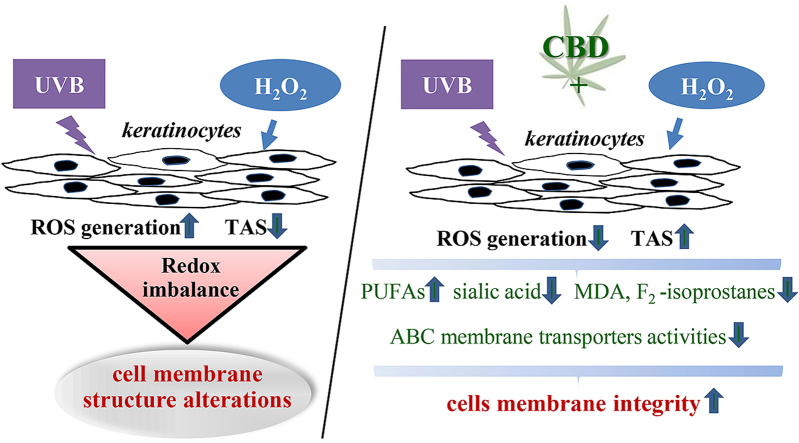
Abstract
Keratinocytes, the major cell type of the epidermis, are particularly sensitive to environmental factors including exposure to sunlight and chemical agents. Since oxidative stress may arise as a result of these factors, compounds are actively sought that can act as protective agents. Recently, cannabidiol (CBD), a phytocannabinoid found in Cannabis Sativa L., has gained increased interest due to its anti-inflammatory and antioxidant properties, and absence of psychoactive effects. This prompted us to analyze the protective effects of CBD on keratinocytes exposed to UVB irradiation and hydrogen peroxide. Here we show, using liquid chromatography mass spectrometry, that CBD was able to penetrate keratinocytes, and accumulated within the cellular membrane. CBD reduced redox balance shift, towards oxidative stress, caused by exposure UVB/hydrogen peroxide, estimated by superoxide anion radical generation and total antioxidant status and consequently lipid peroxidation level. CBD was found to protect keratinocytes by preventing changes in the composition of the cellular membrane, associated with UVB/hydrogen peroxide damages which included reduced polyunsaturated fatty acid levels, increased sialic acid and lipid peroxidation products (malondialdehyde and 8-isoprostanes) levels. This maintains cell membranes integrity and prevents the release of lactate dehydrogenase. In addition, CBD prevented UVB/hydrogen peroxide-induced reduction of keratinocyte size and zeta potential, and also decreased activity of ATP-binding cassette membrane transporters. Together, these findings suggest that CBD could be a potential protective agent for keratinocytes against the harmful effects of irradiation and chemical environmental factors that cause oxidative stress.
Keywords: Keratinocyte cell membrane, Cannabidiol, UVB, Hydrogen peroxide, Oxidative stress, Antioxidative defense
Graphical abstract
Highlights
-
•
UVB/H2O2 stimulates keratinocytes membrane penetration by CBD.
-
•
CBD protects cells against UVB/H2O2 induced redox imbalance.
-
•
CBD maintains membrane integrity by preventing its component modifications.
-
•
CBD decreases activity of ATP-binding cassette membrane transporters.
-
•
CBD could be a potential keratinocytes protector against the harmful factors.
Abbreviations
- 4-HNE
4-hydroxynenenal
- 8-isoPGF2a
8-isoprostaglandin F2a
- ABTS
2,2′-azino-bis-3-ethylbenzthiazoline-6-sulfonic acid
- AKT
protein kinase B
- BACH1
transcription regulator protein/BRCA1 associated C-terminal helicase
- BCRP
breast cancer resistance protein
- CBD
cannabidiol
- CMH
1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethyl-pyrrolidine
- COX2
cyclooxygenase 2
- DCBD
decarbonylated cannabidiol
- DLS
dynamic light scattering
- DMEM
Dulbecco's Modified Eagle's Medium
- DMSO
dimethyl sulfoxide
- ESI
electrospray ionization source
- ESR
electron spin resonance
- FABPs
fatty acid-binding proteins
- FAMEs
fatty acid methyl esters
- FBS
fetal bovine serum
- FID
flame ionization detector
- GC
gas chromatograph
- H2O2
hydrogen peroxide
- HPLC
high-performance liquid chromatography
- IFN-β
interferon beta
- IFN-γ
interferon gamma
- IL-10
interleukin 10
- IL-17
interleukin 17
- IL-1β
interleukin 1 beta
- IL-4
interleukin 4
- IL-6
interleukin 6
- iNOS
inducible nitric oxide synthase
- JNK
Jun N-terminal kinase
- LCMS
liquid chromatography–mass spectrometry
- LDH
lactate dehydrogenase
- LDV
laser doppler velocimetry
- LPS
lipopolysaccharide
- MDA
malondialdehyde
- MDR1
multidrug resistance protein 1
- MRM
multiple-reaction monitoring
- MRP
multidrug resistance proteins
- NADPH
nicotinamide adenine dinucleotide phosphate
- NF-κB
nuclear factor-κB
- NOX
NADPH oxidase
- Nrf2
nuclear factor erythroid 2-related factor 2
- OATP
organic-anion-transporting polypeptide
- p38
p38 mitogen-activated protein kinase
- p53
cellular tumor antigen/tumor-suppressor protein
- PUFAs
polyunsaturated fatty acids
- ROS
reactive oxygen species
- SPE
solid phase extraction
- TAS
total antioxidant status
- TLC
thin-layer chromatography
- TNF-α
tumor necrosis factor alpha
- VDACs
voltage-dependent anion channels
- XO
xanthine oxidase
1. Introduction
The skin is the primary organ protecting the human body against the damaging effects of exogenous factors, and also participates in maintaining internal homeostasis [1]. As a result, skin cells, especially epidermal cells, are constantly exposed to both irradiation (including daily ultraviolet, UV) and chemical agents that contribute to increased production of reactive oxygen species (ROS) and impaired endogenous antioxidant efficacy [2,3]. This leads to redox imbalance and the formation of oxidative stress [4]. The skin cells most sensitive to environmental factors are keratinocytes, which are the basic cells that build the epidermal layer [5].
The cell membranes of keratinocytes, the basic components of which are phospholipids, are the cellular part most exposed to environmental factors. In the event of disruption of redox homeostasis in cells, ROS act as transient signaling molecules [6] and stimulate the production of further lipid mediators, such as lipid peroxidation products [7]. Lipid peroxidation occurs as the result of nonenzymatic and enzymatic processes, and the most sensitive peroxidation molecules are free or phospholipid polyunsaturated fatty acids (PUFAs), including arachidonic, linoleic, linolenic, eicosapentaenoic and docosahexaenoic acids [8]. ROS may lead to oxidative cyclization of phospholipid PUFAs to form F2-isoprostanes or D2/E2-isoprostanes [9]. Fatty acids released by phospholipases undergo oxidative fragmentation to form α, β-unsaturated aldehydes, including malondialdehyde (MDA), also 4-hydroxynenenal (4-HNE), and other aldehydes [10,11]. The structure and function of biological membranes may be affected by oxidative modifications of membrane proteins [12,13]. Moreover, sialic acid - a component of glycolipids and glycoproteins and a carrier of a negative charge on the surface of the membrane - participates in maintaining the physicochemical properties of cell membranes [[14], [15], [16]].
Modification of the chemical composition of biomembranes, including the creation lipid peroxidation products, disturbs lipid asymmetry. This reduces the hydrophobicity of the interior of the lipid membrane, causing its depolarization. Such changes directly disrupt the proper functioning of the cell membrane, potentially leading to a loss of membrane integrity and an increase in its permeability [17]. Additionally, lipid peroxidation products are chemically reactive molecules due to their chemical structure and consequentially, lipid peroxidation products can easily form adducts with most of the cell's nucleophilic components, including proteins, lipids and DNA, leading to cellular metabolism disorders. These reactions reduce the level of free reactive products of lipid peroxidation, while increasing, for example, the level of adducts of formed aldehydes and proteins (e.g. 4-HNE-protein adducts). Such products promote disorders of cell signaling, and thus stimulate metabolic modifications that lead to cellular dysfunction followed by cell death or tumor transformation [18].
As a result of these processes, research is being conducted to search for compounds (preferably of natural origin) that protect skin cells against the effects of exogenous factors, especially those that disturb the redox balance [19,20]. An example of a recently intensively studied compound is cannabidiol (CBD), one of the major phytocannabinoids found in Cannabis sativa L., which has no psychoactive effects [21]. CBD has been shown to be a lipophilic antioxidant with anti-inflammatory properties [22,23]. Observations have shown that treatment of atherosclerotic testicular cells from male Sprague-Dawley rats with CBD causes inhibition of the expression of proxidative and proinflammatory proteins [inducible nitric oxide synthase (iNOS) and cyclooxygenase 2 (COX2)] [24]. CBD treatment also reduced the activity of iNOS activated by lipopolysaccharide (LPS) in mouse macrophages [25]. In addition, CBD has also been found to improve the antioxidant properties of cells by regulating the expression of nuclear factor erythroid 2-related factor 2 (Nrf2) [26,27]. Importantly, CBD possesses neuroprotective capabilities, as an increase in superoxide dismutase mRNA expression was observed in Parkinson's and Huntington's disease models following CBD treatment [23]. This antioxidant/neuroprotective effect has been found to involve the activation of signaling pathways implicated in controlling redox balance, which CBD can restore [28]. The anti-inflammatory effects of CBD have been highlighted in the mouse model of multiple sclerosis, where CBD therapy lowered the level of proinflammatory cytokines such as interleukin 1 beta (IL-1β), tumour necrosis factor alpha (TNF-α), interferon beta (IFN-β), interferon gamma (IFN-γ), interleukin 17 (IL-17) and interleukin 6 (IL-6), and increased the levels of anti-inflammatory cytokines [interleukin 4 (IL-4) and interleukin 10 (IL-10)] [23]. Additionally, in a mouse model of diabetic cardiomyopathy, CBD relieved oxidative/nitrosative stress and inflammation, and protected related cell signaling pathways [29].
Given the currently available data, the purpose of this work was to assess the impact of CBD on the structure and function of the cell membranes of keratinocytes exposed to oxidative stress. UVB radiation and hydrogen peroxide were used as physical and chemical agents, respectively.
2. Material and methods
2.1. Cell culture and treatment
Human keratinocytes (CDD 1102 KERTr) obtained from American Type Culture Collection were cultured in Dulbecco's Modified Eagle's Medium (DMEM) with 10% fetal bovine serum (FBS) supplemented with 50 U/mL penicillin and 50 μg/mL streptomycin. The cells were cultured in a humidified atmosphere of 5% CO2 at 37 °C up to 70% confluence. After reaching the required confluency, keratinocytes were divided into 3 main groups:
2.1.1. Control groups
-
I.
Control cells cultured in standard medium;
-
II.
Cells cultured in medium containing 4 μM CBD (Sigma-Aldrich, MO, USA) in 0.2% ethanol for 24 h;
-
III.
Cells cultured in medium containing 4 μM CBD for 48 h.
2.1.2. UVB-irradiated groups
-
IV.
Cells exposed to UVB irradiation (312 nm) at 60 mJ/cm2 (Bio-Link Crossliner BLX 312; Vilber Lourmat, Germany; 6 lamps at a distance of 15 cm);
-
V.
Cells cultured for 24 h after UVB irradiation in medium containing 4 μM CBD;
-
VI.
Cells cultured for 24 h before and 24 h after UVB irradiation in medium containing 4 μM CBD.
2.1.3. Hydrogen peroxide-exposed groups
-
VII.
Cells cultured for 24 h in medium containing 200 μM hydrogen peroxide (this concentration corresponded to 70% cell viability);
-
VIII.
Cells cultured for 24 h after hydrogen peroxide exposure in medium containing 4 μM CBD.
-
IX.
Cells cultured for 24 h before and for 24 h after hydrogen peroxide exposure in medium containing 4 μM CBD.
All results were normalized to the total level of protein measured using Bradford reagent [30].
2.2. The effect of CBD on redox state in keratinocytes
2.2.1. Oxidative state
NADPH oxidase [NOX - EC 1.6.3.1] activity was analyzed with the method used by Griendling [31]. Lucigenin was used as a luminophore for the luminescence measurement to determine enzyme-specific activity in relative luminescence units (RLU) per milligram of protein. Xanthine oxidase activity [XO - EC1.17.3.2] was determined using the method of Lin [32], through assessing uric acid formation from xanthine. Specific enzyme activity is given in microunits per milligram of protein.
Superoxide anion generation was analyzed using electron spin resonance (ESR) spectrometer e-scan (Noxygen GmbH/Bruker Biospin GmbH, Germany). This method evaluated the selective interaction of the spin probe 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethyl-pyrrolidine (CMH) (200 μM) with ROS, forming a stable CM nitroxide radical with half-life 4 h. Superoxide anions were measured based on the kinetics of nitroxide accumulation in accordance with the ESR amplitude of the low ESR spectral component, as in a study from Kuzkaya [33]. Superoxide anion generation is expressed in nanomoles per minute per milligram of protein.
2.2.2. Antioxidative state
Total antioxidant status (TAS) was determined using 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulfonic acid (ABTS) [34]. A solution of cationic ABTS radicals was prepared in phosphate buffer (PBS, pH 7.4). 3-fold diluted samples (5 μl) were mixed with ABTS radical working solution (245 μl) in a 96 well plate at 37 °C for 10 min. Absorbance at 734 nm was then measured using an Infinite 200 plate reader (TEKAN, Switzerland). Ultimately, TAS is expressed as mg vitamin C/mg protein.
2.3. Characteristics of keratinocyte membrane
2.3.1. Phospholipid fatty acid composition
Phospholipid fatty acids profiles of keratinocyte membranes were determined using gas chromatography (GC) according to the method of Christie [35]. Lipid components were isolated using chloroform/methanol mixture (2:1, v/v) (Folch extraction) in the presence of 0.01% butylated hydroxytoluene–BHT. Using thin-layer chromatography (TLC), phospholipid fatty acids were separated using as mobile phase mixture of heptane-diisoprophyl ether-acetic acid (60:40:3,v/v/v). Next, fatty acids were transmethylated to fatty acid methyl esters (FAMEs) with boron trifluoride in methanol under nitrogen atmosphere at 100 °C for 30 min. FAMEs were analyzed by GC/flame ionization detector (FID) on Clarus 500 Gas Chromatograph (PerkinElmer, MA, USA). The separation of FAMEs was performed on a capillary column coated with Varian CP-Sil88 stationary phase (50 m × 0.25 mm, ID 0.2 μm, Varian). Operating conditions were as follows: the split-splitless injector was used in split mode (split ratio of 1:20); the injection volume of the sample was 2 μL; the temperature of the injector and detector was 260 °C while column temperature was programmed from 150 °C (2 min) to 230 °C (10 min) at 4 °C/min; finally, the carrier gas used was helium (flow rate of 1 ml/min). Identification of FAMEs was performed by comparison retention time using an internal standard (1,2-dinonadecanoyl-sn-glycero-3-phosphocholine (19:0 PC) for phospholipid fatty acids). The phospholipid fatty acid concentration was expressed as a micrograms per milligram of protein.
2.3.2. Sialic acid content
Total sialic acid content was determined by modified Jourdian's resorcinol method [36]. Color intensity was measured at 630 nm using a diode array spectrophotometer (Hewlett Packard). The sialic acid level was read from the standard curve for the N-acetylneuraminic acid solution (31.25–1000 nmol/ml) and normalized for milligrams of protein. Results are expressed as a percentage of sialic acid level in control cells.
2.3.3. Lipid peroxidation products
Malondialdehyde (MDA) was measured as malondialdehyde-thiobarbituric acid adducts by high-performance liquid chromatography (HPLC) with spectrofluorometric detection (λex = 532 nm/λem = 553 nm) [37]. After protein precipitation and removing MDA adducts (centrifugation at 10.000×g for 15 min at 4 °C) with thiobarbituric acid (42 mM) were analyzed. After 60 min incubation at 100 °C, samples were mixed with MetOH-NaOH (1:1) in vials. HPLC separation of MDA-TBA adducts was followed with the mobile phase consisting of 40:60 (v/v) methanol: potassium phosphate buffer (0.05 M, pH 6.8) on RP C18 column. The MDA concentration was determined using a calibration curve range: 1–120 nmol/L (r2- 0.9995) and expressed as micromoles per milligram of protein.
F2-isoprostanes (8-isoPGF2a) were measured by liquid chromatography-mass spectrometry (LCMS) (Nexera X2 ultra high performance liquid chromatograph interfaced with LCMS-8060, Shimadzu, Kyoto, Japan) with electrospray ionization source (ESI) operating negative ion mode with multiple-reaction monitoring (MRM) using the method reported by Coolen [38]. In LC-MS analysis with negative-ion mode, 8-isoPGF2a-d4 was used as an internal standard and mass transition was analyzed as 353.2 → 193.1 for 8-isoPGF2a, 357.2 → 197.1 for 8-isoPGF2a-d4. The limit of detection was 1 pg/ml. Results were normalized for milligrams of protein and expressed as pg/mg protein.
2.3.4. Cell size and zeta potential
Changes in phospholipid composition of the cell membrane affect physicochemical properties of cells such as cells size and membrane zeta potential. To determine these parameters, keratinocytes were suspended in PBS and placed in a measuring vessel to determine cell size and zeta potential of the cell membrane. These parameters were measured using a Zetasizer Nano ZS apparatus (Malvern Instruments, UK). This apparatus uses a process called Dynamic Light Scattering (DLS) and Laser Doppler Velocimetry (LDV).
2.3.5. Membrane permeability
Lactate dehydrogenase (LDH) leakage into medium was used as a measure of plasma membrane permeabilization [39]. The activity of LDH in the medium or cell lysates was estimated by the NADH level decrease in the presence of pyruvate measured spectrophotometrically at 340 nm (Multiskan GO Microplate Spectrophotometer Thermo Scientific, USA). The percentage LDH release from cells was calculated by comparing activity in the medium to the cell lysate.
2.3.6. CBD cytosolic and membrane concentrations
Cytosol and membrane CBD level in keratinocytes was determined using ultra-performing liquid chromatography tandem mass spectrometry (LCMS 8060, Shimadzu, Kioto, Japan) using a recently published method [40]. CBD was extracted using solid phase extraction (SPE) and analyzed in positive-ion mode. CBD-d9 was used as an internal standard for quantification. The precursor to the product ion transition was 315.1 → 193.00 for CBD. The results were normalized for milligrams of protein and are expressed as μg/mg protein.
2.3.7. Transmembrane OATP transporter activity
The activity of OATP transporters were determined using a Multidrug-Resistance assay according to the manufacturer's protocols (eFluxx-ID Multidrug resistance assay kits, Enzo LifeSciences, UK). Keratinocytes with inhibitors of OATP transporters [multidrug resistance protein 1 (MDR1), multidrug resistance-associated proteins (MRP), breast cancer resistance protein (BCRP)] in DMSO, and keratinocytes without inhibitors (containing PBS and DMSO) were incubated in dark 96 well plates for 5 min (37 °C). Next, EFLUXX-ID® green detection reagent was added, and samples were incubated for 30 min (37 °C). Luminescence was measured [λex485 nm/λem535 nm] using EnSpire 2300 Multilable Reader (PerkinElmer, MA, USA). Activities of MDR1, MRP, BCRP were normalized to the total level of protein, and the final results were expressed as a percentage of the activity of transporters compared to control cells.
2.4. Statistics
Data were analyzed using standard statistical analyses, including one-way ANOVA for multivariate analyses. Results are expressed as mean ± standard deviation (SD) for n = 5. P values less than 0.05 were considered significant.
3. Results
3.1. Effect of CBD on keratinocytes redox status
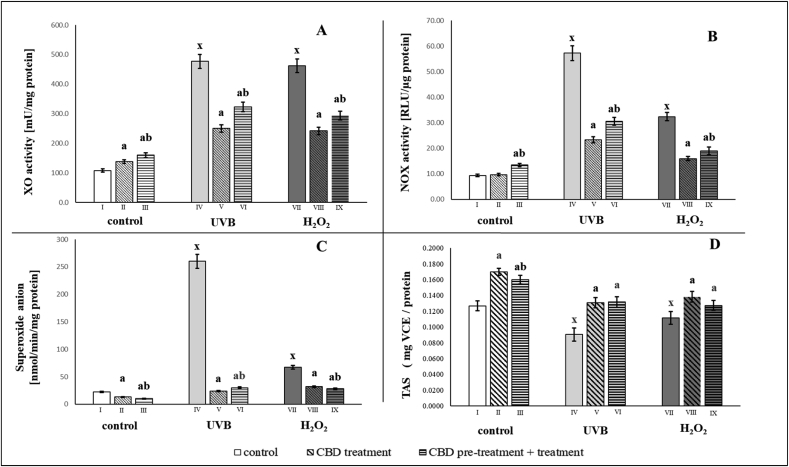
To assess the effects of CBD on redox conditions in keratinocytes, the cells were exposed to UVB irradiation or hydrogen peroxide before measuring XO and NOX activity, superoxide anion generation and total antioxidant status (TAS). After exposure of the cells to UVB irradiation and hydrogen peroxide, keratinocytes showed a 4-fold increase in xanthine oxidase activity, while CBD treatment was followed by a 2-fold decrease in the activity versus mentioned increased activity (Fig. 1). NADPH oxidase activity after UVB irradiation increased approximately 6-fold, and after the use of hydrogen peroxide increased 3-fold. Exposure of the keratinocytes to CBD reduced the NADPH oxidase activity by approximately 50%. As a result, the level of superoxide anions production also decreased after CBD treatment. In cells pretreated and/or treated with CBD, production of UVB-induced superoxide anions was reduced by approximately 90%, while in hydrogen peroxide-treated cells, pretreatment and/or treatment of keratinocytes with CBD led to a reduction in the level of superoxide anion production by almost 50% (Fig. 1).
Fig. 1.
The redox status estimated through pro-oxidant enzymes activity (XO - A; NOX - B), superoxide anion generation (C) and Total Antioxidant Status (D) in groups of keratinocytes: I. control; II. cultured with CBD (4uM) for 24h; III. cultured with CBD (4uM) for 48h; VI. irradiated with UVB [60 mJ/cm2]; V. irradiated with UVB [60 mJ/cm2] and cultured with CBD (4uM) for 24h after irradiation; VI. irradiated with UVB [60 mJ/cm2] and cultured with CBD for 24h befor irradiation and 24h after irradiation; VII. exposed to H2O2 [200μM]; VIII. exposed to H2O2 [200μM] and cultured with CBD (4uM) for 24h after exposer to H2O2; IX. exposed to H2O2 [200μM] and cultured with CBD (4uM) for 24h before and 24h after exposer to H2O2. Mean values ± SD of five independent experiments and statistically significant differences for p < 0.05 are presented: a - differences vs. group I/group IV/group VII; b - differences vs. group II/group V/group VIII; x - differences between group IV/group VII and group I.
CBD antioxidant activity was also revealed by its effect on the total status of keratinocyte antioxidants (TAS), which increased significantly after CBD treatment in all treated groups (Fig. 1D).
3.1.1. Effect of CBD on keratinocyte membrane composition
The effect of CBD on the levels of phospholipid PUFAs, sialic acid and lipid peroxidation products was measured after keratinocytes exposure to UVB irradiation and hydrogen peroxide. The results indicated that the level of phospholipid polyunsaturated fatty acids (PUFAs), especially arachidonic and docosahexaenoic acid, dropped dramatically (by about 65%) in UVB irradiated cells (Table 1). The exposure of keratinocytes to hydrogen peroxide led to an increase in all phospholipid fatty acids. Treatment of keratinocytes with CBD before and after UVB irradiation increased the level of phospholipid PUFAs by 3-fold. The level of docosahexaenoic acid was much higher in cells treated with CBD before and after UVB irradiation compared to cells treated only after UVB irradiation. The use of CBD after hydrogen peroxide exposure resulted in a reduction of approximately 20% in the level of linoleic, γ-linolenic, arachidonic and eicosapentaenoic acid. On the other hand, keratinocytes treated with CBD before and after hydrogen peroxide had a further reduced level of these fatty acids (linoleic acid by about 30%; arachidonic and docosahexaenoic acid by 17%; γ-linolenic, oleic, linolenic and eicosapentaenoic acid by approximately 25%). Consequently, cells treated with CBD after hydrogen peroxide generally had higher levels of fatty acids (linolenic, linoleic, oleic, γ-linolenic and arachidonic) than cells treated with CBD before and after exposure to hydrogen peroxide. The results indicate that CBD did indeed partially prevent redox imbalance and its consequences in keratinocyte membranes despite exposure to exogenous factors.
Table 1.
Unsaturated phospholipid fatty acids profiles in groups of keratinocytes: I. control; II. cultured with CBD (4uM) for 24h; III. cultured with CBD (4uM) for 48h; VI. irradiated with UVB [60 mJ/cm2]; V. irradiated with UVB [60 mJ/cm2] and cultured with CBD (4uM) for 24h after irradiation; VI. irradiated with UVB [60 mJ/cm2] and cultured with CBD for 24h befor irradiation and 24h after irradiation; VII. exposed to H2O2 [200μM]; VIII. exposed to H2O2 [200μM] and cultured with CBD (4uM) for 24h after exposer to H2O2; IX. exposed to H2O2 [200μM] and cultured with CBD (4uM) for 24h before and 24h after exposer to H2O2. Mean values ± SD of five independent experiments and statistically significant differences for p < 0.05 are presented:a - differences vs. group I/group IV/group VII; b - differences vs. group II/group V/group VIII; x - differences between group IV/group VII and group I.
| Control groups |
Keratinocytes treated with UVB |
Keratinocytes treated with H2O2 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Fatty acids μg/mg of protein |
Control (I) | CBD treatment (II) | CBD pretreatment + treatment (III) |
UVB (IV) | CBD treatment (V) | CBD pretreatment + treatment (VI) |
H2O2(VII) | CBD treatment (VIII) | CBD pretreatment + treatment (IX) |
| Oleic (C18:1n9c) | 69.2 ± 3.4 | 80.6 ± 4.0a | 77.7 ± 3.9a | 23.4 ± 1.2x | 67.7 ± 3.4ab | 77.3 ± 3.9ab | 82.6 ± 4.1x | 77.1 ± 3.8 | 70.7 ± 3.5a |
| Linoleic (C18:2n6c) | 21.6 ± 1.1 | 25.3 ± 1.3a | 22.5 ± 1.1b | 7.9 ± 0.4x | 22.4 ± 1.1a | 18.4 ± 0.9ab | 28.2 ± 1.1x | 22.1 ± 1.1a | 18.9 ± 0.9ab |
| γ-Linolenic (C18:3n6) | 7.44 ± 0.37 | 8.86 ± 0.44a | 7.16 ± 0.36b | 2.70 ± 0.13x | 7.57 ± 0.38a | 6.89 ± 0.34a | 10.13 ± 0.51x | 8.40 ± 0.42a | 7.23 ± 0.36ab |
| Linolenic (C18:3n3) | 0.10 ± 0.01 | 0.12 ± 0.01 | 0.11 ± 0.01 | 0.03 ± 0.01x | 0.09 ± 0.01a | 0.11 ± 0.01ab | 0.16 ± 0.01x | 0.13 ± 0.01a | 0.11 ± 0.01a |
| Arachidonic (C20:4n6) | 8.48 ± 0.42 | 9.98 ± 0.50a | 9.15 ± 0.46 | 2.97 ± 0.15x | 8.61 ± 0.43a | 8.72 ± 0.44a | 10.22 ± 0.51x | 9.11 ± 0.46a | 8.10 ± 0.40ab |
| cis-5,8,11,14,17-Eicosapentaenoic (C20:5n3) | 4.40 ± 0.22 | 5.16 ± 0.26a | 4.44 ± 0.22b | 1.51 ± 0.08x | 4.30 ± 0.22a | 4.50 ± 0.22a | 5.33 ± 0.27x | 4.64 ± 0.23a | 4.23 ± 0.21a |
| cis-4,7,10,13,16,19-Docosahexaenoic (C22:6n3) | 2.60 ± 0.13 | 3.23 ± 0.16a | 3.05 ± 0.15a | 0.91 ± 0.05x | 2.67 ± 0.13a | 3.11 ± 0.16ab | 3.20 ± 0.16x | 3.00 ± 0.15 | 2.84 ± 0.14a |
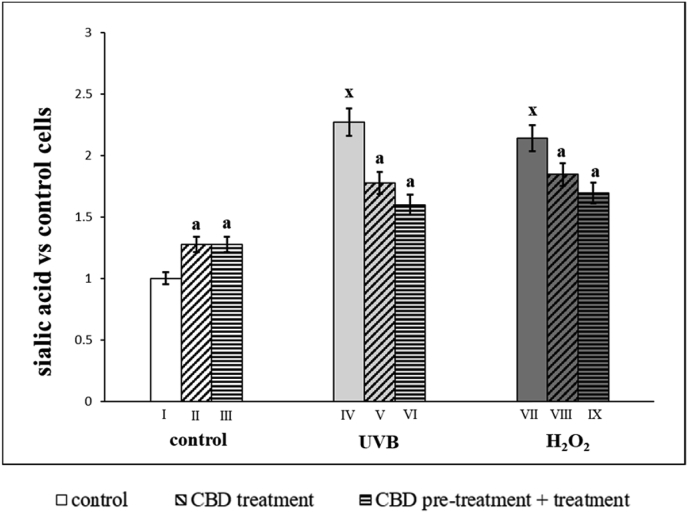
As well as membrane phospholipids, chemical exposure and irradiation cause changes in the level of sialic acid (Fig. 2), which is one of the basic components of cell membrane glycoproteins and glycolipids. Keratinocytes irradiated by UVB or exposed to hydrogen peroxide, had approximately 2 times higher sialic acid levels than control cells. However, CBD treatment enhanced sialic acid levels in control cells, and decreased the levels to a similar degree in keratinocytes irradiated with UVB or exposure to hydrogen peroxide. Changes in sialic acid levels did not depend on whether CBD was used only after or before and after irradiation/exposure.
Fig. 2.
Sialic acid level in groups of keratinocytes: I. control; II. cultured with CBD (4uM) for 24h; III. cultured with CBD (4uM) for 48h; VI. irradiated with UVB [60 mJ/cm2]; V. irradiated with UVB [60 mJ/cm2] and cultured with CBD (4uM) for 24h after irradiation; VI. irradiated with UVB [60 mJ/cm2] and cultured with CBD for 24h befor irradiation and 24h after irradiation; VII. exposed to H2O2 [200μM]; VIII. exposed to H2O2 [200μM] and cultured with CBD (4uM) for 24h after exposer to H2O2; IX. exposed to H2O2 [200μM] and cultured with CBD (4uM) for 24h before and 24h after exposer to H2O2. Mean values ± SD of five independent experiments and statistically significant differences for p < 0.05 are presented:a - differences vs. group I/group IV/group VII; b - differences vs. group II/group V/group VIII; x - differences between group IV/group VII and group I.
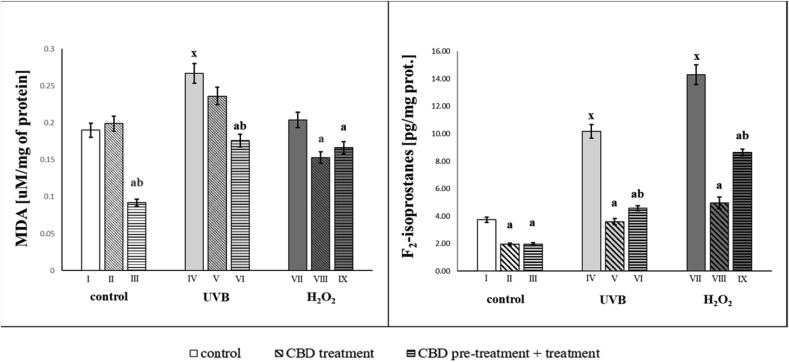
The shift in redox balance towards the oxidant reaction under the influence of UVB and hydrogen peroxide is accompanied by intensified ROS-dependent metabolism of PUFAs. This confirms the increase in the level of lipid peroxidation products such as MDA, formed during oxidative fragmentation, as well as F2-isoprostanes being the product of oxidative cyclization. The strongest increase in MDA level (1.4 times) was observed after UVB irradiation, while hydrogen peroxide exposure led to an increase in MDA level, but not to a statistically significant degree. CBD prevented UVB- and hydrogen peroxide-induced increases in lipid peroxidation products, including MDA and F2-isoprostanes (Fig. 3). CBD treated cells both, before as well as before and after UVB irradiation led to a significant reduction in MDA level, but the treatment of CBD before and after UVB irradiation was more effective. However in CBD-treated keratinocytes both before as well as before and after hydrogen peroxide exposure MDA levels were decreased. Additionally, the level of F2-isoprostanes in UVB-irradiated or hydrogen peroxide-exposed keratinocytes was significantly increased, whereas CBD treatment led to a significant decrease in the level of F2-isoprostanes. Protective use of CBD in both cases promoted the prevention of oxidative stress, with a significant reduction (50%) of F2-isoprostanes level in cells treated with CBD (Fig. 3).
Fig. 3.
Malondialdehyde (MDA) and F2-isoprostanes level in groups of keratinocytes: I. control; II. cultured with CBD (4uM) for 24h; III. cultured with CBD (4uM) for 48h; VI. irradiated with UVB [60 mJ/cm2]; V. irradiated with UVB [60 mJ/cm2] and cultured with CBD (4uM) for 24h after irradiation; VI. irradiated with UVB [60 mJ/cm2] and cultured with CBD for 24h befor irradiation and 24h after irradiation; VII. exposed to H2O2 [200μM]; VIII. exposed to H2O2 [200μM] and cultured with CBD (4uM) for 24h after exposer to H2O2; IX. exposed to H2O2 [200μM] and cultured with CBD (4uM) for 24h before and 24h after exposer to H2O2. Mean values ± SD of five independent experiments and statistically significant differences for p < 0.05 are presented:a - differences vs. group I/group IV/group VII; b - differences vs. group II/group V/group VIII; x - differences between group IV/group VII and group I.
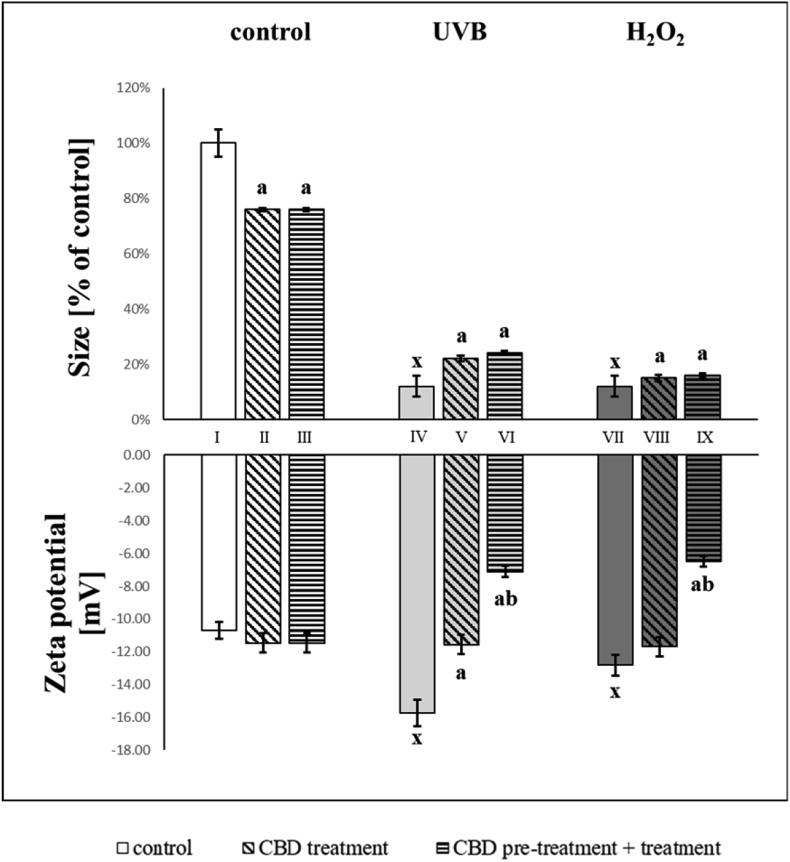
Changes in the composition of membrane phospholipids are accompanied by modifications in the size of keratinocytes. Both UVB and hydrogen peroxide caused up to an 8-fold reduction in cell size (Fig. 4). However, exposure of control keratinocytes to CBD also resulted in a decrease in cell size, of approximately 24%. CBD was partially protective against damage caused by UVB irradiation and hydrogen peroxide exposure, which was observed as an increase in the volume of cells relative to untreated cells exposed to UVB irradiation.
Fig. 4.
Size [% of control cells] and zeta potential [mV] in groups of keratinocytes: I. control; II. cultured with CBD (4uM) for 24h; III. cultured with CBD (4uM) for 48h; VI. irradiated with UVB [60 mJ/cm2]; V. irradiated with UVB [60 mJ/cm2] and cultured with CBD (4uM) for 24h after irradiation; VI. irradiated with UVB [60 mJ/cm2] and cultured with CBD for 24h befor irradiation and 24h after irradiation; VII. exposed to H2O2 [200μM]; VIII. exposed to H2O2 [200μM] and cultured with CBD (4uM) for 24h after exposer to H2O2; IX. exposed to H2O2 [200μM] and cultured with CBD (4uM) for 24h before and 24h after exposer to H2O2. Mean values ± SD of five independent experiments and statistically significant differences for p < 0.05 are presented: a - differences vs. group I/group IV/group VII; b - differences vs. group II/group V/group VIII; x - differences between group IV/group VII and group I.
Due to alterations in membrane phospholipid composition and cell size, changes in zeta potential were also observed (Fig. 4). Negative zeta potential, following UVB or hydrogen peroxide, increased remarkably compared to control cells. Pre- and post-treatment with CBD, significantly decreased (by up to 50%) the negative zeta potential after UVB or hydrogen peroxide.
3.1.2. Penetration of endogenous and exogenous compounds through cell membranes
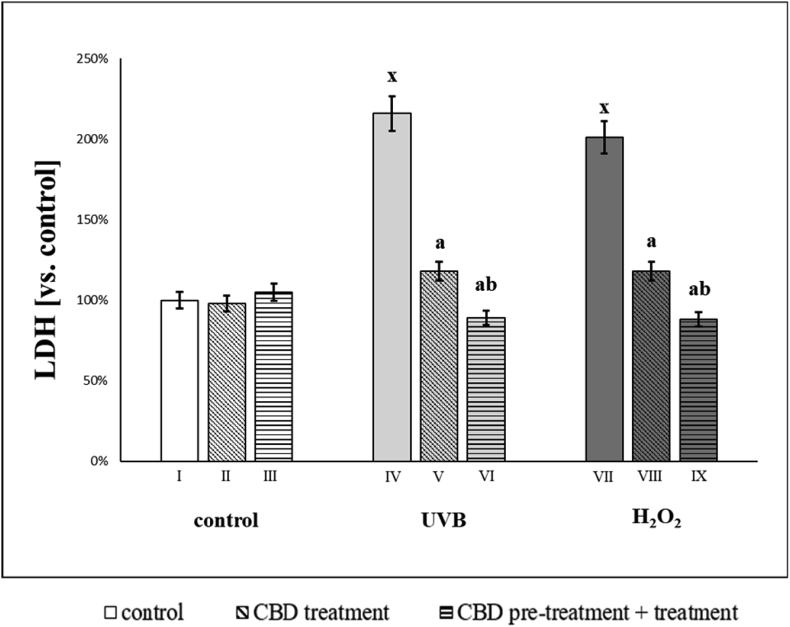
Changes in the composition of the cell membranes of keratinocytes exposed to irradiation or a chemical agent also affected cell function. We observed that after UVB or hydrogen peroxide keratinocyte membrane permeability increased by a factor of 2, based on LDH leakage from cells. The use of CBD after both irradiation and chemical exposure reduced this leakage by about 50%. However, the use of CBD before and after the above-mentioned factors further increased the protective effect of CBD. It can therefore be concluded that CBD reduced the degree of damage to the keratinocyte membrane, most effectively before and after the exposure to irradiation and chemical factors (Fig. 5).
Fig. 5.
Lactate dehydrogenase (LDH) activity in mediums of keratinocytes: I. control; II. cultured with CBD (4uM) for 24h; III. cultured with CBD (4uM) for 48h; VI. irradiated with UVB [60 mJ/cm2]; V. irradiated with UVB [60 mJ/cm2] and cultured with CBD (4uM) for 24h after irradiation; VI. irradiated with UVB [60 mJ/cm2] and cultured with CBD for 24h befor irradiation and 24h after irradiation; VII. exposed to H2O2 [200μM]; VIII. exposed to H2O2 [200μM] and cultured with CBD (4uM) for 24h after exposer to H2O2; IX. exposed to H2O2 [200μM] and cultured with CBD (4uM) for 24h before and 24h after exposer to H2O2. Mean values ± SD of five independent experiments and statistically significant differences for p < 0.05 are presented: a - differences vs. group I/group IV/group VII; b - differences vs. group II/group V/group VIII; x - differences between group IV/group VII and group I.
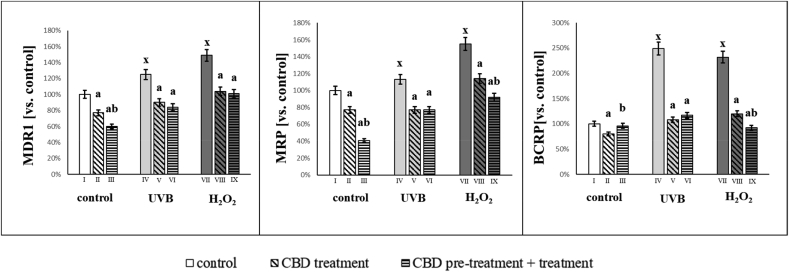
After UVB or hydrogen peroxide exposure the activity of ABC cassette transporters (MDR1, MRP, BCRP) increased significantly (Fig. 6). The use of CBD significantly reduced the activity these transporters. After UVB irradiation, CBD treatment decreased the activity of MDR1, MRP and BCRP to 33%, 32% and 56% (vs values of control UVB irradiated groups), respectively. CBD treatment of cells exposed to hydrogen peroxide reduced the activity of MDR1, MRP and BCRP by approximately 32%, 40% and 60% (compared to the values of the groups exposed to hydrogen peroxide), respectively. CBD treatment pre- and post-exposure of keratinocytes to hydrogen peroxide was more effective at reducing MRP and BCRP transporters activity.
Fig. 6.
Activities of ABC-cassette transporters (MDR1, MRP, BCRP) in groups of keratinocytes: I. control; II. cultured with CBD (4uM) for 24h; III. cultured with CBD (4uM) for 48h; VI. irradiated with UVB [60 mJ/cm2]; V. irradiated with UVB [60 mJ/cm2] and cultured with CBD (4uM) for 24h after irradiation; VI. irradiated with UVB [60 mJ/cm2] and cultured with CBD for 24h befor irradiation and 24h after irradiation; VII. exposed to H2O2 [200μM]; VIII. exposed to H2O2 [200μM] and cultured with CBD (4uM) for 24h after exposer to H2O2; IX. exposed to H2O2 [200μM] and cultured with CBD (4uM) for 24h before and 24h after exposer to H2O2. Mean values ± SD of five independent experiments and statistically significant differences for p < 0.05 are presented: a - differences vs. group I/group IV/group VII; b - differences vs. group II/group V/group VIII; x - differences between group IV/group VII and group I.
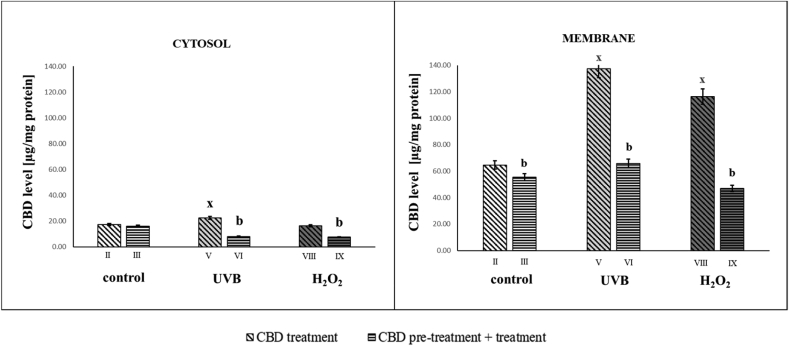
It can therefore be concluded that oxidative stress induced in keratinocytes by UVB irradiation or hydrogen peroxide not only contributed to the modification of phospholipids of the keratinocytes membrane, but also affected membrane proteins (such as transmembrane ABC transporters) that may participate in the supply/removal of exo- and endogenous compounds. Increased activity of ABC membrane transporters was associated with greater membrane permeability allowing CBD to enter the cytosol more efficiently following UVB irradiation (Fig. 7).
Fig. 7.
CBD level [μg/mg protein] in groups of keratinocytes: II. cultured with CBD (4uM) for 24h; III. cultured with CBD (4uM) for 48h; V. irradiated with UVB [60 mJ/cm2] and cultured with CBD (4uM) for 24h after irradiation; VI. irradiated with UVB [60 mJ/cm2] and cultured with CBD for 24h befor irradiation and 24h after irradiation; VIII. exposed to H2O2 [200μM] and cultured with CBD (4uM) for 24h after exposer to H2O2; IX. exposed to H2O2 [200μM] and cultured with CBD (4uM) for 24h before and 24h after exposer to H2O2. Mean values ± SD of five independent experiments and statistically significant differences for p < 0.05 are presented: b - differences vs.group II/group V/group VIII; x - differences between group IV/group VII and group I.
4. Discussion
The skin is the main barrier which provides protection against damaging environmental factors. Among these, the most common is sunlight which is particularly important due to high energy UVB radiation, which disturbs the metabolism of keratinocytes [41]. Chemical factors can also affect cutaneous biology, by altering cellular membrane composition, structure, and functions [42].
Skin cells are characterized by active and diverse lipid metabolism, which is necessary for barrier and signaling functions [43]. The skin's functions are supported by cell membrane proteins, whose biological activities promote the proper interactions of cells with each other and with the environment [44]. Therefore, metabolic alterations caused by external factors that can lead to oxidative stress and oxidative modifications of cellular components may cause changes in membrane structure and biological activity. This can have downstream effects on cellular metabolism, leading to functional skin cell disorders and ultimately contributing to the development of numerous skin diseases such as erythema, photoallergic reactions, autoimmune diseases, psoriasis, neutrophilic disorders, and cancer [45].
CBD, a compound with known anti-inflammatory and antioxidant properties [23,46], could be considered as a potential therapeutic compound to protect against the damaging environmental factors. The evidence presented here supports this notion, as the results indicate that CBD effectively prevents changes in the structure and function of cell membranes of keratinocyte exposed to UVB irradiation or hydrogen peroxide.
The results of this study confirm earlier reports indicating that hydrogen peroxide, and in particular UVB irradiation, contributes to the production of superoxide radicals and reduces the antioxidant capacity of keratinocytes. Mechanistically, this is through increasing the activity of xanthine oxidase and NADPH, [47]. In contrast, the use of CBD counteracts redox imbalances caused by UVB irradiation and hydrogen peroxide in human keratinocytes, both at the level of oxidants and antioxidants. Earlier literature also pointed to the protective role of CBD, but in other pathological conditions [48,49]. Based on the literature, it could be suggested that this effect results from the CBD phenolic structure that provides the ability to terminate free radical chain reactions, capture free radicals, or convert radicals to less active forms [50]. CBD also reduces the generation of ROS by chelating the transition metal ions involved in the Fenton reaction to form extremely reactive hydroxyl radicals [51]. In addition to directly reducing the level of oxidants, CBD also modifies the redox balance by changing the level and activity of endogenous antioxidants [23].
CBD may also modify other metabolic pathways in keratinocytes that alleviate disorders resulting from UVB and hydrogen peroxide exposure. It has been previously shown that UVB irradiation can modify various molecular signals transmitted via phosphorylation of JNK and AKT kinases [52], while hydrogen peroxide affects redox signaling, and modulates the activity of transcription factors, including Nrf2, which is responsible for transcription of cytoprotective genes [[53], [54], [55]]. It has recently been observed that this effect occurs as a result of inhibiting the expression of Bach1 - the regulator of transcription factor Nrf2 - and thereby increasing the expression of Nrf2 target genes such as hemeoxygenase 1 [26]. The changes caused by CBD in the level/activity of cytoprotective proteins result not only from modifications at the mRNA level, but also at the protein level. It is known that UVB and hydrogen peroxide modify the functions of proteins by oxidizing biologically important protein thiol groups [56,57], while CBD - in direct interaction with cysteine - can reduce glutathione or protein disulfide bridges, and thus restore their biological activity [58]. CBD can simultaneously modify the interaction of components of the metabolic pathways of Nrf2 and nuclear factor-κB (NF-κB), and thus simultaneously affect the antioxidant and anti-inflammatory abilities of cells [27].
The changes in ROS levels and antioxidant capabilities presented here, suggest that UVB irradiation disturbs the redox balance more strongly than hydrogen peroxide, which would indicate that the severity of keratinocyte oxidative stress is also greater after UVB irradiation. However, the analysis of the effects of oxidative stress assessed by changes in the composition/structure and function of biological membranes suggests that both UVB and hydrogen peroxide clearly, but distinctly, modify the lipid structure of the membranes.
It is known that oxidative stress associated with UVB irradiation and hydrogen peroxide exposure promotes oxidative modifications of cellular components, including phospholipid polyunsaturated fatty acids [59,60]. This is especially visible in the case of UVB irradiated keratinocytes, which have increased phospholipid oxidative fragmentation and cyclization, demonstrated by an increase in MDA and F2-isoprostane levels. As a result, UVB irradiation significantly reduced the level of phospholipid polyunsaturated fatty acids. Hydrogen peroxide also increased the oxidative cyclization of PUFA's hydrocarbon chains, indicated by an increase in F2-isoprostane levels. However, since an increase in the level of PUFA's has been observed after the use of hydrogen peroxide [61], the increase in the level of these acids, together with the enhanced lipid peroxidation process, may be a consequence of the intensification of PUFA's de novo synthesis in response to metabolic changes caused by exogenous factors.
Another membrane component that undergoes modification during oxidative stress is sialic acid, which is part of glycolipids and glycoproteins. Sialic acid is a carrier of a negative charge on the surface of the membrane, and therefore changes the physicochemical properties and zeta potential of cell membranes [62,63]. UVB and hydrogen peroxide exposure caused an increase in electric charge on the surface of keratinocyte cells, which corresponded to an increase in sialic acid content and a decrease in cell size. Published data confirm the relationship between the increase in sialic acid level and ROS generation but also altered sialylation caused by UV irradiation [16]. In addition, under the influence of UVB or hydrogen peroxide, the barrier function of epidermal cells is impaired, and intracellular water leaves the keratinocytes by osmosis, which leads to cell shrinkage [64]. Maintaining a constant cell volume is critical to normal cell activity, such as growth, migration, and regulation of intracellular metabolism [65,66]. CBD treatment reduced the damaging effects of UVB and hydrogen peroxide, and helped restore proper regulation of keratinocyte metabolism.
CBD treatment significantly prevented membrane phospholipid responses to the exogenous factors. This was evident both in the level of phospholipid PUFAs, lipid peroxidation products (MDA and F2-isoprostanes), as well as in the zeta potential and cell size. The consequence of decreasing changes in the structure of membranes is a significant reduction in membrane permeability, which was assessed on the basis of LDH levels in the extracellular space. Since CBD belongs to lipophilic antioxidants, its main sites of action are biological membranes. This was confirmed by the results of this study, as an accumulation of CBD was observed in the keratinocyte layer. Therefore, CBD protects biomembranes, preventing phospholipid peroxidation. Earlier studies have shown that - regardless of protection against changes caused by the physicochemical effects of exogenous factors - CBD treatment also reduces MDA levels in mouse hippocampal neuronal cell [67], as well as in the heart tissue of Sprague-Dawley rats [68]. In addition, a similar trend of changes in level of lipid peroxidation product (MDA and 4-HNE) was observed in fibroblasts after using other lipophilic antioxidants such as rutin [3,69].
Changes in the structure of the cell membrane may also include modifications of membrane proteins, which can undergo oxidative modifications as a result of reaction with ROS and lipid peroxidation products. This may also apply to membrane transporters (ABC) carrying exogenous substances and their metabolites across membranes. Accordingly, UVB and hydrogen peroxide-induced oxidative stress enhance the activity of transmembrane transporters (MRP, MDR and BCRP), but CBD treatment impedes this activity. Previous reports confirm that the expression of ABC transporters, including MDR1, MRP1, MRP2, MRP4, and BCRP, increases as a result of oxidative stress [70]. Studies on mouse brain cells have shown that knocking out MDR1 or BCRP in transgenic animals does not affect CBD transport, while CBD inhibits both MDR1 and BCRP transporters [71]. In humans, CBD also inhibits the activity of MRP1 in ovarian cancer cells [72], while inducing BCRP expression and reducing MDR1 expression in human trophoblast-like cell lines, BeWo and Jar [73]. CBD, which activates redox-sensitive transcription factor - Nrf2 [27], can thus induce MRP expression as shown in C57BL/6J mouse hepatocytes [74]. Activating Nrf2 also induces the expression and transport function of MDR1, MRP2, and BCRP in the blood-brain and blood-spinal cord barriers of Sprague Dawley rats [75]. However, to increase the expression/activity of transporters by Nrf2, the contribution of additional factors (p53, p38 and NFκB) is necessary [75].
Based on the above data, it is difficult to determine whether and what transporters are involved in the transmission of CBD across cell membranes. However, it is certain that CBD has an inhibitory effect on the activity of these transporters, particularly after UVB irradiation. In addition, it is not known whether its structure is modified during transport of CBD across membranes, which may also promote inhibition of transporter activity. This may also be the reason that in our studies we found significantly lower CBD levels in the cytosol than in membranes, especially in longer treated cells. In addition the level of cytosol free CBD may be decreased by its interaction with fatty acid-binding proteins (FABPs), which mediate anandamide transport to its catabolic enzyme fatty acid amide hydrolase but is also target for CBD [76]. In addition to above, active molecules in the membrane and cytosol, including ROS and lipid peroxidation products may interact with membrane transporters. Moreover the transport of various compounds across cell membranes is also dependent on the sialic acid contained in glycoproteins and membrane glycolipids, the levels of which change under the influence of exogenous factors, as indicated above. Regarding CBD penetration through cell membranes, recent studies also suggest that CBD may interact directly with voltage-dependent anion channels (VDACs) in the outer mitochondrial membrane [77]. VDACs have also been observed in the cell membrane of several cell lines including lymphocytes, epithelial cells, and astrocytes [78]. However, CBD-inducing VDAC1 channel closure may decrease CBD level in keratinocytes [79]. In addition, it should be taken into account that structural and metabolic changes related to CBD activity are exacerbated by the oxidative action of UVB or hydrogen peroxide and can then be further modified as a result of CBD re-action. The results obtained here do not allow to identify changes caused by secondary interactions. In addition, it has previously been shown that cytoprotective compounds can have different time-dependent effects [80]. Therefore, given the two ways of CBD administration (after UVB radiation as well as before and after UVB radiation), it is possible that CBD interactions with cell components may also be modified. It is known that CBD undergoes oxidative metabolism and its concentration in biological material decreases over time [81]. In this, decarbonylation has been observed to form DCBD, a compound with reduced hydrophilic properties and longer retention time relative to CBD (under LCMS analysis), shown in human samples [82].
It should be taken into account that structural and metabolic changes after CBD pretreatment can be modulated by the oxidative action of UVB or hydrogen peroxide and can then be further modified as a result of CBD re-action. The results obtained here do not allow to identify changes caused by secondary interactions. Therefore, the results obtained in this work require further qualitative and quantitative analysis.
5. Conclusion
CBD reduces the harmful effects of UVB and hydrogen peroxide on keratinocytes, which is associated with CBD's antioxidant activity. This phytocannabinoid, by regulating the redox balance of cells, partially prevents oxidative changes affecting phospholipids, proteins and sialic acid in keratinocyte membranes, which helps maintain membrane integrity. CBD regulates the activity of ABC membrane transporters. Due to its beneficial protective effect, CBD can be disclosed as a protective agent for skin keratinocytes against the harmful effects of radiation and chemical environmental factors that cause oxidative stress in skin cells.
Declaration of competing interest
The authors have no conflicts of interest to declare.
Acknowledgements
This research was conducted within the project which has received funding from the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 754432 and the Polish Ministry of Science and Higher Education, from financial resources for science in 2018–2023 granted for the implementation of an international co-financed project.
References
- 1.Baek J., Lee M.G. Oxidative stress and antioxidant strategies in dermatology. Redox Rep. 2016;21:164–169. doi: 10.1179/1351000215Y.0000000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zuliani T., Denis V., Noblesse E., Schnebert S., Andre P., Dumas M., Ratinaud M.H. Hydrogen peroxide-induced cell death in normal human keratinocytes is differentiation dependent. Free Radic. Biol. Med. 2005;38:307–316. doi: 10.1016/j.freeradbiomed.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 3.Gęgotek A., Bielawska K., Biernacki M., Zaręba I., Surażyński A., Skrzydlewska E. Comparison of protective effect of ascorbic acid on redox and endocannabinoid systems interactions in in vitro cultured human skin fibroblasts exposed to UV radiation and hydrogen peroxide. Arch. Dermatol. Res. 2017;309:285–303. doi: 10.1007/s00403-017-1729-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ray P.D., Huang B.W., Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalmau N., Andrieu-Abadie N., Tauler R., Bedia C. Phenotypic and lipidomic characterization of primary human epidermal keratinocytes exposed to simulated solar UV radiation. J. Dermatol. Sci. 2018;92:97–105. doi: 10.1016/j.jdermsci.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Wojtovich A.P., Foster T.H. Optogenetic control of ROS production. Redox Biol. 2014;2:368–376. doi: 10.1016/j.redox.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nita M., Grzybowski A. The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid. Med. Cell. Longev. 2016 doi: 10.1155/2016/3164734. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaschler M.M., Stockwell B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017;482:419–425. doi: 10.1016/j.bbrc.2016.10.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Milne G.L., Dai Q., Roberts L.J. The isoprostanes - 25 years later. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2015;1851:433–445. doi: 10.1016/j.bbalip.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Łuczaj W., Gęgotek A., Skrzydlewska E. Antioxidants and HNE in redox homeostasis. Free Radic. Biol. Med. 2017;111:87–101. doi: 10.1016/j.freeradbiomed.2016.11.033. [DOI] [PubMed] [Google Scholar]
- 11.Gęgotek A., Skrzydlewska E. Biological effect of protein modifications by lipid peroxidation products. Chem. Phys. Lipids. 2019;221:46–52. doi: 10.1016/j.chemphyslip.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 12.Wall S.B., Oh J.Y., Diers A.R., Landar A. Oxidative modification of proteins: an emerging mechanism of cell signaling. Front. Physiol. 2012;3:369. doi: 10.3389/fphys.2012.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dobrzyńska I., Szachowicz-Petelska B., Weresa J., Figaszewski Z.A., Skrzydlewska E. Changes in physicochemical properties of kidney cells membrane as a consequence of hypertension and treatment of hypertensive rats with FAAH inhibitor. Chem. Biol. Interact. 2019;299:52–58. doi: 10.1016/j.cbi.2018.11.024. [DOI] [PubMed] [Google Scholar]
- 14.Eguchi H., Ikeda Y., Ookawara T., Koyota S., Fujiwara N., Honke K., Wang P.G., Taniguchi N., Suzuki K. Modification of oligosaccharides by reactive oxygen species decreases sialyl lewis x-mediated cell adhesion. Glycobiology. 2005;15:1094–1101. doi: 10.1093/glycob/cwj003. [DOI] [PubMed] [Google Scholar]
- 15.Dobrzyńska I., Gęgotek A., Gajko E., Skrzydlewska E., Figaszewski Z.A. Effects of rutin on the physicochemical properties of skin fibroblasts membrane disruption following UV radiation. Chem. Biol. Interact. 2018;282:29–35. doi: 10.1016/j.cbi.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Ren Y., Liu X., Geng R., Lu Q., Rao R., Tan X., Yang X., Liu W. Increased level of α2,6-sialylated glycans on HaCaT cells induced by titanium dioxide nanoparticles under UV radiation. Nanomaterials. 2018;8:E253. doi: 10.3390/nano8040253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Der Paal J., Neyts E.C., Verlackt C.C.W., Bogaerts A. Effect of lipid peroxidation on membrane permeability of cancer and normal cells subjected to oxidative stress. Chem. Sci. 2016;7:489–498. doi: 10.1039/c5sc02311d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dalleau S., Baradat M., Guéraud F., Huc L. Cell death and diseases related to oxidative stress:4-hydroxynonenal (HNE) in the balance. Cell Death Differ. 2013;20:1615–1630. doi: 10.1038/cdd.2013.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Augustyniak A., Bartosz G., Čipak A., Duburs G., Horáková L., Łuczaj W., Majekova M., Odysseos A.D., Rackova L., Skrzydlewska E., Stefek M., Štrosová M., Tirzitis G., Venskutonis P.R., Viskupicova J., Vraka P.S., Žarković N. Natural and synthetic antioxidants: an updated overview. Free Radic. Res. 2010;44:1216–1262. doi: 10.3109/10715762.2010.508495. [DOI] [PubMed] [Google Scholar]
- 20.Xu D.P., Li Y., Meng X., Zhou T., Zhou Y., Zheng J., Zhang J.J., Bin Li H. Natural antioxidants in foods and medicinal plants: extraction, assessment and resources. Int. J. Mol. Sci. 2017;18:96. doi: 10.3390/ijms18010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blessing E.M., Steenkamp M.M., Manzanares J., Marmar C.R. Cannabidiol as a potential treatment for anxiety disorders. Neurotherapeutics. 2015;12:825–836. doi: 10.1007/s13311-015-0387-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borges R.S., da Silva A. Cannabidiol as an antioxidant. In: Preedy V., editor. Handb. Cannabis Relat. Pathol. 2017. pp. 123–130. [Google Scholar]
- 23.Peres F.F., Lima A.C., Hallak J.E.C., Crippa J.A., Silva R.H., Abílio V.C. Cannabidiol as a promising strategy to treat and prevent movement disorders? Front. Pharmacol. 2018;9:482. doi: 10.3389/fphar.2018.00482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen J., Hou C., Chen X., Wang D., Yang P., He X., Zhou J., Li H. Protective effect of cannabidiol on hydrogen peroxide-induced apoptosis, inflammation and oxidative stress in nucleus pulposus cells. Mol. Med. Rep. 2016;14:2321–2327. doi: 10.3892/mmr.2016.5513. [DOI] [PubMed] [Google Scholar]
- 25.Rajan T.S., Giacoppo S., Iori R., De Nicola G.R., Grassi G., Pollastro F., Bramanti P., Mazzon E. Anti-inflammatory and antioxidant effects of a combination of cannabidiol and moringin in LPS-stimulated macrophages. Fitoterapia. 2016;112:104–115. doi: 10.1016/j.fitote.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 26.Casares L., García V., Garrido-Rodríguez M., Millán E., Collado J.A., García-Martín A., Peñarando J., Calzado M.A., de la Vega L., Muñoz E. Cannabidiol induces antioxidant pathways in keratinocytes by targeting BACH1. Redox Biol. 2020;28:101321. doi: 10.1016/j.redox.2019.101321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jastrząb, Gęgotek, Skrzydlewska Cannabidiol regulates the expression of keratinocyte proteins involved in the inflammation process through transcriptional regulation. Cells. 2019;8:827. doi: 10.3390/cells8080827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernández-Ruiz J., Sagredo O., Pazos M.R., García C., Pertwee R., Mechoulam R., Martínez-Orgado J. Cannabidiol for neurodegenerative disorders: important new clinical applications for this phytocannabinoid? Br. J. Clin. Pharmacol. 2013;75:323–333. doi: 10.1111/j.1365-2125.2012.04341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rajesh M., Mukhopadhyay P., Btkai S., Patel V., Saito K., Matsumoto S., Kashiwaya Y., Horvth B., Mukhopadhyay B., Becker L., Hask G., Liaudet L., Wink D.A., Veves A., Mechoulam R., Pacher P. Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J. Am. Coll. Cardiol. 2010;56:2115–2125. doi: 10.1016/j.jacc.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 31.Griendling K.K., Minieri C.A., Ollerenshaw J.D., Alexander R.W. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ. Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 32.Lin C.M., Chen C.S., Chen C.T., Liang Y.C., Lin J.K. Molecular modeling of flavonoids that inhibits xanthine oxidase. Biochem. Biophys. Res. Commun. 2002;294:167–172. doi: 10.1016/S0006-291X(02)00442-4. [DOI] [PubMed] [Google Scholar]
- 33.Kuzkaya N., Weissmann N., Harrison D.G., Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J. Biol. Chem. 2003;278:22546–22554. doi: 10.1074/jbc.M302227200. [DOI] [PubMed] [Google Scholar]
- 34.Lee S.G., Wang T., Vance T.M., Hurbert P., Kim D.O., Koo S.I., Chun O.K. Validation of analytical methods for plasma total antioxidant capacity by comparing with urinary 8-isoprostane level. J. Microbiol. Biotechnol. 2017;27:388–394. doi: 10.4014/jmb.1604.04053. [DOI] [PubMed] [Google Scholar]
- 35.Christie W.W. Preparation of ester derivatives of fatty acids for chromatographic analysis. In: Christie W.W., editor. Adv. Lipid Methodol. – Two. The Oily Press; Dundee: 1993. pp. 69–111. [Google Scholar]
- 36.Jourdian G.W., Dean L., Roseman S. The sialic acids. XI. A periodate-resorcinol method for the quantitative estimation of free sialic acids and their glycosides. J. Biol. Chem. 1971;246:430–435. http://www.ncbi.nlm.nih.gov/pubmed/5542012 [PubMed] [Google Scholar]
- 37.Londero D., Lo Greco P. Automated high-performance liquid chromatographic separation with spectrofluorometric detection of a malondialdehyde-thiobarbituric acid adduct in plasma. J. Chromatogr., A. 1996;729:207–210. doi: 10.1016/0021-9673(95)00959-0. [DOI] [PubMed] [Google Scholar]
- 38.Coolen S.A., van Buuren B., Duchateau G., Upritchard J., Verhagen H. Kinetics of biomarkers: biological and technical validity of isoprostanes in plasma. Amino Acids. 2005;29:429–436. doi: 10.1007/s00726-005-0229-2. [DOI] [PubMed] [Google Scholar]
- 39.Fotakis G., Timbrell J.A. In vitro cytotoxicity assays: comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol. Lett. 2006;160:171–177. doi: 10.1016/j.toxlet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Luque-Córdoba D., Calderón-Santiago M., Luque de Castro M.D., Priego-Capote F. Study of sample preparation for determination of endocannabinoids and analogous compounds in human serum by LC–MS/MS in MRM mode. Talanta. 2018;185:602–610. doi: 10.1016/j.talanta.2018.04.033. [DOI] [PubMed] [Google Scholar]
- 41.Gegotek A., Biernacki M., Ambrozewicz E., Surazyński A., Wroński A., Skrzydlewska E. The cross-talk between electrophiles, antioxidant defence and the endocannabinoid system in fibroblasts and keratinocytes after UVA and UVB irradiation. J. Dermatol. Sci. 2016;81:107–117. doi: 10.1016/j.jdermsci.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 42.Zhu D., Tan K.S., Zhang X., Sun A.Y., Sun G.Y., Lee J.C.M. Hydrogen peroxide alters membrane and cytoskeleton properties and increases intercellular connections in astrocytes. J. Cell Sci. 2005;118:3695–3703. doi: 10.1242/jcs.02507. [DOI] [PubMed] [Google Scholar]
- 43.Gruber F. Ski. Stress Response Pathways Environ. Factors Mol. Oppor. Springer International Publishing; 2016. The skin lipidome under environmental stress—technological platforms, molecular pathways and translational opportunities; pp. 1–27. [DOI] [Google Scholar]
- 44.Palsdottir H., Hunte C. Lipids in membrane protein structures. Biochim. Biophys. Acta Biomembr. 2004;1666:2–18. doi: 10.1016/j.bbamem.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 45.Kruk J., Duchnik E. Oxidative stress and skin diseases: possible role of physical activity. Asian Pac. J. Cancer Prev. APJCP. 2014;15:561–568. doi: 10.7314/apjcp.2014.15.2.561. [DOI] [PubMed] [Google Scholar]
- 46.Oláh A., Tóth B.I., Borbíró I., Sugawara K., Szöllõsi A.G., Czifra G., Pál B., Ambrus L., Kloepper J., Camera E., Ludovici M., Picardo M., Voets T., Zouboulis C.C., Paus R., Bíró T. Cannabidiol exerts sebostatic and antiinflammatory effects on human sebocytes. J. Clin. Invest. 2014;124:3713–3724. doi: 10.1172/JCI64628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hernández A.R., Vallejo B., Ruzgas T., Björklund S. The effect of UVB irradiation and oxidative stress on the skin barrier—a new method to evaluate sun protection factor based on electrical impedance spectroscopy. Sensors. 2019;19:E2376. doi: 10.3390/s19102376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rajesh M., Mukhopadhyay P., Bátkai S., Haskó G., Liaudet L., Drel V.R., Obrosova I.G., Pacher P. Cannabidiol attenuates high glucose-induced endothelial cell inflammatory response and barrier disruption. Am. J. Physiol. Heart Circ. Physiol. 2007;293:610–619. doi: 10.1152/ajpheart.00236.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pan H., Mukhopadhyay P., Rajesh M., Patel V., Mukhopadhyay B., Gao B., Haskó G., Pacher P. Cannabidiol attenuates cisplatin-induced nephrotoxicity by decreasing oxidative/nitrosative stress, inflammation, and cell death. J. Pharmacol. Exp. Therapeut. 2009;328:708–714. doi: 10.1124/jpet.108.147181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Borges R.S., Batista J., Viana R.B., Baetas A.C., Orestes E., Andrade M.A., Honório K.M., Da Silva A.B.F. Understanding the molecular aspects of tetrahydrocannabinol and cannabidiol as antioxidants. Molecules. 2013;18:12663–12674. doi: 10.3390/molecules181012663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hamelink C., Hampson A., Wink D.A., Eiden L.E., Eskay R.L. Comparison of cannabidiol, antioxidants, and diuretics in reversing binge ethanol-induced neurotoxicity. J. Pharmacol. Exp. Therapeut. 2005;314:780–788. doi: 10.1124/jpet.105.085779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Syed D.N., Afaq F., Mukhtar H. Differential activation of signaling pathways by UVA and UVB radiation in normal human epidermal keratinocytes. Photochem. Photobiol. 2012;88:1184–1190. doi: 10.1111/j.1751-1097.2012.01115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Juknat A., Pietr M., Kozela E., Rimmerman N., Levy R., Gao F., Coppola G., Geschwind D., Vogel Z. Microarray and pathway analysis reveal distinct mechanisms underlying cannabinoid-mediated modulation of LPS-induced activation of BV-2 microglial cells. PloS One. 2013;8 doi: 10.1371/journal.pone.0061462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gęgotek A., Skrzydlewska E. The role of transcription factor Nrf2 in skin cells metabolism. Arch. Dermatol. Res. 2015;307:385–396. doi: 10.1007/s00403-015-1554-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sies H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: oxidative eustress. Redox Biol. 2017;11:613–619. doi: 10.1016/j.redox.2016.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rudzka D.A., Cameron J.M., Olson M.F. Reactive oxygen species and hydrogen peroxide generation in cell migration. Commun. Integr. Biol. 2015;8 doi: 10.1080/19420889.2015.1074360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Davies M.J. Protein oxidation and peroxidation. Biochem. J. 2016;473:805–825. doi: 10.1042/BJ20151227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu H.Y., Jan T.R. Cannabidiol hydroxyquinone-induced apoptosis of splenocytes is mediated predominantly by thiol depletion. Toxicol. Lett. 2010;195:68–74. doi: 10.1016/j.toxlet.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 59.Perluigi M., Di Domenico F., Blarzino C., Foppoli C., Cini C., Giorgi A., Grillo C., De Marco F., Butterfield D.A., Schininà M.E., Coccia R. Effects of UVB-induced oxidative stress on protein expression and specific protein oxidation in normal human epithelial keratinocytes: a proteomic approach. Proteome Sci. 2010;8:13. doi: 10.1186/1477-5956-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Birben E., Sahiner U.M., Sackesen C., Erzurum S., Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rysman E., Brusselmans K., Scheys K., Timmermans L., Derua R., Munck S., Van Veldhoven P.P., Waltregny D., Daniëls V.W., Machiels J., Vanderhoydonc F., Smans K., Waelkens E., Verhoeven G., Swinnen J.V. De novo lipogenesis protects cancer cells from free radicals and chemotherapeutics by promoting membrane lipid saturation. Canc. Res. 2010;70:8117–8126. doi: 10.1158/0008-5472.CAN-09-3871. [DOI] [PubMed] [Google Scholar]
- 62.Wang B., Brand-Miller J. The role and potential of sialic acid in human nutrition. Eur. J. Clin. Nutr. 2003;57:1351–1369. doi: 10.1038/sj.ejcn.1601704. [DOI] [PubMed] [Google Scholar]
- 63.Varki A., Schauer R. Sialic acids. In: Varki A E.M., Cummings R.D., Esko J.D., Freeze H.H., Stanley P., Bertozz C.R., Hart G.W., editors. Essentials Glycobiol. second ed. Cold Spring Harbor Laboratory Press; Cold Spring Harbor (NY): 2009. [Google Scholar]
- 64.El-Chami C., Haslam I.S., Steward M.C., O'Neill C.A. Role of organic osmolytes in water homoeostasis in skin. Exp. Dermatol. 2014;23:534–537. doi: 10.1111/exd.12473. [DOI] [PubMed] [Google Scholar]
- 65.Strange K. Cellular volume homeostasis. Adv. Physiol. Educ. 2004;28:155–159. doi: 10.1152/advan.00034.2004. [DOI] [PubMed] [Google Scholar]
- 66.Lang F. Mechanisms and significance of cell volume regulation. J. Am. Coll. Nutr. 2007;26:613–623. doi: 10.1080/07315724.2007.10719667. [DOI] [PubMed] [Google Scholar]
- 67.Sun S., Hu F., Wu J., Zhang S. Cannabidiol attenuates OGD/R-induced damage by enhancing mitochondrial bioenergetics and modulating glucose metabolism via pentose-phosphate pathway in hippocampal neurons. Redox Biol. 2017;11:577–585. doi: 10.1016/j.redox.2016.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fouad A.A., Albuali W.H., Al-Mulhim A.S., Jresat I. Cardioprotective effect of cannabidiol in rats exposed to doxorubicin toxicity. Environ. Toxicol. Pharmacol. 2013;36:347–357. doi: 10.1016/j.etap.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 69.Gȩgotek A., Rybałtowska-Kawałko P., Skrzydlewska E. Rutin as a mediator of lipid metabolism and cellular signaling pathways interactions in fibroblasts altered by UVA and UVB radiation. Oxid. Med. Cell. Longev. 2017 doi: 10.1155/2017/4721352. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grewal G.K., Kukal S., Kanojia N., Saso L., Kukreti S., Kukreti R. Effect of oxidative stress on ABC transporters: contribution to epilepsy pharmacoresistance. Molecules. 2017;22:E365. doi: 10.3390/molecules22030365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brzozowska N., Li K.M., Wang X.S., Booth J., Stuart J., McGregor I.S., Arnold J.C. ABC transporters P-gp and Bcrp do not limit the brain uptake of the novel antipsychotic and anticonvulsant drug cannabidiol in mice. PeerJ. 2016;4 doi: 10.7717/peerj.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Holland M.L., Allen J.D., Arnold J.C. Interaction of plant cannabinoids with the multidrug transporter ABCC1 (MRP1) Eur. J. Pharmacol. 2008;591:128–131. doi: 10.1016/j.ejphar.2008.06.079. [DOI] [PubMed] [Google Scholar]
- 73.Feinshtein V., Erez O., Ben-Zvi Z., Erez N., Eshkoli T., Sheizaf B., Sheiner E., Huleihel M., Holcberg G. Cannabidiol changes P-gp and BCRP expression in trophoblast cell lines. PeerJ. 2013;1:e153. doi: 10.7717/peerj.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maher J.M., Dieter M.Z., Aleksunes L.M., Slitt A.L., Guo G., Tanaka Y., Scheffer G.L., Chan J.Y., Manautou J.E., Chen Y., Dalton T.P., Yamamoto M., Klaassen C.D. Oxidative and electrophilic stress induces multidrug resistance-associated protein transporters via the nuclear factor-E2-related factor-2 transcriptional pathway. Hepatology. 2007;46:1597–1610. doi: 10.1002/hep.21831. [DOI] [PubMed] [Google Scholar]
- 75.Wang X., Campos C.R., Peart J.C., Smith L.K., Boni J.L., Cannon R.E., Miller D.S. Nrf2 upregulates ATP binding cassette transporter expression and activity at the blood-brain and blood-spinal cord barriers. J. Neurosci. 2014;34:8585–8593. doi: 10.1523/JNEUROSCI.2935-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Elmes M.W., Kaczocha M., Berger W.T., Leung K.N., Ralph B.P., Wang L., Sweeney J.M., Miyauchi J.T., Tsirka S.E., Ojima I., Deutsch D.G. Fatty acid-binding proteins (FABPs) are intracellular carriers for Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) J. Biol. Chem. 2015;290:8711–8721. doi: 10.1074/jbc.M114.618447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Olivas-Aguirre M., Torres-López L., Valle-Reyes J.S., Hernández-Cruz A., Pottosin I., Dobrovinskaya O. Cannabidiol directly targets mitochondria and disturbs calcium homeostasis in acute lymphoblastic leukemia. Cell Death Dis. 2019;10:779. doi: 10.1038/s41419-019-2024-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.De Pinto V., Messina A., Lane D.J.R., Lawen A. Voltage-dependent anion-selective channel (VDAC) in the plasma membrane. FEBS Lett. 2010;584:1793–1799. doi: 10.1016/j.febslet.2010.02.049. [DOI] [PubMed] [Google Scholar]
- 79.Rimmerman N., Ben-Hail D., Porat Z., Juknat A., Kozela E., Daniels M.P., Connelly P.S., Leishman E., Bradshaw H.B., Shoshan-Barmatz V., Vogel Z. Direct modulation of the outer mitochondrial membrane channel, voltage-dependent anion channel 1 (VDAC1) by cannabidiol: a novel mechanism for cannabinoid-induced cell death. Cell Death Dis. 2013;4 doi: 10.1038/cddis.2013.471. e949–e949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gęgotek A., Bielawska K., Biernacki M., Dobrzyńska I., Skrzydlewska E. Time-dependent effect of rutin on skin fibroblasts membrane disruption following UV radiation. Redox Biol. 2017;12:733–744. doi: 10.1016/j.redox.2017.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hložek T., Uttl L., Kadeřábek L., Balíková M., Lhotková E., Horsley R.R., Nováková P., Šíchová K., Štefková K., Tylš F., Kuchař M., Páleníček T. Pharmacokinetic and behavioural profile of THC, CBD, and THC+CBD combination after pulmonary, oral, and subcutaneous administration in rats and confirmation of conversion in vivo of CBD to THC. Eur. Neuropsychopharmacol. 2017;27:1223–1237. doi: 10.1016/j.euroneuro.2017.10.037. [DOI] [PubMed] [Google Scholar]
- 82.Kraemer M., Broecker S., Madea B., Hess C. Decarbonylation: a metabolic pathway of cannabidiol in humans. Drug Test. Anal. 2019;11:957–967. doi: 10.1002/dta.2572. [DOI] [PubMed] [Google Scholar]