Abstract
Background
Benign prostatic hyperplasia (BPH) is one of the major causes of lower urinary tract symptoms (LUTS), including storage LUTS such as urinary frequency and urgency. Recently, a growing number of clinical studies indicate that prostatic inflammation could be an important pathophysiological mechanism inducing storage LUTS in BPH patients. Here we aimed to investigate whether non-bacterial prostatic inflammation in a rat model induced by intraprostatic formalin injection can lead to long-lasting bladder overactivity and changes in bladder afferent neuron excitability.
Methods
Male Sprague-Dawley rats were divided into four groups (n=12 each): normal control group, 1-week prostatic inflammation group, 4-week inflammation group and 8-week inflammation group. Prostatic inflammation was induced by formalin (10%; 50 μl per lobe) injection into bilateral ventral lobes of the prostate. Voiding behaviour was evaluated in metabolic cages for each group. Ventral lobes of the prostate and the bladder were then removed for HE staining to evaluate inflammation levels. Continuous cystometrograms (CMG) were recorded to measure intercontraction intervals (ICI) and voided volume per micturition. Whole-cell patch clamp recordings were performed on dissociated bladder afferent neurons labelled by fluorogold (FG) injected into the bladder wall, to examine the electrophysiological properties.
Results
Results of metabolic cage measurements showed that formalin-treated rats exhibited significant (P<0.05) increases in micturition episodes/12h and decrease in voided volume per micturition at every time point post injection. Continuous CMG illustrated the significant (P<0.05) higher number of non-voiding contractions per void and shorter ICI in formalin-treated rats compared to normal rats. HE staining showed significant prostatic inflammation, which declined gradually, in prostate tissues of formalin-induced rats. In patch clamp recordings, capsaicin-sensitive bladder afferent neurons from rats with prostatic inflammation had significantly (P<0.05) lower thresholds for spike activation and a “multiple” firing pattern compared to control rats at every time point post injection.
Conclusions
Formalin-induced prostatic inflammation can lead to long-lasting bladder overactivity in association with bladder afferent neuron hyperexcitability. This long-lasting model could be a useful tool for the study of inflammation-related aspects of male LUTS pathophysiology.
Keywords: bladder overactivity, formalin, prostatic inflammation, rat
Introduction
Benign prostatic hyperplasia (BPH) is one of the major causes of lower urinary tract symptoms (LUTS) in elderly men. The BPH-associated LUTS can be classified as storage and voiding symptoms, and the former includes urgency, frequency, and nocturia, which are overlapped with overactive bladder (OAB) symptoms1. Although the aetiology of LUTS associated with BPH is multifactorial, an increasing number of clinical studies demonstrate that prostatic inflammation is involved in not only the development of histological BPH but also the emergence of male LUTS2–4.
The REDUCE (Reduction by Dutasteride of prostate Cancer Events) trial showed that chronic prostatic inflammation can be detected in 77% of BPH patients who underwent prostate biopsies. A statistically significant correlation was also found between chronic prostatic inflammation and LUTS, especially in storage LUTS2. The MTOPS (Medical Therapy of Prostatic Symptoms) trial also demonstrated that patients with prostatic inflammation in baseline prostate biopsied specimens were more likely to develop symptomatic progression compared to those without inflammation. These findings suggest that prostatic inflammation could be a potential mechanism inducing storage LUTS in BPH patients5.
Reliable and suitable animal models of prostatic inflammation are powerful tools to elucidate the aetio-pathogenic mechanisms and explore effective therapies for patients6. Up to now, various animal models have been developed, including spontaneous, infectious, immune-mediated, and hormone-mediated, chemical agent-induced or stress-induced models7. Each model has unique features and is used in different studies according to their research purposes. Among these models, intraprostatic injection of chemicals (such as formalin, carrageenan, zymosan, or CFA) is a convenient and effective approach. Our previous studies showed that a rat model of formalin-induced prostatic inflammation developed molecular changes similar to those identified in human BPH specimens8,9 and exhibits bladder overactivity and afferent hyperexcitability at 1 week after intraprostatic formalin injection10. Schwartz et al.11 also reported that bladder afferent pathways were sensitized in a 4-week mouse model of prostatitis induced by intraprostatic zymosan injection possibly due to cross-organ sensitization between the prostate and bladder. Nevertheless, most of the previous studies focused on the basic histopathology and inflammatory mechanisms, and a few have evaluated the long-term effect of prostatic inflammation on bladder function and afferent mechanisms.
Since an adequate chronic animal model is necessary for further BPH-related prostatic inflammation studies, we aimed to investigate whether the rat model of formalin-induced prostatic inflammation can lead to long-lasting bladder overactivity and the changes in bladder afferent neuron excitability.
Materials and Methods
Animals and Surgery
Male Sprague-Dawley rats (220–250 g, n=48) were divided into four groups; normal control group (n=12), 1-week prostatic inflammation group (n=12), 4-week inflammation group (n=12) and 8-week inflammation group (n=12). Rats were housed in under a 12/12 hr reversed light–dark cycle. In our previous studies, we utilized 5% formalin solution to induce prostatic inflammation8–10. In this study, we increased the formalin concentration to 10% to examine whether prostatic inflammation induced by injection of 10% formalin solution (50 μl) into each of bilateral ventral lobes of the prostate can induce the long-lasting effects on bladder function and bladder afferent activity.
All experimental procedures were in accordance with NIH (National Institutes of Health) guidelines and approved by the University of Pittsburgh institutional animal care and use committee.
Experiment 1
Metabolic cage study
One third of the rats (n=4 in each group) were placed in metabolic cages and voiding behaviour was evaluated for 12 hours during night (7 p.m. to 7 a.m., light off).
Tissue inflammation
After metabolic cage measurements, the rats were perfused transcardially with cold 0.01M phosphate buffer saline followed by cold PFA (paraformaldehyde) (4% solution in 0.1 M phosphate buffer). The prostate was harvested and embedded in OCT Tissue-Tek compound (Sakura Finetek U.S.A, Torrance, CA), frozen on dry ice, and kept at −80°C until use. The bladder was harvested, postfixed in 4% PFA overnight, then transferred to 30% glucose for 1–2 days and stored in −80°C until use. Frozen tissues were serially sectioned at 10 μm thickness and stained with haematoxylin and eosin.
Experiment 2
Continuous CMG
Another one third of the rats (n=4 in each group) underwent continuous cystometry, and cystometrograms (CMG) were recorded to measure intercontraction intervals (ICI) and voided volume per micturition. Briefly, an incision was made in the lower middle abdomen and then a polyethylene catheter (PE-50; Clay Adams, Parsippany, NJ, USA) was inserted into the bladder through the dome. After recovery from anaesthesia, the animals were placed in a restraining device (Natsume Seisakusho Co., Tokyo, Japan) and the cystostomy catheter was connected to a pressure transducer and an infusion pump via a three-way stopcock. After rats were acclimated in the restraining device for at least 2 hours, physiological saline at room temperature was infused into the bladder at a rate of 0.04 ml/min, and parameters including intercontraction interval (ICI) and non-voiding contractions (NVCs) were evaluated. NVCs were defined as rises in intravesical pressure that exceeded 10 cm H2O over the baseline without fluid elimination from the urethral orifice.
Experiment 3
Fluorogold injection
The remaining one third of the rats (n=4 in each group) were used for patch clamp recordings. One week before sacrifice, 2.5% fluorogold (FG) were injected into the bladder wall via a 30-gauge Hamilton syringe (4 sites, 10μL/site).
Characterization of afferent neuron excitability
At each time point, L6-S1 dorsal root ganglia (DRG) were removed as previously described and dissociated into single neurons by enzymatic methods.12 Then, whole-cell patch clamp recordings were performed at room temperature (20°C to 22°C) on FG-labelled bladder afferent neurons within 24 hours after dissociation. The internal solution contained 140 mM KCl, 1 mM CaCl2, 2 mM MgCl2, 11 mM EGTA, 10 mM HEPES and 2 mM Mg adenosine triphosphate, adjusted to pH 7.4 with KOH. Patch electrodes had 2 to 4 MΩ resistance when filled with the internal solution. Neurons were superfused at a flow rate of 2.0 ml per minute with an external solution containing 150 mM NaCl, 5 mM KCl, 2.5 mM CaCl2, 1 mM MgCl2, 10 mM HEPES and 10 mM D-glucose, adjusted to pH 7.4 with NaOH. Action potential and firing pattern were recorded first in the current clamp mode. Then, in the voltage clamp mode, the sensitivity of neurons to capsaicin was evaluated by bath application of capsaicin (1 μM), which evoked a transient inward current in capsaicin-sensitive neurons, at the holding potential of −60 mV.
Statistical Analysis
Data are presented as mean ± SD. One-way ANOVA followed by LSD was used for statistical analysis through IBM SPSS 22 software. P-values less than 0.05 were considered statistically significant. Graphs were drawn by GraphPad Prism Software.
Results
Metabolic cage study
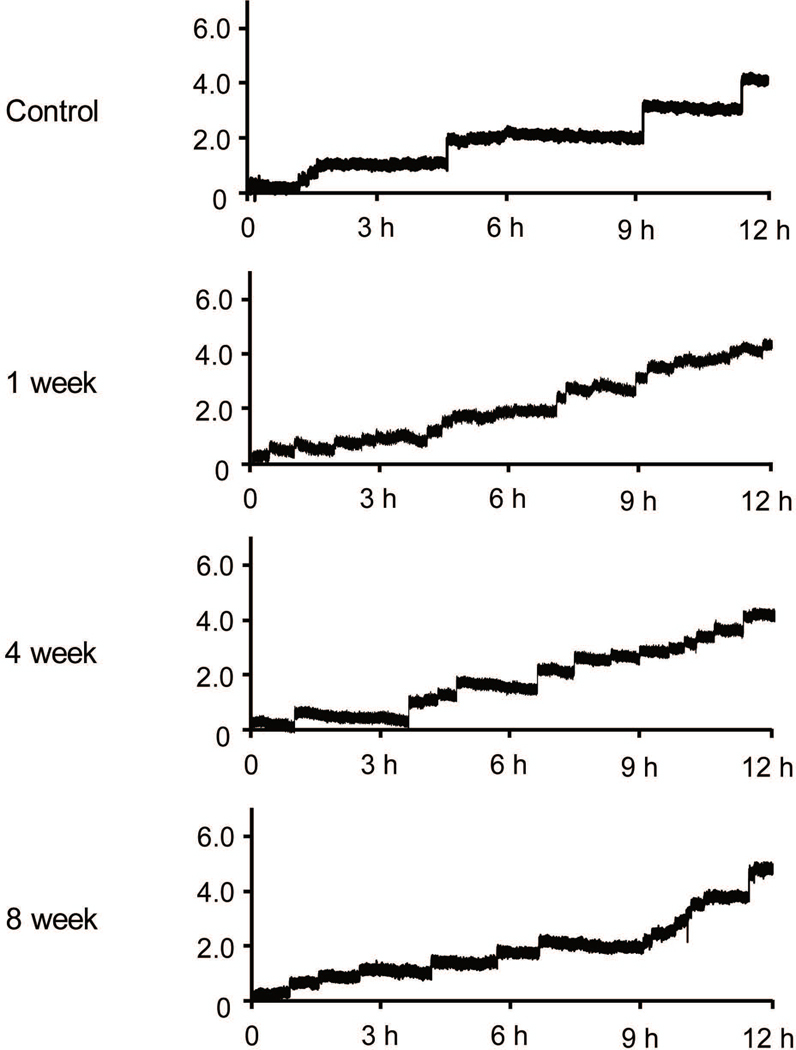
As shown in Figure 1 and Table 1, formalin-treated rats exhibited a significant (P<0.05) increase in micturition episodes/12h and a decrease in voided volume per micturition at each time point (micturition episodes/12h: 14.2 ± 1.7 [1 week], 14.5 ± 1.9 [4 weeks], and 13.8 ± 1.5 [8 weeks] vs. 8.5 ± 1.0 [control]; voided volume per micturition: 0.49 ± 0.10 mL, 0.33 ± 0.03 mL, and 0.32 ± 0.04 mL vs. 0.32 ± 0.03 mL). Meanwhile, formalin-treated rats showed no difference in total voided volume compared to normal rats. These results indicate that this prostatic inflammation model has long-term bladder overactivity, which was maintained for at least 8 weeks.
Figure 1.
Metabolic cage study of voiding behaviour. Formalin-treated rats exhibited an increase in micturition episodes/12h and a decrease in voided volume per micturition at each time point compared to control rats whereas total voiding volume was not altered among groups.
Table 1.
Metabolic cage parameters.
| control | 1 week | 4 weeks | 8 weeks | |
|---|---|---|---|---|
| Total voiding volume/12h | 4.06 ± 0.41 | 4.67 ± 0.53 | 4.6 ± 0.67 | 4.48 ± 0.65 |
| Micturition episodes/12h | 8.5 ± 1.0 | 14.2 ± 1.7* | 14.5 ± 1.9* | 13.8 ± 1.5* |
| Voided volume per void (ml) | 0.49 ± 0.1 | 0.33 ± 0.03* | 0.32 ± 0.04* | 0.32 ± 0.03* |
Results showed that PRO rats have higher number of void per 12 hour and lower voided volume per void than normal rats.
, p<0.05.
Continuous CMG
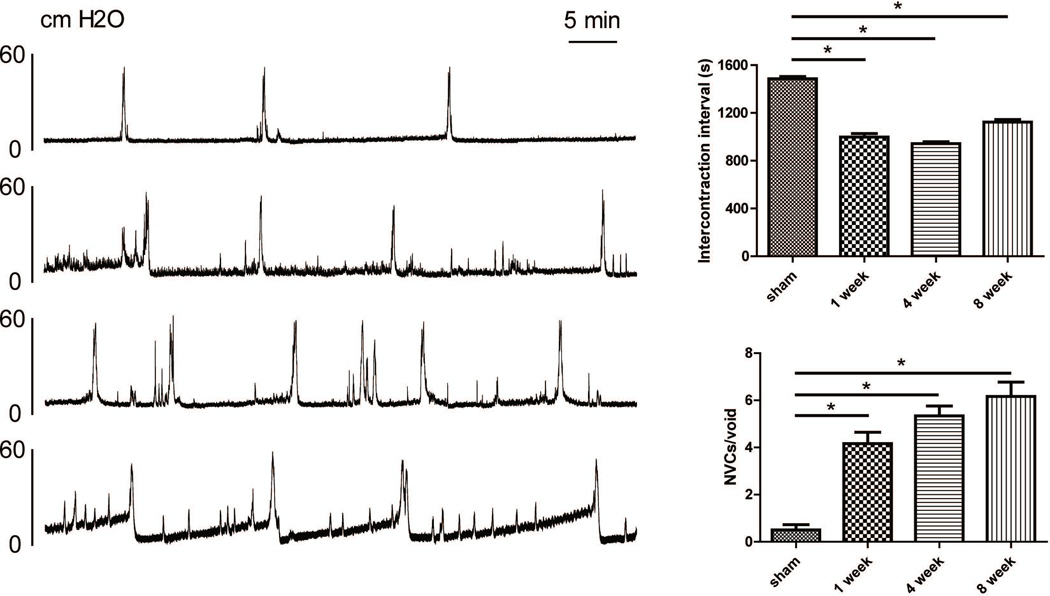
Compared to control rats, formalin-treated rats exhibited a significantly (P<0.05) higher number of NVCs per void (4.2 ± 1.2 [1 week], 5.3 ± 1.0 [4 weeks], and 6.2 ± 1.5 [8 weeks] vs. 0.5 ± 0.5 [control]) and shorter ICI (998.3 ± 71.4 ms [1 week], 943.3 ± 40.3 ms [4 weeks], and 1123.4 ± 53.5 ms [8 weeks] vs. 1486.7 ± 43.2 ms [control]) (Figure 2). These results illustrated that prostatic inflammation in our model can induce long-lasting bladder overactivity for at least 8 weeks.
Figure 2.
Representative cystometrograms (A) and comparison of cystometric parameters (B). Compared to control rats, formalin-treated rats with prostatic inflammation exhibited a significantly (P<0.05) higher number of non-voiding contractions per void and shorter intercontraction intervals.
Tissue inflammation
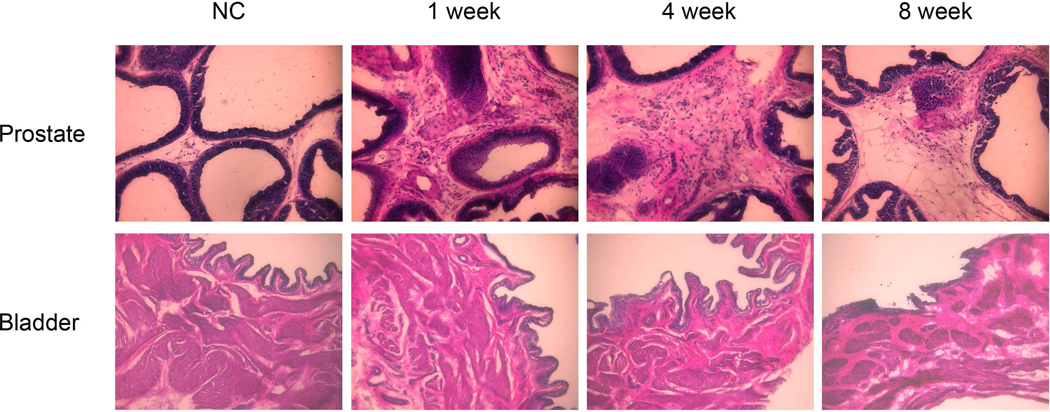
Haematoxylin and eosin staining (Figure 3) showed that there were regular shaped acini and intact epithelial membrane in prostate tissues of control rats whereas inflammatory cell infiltration in stroma and irregular shaped acini were found in prostate tissues of formalin-induced rats. The inflammation responses characterized by inflammatory cell infiltration and thicken stromal area continued up to 8 weeks after formalin injection although the degree of inflammatory changes was reduced gradually with time and become less obvious at 8 weeks. In contrast, inflammation such as inflammatory cell accumulation or epithelium layer changes, was not found in the bladder sections from any of the four groups.
Figure 3.
Haematoxylin and eosin (HE) staining of the prostate and the bladder. HE staining showed that there were regular shaped acini and intact basement membrane in the prostate tissue of normal control rats (NC), whereas stromal infiltration of mast cells and lymphocytes and irregular shaped acini were found in prostate tissues of formalin-treated rats. The inflammation responses continued up to 8 weeks after formalin injection although the degree of inflammatory changes is reduced gradually with time and become weak at 8 weeks. Bladder tissues from any of four groups did not show inflammatory cell accumulation or epithelium layer changes.
Bladder afferent neuron excitability
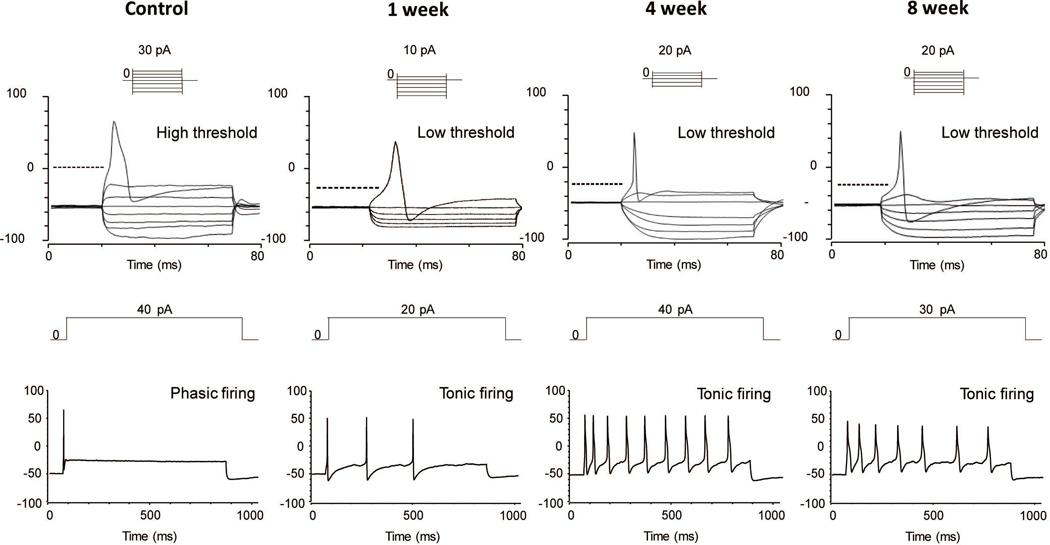
Figure 4 and Table 2 showed representative recordings and parameters of action potentials in capsaicin-sensitive bladder afferent neurons from the four groups of animals. The resting membrane potential of capsaicin-sensitive bladder afferent neurons did not differ between control and prostatic inflammation rats. However, the spike threshold for eliciting action potentials, peak membrane potentials and spike duration in bladder afferent neurons from prostatic inflammation rats were significantly lower than those in bladder afferent neurons from normal rats. In addition, the number of action potentials during an 800-ms membrane depolarization in capsaicin-sensitive bladder afferent neurons from prostatic inflammation rats was significantly higher than that in neurons from normal rats, when the current intensity was set to the value just above the threshold for inducing spike activation with a 50-ms pulse. These results demonstrated that the prostatic inflammation induced long-term bladder afferent neuron hyperexcitability up to 8weeks.
Figure 4.
Patch clamp recordings of bladder afferent neurons. In patch clamp recordings, capsaicin-sensitive bladder afferent neurons from rats with prostatic inflammation had lower thresholds for spike activation compared to control rats. The number of action potentials of bladder afferent neurons during an 800 ms depolarizing pulse was increased after prostatic inflammation at every time point compared to control rats.
Table 2.
Electrophysiological properties of capsaicin sensitive bladder afferent neurons.
| control | 1 week | 4 weeks | 8 weeks | |
|---|---|---|---|---|
| No. of neurons/rats | 8/4 | 9/4 | 8/4 | 7/4 |
| Diameter (μm) | 24.8 ± 1.7 | 27.6 ± 2.3* | 28.5 ± 2.1* | 28.3 ± 2.2* |
| Input capacitance (pF) | 24.3 ± 2.0 | 27.3 ± 2.8 | 28.3 ± 3.1* | 28.5 ± 3.3* |
| Resting membrane potentials (mV) | −49.9 ± 0.4 | −49.7 ± 1.0 | −49.4 ± 1.3 | −49.7 ± 0.9 |
| Spike threshold | −11.0 ± 2.3 | −19.3 ± 1.8* | −22.2 ± 1.7* | −21.0 ± 1.8* |
| Peak membrane potential (mV) | −51.2 ± 2.6 | −45.8 ± 2.3* | −47.7 ± 2.2* | −47.3 ± 2.6* |
| Spike durations (ms) | 4.1 ± 0.2 | 3.5 ± 0.3* | 3.3 ± 0.2* | 3.5 ± 0.2* |
| Number of spikes (800-ms depolarization) | 1.5 ± 0.8 | 5.0 ± 1.4* | 6.8 ± 1.9* | 5.7 ± 1.2* |
, p<0.05 vs control.
Discussion
The results of the present study, indicated that: (1) formalin-induced prostatic inflammation can result in long-lasting bladder overactivity as evidenced by increased number of voiding episodes and reduced voided volume per micturition and intercontraction interval (ICI); (2) intraprostatic injection of formalin can induce chronic inflammation in the prostate without affecting the bladder and (3) prostatic inflammation can induce long-lasting hyperexcitability of capsaicin-sensitive C-fiber bladder afferent neurons. Thus, it is assumed that intraprostatic injection of formalin induces chronic prostatic inflammation leading to bladder overactivity via sensitization of capsaicin-sensitive C-fiber bladder afferent pathways.
Intraprostatic formalin injection has been recognized as a convenient and qualified method establish non-bacteria prostatic inflammation. Previous studies including ours showed that a single intraprostatic injection of 5% or 10% formalin could induce prostatic inflammation and result in bladder overactivity for up to 4 weeks8,9,13, as well as bladder afferent hyperexcitability at 1 week after formalin injection10. Our current study further confirmed that formalin-treated rats exhibit bladder overactivity that lasts for 8 weeks using metabolic cage measurements and continuous CMG recordings. Moreover, we found a gradual decline in the prostatic inflammation at 4 weeks and 8 weeks, which indicates that the bladder overactivity symptoms can still exist even when the prostatic inflammation is partially recovered. In this study, we used 10% formalin solution to induce prostatic inflammation, which was a higher concentration than that in our previous studies8,9. This is because a higher dose of chemical reagent could induce more consistent tissue inflammation. For example, intraprostatic injection of capsaicin was found to induce prostatic inflammation in a dose-dependent fashion14. The present study confirmed that intraprostatic injection of 10% formalin solution can induce long-lasting prostatic inflammation up to 8 weeks in association with bladder overactivity and afferent hyperexcitability.
In addition, our histological results showed that intraprostatic injection of formalin can only induce inflammation in the prostate, but not in the bladder. Recently, cross-organ sensitization among pelvic organs such as bladder, colon and rectum has been proposed as a potential mechanism for overlapped afferent sensitization of different visceral sensory systems. For example, local rectal anesthesia can promptly improve pelvic pain and discomfort in patients with irritable bowel syndrome (IBS) 15,16. Previous studies in rodents also documented that bidirectional colon-bladder cross-organ sensitization can be induced by colon or bladder inflammation17,18. These neural cross-talks have also been found between the prostate and the bladder. Dual neural retrograde tracing studies showed the convergence of the prostate and bladder afferent pathways by labelling neurons in the lumbosacral DRG10,19. Ishigooka et al. also reported that capsaicin-induced bladder and prostate inflammation can lead to an increased expression of c-fos, a functional marker of nociceptive cell activation, at similar region of L6 and S1 spinal cord levels20. Schwartz et al.11 also found that prostatic inflammation significantly increased urinary frequency, along with hypersensitivity to bladder distension due to sensitized bladder afferents. Our recent study also revealed that bladder overactivity and nerve growth factor (NGF) overexpression in the bladder in rats with formalin-induced prostatic inflammation is dependent on activation of primary afferents in the pelvic nerve, which contain dichotomized afferents innervating the bladder and the prostate, because the transection of the pelvic nerve prevented these prostatic inflammation-induced bladder pathophysiology10.
Accordingly, in our recent study, we combined retrograde tracing technique and patch clamp recordings to examine the neuron excitability of C-fiber bladder afferent neurons in rats with prostatic inflammation, and found that C-fiber bladder afferent neurons of these rats exhibited cell hyperexcitability, evident as lower spike thresholds and a multiple firing pattern in rats with 1-week prostatic inflammation compared to normal rats10. In the current study, the afferent hyperexcitability continued for 8 weeks without any decline along with the consistent bladder overactivity although there was a gradual decrease of inflammatory responses in the prostate. Thus, our data supports the cross-organ sensitization from the prostate to the bladder is one of the main underlying mechanisms for bladder overactivity after prostatic inflammation. These findings may explain the fact that approximately 20–50% of BPH/LUTS patients still have persistent OAB symptoms after transurethral resection of the prostate (TURP)21.
Moreover, it has been shown that increased release of proinflammatory neuropeptides and cytokines contributes to cross-organ sensitization in pelvic organs. Pan et al. reported that experimental colitis could lead to up-regulations in gene and protein expression of substance P (SP) and calcitonin gene-related peptide (CGRP) in DRG neurons, and thus influence the function of the urinary bladder via activation of TRPV1 signaling pathways22. Previous studies including ours also showed a significant up-regulation of NGF in the prostate and/or the bladder10,11. In addition, Mizoguchi et al. reported an increase of TRPA1 receptor in the bladder mucosa in rats with 4-weeks prostatic inflammation. NGF has been shown to be involved in pathological changes in C-fiber bladder sensory pathways and a crucial factor in neurogenic detrusor overactivity (DO)23 as well as colitis-induced bladder overactivity24. It has been demonstrated that bladder afferent nerves take up NGF and transport it to DRG, where it alters the expression of ion channels and receptors and induces hyperexcitability of C-fiber bladder afferent neurons, which finally leads to neurogenic bladder dysfunction12,25. Taken together, it is reasonable to assume that C-fiber bladder afferent hyperexcitability after prostate inflammation can promote release of neural factors and upregulate the expression of specific receptors, leading to cross-organ sensitization and afferent hypersensitivity.
The current study has some limitations. First, we only labelled bladder afferent neurons and examined their neuronal excitability. Excitability of double-labelling dichotomized afferent neurons innervating both bladder and prostate needs to be examined for further confirmation of the prostate-to-bladder cross-organ sensitization theory although we recently reported that dichotomized DRG neurons innervating both prostate and bladder from rats with 1-week prostatic inflammation exhibited neuronal hyperexcitability10. Secondly, we did not examine the molecular change in DRG of our 8-weeks prostatic inflammation model. Further studies are anticipated to find out which types of ion channels or receptors are the crucial factors in the long-term cross-organ sensitization process.
Conclusions
Formalin-induced prostatic inflammation can induce long-lasting bladder overactivity in rats evident as frequent micturition and increased non-voiding contractions in association of bladder afferent neuron hyperexcitability. Clinically, chronic prostatic inflammation might contribute to storage LUTS in BPH patients. Thus, our long-lasting model of prostatic inflammation would be useful for the study of inflammation-related aspects of male LUTS pathophysiology.
Acknowledgments
Conflict of interest: The authors have declared that no conflict of interest exists. This work was supported by a grant from the National Institutes of Health National Institute of Diabetes and Digestive and Kidney (U54 DK112079).
Reference
- 1.Eckhardt MD, van Venrooij GE, van Melick HH, Boon TA. Prevalence and bothersomeness of lower urinary tract symptoms in benign prostatic hyperplasia and their impact on well-being. The Journal of urology. 2001;166(2):563–568. [PubMed] [Google Scholar]
- 2.Nickel JC, Roehrborn CG, O’Leary MP, Bostwick DG, Somerville MC, Rittmaster RS. The relationship between prostate inflammation and lower urinary tract symptoms: examination of baseline data from the REDUCE trial. European urology. 2008;54(6):1379–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mishra VC, Allen DJ, Nicolaou C, et al. Does intraprostatic inflammation have a role in the pathogenesis and progression of benign prostatic hyperplasia? BJU international. 2007;100(2):327–331. [DOI] [PubMed] [Google Scholar]
- 4.Gerstenbluth RE, Seftel AD, MacLennan GT, et al. Distribution of chronic prostatitis in radical prostatectomy specimens with up-regulation of bcl-2 in areas of inflammation. The Journal of urology. 2002;167(5):2267–2270. [PubMed] [Google Scholar]
- 5.Roehrborn CG. Definition of at-risk patients: baseline variables. BJU international. 2006;97 Suppl 2:7–11; discussion 21–12. [DOI] [PubMed] [Google Scholar]
- 6.Vykhovanets EV, Resnick MI, MacLennan GT, Gupta S. Experimental rodent models of prostatitis: limitations and potential. Prostate cancer and prostatic diseases. 2007;10(1):15–29. [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Zhong S, Xu T, et al. Histopathological classification criteria of rat model of chronic prostatitis/chronic pelvic pain syndrome. International urology and nephrology. 2015;47(2):307–316. [DOI] [PubMed] [Google Scholar]
- 8.Funahashi Y, O’Malley KJ, Kawamorita N, et al. Upregulation of androgen-responsive genes and transforming growth factor-beta1 cascade genes in a rat model of non-bacterial prostatic inflammation. The Prostate. 2014;74(4):337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mizoguchi S, Mori K, Wang Z, et al. Effects of Estrogen Receptor beta Stimulation in a Rat Model of Non-Bacterial Prostatic Inflammation. The Prostate. 2017;77(7):803–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Funahashi Y, Takahashi R, Mizoguchi S, Suzuki T, Takaoka E, Ni J, Wang Z, DeFranco DB, de Groat WC, Tyagi P, Yoshimura N Bladder overactivity and afferent hyperexcitability induced by prostate-to-bladder cross-sensitization in rats with prostatic inflammation. Journal of Physiology (London).in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz ES, La JH, Young EE, Feng B, Joyce S, Gebhart GF. Chronic Prostatitis Induces Bladder Hypersensitivity and Sensitizes Bladder Afferents in the Mouse. The Journal of urology. 2016;196(3):892–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshimura N, Bennett NE, Hayashi Y, et al. Bladder overactivity and hyperexcitability of bladder afferent neurons after intrathecal delivery of nerve growth factor in rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26(42):10847–10855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park JS, Jin MH, Hong CH. Neurologic Mechanisms Underlying Voiding Dysfunction due to Prostatitis in a Rat Model of Nonbacterial Prostatic Inflammation. International neurourology journal. 2018;22(2):90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chuang YC, Yoshimura N, Wu M, et al. Intraprostatic capsaicin injection as a novel model for nonbacterial prostatitis and effects of botulinum toxin A. European urology. 2007;51(4):1119–1127. [DOI] [PubMed] [Google Scholar]
- 15.Verne GN, Robinson ME, Vase L, Price DD. Reversal of visceral and cutaneous hyperalgesia by local rectal anesthesia in irritable bowel syndrome (IBS) patients. Pain. 2003;105(1–2):223–230. [DOI] [PubMed] [Google Scholar]
- 16.Price DD, Zhou Q, Moshiree B, Robinson ME, Verne GN. Peripheral and central contributions to hyperalgesia in irritable bowel syndrome. The journal of pain : official journal of the American Pain Society. 2006;7(8):529–535. [DOI] [PubMed] [Google Scholar]
- 17.Pezzone MA, Liang R, Fraser MO. A model of neural cross-talk and irritation in the pelvis: implications for the overlap of chronic pelvic pain disorders. Gastroenterology. 2005;128(7):1953–1964. [DOI] [PubMed] [Google Scholar]
- 18.Ustinova EE, Fraser MO, Pezzone MA. Cross-talk and sensitization of bladder afferent nerves. Neurourology and urodynamics. 2010;29(1):77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee S, Yang G, Xiang W, Bushman W. Retrograde double-labeling demonstrates convergent afferent innervation of the prostate and bladder. The Prostate. 2016;76(8):767–775. [DOI] [PubMed] [Google Scholar]
- 20.Ishigooka M, Zermann DH, Doggweiler R, Schmidt RA. Similarity of distributions of spinal c-Fos and plasma extravasation after acute chemical irritation of the bladder and the prostate. The Journal of urology. 2000;164(5):1751–1756. [PubMed] [Google Scholar]
- 21.Nitti VW, Kim Y, Combs AJ. Voiding dysfunction following transurethral resection of the prostate: symptoms and urodynamic findings. The Journal of urology. 1997;157(2):600–603. [PubMed] [Google Scholar]
- 22.Pan XQ, Gonzalez JA, Chang S, Chacko S, Wein AJ, Malykhina AP. Experimental colitis triggers the release of substance P and calcitonin gene-related peptide in the urinary bladder via TRPV1 signaling pathways. Experimental neurology. 2010;225(2):262–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimizu N, Wada N, Shimizu T, et al. Effects of nerve growth factor neutralization on TRP channel expression in laser-captured bladder afferent neurons in mice with spinal cord injury. Neuroscience letters. 2018;683:100–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawamorita N, Yoshikawa S, Kashyap M, et al. Liposome Based Intravesical Therapy Targeting Nerve Growth Factor Ameliorates Bladder Hypersensitivity in Rats with Experimental Colitis. The Journal of urology. 2016;195(6):1920–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Groat WC, Yoshimura N. Plasticity in reflex pathways to the lower urinary tract following spinal cord injury. Experimental neurology. 2012;235(1):123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]