Abstract
The present study reports mosquito larvicidal potential of green synthesized silver nanoparticles by using Annona glabra leaves (An-AgNPs). Synthesized An-AgNPs were characterized by Ultraviolet-Visible spectroscopy (UV-VIS), Scanning Electron Microscopy (SEM), Dynamic Light Scattering (DLS) technique and Fourier transform infrared spectroscopy (FTIR). Colur change from pale yellow to brick red of the plant extract and AgNO3 solution indicated the formation of An-AgNPs initially. Surface Plasmon Resonance (SPR) band at 435 nm in the UV-Vis confirmed the formation of An-AgNPs. SEM images showed that An-AgNPs were spherical in shape. FTIR proved that An-AgNPs were functionalized with biomolecules in A. glabra leaves. Based on DLS analysis the average size range of synthesized An-AgNPs was determine to be 10–100 nm and 100–1000 nm.
Third instar larvae of dengue vector mosquitoes, Aedes aegypti and Aedes albopictus were subjected to larvicidal bioassays in a range of concentrations of An-AgNPs and A. glabra crude aqueous leaf extract (2–10 mg/L). An-AgNPs exhibited very high larvicidal activity against dengue vector mosquito larvae; LC50 value for Ae. aegypti at 24 h exposure to An-AgNPs (Plant extract: AgNO3 1 : 10) 5.29 mg/L; An-AgNPs (Plant extract: AgNO3 2 : 10) 2.43 mg/L while LC50 value for Ae. albopictus at 24 h exposure to An-AgNPs (Plant extract: AgNO31:10) 3.02 mg/L; An-AgNPs (Plant extract: AgNO3 2:10) 2.51 mg/L. LC50 values obtained for A. glabra leaf extract tested against Ae. aegypti and Ae. albopictus are 5.94 mg/L and 5.00 mg/L respectively at 24-hour exposure. This study further revealed that Ae. albopictus is more susceptible than to Ae. aegypti to a given concentration of An-AgNPs and to crude aqueous leaf extract of A. glabra. Larvicidal effect of An-AgNPs is superior to the crude aqueous leaf extract of A. glabra. An-AgNPs is a potent larvicide for dengue vector control.
Keywords: Chemistry, Environmental science, Annona glabra, Aedes aegypti, Aedes albopictus, Green synthesis, Silver nanoparticle, Larvicidal activity
Chemistry, Environmental science; Annona glabra; Aedes aegypti; Aedes albopictus; Green synthesis; Silver nanoparticle; Larvicidal activity.
1. Introduction
Aedes aegypti and Aedes albopictus are the two mosquito vectors of important arboviruses of the two genera of Flavivirus and Togavirus globally. Ae. aegypti is the main competent vector of flaviviruses such as ZIKA, dengue, chikungunya and yellow fever virus. Ae. albopictus is a vector for Flaviviruses such as yellow fever virus, Zika virus, dengue virus, Japanese encephalitis virus, and West Nile virus and Togaviruses such as Eastern equine virus and ross river virus (Paupy et al., 2009).
With the exception of yellow fever, for which an efficient vaccine has been available since the 1940s (Frierson, 2010), no vaccine is currently commercially available against the viral diseases transmitted by Ae. aegypti and Ae. albopictus. Therefore, the prevention of these diseases is mainly achieved through mosquito population control (Bisset et al., 2006; WHO, 2009).
Larvicides are among the main tools in mosquito control programmes. The most widely used larvicides are organophosphates such as temephos, methoprene, growth inhibitors, and bacterial insecticides such as Bacillus thuringiensis israelensis (Bti) and Bacillus sphaericus (Bisset et al., 2006; De Silva and Mendes, 2007; Poopathi and Abidha, 2010; Anupam et al., 2012). Larvicides are applied to either natural or artificial bodies of water, as a result their effect to beneficial and other nontarget organisms, including humans must be harmless (Arjunan et al., 2012; Allgeier et al., 2018).
The persistent and in some cases indiscriminate use of synthetic chemical insecticides has resulted in a reduction of their efficacy due to the dramatic emergence of resistant insect populations during the last decades (Govindarajan et al., 2005). Increasing cost of novel insecticides and annual exportation expenditures, effect on non-target populations particularly on mammals, high toxicity to mammals, entering to food chains and food webs, bioaccumulation in non-target organisms’ bodies, nonbiodegradable nature, ecological imbalance, the emergence of refractory vector behaviour and environmental pollution are occurred due to synthetic chemical insecticides (Anupam et al., 2012). The solution for these arising problems relies on searching for new and effective compounds that are easily degradable, environmental friendly, do not have any adverse effect on non-target populations, easily available and safe products at low cost.
Using botanical extracts is a sound option to avoid the adverse effects of synthetic chemical insecticides. Abd Kadir et al. (2013) reviewed the potential anti-dengue medicinal plants reported from 69 studies from 1997 to 2012. Plant derived insecticides usually contain a combination of several chemical compounds, unlike conventional insecticides which is mainly based on one specific active agent. These botanicals work synergistically and target different biological processes thus reducing the resistance development in their targets. An interesting advancement for the solely plant based insecticides is the development of plant-mediated nano based products combining the insecticidal activity of the botanicals and enhanced efficiency at the nano scale (1–100 nm) due to the high surface area to volume ratio of the nanoparticles. Combinatorial effect of these characteristics have enabled to achieve their insecticide efficacy at very low concentrations (≤30 mg/L) (Benelli et al., 2017). NPs can easily penetrate across the cell membrane of a living organism, due to their minute size thus avoid defense mechanisms. Afterwards, NPs migrate into the cell and reach organelles such as mitochondria, modifying the cell metabolism and leading to cell death. Therefore, NPs could be toxic to both vertebrates and invertebrates.
Recently, green fabrication of silver nanoparticles have acquired considerable attention owing to their mosquito larvicidal activity towards medically challenged pathogens and dreadful vectors (Priya and Santhi, 2014; Soni and Prakash, 2014; Benelli et al., 2017; Madanagopal et al., 2017; Morejón et al., 2018).
A comprehensive review on plant-mediated biosynthesis of nanoparticles as an emerging tool against mosquitoes has been published (Benelli, 2016). Majority of the plant-fabricated mosquitocidal nanoparticles reported to date are based on silver (Benelli, 2016). In the present study we attempt to use Annona glabra leaf extract to fabricate silver nanoparticles (An-AgNPs). Annona glabra (Pond apple) (Family Annonaceae) prefers wetter tropical and sub tropical habitats. Annona glabra is native for North, South and Central America and West Africa. It is regarded as an invasive weed in Australia due to its potential for spread, and negative impacts on environment and economy. Pond apple is also a problem in Sri Lanka and Fiji. Annona glabra is an aggressive and very hardy tree that forms dense thickets (Austin, 2004).
A. glabra is used in traditional medicine against several human ailments and disorders such as constipation, fever, ulcers, and tumour, including cancer because these plants consist with cytotoxic, antitumour, antifungal, antiparasitic, antibacterial, antispasmodic, antimicrobial, anticancer, antioxidant, and hepatoprotective repellent properties (Cave et al., 1997; Biba et al., 2014).
Previous studies on insecticidal properties of Annona glabra leaf extracts against Ae. albopictus and Ae. aegypti, the two dengue vector mosquito species in Sri Lanka showed promising results (Amarasinghe and Ranasinghe, 2017). However, no studies have been reported on use of A. glabra mediated AgNPs to control vector mosquiotoes in Sri Lankan condition. The present study attempts to enhance the bioefficacy of A. glabra leaf extract by green fabricating AgNPs. To the authors knowledge this is the first study on the larvicidal efficacy of An-AgNPs against Ae. albopictus and Ae. Aegypti.
2. Materials and methods
2.1. Plant material collection
Leaves of Annona glabra plant were collected in December 2018, from marshland in Hunupitiya (N 06° 58.904/, E 079° 54.281/), Kelaniya and kept in polythene bags and brought to the laboratory. Authentication of the plant was done with the help of the herbarium collection at the Department of Plant and Molecular Biology, Faculty of Science, University of Kelaniya.
2.2. Preparation of aqueous crude extract of Annona glabra leaves
Two hundred and fifty grams of fresh leaves were washed well with running tap water, rinsed with deionized water and decanted. The leaves were air dried and crushed well by using a pestle and mortar and placed in a conical flask (1000 mL) and 800 mL of deionized water was added. This was kept over night and the residue was filtered and discarded. Filtrate was concentrated under reduced pressure in a rotory evaporator at 70 °C to obtain the crude of aqueous leaf extract. Then the crude was freeze dried. Crude extract of 0.1 g was dissolved in 1000 mL of deionized water and 100 mg/L stock solution was prepared. The stock solution was used for the preparation of a concentration series of aqueous crude extract for bioassays.
2.3. Green synthesis of silver nanoparticles using Annona glabra leaf extract (An-AgNPs)
Fresh leaves weighing 160 g were washed well with running tap water, rinsed with deionized water and decanted. The leaves were crushed using a pestle and mortar and placed in a conical flask (1000 mL). Eight hundred mL of deionized water was added to the flask and covered with an aluminium foil. Then the sample was heated to 70 °C on a hot plate and left for 1 h with a magnetic stirrer to obtain the stock solution (200 g/L) of aqueous leaf extract. Eight hundred mL of the resultant leaf extract was filtered using a Whatman No.1 filter paper. AgNO3 stock solution (1 g/L) was prepared by dissolving 1 g of AgNO3 flakes (Sigma-Aldrich) in 1 L deionized water.
Annona glabra leaf extract and AgNO3 stock solution were mixed to the ratios of leaf extract: AgNO3 1 : 10 and 2 : 10 v/v to obtain two final products. This was performed by mixing 10 mL and 20 mL respectively of Annona glabra aqueous leaf extract separately with 100 mL of AgNO3 stock solution. Two mixtures were incubated at room temperature (27 °C ± 1) for 3 h with continuous stirring on a stirrer mixer until the solution become brick red color.
Each sample was then centrifuged at 5000 rpm for 20 min using a centrifuge machine (HERMLEZ 206 A) and the supernatant was decanted. Five mL deionized water was added to the precipitate and centrifuged again at 5000 rpm for 20 min. This was repeated one more time to wash the NPs thoroughly. The final precipitate was dried in desiccator for two days and dried form was used for further confirmation.
2.4. Characterization of silver nanoparticles (AgNPs)
2.4.1. UV - vis spectroscopy
Formation of AgNPs was confirmed by observing the Surface Plasmon Resonance (SPR) band from 200 to 1000 nm using UV-Vis spectrophotometer (Thermo Scientific, 1800) at a resolution of 5 nm at room temperature (27 °C ± 1) at the Department of Chemistry, Faculty of Science, University of Kelaniya.
2.4.2. SEM analysis
The facility of the Scanning Electron Microscope (SEM) (EVOLS) was obtained to determine the shape of developed nanoparticles at the Faculty of Science, University of Peradeniya. Samples of AgNPs were deposited in dried form on a double conductive tape which was stick on a sample holder at room temperature (27 °C ± 1). A thin layer of gold-platinum was coated to make samples conductive. Then the sample was imaged at 80 kV operation voltage.
2.4.3. Particle size measurements
Particle size was determined by dynamic light scattering technique (DLS) (CILAS NANO DS) at the Faculty of Science, University of Peradeniya, Sri Lanka
2.4.4. Fourier transformed infrared (FTIR) spectrum
FTIR spectrum of the sample was recorded by fourier transformed infrared spectrophotometer (Perkin Elmer) at the Department of Chemistry, University of Kelaniya, Sri Lanka. The FTIR spectrum was recorded from 4000 to 750 cm−1 by placing the dried AgNP powder on the crystal.
2.5. Bioassay on larvicidal activity
Ae. aegypti and Ae. albopictus mosquito larvae were evaluated with the World Health Organization (2005) standard protocols.
2.5.1. Larvicidal activity of Annona glabra AgNano (An-AgNPs)
Healthy two day old third instar larvae of Ae. aegypti were transferred in batches of 20 in 50 mL of water into glass beakers of 150 mL (n = 24). The total volume of water in each beaker was increased to 90.0 mL by adding deionized water. 0.1 g of green synthesized AgNPs (Plant extract: AgNO3; 1:10) compound was dissolved by sonication in 1000 mL of deionized water to produce stock solutions of AgNPs (Plant extract: AgNO3; 1:10). Concentrations of AgNPs (Plant extract: AgNO3; 1:10) 0.002 g/L, 0.004 g/L, 0.006 g/L, 0.008 g/L and 0.01 g/L were prepared by micropipetting (Khader et al., 2017; Khader and Ramesh, 2018). One concentration was prepared by randomly selecting four beakers that each including 20 larvae and adding the required volume of AgNPs (Plant extract: AgNO3; 1:10) solution into each beaker based on C1V1 = C2V2 equation. Then each solution was completed up to 100 mL with distilled water. This was continued for all the concentrations. A control treatment was run with 100 mL of deionized water. A fully randomized design was used (6✕4). The number of dead mosquito larvae was counted after 24 and 48 h of exposure and the percentage mortality was calculated. This was continued for third instar larvae of Ae. albopictus. Same procedure was done for AgNPs (Plant extract: AgNO3; 2:10).
2.5.2. Larvicidal activity of Annona glabra aqueous crude extract
Healthy third instar larvae Ae. aegypti were transferred in batches of 20 into glass beakers of 150 mL containing 75 mL of deionized water (n = 24). Concentrations of crude extract 2 mg/L, 4 mg/L, 6 mg/L, 8 mg/L and 10 mg/L were prepared One concentration was prepared by randomly selecting four beakers that each including 20 larvae and the addition of the required volume of crude extract stock solution into each beaker based on C1V1 = C2V2 equation. Then each solution was top up to 100 mL with distilled water. This was continued for all the concentrations. A control treatment was run with 100 mL of deionized water. A fully randomized design was used (6✕4). The number of dead mosquito larvae was counted after 24 and 48 h of exposure and the percentage mortality was calculated. This was continued for third instar larvae of Ae. albopictus.
In both bioassays, moribund larvae were counted and added to dead larvae for calculating percentage mortality. Dead larvae are those that did not induce to move when they were probed with a needle in the siphon or the cervical region. Moribund larvae were those incapable of rising to the surface or not showing the characteristic diving reaction when the water was disturbed (WHO, 2005).
2.6. Statistical analysis
Anderson – Darling test was used to determine whether the mean percentage of mortality data are normally distributed. P > 0.05 was obtained for all tests. Probit Analysis was used to determine the LC50 for the AgNPs after 24 h and 48 h of exposure. One-Way Analysis of Variance (ANOVA) followed by Tukey's pairwise test was conducted to test if there was any significant difference among Arcsine transformed values of mean percentage mortality of mosquito larvae in each concentrations using MINITAB 14.
3. Results
3.1. Characterization of AgNPs
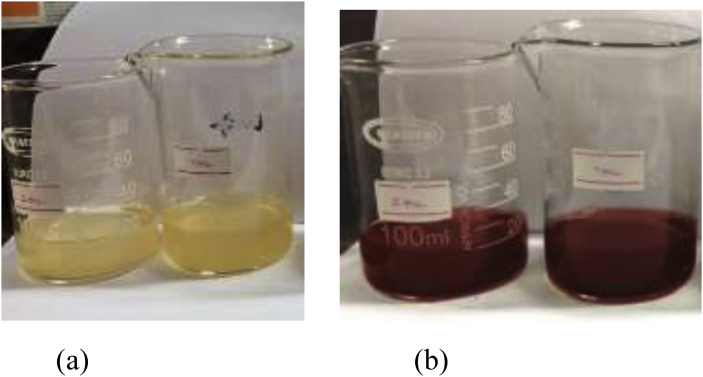
Three hours of incubation of the mixture, Annona glabra leaf extract and AgNO3 stock solution, to the ratios of leaf extract: AgNO3 1: 10 and 2: 10 v/v gave brick colour in the solution mixture.
Initial color and color changes after 3 h of incubation time of samples (Figure 1 a & b).
Figure 1.
(a) Initial color and (b) color changes after 3 h of incubation time of samples containing Plant extract: AgNO3 1:10 (left) and Plant extract: AgNO3 2:10 (Right).
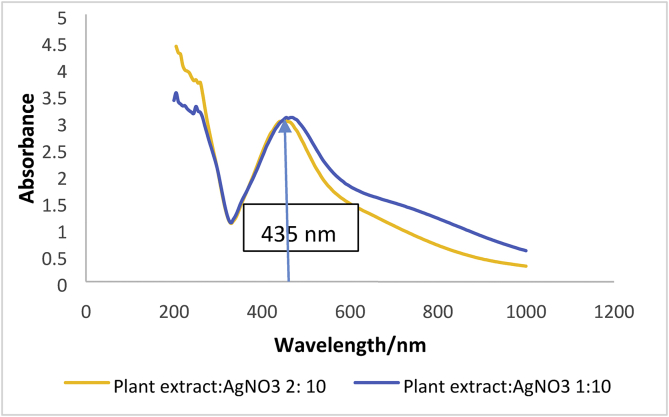
The formation of AgNPs was established by observing the Surface Plasmon Resonance (SPR) band at 435 nm using a UV-Vis spectrophotometer (Figure 2).
Figure 2.
UV–vis spectrum shows a characteristics peak between 400 nm–500 nm.
Image obtained using the Scanning Electron Microscope (SEM) (EVOLS) to determine the shape of developed nanoparticles is shown in Figure 3. This shows the spherical shape of the particles confirming that they are nano particles.
Figure 3.
SEM (Scanning electron microscope) image showing the spherical morphology and agglomeration of synthesized AgNPs.
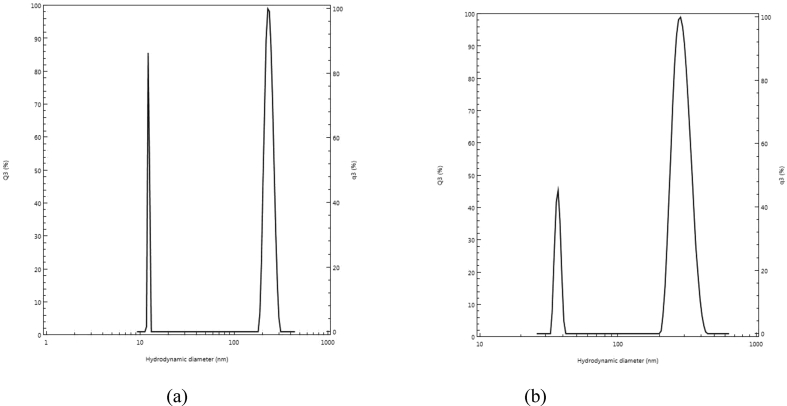
Particle size distribution analysis done using dynamic light scattering technique (DLS) (CILAS NANO DS) resulted in particle size ranges between 10-100 nm and 100–1000 nm (Figure 4 a & b) This confirms the products are nanoparticles.
Figure 4.
The graph of particle size distribution of AgNPs: (a) Plant extract: AgNO3 2:10; (b) Plant extract: AgNO3 1:10.
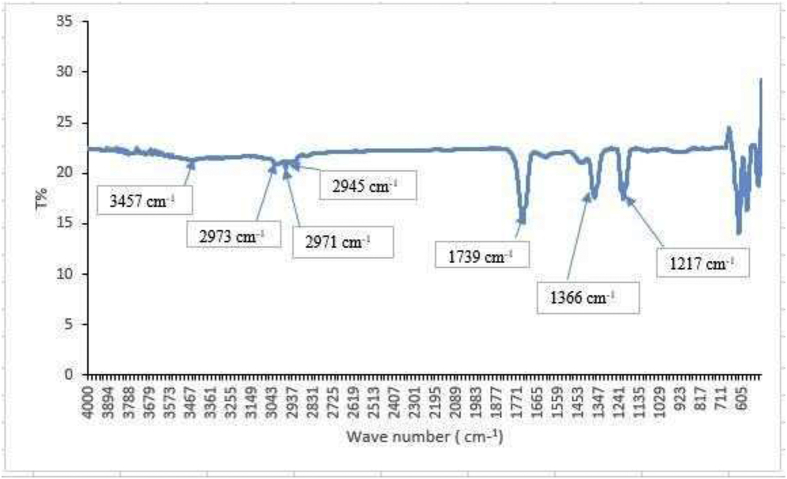
FT-IR spectrum of the sample showed three prominent peaks at 1739 cm−1, 1366 cm−1 and 1217 cm−1 indicating the characteristic functional groups of the phytochemicals (Figure 5). This confirms the AgNPs have uncapped the phytochemicals of A. glabra and the formation of An-AgNPs.
Figure 5.
FT-IR spectrum shows different functional peaks.
3.2. Larvicidal activity of An-AgNPs and determination of the 24 h and 48 h LC50 for AgNPs
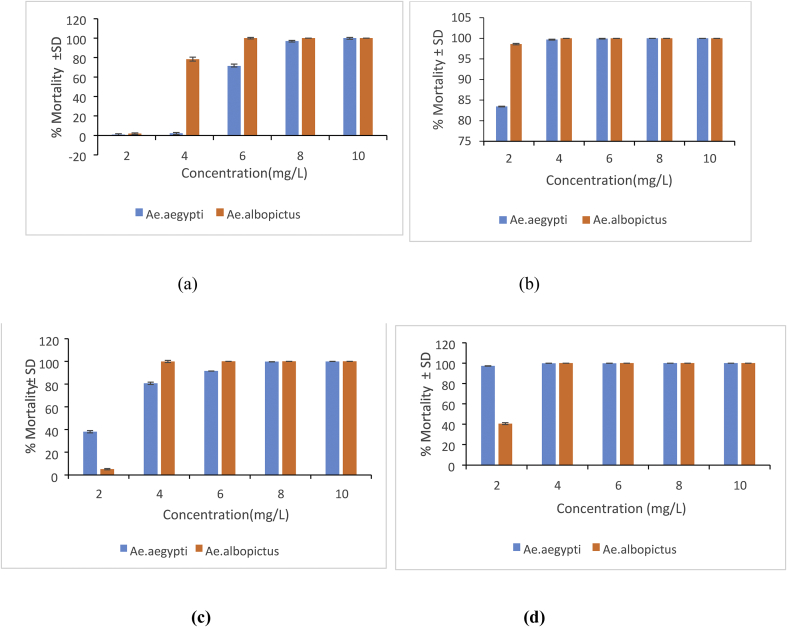
The percentage mortality of Ae. aegypti and Ae. albopictus mosquito larvae exposed to varying concentrations of An-AgNPs (ratio 1:10 and 2:10) for 24 h and 48 h are shown in Figure 6 (a,b,c and d). They indicate that An-AgNPs demonstrated potential larvicidal activity even at lower concentrations of the treatment and larval mortalities increased with the increasing concentration of AgNPs and increasing exposure time from 24 h to 48 h. The highest mortalities were found in larvae of Ae. albopictus (LC50 = 2.51 mg/L) and larvae of Ae. aegypti (LC50 = 2.43 mg/L) exposued to An-AgNP (plant extract: AgNO3 2:10) (Table 1). The mortality was increased comparatively when their exposure time increased from 24 h to 48 h; Ae. albopictus (LC50 = 2.10 mg/L);Ae. aegypti (LC50 = 1.17 mg/L) (Table 1).
Figure 6.
Percentage mortality ±SD of green synthesized AgNPs on Ae. aegypti and Ae. albopictus at different concentrations: (a) Plant extract: AgNO3 1:10 after 24 h exposure. (b) Plant extract: AgNO3 1:10 after 48 h exposure (c) Plant extract: AgNO3 2:10 after 24 h exposure (d) Plant extract: AgNO3 2:10 after 48 h exposure (There was no % mortality in 0 mg/L control treatment).
Table 1.
Larvicidal activity of green synthesized AgNPs against Ae. aegypti and Ae. albopictus.
| Product | Mosquito species | Exposure period (hours) | LC50 (mg/L) | 95 % confidence interval for LC50 |
|
|---|---|---|---|---|---|
| LCL (mg/L) | UCL (mg/L) | ||||
| (Plant extract: AgNO3 1: 10) | Ae. aegypti | 24 | 5.29 | 5.08 | 5.49 |
| 48 | 1.51 | 1.34 | 1.65 | ||
| Ae. albopictus | 24 | 3.02 | 2.86 | 3.17 | |
| 48 | 1.14 | 1.01 | 1.33 | ||
| (Plant extract: AgNO3 2: 10) | Ae. aegypti | 24 | 2.43 | 2.19 | 2.45 |
| 48 | 1.17 | 1.01 | 1.36 | ||
| Ae. albopictus | 24 | 2.51 | 2.4 | 2.64 | |
| 48 | 2.10 | 2.01 | 2.18 | ||
LCL - Lower Confidence Limit UCL - Upper Confidence Limit.
3.3. Larvicidal activity of Annona glabra leaf extract and determination of the 24 h and 48 h LC50 for Annona glabra leaf extract
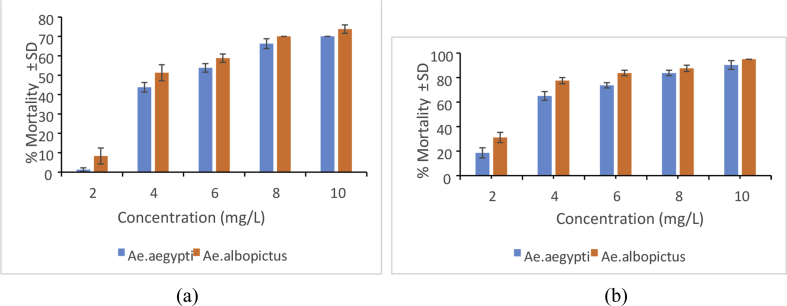
Mosquito larvicidal effect of aqueous crude extract of A. glabra after 24 h and 48 hore exposure is shown in Figure 7 a & b. There is a significant reduction of laraval mortality in crude extracts compared to that of An-AgNP treatment (F = 3.64; p = 0.014) at 24 h (F = 119.72; p ≤ 0.000) at 48 h exposure. The larval mortality was increased in increasing concentration with increased exposure time showing the LC50 values of 5.00402 mg/L for Ae. albopictus and 5.94555 mg/L for Ae. aegypti at 24 h exposure (Table 2; Figure 7a) whereas 2.73467 mg/L for Ae. albopictus and 3.5485 mg/L for Ae. aegypti at 48 h exposure (Table 2; Figure 7b). This result also indicates that LC50 values are always higher compared to the LC50 values of the larvae exposed to An-AgNP treatment and LC50 values obtained for Ae. aegypti are always higher than to those obtained for Ae. albopictus.
Figure 7.
Mean mortality percentage of Annona glabra leaf extract on Ae. aegypti and Ae. albopictus at different concentrations; (a) after 24 h and (b) after 48 h exposure.
Table 2.
Larvicidal activity of A. glabra leaf extract against Ae. aegypti and Ae. albopictus.
| Mosquito species | Exposure period (hours) | LC50 (mg/L) | 95 % confidence interval for LC50 |
|
|---|---|---|---|---|
| LCL (mg/L) | UCL (mg/L) | |||
| Ae. aegypti | 24 | 5.94555 | 5.33 | 6.5585 |
| 48 | 3.5485 | 3.06765 | 3.99527 | |
| Ae. albopictus | 24 | 5.00402 | 4.43068 | 5.55518 |
| 48 | 2.73467 | 2.31874 | 3.11563 | |
LCL - Lower Confidence Limit UCL - Upper Confidence Limit.
4. Discussion
Green approach consumes extracts from botanicals which act both as reducing and capping agents in nanoparticle synthesis (Landage and Wasif, 2012). Controlling vector mosquito larvae causing many diseases by using green synthesized AgNPs is an emerging technique (Priya and Santhi et al., 2014). The present study accomplished green synthesizing of AgNPs by using Annona glabra plant leaf extract for controlling Ae. Aegypti and Ae. albopictus as first attempt in Sri Lanka. A. glabra secondary metabolites and their synthetic derivatives in plants serve as a defense mechanism against insect attacks, provide an alternative sources as larvicides (Isman and Seffrin, 2014). Recent research has proved that the effectiveness of plant derived biologically important compounds, such as saponine, steroids, isoflavonoids, essential oils, alkaloids and tannins obtained from crude extracts of seeds, leaves, fruits, bark and twigs may act as larvicides, insecticides, repellents, antifeedants, moulting hormones, antimoulting hormones, oviposition deterrents, juvenile hormone mimics, growth inhibitors as well as attractants (Shad and Andrew, 2017).
In the current study, we focused on eco-friendly nano-synthetic approach to mosquitocidal AgNPs. Color changes, UV-VIS, FTIR spectroscopic measurements, SEM analysis, and particle size distribution analysis characterized biosynthesized An-AgNPs. Formation of AgNPs evidents from a remarkable change of solution color from pale to brown, reddish-brown and brick red (Madanagopal et al., 2017). The appearance of the SPR band at 435 nm in the UV-Vis absorption spectra confirmed the formation of An-AgNPs (Arjunan et al., 2012).
Distinct IR bands characteristics of O–H stretching (3457 cm−1), CH3/CH2 stretching (2945 - 2973 cm−1), C–N stretching (1217 cm−1) and C=O stretching (1739 cm−1) vibration were observed in the FT-IR spectrum of the An-AgNPs. These bands confirms the the presence of biomolecules on NPs as capping agents. Having these biomolecules such as flavones and reducing sugars in the plant extract make them to act as reducing agents in the synthesis of AgNPs.
The surface morphology of An-AgNPs was investigated using SEM and showed that most of the nanoparticles were roughly spherical in shape with smooth edges. Particle size distribution analysis in this study showed that the mixture of several sizes of nanoparticles was formed in both approaches. Particles of sizes between 1 and 100 nm are called nanoparticles (Elechiguerra et al., 2005). Although modern definitions say particulate dispersions or solid particles with a size range between 10 and 1000 nm are nanoparticles (Priya and Santhi, 2014). Particles of both 1–100 nm size range and 100–1000 nm size range resulted in two approaches of the present study. As the plant material concentration is increased; the size of nanoparticles is decreased because agglomeration is prevented by a high amount of available capping agents in the plant extract and this was evident from Figure 4 a,b.
In the present study, two approaches were tested to synthesize AgNPs using Annona glabra leaves (An-AgNPs); plant extract: AgNO3 1:10 mixture and plant extract: AgNO3 2:10 mixture. Both An-AgNPs products showed larvicidal activity to Ae. aegypti and Ae. albopictus mosquito larvae which is manifested by a high percentage of mortality in comparison to those in the control treatments. This reveals that the mortality of mosquito larvae is not due to the natural or external reasons, but instead, the mortality of mosquito larvae is due to the An-AgNPs in the solution. Present study indicates that the An-AgNPs (plant extract: AgNO3 2:10) are toxicity to Ae. albopictus and Ae. aegypti larvae (LC50 = 2.51 mg/L and LC50 = 2.43 mg/L respectively for 24 h exposure) superior to An-AgNPs (plant extract: AgNO3 1:10) (LC50 = 3.02 g/L; 5.29 mg/L respectively). When the exposure time is increased; comparatively larval mortality is also increased (Araj et al., 2015).
Biological synthesized stable silver nanoparticles using Annona squamosa leaf broth has been tested against Anopheles stephensi, Aedes aegypti, and Culex quinquefasciatus showing lethal effect on fourth instars larvae and decline the longevity of adults (days) in male and female mosquitoes (Arjunan et al., 2012). Green synthesized nanoparticles have been successfully used to reduce mosquito young instar populations in the field with moderate larvicidal effects (Benelli & Govindarajan, 2016; Benelli et al., 2017) and the maximum efficacy (60.18%) was observed with the synthesized silver nanoparticles against the larvae of Cx. quinquefasciatus (Mondal et al., 2014; Rajasekharreddy and Rani, 2014). Gnanadesigan et al. (2011) reported that AgNPs synthesized using Rhizophora mucronata (Family: Rhizophoraceae) leaf extract given 0.585 mg/L (LC50) and as 2.615 mg/L (LC90) values for Ae. aegypti while 0.891 mg/L (LC50) and 6.291 mg/L (LC90) values for Cx. quinquefasciatus. Arjunan et al. (2012) reported that AgNPs synthesized using Annona squamosa leaf broth resulted a higher mortality of fourth instar larvae of Ae. aegypti, Cx. quinquefasciatus and Anopheles stephensi (LC50 = 0.30, 0.41, and 2.12 ppm respectively) and has declined the longevity of adult male and female mosquitoes. Sareen et al. (2012) reported that the larvicidal efficacy of AgNPs synthesized from aqueous leaf extract of Hibiscus rosasinensis (Family: Malvaceae) to control the larvae of Aedes albopictus. Patil et al. (2012) synthesized AgNPs using Pergularia daemia plant latex to use against Ae. aegypti and An. stephensi. Hence, green synthesized Ag-NPs could be considered to be used as possible alternative over physical and chemical methods.
Food consumption development is retarded in the animal exposed to AgNPS that influence their death. The biotoxicity against mosquito young instars related to the ability of nanoparticles to penetrate through the exoskeleton (Benelli, 2016). In the intracellular space, nanoparticles can bind to sulfur from proteins or to phosphorus from DNA, leading to the rapid denaturation of organelles and enzymes leads to death of mosquito larvae and pupae. Subsequently, the decrease in membrane permeability and disturbance in proton motive force may cause loss of cellular function and cell death (Benelli, 2016; Subramaniam et al., 2015). Generally, the active toxic ingredients of plant extracts are secondary metabolites that are evolved to protect from herbivores. The mode of action of phytochemicals in the target insect bodies could be of several types (Koul, 2008). The insects that feed on these secondary metabolites potentially encountering toxic substances with relatively non-specific effects on a wide range of molecular targets. The targets are range from proteins such as receptors, enzymes, signaling molecules, ion-channels, and structural proteins; nucleic acids, bio membranes and other cellular components (Rattan, 2010).
In the case of the Family Annonaceae, the literature search indicated that only seven annonaceous plant species have been studied for their mosquito larvicidal properties with extracts from the genus Annona showing strong insecticidal activities. However, with about 150 plant species known from the genus Annona only 4 species; A. crassiflora, A. glabra, A. muricata, A. squamosa have been studied for mosquito larvicidal activities (Das et al., 2007). A. crassiflora showed larvicidal activity against Ae. aegypti (De Omena et al., 2007). A. squamosa have a larvicidal activity against Ae. albopictus and Culex quinquefasciatus (Das et al., 2007). Seed extract of A. muricata showed larvicidal activity against Ae. aegypti (Promisiri et al., 2006). De Omena et al. (2007) reported the larvicidal efficacy of ethanol stem bark extract of A. glabra against Ae. aegypti. As reported by Amarasinghe and Ranasinghe (2017), Annona glabra leaves showed a mosquito larvicidal effect. Present study revealed that the An-AgNPs (Plant extract: AgNO3 1:10) has enhanced significantly the efficacy of A. glabra showing LC50 values for Ae. albopictus and Ae. aegypti larvae as 3.02 mg/L and 5.29 mg/L respectively for 24 h exposure compared to the LC50 values for A. glabra crude leaf aqueous extract (Figure 6 a-d; Table 2). Comparable results were reported by Khader et al. (2017) from a study conducted on Annona squamosa crude extractand its biosynthesized AgNPs against different species of mosquito larvae. Among the nano products tested in this study; An-AgNPs (Plant extract: AgNO3 2:10) is more suitable than An-AgNPs (Plant extract: AgNO3 1:10) for its further development as a mosquito larvicide.
5. Conclusions
Present study achieved a successful synthesis of AgNPs using A. glabra aqueous plant extract (An- AgNPs). There is a strong larvicidal activity of An-AgNPs aqueous leaf extract against dengue vector mosquitoes, Ae. aegypti and Ae. albopictus. An-AgNPs synthesized to the ratio of 2:10 (plant extract: AgNO3) found to be more effective than that synthesized to the ratio 1:10. This further concludes that A. glabra leaf extract can be used as an effective capping agent as well as the reducing agent for the synthesis of silver nanoparticles as a larvicide.
Declarations
Author contribution statement
L.D. Amarasinghe, P.A.S.R. Wickramarachchi: Conceived and designed the experiments; Wrote the paper.
A.A.A.U. Aberathna: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
W. S. Sithara: Performed the experiments; Analyzed and interpreted the data.
C. R. De Silva: Analyzed and interpreted the data.
Funding statement
This work was supported by the University of Kelaniya Grant: RP/03/02/06/02/2019.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Abd Kadir S.L., Yaakob H., Mohamed Zulkifli R. Potential anti-dengue medicinal plants: a review. J. Nat. Med. 2013;67:677–689. doi: 10.1007/s11418-013-0767-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allgeier S., Kästel A., Brühl C.A. Adverse effects of mosquito control using Bacillus thuringiensis var. israelensis: reduced chironomid abundances in mesocosm, semi-field and field studies. Ecotoxicol. Environ. Saf. 2018;169:786–796. doi: 10.1016/j.ecoenv.2018.11.050. 2019 Mar, Epub 2018 Nov 29. [DOI] [PubMed] [Google Scholar]
- Amarasinghe L.D., Ranasinghe H.A.K. Faculty of Science, University of Kelaniya; 2017. Herbal Extracts and Extracellular Metabolites of Antagonistic Fungi as Larval Killing Agents of Dengue Vector Mosquitoes. Gloria Scientiam – Golden Jubilee Commemorative Volume; pp. 72–86. [Google Scholar]
- Anupam G., Chowdhury N., Chandra G. Plant extracts as potential mosquito larvicides. Indian J. Med. Res. 2012;135(5):581–598. [PMC free article] [PubMed] [Google Scholar]
- Araj S.E.A., Salem N.M., Ghabeish I.H., Awwad A.M. Toxicity of nanoparticles against Drosophila melanogaster (Diptera: drosophilidae) J. Nanomater. 2015;2015 [Google Scholar]
- Arjunan N.K., Murugan K., Rejeeth C., Madhiyazhagan P., Barnard D.R. Green synthesis of silver nanoparticles for the control of mosquito vectors of malaria, filariasis, and dengue. Vector Borne Zoonotic Dis. 2012;12(3):262–268. doi: 10.1089/vbz.2011.0661. [DOI] [PubMed] [Google Scholar]
- Austin D.F. Oaks, live and otherwise. Discovering Florida’s ethanobotany. The people and plant interaction series. The Palmetto. 2004;23(1):87. [Google Scholar]
- Benelli G. Plant-mediated biosynthesis of nanoparticles as an emerging tool against mosquitoes of medical and veterinary importance: a review. Parasitol. Res. 2016;115:23–34. doi: 10.1007/s00436-015-4800-9. [DOI] [PubMed] [Google Scholar]
- Benelli G., Govindarajan M. Green-synthesized mosquito oviposition attractants and ovicides: towards a nanoparticle-based “lure and kill” approach? J. Cluster Sci. 2016;28:287–308. [Google Scholar]
- Benelli G., Caselli A., Canale A. Nanoparticles for mosquito control: challenges and constraints. King Saud Univ. J. King Saud Univ. – Sci. 2017;29(4):424435. [Google Scholar]
- Biba V.S., Amily A., Sangeetha S., Remani P. Anticancer, antioxidant and antimicrobial activity of Annonaceae family. World J. Pharm. Pharmaceut. Sci. 2014;3:1595–1604. [Google Scholar]
- Bisset J., Rodríguez M., Fernández D. Selection of insensitive acetyl cholinesterase of Medical Entomology. 2006;43(6):1185–1189. [PubMed] [Google Scholar]
- Cave A., Figadere B., Laurens A., Cortes D. Acetogenins from Annonaceae. Prog. Chem. Org. Nat. Prod. 1997;70:81–288. doi: 10.1007/978-3-7091-6551-5_2. [DOI] [PubMed] [Google Scholar]
- Das N.G., Goswami D., Rabha B. Preliminary evaluation of mosquito larvicidal efficacy of plant extracts. J. Vector Borne Dis. 2007;(2):145–148. PubMed44. [PubMed] [Google Scholar]
- De Omena M.C., Navarro D.M., de Paula J.E., Luna J.S., Ferreira de Lima M.R., Sant'Ana A.E. Larvicidal activities against Aedes aegypti of some Brazilian medicinal plants. Bioresour. Technol. 2007;98(13):2549–2556. doi: 10.1016/j.biortech.2006.09.040. 2007 Sep, Epub 2006 Nov 28. [DOI] [PubMed] [Google Scholar]
- De Silva J., Mendes J. Susceptibility of Aedes aegypti (L) to the insect growth regulators diflubenzuron and methoprene in Uberlândia, State of Minas Gerais. Revista da Sociedade Brasileira de Medicina Troipcal. 2007;40(6):612–616. doi: 10.1590/s0037-86822007000600002. [DOI] [PubMed] [Google Scholar]
- Elechiguerra J.L., Burt J.L., Morones J.R., Camacho-Bragado A., Gao X., Lara H.H., Yacaman M.J. Interaction of silver nanoparticles with HIV-1. J. Nanobiotechnol. 2005;3:6. doi: 10.1186/1477-3155-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frierson J. The yellow fever vaccine: a history. Yale J. Biol. Med. 2010;83(2):77–85. [PMC free article] [PubMed] [Google Scholar]
- Gnanadesigan M., Anand M., Ravikumar S., Maruthupandy M., Vijayakumar V., Selvam S., Dhineshkumar M., Kumaraguru A.K. Biosynthesis of silver nanoparticles by using mangrove plant extract and their potential mosquito larvicidal property. Asian Pac. J. Trop. Med. 2011;4:799–803. doi: 10.1016/S1995-7645(11)60197-1. [DOI] [PubMed] [Google Scholar]
- Govindarajan M., Jebanesan A., Reetha D. Larvicidal effect of extracellular secondary metabolites of different fungi against the mosquito,Culex quinquefasciatus. Trop. Biomed. 2005;22(1):1–3. [PubMed] [Google Scholar]
- Isman M.B., Seffrin R. In: Natural Insecticides from the Annonaceae: A Unique Example for Developing Biopesticides. Singh D., editor. © Springer India; 2014. pp. 21–33. (Advances in Plant Biopesticides). [Google Scholar]
- Khader S.Z.A., Syed Zameer Ahmed Sidhra, Sathyan Jagadeeswari, Rafi Mahboob Mohamed, Venkatesh Kisore P., Ramesh Kishore. A comparative study on larvicidal potential of selected medicinal plants over green synthesized silver nano particles. Egypt. J. Basic Appl. Sci. 2018;5(1):54–62. [Google Scholar]
- Khader Syed Zameer Ahmed, Syed Zameer Ahmed Sidhra, Balasubramanian Senthil Kumar, kumarArunachalam Thanga, Kannappan Geetha, Mahboob Mohamed Rafi, Ponnusam Ponmurugan, Ramesh Kishore. Modulatory effect of dianthrone rich alcoholic flower extract of Cassia auriculata L. on experimental diabetes. Integr. Med. Res. 2017;6(2):131–140. doi: 10.1016/j.imr.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koul O. Phytochemicals and insect control: an antifeedant approach. Crit. Rev. Plant Sci. 2008;27(1):1–24. [Google Scholar]
- Landage S.M., Wasif A.I. Nanosilver – an effective antimicrobial agent for finishing of textiles. Int. J. Eng. Sci. Emerg. Technol. 2012;4(1):66–78. [Google Scholar]
- Madanagopal N., Lena M., Sumathi P., Sundaravadivelan C. Effect of phyto synthesized silver nanoparticles on developmental stages of malaria vector, Anopheles stephensi and dengue vector, Aedes aegypti. Egypt. J. Basic Appl. Sci. 2017;4:212–218. [Google Scholar]
- Mondal N.K., Chowdhury A., Dey U., Mukhopadhya P., Chatterjee S., Das K., Datta J.K. Green synthesis of silver nanoparticles and its application for mosquito control. Asian Pac. J. Trop. Dis. 2014;4:S204–S210. [Google Scholar]
- Morejon B., Pilaquinga F., Domenech F., Ganchla D., Debut A., Neira M. Larvicidal activity of silver nanoparticles synthesized using extracts of Ambrosia arborescens (Asteraceae) to control Aedes aegypti L. (Diptera: Culicidae) J. Nanotechnol. 2018:8. Article ID 6917938. [Google Scholar]
- Patil C.D.,, Borase H.P., Patil S.V., Salunkhe R.B., Salunke B.K. Larvicidal activity of silver nanoparticles synthesized using Pergularia daemia plant latex againstAedes aegypti and Anopheles stephensi and nontarget fish Poecillia reticulate. Parasitol. Res. 2012;111(2):555–562. doi: 10.1007/s00436-012-2867-0. [DOI] [PubMed] [Google Scholar]
- Paupy C., Delatte H., Bagny L., Corbel V., Fontenille D. Aedes albopictus, an arbovirus vector: from the darkness to the light. Microb. Infect. 2009;11:1177–1185. doi: 10.1016/j.micinf.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Poopathi S., Abidha S. Mosquitocidal bacterial toxins (Bacillus sphaericus and Bacillus thuringiensis serovar israelensis): mode of action, cytopathological effects and mechanism of resistance. J. Physiol. Pathophysiol. 2010;1(3):22–38. 2010. [Google Scholar]
- Priya S., Santhi S. A review on nanoparticles in mosquito control - a green revolution in future. Int. J. Res. Appl. Sci. Eng. Technol. 2014:378–387. [Google Scholar]
- Promisiri S., Naksathit A., Kruatrachue M., Thavara U. Evaluations of larvicidal activity of medicinal plant extracts to Aedesaegypti (Diptera: Culicidae) and other effects on a non target fish. Insect Sci. 2006;13:179–188. [Google Scholar]
- Rajasekharreddy, Rani Pathipati Usha. Biofabrication of Ag nanoparticles using Sterculia foetida L. seed extract and their toxic potential against mosquito vectors and, HeLa cancer cells. Mater. Sci. Eng. C. 2014;39:203–212. doi: 10.1016/j.msec.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Rattan R.S. Mechanism of action of insecticidal secondary metabolites of plant origin. Crop Protect. 2010;29:913–920. [Google Scholar]
- Sareen S.J., Pillai R.K., Chandramohanakumar N., Balagopalan M. Larvicidal potential of biologically synthesised silver nanoparticles against Aedes Albopictus. Res. J. Recent Sci. 2012;1:52–56. [Google Scholar]
- Shad A., Andrew J. Larvicidal efficacy of ethanolic extracts of Annona squamosal (Annonaceae) over the filarial vector, Culex quinquefasciatus Say (Culicidae) J. Entomol. Zool. Stud. 2017;5:373–377. [Google Scholar]
- Soni N., Prakash S. Microbial synthesis of spherical nanosilver and nanogold for mosquito control. Ann. Microbiol. 2014;64(3):1099–1111. [Google Scholar]
- Subramaniam J., Murugan K., Panneerselvam C., Kovendan K., Madhiyazhagan P., Mahesh Kumar P., Dinesh D., Chandramohan B., Suresh U., Nicoletti M., Higuchi A., Hwang J.S., Kumar S., Alarfaj A.A., Munusamy M.A., Messing R.H., Benelli G. Environ. Sci. Pollut. Res. 2015;22:20067. doi: 10.1007/s11356-015-5253-5. [DOI] [PubMed] [Google Scholar]
- WHO . WHO Publication; Geneva, Switzerland: 2005. Guidelines for Laboratory and Field Testing of Mosquito Larvicides; p. 41. [Google Scholar]
- WHO . WHO Publications; Geneva, Switzerland: 2009. Dengue Guidelines for Diagnosis, Treatment, Prevention and Control. [PubMed] [Google Scholar]