Abstract
Objectives
In Western populations, patients with depression die 10–25 years prematurely compared to controls, mainly due to lifestyle-related diseases. Tobacco smoking, excessive alcohol intake, poor diets and physical inactivity are among the major contributors to disease comorbidities. The objective of this research is to assess the dietary and lifestyle behaviours for Bahraini patients with depression and to determine their associations with different medical comorbidities.
Methods
A case-control study was conducted from March to December 2019. A sample of 96 diagnosed patients with depression was recruited from the Psychiatric Hospital/Bahrain, and 96 age- and sex-matched controls were recruited from primary health centres. Assessment of anthropometrics, dietary and alcohol intakes, tobacco smoking and physical activity levels were undertaken for both cases and controls. National electronic medical records were reviewed retrospectively for medical comorbidities for the recruited cases. Logistic regression analysis was used to identify associations between lifestyle behaviours and medical comorbidities after controlling for confounding factors.
Results
Patients with depression reported higher intakes of energy and energy-yielding macronutrients (e.g., carbohydrates, protein, and fat); three-fold higher rates of tobacco smoking; and significantly lower levels of physical activity. Cases appeared to be at a doubled risk for developing obesity, diabetes type 2, hypertension, and musculoskeletal disorders. The risk for cardiovascular problems was similar for cases and controls.
Conclusions
Poor dietary intakes, increased prevalence of smoking, and low levels of physical activity were evident in patients with depression in Bahrain; these factors were associated with some medical comorbidities.
Keywords: Biological sciences, Health sciences, Cardiovascular disease, Depression, Dietary habits, Lifestyle behaviors, Physical activity, Smoking
Biological sciences; Health sciences; Cardiovascular disease; Depression; Dietary habits; Lifestyle behaviors; Physical activity; Smoking.
1. Introduction
Globally, depression is a common mental disorder, with about 265 million people of all ages suffer from depression (Lim et al., 2018). Depression is a principal cause of disability worldwide and is a leading contributor to the overall global burden of disease (Charlson et al., 2019). Patients with serious and persistent mental illness (SPMI) experience up to 20 years reduction in life expectancy compared with controls, even after controlling for the risk of accidents and suicide (Daumit et al., 2013; Teasdale et al., 2017). The vast majority of these premature deaths are ascribed to physical health ailments, mainly increased risk for cardiovascular disease (CVD) (Correll et al., 2017).
Understanding modifiable risk factors that mitigate or prevent premature mortality is, therefore, essential (Walker et al., 2015). Among the general population, there is wealth of evidence that inadequate diet and excessive energy intake, along with unhealthy behaviours like alcohol, smoking, or substance abuse, and lack physical activity (PA) are associated with adverse physical health including certain types of cancers, CVD, and premature mortality (GBD, 2017 Risk Factor Collaborators, 2018). This evidence supports the idea that population-tailored interventions that focus on lifestyle modifications are a keystone of health outcome determination (Teasdale et al., 2017).
Patients with depression have a remarkably high prevalence of overweight and obesity, estimated up to four times compared with controls (Ali and Jahrami, 2018; Mulugeta et al., 2018). Consequently, they have an increased burden of metabolic abnormalities, including type 2 diabetes, hyperlipidemia, hypertension, CVD, certain types of cancers, and musculoskeletal disorders (MSD) (Köhler et al., 2017; Vancampfort et al., 2016a, 2015b). Among patients with depression, obesity is an outcome of multifactorial variables, including unhealthy diets that are high in calories, carbohydrates and fats (Teasdale et al., 2017) besides sedentary behaviour (Stubbs et al., 2018; Vancampfort et al., 2018). Several studies have indicated to a decreased intake of healthy, nutrient-dense foods such as vegetables and fruits, with an above-average caloric intake from a diet rich in sugars and saturated fats; a matter that seems to disrupt in patients with schizophrenia (Phillips et al., 2018; Shivappa et al., 2018). Patients with depression are at higher risk of being sedentary. According to two recent meta-analyses, depression patients also involved in less vigorous, moderate, and light, physical activities compared to their corresponding controls (Stubbs et al., 2018; Vancampfort et al., 2018).
Latest research also indicates that psychotropic medications may exert a remarkable effect on weight gain, particularly those that are the first options of treatment for depression including specific serotonin selective reuptake inhibitors (SSRIs), tricyclic antidepressants (TCA's), and serotonin-norepinephrine reuptake inhibitors (SNRIs) (Dent et al., 2012). Patients on psychotropic medications report decreased satiety, increased appetite, and increased cravings for sugary foods and sweetened beverages (Firth et al., 2019b). Smoking has been linked with depression in 50–80% of the patients; they are also less likely to stop smoking compared with controls (Fluharty et al., 2017).
The 2019 Lancet Psychiatry Commission concluded that patients with depression are at higher risk for premature mortality and morbidity due to their unhealthy food choices, adverse effects from some offered treatments, and effects of their symptoms; thus, identifying advanced knowledge areas to reduce mortality and morbidity in this susceptible group of people is becoming a prioritized health issue (Firth et al., 2019a).
Investigating the previously published research unravels that investigators have mostly focused on the impact of a single lifestyle or dietary factor on the general health of depression patients in any given study, with no published works to date has comprehensively examined the possible interactions between different lifestyle and dietary behaviours of a single sample of patients in comparison with the control group and their impact on disease risk. Further, most of the published research had been executed in Western countries, with their distinct environmental contexts and cultural that uniquely affect the health outcomes of their mentally ill patients. Thus, the current research stems from the hypothesis that the presence of one or more lifestyle and dietary risk factors will raise the presence of comorbidities among depression patients. Accordingly, the current study was designed to characterize the lifestyle habits and dietary behaviours of patients with depression within Bahraini socio-cultural and environmental contexts, and to examine the effect of the presence of one or more lifestyle and dietary risk factors on the comorbidities among the cases with depression in comparison to sex- and age-matched controls.
2. Materials and methods
2.1. Study design
The study was planned, executed, and reported using the guidelines of the strengthening the reporting of observational studies in epidemiology (STROBE) statement (Elm et al., 2014). The study was executed between March–December 2019, using a matched case-control design.
Recruitment of patients with depression took place in the Psychiatric Hospital, Ministry of Health, Manama, Kingdom of Bahrain, which is the national center for diagnosis, treatment, and rehabilitation of patients with mental illnesses. Controls were recruited from community health centres during routine examination/investigation visits.
Bahrain is a country with an area of less than 800 square kilometres and a population of 1.5 million. The majority of the population is Muslim and the official language is Arabic. According to the World Bank report, Bahrain is classified as a high-income country, the costs of healthcare account for approximately 5% of Gross Domestic Product (“World Bank,” 2020). Bahrain is part of the Eastern Mediterranean Region (EMR) health region (“WHO EMRO,” 2020). Over the past three decades, Bahrain has undergone significant improvements in health care status, including increased life expectancy (~75 years), reductions in child mortality, and the prevalence of communicable diseases (Charara et al., 2017). Nonetheless, as people in Bahrain are living longer, the burden of chronic diseases, including mental disorders, are on the rise (Jahrami et al., 2019, 2014).
2.2. Participants
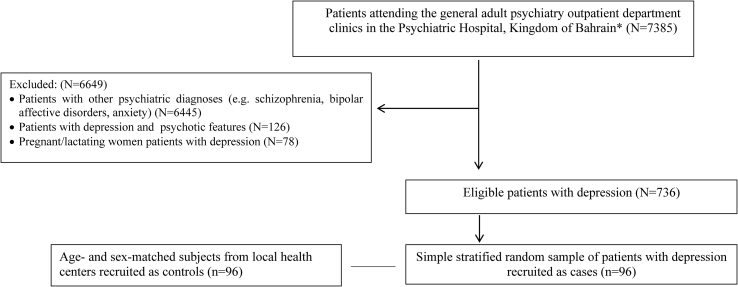
In our case-control study, the cases aged between 20-60 years, diagnosed with depression (major depressive disorder, single episode, unspecified) according to the International Statistical Classification of Diseases, 10th Revision (World Health Organization, 2005). The 96 cases with depression were recruited using simple random sampling from the overall cases attending the out-patient department (OPD) clinics. Similarly, the control group was obtained by matching each case with one randomly selected corresponding control after being matched with demographic characteristics (sex and age) from the primary health care centres. Age matching was defined in this study by matching the year of birth. Exclusion criteria for cases were being pregnant/lactating women, the coexistence of depression with psychotic features or any other psychiatric comorbidity, those who were dieting, or recruited in lifestyle interventions studies or clinical trials. Exclusion criteria for the control group were being pregnant/lactating women, the presence of a positive history of psychiatric illness, and those who had been following a special diet during the past six months, or recruited in lifestyle interventions studies or clinical trials (Figure 1).
Figure 1.
Flowchart depicting the recruitment procedure of the study participants. ∗The Psychiatric Hospital, Ministry of Health, Kingdom of Bahrain is the National Centre for Mental Health.
2.3. Clinical assessments and data collection
Collected information included basic sociodemographic variables, medical history, the validated Arabic version of International Physical Activity Questionnaire-Short Form (IPAQ-SF) (Helou et al., 2017), psychiatric history for cases, smoking status, and dietary intake using a quantitative food frequency questionnaire (FFQ) covering 102 foods distributed on 38 items/groups.
2.3.1. Sociodemographic and anthropometric measurements
A structured data collection questionnaire was used to collect information from the participants during an interview, and anthropometric measurements (weight and height) were taken at that time. Weight was measured using digital scales with height rod attachment that was kept on a firm horizontal floor. Participants wore stood upright without shoes, light clothing, and their weights were recorded to the nearest 0.1 kg. Height was measured with the rod attached to the weighing scale to the nearest 1.0 cm. Body composition analysis was performed using a bioelectrical impedance technique (InBody 230 model: MW160, Seoul/Korea) that measured body mass (to the nearest 0.1 kg), fat mass, and body fat percentage. Body mass index (BMI) (kg/m2) was calculated accordingly and classified according to the World Health Organisation (WHO) categories of underweight, normal, overweight, or obese.
2.3.2. Physical activity measurement
The IPAQ-SF is a validated assessment tool for assessing PA among adults. We evaluated the past seven days' recall, which assesses the frequency and duration of light-, moderate- and vigorous-intensity PA.
2.3.3. Dietary measurement
The FFQ was used to estimate the frequency of food consumption from the seven main food groups in our sample. Participants were asked how frequently, on average, during the past month they had consumed a standard serving of a specific food item in six categories (1 time/day, ≥2 times/day, 1–2 times/week, 3–6 times/week, 1–3 times/month, rarely or never). The responses on the frequent consumption of a specific serving size were standardized using food photographs to determine a standard unit for portions. Dietary intake assessed using the FFQ were analysed using nutrition and fitness software (ESHA Food Processor SQL, version 10.1.1, Salem, OR, USA). ESHA was used to estimate a gross mean of daily total energy intake, macro- and selected essential micronutrients.
We computed estimated energy requirement (EER) for each subject based on sex, age, weight, height, and PA level as per the Institute of Medicine's (IOM) formulas (Gerrior et al., 2006).
2.3.4. Medical and psychiatric history
Medical information was collected for the participants from the National Health Information System, Bahrain. We focused on CVD, hypertension, diabetes type 2, and MSD as major outcomes. Beck Depression Inventory-II (BDI-II) is a 21-question multiple-choice inventory for assessing the severity of depression symptoms (Beck et al., 1996). The validated Arabic version translation was used in the current research (Naja et al., 2019).
2.4. Statistical analyses
Descriptive statistics were summarized for the demographic characteristics, lifestyle behaviours, and dietary intakes of the participants. The mean and standard deviation (SD) were used to report continuous variables, and count and percentage were used to report categorical variables. The study comprised a matched case-control analysis, and analytical statistics were conducted to figure out the association between lifestyle and dietary behaviours with the health outcomes. Logistic regression was performed, and the Odds ratio (OR) was calculated, and significance was considered at P value < 0.05. All statistical analyses were performed using Stata 16.0 software.
2.5. Ethical considerations
The Research Ethics Committee/Ministry of Health, Kingdom of Bahrain approved the study (No.2018/REC/EF023). Written informed consent was sought from each participant before starting data collection. Participation was voluntary, with no monetary or non-monetary incentives.
3. Results
3.1. Characteristics of the participants
A total of 192 participants (96 cases and 96 age- and sex-matched controls) were recruited in the current study. The mean age was 44 years for the cases and 42 years for the controls. Sex distribution was similar due to matching with approximately 40% being male and approximately 60% being female. Table 1 shows the sociodemographic and anthropometric characteristics and general health status of the study participants. All the participants were affiliated to the Islam religion. In the current work, it was clear that where cases were including a significantly higher number of singles, divorced, and widows than controls. Further, the number of unemployed cases was significantly higher than the number in their corresponding controls.
Table 1.
Differences in general sociodemographic and anthropometric characteristics, and general health status of the study participants.
| Variable |
Cases, n = 96 |
Controls, n = 96 |
P-value∗∗ |
|---|---|---|---|
| Sex | |||
| Male | 37 (38.54%) | 37 (38.54%) | 1.0 |
| Female | 59 (61.46%) | 59 (61.46%) | |
| Age (year) | 44.13 ± 12.57 | 42.47 ± 13.50 | 0.40 |
| Education | |||
| Primary school | 27 (28.42%) | 12 (12.5%) | 0.001 |
| High school | 40 (42.11 %) | 23 (23.96%) | |
| Associate diploma/B.Sc. | 25 (26.31%) | 50 (52.08%) | |
| Postgraduates | 3 (3.16%) | 11 (11.46%) | |
| Job status | |||
| Employed/student | 27 (28.13%) | 69 (71.87%) | 0.001 |
| Retired | 7 (7.29 %) | 15 (15.63 %) | |
| Housewife for women | 18 (18.75%) | 10 (10.42 %) | |
| Unemployed | 44 (45.83 %) | 2 (2.08%) | |
| Marital status | |||
| Single | 31 (32.29%) | 17 (17.71%) | 0.001 |
| Married | 48 (50%) | 73 (76.04%) | |
| Divorced | 15 (15.63%) | 2 (2.08%) | |
| Widow | 2 (2.08%) | 4 (4.17%) | |
| Weight (kg) | 76.36 ± 18.58 | 75.16 ± 16.48 | 0.63 |
| Height (cm) | 162.68 ± 9.53 | 164.73 ± 9.55 | 0.13 |
| BMI (kg/m2) | 28.99 ± 7.32 | 27.44 ± 6.12 | 0.11 |
| BMI Classification | |||
| Underweight | 4 (4.17 %) | 2 (2.08%) | |
| Normal | 26 (27.08 %) | 34 (35.42%) | |
| Overweight | 30 (31.25%) | 35 (36.46%) | |
| Obese | 36 (37.5 %) | 25 (26.04%) | 0.25 |
| Body fat percentage (%) | 35.38 ± 12.03 | 32.67 ± 10.03 | 0.09 |
| Total body water percentage (%) | 35.98 ± 6.35 | 36.08 ± 6.6 | 0.91 |
| Body surface area (m2) | 1.84 ± 0.24 | 1.85 ± 0.23 | 0.98 |
| Lean mass (kg) | 48.41 ± 8.02 | 48.9 ± 8.38 | 0.68 |
| Fat mass (kg) | 27.94 ± 13.02 | 26.26 ± 10.19 | 0.31 |
| Beck Depression Inventory-II (BDI-II) | |||
| Mild | 13 (13.54%) | NA | NA |
| Moderate | 40 (41.67%) | ||
| Severe | 43 (44.79%) | ||
| Diabetes type 2∗∗∗ | 26 (27.08%) | 7 (7.29%) | 0.001 |
| Hypertension∗∗∗ | 27 (28.13%) | 11 (11.46%) | 0.004 |
| Cardiovascular problems∗∗∗ | 6 (6.25%) | 7 (7.29%) | 0.78 |
| Musculoskeletal disorders∗∗∗ | 39 (40.63%) | 24 (25%) | 0.020 |
∗ Mean ± SD or n(%).
∗∗ t-test or Pearson's/McNemar's Chi2 test.
∗∗∗ Diagnosis according to ICD-10.
Cardiovascular disease included: angina, myocardial infarction, stroke, heart failure; cardiomyopathy, heart arrhythmia, carditis, and venous thrombosis.
Musculoskeletal disorders included: osteoarthritis, gout, rheumatoid arthritis, fibromyalgia, plantar fasciitis, heel spurs, and tendonitis.
All cases were regularly attending the outpatient department with a median of one visit/month for follow-up. According to BDI-II, about 85% of the cases were of moderate to severe depression. At the time of the study, all cases were on psychopharmacological treatment, 42% were on SSRIs, 35% were on SNRIs, 12% were on TCA, and the remaining 11% were on combined antidepressants therapy.
3.2. Health status of the participants
Using matched analysis controlling for sex and age, we found that the most common physical-health morbidities among the depression cases in comparison with the controls were: type 2 diabetes mellitus (OR 4.7, 95% CI 1.8–13.0), hypertension (OR 3.1, 95% CI 1.4–7.2), MSD (OR 2.0, 95% CI 1.1–4.0), and obesity (OR 1.7, 95% CI 0.9–3.3).
Compared with controls, cases with depression were significantly more likely to have one physical-health comorbidity (OR 2.9; 95% CI 1.6–5.7), two physical-health comorbidities (OR 2.6; 95% CI 1.3–5.3), and three or more physical-health comorbidities (OR 3.1; 95% CI 1.2–8.4).
3.3. Dietary intakes
Intake frequency of 30 of the most frequently consumed food items is given in Table 2. Results of the FFQ showed that cases with depression generally reported higher food consumption for 11 items out of 30; these items were mostly energy-dense. Controls showed few items (six out of 30) to be of a higher intake frequency.
Table 2.
Differences in frequency of food intake of the study participants.
| Food | Cases, n = 96 |
Controls, n = 96 |
Chi2 (X2), P Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 time/day | 2 + times/day | 1-2 times/week | 3-6 times/week | 1-3 times/month | Rarely | 1 time/day | 2 + times/day | 1-2 times/week | 3-6 times/week | 1-3 times/month | Rarely | ||
| White “Lebanese/Arabic” bread (30 g) | 14 (14.58%) | 35 (36.46%) | 19 (19.79%) | 17 (17.71%) | 1 (1.04%) | 10 (10.42%) | 31 (32.29%) | 19 (19.79%) | 17 (17.71) | 10 (10.42%) | 9 (9.38%) | 10 (10.42%) | Chi2 = 19.48 P = 0.002 |
| Brown “Lebanese/Arabic” bread (30 g) | 1 (1.06%) | 5 (5.32%) | 8 (8.51%) | 7 (7.45%) | 14 (14.89%) | 59 (62.77%) | 13 (13.54%) | 3 (3.13%) | 13 (13.54%) | 6 (6.25%) | 26 (27.08%) | 35 (36.46%) | Chi2 = 21.76 P = 0.001 |
| Biscuits, rusks, cookies (30 g) | 4 (4.35%) | 8 (8.7%) | 19 (20.65%) | 30 (32.61%) | 15 (16.30%) | 16 (17.39) | 13 (13.54%) | 7 (7.29%) | 38 (39.58%) | 12 (12.50%) | 11 (11.46%) | 15 (15.63%) | Chi2 = 19.45 P = 0.002 |
| Cereals, cereals bar (30 g/1 bar) | 0 | 8 (9.09%) | 3 (3.41%) | 18 (20.45%) | 14 (15.91%) | 45 (51.14%) | 10 (10.42%) | 3 (3.13%) | 13 (13.54%) | 4 (4.17%) | 23 (23.96%) | 43 (44.79%) | Chi2 = 29.37 P = 0.001 |
| Beef (steak or cubes) (150 g) | 0 | 1 (1.04%) | 18 (18.75%) | 23 (23.96%) | 23 (23.96%) | 31 (32.29%) | 3 (3.13%) | 3 (3.13%) | 35 (36.46%) | 7 (7.29%) | 26 (27.08%) | 22 (22.92%) | Chi2 = 19.69 P = 0.001 |
| Burgers, meatballs, minced meat (75 g) | 0 | 3 (3.13%) | 24 (25%) | 28 (29.17%) | 13 (13.54%) | 28 (29.17%) | 1 (1.04%) | 2 (2.08%) | 32 (33.33%) | 8 (8.33%) | 26 (27.08%) | 27 (28.13%) | Chi2 = 17.80 P = 0.001 |
| Chicken (all types of preparation) (150 g) | 5 (5.26%) | 15 (15.79%) | 44 (46.32%) | 24 (25.26%) | 2 (2.11%) | 5 (5.26%) | 19 (19.79%) | 7 (7.29%) | 37 (38.54%) | 25 (26.04%) | 5 (5.21%) | 3 (3.13%) | Chi2 = 13.48 P = 0.019 |
| Lamb, goat, deer, rabbit, lamb (150 g) | 0 | 1 (1.12%) | 12 (13.48%) | 20 (22.47%) | 22 (24.72%) | 34 (38.2%) | 2 (2.08%) | 1 (1.04%) | 15 (15.63%) | 4 (4.17%) | 17 (17.17%) | 57 (59.38%) | Chi2 = 19.21 P = 0.002 |
| Fish (150 g) | 2 (2.08%) | 5 (5.21%) | 39 (40.63%) | 25 (26.04%) | 16 (16.67%) | 9 (9.38%) | 8 (8.33%) | 4 (4.17%) | 40 (41.67%) | 25 (26.04%) | 9 (9.38%) | 10 (10.42%) | Chi2 = 5.73 P = 0.333 |
| Seafood (octopus, squid, shrimps) (150 g) | 0 | 1 (1.05%) | 11 (11.58%) | 23 (24.21%) | 31 (32.63%) | 29 (30.53%) | 0 | 1 (1.04%) | 10 (10.4%) | 3 (3.13%) | 31 (32.29%) | 51 (53.13%) | Chi2 = 21.47 P = 0.001 |
| Lentils, beans, chickpeas (150 g/1 cup) | 0 | 4 (4.21%) | 16 (16.84%) | 22 (23.16%) | 34 (35.79%) | 19 (20%) | 3 (3.13%) | 3 (3.13%) | 32 (33.33%) | 3 (3.13%) | 44 (45.83%) | 11 (11.46%) | Chi2 = 26.32 P = 0.001 |
| Fresh soup (250 ml/cup) | 4 (4.17%) | 4 (4.17%) | 17 (17.71%) | 16 (16.67%) | 22 (22.92%) | 33 (34.38%) | 5 (5.21%) | 1 (1.04%) | 26 (27.08%) | 6 (6.25%) | 31 (32.29%) | 27 (28.13%) | Chi2 = 10.46 P = 0.063 |
| Soup with pasta (e.g. noodles) (250 ml/cup) | 0 | 1 (1.04%) | 13 (13.54%) | 22 (22.92%) | 26 (27.08%) | 34 (35.42%) | 0 | 1 (1.04%) | 14 (14.58%) | 3 (3.13%) | 23 (23.96%) | 55 (57.29%) | Chi2 = 19.61 P = 0.001 |
| Rice (all types) (1 cup/medium plate)) | 18 (19.15%) | 44 (46.81%) | 13 (13.83%) | 11 (11.70%) | 3 (3.19%) | 5 (5.32%) | 63 (65.63%) | 3 (3.13%) | 8 (8.33%) | 12 (12.5%) | 8 (8.33%) | 2 (2.08%) | Chi2 = 65.54 P = 0.001 |
| Boiled potatoes, mashed potatoes (1 medium) | 1 (1.08%) | 6 (6.45%) | 20 (21.51%) | 22 (23.66%) | 10 (10.75%) | 34 (36.56%) | 5 (5.21%) | 3 (3.13%) | 26 (27.08%) | 8 (8.33%) | 36 (37.5%) | 18 (.75%) | Chi2 = 30.56 P = 0.001 |
| French fries regular portion | 4 (4.26%) | 2 (2.13%) | 18 (19.15%) | 28 (29.79%) | 16 (17.02%) | 26 (27.66%) | 5 (5.21%) | 3 (3.13%) | 24 (25%) | 13 (13.54%) | 39 (40.63%) | 12 (12.5%) | Chi2 = 21.41 P = 0.001 |
| Fresh Vegetables (any kind) (1 piece) | 17 (17.71%) | 36 (37.50%) | 16 (16.67%) | 15 (15.63%) | 5 (5.21%) | 7 (7.29%) | 33 (34.38%) | 20 (20.83%) | 16 (16.67%) | 14 (14.58%) | 10 (10.42%) | 3 (3.13%) | Chi2 = 12.99 P = 0.023 |
| Fresh Fruits (any kind) (1 piece) | 18 (18.95%) | 31 (32.63%) | 18 (18.95%) | 16 (16.84%) | 9 (9.47%) | 3 (3.16%) | 32 (33.33%) | 29 (30.21%) | 18 (18.75%) | 12 (12.50%) | 4 (4.17%) | 1 (1.04%) | Chi2 = 7.47 P = 0.188 |
| Dried fruits (¼ cup) | 1 (1.06%) | 3 (3.19%) | 2 (2.13%) | 9 (9.57%) | 19 (20.21%) | 60 (63.83%) | 14 (14.58%) | 5 (5.21%) | 10 (10.42%) | 9 (9.38%) | 24 (25%) | 34 (35.42%) | Chi2 = 24.85 P = 0.001 |
| Dried nuts, nuts (¼ cup) | 0 | 12 (12.63%) | 13 (13.68%) | 25 (26.32%) | 28 (29.47%) | 17 (17.89%) | 15 (15.63%) | 10 (10.42%) | 24 (25%) | 9 (9.38%) | 31 (32.29%) | 7 (7.29%) | Chi2 = 30.29 P = 0.001 |
| Yoghurt complete or light (170 g) | 6 (6.38%) | 17 (18.09%) | 19 (20.21%) | 24 (25.53%) | 10 (10.64%) | 18 (19.15%) | 19 (19.79%) | 6 (6.25%) | 36 (37.50%) | 12 (12.5%) | 15 (15.63%) | 8 (8.33%) | Chi2 = 26.10 P = 0.001 |
| Cream cheese “Glasses” (25 g) | 7 (7.37%) | 22 (23.16%) | 20 (21.05%) | 20 (21.05%) | 4 (4.21%) | 22 (23.16%) | 21 (21.88%) | 6 (6.25%) | 28 (29.17%) | 12 (12.50%) | 17 (17.71%) | 12 (12.5%) | Chi2 = 30.46 P = 0.001 |
| Feta, white cheese, hard cheese (25 g) | 3 (3.16%) | 7 (7.37%) | 16 (16.84%) | 18 (18.95%) | 24 (25.26%) | 27 (28.42%) | 13 (13.54%) | 8 (8.33%) | 31 (32.29%) | 11 (11.46%) | 17 (17.71%) | 16 (16.67%) | Chi2 = 16.79 P = 0.005 |
| Egg (boiled, fried, omelette) (1 medium egg) | 3 (3.16%) | 6 (6.32%) | 30 (31.58%) | 42 (44.21%) | 8 (8.42%) | 6 (6.32%) | 12 (12.5%) | 4 (4.17%) | 53 (55.21%) | 14 (14.58%) | 11 (11.46%) | 2 (2.08%) | Chi2 = 28.64 P = 0.001 |
| Pies (e.g. cheese pie, spinach pie) (1 pie) | 0 | 2 (2.08%) | 18 (18.75%) | 35 (36.46%) | 20 (20.83%) | 21 (21.88%) | 6 (6.25%) | 2 (2.08%) | 31 (32.29%) | 9 (9.38%) | 28 (29.17%) | 20 (90.83%) | Chi2 = 26.17 P = 0.001 |
| Ice cream, milk shake, pudding (1 scoop) | 4 (4.17%) | 7 (7.29%) | 18 (18.75%) | 22 (22.92%) | 19 (19.79%) | 26 (27.08%) | 3 (3.13%) | 4 (4.17%) | 27 (28.13%) | 8 (8.33%) | 35 (36.46%) | 19 (19.79%) | Chi2 = 15.12 P = 0.010 |
| Honey, jam (10 ml) | 7 (7.37%) | 6 (6.32%) | 12 (12.63%) | 14 (14.74%) | 19 (20%) | 37 (38.95%) | 11 (11.46%) | 6 (6.25%) | 20 (20.83%) | 5 (5.21%) | 35 (36.46%) | 19 (19.79%) | Chi2 = 17.67 P = 0.003 |
| Olives (10 drupes) | 3 (3.13%) | 8 (8.33%) | 10 (10.42%) | 22 (22.92%) | 22 (22.92%) | 31 (32.29%) | 10 (10.42%) | 3 (3.13%) | 26 (27.08%) | 12 (12.50%) | 20 (20.83%) | 25 (26.04%) | Chi2 = 16.83 P = 0.005 |
| Chocolate (any type) (60 g) | 2 (2.08%) | 16 (16.67%) | 14 (14.58%) | 23 (23.96%) | 12 (12.50%) | 29 (30.21%) | 17 (17.71%) | 6 (6.25%) | 28 (29.17%) | 14 (14.58%) | 19 (19.79%) | 12 (12.5%) | Chi2 = 31.87 P = 0.001 |
| Chips packs, popcorn (70 g) | 6 (6.25%) | 13 (13.54%) | 12 (12.50%) | 22 (22.92%) | 13 (13.54%) | 30 (31.25%) | 18 (18.75%) | 8 (8.33%) | 19 (19.79%) | 9 (9.38%) | 25 (26.04%) | 16 (16.67%) | Chi2 = 23.27 P = 0.001 |
The intake frequency of eight main frequently consumed beverages was also assessed and is given in Table 3. Depression cases showed different consumption patterns of beverages compared to controls.
Table 3.
Beverages frequency intake of study participants.
| Beverage | Cases, n = 96 |
Controls, n = 96 |
Chi2 (X2), P Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-2 times/day | 3-4 times/day | 5 times/day | 1-2 times/week | 3-6 times/week | Rarely/never | 1-2 times/day | 3-4 times/day | 5 times/day | 1-2 times/week | 3-6 times/week | Rarely | ||
| Fruit juice (Packs) 1 cup/pack | 10 (10.42%) | 5 (5.21%) | 23 (23.96%) | 13 (13.54%) | 24 (25%) | 20 (20.83%) | 37 (38.54%) | 5 (5.21%) | 0 | 32 (33.33%) | 9 (9.38%) | 13 (13.54%) | Chi2 = 55.83 P = 0.001 |
| Soft (carbonated) drinks 1 can | 4 (4.17%) | 3 (3.13%) | 9 (9.38%) | 16 (16.67%) | 8 (8.33%) | 56 (58.33%) | 13 (13.54%) | 2 (2.08%) | 2 (2.08%) | 22 (22.92%) | 5 (5.21%) | 52 (54.17%) | Chi2 = 11.20 P = 0.047 |
| Milk, milk shake 1 glass | 3 (3.13%) | 3 (3.13%) | 33 (34.38%) | 14 (14.58%) | 16 (16.67%) | 27 (28.13%) | 33 (34.38%) | 2 (2.08%) | 3 (3.13%) | 29 (30.21%) | 7 (7.29%) | 22 (22.92%) | Chi2 = 59.46 P = 0.001 |
| Coffee in a cup (e.g. Americano) 1 cup | 2 (2.08%) | 3 (3.13%) | 12 (12.50%) | 12 (12.50%) | 8 (8.33%) | 57 (59.38%) | 25 (26.04%) | 3 (3.13%) | 1 (1.04%) | 15 (15.63%) | 2 (2.08%) | 50 (52.08%) | Chi2 = 35.29 P = 0.001 |
| Arabic Coffee 1 shot | 2 (2.08%) | 5 (5.21%) | 4 (4.17%) | 5 (5.21%) | 23 (23.96%) | 57 (59.38%) | 22 (22.92%) | 3 (3.13%) | 1 (1.04%) | 24 (25%) | 6 (6.25%) | 40 (41.67%) | Chi2 = 44.35 P = 0.001 |
| Tea, other herbal teas 1 cup | 18 (18.75%) | 17 (17.71%) | 35 (36.46%) | 4 (4.17%) | 7 (7.29%) | 14 (14.58%) | 42 (43.75%) | 12 (12.50%) | 5 (5.21%) | 13 (13.54%) | 5 (5.21%) | 19 (19.79%) | Chi2 = 39.81 P = 0.001 |
| Isotonic/energy drinks 1 glass | 0 | 0 | 2 (2.08%) | 0 | 6 (6.25%) | 86 (89.58%) | 1 (1.04%) | 0 | 0 | 6 (6.25%) | 0 | 89 (92.71%) | Chi2 = 17.05 P = 0.004 |
| Alcoholic drinks (e.g. wine, beer, whisky) 1 unit | 0 | 0 | 0 | 0 | 0 | 96 (100%) | 0 | 0 | 0 | 0 | 0 | 96 (100%) | NA |
Table 4 shows the average daily intakes of energy, macro- and selected micro-essential nutrients for the study participants. Cases showed statistically significantly higher intakes of energy, protein, fats, saturated fats, monounsaturated fats, polyunsaturated fats, cholesterol, riboflavin, vitamin E, iron, zinc, calcium, and potassium.
Table 4.
Average daily intakes of energy, macro- and selected essential micro-nutrients for the study participants.
| Nutrient | Cases, n = 96∗ | Controls, n = 96∗ | DRIs∗∗ | P-Value∗∗∗ |
|---|---|---|---|---|
| Energy (Kcal) | 2650.4 ± 883.78 | 2266.63 ± 520.1 | 2000–2500 | 0.001 |
| Protein (g) | 109.30 ± 38.21 | 87.04 ± 24.56 | 56 | 0.001 |
| Carbohydrates (g) | 275.39 ± 104.37 | 269.56 ± 64.45 | 130 | 0.642 |
| Fibres (g) | 20.85 ± 14.66 | 18.03 ± 6.59 | 38 | 0.088 |
| Fats (g) | 72.0 ± 26.99 | 58.94 ± 18.03 | ND | 0.001 |
| Saturated fats (g) | 34.72 ± 13 | 26.35 ± 8.52 | ND | 0.001 |
| Monounsaturated fats (g) | 24.21 ± 10.18 | 19.33 ± 6.2 | ND | 0.001 |
| Polyunsaturated fats (g) | 17.02 ± 8 | 14.7 ± 6.4 | ND | 0.027 |
| Trans-fats (g) | 0.36 ± 0.22 | 0.40 ± 0.35 | ND | 0.396 |
| Cholesterol (mg) | 360.24 ± 148.2 | 232.29 ± 86.95 | ND | 0.001 |
| Omega-3 (g) | 0.82 ± 0.51 | 0.75 ± 0.35 | 1.59 | 0.249 |
| Omega-6 (g) | 10.47 ± 6.28 | 9.16 ± 4.78 | 15 | 0.106 |
| Thiamine, B1 (mg) | 1.42 ± 0.56 | 1.45 ± 0.5 | 1.2 | 0.746 |
| Riboflavin, B2 (mg) | 1.59 ± 0.56 | 1.43 ± 0.46 | 1.3 | 0.045 |
| Niacin (mg) | 20.38 ± 7.75 | 18.93 ± 6.53 | 15 | 0.164 |
| Vitamin B6 (mg) | 2.07 ± 0.83 | 2.11 ± 0.56 | 1.7 | 0.738 |
| Vitamin B12 (μg) | 3.08 ± 2.31 | 3.16 ± 2.08 | 2.5 | 0.821 |
| Vitamin C (mg) | 90.84 ± 58.83 | 113.7 ± 63.14 | 90 | 0.010 |
| Vitamin D (IU) | 80.84 ± 64.64 | 87.15 ± 65.98 | 400 | 0.504 |
| Vitamin E (mg) | 3.03 ± 1.64 | 2.57 ± 1.36 | 10 | 0.034 |
| Folic Acid (μg) | 431.05 ± 200.78 | 423.48 ± 218.8 | 400 | 0.803 |
| Vitamin A (IU) | 993.98 ± 400.36 | 807.46 ± 260.5 | 900 | 0.418 |
| Beta-carotene (μg) | 1915.05 ± 944.52 | 1809.83 ± 848.7 | 1500 | 0.518 |
| Iron (mg) | 39.28 ± 17.81 | 31.47 ± 12.2 | 18 | 0.001 |
| Magnesium (mg) | 319.53 ± 187.18 | 336.92 ± 155.23 | 420 | 0.485 |
| Selenium (μg) | 64.43 ± 25.04 | 57.94 ± 21.79 | 100 | 0.057 |
| Zinc (mg) | 9.52 ± 4.22 | 7.65 ± 2.66 | 11 | 0.001 |
| Calcium (mg) | 665.27 ± 313.64 | 637.5 ± 279.29 | 1200 | 0.001 |
| Sodium (mg) | 2136.25 ± 867.11 | 1699.6 ± 625.1 | 1500 | 0.074 |
| Phosphorous (mg) | 913.2 ± 305.9 | 839.28 ± 261.8 | 700 | 0.254 |
| Potassium (mg) | 3185.83 ± 1163.5 | 3020.6 ± 805.5 | 4700 | 0.001 |
| Alcohol (g) | 0 ± 0 | 0 ± 0 | ND | NA |
| Caffeine (mg) | 164.35 ± 182.51 | 239.89 ± 275.7 | ND | 0.001 |
∗Mean ± SD.
∗∗Dietary Reference Intakes, Food and Nutrition Board, Institute of Medicine (Ref).
∗∗∗Difference between cases and controls.
ND – Not Defined.
3.4. General dietary habits, smoking, alcohol intake, caffeine intake, and PA
General dietary habits, smoking, alcohol intake, caffeine intake, and PA are presented in Table 5. Cases with depression appeared more likely to skip meals with 34.4% consuming less than three meals, compared to only 23.9% of controls. The prevalence of smoking was approximately four-times higher among cases with depression; with 37.2% were current tobacco smokers, compared with 10.4% of the controls. Neither the cases nor the controls were drinking alcohol; this is perhaps attributed to religion and Islamic laws that prohibit alcohol. Caffeine intake was significantly lower among cases, especially those of excessive intake pattern; approximately 11% of cases consumed ≥400 mg/day, compared to approximately 30% of controls.
Table 5.
Differences in average values for general dietary behaviours, smoking, and physical activity practiced per week, and daily sleeping hours of the study participants.
| Variable |
Cases n = 96 |
Controls n = 96 |
P-Value∗ |
|---|---|---|---|
| Number of meals/day | |||
| Less than 3 meals | 33 (34.4%) | 23 (24%) | 0.20 |
| Three meals | 52 (54.2%) | 64 (66.7%) | |
| More than 3 meals | 11 (11.5%) | 9 (9.48%) | |
| Alcohol intake (regular intake) | 0 (0%) | 0 (0%) | NA |
| Caffeine intake (≥400 mg/day) | 11 (11.5%) | 28 (29.5%) | P = 0.003∗ |
| Smoking | 35 (37.2%) | 10 (10.4%) | P = 0.001∗ |
| Vigorous intensity PA (minutes/day) | 3.23 ± 14.16 | 12.84 ± 29.39 | P = 0.004∗ |
| Vigorous intensity (times/week) | 0.22 ± 1.13 | 0.91 ± 1.88 | P = 0.002∗ |
| Moderate intensity PA (minutes/day) | 4.43 ± 13.47 | 6.04 ± 19.67 | P = 0.508 |
| Moderate intensity (times/week) | 0.74 ± 2.04 | 0.52 ± 1.63 | P = 0.412 |
| Light intensity PA (minutes/day) | 23.28 ± 31.01 | 29.32 ± 26.39 | P = 0.148 |
| Light intensity PA (times/week) | 2.66 ± 2.78 | 4.21 ± 2.75 | P = 0.001∗ |
| Sitting (minutes/day) | 228.35 ± 169.85 | 96.62 ± 94.44 | P = 0.001∗ |
| Sitting intensity (times/week) | 6.94 ± 0.61 | 5.5 ± 2.28 | P = 0.001∗ |
| Vigorous intensity PA (MET minutes/week) | 103.33 ± 542.73 | 374.83 ± 1004.93 | P = 0.021∗ |
| Moderate intensity PA (MET minutes/week) | 138.93 ± 462.53 | 150.96 ± 540.16 | P = 0.869 |
| Light intensity PA (MET minutes/week) | 378.98 ± 628.48 | 508.75 ± 494.82 | P = 0.114 |
| Total PA (MET minutes/week) | 621.25 ± 1013.91 | 1034.55 ± 1385.72 | P = 0.019 |
| Total MET activity categories | |||
| Inactive | 65 (67.71%) | 47 (48.96%) | P = 0.023 |
| Minimally active | 26 (27.08%) | 44 (45.83%) | |
| Hyper? active | 5 (5.21%) | 5 (5.21%) | |
∗Significant P < 0.05.
PA = Physical activity.
MET = Metabolic Equivalent Task.
The mean duration of PA/week and the metabolic equivalent for task (MET) (in minutes/week) at various intensity levels are shown for the cases and controls in Table 5. Cases with depression appeared to have a similar PA activity level of controls for moderate-intensity PA, and light intensity. Controls spent more time in vigorous-intensity PA, and cases spent more time sitting. The overall assessment of PA revealed that more cases are inactive compared to controls, approximately 68% vs. 49%, respectively.
3.5. Association between dietary and lifestyle behaviours and disease morbidities in cases
Using logistic regression, the associations between the predictive variables of lifestyle behaviours (excessive caloric intake, PA, and smoking) and outcome variables having: one medical comorbidity, two medical comorbidities, or three or more medical comorbidities after adjusting for age and sex are shown in Table 6. The first and third models (outcome one medical comorbidity and outcome three or more medical comorbidities) showed that no variable predicted the outcome. The second model (outcome two medical comorbidities) showed that smoking was statistically significant (P = 0.005) a risk factor. For the controls, age and female sex were the main risk factors in predicting one-, two-, and three or more-comorbidities (Table 6).
Table 6.
The association between lifestyle behaviours and medical comorbidities in the study participants.
| Independent variable | Dependent variable |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 comorbidity |
2 comorbidities |
≥3 comorbidities |
|||||||
| OR |
P-vale |
95% CI |
OR |
P-vale |
95% CI |
OR |
P-vale |
95% CI |
|
| Cases with depression (n = 96) | |||||||||
| Age | 1.09 | 0.001 | (1.04–1.14) | 1.10 | 0.001 | (1.05–1.16) | 1.09 | 0.001 | (1.03–1.15) |
| Sex | 3.81 | 0.05 | (1–14.50) | 6.87 | 0.01 | (1.66–28.42) | 3.45 | 0.07 | (0.89–13.34) |
| Smoking | 3.21 | 0.12 | (0.74–13.87) | 8.63 | 0.01 | (1.93–38.50) | 1.44 | 0.60 | (0.37–5.66) |
| Inadequate physical activity (inactivity)∗ | 0.43 | 0.15 | (0.14–1.37) | 0.46 | 0.18 | (0.15–1.45) | 1.03 | 1 | (0.31–3.38) |
| Excessive caloric intake∗∗ | 0.38 | 0.18 | (0.09–1.54) | 1.28 | 0.68 | (0.39–4.14) | 1.26 | 0.72 | (0.36–4.41) |
|
Controls (n=96) | |||||||||
| Age | 1.13 | 0.001 | (1.08–1.19) | 1.17 | 0.001 | (1.09–1.25) | 1.10 | 0.01 | (1.03–1.17) |
| Sex | 1.97 | 0.33 | (0.51–7.56) | 4.50 | 0.08 | (0.83–24.53) | 3.45 | 1 | - |
| Smoking | 0.80 | 0.82 | (0.12–5.54) | 1.68 | 0.74 | (0.79–35.99) | 3.54 | 1 | - |
| Inadequate physical activity (inactivity)∗ | 0.77 | 0.64 | (0.25–2.35) | 1.10 | 0.90 | (0.26–4.59) | 1.37 | 0.73 | (0.23–8.08) |
| Excessive caloric intake∗∗ | 0.82 | 0.72 | (0.28–2.42) | 0.38 | 0.21 | (0.085–1.73) | 0.83 | 0.83 | (0.15–4.72) |
∗ <600 MET-min/week or <150 min/week.
∗∗ Excessive caloric intake = actual caloric intake ≥ estimated energy requirement (EER).
Multiple logistic regression – adjusting for age and sex.
4. Discussion
The findings of the current study show that patients with depression have a significantly higher prevalence of smoking, marked unhealthy dietary intake, and decreased PA compared with controls. Such factors were significantly associated with several comorbidities, and patients with depression appeared to be at increased risk for medical conditions such as obesity, diabetes type 2, hypertension, and MSD. Interestingly, the prevalence of CVD was the same in both cases, with depression and controls. Cases with depression were more at risk of having up to three or more physical-health conditions compared with controls.
From public health perspective, our research highlights the poor lifestyle choices of patients with depression. For example, one-third of our cases were current smokers. Previous research consistently showed that patients SPMI are three times more likely to be smokers than controls (Gurillo et al., 2015; Hamadeh et al., 2016). Smoking increases the risk of smoking-related health complications and premature death, including death from CVD and some cancers (Mitchell et al., 2015). Programs to prevent or aid patients to quit smoking need to be activated in psychiatric care settings.
Nutrition plays a fundamental role in the development and progression of depression This relationship takes two-directional effects; that depression adversely affect the dietary behaviours, while the unhealthy, proinflammatory food choices would trigger the inflammation-related changes associated with depression (Air et al., 2016; Baune and Tully, 2016).
Excessive caloric intake was also found to be more prevalent in cases with depression compared to controls; which is found to be associated with an increased risk of overweight and obesity. Numerous studies among the general population have associated different measures of adiposity and excess body weight with an increased morbidity, mortality and disability from diabetes type 2, CVD, certain types of cancers, chronic kidney disease, and musculoskeletal disorders (Flegal et al., 2010; Li et al., 2019; Trafford et al., 2019). Further, excessive caloric intake has been linked with increased bodily inflammation, with the latter has reported as one of the etiopathogenesis factors that underground the development of depression (Shivappa et al., 2018; Wirth et al., 2017). The larger intake of total calories and macronutrients by depression patients in the current study is consistent with the findings of a large-scale study on a total of 69,843 subjects from the UK. In that study, multivariable linear regression on 14,619 individuals diagnosed with major depressive disorders (MDD) revealed that the MDD sample showed significantly greater (all p < 0.001) age- and gender-adjusted intake in comparison to controls, for total energy, total fat, saturated fat, carbohydrates, sugar, and protein, along with a small difference in dietary fibre intake (Firth et al., 2018).
The reported higher intakes by of depression cases for processed meats, refined carbohydrates, and energy-dense foods goes in line with the findings of one large-scale study by Lucas and colleagues who examined the association between an “inflammatory dietary pattern” and depression in women from the Nurses' Health Study (n = 43,685) (Lucas et al., 2014) They found that the inflammatory dietary pattern was characterized by high intakes of refined grains, red meat, margarine, and soft drinks, and as well as low intakes of green and yellow vegetables, olive oil, coffee, and wine. This Westernized dietary pattern was associated with the inflammatory biomarkers' tumor necrosis factor alpha (TNF- α), IL-6 and CRP. These findings led to the conclusions that the inflammatory dietary pattern is significantly associated with an increased risk for the development of depression, suggesting that inflammation may underlie the diet-depression relationship (Lucas et al., 2014).
Comparing dietary patterns of depressed patients versus healthy people received few highlights in the research literature. Few studies focused on patients with a clinical diagnosis of depression made against established diagnostic criteria (e.g., ICD-10). Previous research showed that intakes of patients with depression were similar to those reported in the National Nutrition Surveys (Forsyth et al., 2012). Patients with depression also appeared to be less likely complying with the recommended daily intake of fruits and vegetables (Wolniczak et al., 2017). Results of a recent meta-analysis also showed that low intakes of whole grain, fish, olive oil, low-fat dairy, and antioxidants and low intakes of animal foods are associated with depression (Li et al., 2017).
Close examination of the energy, macro- and selected essential micro-nutrients for the study participants reveals that patients with depression depend more on energy-dense diets than controls, with more focus on fats and protein. These results are in line with previous work on depression patients, where depression was found to lead to changes in dietary patterns, including eating comfort foods, which tend to be calorie-dense foods high in sugar and fat (Finch and Tomiyama, 2015).
In our study, alcoholic beverages items were not regularly consumed by patients; this is perhaps because alcohol is considered haram (prohibited or sinful) by Muslims. An interesting finding was that the caffeine intake of patients with depression was significantly less in cases with depression compared to controls. While one explanation is that coffee and caffeine consumption were significantly associated with decreased risk of depression (Grosso et al., 2016; Wang et al., 2016). A more plausible explanation is that patients with depression intentionally reduce their caffeine intake as a practical measure to reduce their troubles with insomnia.
Physical inactivity was more prevalent among cases with depression; overall, two-third of the cases did not meet the minimum recommendation for PA. Furthermore, patients with depression had a significantly lower MET minute/week compared to controls (621 and 1034 MET minutes/week, respectively). Several meta-analyses documented that patients with depression are at increased risk of engaging in high levels of sedentary behaviours (Stubbs et al., 2018; Vancampfort et al., 2016b). Nonetheless, patients with depression appeared to be more active compared to patients with schizophrenia (Jahrami et al., 2017; Vancampfort et al., 2016b).
Among patients with depression, the most significant fraction of the causes of sedentary behaviour were mobility limitations, followed by impairments in sleep or lethargy, pain/or discomfort, social anxiety, disability, cognitive problems, and sensory problems (Stubbs et al., 2018). More actions are needed prevent discrimination and reduce stigma of mental illness and ensure equitable access to all aspects of healthcare for those with SPMI.
Previous research emphasized the burden of CVD among cases with depression (Correll et al., 2017; Stubbs et al., 2016; Vancampfort et al., 2015a). Nonetheless, our findings showed that the prevalence of CVD among cases with depression was similar to those of controls. We explain that previous research focused on older adults and the elderly, while our research focused on middle-aged patients. It must be, however, highlighted that the risk factors for CVD among cases were statistically significant compared to controls, particularly diabetes type 2, hypertension, and increased BMI. This has significant clinical implications in the sense that for best protective results, lifestyle modifications should be well-timed and offered as comprehensive interventions to address various components. Thus, we recommend that offering smoking cessation therapy, exercise education, and nutritional interventions by qualified health-care professionals should be prioritized in daily clinical practice to maintain an active lifestyle by the patient with depression and other SPMI (Jahrami et al., 2019).
Our study aimed to identify key behavioural risk factors for depression, the results suggests that concurrently considering multiple lifestyle factors is more appropriate in understanding and managing risk factors for physical health comorbidities. Such transdiagnostic, multifactorial approaches have not been widely reflected in the published literature, which generally focuses on specific factors for individual disorders (Firth et al., 2019a).
Thus, to comprehensively promote or maintain the physical health of people with depression and other SPMI, the development of sensitive, reliable and time-efficient tools for life screening is needed. These tools need to be holistic and comprehensive covering exercise, diet, substance use, sleep; and sexual health at on go. The use of online tools and mobile based applications might be worthy to examine.
Mounting research highlighted the role of diet and inflammation in the initiation or progression of mental health illness including depression (Akbaraly et al., 2016; Doyle et al., 2013; Lucas et al., 2014; Moieni et al., 2015; Poole et al., 2011; Sánchez-Villegas et al., 2015; Shivappa et al., 2018; Zunszain et al., 2013). It is believed that those living with depressive symptoms and eating a pro-inflammatory diet, possibly due to the depressive symptoms, may be putting themselves at increased risk for future mental illness (Wirth et al., 2017). This relationship is supported by the fact that depression has been associated with inflammation-related conditions such as cardiovascular disease (CVD), cancer, stroke, and diabetes (Berge and Riise, 2015; Kang et al., 2015; Reich, 2008; Yousofpour et al., 2015); findings that are confirmed in our current research Thus, further research is needed to figure out how dietary inflammation (as measured by dietary inflammatory indices) correlates with the presence of depression in our case-control study.
5. Strengths and limitations
The main strength of the current study is that the research was done on a clinical sample of depression, where the diagnosis was made against the ICD-10 criteria. The other strength of the current research stems from the nature of comprehensive assessments of the significant lifestyle behaviours, including dietary and alcohol intakes, PA, and smoking in one sample.
One limitation of the current research is that we included patients who were regularly attending OPD facilities; this perhaps might not be generalizable to those who were not adherent to their therapy and follow-ups. Also, because the study did not initially focus on CVD, we did not have information on several biological CVD markers, including lipid abnormalities, inflammation, and elevated blood pressure. Future research is encouraged to include biomarkers to assess the association between lifestyle factors and those pointers. Pharmacological studies to determine if adverse health behaviours or outcomes were associated with any specific classes of antidepressant medications in our population are needed in the future. Finally, as with any case-control study recall bias might be an issue when self-reporting lifestyle factors.
6. Conclusion
Patients with depression had a higher prevalence of smoking, excessive dietary intakes, and decreased physical activity compared with their corresponding age- and sex-matched controls. Patients with depression appeared to be at higher risk for developing chronic medical conditions such as obesity, type 2 diabetes, hypertension, and MSD.
Declarations
Author contribution statement
H. Jahrami: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Z. Saif: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
M. Faris: Analyzed and interpreted the data; Wrote the paper.
M. AlHaddad: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
L. Hammad and B. Ali: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
None.
References
- Air T., Tully P.J., Sweeney S., Beltrame J. Epidemiology of cardiovascular disease and depression. In: Baune B.T., Tully P.J., editors. Cardiovascular Diseases and Depression: Treatment and Prevention in Psychocardiology. Springer International Publishing; Cham: 2016. pp. 5–21. [Google Scholar]
- Akbaraly T., Kerlau C., Wyart M., Chevallier N., Ndiaye L., Shivappa N., Hébert J.R., Kivimäki M. Dietary inflammatory index and recurrence of depressive symptoms: results from the Whitehall II Study. Clin. Psychol. Sci. 2016;4:1125–1134. doi: 10.1177/2167702616645777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M.K., Jahrami H.A. Prevalence of metabolic syndrome and metabolic abnormalities among patients with depression. Bahrain Med. Bull. 2018;40:86–89. [Google Scholar]
- Baune B.T., Tully P.J., editors. Cardiovascular Diseases and Depression: Treatment and Prevention in Psychocardiology. Springer International Publishing; 2016. [Google Scholar]
- Beck A.T., Steer R.A., Ball R., Ranieri W. Comparison of beck depression inventories -IA and -II in psychiatric outpatients. J. Pers. Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Berge L.I., Riise T. Comorbidity between type 2 diabetes and depression in the adult population: directions of the association and its possible pathophysiological mechanisms [WWW Document] Internet J. Endocrinol. 2015 doi: 10.1155/2015/164760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charara R., Forouzanfar M., Naghavi M., Moradi-Lakeh M., Afshin A., Vos T., Daoud F., Wang H., Bcheraoui C.E., Khalil I., Hamadeh R.R., Khosravi A., Rahimi-Movaghar V., Khader Y., Al-Hamad N., Obermeyer C.M., Rafay A., Asghar R., Rana S.M., Shaheen A., Abu-Rmeileh N.M.E., Husseini A., Abu-Raddad L.J., Khoja T., Rayess Z.A.A., AlBuhairan F.S., Hsairi M., Alomari M.A., Ali R., Roshandel G., Terkawi A.S., Hamidi S., Refaat A.H., Westerman R., Kiadaliri A.A., Akanda A.S., Ali S.D., Bacha U., Badawi A., Bazargan-Hejazi S., Faghmous I.A.D., Fereshtehnejad S.-M., Fischer F., Jonas J.B., Defo B.K., Mehari A., Omer S.B., Pourmalek F., Uthman O.A., Mokdad A.A., Maalouf F.T., Abd-Allah F., Akseer N., Arya D., Borschmann R., Brazinova A., Brugha T.S., Catalá-López F., Degenhardt L., Ferrari A., Haro J.M., Horino M., Hornberger J.C., Huang H., Kieling C., Kim D., Kim Y., Knudsen A.K., Mitchell P.B., Patton G., Sagar R., Satpathy M., Savuon K., Seedat S., Shiue I., Skogen J.C., Stein D.J., Tabb K.M., Whiteford H.A., Yip P., Yonemoto N., Murray C.J.L., Mokdad A.H. The burden of mental disorders in the eastern mediterranean region, 1990-2013. PloS One. 2017;12 doi: 10.1371/journal.pone.0169575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlson F., van Ommeren M., Flaxman A., Cornett J., Whiteford H., Saxena S. New WHO prevalence estimates of mental disorders in conflict settings: a systematic review and meta-analysis. Lancet Lond. Engl. 2019;394:240. doi: 10.1016/S0140-6736(19)30934-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll C.U., Solmi M., Veronese N., Bortolato B., Rosson S., Santonastaso P., Thapa-Chhetri N., Fornaro M., Gallicchio D., Collantoni E., Pigato G., Favaro A., Monaco F., Kohler C., Vancampfort D., Ward P.B., Gaughran F., Carvalho A.F., Stubbs B. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatr. 2017;16:163–180. doi: 10.1002/wps.20420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daumit G.L., Dickerson F.B., Wang N.-Y., Dalcin A., Jerome G.J., Anderson C.A.M., Young D.R., Frick K.D., Yu A., Gennusa J.V., Oefinger M., Crum R.M., Charleston J., Casagrande S.S., Guallar E., Goldberg R.W., Campbell L.M., Appel L.J. A behavioral weight-loss intervention in persons with serious mental illness. N. Engl. J. Med. 2013;368:1594–1602. doi: 10.1056/NEJMoa1214530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent R., Blackmore A., Peterson J., Habib R., Kay G.P., Gervais A., Taylor V., Wells G. Changes in body weight and psychotropic drugs: a systematic synthesis of the literature. PloS One. 2012;7 doi: 10.1371/journal.pone.0036889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle T.A., de Groot M., Harris T., Schwartz F., Strotmeyer E.S., Johnson K.C., Kanaya A. Diabetes, depressive symptoms, and inflammation in older adults: results from the Health, Aging, and Body Composition Study. J. Psychosom. Res. 2013;75:419–424. doi: 10.1016/j.jpsychores.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int. J. Surg. 2014;12:1495–1499. doi: 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- Finch L.E., Tomiyama A.J. Comfort eating, psychological stress, and depressive symptoms in young adult women. Appetite. 2015;95:239–244. doi: 10.1016/j.appet.2015.07.017. [DOI] [PubMed] [Google Scholar]
- Firth J., Siddiqi N., Koyanagi A., Siskind D., Rosenbaum S., Galletly C., Allan S., Caneo C., Carney R., Carvalho A.F., Chatterton M.L., Correll C.U., Curtis J., Gaughran F., Heald A., Hoare E., Jackson S.E., Kisely S., Lovell K., Maj M., McGorry P.D., Mihalopoulos C., Myles H., O’Donoghue B., Pillinger T., Sarris J., Schuch F.B., Shiers D., Smith L., Solmi M., Suetani S., Taylor J., Teasdale S.B., Thornicroft G., Torous J., Usherwood T., Vancampfort D., Veronese N., Ward P.B., Yung A.R., Killackey E., Stubbs B. The Lancet Psychiatry Commission: a blueprint for protecting physical health in people with mental illness. Lancet Psychiatr. 2019;6:675–712. doi: 10.1016/S2215-0366(19)30132-4. [DOI] [PubMed] [Google Scholar]
- Firth J., Stubbs B., Teasdale S.B., Ward P.B., Veronese N., Shivappa N., Hebert J.R., Berk M., Yung A.R., Sarris J. Diet as a hot topic in psychiatry: a population-scale study of nutritional intake and inflammatory potential in severe mental illness. World Psychiatry off. J. World Psychiatr. Assoc. WPA. 2018;17:365–367. doi: 10.1002/wps.20571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth J., Ward P.B., Stubbs B. Editorial: lifestyle psychiatry. Front. Psychiatr. 2019;10 doi: 10.3389/fpsyt.2019.00597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal K.M., Carroll M.D., Ogden C.L., Curtin L.R. Prevalence and trends in obesity among US adults, 1999-2008. J. Am. Med. Assoc. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- Fluharty M., Taylor A.E., Grabski M., Munafò M.R. The association of cigarette smoking with depression and anxiety: a systematic review. Nicotine Tob. Res. 2017;19:3–13. doi: 10.1093/ntr/ntw140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth A.K., Williams P.G., Deane F.P. Nutrition status of primary care patients with depression and anxiety. Aust. J. Prim. Health. 2012;18:172–176. doi: 10.1071/PY11023. [DOI] [PubMed] [Google Scholar]
- GBD 2017 Risk Factor Collaborators Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Lond. Engl. 2018;392:1923–1994. doi: 10.1016/S0140-6736(18)32225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrior S., Juan W., Peter B. An easy approach to calculating estimated energy requirements. Prev. Chronic Dis. 2006;3(4):A129. [PMC free article] [PubMed] [Google Scholar]
- Grosso G., Micek A., Castellano S., Pajak A., Galvano F. Coffee, tea, caffeine and risk of depression: a systematic review and dose-response meta-analysis of observational studies. Mol. Nutr. Food Res. 2016;60:223–234. doi: 10.1002/mnfr.201500620. [DOI] [PubMed] [Google Scholar]
- Gurillo P., Jauhar S., Murray R.M., MacCabe J.H. Does tobacco use cause psychosis? Systematic review and meta-analysis. Lancet Psychiatr. 2015;2:718–725. doi: 10.1016/S2215-0366(15)00152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamadeh R.R., Ansari A.A., Jahrami H., Offi A.A. Cigarette and waterpipe smoking among adult patients with severe and persistent mental illness in Bahrain: a comparison with the National Non-communicable Diseases Risk Factors Survey. BMC Res. Notes. 2016;9 doi: 10.1186/s13104-016-1894-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helou K., El Helou N., Mahfouz M., Mahfouz Y., Salameh P., Harmouche-Karaki M. Validity and reliability of an adapted Arabic version of the long international physical activity questionnaire. BMC Publ. Health. 2017;18:49. doi: 10.1186/s12889-017-4599-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahrami H., Faris M.A.-I., Ghazzawi H.A., Saif Z., Habib L., Shivappa N., Hébert J.R. Increased dietary inflammatory index is associated with schizophrenia: results of a case–control study from Bahrain. Nutrients. 2019;11 doi: 10.3390/nu11081867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahrami H., Saif Z., Qaheri S., Asad A., Panchasharm G. The disability profile of individuals with schizophrenia in Bahrain using the life skills profile 39. Arab J. Psychiatr. 2014;25:190–200. [Google Scholar]
- Jahrami H.A., Faris M.A.-I.E., Saif Z.Q., Hammad L.H. Assessing dietary and lifestyle risk factors and their associations with disease comorbidities among patients with schizophrenia: a case–control study from Bahrain. Asian J. Psychiatr. 2017;28:115–123. doi: 10.1016/j.ajp.2017.03.036. [DOI] [PubMed] [Google Scholar]
- Kang H.-J., Kim S.-Y., Bae K.-Y., Kim S.-W., Shin I.-S., Yoon J.-S., Kim J.-M. Comorbidity of depression with physical disorders: research and clinical implications. Chonnam Med. J. 2015;51:8–18. doi: 10.4068/cmj.2015.51.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler C.A., Freitas T.H., Maes M. d, De Andrade N.Q., Liu C.S., Fernandes B.S., Stubbs B., Solmi M., Veronese N., Herrmann N. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr. Scand. 2017;135:373–387. doi: 10.1111/acps.12698. [DOI] [PubMed] [Google Scholar]
- Li J., Zou B., Yeo Y.H., Feng Y., Xie X., Lee D.H., Fujii H., Wu Y., Kam L.Y., Ji F., Li X., Chien N., Wei M., Ogawa E., Zhao C., Wu X., Stave C.D., Henry L., Barnett S., Takahashi H., Furusyo N., Eguchi Y., Hsu Y.-C., Lee T.-Y., Ren W., Qin C., Jun D.W., Toyoda H., Wong V.W.-S., Cheung R., Zhu Q., Nguyen M.H. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999-2019: a systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2019;4:389–398. doi: 10.1016/S2468-1253(19)30039-1. [DOI] [PubMed] [Google Scholar]
- Li Y., Lv M.-R., Wei Y.-J., Sun L., Zhang J.-X., Zhang H.-G., Li B. Dietary patterns and depression risk: a meta-analysis. Psychiatr. Res. 2017;253:373–382. doi: 10.1016/j.psychres.2017.04.020. [DOI] [PubMed] [Google Scholar]
- Lim G.Y., Tam W.W., Lu Y., Ho C.S., Zhang M.W., Ho R.C. Prevalence of depression in the community from 30 countries between 1994 and 2014. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-21243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas M., Chocano-Bedoya P., Schulze M.B., Shulze M.B., Mirzaei F., O’Reilly É.J., Okereke O.I., Hu F.B., Willett W.C., Ascherio A. Inflammatory dietary pattern and risk of depression among women. Brain Behav. Immun. 2014;36:46–53. doi: 10.1016/j.bbi.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell A.J., Vancampfort D., Hert M.D., Stubbs B. Do people with mental illness receive adequate smoking cessation advice? A systematic review and meta-analysis. Gen. Hosp. Psychiatr. 2015;37:14–23. doi: 10.1016/j.genhosppsych.2014.11.006. [DOI] [PubMed] [Google Scholar]
- Moieni M., Irwin M.R., Jevtic I., Olmstead R., Breen E.C., Eisenberger N.I. Sex differences in depressive and socioemotional responses to an inflammatory challenge: implications for sex differences in depression. Neuropsychopharmacol. Off. Publ. Am. Coll. 2015;40:1709–1716. doi: 10.1038/npp.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulugeta A., Zhou A., Power C., Hyppönen E. Obesity and depressive symptoms in mid-life: a population-based cohort study. BMC Psychiatr. 2018;18:297. doi: 10.1186/s12888-018-1877-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naja S., Al-Kubaisi N., Chehab M., Al-Dahshan A., Abuhashem N., Bougmiza I. Psychometric properties of the Arabic version of EPDS and BDI-II as a screening tool for antenatal depression: evidence from Qatar. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2019-030365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips C.M., Shivappa N., Hébert J.R., Perry I.J. Dietary inflammatory index and mental health: a cross-sectional analysis of the relationship with depressive symptoms, anxiety and well-being in adults. Clin. Nutr. Edinb. Scotl. 2018;37:1485–1491. doi: 10.1016/j.clnu.2017.08.029. [DOI] [PubMed] [Google Scholar]
- Poole L., Dickens C., Steptoe A. The puzzle of depression and acute coronary syndrome: reviewing the role of acute inflammation. J. Psychosom. Res. 2011;71:61–68. doi: 10.1016/j.jpsychores.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich M. Depression and cancer: recent data on clinical issues, research challenges and treatment approaches. Curr. Opin. Oncol. 2008;20:353–359. doi: 10.1097/CCO.0b013e3282fc734b. [DOI] [PubMed] [Google Scholar]
- Sánchez-Villegas A., Ruíz-Canela M., de la Fuente-Arrillaga C., Gea A., Shivappa N., Hébert J.R., Martínez-González M.A. Dietary inflammatory index, cardiometabolic conditions and depression in the Seguimiento Universidad de Navarra cohort study. Br. J. Nutr. 2015;114:1471–1479. doi: 10.1017/S0007114515003074. [DOI] [PubMed] [Google Scholar]
- Shivappa N., Hébert J.R., Veronese N., Caruso M.G., Notarnicola M., Maggi S., Stubbs B., Firth J., Fornaro M., Solmi M. The relationship between the dietary inflammatory index (DII®) and incident depressive symptoms: a longitudinal cohort study. J. Affect. Disord. 2018;235:39–44. doi: 10.1016/j.jad.2018.04.014. [DOI] [PubMed] [Google Scholar]
- Stubbs B., Aluko Y., Myint P.K., Smith T.O. Prevalence of depressive symptoms and anxiety in osteoarthritis: a systematic review and meta-analysis. Age Ageing. 2016;45:228–235. doi: 10.1093/ageing/afw001. [DOI] [PubMed] [Google Scholar]
- Stubbs B., Vancampfort D., Firth J., Schuch F.B., Hallgren M., Smith L., Gardner B., Kahl K.G., Veronese N., Solmi M., Carvalho A.F., Koyanagi A. Relationship between sedentary behavior and depression: a mediation analysis of influential factors across the lifespan among 42,469 people in low- and middle-income countries. J. Affect. Disord. 2018;229:231–238. doi: 10.1016/j.jad.2017.12.104. [DOI] [PubMed] [Google Scholar]
- Teasdale S.B., Ward P.B., Rosenbaum S., Samaras K., Stubbs B. Solving a weighty problem: systematic review and meta-analysis of nutrition interventions in severe mental illness. Br. J. Psychiatry. 2017;210:110–118. doi: 10.1192/bjp.bp.115.177139. [DOI] [PubMed] [Google Scholar]
- Trafford A.M., Parisi R., Kontopantelis E., Griffiths C.E.M., Ashcroft D.M. Association of psoriasis with the risk of developing or dying of cancer: a systematic review and meta-analysis. JAMA Dermatol. 2019 doi: 10.1001/jamadermatol.2019.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vancampfort D., Correll C.U., Galling B., Probst M., De Hert M., Ward P.B., Rosenbaum S., Gaughran F., Lally J., Stubbs B. Diabetes mellitus in people with schizophrenia, bipolar disorder and major depressive disorder: a systematic review and large scale meta-analysis. World Psychiatry off. J. World Psychiatr. Assoc. WPA. 2016;15:166–174. doi: 10.1002/wps.20309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vancampfort D., Firth J., Schuch F., Rosenbaum S., De Hert M., Mugisha J., Probst M., Stubbs B. Physical activity and sedentary behavior in people with bipolar disorder: a systematic review and meta-analysis. J. Affect. Disord. 2016;201:145–152. doi: 10.1016/j.jad.2016.05.020. [DOI] [PubMed] [Google Scholar]
- Vancampfort D., Mitchell A.J., De Hert M., Sienaert P., Probst M., Buys R., Stubbs B. Type 2 diabetes in patients with major depressive disorder: a meta-analysis of prevalence estimates and predictors. Depress. Anxiety. 2015;32:763–773. doi: 10.1002/da.22387. [DOI] [PubMed] [Google Scholar]
- Vancampfort D., Stubbs B., Mitchell A.J., De Hert M., Wampers M., Ward P.B., Rosenbaum S., Correll C.U. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta-analysis. World Psychiatr. 2015;14:339–347. doi: 10.1002/wps.20252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vancampfort D., Stubbs B., Mugisha J., Firth J., Schuch F.B., Koyanagi A. Correlates of sedentary behavior in 2,375 people with depression from 6 low- and middle-income countries. J. Affect. Disord. 2018;234:97–104. doi: 10.1016/j.jad.2018.02.088. [DOI] [PubMed] [Google Scholar]
- Walker E.R., McGee R.E., Druss B.G. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatr. 2015;72:334–341. doi: 10.1001/jamapsychiatry.2014.2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Shen X., Wu Y., Zhang D. Coffee and caffeine consumption and depression: a meta-analysis of observational studies. Aust. N. Z. J. Psychiatr. 2016;50:228–242. doi: 10.1177/0004867415603131. [DOI] [PubMed] [Google Scholar]
- WHO EMRO WWW Document. 2020. http://www.emro.who.int/index.html
- Wirth M.D., Shivappa N., Burch J.B., Hurley T.G., Hébert J.R. The dietary inflammatory index, shift work, and depression: results from NHANES. Health Psychol. Off. J. Div. Health Psychol. Am. Psychol. Assoc. 2017;36:760–769. doi: 10.1037/hea0000514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolniczak I., Cáceres-DelAguila J.A., Maguiña J.L., Bernabe-Ortiz A. Fruits and vegetables consumption and depressive symptoms: a population-based study in Peru. PloS One. 2017;12 doi: 10.1371/journal.pone.0186379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Bank WWW Document. 2020. https://data.worldbank.org/country/bahrain
- World Health Organization . World Health Organization; Geneva: 2005. International Classification of Diseases (ICD) [Google Scholar]
- Yousofpour M., Kamalinejad M., Esfahani M.M., Shams J., Tehrani H.H., Bahrami M. Role of heart and its diseases in the etiology of depression according to avicenna’s point of view and its comparison with views of classic medicine. Int. J. Prev. Med. 2015;6:49. doi: 10.4103/2008-7802.158178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zunszain P.A., Hepgul N., Pariante C.M. Inflammation and depression. Curr. Top. Behav. Neurosci. 2013;14:135–151. doi: 10.1007/7854_2012_211. [DOI] [PubMed] [Google Scholar]