Abstract
This research focused on studying the effects of orally administered pressure-blanched white saffron on the antioxidative properties and lipid profiles of wistar rats. White saffron was blanched in autoclave for 2.5, 5, 7.5, and 10 min at 100, 105, 110, 115, and 120 °C, which are equivalent to 14.71, 17.53, 20.79, 24.54, and 28.81 psia, respectively. A total of 30 male wistar rats aged four weeks were fed with a standard diet (N), oxidized peanut oil diet + unblanched white saffron (A), oxidized peanut oil diet + blanched white saffron (B), oxidized peanut oil diet + pressure-blanched white saffron (C), and oxidized peanut oil diet + aquadest (NC), for two weeks after pre-treatment with the standard diet for a week. Invivo study showed treatment with pressure-blanched white saffron could significantly improve SOD, Vitamin E, and HDL levels compared to the negative control (NC); 686.44 U/g Hb, 10.87 μg/mL, and 94.17 mg/dL versus 405.37 U/g Hb, 7.44 μg/mL, and 43.47 mg/dL, respectively. Meanwhile, treatment with pressure-blanched white saffron could significantly reduce MDA, total cholesterol, LDL, and triglyceride levels in the blood compared to the negative control (NC); 1.98 mmol/L, 108.74 mg/dL, 40.99 mg/dL, and 78.06 mg/dL versus 8.54 mmol/L, 232.46 mg/dL, 149.17 mg/dL, and 172.61 mg/dL, respectively. The results showed that pressurized blanching could significantly increase antioxidant levels of white saffron, and its dried form could improve antioxidative properties and lipid profiles in vivo.
Keywords: Food science, White saffron, Pressurized blanching, Total phenols, Antioxidant activities, Lipid profiles
Food science; White saffron; Pressurized blanching; Total phenols; Antioxidant activities; Lipid profiles.
1. Introduction
Antioxidants are usually obtained from natural resources and synthetic materials. Those from synthetic materials are more effective, but less safe health-wise. Blanching as preparatory heating is conducted to deactivate enzymes that play a role in polyphenol degradation and to increase the antioxidant levels of agricultural products. At 100 °C, pressurized blanching on wheat could increase total wheat flour phenols (Cheng et al., 2006). Corn blanched in autoclave showed an increased total phenol level (Randhir et al., 2008). On black beans, pressurized blanching showed higher ORAC (Oxygen Radical Absorbance Capacity) value than unpressurized (Xu and Chang, 2008b). Likewise, steaming of lentils at 15 psi for 15 min could significantly improve the ORAC value due to Maillard reaction (Xu and Chang, 2008a).
Furthermore, pressurized tea extract, heated fruits, and heated vegetables showed an increased antioxidant activity (Manzocco et al., 2000; Nicoli et al., 1999). In addition, heated bilberry extract had higher antioxidant activity than its unheated form due to the degradation of glycosides to aglycons and sugars (Yue and Xu, 2008). According to Sadilova et al. (2006), anthocyanins (glycoside compounds) could hydrolyze into anthocyanidins under acidic conditions.
White saffron as a major source of antioxidants is very easy to cultivate and do not require special treatment. Interestingly, the rhizomes can be processed into different kinds of food products, such as instant powder, syrup, and wet and dried sweets. Pujimulyani et al. (2013) have studied the effects of blanching on the antioxidant activity and phenolic contents of white saffron. Blanching in 0.05% citric acid media at boiling temperature for 5 min could escalate total phenolics of white saffron from 1.01 ± 0.04 to 1.33 ± 0.15 mg/g dried extract, with a significant rise in catechin, epigallocatechin, and epigallocatechingallat (EGCG) levels. In addition, the epicatechin and gallocatechingallat levels were similar to those of the unblanched white saffron, while the gallic acid level of the blanched white saffron was slightly lower than that of the unblanched.
This research was focused on examining the effects of pressure-blanched white saffron on the antioxidative properties and lipid profiles of oxidized peanut oil-treated wistar rats. Initially, blanched white saffron with appropriate levels of total phenols, EGCG, 2,2,1-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging, and ferric reducing antioxidant power (FRAP) was chosen for in vivo research. Theoretically, it was assumed that administration of pressure-blanched white saffron to the oxidized peanut oil-treated wistar rats could improve their superoxide dismutase (SOD), Vitamin E, and high-density lipoprotein (HDL) levels, while reducing the malondialdehyde (MDA), total cholesterol, low-density lipoprotein (LDL), and triglyceride levels.
2. Materials and methods
2.1. Materials
The plant, white saffron (Curcuma mangga Val.), was freshly harvested from Sedayu, Bantul, in Yogyakarta. The reagents were ethanol, methanol, HCl, acetate buffer, FeCl3.6H2O, Na2CO3, Na3NO2, AlCl3. 6H2O, NaOH, acetone, acetic acid, vanillin, H3PO4, N2 gas, CH3CN, ethyl acetate (E Merck), aquabidestilata (Ika Pharmindo), EDTA, ketamine, and distilled water (aquadest).
Some instruments, such as autoclave, vacuum rotary evaporator (Heidolph VV, 2000), UV-Vis spectrophotometer (Genesys-20), incubator, vacuum filter, centrifuge, 0.45-μm-milex filter, microfactor, sartorius scale, homogenizer, blender, High-Performance Liquid Chromatography (HPLC) Knauer with C18 column, Photodiode Array Detector (DAD) UV 6000LP, Smartline pump, and ChromGate 3.1.6 software, were used in this study.
2.2. Preparation of pressure-blanched white saffron and animal study
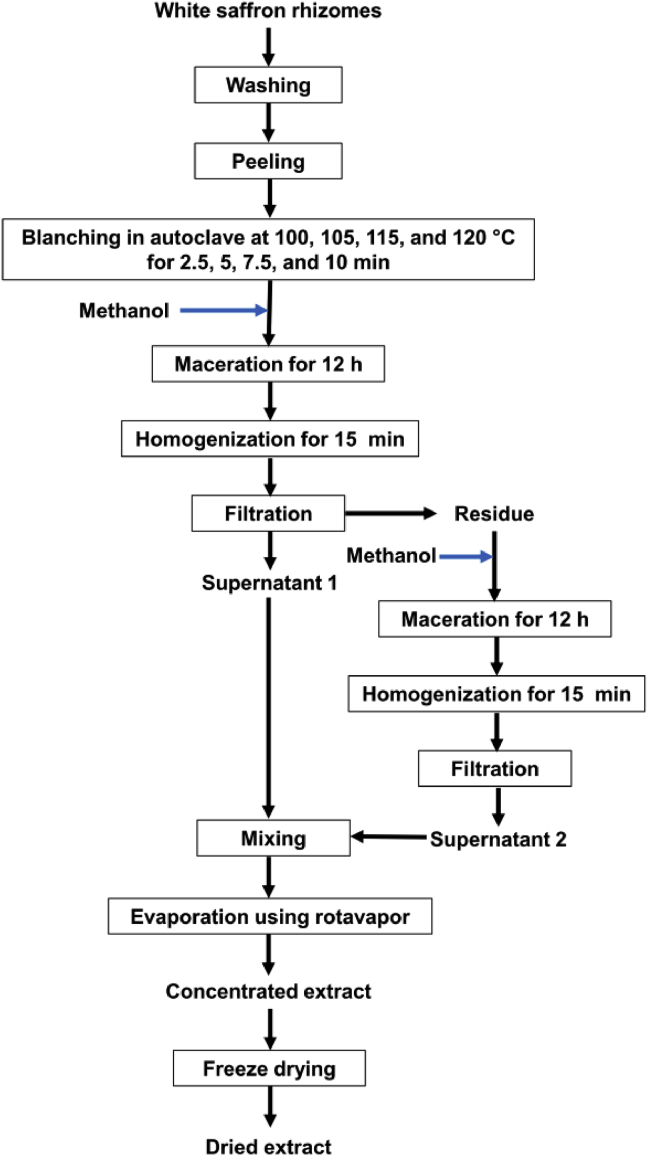
White saffron rhizomes were washed, peeled, and blanched in autoclave at different temperatures (100, 105, 110, 115, and 120 °C; equivalent to 14.71, 17.53, 20.79, 24.54, and 28.81 psia, respectively) for 2.5, 5, 7.5, and 10 min. Then, it was extracted, evaporated, and freeze-dried to get the dried extract (Figure 1).
Figure 1.
Flow chart of preparation steps for making dried extract from raw materials of white saffron.
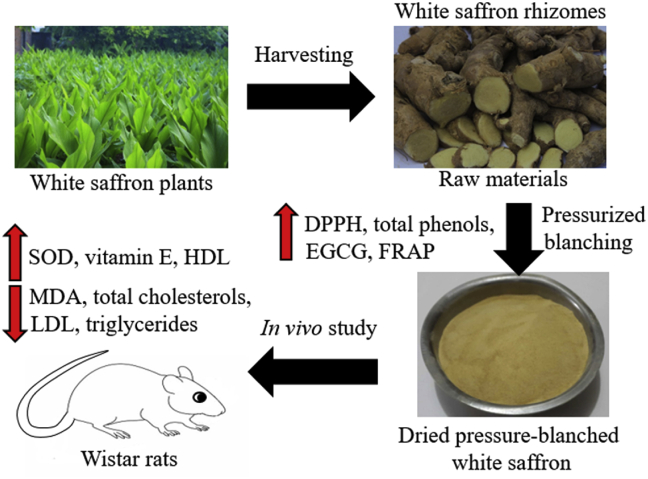
The dried white saffron powder with the highest total phenols and EGCG and the appropriate DPPH and FRAP values was chosen to feed wistar rats. Figure 2 showed the schematic diagram of the research design.
Figure 2.
A schematic diagram of the research design. White saffron was freshly harvested and given pressurized blanching before extraction. Dried powder of pressure-blanched white saffron was given to the oxidized peanut oil-treated wistar rats for two weeks. Blood serum and plasma were collected to measure the SOD, Vitamin E, MDA, total cholesterol, HDL, LDL, and triglyceride levels during treatment.
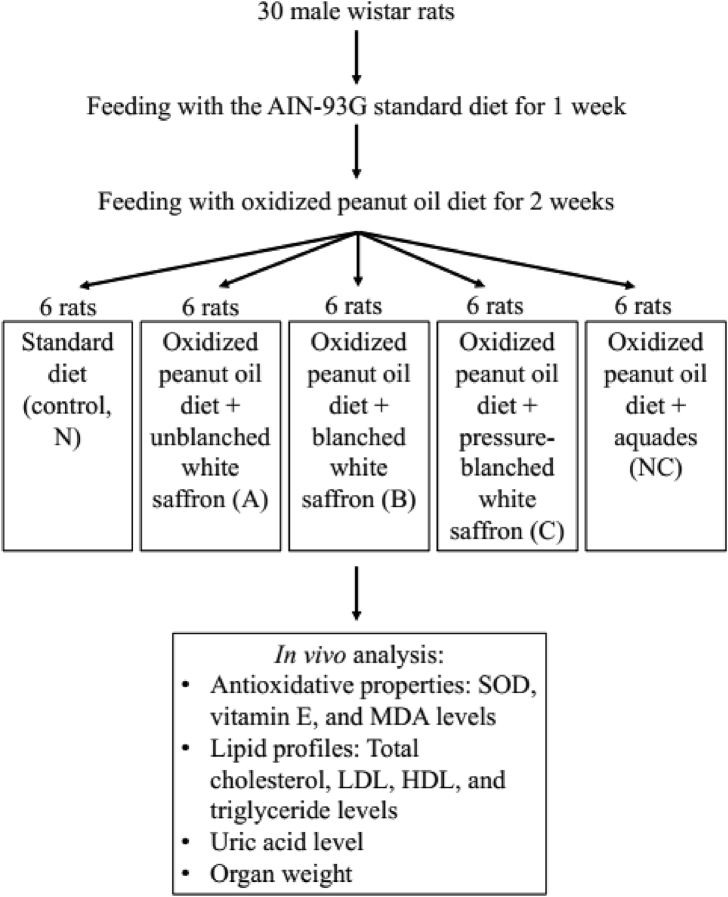
A total of 30 male wistar rats, aged four weeks, were fed with the American Institute of Nutrition (AIN) standard diet (N), oxidized peanut oil diet + unblanched white saffron (A), oxidized peanut oil diet + blanched white saffron (B), oxidized peanut oil diet + pressure-blanched white saffron (C), and oxidized peanut oil diet + aquadest (NC) for two weeks after pre-treatment with the standard diet for a week. The composition of the oxidized peanut oil diet has been adapted to the AIN standard (Reeves et al., 1993) (Table 1).
Table 1.
The composition of the standard diet and oxidized peanut oil diet.
| Ingredients | AIN-93G diet (g/kg diet) | Oxidized peanut oil diet (g/kg diet) |
|---|---|---|
| Cornstarch | 387.486 | 397.500 |
| Casein (≥85% protein) | 200.000 | 200.000 |
| Dextrinized cornstarch (90–94% tetrasaccharides) | 132.000 | 132.000 |
| Sucrose | 100.000 | 100.000 |
| Soybean oil (no additives) | 70.000 | - |
| Oxidized peanut oil | - | 70.000 |
| Fiber | 50.000 | 50.000 |
| Mineral mix (AIN-39G-MX) | 35.000 | 35.000 |
| Vitamin mix (AIN-93M-VX) | 10.000 | 10.000 |
| L-Cystine | 3.000 | 3.000 |
| Choline bitartrate (41.1% choline) | 2.500 | 2.500 |
| Tert-butylhyroquoinone | 0.014 | - |
The animal experiments were conducted in UGM Center for Food and Nutrition Studies (PSPG), Gadjah Mada University, Indonesia, from March to October 2012, in compliance with relevant laws and approved by the ethical committee led by Prof. Dr. Ir. Endang Sutriswati Rahayu, M.S. The experimental animals were separately kept in a cage at room temperature with a diurnal system. Feed and drinking water were given on ad libitum basis, and their body weights were monitored every 2 days. The feed intake was weighed every day. The treatments were given orally using blunt micro syringes (white saffron powder was diluted with water), and it lasted for a period of 14 days. For blood sampling purposes, the wistar rats were anesthetized with ketamine (60 mg/kg) and their blood was drawn through the orbital sinus, given ethylenediaminetetraacetic acid (EDTA) anticoagulant, and the liver, kidney, and testes were harvested and weighed accordingly. To analyze the antioxidative properties and lipid profiles, blood samples were taken before and after treatments with the extract (Figure 3).
Figure 3.
A schematic diagram of the animal study. The administered white saffron was equivalent to 3 g per day of human consumption with a conversion factor of 0.018 for rats (3 g × 0.018 = 0.054 g per day).
2.3. Determination of DPPH value
The DPPH free radical scavenging capacity of white saffron extract was determined using Xu and Chang (2008a) method with slight modification. Briefly, 0.2 mL of the sample (100 mg dried extract dissolved in 3 mL ethanol) was added to 3.8 mL of ethanol solution containing DPPH radical (0.05 mM). The mixture solution was swirled for 1 min and maintained in the dark at room temperature for 30 min. A spectrophotometer was used to measure the absorbance of samples against ethanol as blank (λ = 517 nm). The calibration curve of trolox was used to determine the DPPH value of the samples as mg trolox/g dried extract.
2.4. Determination of total phenols
The Folin-Ciocalteu method (Singleton et al., 1999) was used to quantify the total phenols using a standard gallic acid. Briefly, a sample of 50 μL (100 mg dried extract dissolved in 3 mL ethanol) was mixed with 250 μL of Folin-ciocalteu solution and swirled for 1 min. Then, 750 μl of NaCO3 20% solution was added into it and swirled for another 1 min, and distilled water was subsequently added until 5 mL. After 2 h of incubation at room temperature, the absorbance of the solution was measured at λ of 760 nm. The standard gallic acid was generated from different concentrations of gallic acid ranging from 31.875 to 510 mg/L with R = 0.99. Total phenols were determined as mg gallic acid equivalent (GAE) per gram of dried extract.
2.5. Determination of EGCG level
The quantification of EGCG (mg/g dried extract) was performed using HPLC (Monagas et al., 2007). Briefly, 500 μL of extract solution (100 mg dried extract dissolved in 3 mL methanol:HCl with a ratio of 1000:1) was evaporated using N2 followed with the addition of 1 mL of H3PO3, filtered using millex filter 0.45 μm, and then injected to the HPLC with C18 column (4.6 × 250 mm, dp 5 μm). Quercetin-3-rutinoside was used as a standard (λ of 256 nm, 50 °C), and a solution of H3PO3:CH3CN:acetic ethyl with a ratio of 84:12:4 was used as the eluent.
2.6. Determination of FRAP value
Antioxidant's capability to reduce Fe3+ was determined using FRAP method (Benzie and Strain, 1996). Initially, the FRAP reagent was prepared by gently adding 300 mM acetic buffer (pH 3.6) into as prepared solution of 10 mM TPTZ in 40 mM HCl and 20 mM FeCl3.6H2O, with a ratio of 10:1:1. Then, 3 mL of the FRAP reagent was preheated at 37 °C for 10 min. Immediately, 100 μL of extract solution (100 mg dried extract dissolved in 3 mL ethanol) was mixed with 300 μL of distilled water followed with the addition of as prepared FRAP reagent. It was then swirled for 1 min and left for 4 min. The λ of 593 nm was used to measure the absorbance. Thereafter, FRAP value was calculated in mg ferro equivalent/g dried extract using a calibration curve of Fe2+ (from 4.3 to 137.5 mg ferro/L, R = 0.99).
2.7. Determination of SOD level
Initially, blood was drawn from the rat's orbital sinus, given EDTA anticoagulant and through centrifugation at 3,000 rpm for 10 min, the red blood cells were isolated from the plasma. Thereafter, 50 μL of hemolysate with a ratio of 1:200 (v/v) of red blood cells and distilled water (equivalent to about 75 μg Hb) was prepared to measure the SOD level in erythrocytes by the RANSOD Kit (Randox Laboratories, Ltd., Crumlin, UK), following the manufacturer instructions. The SOD level was expressed as unit per gram of hemoglobin (U/g Hb), in which one unit was defined as the amount of protein that inhibits the rate of 2-para (iodophenyl)-3 (nitrophenyl)-5(phenyl) tetrazolium chloride (INT) reduction by 50%.
2.8. Determination of vitamin E level
Blood plasma (20 μL) was mixed with 100 μL of extract solution (5 mg of BHT/mL in ethanol:butanol, 50:50 v/v). The mixture was centrifuged at 12,000 rpm for 5 min. The supernatant (20 μL) was injected to the HPLC (flowrate of 1 mL/min) with C18 column, and detected using Uv-Vis at λ of 292 nm with the retention time of 3–6 min.
2.9. Determination of MDA level
MDA activity as an indicator of free radicals (highly reactive molecules) generation or lipid peroxidation was determined by quantifying the concentration of thiobarbituric acid (TBA) reactive substances (TBARS). Briefly, 750 μL of phosphoric acid was pipetted into a 13-mL polypropylene tube and 50 μL of plasma was then added. The solution was gently mixed and 250 μL of 40 mM TBA solution was subsequently added. Finally, 450 μL of distilled water was added and covered tightly, and was heated for 1 h. After cooling into an ice bath, the solution was subsequently mixed gently and applied to Sep-Pak C18 column (prewashing was conducted with 5 mL of methanol and double distilled water (dd H2O)). The TBARS was eluted from the column with 4 mL of methanol. The absorbance was measured spectrophotometrically at 532 nm using tetraethoxypropane (TEP) as the external standard and the level of lipid peroxides was indicated as nmol of MDA.
2.10. Determination of lipid profiles
Total cholesterol, LDL, HDL, and triglycerides were measured from blood plasma using DiaSys Diagnostic Systems GmbH kits (Germany), with an enzymatic photometric method (Cholesterol FS, “CHOD-PAP”; LDL Precipitant, “CHOD-PAP”; HDL Precipitant, “CHOD-PAP”), and a colorimetric enzymatic method (Triglyceride FS, “glycerol-3-phosphate-oxidase (GPO)”). To measure the cholesterol level, 1 mL of the enzymatic triglyceride reagent was pipetted into test tubes and added with 10 μL of plasma or glycerol standard, and mixed gently. The mixtures were then incubated at 37 °C for 15 min, and the absorbance was detected at 500 nm within 60 min, against the reagent blank in which distilled water was substituted for the sample. The absorbance of the sample was divided by the standard and multiplied by its concentration to obtain total cholesterol in the blood (similar calculation for HDL, LDL, and triglycerides).
To measure the HDL level, 200 μL of plasma or the standard was added and mixed gently with 500 μL of precipitation reagent, prepared from phosphotungstic acid and magnesium chloride. Thereafter, it was incubated for 15 min at room temperature and then centrifuged at 2500 g for 20 min. Within 2 h after the centrifugation, 100 μL of the clear supernatant or the standard was mixed with 1 mL of cholesterol reagent. The mixtures were then incubated at 37 °C for 5 min, and the absorbance was detected at 500 nm within 45 min, against the reagent blank.
A similar procedure with HDL level measurement was used to measure the LDL level. Briefly, 100 μL of serum or the standard was added with 1 mL of precipitation reagent (prepared from heparin and sodium citrate). The mixtures were then incubated at room temperature for 15 min and then centrifuged at 2500 g for 20 min. Within 1 h after the centrifugation, 100 μL of the clear supernatant or the standard was mixed with 1 mL of cholesterol reagent. The mixtures were then incubated at 37 °C for another 5 min, and the absorbance was detected at 500 nm within 45 min, against the reagent blank.
Finally, to measure the triglyceride level, 10 μL of plasma or the standard was added with 1 mL of as prepared reagent containing Good's buffer, 4-Chlorophenol, ATP, Mg2+, glycerol kinase, peroxidase, lipoprotein lipase, 4-Aminoantipyrine, and GPO. Thereafter, the mixtures were then incubated at 37 °C for 10 min, and the absorbance was detected at 500 nm within 60 min, against the reagent blank in which distilled water was substituted for the sample. Total cholesterol, LDL, HDL, and triglycerides were expressed in mg/dL.
2.11. Statistical analysis
Statistical analysis was conducted using SPSS software (version 16). The data were analyzed by Randomized Complete Block Design (RCBD). Furthermore, Duncan's multiple range test was involved to determine a significant difference between the sample means (p ≤ 0.05).
3. Results and discussions
3.1. In vitro study of pressure-blanched white saffron
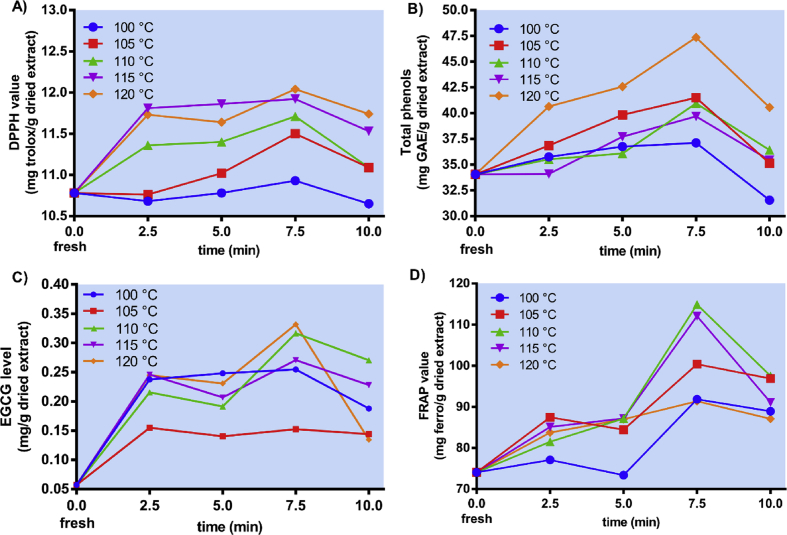
In an in vitro study conducted, white saffron was blanched at different temperatures and time points to select the optimum condition of blanching, as seen in Figure 4A-D. In general, blanching in autoclave at 120 °C for 7.5 min showed the optimum condition for increasing total phenols, DPPH value, and EGCG level of white saffron, thereby being selected for the pressure-blanched treatment (C). However, the FRAP value was much lower than blanching in lower temperatures at the same time (7.5 min). It was notable that the total phenols of white saffron after pressurized blanching obviously increased compared to that of fresh ones (Figure 4B). The increase might be due to the degradation of glycosides into aglycons and complex phenolic compounds into simple substances. The result was similar to the study by Nickel et al. (2016) and Geetha et al. (2018) that examined pressurized blanching in Chenopodium quinoa (Willd.) grains and tomatoes, respectively. In addition, Aisyah et al. (2014) examined the antioxidant activity of blanched vegetables, which could increase the yield of extraction due to the easy separation of antioxidants from the cell matrixes.
Figure 4.
In vitro study of pressure-blanched white saffron. A) DPPH value, B) total phenols, C) EGCG, and D) FRAP value of fresh white saffron and pressure-blanched white saffron at 100, 105, 110, 115, and 120 °C for 2.5, 5, 7.5, and 10 min. Data are presented as mean ± SD.
Similarly, the DPPH value of white saffron increased noticeably compared to that of fresh ones (Figure 4A). A minimum temperature of 110 °C was required to increase free radical scavenging ability (RSA) of white saffron. The increased antioxidant activity was suspected due to the release of phenolic compounds. Furthermore, the EGCG level of pressure-blanched white saffron rose significantly compared to that of fresh ones (Figure 4C). This catechin-based compound was very important due to its potential role in managing oxidative stress, as proven by the high FRAP value of white saffron during blanching under pressure (Figure 4D).
A correlation analysis was conducted based on the data from Figure 4A-D. The results showed that at blanching temperature of 120 °C, total phenols and DPPH value would rise as the increased time of blanching (R = 0.93). Meanwhile, at 10 min, they would rise as the increased temperature of blanching (R = 0.87) (Table 2). In addition, at 120 °C, total EGCG level and FRAP value would increase as the increased time of blanching (R = 0.82), while there was no significant correlation between the two variables at blanching time of 10 min with the increased temperature of blanching (R = 0.42) (Table 3).
Table 2.
Correlation analysis between total phenols and DPPH value.
| Temperature (°C) | Different time |
Time (min) | Different temperature |
||
|---|---|---|---|---|---|
| R-squared (R2) | Pearson's r (R) | R-squared (R2) | Pearson's r (R) | ||
| 100 | 0.48 | 0.69 | 0 | 0.00 | 0.00 |
| 105 | 0.54 | 0.73 | 2.5 | 0.02 | 0.14 |
| 110 | 0.70 | 0.84 | 5 | 0.07 | 0.26 |
| 115 | 0.39 | 0.62 | 7.5 | 0.55 | 0.74 |
| 120 | 0.86 | 0.93 | 10 | 0.76 | 0.87 |
Table 3.
Correlation analysis between EGCG level and FRAP value.
| Temperature (°C) | Different time |
Time (min) | Different temperature |
||
|---|---|---|---|---|---|
| R-squared (R2) | Pearson's r (R) | R-squared (R2) | Pearson's r (R) | ||
| 100 | 0.12 | 0.35 | 0 | 0.00 | 0.00 |
| 105 | 0.64 | 0.80 | 2.5 | 0.27 | 0.52 |
| 110 | 0.79 | 0.89 | 5 | 0.20 | 0.45 |
| 115 | 0.60 | 0.77 | 7.5 | 0.01 | 0.10 |
| 120 | 0.68 | 0.82 | 10 | 0.18 | 0.42 |
However, regardless of the beneficial effects of pressurized blanching, the duration should be controlled up to the optimal time, because long exposure showed a significant reduction in DPPH, total phenols, EGCG, and FRAP value of white saffron after reaching their peaks (Figure 4A-D).
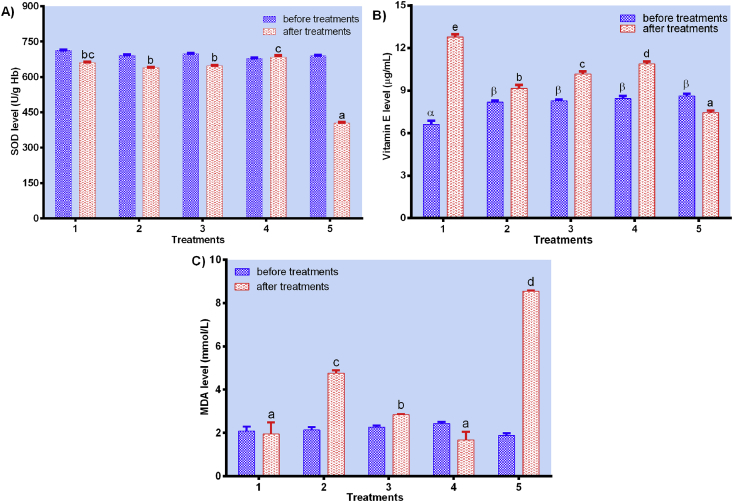
3.2. In vivo study of antioxidative properties
After two weeks of treatment, SOD, Vitamin E, and MDA levels of wistar rats were evaluated for their antioxidative properties, shown in Figure 5A-C. SOD is one of the biological antioxidants in humans or animals, and its high level in blood indicates the body is in healthy condition. In vivo study revealed that the SOD level of wistar rats fed with pressure-blanched white saffron was much higher than that of the negative control (NC), and slightly higher than that of unblanched (A) and blanched (B) (Figure 5A). The result was in accordance with Ahsan (2013), that studied the effect of pressurized blanching on the inhibition of peroxide formation. In another study, water blanching could also increase the antioxidant activity of white saffron as measured by 2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) method, with IC50 value of 24.23 ± 2.77 μg/mL (Pujimulyani et al., 2018). The presence of antioxidants increases the activity of SOD enzymes to suppress the generation of reactive oxygen species (ROS) (Palido-Moran et al., 2016).
Figure 5.
In vivo study of antioxidative properties. A) SOD, B) Vitamin E, and C) MDA levels of rats treated with the standard diet for a week followed with a two-week treatment of the standard diet (1), oxidized peanut oil diet + unblanched white saffron (2), oxidized peanut oil diet + blanched white saffron (3), oxidized peanut oil diet + pressure-blanched white saffron (4), and oxidized peanut oil diet + aquadest (5). Data are presented as mean ± SD. Different notations indicate a significant difference, p ≤ 0.05.
Vitamin E is an essential antioxidant required by the human body. Figure 5B showed Vitamin E level of pressure-blanched white saffron (C) was much higher than that of blanched (B), unblanched (A), and the negative control (NC), respectively. Its increase in the blood was related to the high amount of curcumin after blanching. According to Rai et al. (2010), curcumin could induce its production and prevent deoxyribonucleic acid (DNA) damage by reducing oxidative stress.
Interestingly, the MDA level of pressure-blanched white saffron (C) was similar to that of the positive control (N) and much lower than that of the negative (NC), unblanched (A), and blanched (B), respectively (Figure 5C). The ability to prevent the formation of MDA by samples blanched under pressure was superior to another. This might be due to the presence of curcumin that can significantly reduce MDA formation. Also, the degraded form of glycosides (aglycones) has been shown to be higher in blanched white saffron than in unblanched (Pujimulyani et al., 2012), leading to an increase of MDA inhibition. Therefore, the MDA level of the negative control (NC) was the highest due to the fat oxidation from oxidized oils.
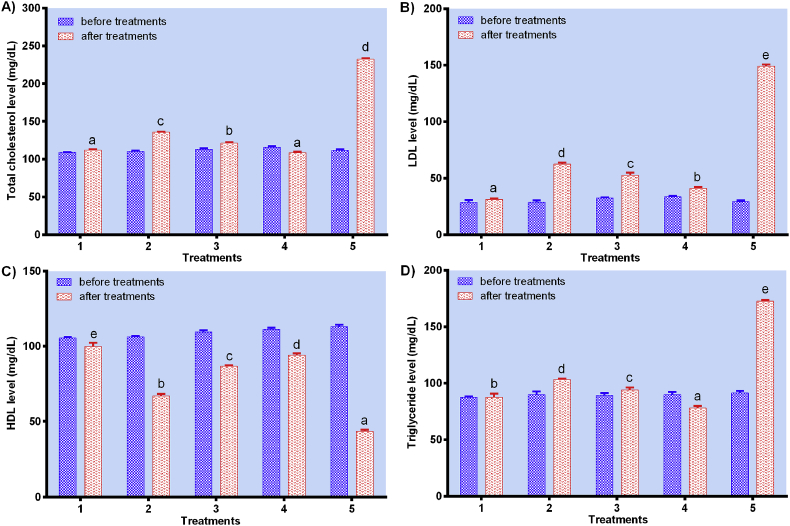
3.3. In vivo study of lipid profiles
Blood lipid profiles of oxidized peanut oil-treated wistar rats, including total cholesterol, LDL, HDL, and triglyceride, were evaluated after treatment as shown in Figure 6A-D. Pressure-blanched treatment (C) effectively suppressed the cholesterol levels as compared to the negative control (NC) (more than twice) (Figure 6A). According to Pujimulyani et al. (2010), water blanched white saffron showed higher antioxidant activity than unblanched, as measured by DPPH and FRAP assay. The bioactive compounds in pressure-blanched white saffron might reduce oxidation-induced cholesterol accumulation.
Figure 6.
In vivo study of lipid profiles. A) Total cholesterol, B) LDL, C) HDL, and D) triglyceride levels of wistar rats treated with the standard diet for a week followed with a two-week treatment of the standard diet (1), oxidized peanut oil diet + unblanched white saffron (2), oxidized peanut oil diet + blanched white saffron (3), oxidized peanut oil diet + pressure-blanched white saffron (4), and oxidized peanut oil diet + aquadest (5). Data are presented as mean ± SD. Different notations indicate a significant difference, p ≤ 0.05.
It is believed that a high level of LDL in the blood can cause harm to the body. However, the LDL level of wistar rats fed with pressure-blanched white saffron (C) was thrice lower than that of the negative control (NC) (Figure 6B). This might be due to the presence of curcumin (Pujimulyani and Sutardi, 2003), polyphenols (Pujimulyani et al., 2010), and dietary fibers (Rezki, 2017) in white saffron. Curcumin-rich diet could reduce the LDL level in the blood (Su et al., 2017). Furthermore, a study by Gani et al. (2013) showed that polyphenol compounds and dietary fibers in red gedi could also reduce the LDL level in the blood.
Conversely, the HDL level of wistar rats fed with pressure-blanched white saffron (C) was twofold higher than that of the negative control (NC) (Figure 6C). This might be due to the presence of curcumin in white saffron (Ganjali et al., 2017). Regarding its effect on HDL function, the results of Syaefudin et al. (2016) showed that feeding Peking broiler ducks with black saffron flour could increase their HDL level. The active substances of essential oils and curcumin could help improve intestinal peristalsis (Rositawati et al., 2010) and facilitate loss of bile salts in the duodenum, which subsequently triggers livers to produce more bile salts. Bile salt's production requires cholesterol, therefore when it is not sufficient, the production of HDL will increase to help the cholesterol transport from tissues to the liver (Hartoyo et al., 2005). In addition, the presence of quercetin in white saffron (Pujimulyani et al., 2012) could increase the HDL level (Made Harumi et al., 2015). Furthermore, this antioxidant could further inhibit atherosclerosis progression through the improvement of blood transport due to reduction of cholesterol levels (Fernandes-Silva et al., 2012).
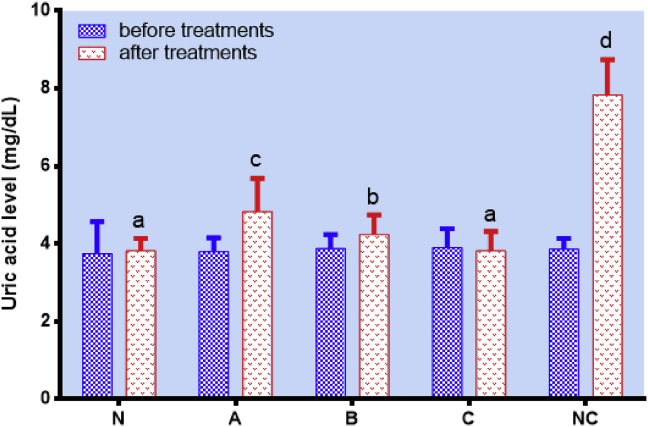
On the other hand, the triglyceride level of wistar rats fed with pressure-blanched white saffron (C) was twice lower than that of the negative control (NC) (Figure 6D). Again, this might be due to the presence of curcumin. According to Su et al. (2017) and Mohammadi et al. (2013), curcumin-rich diet could effectively reduce the triglyceride level as studied in vivo. Furthermore, the uric acid level of pressure-blanched white saffron (C) was examined before and after treatment, and it was twice lower than that of the negative control (NC) (Figure 7).
Figure 7.
Uric acid level of wistar rats treated with the standard diet for a week followed with a two-week treatment of the standard diet (N), oxidized peanut oil diet + unblanched white saffron (A), oxidized peanut oil diet + blanched white saffron (B), oxidized peanut oil diet + pressure-blanched white saffron (C), and oxidized peanut oil diet + aquadest (NC). Data are presented as mean ± SD. Different notations indicate a significant difference, p ≤ 0.05.
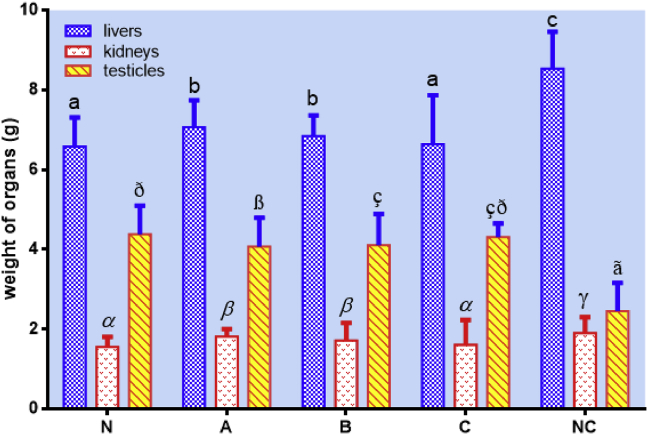
Surprisingly, livers' and kidneys' weight of the negative control (NC) were much higher than those of pressure-blanched white saffron (C) and the positive control (N), indicating the swelling of livers and kidneys due to oxidation. Meanwhile, the testicles' weight of the negative control (NC) was much smaller than those of pressure-blanched (C) and the positive control (N). This might be due to the oxidation and cell damages, leading to the shrinking of testicles’ weight (Figure 8).
Figure 8.
Organs' weight of wistar rats, including livers, kidneys, and testicles, after treatment with the standard diet (N), oxidized peanut oil diet + unblanched white saffron (A), oxidized peanut oil diet + blanched white saffron (B), oxidized peanut oil diet + pressure-blanched white saffron (C), and oxidized peanut oil diet + aquadest (NC). Data are presented as mean ± SD. Different notations indicate a significant difference, p ≤ 0.05.
Results of the in vivo study showed antioxidants induced the improvement of lipid profiles of oxidized peanut oil-treated wistar rats. This might be correlated to the antioxidant metabolic pathways of Vitamin E and SOD. Primarily, Vitamin E metabolism is initiated with a CYP4F2/CYP3A4-dependent ω-hydroxylation cycle followed by five cycles of subsequent β-oxidation, forming water-soluble metabolites, including long-chain metabolites (e.g., carboxydimethyldecylhydroxychromanol (CDMDHC, 11′-COOH) and carboxymethyloctylhydroxychromanol (CDMOHC, 9′-COOH)); intermediate-chain metabolites (e.g., carboxymethylhexylhydroxychromanol (CDMHHC, 7′-COOH) and carboxymethylbutylhydroxychromanol (CMBHC, 5′-COOH)); and short-chain metabolites (e.g., carboxyethylhydroxychromanol (CEHC, 3′-COOH)), excreted through urine or feces (Schmölz et al., 2016). In blood circulation, Vitamin E is transported by the plasma lipoproteins and erythrocytes. In erythrocytes, it can be mediated by either passive diffusion or receptor-mediated transport. Interestingly, it has been studied in vivo for its role in up-regulating the ATP-binding cassette transporters ABCG5/ABCG8, and ABC1, which are responsible for maintaining cholesterol influx and efflux for balancing the cholesterol level in the body (Rogi et al., 2011). Furthermore, a clinical study showed the effects of vitamin E as a natural antioxidant combined with fish oil in improving lipid profiles by reducing total cholesterol and LDL levels and reducing anti-oxidized LDL autoantibodies by protecting LDL against oxidation to prevent cardiovascular-related diseases, especially atherosclerosis (Alves Luzia et al., 2015; Maruf et al., 2019). Another recent clinical study showed that vitamin E supplementation led to a significant reduction in mortality of 29,092 patients with cardiovascular, heart, stroke, cancer, and respiratory diseases, with a risk reduction of 30% (Huang et al., 2019).
On the other hand, SOD is an important enzyme in the body that can catalyze the dismutation of superoxide radical into oxygen and hydrogen peroxide, leading to the moderation of oxidative stress. Mondola et al. (2002) studied the effects of different types of SODs on cholesterol metabolism. The result showed that all forms of SODs affected cholesterol metabolism independently by decreasing microsomal enzyme 3-hydroxy-3-methylglutaryl CoA (HMG-CoA) reductase activity and its protein levels, leading to protein kinase C-mediated reduction of cholesterol synthesis. Additionally, a clinical study showed that an imbalance in SOD and thioredoxin reductase (TrxR-1) activities was significantly correlated to LDL oxidation, a marker of oxidative stress (Augusti et al., 2012). Therefore, SOD plays a pivotal role in maintaining low levels of oxygen metabolites in tissues and in defense against oxidative stress.
4. Conclusions
In conclusion, white saffron blanched under pressure showed the ability to improve SOD, Vitamin E, and HDL levels, while reducing MDA, total cholesterol, LDL, and triglyceride levels in the blood. Initially, the optimal blanching condition was at 120 °C for 7.5 min, which was equivalent to the pressure of 28.81 psia, giving the highest total phenols, EGCG, and DPPH of 47.35 mg GAE/g dried extract, 0.3317 mg/g dried extract, and 12.04 mg trolox/g dried extract, respectively. In vivo study showed that treatment with the pressure-blanched white saffron could significantly improve SOD, Vitamin E, and HDL levels in the blood compared to the negative control (NC); 686.44 U/g Hb, 10.87 μg/mL, and 94.17 mg/dL versus 405.37 U/g Hb, 7.44 μg/mL, and 43.47 mg/dL, respectively. Meanwhile, it could significantly reduce MDA, total cholesterol, LDL, and triglyceride levels compared to the negative control (NC); 1.98 mmol/L, 108.74 mg/dL, 40.99 mg/dL, and 78.06 mg/dL versus 8.54 mmol/L, 232.46 mg/dL, 149.17 mg/dL, and 172.61 mg/dL, respectively. The results showed that pressurized blanching could significantly increase the antioxidant levels of white saffron and its dried form could improve antioxidative properties and lipid profiles in vivo.
Declarations
Author contribution statement
D. Pujimulyani: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
U. Santoso: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
S. Luwihana D.: Performed the experiments; Contributed reagents, materials, analysis tools or data.
A. Maruf: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by the Directorate General of Higher Education, the Ministry of Research, Technology and Higher Education of the Republic of Indonesia (Hibah Fundamental [0541/023-04.1/00/2012]).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Ahsan M.U. Faculty of Agroindustry, University of Mercu Buana Yogyakarta; 2013. Pengaruh Waktu Blanching Bertekanan pada Suhu 120 °C terhadap Kemampuan Penghambatan Pembentukan Peroksida Kunir Putih (Curcuma mangga Val.) Thesis. [Google Scholar]
- Aisyah Y., Rasdiansyah R., Muhaimin M. Pengaruh pemanasan terhadap aktivitas antioksidan pada beberapa jenis sayuran. J. Teknol. Dan. Ind. Pertan. Indones. 2014;6:2. [Google Scholar]
- Alves Luzia L., Mendes Aldrighi J., Teixeira Damasceno N.R., Rodrigues Sampaio G., Aparecida Manólio Soares R., Tande Silva I. Fish oil and vitamin e change lipid profiles and anti-LDL-antibodies in two different ethnic groups of women transitioning through menopause. Nutr. Hosp. 2015;32:165–174. doi: 10.3305/nh.2015.32.1.9079. [DOI] [PubMed] [Google Scholar]
- Augusti P.R., Ruviaro A.R., Quatrin A., Somacal S., Conterato G.M., Vicentini J.T. Imbalance in superoxide dismutase/thioredoxin reductase activities in hypercholesterolemic subjects: relationship with low density lipoprotein oxidation. Lipids Health Dis. 2012;11:79. doi: 10.1186/1476-511X-11-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzie I.F.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power” the FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Cheng Z., Su L., Moore J., Zhou K., Luther M., Yin J.J. Effects of postharvest treatment and heat stress on availability of wheat antioxidants. J. Agric. Food Chem. 2006;54:5623–5629. doi: 10.1021/jf060719b. [DOI] [PubMed] [Google Scholar]
- Fernandes-Silva M.M., Carvalho V.O., Guimarães G.V., Bacal F., Bocchi E.A. Physical exercise and microRNAs: new frontiers in heart failure. Arq. Bras. Cardiol. 2012;98:459–466. doi: 10.1590/s0066-782x2012000500012. [DOI] [PubMed] [Google Scholar]
- Gani N., Momuat L.I., Pitoi M.M. Profil lipida plasma tikus wistar yang hiperkolesterolemia pada pemberian gedi merah (Abelmoschus manihot L.) J. MIPA Unsrat. Online. 2013;2:44–49. [Google Scholar]
- Ganjali S., Blesso C.N., Banach M., Pirro M., Majeed M., Sahebkar A. Effects of curcumin on HDL functionality. Pharmacol. Res. 2017;119:208–218. doi: 10.1016/j.phrs.2017.02.008. [DOI] [PubMed] [Google Scholar]
- Geetha K., Hulamani S., Shivaleela H.B. Effect of cooking on total antioxidant activity, polyphenols and flavonoid content in commonly consumed vegetables. Int. J. Curr. Microbiol. Appl. Sci. 2018;7:1459–1466. [Google Scholar]
- Hartoyo B., Irwan I., Iriyanti N. Effect of fatty acids fiber concentration in broiler ration to cholesterol, HDL and LDL blood serum. Anim. Prod. 2005;7:27–33. https://animalproduction.net/index.php/JAP/article/viewFile/74/61 [Google Scholar]
- Huang J., Weinstein S.J., Yu K., Männistö S., Albanes D. Relationship between serum alpha-tocopherol and overall and cause-specific mortality. Circ. Res. 2019;125:29–40. doi: 10.1161/CIRCRESAHA.119.314944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Made Harumi P., Trisna G., Nyoman Odiyana P.G., Deby Aulia R., Putri Khrisnawati A.A.A., Farmawati A. Quercetin and curcumin prevent decreasing of ldl-cholesterol and increasing of HDL-cholesterol in high fat diet rats. Media Farmasi. 2015;12:225–232. [Google Scholar]
- Manzocco L., Calligaris S., Masrrocola D., Nicoli M.C., Lerici C.R. Review of non-enzymatic browning and antioxidant capacity in processed foods. Trends Food Sci. Technol. 2000;11:340–346. [Google Scholar]
- Maruf A., Wang Y., Yin T., Huang J., Wang N., Durkan C. Atherosclerosis treatment with stimuli-responsive nanoagents: recent advances and future perspectives. Adv. Healthc. Mater. 2019;8 doi: 10.1002/adhm.201900036. [DOI] [PubMed] [Google Scholar]
- Mohammadi A., Sahebkar A., Iranshahi M., Amini M., Khojasteh R., Ghayour-Mobarhan M. Effects of supplementation with curcuminoids on dyslipidemia in obese patients: a randomized crossover trial. Phytother Res. 2013;27:374–379. doi: 10.1002/ptr.4715. [DOI] [PubMed] [Google Scholar]
- Monagas M., Garrido I., Lebron-Aguilar R., Bartolome B., Gomez-Cordoves C. Almond (Prunus dulcis (Mill.) D.A. Webb) skins as a potential source of bioactive polyphenols. J. Agric. Food Chem. 2007;55:8498–8507. doi: 10.1021/jf071780z. [DOI] [PubMed] [Google Scholar]
- Mondola P., Serù R., Santillo M., Damiano S., Bifulco M., Laezza C. Effect of Cu,Zn superoxide dismutase on cholesterol metabolism in human hepatocarcinoma (HepG2) cells. Biochem. Biophys. Res. Commun. 2002;295:603–609. doi: 10.1016/s0006-291x(02)00720-9. [DOI] [PubMed] [Google Scholar]
- Nickel J., Spanier L.P., Botelho F.T., Gularte M.A., Helbig E. Effect of different types of processing on the total phenolic compound content, antioxidant capacity, and saponin content of Chenopodium quinoa Willd grains. Food Chem. 2016;209:139–143. doi: 10.1016/j.foodchem.2016.04.031. [DOI] [PubMed] [Google Scholar]
- Nicoli M.C., Anese M., Parpinel M. Influence of processing on the antioxidant properties of fruit and vegetables. Trends Food Sci. Technol. 1999;10:94–100. [Google Scholar]
- Palido-Moran M., Moreno-Fernandez J., Ramirez-Tortosa C., Ramirez-Tortosa M. Curcumin and health. Molecules. 2016;21:264. doi: 10.3390/molecules21030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujimulyani D., Sutardi . Proceeding International Conference on Redesigning Sustainable Development on Food and Agricultural System for Developing Countries. 2003. Curcuminoid content and antioxidative properties on white saffron extract (Curcuma mangga Val.) [Google Scholar]
- Pujimulyani D., Raharjo S., Marsono Y., Santoso U. Aktivitas antioksidan dan kadar senyawa fenolik pada kunir putih (Curcuma mangga Val.) segar dan setelah blanching. Agri. 2010;30:2. [Google Scholar]
- Pujimulyani D., Raharjo S., Marsono Y., Santoso U. The effect of blanching on antioxidant activity and glycosides of white saffron (Curcuma mangga Val.) Int. Food Res. J. 2012;19:617–621. https://ifrj.upm.edu.my/19%20(02)%202012/(37)IFRJ-2012%20Dwiyati.pdf [Google Scholar]
- Pujimulyani D., Raharjo S., Marsono Y., Santoso U. The phenolic substances and antioxidant activity of white saffron (Curcuma mangga Val.) as affected by blanching methods. World Acad. Sci. Eng. Technol. 2013;7:947–950. [Google Scholar]
- Pujimulyani D., Yulianto W.A., Setyowati A., Arumwardana S., Amalia A., Kusuma H.S.W. Amylase inhibition and free radical scavenging activities of white turmeric extract and fractions. J. Food Technol. Industry. 2018;29:10–18. [Google Scholar]
- Rai B., Kaur J., Jacobs R., Singh J. Possible action mechanism for curcumin in pre-cancerous lesions based on serum and salivary markers of oxidative stress. Int. J. Oral Sci. 2010;52:251–256. doi: 10.2334/josnusd.52.251. [DOI] [PubMed] [Google Scholar]
- Randhir R., Kwon Y., Shetty K. Effect of thermal processing on phenolics, antioxidant activity and health-relevant functionality of select grain sprouts and seedlings. Innovat. Food Sci. Emerg. Technol. 2008;9:355–364. [Google Scholar]
- Reeves P.G., Nielsen F.H., Fahey G.C., Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- Rezki R. Faculty of Agroindustry, University of Mercu Buana Yogyakarta; 2017. Pengaruh Penyimpanan Bahan Segar terhadap Aktivitas Antioksidan IC50 dan Kadar Serat Kasar Kunir Putih (Curcuma mangga Val.)https://eprints.mercubuana-yogya.ac.id/id/eprint/1486 Thesis. [Google Scholar]
- Rogi T., Tomimori N., Ono Y., Kiso Y. The mechanism underlying the synergetic hypocholesterolemic effect of sesamin and α-tocopherol in rats fed a high-cholesterol diet. J. Pharmacol. Sci. 2011;115:408–416. doi: 10.1254/jphs.10287fp. [DOI] [PubMed] [Google Scholar]
- Rositawati I., Saifut N., Muharlien Upaya peningkatan performa itik Mojosari periode starter melalui penambahan temulawak (Curcuma xanthoriza, Roxb) pada pakan. J. Ternak Tropika. 2010;11:32–40. https://ternaktropika.ub.ac.id/index.php/tropika/article/view/100/96 [Google Scholar]
- Sadilova E., Stintzing F.C., Carle R. Thermal degradation of acylated and nonacylated anthocyanins. J. Food Sci. 2006;71:C504–C512. [Google Scholar]
- Schmölz L., Birringer M., Lorkowski S., Wallert M. Complexity of vitamin E metabolism. World J. Biol. Chem. 2016;7:14–43. doi: 10.4331/wjbc.v7.i1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton V.L., Orthofer R., Lamuela-Raventós R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999;299:152–178. [Google Scholar]
- Su L.Q., Wang Y.D., Chi H.Y. Effect of curcumin on glucose and lipid metabolism, FFAs and TNF-α in serum of type 2 diabetes mellitus rat models. Saudi J. Biol. Sci. 2017;24:1776–1780. doi: 10.1016/j.sjbs.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syaefudin A.A., Murwani R., Isroli I. Tepung temu hitam (Curcuma aeruginosa Roxb) dalam ransum memperbaiki produktifitas dan high density lipoprotein (HDL) serum itik pedaging peking. J. Ilmu-Ilmu Peternakan. 2016;26:1–5. [Google Scholar]
- Xu B., Chang S.K. Effect of soaking, boiling, and steaming on total phenolic content and antioxidant activities of cool season food legumes. Food Chem. 2008;110:1–13. doi: 10.1016/j.foodchem.2008.01.045. [DOI] [PubMed] [Google Scholar]
- Xu B., Chang S.K. Total phenolic content and antioxidant properties of eclipse black beans (Phaseolus vulgaris L.) as affected by processing methods. J. Food Sci. 2008;73:H19–H27. doi: 10.1111/j.1750-3841.2007.00625.x. [DOI] [PubMed] [Google Scholar]
- Yue X., Xu Z. Changes of anthocyanins, anthocyanidins, antioxidant activity in bilberry extract during dry heating. J. Food Sci. 2008;73:C494–C499. doi: 10.1111/j.1750-3841.2008.00845.x. [DOI] [PubMed] [Google Scholar]