dell TJ, Black DL. Rbfox1 Regulates Synaptic Transmission through the Inhibitory Neuron-Specific vSNARE Vamp1. Neuron 98: 127–141.e7, 2018. doi: 10.1016/j.neuron.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Walters R, Parker R. Quality control: is there quality control of localized mRNAs? J Cell Biol 204: 863–868, 2014. doi: 10.1083/jcb.201401059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wang ET, Ward AJ, Cherone JM, Giudice J, Wang TT, Treacy DJ, Lambert NJ, Freese P, Saxena T, Cooper TA, Burge CB. Antagonistic regulation of mRNA expression and splicing by CELF and MBNL proteins. Genome Res 25: 858–871, 2015. doi: 10.1101/gr.184390.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wang GS, Kearney DL, De Biasi M, Taffet G, Cooper TA. Elevation of RNA-binding protein CUGBP1 is an early event in an inducible heart-specific mouse model of myotonic dystrophy. J Clin Invest 117: 2802–2811, 2007. doi: 10.1172/JCI32308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wang H, Chen Y, Li X, Chen G, Zhong L, Chen G, Liao Y, Liao W, Bin J. Genome-wide analysis of alternative splicing during human heart development. Sci Rep 6: 35520, 2016. doi: 10.1038/srep35520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wang J, Li G, Yu D, Wong YP, Yong TF, Liang MC, Liao P, Foo R, Hoppe UC, Soong TW. Characterization of CaV1.2 exon 33 heterozygous knockout mice and negative correlation between Rbfox1 and CaV1.2 exon 33 expressions in human heart failure. Channels (Austin) 12: 51–57, 2018. doi: 10.1080/19336950.2017.1381805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Wang W. HuR and post-transcriptional regulation in vascular aging. Sci China Life Sci 57: 863–866, 2014. doi: 10.1007/s11427-014-4706-2. [DOI] [PubMed] [Google Scholar]
- 219.Wang Y, Gogol-Döring A, Hu H, Fröhler S, Ma Y, Jens M, Maaskola J, Murakawa Y, Quedenau C, Landthaler M, Kalscheuer V, Wieczorek D, Wang Y, Hu Y, Chen W. Integrative analysis revealed the molecular mechanism underlying RBM10-mediated splicing regulation. EMBO Mol Med 5: 1431–1442, 2013. doi: 10.1002/emmm.201302663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Wang YC, Rubenstein PA. Splicing of two alternative exon pairs in beta-tropomyosin pre-mRNA is independently controlled during myogenesis. J Biol Chem 267: 12004–12010, 1992. [PubMed] [Google Scholar]
- 221.Ward AJ, Rimer M, Killian JM, Dowling JJ, Cooper TA. CUGBP1 overexpression in mouse skeletal muscle reproduces features of myotonic dystrophy type 1. Hum Mol Genet 19: 3614–3622, 2010. doi: 10.1093/hmg/ddq277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Warf MB, Berglund JA. MBNL binds similar RNA structures in the CUG repeats of myotonic dystrophy and its pre-mRNA substrate cardiac troponin T. RNA 13: 2238–2251, 2007. doi: 10.1261/rna.610607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Warren CM, Krzesinski PR, Campbell KS, Moss RL, Greaser ML. Titin isoform changes in rat myocardium during development. Mech Dev 121: 1301–1312, 2004. doi: 10.1016/j.mod.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 224.Watanabe T, Kimura A, Kuroyanagi H. Alternative Splicing Regulator RBM20 and Cardiomyopathy. Front Mol Biosci 5: 105, 2018. doi: 10.3389/fmolb.2018.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wei C, Qiu J, Zhou Y, Xue Y, Hu J, Ouyang K, Banerjee I, Zhang C, Chen B, Li H, Chen J, Song L-S, Fu X-D. Repression of the Central Splicing Regulator RBFox2 Is Functionally Linked to Pressure Overload-Induced Heart Failure. Cell Rep 10: 1521–1533, 2015. doi: 10.1016/j.celrep.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wei C, Xiao R, Chen L, Cui H, Zhou Y, Xue Y, Hu J, Zhou B, Tsutsui T, Qiu J, Li H, Tang L, Fu X-D. RBFox2 Binds Nascent RNA to Globally Regulate Polycomb Complex 2 Targeting in Mammalian Genomes. [Correction in Mol Cell 62: 982, 2016.] Mol Cell 62: 875–889, 2016. doi: 10.1016/j.molcel.2016.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Wells QS, Becker JR, Su YR, Mosley JD, Weeke P, D’Aoust L, Ausborn NL, Ramirez AH, Pfotenhauer JP, Naftilan AJ, Markham L, Exil V, Roden DM, Hong CC. Whole exome sequencing identifies a causal RBM20 mutation in a large pedigree with familial dilated cardiomyopathy. Circ Cardiovasc Genet 6: 317–326, 2013. doi: 10.1161/CIRCGENETICS.113.000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Wheeler EC, Van Nostrand EL, Yeo GW. Advances and challenges in the detection of transcriptome-wide protein-RNA interactions. Wiley Interdiscip Rev RNA 9: e1436, 2018. doi: 10.1002/wrna.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Wu K, He J, Pu W, Peng Y. The Role of Exportin-5 in MicroRNA Biogenesis and Cancer. Genomics Proteomics Bioinformatics 16: 120–126, 2018. doi: 10.1016/j.gpb.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Xu X, Yang D, Ding J-H, Wang W, Chu P-H, Dalton ND, Wang H-Y, Bermingham JR Jr, Ye Z, Liu F, Rosenfeld MG, Manley JL, Ross J Jr, Chen J, Xiao R-P, Cheng H, Fu X-D. ASF/SF2-regulated CaMKIIdelta alternative splicing temporally reprograms excitation-contraction coupling in cardiac muscle. Cell 120: 59–72, 2005. doi: 10.1016/j.cell.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 232.Yablonovitch AL, Deng P, Jacobson D, Li JB. The evolution and adaptation of A-to-I RNA editing. PLoS Genet 13: e1007064, 2017. doi: 10.1371/journal.pgen.1007064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Ye B, Kroboth SL, Pu JL, Sims JJ, Aggarwal NT, McNally EM, Makielski JC, Shi NQ. Molecular identification and functional characterization of a mitochondrial sulfonylurea receptor 2 splice variant generated by intraexonic splicing. Circ Res 105: 1083–1093, 2009. doi: 10.1161/CIRCRESAHA.109.195040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Ye J, Beetz N, O’Keeffe S, Tapia JC, Macpherson L, Chen WV, Bassel-Duby R, Olson EN, Maniatis T. hnRNP U protein is required for normal pre-mRNA splicing and postnatal heart development and function. Proc Natl Acad Sci USA 112: E3020–E3029, 2015. doi: 10.1073/pnas.1508461112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Ye J, Llorian M, Cardona M, Rongvaux A, Moubarak RS, Comella JX, Bassel-Duby R, Flavell RA, Olson EN, Smith CWJ, Sanchis D. A pathway involving HDAC5, cFLIP and caspases regulates expression of the splicing regulator polypyrimidine tract binding protein in the heart. J Cell Sci 126: 1682–1691, 2013. doi: 10.1242/jcs.121384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Yin Z, Ren J, Guo W. Sarcomeric protein isoform transitions in cardiac muscle: a journey to heart failure. Biochim Biophys Acta 1852: 47–52, 2015. doi: 10.1016/j.bbadis.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Zander G, Krebber H. Quick or quality? How mRNA escapes nuclear quality control during stress. RNA Biol 14: 1642–1648, 2017. doi: 10.1080/15476286.2017.1345835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Zhai LT, Xiang S. mRNA quality control at the 5′ end. J Zhejiang Univ Sci B 15: 438–443, 2014. doi: 10.1631/jzus.B1400070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Zhang B, Herman PK. It is all about the process(ing): P-body granules and the regulation of signal transduction. Curr Genet 2019. doi: 10.1007/s00294-019-01016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Zhang J, Bahi N, Llovera M, Comella JX, Sanchis D. Polypyrimidine tract binding proteins (PTB) regulate the expression of apoptotic genes and susceptibility to caspase-dependent apoptosis in differentiating cardiomyocytes. Cell Death Differ 16: 1460–1468, 2009. doi: 10.1038/cdd.2009.87. [DOI] [PubMed] [Google Scholar]
- 241.Zhang M, Zhang Y, Xu E, Mohibi S, de Anda DM, Jiang Y, Zhang J, Chen X. Rbm24, a target of p53, is necessary for proper expression of p53 and heart development. Cell Death Differ 25: 1118–1130, 2018. doi: 10.1038/s41418-017-0029-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Zhang T, Lin Y, Liu J, Zhang ZG, Fu W, Guo LY, Pan L, Kong X, Zhang MK, Lu YH, Huang ZR, Xie Q, Li WH, Xu XQ. Rbm24 Regulates Alternative Splicing Switch in Embryonic Stem Cell Cardiac Lineage Differentiation. Stem Cells 34: 1776–1789, 2016. doi: 10.1002/stem.2366. [DOI] [PubMed] [Google Scholar]
- 243.Zhang Y, Si Y, Ma N, Mei J. The RNA-binding protein PCBP2 inhibits Ang II-induced hypertrophy of cardiomyocytes though promoting GPR56 mRNA degeneration. Biochem Biophys Res Commun 464: 679–684, 2015. doi: 10.1016/j.bbrc.2015.06.139. [DOI] [PubMed] [Google Scholar]
- 244.Zhou A, Shi G, Kang G-J, Xie A, Liu H, Jiang N, Liu M, Jeong E-M, Dudley SC Jr. RNA Binding Protein, HuR, Regulates SCN5A Expression Through Stabilizing MEF2C transcription factor mRNA. J Am Heart Assoc 7: e007802, 2018. doi: 10.1161/JAHA.117.007802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Zhou A, Xie A, Kim TY, Liu H, Shi G, Kang G-J, Jiang N, Liu M, Jeong E-M, Choi B-R, Dudley SC Jr. HuR-mediated SCN5A messenger RNA stability reduces arrhythmic risk in heart failure. Heart Rhythm 15: 1072–1080, 2018. doi: 10.1016/j.hrthm.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Zhou B, Liu J, Ren Z, Yao F, Ma J, Song J, Bennett B, Zhen Y, Wang L, Hu G, Hu S. Cnot3 enhances human embryonic cardiomyocyte proliferation by promoting cell cycle inhibitor mRNA degradation. Sci Rep 7: 1500, 2017. doi: 10.1038/s41598-017-01628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Zigáčková D, Vaňáčová Š. The role of 3′ end uridylation in RNA metabolism and cellular physiology. Philos Trans R Soc Lond B Biol Sci 373: 20180171, 2018. doi: 10.1098/rstb.2018.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]