Abstract
The extensive use of di--n-butyl phthalate (DBP) as a plasticizer in medical devices, personal care products, and industries, which is a major threat to humankind as it leaches out easily from the plastic matrix into the environment. Health risks posed to adults and children from the broad usage of DBP in cosmetics and infant toys observed predominantly due to repeated and prolonged exposure. Hence, this study was undertaken to evaluate the potential effect of DBP in the hepatic tissue of rats up to three generations. Wistar rats were induced at a dose of 500 mg DBP /kg body weight dissolved in olive oil by oral gavage throughout gestation (GD 6–21), lactation and post-weaning and reared by crossing intoxicated rats up to three generations. Results of the present study showed a significant increase in the relative weight of liver, while decreased levels of antioxidant enzymes viz., superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and reduced glutathione (GSH) was evident in DBP treated rats at P < 0.05. Besides hepatic marker enzymes viz., alanine transaminase (ALT) and aspartate transaminase (AST) were elevated significantly in experimental rats compared to those of the control group. Furthermore, histological studies revealed congested central veins and dilated sinusoids in F1 progeny while mild to severe focal inflammatory infiltrations were evident in F2 & F3 rats. Negative correlation observed between the levels of antioxidant enzymes and transaminase activity. In brief, DBP exposure elicits oxidative stress and alters the transaminase activity levels causing damage in hepatic tissue. F3 progeny found to high vulnerability to the exposure of DBP than F2 & F1 rats.
Keywords: Di-n-butyl phthalate, Multigenerational assessment, Hepatotoxicity, Oxidative stress
1. Introduction
Phthalate esters (PE) are synthetic organic molecules extensively used as plasticizers in consumer products and has become indispensable in the human routine lifestyle. They are utilised as additives in production of PVC products, cosmetics and perfume industries owing to their nature of flexibility, stabilizer [1], adhesiveness [2], fixative and denaturing property [3]. Phthalate esters are classified into high molecular weight (MW) phthalate esters with 7–13 carbon atoms [Diisodecyl phthalate (DIDP), diisononyl phthalate (DINP), di-2-propylheptyl phthalate (DPHP), diisoundecyl phthalate (DIUP) and diisotridecyl phthalate (DTDP)] and low molecular weight phthalate esters with 3–6 carbon atoms [di-n-butyl phthalate (DBP), diisobutyl phthalate (DIBP), butyl benzyl phthalate (BBP) and di-2-ethylhexyl phthalate (DEHP)] in their backbone. As the usage of plasticizers is on the rise and their non-degradability has led to their ubiquitous presence in the environment. This has caused humans’ exposure of phthalates through air, water, food, and dermal contact leading to many health hazards [[4], [5], [6], [7], [8]]. Recent investigations by Bi et al. [9] detected the presence of phthalates viz., DEHP, BBP, DBP, and DIBP in the dust of various indoor environment. Once phthalates gain entry into the body through air [4], water [5], and food [6], dermal contact [8], later transform into their corresponding metabolites rapidly and eliminate through urine and feces. However their presence is detected in body fluids namely plasma, amniotic fluid, breast’s milk and urine of humans [10,11]. The latest investigations indicated that the frequent usage of cosmetics during and before pregnancy was linked with the detection of phthalate metabolites in the hair [12]. Furthermore, many studies have shown that phthalates interfere with the endocrine system by acting as anti-androgens or mimic hormone (estrogen and androgen) which bring alteration(s) in the normal functioning of the reproductive system [[13], [14], [15], [16]]. Phthalates along with their metabolites have the potential to cause toxicity in the reproductive system. For instance, a decline in sperm count, incidence of cryptorchidism, and hypospadias have been reported [16,17]. Besides, phthalate esters are developmental toxicants resulted in low birth weight in infants [18], shortened anogenital distance (AGD) in both male and female rats [19] and observed skeletal as well as craniofacial anomalies, and growth retardation in rat fetuses [19,20].
Guo et al. [21] experiments on human subjects have shown the presence of 14 phthalate metabolites in urine samples collected from seven Asian countries (viz., China, India, Japan, Korea, Kuwait, Malaysia, and Vietnam). For comparison, samples collected from population residing in Kuwait (median: 1050 ng/mL), India (389 ng/ mL), China (234 ng/mL), Vietnam (133 ng/mL), Japan (120 ng/mL), Korea (117 ng/mL), and Malaysia (94.9 ng/mL) possess phthalate metabolites as shown in parenthesis. In comparison to other phthalates, exposure to DBP, a low molecular weight phthalate found to cause low toxicity [22] while its prolonged exposure may have compounded effects. India stands to be in second position for the exposure to phthalates [21]. In Indian scenario, the restaurants pack hot food in polythene bags and store in plastics, wherein the phthalate migration is a common phenomenon.
The liver, a vital organ known for detoxification of xenobiotics that enters the body besides metabolizing many chemical compounds [16] resulting in the regulation of homeostasis of the physiological system. Several investigations have indicated the vulnerability of the liver to phthalate exposure. For instance, Reddy and Lalwai [23], have shown liver tumors in rodents’ exposure to DEHP; in addition, the disruption caused to the endocrine system has induced hepatocellular carcinoma in animals [24]. Studies of Lake et al. [25] on rats upon exposure to DEHP indicated hepatomegaly, besides proliferation of smooth endoplasmic reticulum, increase in microsomal cytochrome P-450. Phthalates being novel compounds of peroxisome proliferators (PP) shown to activate peroxisome proliferator-activated receptor (PPAR) in rat liver leading to the metabolic changes. Recent studies of Ma et al. [26] have indicated excessive ROS generation leading to oxidative stress followed by liver damage upon exposure of diisononyl phthalate (DINP) at 20 mg/kg BW (body weight) dose in Kunming mice while 200 mg/kg/day dose shown to cause edema, central vein dilation, congestion and narrowing sinusoids with loose cytoplasm. Likewise, findings of Seo et al. [27] indicated hepatomegaly and severe oxidative stress wherein increased catalase (CAT), lipid peroxidation (LPO) and decrease in glutathione-S- transferase (GST) activities witnessed in rodent model upon exposure to DBP. The case studies of Bhatia et al. [28] specified the involvement of oxidative stress as a contributory factor in the damage of hepatocytes along with the depletion of antioxidant enzymes in patients suffering from acute liver disease. Chronic exposure to DEP in rats at a dose of 10 mg/kg caused changes in hepatic architecture as evidenced by severe intra- and intercellular vacuolations and fatty degeneration in hepatocytes of centrilobular as well as periportal areas besides shreds of electron microscopic evidence indicate increased peroxisomal number in a dose-dependent manner [29]. Later studies of Pereira et al. [30] on male rats upon exposure to DEP for three generations observed compounded toxic effects in F2 progeny ascertained by the substantial increase in liver weight to BW ratio and variations in the level of both serum and tissue transaminase activities.
Fetus and neonates are the most susceptible population to endocrine-disrupting chemicals (EDC) while the hormones exert their action specifically during the developmental programming window [31]. Exposure to androgenic toxic substances in early life affects subsequent generations via parental lineage. The parental effects that can be linked to their environment in ‘early life’, said to be the period from before conception to the end of juvenile growth and the start of sexual maturation. The function of the liver is particularly affected by the unique physiologic milieu during pregnancy. Gestation and the postpartum period that results in abnormal liver function due to lack of adequacy of enzymes for biotransformation of xenobiotics to their intermediates, leading to their accumulation might impose adverse effects in organs and tissues [32,33]. In a given situation, if the phthalate exposures continue over gestation and perinatal period, the developing offspring might have dyshomeostasis to cope with liver functions. Because of these concerns, DBP was selected as one of the phthalates to be examined in a protocol using albino rats to evaluate multigenerational effects with the presumption that the delayed or latent manifestation of toxicity if absent in the first generation can be observed with the enhancement of toxic effect in the subsequent generations. Hence, this study was undertaken to evaluate the potential effect of DBP in the hepatic tissue of rats up to three generations.
The European Union (EU) has imposed restrictions on four-phthalates viz., DEHP, DBP and BBP further DIBP was also added to the final list of restricted phthalates and is being effective from July 7, 2020, under REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) [34]. Besides, these four phthalates were also banned in electronics under the EU Restriction of Hazardous Substances (RoHS) Directive effective from July 2019 and US Consumer Product Safety Commission, which also extended their restriction in using these four phthalates in toys and childcare articles [35] (CPSC, 2017). The European Union also banned di-n-octyl phthalate (DNOP) in toys and childcare articles since 1999 [36].
2. Materials and methods
2.1. Chemicals
Di-n-butyl phthalate, 2,4-dinitrophenyl hydrazine (DNPH), 1-chloro-2,4-dinitrobenzoic acid (CDNB), reduced nicotinamide diphosphate (NADPH), 5, 5- dithio-bis-2-nitrobenzoic acid (DTNB), epinephrine, thiobarbituric acid (TBA), trichloroacetic acid (TCA), bovine serum albumin (BSA) and reduced glutathione (GSH) were purchased from Sigma-Aldrich Ltd. Standards and other chemicals (AR grade) procured from Merck Ltd. Jenway-6405 UV–vis spectrophotometer (UK) were used in biochemical assays. Olympus microscope (CX-61) and Olympus camera (E-330) were used for histological studies.
2.2. The rationale for dose selection
The present study assessed the multigenerational effects, by exposing the rats to DBP for two weeks during gestation and four weeks during the lactation periods. In the pilot study, the LD50 of DBP in exposed rats was 8012 mg/ kg BW. In the present study, 1/16 LD50 value – 500 mg/kg BW was selected with no mortality in rats and demonstrated the safe dose in the test animal.
2.3. Animals
Institutional Animals Ethics Committee, Bangalore University, Bangalore (CPCSEA No.402, File No.25/525/2009 dated 23.03.2011) approved the protocol for the use of animals. Albino rats (Wistar strain) were procured from Sri Raghavendra Enterprises, Bangalore and acclimatized for a week and maintained at room temperature 25 ± 20 °C with the light-dark cycle. They were fed with standard rodent diet (Amrut feeds, India) and water ad libitum throughout the experimental study.
2.4. Experimental design
The study was carried out using forty-eight healthy adult Wistar rats, of which 36 females:12 males (3 females: 1male ratio) kept in cages for a week and females were examined for the vaginal plugs, of which 30 females were recorded positive for pregnancy. They were divided into two groups, group I was labeled as the control group (n = 15) administered with olive oil and group II (n = 15) was the experimental group administered with DBP (500 mg/kg BW) dissolved in olive oil through oral gavage from gestational day (GD) 6−18. The pregnant rats were considered parental generation (F0) dams and the pups born to them were considered F1 progeny and recorded the sex ratio (male: female). F1 and F2 pregnant rats (n = 15) numbers were maintained the same in both groups (I and II). The same dosing regimen was followed to raise F2 and F3 progeny and mating was avoided between siblings of each generation. At postnatal day (PND)-30, the control group and experimental rats were euthanized by cervical dislocation under 1% pentobarbital sodium (0.4 mL/100 g bw) anaesthesia, to excise liver tissue, which was further used to assess biochemical and histological parameters.
2.5. Hepatosomatic index
The relative weight of the liver was calculated using the formula,
2.6. Biochemical analyses
The homogenate (10 % -w/v) of the liver was prepared in ice-cold 0.1 M phosphate buffer using Potter Elvehjem tissue grinder and later centrifuged at 10,000 rpm. The supernatant was isolated and further used for biochemical assessments.
2.6.1. Total protein assessment
The total protein content in liver tissue was estimated by employing the method of Lowry et al. [37] using BSA as standard. The intensity of color formed was directly proportional to the amount of protein present which was measured by UV/Vis spectrophotometer at 660 nm.
2.6.2. Malondialdehyde (MDA) assay
Peroxidation of unsaturated fatty acids or tissue lipids leads to the formation of MDA which reacts with TBA to form a stable chromophoric product (pink in colour) detected in UV/Vis spectrophotometer by measuring the absorbance of MDA at 535 nm as described by Niehaus and Samuelsson [38].
2.6.3. Antioxidant enzyme assays
The superoxide dismutase (SOD) activity was measured by the inhibition of epinephrine auto-oxidation at 480 nm [39]. While the activity of catalase was determined by assaying the decomposition of hydrogen peroxide at 240 nm adopting the method of Aebi [40]. Further glutathione peroxidase (GPx) activity was assayed at 340 nm according to Lawrence and Burk [41] using NADPH and hydrogen peroxide (H2O2) as substrates.
2.6.4. Reduced glutathione assay
The reduced glutathione (GSH) content was determined by following the protocol of Ellman [42] and assayed the formation of yellow-colored 5-thio-2 nitro benzoic acid (TNB) due to the reaction between GSH and TNB measured at 420 nm.
2.6.5. Hepatic biomarker enzyme assay
The levels of transaminase activity viz., AST and ALT activities in hepatic tissue were measured at 505 nm by following Reitman and Frankel’s [43] method.
2.7. Histopathological evaluation
Liver tissue was fixed in 10 % phosphate-buffered formalin, later processed for fixation, dehydration, embedding, sectioning and finally stained with hematoxylin and eosin stains. The images of stained tissue sections were captured with the aid of Olympus microscope (CX-61) and Olympus camera (E-330) at the magnification of 10X and 40 × .
2.8. Statistical analysis
The data were analysed statistically using Student’s t-test to compare control and their respective experimental groups while the experimental group of each generation was compared using one-way ANOVA followed by Tukey’s HSD post-hoc test and the results were considered significant at P < 0.05 using SPSS software (version 20.0). The data were expressed as mean ± S.E. and the correlation analysis was done using the Pearson correlation coefficient (r) and the values were considered statistically significant at P < 0.01 and P < 0.05.
3. Results
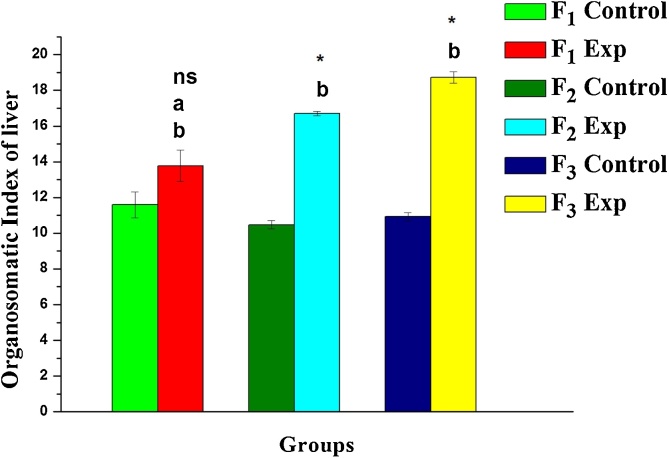
There was no mortality observed in gestational rats with the comparative assessments made for three-generations upon DBP exposure. As shown in Fig. 1, the organosomatic index (relative weight) of the liver in DBP treated rats (F1-F3) was significantly higher than the control group (P < 0.05) indicating enlargement of the liver. The extent of increment in relative weight found to be +18.9, +59.6 and +71.4 in F1, F2 and F3 progeny when compared to their corresponding control groups.
Fig. 1.
Variations in organosomatic index in one-month old rats upon DBP exposure: A three-generational comparative study. Values are mean ± SE of six rats. Symbol ‘*’ - significantly different and ‘ns’ - non-significant from their corresponding controls as determined by Student’s t-test, P < 0.05; alphabets ‘a’ and ‘b’ are significant different among experimental groups as determined by one-way ANOVA followed by post-hoc Tukey’s HSD test, P < 0.05. F1, F2 and F3 represent first, second and third generation respectively.
3.1. Evaluation of oxidative stress indices and hepatic injury biomarkers
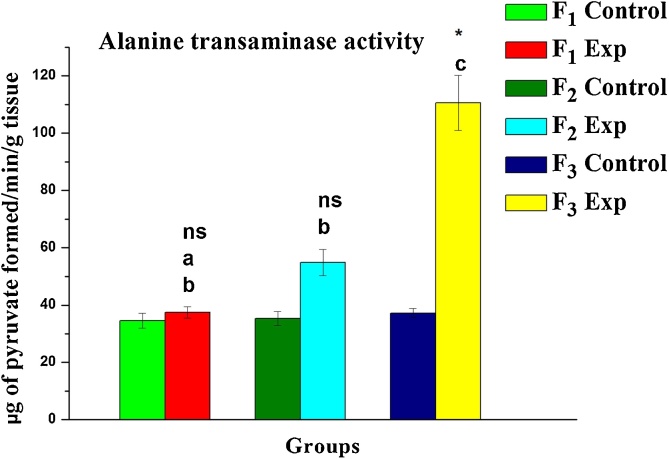
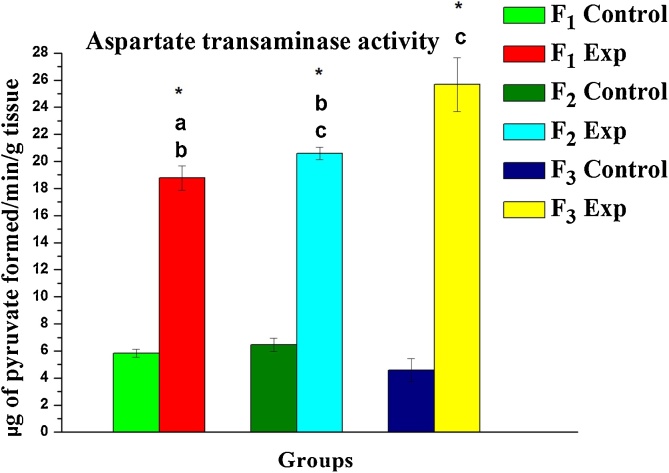
Following exposure to DBP, the oxidative stress in terms of lipid peroxidation was notably elevated. A significant increase in MDA (a by-product of lipid peroxidation) levels by +0.78, +21.74 and +9.63 % was evident in F1, F2, F3 progeny respectively compared to their corresponding control group (Table 1) while the activities of SOD, CAT, GPx as well as GSH levels found inhibition by -32.49, -56.44; -24.74 %; -57.35, -31.93−75.20%; -3.70, +61.90, – 50.00 %; - 41.25, - 52.53, - 83.61 % in F1, F2 and F3 respectively. Contrarily, elevated transaminase activity levels (ALT and AST) were evident in exposed progeny (F1, F2, and F3) and observed % changes being +221.81, +252.99, +340.19 % and +8.52, +58.91, +220.15 % respectively when compared to their corresponding control groups (Fig. 2, Fig. 3).
Table 1.
DBP induced alterations in oxidative stress parameters in the liver tissue of rats studied up to three-generations.
| Groups | LPO (μmoles of MDA/g wet wt. tissue) | SOD (U/mg protein) | CAT (μmoles H2O2 hydrolyzed/ min/mg protein) | GPx (μmoles of NADPH oxidized/min/mg protein) | GSH (μmoles/ min/g wet wt. tissue) | |
|---|---|---|---|---|---|---|
| F1 | C | 1.29 ± 0.17 | 2.37 ± 0.61 | 1.36 ± 0.43 | 0.27 ± 0.05 | 4.17 ± 0.38 |
| E | 1.30 ± 0.20a,ns (+0.78) | 1.60 ± 1.35a,ns (-32.49) | 0.58 ± 0.16a,ns (-57.35) | 0.26 ± 0.06a,ns (-3.70) | 2.45 ± 0.19b,ns (-41.25) | |
| F2 | C | 1.15 ± 0.11 | 2.64 ± 0.36 | 1.19 ± 0.46 | 0.21 ± 0.02 | 4.34 ± 0.38 |
| E | 1.40 ± 0.09a,ns (+21.74) | 1.15 ± 0.32a,ns (-56.44) | 0.81 ± 0.38a,ns (-31.93) | 0.34 ± 0.05a,ns (+61.90) | 2.06 ± 0.15b,ns (-52.53) | |
| F3 | C | 1.35 ± 0.12 | 1.90 ± 0.19 | 1.25 ± 0.45 | 0.22 ± 0.01 | 4.15 ± 0.39 |
| E | 1.48 ± 0.10a,ns (+9.63) | 1.43 ± 0.41a,ns (-24.74) | 0.31 ± 0.11a,ns (-75.20) | 0.11 ± 0.03a,ns (-50.00) | 0.68 ± 0.08a,ns (-83.61) |
Values are mean ± SE of six observations.
Symbol ‘*’ significantly different and ‘ns’ non-significant from their corresponding control as determined by Student’s t-test, P < 0.05; alphabets ‘a’ and ‘b’ are significantly different among experimental groups in the inter-generational analysis as determined by one-way ANOVA followed by post-hoc Tukey’s HSD test, P < 0.05. F1, F2 and F3 represent first, second and third generation respectively. C- Control, E- Experimental.
Fig. 2.
Variations measured in the level of Alanine transaminase activity in liver tissue regions of rats upon gestational DBP exposure: A three-generational toxicity assessment. Values are mean ± SE of six observations. Symbol ‘*’ significantly different and ‘ns’ non-significant from their corresponding controls as determined by Student’s t-test, P < 0.05; different alphabets ‘a’, ‘b’ and ‘c’ are significantly different among experimental groups in the inter-generational analysis as determined by one-way ANOVA followed by post-hoc Tukey’s HSD test, P < 0.05. F1, F2 and F3 represent first, second and third generation respectively.
Fig. 3.
Variations measured in the level of Aspartate transaminase activity in liver tissue of rats upon gestational DBP exposure: A three-generational toxicity assessment. Values are mean ± SE of six observations. Symbol ‘*’ significantly different from their corresponding controls as determined by Student’s t-test, P < 0.05; different alphabets ‘a’, ‘b’ and ‘c’ are significantly different among experimental groups in the inter-generational analysis as determined by one-way ANOVA followed by post-hoc Tukey’s HSD test, P < 0.05. F1, F2 and F3 represents first, second and third generation respectively.
3.2. Histopathological evaluation
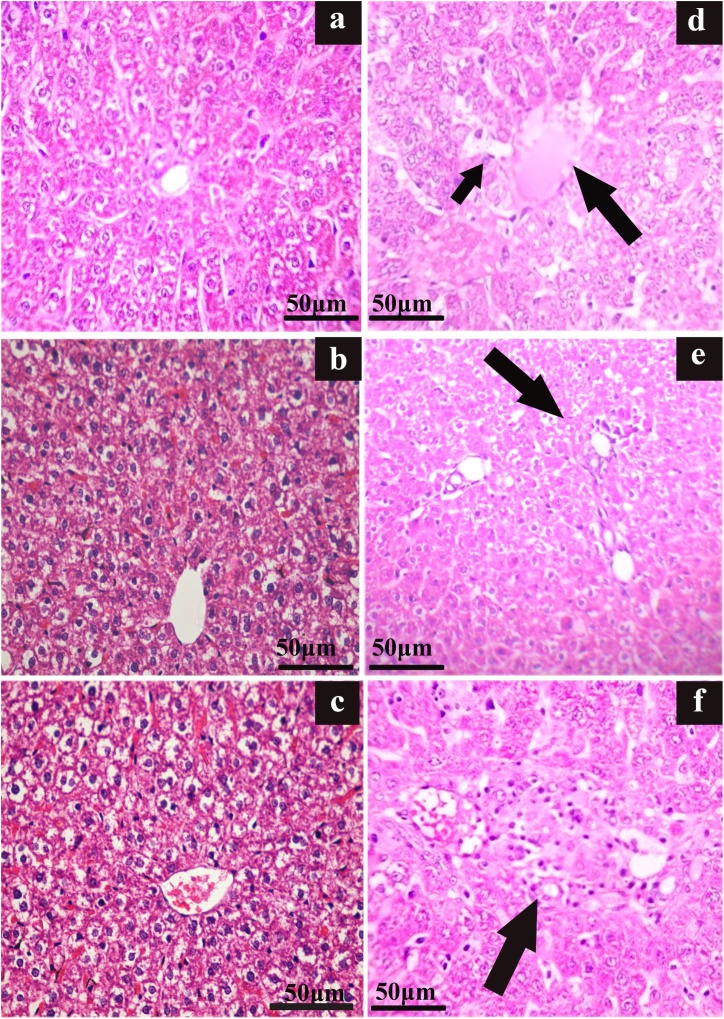
The photomicrographs of the liver histology (F1- F3 rats) are shown in Fig. 4(a–f). They are indicative of remarkable loss of hepatocellular-architecture [Fig. 4(d–f)] with congested and dilated sinusoids (incidental rate- 33.3 %) in F1 progeny; the mild focal inflammatory infiltrations with the incidental rate of 50.0 % in F2 progeny and the severe focal inflammatory infiltrations with 16.7 % incidental rate in F3 progeny were witnessed Fig. 4(a–c) while the control subjects (F1 - F3) had an intact cytoarchitecture of the liver.
Fig. 4.
Photomicrograph of liver sections showing DBP induced alterations in hepatocellular architecture in Wistar rats. (H&E staining, Scale bar-50 μm).
Impression ( ): ‘a’, ‘b’, ‘c’ – control of F1, F2 and F3 with liver parenchyma shows intact architecture. ‘d’ – F1 toxicated showing central veins congested and dilated sinusoids (small arrow), ‘e’ - F2 toxicated showing mild focal inflammatory infiltrations and ‘f’ –F3 toxicated showing the focal inflammatory infiltrations in the periportal region.
): ‘a’, ‘b’, ‘c’ – control of F1, F2 and F3 with liver parenchyma shows intact architecture. ‘d’ – F1 toxicated showing central veins congested and dilated sinusoids (small arrow), ‘e’ - F2 toxicated showing mild focal inflammatory infiltrations and ‘f’ –F3 toxicated showing the focal inflammatory infiltrations in the periportal region.
3.3. Correlation analysis
The correlates (‘r’) analysed between the levels of antioxidant enzymes and activity levels of transaminases are shown in Table 2. A negative association was established between antioxidant enzymes and transaminases (ALT & AST) in the hepatic tissue of the experimental rats tested generationally. The ALT/SOD correlates showed a negative trend (with r-values, -0.851, -0.929 and -0.529) in F1, F2 and F3 rats respectively. Likewise other indices showed a negative correlation which include AST/SOD (P < 0.01) [with r-values, 0.364−0.776 and -0.538]; ALT/CAT [with r-values, -0.922, -0.594, -0.622]; AST/CAT [with r-values, -0.575, -0.315, -0.659]; ALT/GPx [with r-values, -0.813, -0.002, - 0.894]; and AST/GPx [with r-values, -0.216, -0.499, -0.899) in F1, F2 and F3 intoxicated rats respectively.
Table 2.
Effect of DBP on the correlation between the transaminase (ALT/AST) and antioxidant enzyme levels in rats with a comparative assessment of three-generations.
| Generations | Pearson Correlation Coefficient (r) |
|||||
|---|---|---|---|---|---|---|
| ALT/SOD | AST/SOD | ALT/CAT | AST/CAT | ALT/GPx | AST/GPx | |
| F1 | −0.851** | −0.364 | −0.922** | −0.575 | −0.813** | −0.216** |
| F2 | −0.929** | −0.776** | −0.594* | −0.315 | −0.002 | −0.499 |
| F3 | −0.529 | −0.538 | −0.622* | −0.659* | −0.894** | −0.899** |
F1, F2 and F3 represent first, second and third generations; ‘**’ and ‘*’ represent ‘r’ is significant at P < 0.01 and P < 0.05 respectively.
4. Discussion
The extensive use of plasticizers in a wide variety of industrial and domestic products such as PVC, personal care and medical devices have led to the prevalence of phthalate metabolites in the environment. The liver is a primary organ to regulate many metabolic pathways and a key organ to filter the toxic substances. Any damage caused to the liver may lead to complications in the regulation of metabolic pathways. The present study was carried out to understand the role of DBP exposure in the biochemical and histological outcomes of the hepatic tissue and implications of the study was better understood by exposing Wistar rats to DBP for three consecutive generations. The experimental rats (F1-F3) showed a significant increase in the hepato-somatic index (P < 0.05) upon exposure to DBP, indicating hepatomegaly which is a remarkable evidence of liver damage. The hepato-somatic index is usually controlled by two factors, the first being genetic and the second one is the biochemical activity [44]. Following the toxic insult or any infection, the liver tries to restore the hepatic mass, nevertheless, prolonged exposure to xenobiotic agents leads to an adaptive response by increasing its size and influence the enzyme activity to elevate resulting in an alteration of the homeostasis and indicative of liver damage. In the present study, the liver size was increased as an adaptive response which is a compensatory mechanism. Further, elevated level (s) of the transaminases (AST and ALT) significantly (P < 0.05) which are biomarkers of liver injury in the F3 intoxicated rats compared to F2 and F1 progeny to maintain the homeostasis. Similarly, Kwack et al. [45] experiments on Sprague Dawley (SD) rats resulted in liver weight increase due to the exposure of phthalate diesters and monoesters for a short period. Further, their findings observed the significant increase in the level(s) of AST of hepatic tissue in SD rats upon exposure to phthalates such as DBP, diundecyl phthalate (DUP), DINP, monobutyl phthalate (MBuP/MBP), monobenzyl phthalate (MBeP). While the level(s) of ALT increased significantly in rats exposed specifically to DEHP and mono-(2-ethylhexyl) phthalate (MEHP). Similar findings of Mapuskar et al. [46] and Kang et al. [47] demonstrated increased levels of hepatic enzymes in female Swiss mice and Paralichthys olivaceus (a marine culture fish) causing the extensive damage to liver upon DEP exposure at specific dose respectively. The elevated level of transaminases in the present study indicates the liver injury due to the exposure of DBP, which was profoundly observed in F3 experimental rats compared to the other two generations.
Oxidative stress, which is an altered state of equilibrium due to the generation of the excess of free radicals and depletion of endogenous antioxidants. In the present study, the oxidative stress was induced leading to excessive generation of free radicals resultantly lipid peroxidation was witnessed in the form of raised MDA level, the by-product of lipid peroxidation in hepatic tissue due to continuous DBP toxic insult carried out in experimental rats for three consecutive generations. As a consequence, the increase in the cellular levels of antioxidant enzymes would have been initiated in the liver tissue of experimental rats, while the results of the present study indicate the decrement in the antioxidant enzyme activities indicating the utilization of enzymes in scavenging the free radicals. The decreased levels of antioxidant enzymes viz., GST, SOD, CAT, and GPx thereby indicating the inefficiency/ utilization of antioxidant enzymes to combat oxidative stress in the hepatic tissue of intoxicated rats (F1-F3) compared to the control group. Besides, a decrement of GSH was observed, a major radical scavenger of free radicals in the experimental rats. In agreement with the present study, Yavasoglu et al. [48] investigations in the liver tissue of mice indicated the butyl cyclohexyl phthalate (BCP) exposure led to increased MDA level, indicative of lipid peroxidation while SOD and CAT activity were decreased and Erkekoglu et al. [49] investigations in the rodent showed the DEHP exposure caused increased MDA level while decreased SOD, GPx, GST and GSH activities in liver tissue. Furthermore, phthalates are considered peroxisome proliferators (PPs) identified as chemical carcinogens [50]. Among them, DBP is classified as weak PP causes oxidative damage in the liver along with the concomitant increase in the MDA levels at high doses [27]. Besides, DEHP a strong peroxisome proliferator increased the incidence of liver tumors in rodents and animals treated with PP increased peroxisomal β-oxidation enzymes activity while decreased the H2O2 decomposition [51]. The excessive production of H2O2 led to oxidative damage, which caused carcinogenesis in the livers of rodents exposed to phthalates [27]. The investigations of Moody and Reddy [52] reported plasticizers such as DEHP, DEHA, and DEHS in rats induced hepatic peroxisome proliferation along with the increase in relative liver weight. To summarize from the present findings, plasticizers induce hepatomegaly, altered the levels of transaminases and oxidation of fatty acids subsequently leading to oxidative stress, which were detected and caused extensive damage to the liver.
The intact cellular architecture of the liver is required for normal functioning as major metabolic pathways are carried out in the liver. The histological investigations of the liver showed moderate changes in the hepatocellular architecture of F1 rats with the central veins congested and sinusoids dilated; mild and severe focal inflammatory infiltrations in the periportal region of F2 and F3 intoxicated rats were remarkable. The generation of free radicals led to the oxidation of cellular constituents with polyunsaturated fatty acids leading to oxidative damage which mediated loss of cell membrane integrity as well as hepatic cells. This resulted in the disturbance of hepatoarchitecture which in turn increased the AST and ALT levels. Mapuskar et al. [46] and Pereira et al. [30] indicated DEP at high dose altered the liver tissue architecture with vacuolations and degeneration in Swiss mice as well in Wistar rats. Yavasoglu et al. [48] also reported butyl cyclohexyl phthalate (BCP) altered the histological architecture of the liver such as congestions in vena centralis, an enlargement of the sinusoids, degeneration in hepatocytes, vacuole formations and presence of lipid droplets in hepatocytes along with eosinophilic cytoplasm were observed in mice. Recent findings of Erkekoglu et al. [49] demonstrated the remarkable focal necrosis and inflammatory cell infiltration in Selenium deficient rats upon DEHP exposure. The implication of the current study can be drawn as such with the depletion of antioxidant enzymes and increased liver AST and ALT levels causing the severe damage in the liver and ascertained with the histological studies of the liver.
5. Conclusion
The comparative evaluation of three-generations demonstrated that Wistar rats upon DBP exposure from the early embryonic stage until the lactation elevated hepato-somatic index. Besides elicited the oxidative stress in hepatic tissue by decreasing the activities of the antioxidant enzymes while increasing lipid peroxidation. The oxidative stress caused loss in the integrity of the hepatocytes’ membrane which was manifested through biochemical alterations by the leakage of free radicals. This stress corroborated with the altered hepatic-architecture in the form of severe infiltrations (F3 experimental group) which is the remarkable evidence to show the liver damage; an increment in the level(s) of transaminases (ALT and AST) which caused the hepatic tissue to undergo congested and dilated sinusoids and infiltrations alterations. Under the intense oxidative stress, the free radicals surpassed the antioxidant defence ability and resulted in the depletion of antioxidant enzyme activity. The investigations of the study highlight the hepatotoxic potential of DBP in rats conserved up to three-generations with a severe hepatotoxicity observed in F3 experimental rats.
Authorship statement
The design of the study was done by Mahaboob Basha P and data analysis, writing, practical aspects were carried out by Radha MJ and revision of manuscript are done by both the authors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowlegment
We thank UGC, New Delhi, India, for providing project grant (F.No.41-31 / 2012 (SR) dated 10-07-2012).
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.toxrep.2020.06.008.
Contributor Information
M.J. Radha, Email: radha_varvan@yahoo.co.in.
P. Mahaboob Basha, Email: pmbashabub@rediffmail.com.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Guerra M.T., Scarano Wellerson R., C de Toledo Fabíola, Franci Janete A.A., Kempinas W.D.G. Reproductive development and function of female rats exposed to di-η-butylphthalate (DBP) in utero and during lactation. Reprod. Toxicol. 2010;29:99–105. doi: 10.1016/j.reprotox.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Koo H.J., Lee B.M. Estimated exposure to phthalates in cosmetics and risk assessment. J. Toxicol. Environ. Health A. 2004;67:1901–1914. doi: 10.1080/15287390490513300. [DOI] [PubMed] [Google Scholar]
- 3.Hauser R., Calafat A.M. Phthalates and human health. Occup. Environ. Med. 2005;62:806–818. doi: 10.1136/oem.2004.017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song M., Chi C., Guo M., Wang X., Cheng L., Shen X. Pollution levels and characteristics of phthalate esters in indoor air of offices. J. Environ. Sci. (China) 2015;28:157–162. doi: 10.1016/j.jes.2014.05.051. [DOI] [PubMed] [Google Scholar]
- 5.Selvaraj K.K., Sundaramoorthy G., Ravichandran P.K., Girijan G.K., Sampath S., Ramaswamy B.R. Phthalate esters in water and sediments of the Kaveri River, India: environmental levels and ecotoxicological evaluations. Environ. Geochem. Health. 2014;37:83–96. doi: 10.1007/s10653-014-9632-5. [DOI] [PubMed] [Google Scholar]
- 6.Sun J., Wu X., Gan J. Uptake and metabolism of phthalate esters by edible plants. Environ. Sci. Technol. 2015;49:8471–8478. doi: 10.1021/acs.est.5b01233. [DOI] [PubMed] [Google Scholar]
- 7.Yen T.H., Lin-Tan D.T., Lin J.L. Food safety involving ingestion of foods and beverages prepared with phthalate-plasticizer-containing clouding agents. J. Formos. Med. Assoc. 2011;110:671–684. doi: 10.1016/j.jfma.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Sonde V., D’Souza A., Tarapore R., Pereira L., Khare M.P., Sinkar P., Krishnan S., Rao C.V. Simultaneous administration of diethylphthalate and ethyl alcohol and its toxicity in male Sprague-Dawley rats. Toxicology. 2000;147:23–31. doi: 10.1016/s0300-483x(00)00164-5. [DOI] [PubMed] [Google Scholar]
- 9.Bi X., Yuan S., Pan X., Winstead C., Wang Q. Comparison, association, and risk assessment of phthalates in floor dust at different indoor environments in Delaware, USA. J. Environ. Sci. Health A. Tox. Subst. Environ. Eng. 2015;50:1428–1439. doi: 10.1080/10934529.2015.1074482. [DOI] [PubMed] [Google Scholar]
- 10.Silva M.J., Reidy J.A., Herbert A.R., Preau J.L., Jr., Needham L.L., AM C. Detection of phthalate metabolites in human amniotic fluid. Bull. Environ. Contam. Toxicol. 2004;72:1226–1231. doi: 10.1007/s00128-004-0374-4. [DOI] [PubMed] [Google Scholar]
- 11.Dostal L.A., Weaver R.P., Schwetz B.A. Transfer of di(2-ethylhexyl) phthalate through rat milk and effects on milk composition and the mammary gland. Toxicol. Appl. Pharmacol. 1987;91:315–325. doi: 10.1016/0041-008x(87)90054-8. [DOI] [PubMed] [Google Scholar]
- 12.Katsikantami I., Tzatzarakis M.N., Karzi V., Stavroulaki A., Xezonaki P., Vakonaki E., Alegakis A.K., Sifakis S., Rizos A.K., Tsatsakis A.M. Biomonitoring of bisphenols A and S and phthalate metabolites in hair from pregnant women in Crete. Sci. Total Environ. 2020 doi: 10.1016/j.scitotenv.2019.135651. [DOI] [PubMed] [Google Scholar]
- 13.Parks L.G., Ostby J.S., Lambright C.R., Abbott B.D., Klinefelter G.R., Barlow N.J., Gray L.E., Jr The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicol. Sci. 2000;58(2):339–349. doi: 10.1093/toxsci/58.2.339. [DOI] [PubMed] [Google Scholar]
- 14.Swan S.H., Main K.M., Liu F., Stewart S.L., Kruse R.L., Calafat A.M., Mao C.S., Redmon J.B., Ternand C.L., Sullivan S., Teague J.L. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ. Health Perspect. 2005;113:1056–1061. doi: 10.1289/ehp.8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Struve M.F., Gaido K.W., Hensley J.B., Lehmann K.P., Ross S.M., Sochaski M.A., Willson G.A., Dorman D.C. Reproductive toxicity and pharmacokinetics of di-n-butyl phthalate (DBP) following dietary exposure of pregnant rats. Birth Defects Res. B Dev. Reprod. Toxicol. 2009;86:345–354. doi: 10.1002/bdrb.20199. [DOI] [PubMed] [Google Scholar]
- 16.Pan G., Hanaoka T., Yu L., Na J., Yamano Y., Hara K., Ichiba M.N., TK R., Wang P., Yin H., Zhang S., Feng Y. Associations between hazard indices of di-n-butyl phthalate and di-2- ethylhexylphthalate exposure and serum reproductive hormone levels among occupationally exposed and unexposed Chinese men. Int. J. Androl. 2011;34:e397–e406. doi: 10.1111/j.1365-2605.2011.01201.x. [DOI] [PubMed] [Google Scholar]
- 17.Hauser R., Meeker J.D., Duty S., Silva M.J., Calafat A.M. Altered semen quality in relation to urinary concentrations of phthalate monoester and oxidative metabolites. Epidemiology. 2006;17:682–691. doi: 10.1097/01.ede.0000235996.89953.d7. [DOI] [PubMed] [Google Scholar]
- 18.Huang Li J., Garcia J.M., Lin H., Wang Y., Yan P., Wang L., Tan Y., Luo J., Qiu Z., Chen J., Shu W. Phthalate levels in cord blood are associated with preterm delivery and fetal growth parameters in Chinese women. PLoS One. 2014;9(2) doi: 10.1371/journal.pone.0087430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katsikantami I., Sifakis S., Tzatzarakis M.N., Vakonaki E., Kalantzi O.I., Tsatsakis A.M., Rizos A.K. A global assessment of phthalates burden and related links to health effects. Environ. Int. 2016;97:212–236. doi: 10.1016/j.envint.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 20.Mahaboob Basha P., Radha M.J. Gestational di-n-butyl phthalate exposure induced developmental and teratogenic anomalies in rats: a multigenerational assessment. Environ. Sci. Pollut. Res. Int. 2017;24:4537–4551. doi: 10.1007/s11356-016-8196-6. [DOI] [PubMed] [Google Scholar]
- 21.Guo Y., Alomirah H., Cho H.-S.-S., Minh T.B., Mohd M.A., Nakata H., Kannan K. Occurrence of phthalate metabolites in human urine from several Asian countries. Environ. Sci. Technol. 2011;45:3138–3144. doi: 10.1021/es103879m. [DOI] [PubMed] [Google Scholar]
- 22.Kavlock R., Boekelheide K., Chapin R., Cunningham M., Faustman E., Foster P., Golub M., Henderson R., Hinberg I., Little R., Seed J., Shea K., Tabacova S., Tyl R., Williams P., Zacharewski T. NTP Center for the evaluation of risks to human reproduction: phthalates expert panel report on the reproductive and developmental toxicity of di-n-butyl phthalate. Reprod. Toxicol. 2002;16:489–527. doi: 10.1016/s0890-6238(02)00033-3. [DOI] [PubMed] [Google Scholar]
- 23.Reddy J.K., Lalwai N.D. Carcinogenesis by hepatic peroxisome proliferators: evaluation of the risk of hypolipidemic drugs and industrial plasticizers to humans. Crit. Rev. Toxicol. 1983;12:1–58. doi: 10.3109/10408448309029317. [DOI] [PubMed] [Google Scholar]
- 24.Ito Y., Yamanoshita O., Kurata Y., Kamijima M., Aoyama T., Nakajima T. Induction of peroxisome proliferator-activated receptor alpha (PPAR alpha)-related enzymes by di(2- ethylhexyl) phthalate (DEHP) treatment in mice and rats, but not marmosets. Arch. Toxicol. 2007;81:219–226. doi: 10.1007/s00204-006-0141-x. [DOI] [PubMed] [Google Scholar]
- 25.Lake B.G., Gangolli S.D., Grasso P., Lloyd A.G. Studies on the hepatic effects of orally administered di-(2-ethylhexyl) phthalate in the rat. Toxicol. Appl. Pharmacol. 1975;32:355–367. doi: 10.1016/0041-008x(75)90226-4. [DOI] [PubMed] [Google Scholar]
- 26.Ma P., Yan Biao, Zeng Qiang, Liu Xudong, Wu Yang, Jiao Ming, Liu Chao, Wu Jiliang, Yang X. Oral exposure of Kunming mice to diisononyl phthalate induces hepatic and renal tissue injury through the accumulation of ROS. Protective effect of melatonin. Food Chem. Toxicol. 2014;68:247–256. doi: 10.1016/j.fct.2014.03.027. [DOI] [PubMed] [Google Scholar]
- 27.Seo K.W., Kim K.B., Kim Y.J., Choi J.Y., Lee K.T., Choi K.S. Comparison of oxidative stress and changes of xenobiotic metabolizing enzymes induced by phthalates in rats. Food Chem. Toxicol. 2004;42:107–114. doi: 10.1016/j.fct.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 28.Vikram Bhatia, Bhardwaj Payal, Elikkottil Jessina, Batra Jyoti, Saraya Anoop. A 7-day profile of oxidative stress and antioxidant status in patients with acute liver failure. Hepatol. Int. 2008;2:465–470. doi: 10.1007/s12072-008-9098-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pereira C., Mapuskar K., Rao C.V. Chronic toxicity of diethyl phthalate in male Wistar rats- -a dose-response study. Regul. Toxicol. Pharmacol. 2006;45:169–177. doi: 10.1016/j.yrtph.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 30.Pereira C., Mapuskar K., Rao C.V. Chronic toxicity of diethyl phthalate-A three generation lactational and gestational exposure study on male Wistar rats. Environ. Toxicol. Pharmacol. 2007;23:319–327. doi: 10.1016/j.etap.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 31.Xin F., Susiarjo M., Bartolomei M.S. Multigenerational and transgenerational effects of endocrine disrupting chemicals: a role for altered epigenetic regulation? Semin. Cell Dev. Biol. 2015;43:66–75. doi: 10.1016/j.semcdb.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Wildt S.N., Kearns G.L., Leeder J.S., van den Anker J.N. Glucuronidation in humans. Pharmacogenetic and developmental aspects. Clin. Pharmacokinet. 1999;36:439-–452. doi: 10.2165/00003088-199936060-00005. [DOI] [PubMed] [Google Scholar]
- 33.Choudhary D., Jansson I., Schenkman J.B., Sarfarazi M., Stoilov I. Comparative expression profiling of 40 mouse cytochrome P450 genes in embryonic and adult tissues. Arch. Biochem. Biophys. 2003;414:91–100. doi: 10.1016/s0003-9861(03)00174-7. [DOI] [PubMed] [Google Scholar]
- 34.Official Journal of the European Union . 2018. Commission Regulation (eu) 2018/2005.http://data.europa.eu/eli/reg/2018/2005/oj) [Google Scholar]
- 35.2017. United States CPSC Prohibits Certain Phthalates in Children’s Toys and Child Care Products.https://www.cpsc.gov/Newsroom/News-Releases/2018/CPSC-Prohibits-Certain-Phthalates-in-Childrens-Toys-and-Child-Care-Products) [Google Scholar]
- 36.Antonios TRAKATELLIS (EPP-ED, EL) Daily notebook Strasbourg; 2005. Official Journal of the European Union.http://data.europa.eu/eli/reg/2018/2005/oj) [Google Scholar]
- 37.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 38.Niehaus W.G., Jr, Samuelsson B. Formation of malonaldehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur. J. Biochem. 1968;6:126–130. doi: 10.1111/j.1432-1033.1968.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 39.Misra H.P., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- 40.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 41.Lawrence R., Burk R.F. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem. Biophys. Res. Commun. 1976;71:952–958. doi: 10.1016/0006-291x(76)90747-6. [DOI] [PubMed] [Google Scholar]
- 42.Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 43.Reitman S., Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 44.Maronpot R.R., Yoshizawa K., Nyska A., Harada T., Flake G., Mueller G., Singh B., Ward J.M. Hepatic enzyme induction: histopathology. Toxicol. Pathol. 2010;38:776–795. doi: 10.1177/0192623310373778. [DOI] [PubMed] [Google Scholar]
- 45.Kwack S.J., Han E.Y., Park J.S., Bae J.Y., Ahn I.Y., Lim S.K., Kim D.H., Jang D.E., Choi L., Lim H.J., Kim T.H., Patra N., Park K.L., Kim H.S., Lee B.M. Comparison of the short-term toxicity of phthalate diesters and monoesters in sprague-dawley male rats. Toxicol. Res. 2010;26:75–82. doi: 10.5487/TR.2010.26.1.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mapuskar K., Pereira Contzen, Rao C.V. Dose-dependent sub-chronic toxicity of diethyl phthalate in female Swiss mice. Pestic. Biochem. Phys. 2007;87:156–163. [Google Scholar]
- 47.Kang J.C., Jee J.H., Koo J.G., Keum Y.H., Jo S.G., Park K.H. Anti-oxidative status and hepatic enzymes following acute administration of diethyl phthalate:in olive flounder Paralichthys olivaceus, a marine culture fish. Ecotoxicol. Environ. Saf. 2010;73:1449–1455. doi: 10.1016/j.ecoenv.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 48.Yavasoglu N.U., Koksal C., Dagdeviren M., Aktug H., Yavasoglu A. Induction of oxidative stress and histological changes in liver by subacute doses of butyl cyclohexylphthalate. Environ. Toxicol. 2012;29:345–353. doi: 10.1002/tox.21813. [DOI] [PubMed] [Google Scholar]
- 49.Erkekoglu P., Kocer-Gumusel B. Genotoxicity of phthalates. Toxicol. Mech. Methods. 2014;24:616–626. doi: 10.3109/15376516.2014.960987. [DOI] [PubMed] [Google Scholar]
- 50.Reddy J.K., Azarnoff D.L., Hignite C.E. Hypolipidaemic hepatic peroxisome proliferators form a novel class of chemical carcinogens. Nature. 1980;283:397–398. doi: 10.1038/283397a0. [DOI] [PubMed] [Google Scholar]
- 51.Caldwell J.C. DEHP: genotoxicity and potential carcinogenic mechanisms—a review. Mutat. Res-Rev. Mutation. 2012;751(2):82–157. doi: 10.1016/j.mrrev.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 52.Moody D.E., Reddy J.K. Hepatic peroxisome (Microbody) proliferation in rats fed plasticizers and related compounds. Toxicol. Appl. Pharm. 1978;45:491–504. doi: 10.1016/0041-008x(78)90111-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.