Abstract
Objective
Disparities in the assessment and treatment of chronic pain among racial/ethnic may lead to self-treatment for undertreated pain. This study examines whether pain intensity among US racial/ethnic groups’ influences rates of psychotherapeutic prescription drug misuse.
Methods
Data included civilian, non-institutionalized adults (age 18–99 years) residing in the United States (n = 34,653) from Waves 1 and 2 of the National Epidemiological Survey on Alcoholism and Related Conditions (NESARC; 2004–2005). The primary outcome variable was prescription drug misuse/PDM (i.e., use without a prescription or other than as prescribed) including tranquilizers, sedatives, stimulants, or opioids. Predictor variables included self-reported race/ethnicity (American Indian, Black, Hispanic, or White) and pain intensity. Data were analyzed in 2019.
Results
Overall, White and Hispanic participants’ pain intensity had a significantly curvilinear relationship with frequency of prescription medication (p < 0.01). PDM rose with pain intensity until pain levels reached “severe,” then PDM rates fell, not significantly differing from the “no pain” levels (χ2(1) = 0.65, p = 0.42). PDM rates for Black participants remained lowest of all other racial/ethnic groups and plateaued with increasing pain intensity.
Conclusions
Our results indicate that undertreated chronic pain may drive rates of PDM among varying racial/ethnic groups. Providing equitable assessment and treatment of pain intensity remains critical. Additional research is needed to examine provider decision-making and unconscious bias, as well as patient health beliefs surrounding perceived need for prescription pain medications.
Keywords: Prescription drug misuse, US racial/Ethnic groups, Chronic non cancer pain, Under treated pain
Highlights
-
•
Prescription drug misuse (PDM) rates vary by racial/ethnic groups.
-
•
Pain intensity appears to affect PDM.
-
•
PDM is not more likely in Black patients than Whites.
-
•
PDM reduction in racial/ethnic populations must address provider implicit bias.
-
•
Provider education needs include differences in cultural pain expression.
1. Introduction
High frequency of prescription medication misuse creates an increased burden on healthcare, criminal justice and employment systems in the United States (US). Overdoses further stress the present public health system and remain a leading cause of death (Centers for Disease Control and Prevention, 2017, 2018). For instance, more than 47,000 people died from opioid overdoses in 2017, with prescription opioids accounting for more than 35% of those deaths (Scholl, Seth, Kariisa, Wilson, & Baldwin, 2018). In addition, one in five Americans currently report at least one lifetime incident of misusing prescription medication (Peteet, 2019). The nonmedical, nonprescribed use or abuse of prescription medications is often described as prescription drug misuse (PDM) (Substance Abuse and Mental Health Services Administration, 2002). The most commonly misused prescription medications include psychotherapeutics (i.e., opioids, sedatives, tranquilizers, and stimulants), especially those used to treat pain (Gauntlett-Gilbert, Gavriloff, & Brook, 2016; Manchikanti, Fellows, & Ailinani, 2010). Contrary to common presumptions, patients may misuse psychotherapeutics for reasons other than to obtain a high. A significant proportion of patients who misuse prescription medication also report chronic non-cancer pain (CNCP) (Edlund et al., 2010; Wachholtz, Ziedonis, Gonzalez, Wachholtz, & Ziedonis, 2011) and reportedly resort to PDM for pain relief (Levi-Minzi, Surratt, Kurtz, & Buttram, 2013; Manchikanti et al., 2010; Merlo, Singhakant, Cummings, & Cottler, 2013). Thus, PDM rates may directly correlate to rates of undertreated CNCP (Edlund et al., 2010; Levi-Minzi et al., 2013; Manchikanti et al., 2010; Merlo et al., 2013; Wachholtz et al., 2011) suggesting that both patient and provider factors contribute to the PDM phenomenon. In order to prevent PDM, factors related to the provider and patient should be considered in pain care (Johnson-Jennings, Walters, & Little, 2017). Current clinical pain assessment guidelines encourage providers to suspect and screen for potential PDM among patients (American Society of Anesthesiologists Task Force on Chronic Pain Management, 2010; Kemp, Clark, Sherman, & Dahl-Smith, 2013). Yet, concern about PDM may interfere with effective CNCP treatment and introduce related inequities.
1.1. Inequities in prescription drug misuse
Healthcare disparities have been found for CNCP and may influence existing PDM rates. The Institute of Medicine (IoM) Unequal Treatment committee defines healthcare disparities as “racial or ethnic differences in the quality of healthcare that are not due to access-related factors or clinical needs, preferences, and appropriateness of intervention” (Smedley, Stith, & Nelson, 2003). Racial disparities have been found in pain medication prescriptions for racial/ethnic minority patients as compared to Whites (Chibnall and Tait, 1989; Cintron & Morrison, 2006; Ezenwa, Ameringer, Ward, & Serlin, 2006; Green et al., 2003; Phinney & Ong, 2007; Tait & Chibnall, 2014). These disparities likely relate to the subjective components of pain, which can defy objective medical assessment and are prone to provider and patient social and psychological factors (Bates & Rankin-Hill, 1994). Therefore, provider unconscious stereotypes/biases likely contribute to racial/ethnic disparities in the assessment and treatment of chronic pain and should be considered (Tait & Chibnall, 2014). Because race is defined as a historical, sociopolitical construct between groups that is often identified via physical appearance and ethnicity, race can serve as a proxy for an individual's cultural, national, and political affiliations (Ezenwa et al., 2006; Phinney & Ong, 2007). Further, racial/ethnic disparities exist regarding access to effective pain treatment, with significant disparities in treating severe levels of pain (Cintron & Morrison, 2006). Hence, undertreated racial/ethnic minority patients in severe pain may be more likely to resort to PDM, as compared to Whites. However, research has yet to determine if those with CNCP vary in their PDM based on pain intensity, individual risk factors and racial/ethnic group status.
Racial/ethnic group status has further been found to correlate with increased individual risk factors for pain (Ezenwa et al., 2006). Therefore, Andersen's Health Behavior Model (Andersen, Harada, Chiu, & Makinodan, 1995) serves as the theoretical foundation for our study as it provides a framework to consider predisposing individual factors (e.g., age, sex, education, occupation, ethnicity), enabling (e.g., income, health insurance status, reliable source of timely healthcare, transportation, etc.) and need-based factors for healthcare (e.g., person's perceived need for pain treatment as well as professional assessment of a person's need for pain treatment) affecting the use of health services. The purpose of this study is to examine if PDM rates vary by racial/ethnic group as opposed to other predisposing or enabling factors and whether reported pain intensity could influence PDM rates.
2. Methods
This study was conducted using data from the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) in order to measure alcohol, tobacco, medicine, and illicit drug use, and allow epidemiological measurement of associations with respondent family histories, socio-economic and demographic characteristics, and physical wellbeing and mental health. Waves 1 and 2 of the program surveyed respondents twice, in 2001–2002 and 2004–2005, so that changes in substance usage and the associated factors could be observed over time. The US Department of Health and Human Services (DHHS) and the National Institute on Alcohol Abuse and Alcoholism (NIAAA) sponsored the collection of this data (Hasin & Grant, 2015).
2.1. Participants
Participants included civilian, non-institutionalized adults (age 18–98 years) residing in the US (n = 34,500) from NESARC Wave 2 (2004–2005, following the Wave 1 interviews in 2001–2002). The sampling design oversampled younger adults and Blacks and Hispanics. These data were collected using a face-to-face, computer-assisted interviewing technique, with observations shortened and appropriate estimates weighted for the US adult non-institutionalized civilian population.1* Data were analyzed in 2019. The five subsample-groups analyzed are self-reported mutually exclusive groups: White (n = 20,000), American Indian/Alaska Natives (AI/AN, n = 700), Black (n = 6400), Asian/Pacific Islanders (A/PI, n = 1000), and Hispanics (n = 6400). All people who reported Hispanic were counted as such, regardless of their race; all other groups are non-Hispanic.
2.2. Main outcome
Two outcomes were measured. First, psychotherapeutic PDM defined by NESARC as using most commonly misused pain related prescription drug (i.e., tranquilizers, sedatives, stimulants and opioids)2* since last interview (PDMSLI) without a prescription, in greater amounts, more often, or longer than prescribed, or for reasons other than a doctor said you should use them. Opioids alone did not have enough statistical power for individual analysis, and statistical differences when combined with tranquilizers, sedatives, and stimulants were minor, so they were combined. Second, PDM frequency (PDMF) was coded as five categories in decreasing order: (4) daily (or nearly-daily) use; (3) weekly use (1–4 times a week); (2) monthly use (1–3 times a month); (1) infrequent use (1–11 times a year); and (0) non-use. The variable was coded at the highest individual response for any of the eight items.3†
2.3. Primary predictors: Individual need factor
White operated as the reference group in order to estimate racial/ethnic pain care disparities (Anderson, Green, & Payne, 2009; Green et al., 2003; Jimenez, Garroutte, Kundu, Morales, & Buchwald, 2011). Pain level intensity was measured by the question: during the past 4 weeks, how often did pain interfere with normal work including both work outside the home and housework? Please answer on a five-point scale (1 = not at all; 5 = extremely) to constitute an individual need factor (i.e., a person's perceived need for health services). In Andersen's health behavior model (Andersen & Davidson, 2001) individual predisposing factors include race/ethnicity, age, sex, and education. Enabling factors included family income and health insurance coverage. We retained the values assigned and allocated by NIAAA to replace missing values (see NESARC Source and Accuracy Statements) (Grant & Kaplan, 2005; Grant, Moore, Shepard, & Kaplan, 2003). The descriptive characteristics for all five racial/ethnic groups are reported in Table 1.
Table 1.
Descriptive characteristics, among respondents to Wave 2 of the National Epidemiology Study on Alcohol and Related Conditions (NESARC), by racial/ethnic group.
| White | Black | America Indian/Alaskan Native | Asian/Pacific Islander | Hispanic | |
|---|---|---|---|---|---|
| Observations (N) | 20000 | 6400 | 700 | 1000 | 6400 |
| Means/Proportionsa | Mean (s.e.) | Mean (s.e.) | Mean (s.e.) | Mean (s.e.) | Mean (s.e.) |
| Prescription Drug Misuse (PDM) | |||||
| PDM Since Last Interview (PDMSLI | 5.35 (0.09) | 3.35 (0.13) | 4.41(0.66) | 2.99 (0.18) | 4.04 (0.06) |
| PDM Frequency level among users (PDMF | PSMSLI = 1)d | 1.80 (0.05) | 1.87 (0.11) | N/A | N/A | 1.81 (0.09) |
| Reported Pain | |||||
| Pain level 1 “None” | 63.2 (0.18) | 60.7 (0.34) | 52.5 (1.35) | 67.9 (0.40) | 69.0 (0.29) |
| Pain level 2 “Low” | 19.5 (0.18) | 19.8 (0.28) | 23.8 (1.01) | 19.6 (0.37) | 16.8 (0.19) |
| Pain level 3 “Mid” | 8.2 (0.11) | 7.9 (0.16) | 8.6 (0.90) | 6.6 (0.39) | 7.0 (0.14) |
| Pain level 4 “High” | 6.2 (0.10) | 7.9 (0.16) | 8.2 (1.01) | 4.2 (0.15) | 5.0 (0.11) |
| Pain level 5 “Extreme” | 2.9 (0.08) | 3.8 (0.11) | 6.9 (1.07) | 1.7 (0.41) | 2.2 (0.13) |
| Structural Risk Factors | |||||
| Family Incomeb | $63,000 (229) | $40,500 (269) | $46,000 (1150) | $70,000 (627) | $44,500 (226) |
| No health insurance | 14.1 (0.17) | 21.4 (0.31) | 23.4 (1.10) | 22.1 (0.36) | 36.1 (0.42) |
| Medicare | 20.3 (0.14) | 16.4 (0.24) | 17.5 (1.38) | 11.4 (0.50) | 9.6 (0.20) |
| Medicaid | 3.9 (0.10) | 12.2 (0.27) | 9.1 (0.86) | 5.5 (0.20) | 9.9 (0.14) |
| Military/VA insurance | 3.6 (0.13) | 4.6 (0.21) | 6.1 (0.73) | 1.7 (0.29) | 1.9 (0.08) |
| Private insurance | 75.5 (0.20) | 57.3 (0.37) | 58.4 (1.52) | 67.6 (0.41) | 49.4 (0.38) |
| Individual Risk Factors | |||||
| Female | 51.9 (0.21) | 56.6 (0.34) | 54.3 (1.30) | 50.9 (0.39) | 49.1 (0.35) |
| Male | 48.1 (0.21) | 43.4 (0.34) | 45.7 (1.30) | 49.1 (0.39) | 50.9 (0.35) |
| Age 18-25 | 13.0 (0.19) | 17.8 (0.42) | 11.9 (0.90) | 18.5 (0.48) | 22.2 (0.25) |
| Age 26-35 | 16.4 (0.13) | 20.4 (0.30) | 17.6 (0.82) | 24.2 (0.42) | 28.0 (0.26) |
| Age 36-50 | 30.9 (0.24) | 32.8 (0.30) | 34.9 (1.34) | 29.8 (0.39) | 29.8 (0.27) |
| Age 51-64 | 20.8 (0.18) | 17.5 (0.18) | 22.2 (1.17) | 17.8 (0.34) | 12.6 (0.19) |
| Age 65 and over | 18.9 (0.13) | 11.5 (0.19) | 13.3 (1.02) | 9.7 (0.45) | 7.5 (0.12) |
| High school dropout | 10.1 (0.16) | 17.5 (0.28) | 19.2 (1.43) | 12.0 (0.51) | 34.7 (0.35) |
| HS diploma/GED | 60.3 (0.21) | 64.3 (0.39) | 63.2 (1.51) | 39.9 (0.43) | 51.5 (0.33) |
| College education | 29.6 (0.19) | 18.1 (0.24) | 17.6 (1.30) | 48.1 (0.70) | 13.8 (0.14) |
Notes: N = 34,500. Sample counts are rounded according to the U.S. Census Bureau Disclosure Review Board Disclosure Avoidance Guidelines. All variables and groups (sex, age, education, health insurance, pain level) of variables showed significant differences in means and proportions between ethnicity/races using Rao-Scott adjusted chi-squared tests of equality at p < 0.001. Income disparities were tested with the F-test for joint significance in a regression of family income on four race/ethnicity indicators. The analyses corrected for observation weighting and survey design effects. “N/A” means that the estimate would be based on fewer than 15 observations, and cannot be disclosed.
c The variable is a (0/1) indicator where true represents endorsement of two items on the list of traumatic childhood experiences recorded in the NESARC survey instrument.
The indicator variables used in our analysis are coded as “1” for true and “0” for false. For ease of reading, these are presented as proportions (0–100 percent) of trues rather than means (between 0.0 and 1.0). The standard errors reported are for the mean or proportion.
The regression analyses used ln(family income) to adjust for the skewed distribution of incomes. In cases where family income was zero or negative, we set ln(family income) to 0.
This is the mean PDM frequency level among people with PDMSLI = 1. The level is 1 for occassional users, 2 for monthly users, 3 for weekly users, and 4 for daily users.--- The results in this table have been cleared by the Census Bureau's Disclosure Review Board release authorization number CBDRB-FY19-124.
2.4. Data analysis
Two outcomes, binary PDMSLI and the PDMF, were estimated separately. PDM in the Wave 2 NESARC survey (measured over the period since the previous interview) was examined using three multivariate logistic and ordered logistic regression models. Table 2 reports adjusted odds ratios (AOR) for each regressor in the model and reports likelihoods of PDMSLI or PDMF as percentages by reported pain level and racial/ethnic group. Model 1 (Table 2) estimates how the likelihood of PDMSLI changes with respect to pain levels (with pain level 1 “not at all” or “none” as reference) and racial/ethnic groups (with White as reference) while also estimating the effects of the predisposing, and enabling variables described above.
Table 2.
Multivariate logistic regression results from NESARC estimating psychotherapeutic misuse (0/1) since the last year's interview, (N = 34,500) and estimating frequency of psychotherapeutic misuse since the last year's interview, (N = 34,500).
| Individual Risk Factors | Model 1: Adjusted Odds Ratios (AOR) for Prescription Drug Misuse Since Last Interview (PDMSLI), (95% Conf. Interval) | Model 2: Adjusted Odds Ratios (AOR) for PDMSLI with Control for structural risk factors(95% Conf. Interval) | Model 3:Prescription Drug Misuse Frequency (PDMF) |
|---|---|---|---|
| Reported Pain and Race/Ethnicity | |||
| Pain level 2 (“little bit”) | 1.98*** (1.82, 2.16) | 1.91*** (1.73, 2.10) | 2.03*** (1.81, 2.27) |
| Pain level 3 (“moderate”) | 2.04*** (1.88, 2.23) | 1.86*** (1.68, 2.05) | 2.74*** (2.40, 3.12) |
| Pain level 4 (“quite a bit/severe”) | 2.75*** (2.40, 3.15) | 2.68*** (2.29, 3.14) | 3.59*** (2.96, 4.36) |
| Pain level 5 (“extreme”) | 1.39 ** (1.14, 1.70) | 1.11 (0.86, 1.43) | 1.54*** (1.20, 1.98) |
| Black | 0.48*** (0.43, 0.53) | 0.37*** (0.33, 0.42) | 0.45*** (0.39, 0.51) |
| AI | 0.64 ** (0.46, 0.89) | 0.65 ** (0.47, 0.90) | 0.48 ** (0.31, 0.75) |
| A/PI | 0.50*** (0.44, 0.57) | 0.50*** (0.44, 0.57) | 0.44*** (0.36, 0.52) |
| Hispanic | 0.58*** (0.55, 0.61) | 0.52*** (0.48, 0.55) | 0.53*** (0.49, 0.57) |
| Interactions of Reported Pain and Race/Ethnicity | |||
| Black and Pain level 2 | 1.58*** (1.35, 1.84) | ||
| Black and Pain level 3 | 2.09*** (1.62, 2.69) | ||
| Black and Pain level 4 | 0.81 (0.52, 1.25) | ||
| Black and Pain level 5 | 3.78*** (2.55, 5.59) | ||
| Hispanic and Pain level 2 | 1.04 (0.91, 1.18) | ||
| Hispanic and Pain level 3 | 1.45*** (1.22, 1.74) | ||
| Hispanic and Pain level 4 | 1.69*** (1.42, 2.02) | ||
| Hispanic and Pain level 5 | 2.32*** (1.64, 3.27) | ||
| Individual Controls | |||
| Female | 0.75*** (0.70, 0.80) | 0.75*** (0.70, 0.79) | 0.66*** (0.60, 0.73) |
| Age 18-25 | 4.40*** (3.93, 4.92) | 4.41*** (3.95, 4.92) | 5.15*** (4.43, 5.99) |
| Age 26-35 | 2.53*** (2.31, 2.79) | 2.53*** (2.31, 2.78) | 3.29*** (2.89, 3.75) |
| Age 36-50 | 1.97*** (1.76, 2.20) | 1.96*** (1.76, 2.19) | 2.41*** (2.08, 2.79) |
| Age 65-99 | 0.71 ** (0.57, 0.88) | 0.72 ** (0.58, 0.89) | 0.64*** (0.52, 0.79) |
| No diploma | 0.87 * (0.77, 0.97) | 0.86 ** (0.77, 0.97) | 0.93 (0.76, 1.13) |
| College degree | 0.95 (0.89, 1.01) | 0.94 (0.88, 1.00) | 0.80*** (0.73, 0.87) |
| Income | 0.82*** (0.79, 0.85) | 0.82*** (0.80, 0.85) | 0.83*** (0.80, 0.86) |
| Structural/Insurance Controls | |||
| Medicare | 0.82 * (0.70, 0.97) | 0.82 * (0.69, 0.96) | 0.80 * (0.66, 0.98) |
| Medicaid | 1.02 (0.87, 1.19) | 1.02 (0.87, 1.19) | 0.93 (0.76, 1.15) |
| Military/VA insurance | 1.29 ** (1.08, 1.54) | 1.28 ** (1.07, 1.53) | 0.81 (0.61, 1.07) |
| Private insurance | 0.90 ** (0.83, 0.97) | 0.90 ** (0.83, 0.97) | 0.79*** (0.70, 0.90) |
Notes: Multivariate logistic regressions control for age group (ages 18–25, 26–35, 36–50, 51–64, 65+), sex (0 = Male /1 = Female), race (0/1 indicators for White, Black, Asian/Pacific Islander, American Indian/Alaskan Native [AI/AN], and Hispanic), educational attainment (indicators for no diploma, high school diploma with no college degree, college graduate), childhood trauma (0 = No /1 = Yes), and insurance coverage (indicators for Private insurance, Medicare, Medicaid, Military/VA, none reported). The regressions also control for (logged) family income, entering zero or negative income families as having a logged value of 0.
Sample sizes only allowed interaction calculations for Blacks and Hispanics and their pain levels.
Regressions use as a referent (baseline) risk a white male with pain level 1 (“no pain”), aged 51–64, a high school diploma, no childhood trauma, and no reported insurance coverage. Odds ratios hold all other variables constant relative to the referent, changing the labeled variable by one unit.
Adjusted Odds Ratios are presented.
Notes: Both regression models had Wald F-tests with p < 0.001, and Model 2 likelihood ratio test against the nested Model 1 (8 d.f.) had p < 0.001.
The results in this table have been cleared by the Census Bureau's Disclosure Review Board release authorization number CBDRB-FY19-124.
*** - significant at 0.001 level.
** - significant at 0.01 level.
* - significant at 0.05 level.
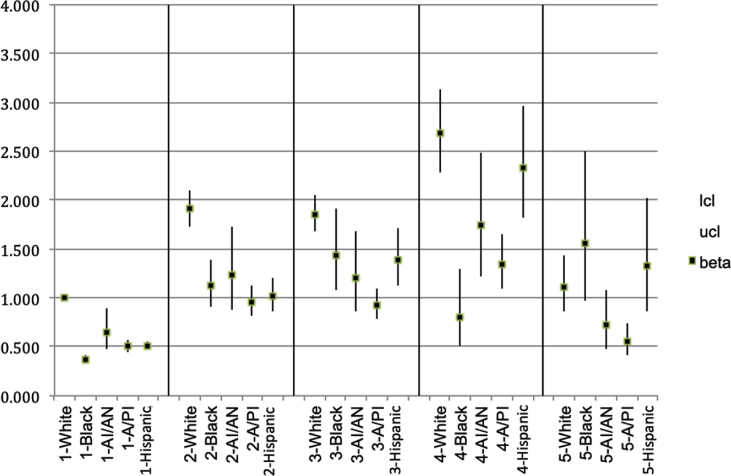
For the subsample groups with enough observations to make valid estimates possible (White, Black, and Hispanic), Model 2 (Table 2) also estimates differential slope terms for each increase in reported pain level for Black and Hispanics relative to Whites in addition to the estimates for the univariate differences within each of those two variables across the sample. The control variables are the same as in Models 1 and 3. The total effects of the two, univariate differences and their joint differential slope were analyzed. This included combining the effects of the four reported pain level estimates [relative to “none”], the Black and Hispanic [constant] difference estimates, and the two estimated sets of interactions between [Black*Reported pain level] and [Hispanic*Reported pain level]) to examine the marginal effects. The addition of the differential slopes allows the White, Black, and Hispanic groups to have fully independent curvilinear relationships between reported pain and PDMSLI rates (Fig. 1).
Fig. 1.
Adjusted Odds Ratios (AORs) for likelihood of non-medical psychotherapeutic misuse since last interview, by racial/ethnic group and self-reported pain intensity level.
The results in this figure have been cleared by the Census Bureau's Disclosure Review Board release authorization number CBDRB-FY19-124.
Model 3 (Table 2) is similar in that it uses the same group of regressors, but estimates a multivariate ordered logistic regression upon the five ascending levels of PDMF. The univariate differences estimated in the model do not measure how reported pain level differences in PDM may differ between racial/ethnic groups. The ordered logistic model, however, also does not estimate separate parameters for each level of the dependent (PDMF) variables, but instead estimates four cut points of the fitted respondent coefficient so the respondent can be assigned to one of the five predicted PDMF categories.
Subsample group sizes were regrettably too small to permit accurate estimation of differential slopes of PDMSLI for AI/ANs or A/PIs, or PDMF for any subpopulation. The AORs in Model 1 and expected percentage rates were calculated as the best estimates of differences in PDMSLI for AI/ANs and A/PIs, and the AOR estimates (Table 2) and expected frequencies were calculated as the best estimates of PDMF differences. Estimation was performed using Stata (version 15.1), and the results were verified through statistical review at the U.S. Census Bureau.
3. Results
3.1. Descriptive characteristics: Individual predisposing factors
The descriptive statistics for each of the subsample groups (Table 1) were evaluated as differing for all reported characteristics using Rao-Scott chi-squared tests of independence at the α = 0.001 level. Of those differences among the risk factors, we wish to note a higher proportion of Black respondents were female (t = 5.8; p < 0.001); Hispanics had the largest proportions of age 18–25 (t = 10.1; p < 0.001) and age 26–35 (t = 12.0; p < 0.001) persons, and also the highest proportion of high school dropouts (t = 9.3; p < 0.001); A/PIs (t = 11.5; p < 0.001), followed by Whites (t = 24.6 among non-A/PIs; p < 0.001), had the highest proportion of college education.
3.2. Individual enabling factors
Individual enabling factors included family income and type of health insurance. Average family income ranged from $40,500 (s.e. = $269) for Black to $70,000 (s.e. = $627) for A/PI respondents. Private insurance was common among all racial/ethnic groups, but was most frequent among Whites (75.5%; s.e. = 0.20%). The lack of any health insurance was highest among Hispanics, and lowest among Whites. The highest rates for public insurance included Medicare for Whites 20.3%; s.e. = 0.14%, Medicaid for Black respondents 12.2%; s.e. = 0.27%, and VA insurance for AI/ANs 6.1%; s.e. = 0.73%.
3.3. Individual need factor
Reported pain levels were used to constitute an individual need factor (i.e., a person's perceived need for health services). AI/ANs reported the lowest level 1 pain (“none” or “no pain whatsoever”) at 52.5% (s.e. = 1.35%). For the middle reported pain levels 2, 3, and 4, AI/ANs reported the highest point estimates for PDMSLI rates and reported pain level 5 “extreme”, at a substantially higher rate (6.9%,t = 2 0.79, p = 0.005) than the other groups.
3.4. PDMSLI: Controlling for Andersen's predisposing factors
Table 2 reports the results of two multivariate logistic regression models estimating the likelihood of PDMSLI as a function of race/ethnicity. The AORs for each group are estimated while controlling for the effects of Andersen (Andersen et al., 1995) predisposing individual and enabling factors measured with information on sex, age, education, income, and health insurance status (Models 1 and 2).
The relationship between reported pain and PDMSLI is complex and bears additional scrutiny. In, Model 1, the AORs for pain levels for White describe a curvilinear relationship as rate of misuse increased as reported pain increased. This relationship peaks at the quite a bit level, and misuse then drops at the level of extreme pain, which is significantly lower than that for the a little bit group (χ2(1) = 12.4, p = 0.0004). The pattern for White respondents in Model 2 was similar, except that misuse among patients experiencing extreme pain declined until misuse was not significantly different from misuse among patients reporting no pain whatsoever (χ2(1) = 0.65, p = 0.42). While A/PI and AI/AN low response rates did not permit calculations of interactions, Hispanic respondents’ interaction terms showed that PDMSLI increased significantly at each of the four increasing levels of reported pain (p < 0.001) until misuse dropped significantly for the extreme pain group (χ2(1) = 267, 13.4, 52.6, 17.6).
For Black respondents, the pattern between PDMSLI and increasing levels of reported pain varied from the other racial/ethnic groups studied. Black respondents’ misuse rates do not significantly differ between moderate pain (level 3) and extreme pain (level 5) (χ2(1) = 0.15, p = 0.69). Fig. 1, reports the estimated AOR and 95% confidence interval from Model 2 for each combination of pain level and race/ethnicity.
3.5. PDMF: Controlling for Andersen's factors
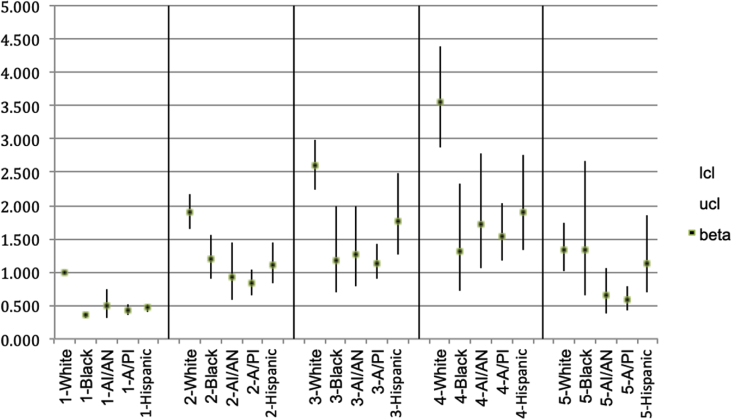
Model 3 (Table 2) represents the multivariate ordinal logistic regression estimating PDMF for each group by sex, age, education, income and health insurance status. In general, the rates for PDMF followed a curvilinear path. The sample sizes for PDMF by reported pain level were not large enough to estimate separate curves for different groups. Thus, the shape of the relationship described by the AORs for pain levels 2 through 4, (relative to the reference level 1.0 for pain level 1) are the best available estimate for the entire sample. That curvilinear shape is similar to that for PDM rates among Whites in Model 1, in that Model 3 estimates higher PDMF as pain increases through the “quite a bit/severe” level (“none” against “little bit” has χ2(1) = 154, p < 0.001; “little bit” against “moderate” has χ2(1) = 20.4, p < 0.001; “moderate” against “quite a bit/severe” has χ2(1) = 8.94, p = 0.004), and then drops for those reporting “extreme” pain (“quite a bit/severe” against “extreme” has χ2(1) = 37.9, p=<0.001). The difference with Model 1 is that PDMF for those reporting “extreme” pain remains higher than for those reporting “none” (χ2(1) = 12.0, p=<0.001).
With two exceptions, the PDMF covariates follow the same pattern as in Model 1 for PDMSLI. The first exception is that respondents with a college education have lower PDMF than those in the baseline high school diploma group. Second, PDMF among those with military/VA insurance is not significantly different than for the non-insured, despite non-insured's higher rate of PDMSLI (See Fig. 2.).
Fig. 2.
AORs for frequency of non-medical psychotherapeutic misuse since last interview, by racial/ethnic group and pain intensity level.
Note: Reference group for AORs is White persons reporting no pain. Pain levels: 1-none; 2-a little bit; 3-moderately; 4-quite a bit; 5-extremely.
The results in this table have been cleared by the Census Bureau's Disclosure Review Board release authorization number CBDRB-FY19-124.
The four groups each have similar AORs for PDMF that are significantly smaller than, and roughly one-half the AOR of the reference group (Whites = 1.00). The only statistically significant differences in AORs between groups (using α = 0.05) is for the Black and A/PI groups, which both have lower AORs than the Hispanic group (Black compared to Hispanic has χ2(1) = 7.34, p = 0.009. A/PI compared to Hispanic has χ2(1) = 4.28, p = 0.042).
4. Discussion
This is the first known study to examine the interactions between of pain intensity and prescription drug misuse among American Indian/Alaska Native, Black, White, Asian Pacific Islander and Hispanic populations. All people who reported Hispanic are counted as such, regardless of their race. We found that prescription drug misuse rates do vary by racial/ethnic groups. Following the Institute of Medicine guidelines for racial/ethnic disparities, we sought to examine if these differences exist not as related to predisposing factors related to access (Smedley et al., 2003). Overall, we found that pain intensity appears to interact with prescription drug misuse/PDM rates in relationship to predisposing and enabling factors from Andersen's Behavioral Model of Health Services Use (Andersen & Davidson, 2001).
Our descriptive findings are consistent with current research that White and AI/AN, as well as other Indigenous populations, experience the highest rates of misuse for all categories of prescription drugs (Blazer & Wu, 2009; Edlund et al., 2010; Kelly et al., 2013; Substance Abuse and Mental Health Services Administration, 2010). As previously supported in the literature (Green, Ndao-Brumblay, West, & Washington, 2005; McCabe et al., 2007), PDM rates were lower among Black and Asian/Pacific Islander respondents. We found that most racial groups experienced a significant decline in PDM rates as pain reached severe levels. This finding may indicate patient agency in the form of requesting medication adjustment or use of other, complementary pain treatments. Moreover, our findings directly contradict the stereotype that Black patients are more likely to misuse prescription drugs as their pain levels increase (Tait & Chibnall, 2014). In fact, Black respondents' PDM did not increase and plateaued at a low rate. Regardless, Black patients may still be undertreated with pain medications due to providers’ unconscious cultural biases and perceptions of misuse.
Though we sought to focus on racial/ethnic disparities while controlling for predisposing factors (e.g., socioeconomic status, education and healthcare coverage), we did find two predisposing factors that may influence the frequency of PDM. Our study found that people who have a college education might misuse prescriptions drugs less than those in the high school diploma group. Our findings support other research that individuals with a college degrees tend misuse prescription drugs at a lower rate than those with less education (Martins et al., 2015). Thus, education may be a protective factor that should be explored in future research examining racial/ethnic disparities in pain research. Secondly, those with military/VA insurance are not significantly different than the non-insured in PDM (See Fig. 2). Thus, veterans who have previously been found more likely to misuse prescriptions drugs for chronic pain (Becker et al., 2009), may have other risk factors than healthcare access that influence their PDM. More research is needed to determine how education and veteran status affects racial/ethnic disparities.
4.1. Provider bias
Research shows that racial/ethnic groups other than whites often receive less effective pain treatment, despite having equivalent reported pain levels (Anderson et al., 2009; Bonham, 2001; Ezenwa et al., 2006; Smedley et al., 2003). The IoM suggests that providers may undertreat certain racial/ethnic persons and prescribe fewer medications than they do for whites (Smedley et al., 2003), which may explain pain care disparities. Providers' implicit racial stereotypes have been found to contribute to these pain treatment disparities (Smedley et al., 2003), perhaps due to providers’ inaccurate assumptions that certain races (i.e., Black and Indigenous persons) may misuse at higher rates (Blair, Steiner, & Havranek, 2011; Tait & Chibnall, 2014; van Ryn & Saha, 2011). Thus, provider treatment decisions may begin with disbelief towards patient pain reporting, especially for ambiguous chronic pain among Black and Indigenous patients (Miner, Biros, Trainor, Hubbard, & Beltram, 2006; Singhal, Tien, & Hsia, 2016). This disbelief may then drive inadequate treatment, especially given that different cultures express severe pain differently (Bates & Rankin-Hill, 1994). The resulting treatment disparities may explain why PDM rates decreased significantly among Whites and Hispanics, once they reached severe pain levels, while PDM rates among Blacks plateaued. More research is needed to determine if this is so.
4.2. Limitations
Our study has several limitations. The NESARC data did not allow for analysis of exact misuse patterns, such as those who misused prescription medications from their healthcare providers and pain intensity levels across time intervals. Tranquilizers, sedatives, stimulants and opioids, the most commonly misused prescription medications, (Manchikanti et al., 2010) and interactions were combined as opioids and did not have enough statistical power for individual analysis. However, their statistical differences when combined were minor. Additionally, the patient's actual healthcare provider referrals, pain assessments and treatment decisions remain unknown. Furthermore, this study focused on racial/ethnic disparities that exist after controlling for predisposing factors, including healthcare access or income, among commonly misused pain related psychotherapeutics (Manchikanti et al., 2010). Though analysis of individual classes of prescriptions were not possible with our data, future research may wish to examine specific classes in regards to racial/ethnic disparities. Nevertheless, the study has several strengths including the large sample size, the diversity of the sample, and the relationship of pain intensity levels to racial/ethnic PDM' making it the first known study of its kind.
4.3. Future research
This study focused on racial/ethnic disparities and controlled for other factors in Andersen's model; future research could examine these additional factors (e.g., health insurance) more thoroughly. Further investigation is needed to explore the causes and barriers within the patient-provider relationship that contribute to pain treatment disparities, including identifying health interventions to prevent discrepancies and bias. Given that Indigenous groups have among the highest mortality rates due to unintentional prescription drug overdose as well as, the highest rates of chronic pain (Jimenez et al., 2011), research is needed to identify the relationship between chronic pain management and PDM risks for Indigenous populations; this was not possible in the current study due to limited data. More research is needed to identify relationships between providers' actual treatment and patient reactions. For instance, initial studies suggest a higher prevalence of genetic polymorphism of CYP2D6 may exist for some groups, causing them to be poor metabolizers of codeines, thus lowering its therapeutic effectiveness (Green et al., 2005). Consequently, these individuals may experience less relief from prescribed medication, frustrate their providers, and may self-manage through increasing their dosage.
5. Conclusion
This is the first known study to explore how chronic pain may relate to prescription drug misuse in relationship to race/ethnicity. This study highlights the need for future research to examine patient health beliefs surrounding perceived need for prescription pain medications and provider medical decision-making for patients from different racial/ethnic groups and to lower health disparities.
Ethical statement
This research article is not under review with any other journals. All authors have read and approved this manuscript. We have no financial disclosures or conflicts of interests to report and acknowledgements are included. No related papers have been submitted for this study.
A University Institutional Review Board deemed this study exempt.
CRediT authorship contribution statement
Michelle Johnson-Jennings: Writing - review & editing, Conceptualization, Methodology, Writing - original draft, Supervision, Visualization. Bonnie Duran: Conceptualization, Writing - review & editing. Jahn Hakes: Validation, Formal analysis, Visualization, Resources, Data curation, Writing - review & editing. Alexandra Paffrath: Project administration, Writing - review & editing, Resources. Meg M. Little: Writing - review & editing, Resources, Project administration, Visualization, Supervision.
Declaration of competing interest
The authors have no conflicts of interests or commercial associations to report for this paper.
Acknowledgements
This work was supported by the following: NIH grants 5R25MH084565-03; 5R25MH084565-03; 5R01DA034466 02 We would also like to acknowledge Dr. Mustafa al’Absi who gave feedback during the earlier drafts of this manuscript and offered support. Dr. Jahn Hakes, from the U.S. Census Bureau Center for Economic Studies, had access to the complete data and assumed responsibility for the data analysis. Any opinions and conclusions expressed herein are those of the authors and do not necessarily reflect the views of the U.S. Census Bureau or the NIAAA. All results have been reviewed to ensure that no confidential information is disclosed. The statistical summaries reported in this document have been cleared by the Census Bureau's Disclosure Review Board release authorization number CBDRB-FY19-124.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ssmph.2020.100563.
Contributor Information
Michelle Johnson-Jennings, Email: mjohnsonjennings@usask.ca.
Bonnie Duran, Email: bonduran@uw.edu.
Jahn Hakes, Email: jahn.k.hakes@census.gov.
Alexandra Paffrath, Email: paffr007@umn.edu.
Meg M. Little, Email: littlem@d.umn.edu.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- American Society of Anesthesiologists Task Force on Chronic Pain Management Practice guidelines for chronic pain management. Anesthesiology. 2010;112(4):810–833. doi: 10.1097/ALN.0b013e3181c43103. [DOI] [PubMed] [Google Scholar]
- Andersen R., Davidson P.L. Improving access to care in America: Individual and contextual indicators. In: Andersen R., Rice T.H., Kominski G.F., editors. Changing the U.S. Health care System : Key issues in health services, policy, and management. 2nd ed. Jossey-Bass; San Francisco: 2001. pp. 3–31.http://web.a.ebscohost.com.ezp1.lib.umn.edu/ehost/ebookviewer/ebook/bmxlYmtfXzU3MDM4X19BTg2?sid=d9420d2a-a998-4d01-8b8c-13cda80e6375@sdc-v-sessmgr03&vid=0&format=EB&lpid=lp_3&rid=0 Retrieved from. [Google Scholar]
- Andersen R., Harada N., Chiu V., Makinodan T. Application of the behavioral model to health studies of Asian and Pacific islander Americans. Asian American and Pacific Islander Journal of Health. 1995;3(2):128–141. http://www.ncbi.nlm.nih.gov/pubmed/11567308 Retrieved from. [PubMed] [Google Scholar]
- Anderson K.O., Green C.R., Payne R. Racial and ethnic disparities in pain: Causes and consequences of unequal care. The Journal of Pain. 2009;10(12):1187–1204. doi: 10.1016/j.jpain.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Bates M.S., Rankin-Hill L. Control, culture and chronic pain. Social Science & Medicine. 1994;39(5):629–645. doi: 10.1016/0277-9536(94)90020-5. [DOI] [PubMed] [Google Scholar]
- Becker W.C., Fiellin D.A., Gallagher R.M., Barth K.S., Ross J.T., Oslin D.W. The association between chronic pain and prescription drug abuse in veterans. Pain Medicine. 2009;10(3):531–536. doi: 10.1111/j.1526-4637.2009.00584.x. [DOI] [PubMed] [Google Scholar]
- Blair I.V., Steiner J.F., Havranek E.P. Unconscious (implicit) bias and health disparities: Where do we go from here? The Permanente Journal. 2011;15(2):71–78. doi: 10.7812/tpp/11.979. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3140753/pdf/i1552-5775-15-2-71.pdf Retrieved from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazer D.G., Wu L.-T. Nonprescription use of pain relievers by middle-aged and elderly community-living adults: National survey on drug use and health. Journal of the American Geriatrics Society. 2009;57(7):1252–1257. doi: 10.1111/j.1532-5415.2009.02306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonham V.L. Race, ethnicity, and pain treatment: Striving to understand the causes and solutions to the disparities in pain treatment. Journal of Law Medicine & Ethics. 2001;28(4_):52–68. doi: 10.1111/j.1748-720x.2001.tb00039.x. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . 2017. Annual surveillance report of drug-related risks and outcomes United States, 2017. [Google Scholar]
- Centers for Disease Control and Prevention . 2018. 2018 annual surveillance report of drug-related risks and outcomes-United States.https://www.cdc.gov/drugoverdose/pdf/pubs/2018- cdc-drug-surveillance-report.pdf Retrieved from. [Google Scholar]
- Chibnall J.T., Tait R.C. The Psychosomatic Symptom Checklist revisited: Reliability and validity in a chronic pain population. Journal of Behavioral Medicine. 1989;12(3):297–307. doi: 10.1007/bf00844873. [DOI] [PubMed] [Google Scholar]
- Cintron A., Morrison R.S. Pain and ethnicity in the United States: A systematic review. Journal of Palliative Medicine. 2006;9(6):1454–1473. doi: 10.1089/jpm.2006.9.1454. [DOI] [PubMed] [Google Scholar]
- Edlund M.J., Martin B.C., Fan M.-Y., Devries A., Braden J.B., Sullivan M.D. Risks for opioid abuse and dependence among recipients of chronic opioid therapy: Results from the TROUP Study. Drug and Alcohol Dependence. 2010;112(1–2):90–98. doi: 10.1016/j.drugalcdep.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezenwa M.O., Ameringer S., Ward S.E., Serlin R.C. Racial and ethnic disparities in pain management in the United States. Journal of Nursing Scholarship. 2006;38(3):225–233. doi: 10.1111/j.1547-5069.2006.00107.x. [DOI] [PubMed] [Google Scholar]
- Gauntlett-Gilbert J., Gavriloff D., Brook P. Benzodiazepines may Be worse than opioids: Negative medication effects in severe chronic pain. Clinical Pain. 2016;32(4):285–291. doi: 10.1097/AJP.0000000000000253. http://ovidsp.dc2.ovid.com.ezp3.lib.umn.edu/sp-4.04.0a/ovidweb.cgi?&S=DEJAFPDLMCEBMKILJPBKEGBFAOPOAA00&Link+Set=S.sh.56%7C16%7Csl_10&Counter5=SS_view_found_article%7C25968447%7Cppezv%7Cmedline%7Cmed13&Counter5Data=25968447%7Cppezv%7Cmedline%7Cmed13 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Grant B.F., Kaplan K.D. 2005. Source and accuracy statement for the 2004–2005 Wave 2 national epidemiologic survey on alcohol and related Conditions. Bethesda, MD. [Google Scholar]
- Grant B.F., Moore T.C., Shepard J., Kaplan K. NESARC); Bethesda, MD: 2003. Source and accuracy statement: Wave 1 national epidemiologic survey on alcohol and related Conditions. [Google Scholar]
- Green C.R., Anderson K.O., Baker T.A., Campbell L.C., Decker S., Fillingim R.B. The unequal burden of pain: Confronting racial and ethnic disparities in pain. Pain Medicine. 2003;4(3):277–294. doi: 10.1046/j.1526-4637.2003.03034.x. [DOI] [PubMed] [Google Scholar]
- Green C.R., Ndao-Brumblay S.K., West B., Washington T. Differences in prescription opioid analgesic availability: Comparing minority and white pharmacies across Michigan. The Journal of Pain. 2005;6(10):689–699. doi: 10.1016/j.jpain.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Hasin D.S., Grant B.F. The national epidemiologic survey on alcohol and related Conditions (NESARC) Waves 1 and 2: Review and summary of findings. Social Psychiatry and Psychiatric Epidemiology. 2015;50(11):1609–1640. doi: 10.1007/s00127-015-1088-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez N., Garroutte E., Kundu A., Morales L., Buchwald D. A review of the experience, Epidemiology, and management of pain among American Indian, Alaska native, and aboriginal Canadian peoples. The Journal of Pain. 2011;12(5):511–522. doi: 10.1016/j.jpain.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Jennings M., Walters K., Little M. And [they] even followed her into the hospital: Primary care providers' attitudes toward referral for traditional healing practices and integrating care for indigenous patients. Journal of Transcultural Nursing. 2017 doi: 10.1177/1043659617731817. [DOI] [PubMed] [Google Scholar]
- Kelly B.C., Wells B.E., LeClair A., Tracy D., Parsons J.T., Golub S.A. Prevalence and correlates of prescription drug misuse among socially active young adults. International Journal of Drug Policy. 2013;24(4):297–303. doi: 10.1016/j.drugpo.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp A., Clark M., Sherman J., Dahl-Smith J. Prescription drug abuse: When the treatment becomes the problem. Journal of the Mississippi State Medical Association. 2013;54(5) http://www.ncbi.nlm.nih.gov/pubmed/23909210 134, 139–140. Retrieved from. [PubMed] [Google Scholar]
- Levi-Minzi M.A., Surratt H.L., Kurtz S.P., Buttram M.E. Under treatment of pain: A prescription for opioid misuse among the elderly? Pain Medicine. 2013;14(11):1719–1729. doi: 10.1111/pme.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchikanti L., Fellows B., Ailinani H., Pampati V. Therapeutic use, abuse, and nonmedical use of opioids: A ten-year perspective. Pain Physician. 2010;13(5):401–435. https://www.painphysicianjournal.com/current/pdf?article=MTM4Mg%3D%3D&journal=57 Retrieved from. [PubMed] [Google Scholar]
- Martins S.S., Kim J.H., Chen L.Y., Levin D., Keyes K.M., Cerdá M. Nonmedical prescription drug use among US young adults by educational attainment. Social Psychiatry and Psychiatric Epidemiology. 2015;50(5):713–724. doi: 10.1007/s00127-014-0980-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe S.E., Morales M., Cranford J.A., Delva J., McPherson M.D., Boyd C.J. Race/ethnicity and gender differences in drug use and abuse among college students. Journal of Ethnicity in Substance Abuse. 2007;6(2):75–95. doi: 10.1300/j233v06n02_06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo L.J., Singhakant S., Cummings S.M., Cottler L.B. Reasons for misuse of prescription medication among physicians undergoing monitoring by a physician health program. Journal of Addiction Medicine. 2013;7(5):349–353. doi: 10.1097/adm.0b013e31829da074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner J., Biros M.H., Trainor A., Hubbard D., Beltram M. Patient and physician perceptions as risk factors for oligoanalgesia: A prospective observational study of the relief of pain in the emergency department. Academic Emergency Medicine. 2006;13(2):140–146. doi: 10.1111/j.1553-2712.2006.tb01662.x. [DOI] [PubMed] [Google Scholar]
- Peteet B.J. Psychosocial risks of prescription drug misuse among U.S. Racial/ethnic minorities: A systematic review. Journal of Ethnicity in Substance Abuse. 2019;18(3):476–508. doi: 10.1080/15332640.2017.1381662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phinney J.S., Ong A.D. Conceptualization and measurement of ethnic identity: Current status and future directions. Journal of Counseling Psychology. 2007;54(3):271–281. doi: 10.1037/0022-0167.54.3.271. [DOI] [Google Scholar]
- van Ryn M., Saha S. Exploring unconscious bias in disparities research and medical education. Journal of the American Medical Association. 2011;306(9) doi: 10.1001/jama.2011.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl L., Seth P., Kariisa M., Wilson N., Baldwin G. Drug and opioid-involved overdose deaths - United States, 2013-2017. MMWR. Morbidity and Mortality Weekly Report. 2018;67(5152):1419–1427. doi: 10.15585/mmwr.mm675152e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal A., Tien Y., Hsia R. Racial-ethnic disparities in opioid prescriptions at emergency department visits for Conditions commonly associated with prescription drug abuse. PloS One. 2016;11(8) doi: 10.1371/journal.pone.0159224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smedley B., Stith A., Nelson A. National Academies Press; Washington, D.C: 2003. Unequal treatment: Confronting racial and ethnic disparities in health care. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . DHHS Publication No. SMA 02-3759; Rockville, MD: 2002. Results from the 2001 national household survey on drug abuse: Volume II. Technical appendices and selected data tables, NHSDA series H-18. [Google Scholar]
- Substance Abuse and Mental Health Services Administration . NSDUH Series H-38A, HHS Publication No. SMA 10-4586Findings; Rockville, MD: 2010. Results from the 2009 national survey on drug use and health: Volume I. Summary of national findings.http://www.oas.samhsa.gov Retrieved from. [Google Scholar]
- Tait R.C., Chibnall J.T. Racial/ethnic disparities in the assessment and treatment of pain. American Psychologist. 2014;69(2):131–141. doi: 10.1037/a0035204. [DOI] [PubMed] [Google Scholar]
- Wachholtz A., Ziedonis D., Gonzalez G. Comorbid pain and opioid addiction: Psychosocial and pharmacological treatments. Substance Use & Misuse. 2011;46(12):1536–1552. doi: 10.3109/10826084.2011.559606. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.