Introduction
Providing medically competent care for gender minorities has become a public health focus. Body-gender incongruence, antitransgender biases, and social isolation contribute to a lifetime risk of suicide attempts in this population greatly exceeding that of the overall US population (25%-43% vs 4.6%).1 Gender-affirming hormone therapy (HT) provides several desired physical and physiologic changes and can reduce suicidal ideation by nearly one half2; however, it can also induce unwanted dermatologic side effects.3 A growing body of literature is emerging to define these effects and their management, which commonly include acne, changes in hair distribution or density, and hyperpigmentation.3,4 An increased susceptibility to female predominant autoimmune conditions has also been suggested,5 raising concern that transgender females on long-term HT may additionally be at risk for other conditions commonly linked to estrogen exposure, such as porphyria cutanea tarda (PCT), erythema nodosum, vascular lesions, and cutaneous manifestations of changes in bile or lipid metabolism.6
We report the case of a 55-year-old woman (male-to-female transgender) who presented with PCT following a change in HT that led to supratherapeutic estrogen. To our knowledge, PCT arising in a transgender patient taking HT has not been reported. The current lack of clear evidence-based HT treatment algorithms and barriers to HT access foster therapeutic inconsistency and hormone level fluctuations, which increase the risk of PCT and other cutaneous side effects of HT in transgender females.
Case report
A 55-year-old woman (transgender male-to-female) maintained on oral estradiol for 23 years presented with a 3-month history of burning pain, pruritus, and recurrent blisters on her forearms and hands after exposure to sunlight. One month before symptom onset, the patient initiated a trial of micronized progesterone (100 mg/d) and increased her daily estradiol from 2 mg to 4 mg in an effort to better control gender dysphoria. Her subsequent laboratory findings were notable for a supratherapeutic total estrogen level (1945 pg/mL). Other medications included lisinopril, spironolactone, medroxyprogesterone, and occasional ibuprofen. She denied a personal or family history of liver disease, hepatitis, iron abnormalities, or blistering eruptions. She consumed 2 beers daily and had a 30-pack-year smoking history.
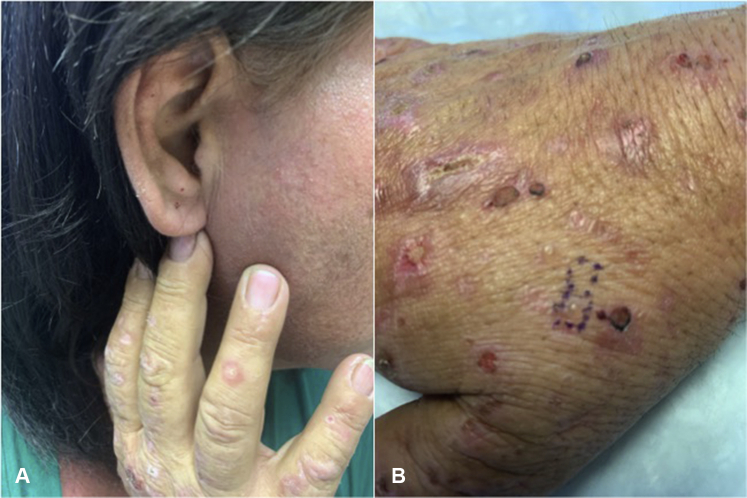
Physical examination confirmed the presence of vesicles and bullae on erythematous bases with scattered milia over the bilateral dorsal forearms, hands, and ears (Figs 1 and 2). Hypertrichosis was not observed or endorsed by the patient. Punch biopsy found a subepidermal blister overlying festooning of the dermal papillae with minimal superficial perivascular lymphohistiocytic infiltrate and a negative immunofluorescence examination (Fig 3). A periodic acid–Schiff stain was negative. Laboratory findings revealed mild hepatic transaminitis, negative HIV and hepatitis C antibodies, unremarkable iron studies, elevated total serum porphyrins, urine uroporphyrin and heptaporphyrin, and HFE C282Y heterozygosity (Table I). A liver ultrasound scan was recommended but not obtained. A diagnosis of PCT was discussed with the patient. She was counseled on smoking cessation, limiting alcohol consumption, and photoprotection. She became distressed upon learning that estrogen may be contributing to her symptoms but ultimately decided to temporarily hold estrogen therapy. Hydroxychloroquine, 100 mg twice weekly, was initiated in lieu of repeated phlebotomy due to patient preference. She achieved clinical remission within 5 months without restricting her alcohol or tobacco use. At that time, estradiol was reintroduced via 0.025-mg patch twice weekly without recurrence.
Fig 1.
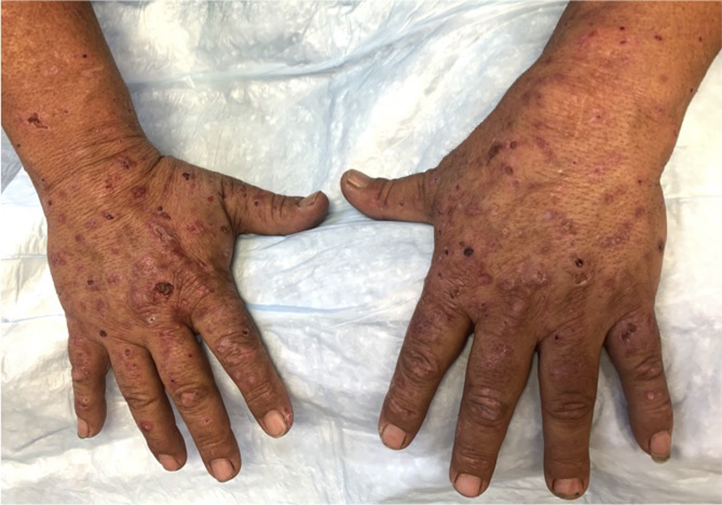
PCT. Vesicles and ruptured bullae on erythematous bases with scattered milia over the bilateral dorsal forearms and hands.
Fig 2.
PCT. Vesicles and ruptured bullae on erythematous bases with scattered milia over the (A) ear lobule and (B) dorsal hand.
Fig 3.
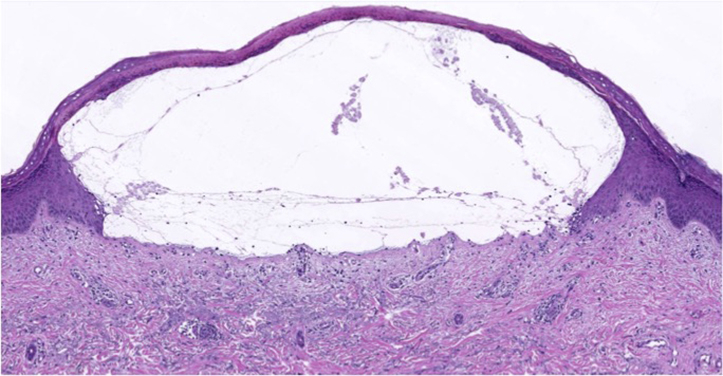
PCT. Hematoxylin-eosin stain shows subepidermal blister overlying festooning of the dermal papillae with minimal superficial perivascular lymphohistiocytic infiltrate. Direct immunofluorescence was negative.
Table I.
Laboratory values from a woman with PCT and HFE C282Y heterozygosity
| Level | Reference range | ||
|---|---|---|---|
| Plasma Porphyrins | |||
| Total | 205.8 (H) | 1.0-5.6 μg/L | |
| Random urine porphyrins | |||
| Uroporphyrin I | 1715.9 (H) | 3.6-21.1 μg/g creat | |
| Uroporphyrin | 1084.3 (H) | ≤ 5.6 μg/g creat | |
| Heptaporphyrin | 934.8 (H) | ≤ 3.4 μg/g creat | |
| Hexaporphyrin | 3.1 | ≤ 6.3 μg/g creat | |
| Pentaporphyrin | 44.1 (H) | ≤ 4.1 μg/g creat | |
| Coproporphyrin I | 75.1 (H) | 6.5-33.2 μg/g creat | |
| Coproporphyrin III | 14.2 | 4.8-88.6 μg/g creat | |
| Total porphyrins | 3871.5 (H) | 27-153.6 μg/g creat | |
| Hemoglobin and iron studies | |||
| HgB | 14.1 | 11.7-15.5 g/dL | |
| Hct | 41.4 | 35%-45 % | |
| Total iron | 101 | 45-160 μg/dL | |
| Iron binding capacity | 314 | 250-450 μg/dL | |
| % Saturation | 32 | 16-45 | |
| Ferritin | 189 | 16-232 ng/mL | |
| Hepatic enzymes | |||
| Alk Phos | 77 | 33-130 U/L | |
| AST | 29 | 10-35 U/L | |
| ALT | 42 (H) | 6-29 U/L | |
| Infectious diseases | |||
| HIV Ag/Ab | Nonreactive | Nonreactive | |
| Hepatitis C Ab | Nonreactive | Nonreactive | |
| Hemochromatosis alleles | |||
| HFE C282Y | Heterozygous | ||
| HFE 63D |
Negative |
||
| Hormone levels |
Estradiol (pg/mL) |
Total estrogen (pg/mL) |
Other |
| 02/2019 | 170 | — | — |
| 03/2019 | 170 | 1949 | Prolactin,10.2 ng/mL |
| 04/2019 | — | 958 | — |
| 06/2019 | 61 | — | Total testosterone, 23 ng/dL |
| 08/2019 | — | 173 | — |
Alk Phos, Alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; Creat, creatinine; H, high; Hct, hematocrit; HgB, hemoglobin.
Discussion
Here we report a case of PCT arising in a male-to-female transgender patient on gender-affirming HT. In PCT, decreased uroporphyrinogen III decarboxylase activity in hepatocytes leads to the accumulation of porphyrins in the skin, provoking photosensitivity. PCT is uncommon but has been associated with estrogen, iron accumulation, hepatotoxins, alcohol consumption, tobacco use, hepatitis C, HIV, and mutations in the hemochromatosis alleles, HFE C282Y and H63D.7
A growing number of patients are seeking out gender-affirming HT to achieve physical, emotional, and social characteristics consistent with their gender identity.8 The prolonged need to suppress endogenous sex hormone secretion and maintain hormone levels within the range consistent with gender identity exposes transgender patients to higher hormone doses relative to other indications for hormone administration.9,10 Lack of strong evidence-based guidelines and inadequate provider training regarding the optimal drugs, dosage, and routes lead to significant variability in gender-affirming HT regimens.11 Treatment is often guided by patients eager to maximize results8 and based on social media reports of what has worked for others.12,13 Pharmacokinetic variability is further driven by cost and drug shortages, which prompt changes in formulation and potential use of black-market products.8,14,15
Our patient had PCT after 23 years taking 1 to 4 mg oral estradiol therapy daily. She had intermittently been on progesterone, and immediately prior to the onset of her symptoms simultaneously increased her estradiol and began a trial of oral micronized progesterone. Her subsequent supratherapeutic serum estrogen prompted discontinuation of micronized progesterone and a 1-month break in estradiol followed by dose reduction. The timeline suggests that supratherapeutic estrogen may have combined with other risk factors (HFE heterozygosity and tobacco and alcohol use) to trigger PCT. She previously tolerated 4 mg estradiol daily, raising concern that combination with micronized progesterone (selected for promotional pricing and reported anxiolytic effects) may have also contributed. Although total estrogen was elevated (goal, 600-1000 pg/mL), serum estradiol and prescribed HT dosing were within published expert opinion–based guidelines.8,14,15
The treatment of PCT requires removing or reducing known risk factors. Although it was not an easy decision, our patient achieved clinical remission by temporary estrogen discontinuation followed by change in route of delivery. When gender-affirming HT is potentially contributing to PCT, a patient-centered discussion of risk versus benefits is paramount. HT for transgender patients is life long and addresses physical and psychological aspects of gender dysphoria.8 General distrust of the health care community among transgender patients may make conversations about adjusting HT better received when they involve the prescribing provider, with whom the patient has an established relationship of trust.16 Although our patient did not maintain other recommended lifestyle changes, such as smoking and alcohol reduction, lifestyle modifications should be encouraged.7
HT and consideration of its cutaneous side effects will continue to grow as the number of transgender patients presenting for health care continues to increase. It is important to recognize the potential risk for PCT in this growing demographic and consider a multifaceted treatment approach that includes HT adjustment as a therapeutic option, while being mindful of its important role in affirming gender identity.
Footnotes
Funding sources: None.
Conflicts of interest: Dr Cullison has served as a consultant for DermTech. The rest of the authors have no conflicts to disclose.
References
- 1.Haas A.P., Rodgers P.L., Herman J.L. Suicide attempts among transgender and gender non-conforming adults: findings of the national transgender discrimination survey. American Foundation for Suicide Prevention, Williams Institute 2014. https://williamsinstitute.law.ucla.edu/wp-content/uploads/AFSP-Williams-Suicide-Report-Final.pdf Available at:
- 2.Bauer G.R., Scheim A.I., Pyne J. Intervenable factors associated with suicide risk in transgender persons: a respondent driven sampling study in Ontario, Canada. BMC Public Health. 2015;15:525. doi: 10.1186/s12889-015-1867-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sullivan P., Trinidad J., Hamann D. Issues in transgender dermatology: a systematic review of the literature. J Am Acad Dermatol. 2019;81(2):438–447. doi: 10.1016/j.jaad.2019.03.023. [DOI] [PubMed] [Google Scholar]
- 4.Yeung H., Kahn B., Ly B.C. Dermatologic conditions in transgender populations. Endocrinol Metab Clin North Am. 2019;48(2):429–440. doi: 10.1016/j.ecl.2019.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mundluru S.N., Larson A.R. Medical dermatologic conditions in transgender women. Int J Womens Dermatol. 2018;4(4):212–215. doi: 10.1016/j.ijwd.2018.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolognia J., Jorizzo Joseph L., Schaffer Julie V., editors. Dermatology. Elsevier Suanders; Philadelphia: 2012. [Google Scholar]
- 7.Bissell D.M., Anderson K.E., Bonkovsky H.L. Porphyria. N Engl J Med. 2017;377(9):862–872. doi: 10.1056/NEJMra1608634. [DOI] [PubMed] [Google Scholar]
- 8.Deutsch M. Overview of feminizing hormone therapy. UCSF Center of Excellence for Transgender Health: Transgender Care and Treatment Guidelines 2016. https://transcare.ucsf.edu/guidelines/feminizing-hormone-therapy Available at:
- 9.Modrono Mostoles N., Pavon de Paz I., Guijarro de Armas G. A case of hypopituitarism and porphyria cutanea tarda in relation to estrogen therapy in a patient with empty sella syndrome. Rev Clin Esp. 2015;215(2):e11–e13. doi: 10.1016/j.rce.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 10.Barton J.C., Edwards C.Q. Porphyria cutanea tarda associated with HFE C282Y homozygosity, iron overload, and use of a contraceptive vaginal ring. J Community Hosp Intern Med Perspect. 2016;6(1):30380. doi: 10.3402/jchimp.v6.30380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geffen S., Horn T., Smith K.J. Advocacy for gender affirming care: learning from the injectable estrogen shortage. Transgend Health. 2018;3(1):42–44. doi: 10.1089/trgh.2017.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grant J., Mottet L., Tanis J. National Center for Transgender Equality and National Gay and Lesbian Task Force; Washington: 2011. Injustice at every turn: a report of the national transgender discrimination survey. [Google Scholar]
- 13.Powers W. Healthcare of the transgender patient and the powers method of hormonal transitioning, version 5.4. Powers Family Medicine 2019. https://drive.google.com/drive/folders/112o11ykp0H-8tU_SbIToT1aZwL6LCK0S?fbclid=IwAR3A9mia4qCWknAqXjecF5rJnMX8bgD5AuLoJ8zdnBBW31_lh9EuOxepSLQ Available at:
- 14.Coleman E., Bockting W.O., Botzer M.C. 7th ed. World Professional Association for Transgender Health; 2012. Standards of care for the health of transsexual, transgender, and gender nonconforming people.https://wpath.org/publications/soc Available at: [Google Scholar]
- 15.Hembree W.C., Cohen-Kettenis P.T., Gooren L. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2017;102(11):3869–3903. doi: 10.1210/jc.2017-01658. [DOI] [PubMed] [Google Scholar]
- 16.Rosendale N., Goldman S., Ortiz G.M. Acute clinical care for transgender patients: a review. JAMA Intern Med. 2018;178(11):1535–1543. doi: 10.1001/jamainternmed.2018.4179. [DOI] [PubMed] [Google Scholar]