Abstract
In everyday life, children often need to engage control in emotionally or motivationally relevant contexts. This study disentangled and directly compared the respective influences of external rewards and positive stimuli on childhood cognitive control. We expected external rewards to promote proactive cognitive control and positive stimuli to impair proactive control, especially in younger age. EEG data were recorded while children (5–6 years old and 9–10 years old) and adults completed a cued task-switching paradigm in three conditions: positive-stimulus, external-reward and control conditions. Provision of reward resulted in more accurate but slower responses, and more pronounced cue-locked posterior positivity, potentially suggesting general proactive mobilisation of attention (i.e., readiness). Despite no effects on behaviour, the presentation of positive stimuli was unexpectedly associated with a greater cue-locked extended slow-wave when task cues were presented ahead of targets (i.e. proactive-control possible) in younger children, suggesting greater proactive cue preparation. In contrast to our hypothesis, both external rewards and positive stimuli seem to promote different types of proactive approaches in children.
Keywords: Children, Cognitive control, Event-related potentials, Proactive control, Positive stimuli, Reward motivation
1. Introduction
Cognitive control – the goal-directed regulation of attention and actions – is often carried out in affective contexts. Children may be more likely to help with housework if they are in a good mood or promised pocket money in exchange. Cognitive control in motivationally or emotionally significant contexts is often referred to as “hot” cognitive control (e.g. Zelazo and Carlson, 2012; Zelazo et al., 2010). Studies so far have usually lumped motivational and emotional states together, overlooking their potentially distinct contributions to cognitive control in children. Therefore, the current study aimed to dissociate and directly compare motivational and emotional influences on childhood cognitive control, which will not only help better understand how cognitive control develops from the perspective of affective-cognitive interactions, but may also inform on practical implications (e.g., how to keep children on task).
Although positive emotion and reward motivation are closely related to each other and both may reflect increased dopamine releasing (Ashby et al., 1999; Schultz, 1992), they may be functionally dissimilar (Chiew and Braver, 2014; Dreisbach and Fröber, 2019; Goschke and Bolte, 2014). Empirical evidence, albeit scarce, supports the dissociable effects of positive emotion and reward motivation on children’s cognitive control. Positive emotion induced by happy faces facilitates task switching (Qu and Zelazo, 2007; Wong et al., 2008), but impairs inhibition (Kramer et al., 2015; Tottenham et al., 2011). Conversely, the expectation of external reward can enhance children’s inhibition (Qu et al., 2013) and working memory (Atkinson et al., 2019), while results are mixed regarding task switching (Qu et al., 2013; Somerville and Casey, 2010; Strang and Pollak, 2014). However, as emotion or motivation have been investigated separately in these previous studies, it is difficult to directly compare their respective effects on children’s cognitive control.
Positive emotion and reward motivation have been argued to serve different adaptive functions (Goschke and Bolte, 2014; Dreisbach and Fröber, 2019). As positive emotion signals safety and security in the surrounding environment, it may encourage spreading attention to new opportunities, which could then promote flexibility (i.e. shifting of goals and task-sets) (Fredrickson, 2013; Pessoa, 2009). In contrast, expectation of external rewards may serve as a motivational signal that promotes cognitive effort, hence enhancing cognitive stability (i.e. maintenance of goals and task-sets) (Braver, 2012; Hefer and Dreisbach, 2017).
Cognitive flexibility and stability map onto the distinction between reactive and proactive control from the Dual Mechanisms of Control (DMC) framework (Braver, 2012; Braver et al., 2007). Proactive control refers to the anticipation and preparation for an upcoming task through sustained activation of task-relevant information (e.g. task-related cues), while reactive control is less effortful and works as a transient “late-correction” mechanism while actually performing the task. The event-related potential (ERP) technique is a frequently used approach for measuring this temporal dynamic of control, especially for proactive control. Proactive cue preparation can be indicated by a cue-locked late posterior positivity over parietal channels: an initial peak attributed to task selection (e.g. Jamadar et al., 2010; Manzi et al., 2011) and an extended slow-wave positivity reflecting cue maintaining and updating in working memory (e.g. Manzi et al., 2011). In childhood, similar ERP effects can be detected in 5 years old: higher amplitude of the initial peak (Chevalier et al., 2015; Manzi et al., 2011) and the slow wave (Elke and Wiebe, 2017; Troller‐Renfree et al., 2020) were observed when children engaged more proactive control.
In adults, positive emotion favours flexibility/reactive control while motivation supports stability/proactive control (for a review, see Goschke and Bolte, 2014). Specifically, monetary reward facilitates proactive control through stable maintenance of task-relevant information and greater use of task-cues in preparation for upcoming tasks (Chiew and Braver, 2013, 2014; Fröber and Dreisbach, 2014, 2016; Walsh et al., 2019). By contrast, the presentation of positive stimuli is associated with a trend towards decreased proactive control (Dreisbach, 2006), helping to solve unexpected conflicts through more flexible exploratory but less task-related processing (Bolte and Goschke, 2010; Fröber and Dreisbach, 2014).
An open question is whether emotion and motivation may similarly affect reactive and proactive control engagement in children, who often struggle to coordinate these control modes (Chevalier, 2015). Unlike adults who flexibly engage either control mode based on task demands (Botvinick and Braver, 2015), children under 6 years tend to over rely on reactive control, despite being capable of proactive engagement when encouraged to do so (Chevalier et al., 2015; Hadley et al., 2020). As growing older, children engage proactive control with growing flexibility (Chatham et al., 2009; Chevalier et al., 2015, 2014).
The present study tested the hypothesis that reward motivation scaffolds stable cognitive effort and proactive control, while positive-emotional stimuli may distract children’s attention away from the task, decreasing proactive control. Critically, these effects may change as proactive engagement improves during childhood. Based on previous findings, we targeted three age groups: 5- to 6-year-olds, who seem to engage mostly reactive control despite being capable of proactive control (e.g. Chevalier et al., 2015; Hadley et al., 2020); 9- to 10-year-olds, who seem to more spontaneously engage proactive control (e.g. Chatham et al., 2009; Chevalier et al., 2014); and young adults, who flexibly engage either control mode (Botvinick and Braver, 2015). Participants completed a cued task-switching paradigm, requiring them to switch between matching targets by age and gender according to a task cue. The cue was presented either ahead of the target, making proactive cue preparation possible, or on task onset, to make preparation and to encourage reactive engagement (Chevalier et al., 2015). As no overt response was expected before target onset, proactive engagement was measured through a cue-locked late posterior positivity: an initial positive peak (e.g. Chevalier et al., 2015), and an extended slow-wave positivity (e.g. Elke and Wiebe, 2017). Positive emotion and motivation were manipulated by varying the type of stimuli (positive vs. neutral faces) and offering reward for correct response. We expected external rewards to promote proactive control, as evidenced by more pronounced cue-locked posterior positivity and faster response, whereas the presentation of happy faces should impair proactive control. These effects should be more pronounced in younger children, as proactive engagement is more dependent on contextual incentives at that age than later in childhood and adulthood (Chevalier et al., 2015; Hadley et al., 2020). The current study could provide empirically evidence on whether and (if so) how different affective factors (i.e. reward vs. positive stimulus) differently influence cognitive control during childhood.
2. Method
2.1. Participants
Study participants included 30 5- to 6-year-old children (Mage = 6.25 years, SD = 0.34 years, 14 female), 29 9- to 10-year-old children (Mage = 9.71 years, SD = 0.62 years, 14 female) and 32 adults (Mage = 18.38 years, SD = 0.71 years, 25 female). Two additional 5-year-old children, one additional 9-year-old children and four adults were excluded due to technical errors or incomplete data. Before participating, written consents were obtained from all adult participants and parents of child participants. During the EEG data processing, due to limited number of good segments caused by head movements during the experiment, 10 younger children, 5 older children and 2 adults were additionally excluded from ERPs analyses (with details described in Section 2.3.2). In total, for ERPs analyses, 20 younger children (5- to 6-year-olds, Mage = 6.25 years, SD = 0.31 years, 10 female), 24 older children (9- to 10-year-olds, Mage = 9.83 years, SD = 0.62 years, 12 female) and 30 adults (Mage = 18.33 years, SD = 0.66 years, 24 female) were included.
2.2. Procedure and materials
A trained experimenter tested all participants individually in a single 90-minute session in the laboratory. After fitting the EEG cap, all participants completed a cued task-switching paradigm (introduced as the “Finding a Friend” game) in three conditions (counterbalanced order): control, positive-stimulus (emotional) and external-reward (motivational) conditions. Participants threaded colourful beads for 3 min in between conditions to ensure their arousal states returned to baseline and did not carry over to the next condition. In the positive-stimulus condition, happy faces were used as stimuli expected to induce a positive mood, whereas neutral faces were used in the control and external-reward conditions. A total of 24 pictures of both male and female children and adults’ happy and neutral faces from Developmental Emotional Faces Stimulus Set (DEFSS, Meuwissen et al., 2017) were used in the task. The pictures were drawn from DEFSS (Meuwissen et al., 2017) according to identification accuracy (“What emotion do you think this face is showing?”) and intensity (“How strong is the emotion?”). Happy faces were chosen if they were identified frequently as Happy (average percentage of correct identification ratings: M = 96.25 %) and rated high on happy emotion (average intensity – “How strong is the happy emotion?” – M = 5.48, out of 7). Neutral faces were chosen if they were identified frequently as Neutral (average percentage of correct identification ratings: M = 88.08 %) and rated high on neutral emotion (average intensity – “How strong is the neutral emotion?” – M = 4.44, out of 7). 15 undergraduate students were then recruited to normatively rate the selected photographs by answering “How much do you feel happy when looking at the picture?” on a 10-point Likert-scale (1 = very unhappy, 10 = very happy). The normative intensity ratings differed significantly for happy faces (M = 7.86) and neutral faces (M = 4.58), t (22) = −47.50, p < .001. Additionally, in the external-reward condition, participants were rewarded with a virtual candy after each correct response. Participants were told that they could trade all the virtual candies they accumulated for real candies (children) or money (adults) at the end of the session. After completing the task, children received a £3 candy voucher (regardless of actual performance) and their accompanying parents received £10 as compensation for their time and travel expenses. Adult participants received 1.5-course credits and an extra £3 compensation (regardless of actual performance).
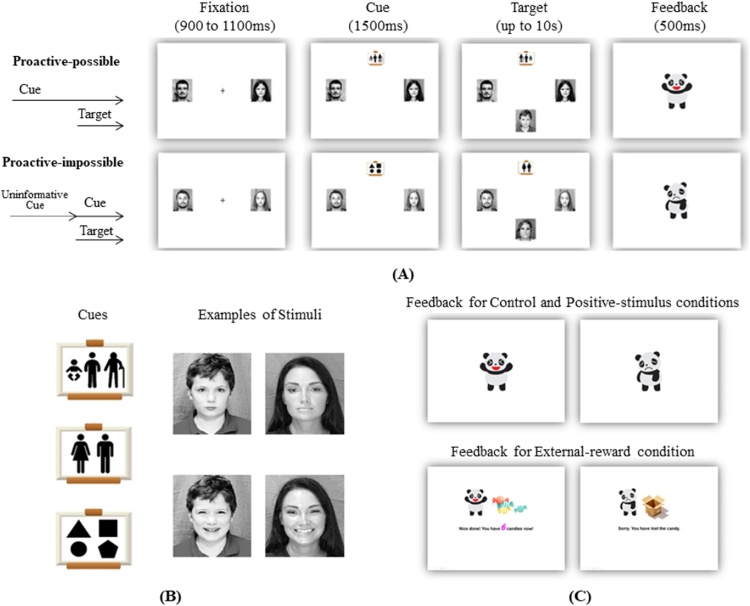
The task was run with E-Prime 2.0 (Psychology Software Tools, Pittsburgh, PA). On each trial, participants matched a target face (central bottom) with one of the two response faces (left and right side of the screen) by pressing the corresponding key on the keyboard (“f” for left side picture, “j” for right side picture) (Fig. 1). To reduce head movements and to respond as fast as possible, during the task, participants were asked to constantly keep their index fingers on the keyboard. Each trial started with a fixation cross presented for 900–1100 ms (jittered inter-trial interval) with two response faces on the two sides, which was then followed by either a task cue (age or gender cue) or an uninformative cue (geometrical shapes). After 1500 ms, the target face showed up on the central bottom of the screen until a response was detected or for up to 10 s (whichever came first). Finally, feedback on response accuracy was presented for 500 ms. Critically, the timing of cue presentation was manipulated across blocks. In proactive-possible blocks, the task cue was presented for 1500 ms before the target onset and remained visible afterwards, hence enabling participants to proactively process the cue information in preparation for the upcoming target. In contrast, in proactive-impossible blocks, an uninformative cue was presented ahead of the target, and the task cue was presented on target onset, hence making proactive cue processing impossible, although participants could still proactively mobilise attention for the upcoming trial. To ensure that there was a perceptual change at the level of the cue in both block types, mirror images of the two cues were used and presented for target onset phase in proactive-possible blocks. The order of cue block types was counterbalanced across participants. All participants were visually and orally informed about the change in cue presentation before they started each block. Within blocks, the two tasks (i.e. age-matching or gender-matching) switched unpredictably.
Fig. 1.
(A) Illustration of the cued task-switching paradigm used in each condition. Participants sorted pictures by age or gender. In proactive-possible blocks, the task cue was released before the target, whereas it was presented on target onset in the proactive-impossible blocks. (B) Examples of cues and face targets used in the task. Neutral faces were used in the control and external-reward conditions, while happy faces were used in the positive-stimulus condition. (C) Feedback used for three conditions: (un)happy panda used in the control and positive-stimulus conditions, additional candy counting used in the external-reward condition.
Participants first completed three demonstration trials for age matching, three demonstration trials for gender matching, four demonstration trials for mixed matching, and six practice trials for mixed matching (without guidance from the experimenter). The practice could be repeated when necessary. Then, participants completed two test blocks (one proactive-possible and one proactive-impossible block) of 34 trials each in all three conditions (control, positive-stimulus, external-reward conditions; 204 test trials in total). Each block contained 16 switch trials and 16 non-switch trials, which were presented randomly, as well as two start trials (due to a short break in each block).
2.3. Data processing
2.3.1. Behavioural
Reaction times (RTs) were only analysed for correct trials after removing values below 200 ms or over M + 3SD (total 6.9 % of trials). All analyses were conducted on log-transformed RTs to correct for skew and minimise age-related baseline differences (Meiran, 1996). As more trials were excluded during the processing of ERP data (as detailed below) and previous research did not observe any interaction between cue presentation timing and trial type (Chevalier et al., 2015), switch and non-switch trials were collapsed for ERP data analysis in order to maximise signal-to-noise ratio. For the sake of consistency, trial types were also collapsed for behavioural data analysis (see Supplementary Material for analyses separating trial types).
2.3.2. Event-related potentials
EEG data were recorded at a 512 Hz sampling rate using 64-channel BioSemi ActiveTwo system (BioSemi, Amsterdam, Netherlands). Impedances were kept under 50 kΩ during recording. The EEG data were processed using EEGLAB 14 (Delorme and Makeig, 2004) and EP Toolkit 2.75 (Dien, 2010a) in MATLAB R2013b (The Mathworks Inc., Natick, MA). A 0.1−30 Hz bandpass filter was first applied to the raw data. The continuous data were then segmented into 5000 ms intervals (1000 ms before and 4000 ms after the cue/pre-cue onset). Bad channels were identified and removed using EEGLAB automatic channel rejection algorithms (spectrum criteria in a range 1−250 Hz on normalized data with 5SD threshold). An average of 0.9 channels was removed per participant (range from 1 to 5 channels). The number of removed channels did not differ between age groups, F (2, 88) = .284, p = .754. Second, independent components analysis (ICA) was implemented on the data excluding bad channels by using the “runica” function and ADJUST 1.1.1 (Mognon et al., 2011) in EEGLAB to remove eye blink artefacts. Third, removed channels were spherically interpolated and the data were re-referenced to the average of all channels.
The epochs of correct trials were further divided into 1700 ms segments. They encompassed the time window of cue processing (200 ms before and 1500 ms after the cue onset), indicating proactive control engagement. The segments were baseline corrected using the respective 200 ms pre-event baselines. Switch and non-switch trials were collapsed to increase signal-to-noise ratio. Participants needed at least 10 good segments in each experimental cell (e.g. DeBoer et al., 2005) to be included in the analyses. Therefore, 10 younger children, 5 older children and 2 adults with not enough good segments were excluded from the ERP analyses (i.e. less than 10 good segments in each cell, mostly due to low response accuracy and bad signals caused by motion artefacts). On average, younger children (N = 20) had 26 good segments (M = 26.07, SD = 2.78), older children (N = 24) had 28 good segments (M = 28.21, SD = 2.64) and adults (N = 30) had 30 good segments (M = 30.22, SD = 1.95) per experimental cell (out of 32).
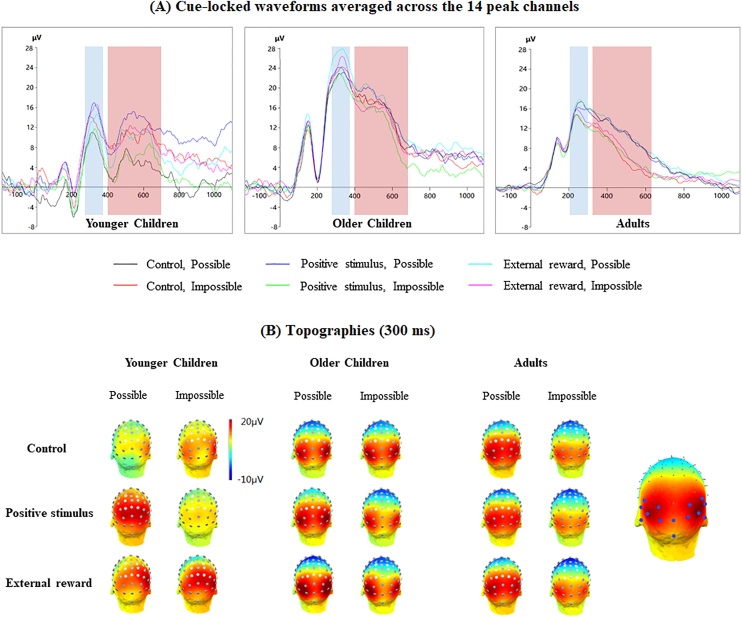
Principal component analyses (PCAs) were conducted in EP Toolkit (Dien, 2010a) to circumscribe the peak channels. This approach ensures channel selection in a more objective fashion than mere visual detection (Dien, 2012). Following recommendations for this approach (Dien, 2010b; Dien and Frishkoff, 2005), first, the data were analysed in temporal mode with Promax rotation. Based on the Scree Plot, six temporal factors were retained in this step, which accounted for 97.03 % of the temporal variance in the ERPs data. Second, a spatial mode with Infomax rotation was used, which further retained two spatial factors (accounted for 83.02 % of the spatial variance). Following topographical check showed that the second factor, which accounted for 16.02 % of the variance, matched the detected late posterior positivity suggested by previous reports (e.g. Chevalier et al., 2015; Manzi et al., 2011). Therefore, waveforms for each age groups were averaged across the 14 channels that loaded on the second factor over 0.6 (Dien, 2010a): 22, 23, 24, 25, 26, 27, 28, 29, 59, 60, 61, 62, 63 and 64 (Fig. 2B right).
Fig. 2.
(A) the cue-locked waveforms of younger children, older children and adults (averaged across the 14 peak channels) are shown in the left, middle and right panel, respectively. The time windows of the posterior positive peak and extended slow-wave are highlighted in blue and red, respectively. (B) Topographies of the posterior positivity for each age group are shown on the left. The topography of peak channels (factor loading > 0.6) is shown on the right (used channels are marked in blue) (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
The cue-locked waveforms showed an initial posterior peak (known to reflect task selection, e.g. Chevalier et al., 2015; Manzi et al., 2011) at around 250 ms in adults and 320 ms in children, which then extended into a positive-going slow-wave (reflecting cue information maintenance and updating in working memory, e.g. Elke and Wiebe, 2017) around 400 ms–700 ms. Therefore, by adding and subtracting 50 ms from the time point of the initial posterior peak to choose the time window, the initial posterior peak was determined as the mean amplitude between 200 ms and 300 ms in adults and the mean amplitude between 270 ms and 370 ms in children after the cue onset within each condition. Then, to better capture the extended slow wave, the time window was chosen as between 400 ms and 700 ms after cue onset in children and the time window between 320 ms and 620 ms after cue onset in adults by visual inspection. The extended slow-wave amplitude was indexed by the mean amplitude of this 300-ms time window after the initial posterior peak.
2.4. Data analysis
Behavioural and EEG data analyses were conducted in IBM SPSS Statistics (Version 24; SPSS Ins., Chicago, IL). Repeated measures ANOVAs were used to identify differences of accuracy, RTs and ERPs data as a function of age group (younger children, older children, adults), condition (control, positive stimulus, external reward), and cue block type (proactive-possible, proactive-impossible). Significant interactions were examined with post hoc tests using Bonferroni correction. Greenhouse-Geisser corrections were also used if the assumption of sphericity was violated. Given the gender imbalance among adult participants, preliminary analyses were conducted separately in each age group to check the effect of gender on both behavioural performance and ERPs. There was no significant difference between females and males in each age group (ps > .054). Therefore, female and male participants were collapsed in all the reported analyses.
3. Results
3.1. Behavioural analyses
3.1.1. Accuracy
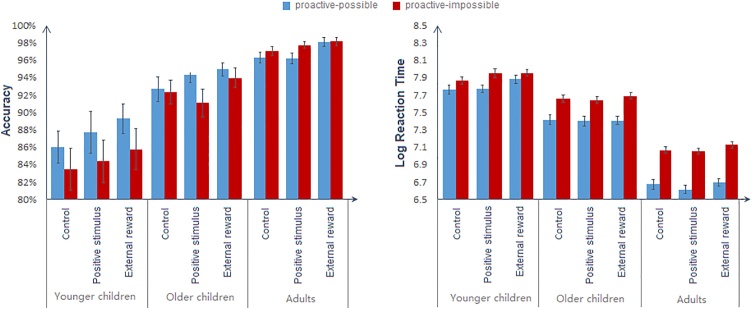
The ANOVA on accuracy revealed significant main effects of affective condition, F (2, 87) = 5.261, p = .006, ηp2 = .125, age group, F (2, 88) = 24.605, p < .001, ηp2 = .359, and block type, F (1, 88) = 8.532, p = .004, ηp2 = .088 (Fig. 3). Accuracy was significantly higher in the external-reward condition (93.3 %) than the control condition (91.3 %), p = .005, and marginally so than the positive-stimulus condition (91.9 %), p = .051. As expected, adults (97.2 %) responded more accurately than both groups of children (ps < .05), while older children (93.2 %) showed higher accuracy than younger children (86.1 %, p < .001). Accuracy was slightly but significantly higher in proactive-possible blocks (92.8 %) than proactive-impossible blocks (91.5 %, p = .004). However, block type interacted with age group, F (2, 88) = 7.044, p = .001, ηp2 = .138. Only younger children had higher accuracy in proactive-possible blocks (87.7 %) than in proactive-impossible blocks (84.5 %), F (1, 88) = 17.039, p < .001, ηp2 = .162, while no differences were found in older children (p = .056) and adults (p = .275).
Fig. 3.
Accuracy rates (left) and log-transformed reaction times (right) for younger children, older children and adults by affective conditions and block types. Bars represent standard errors of the mean. The external-reward condition yielded more accurate but slower responses than the other two conditions. Early cue presentation (i.e. proactive possible) was associated with faster responses in all three age groups and greater accuracy in younger children. There were no interactions between condition and block type.
In addition, there was a significant main effect of trial type (i.e. switch vs non-switch) on accuracy, qualified by a two-way interaction with affective conditions (see Supplementary Material for detailed results).
3.1.2. Response times
Results on RTs showed the opposite pattern to accuracy (Fig. 3). The main effect of the affective condition, F (2, 87) = 9.095, p < .001, ηp2 = .173, was due to slower responses in the external-reward condition (7.46 ln ms) than both control (7.41 ln ms, p = .008) and positive-stimulus conditions (7.40 ln ms, p = .001). Age group, F (2, 88) = 184.126, p < .001, ηp2 = .807, and block type, F (1, 88) = 284.288, p < .001, ηp2 = .764, also significantly affected RTs. RTs decreased across all three age groups (younger children: 7.863 ln ms, older children: 7.534 ln ms, and adults: 6.870 ln ms, ps < .001). Responses were faster in proactive-possible blocks than proactive-impossible blocks overall. There was a significant two-way interaction between block type and age group, F (2, 88) = 32.964, p < .001, ηp2 = .428. Although all three age groups responded faster when proactive control was possible (younger children: F (1, 88) = 17.869, p < .001, ηp2 = .169; older children: F (1, 88) = 83.311, p < .001, ηp2 = .489; adults: F (1, 88) = 256.30, p < .001, ηp2 = .744), the differences between proactive-possible and proactive-impossible blocks increased with age (younger children: Mdifference = 0.116; older children: Mdifference = 0.256; adults: Mdifference = 0.425).
Similar to results on accuracy, there was a significant main effect of trial type on RTs which was also interacted with the effect of affective condition (see Supplementary Material for detailed results).
3.2. ERP analyses
3.2.1. Initial posterior peak
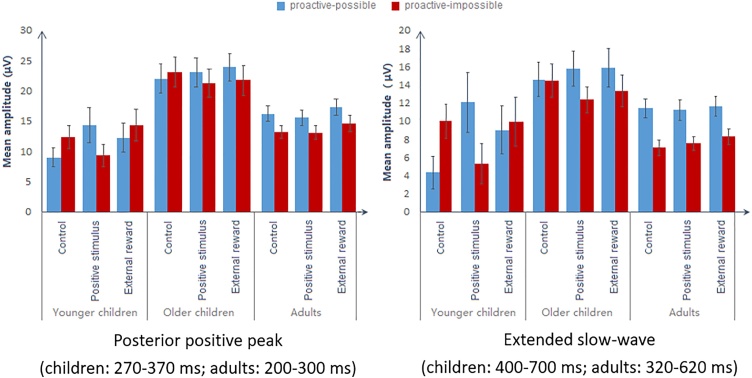
Consistent with the behavioural findings, the amplitude of the initial posterior peak varied across affective conditions, F (2, 70) = 4.617, p = .011, ηp2 = .061, age groups, F (2, 71) = 13.758, p < .001, ηp2 = .279, and block types, F (1, 71) = 4.763, p = .032, ηp2 = .063 (Fig. 4). Amplitude was significantly greater in the external-reward condition than the other two conditions (ps < .046), suggesting that both children and adults generally engaged more cognitive effort when expecting external rewards. As expected, greater amplitude was observed in proactive-possible than proactive-impossible blocks (p = .032). Due to the nature of the current task, all participants engaged more attentional resources after the informative cue than the uninformative cue. Amplitude was greater in older children than the other two age groups (ps < .001). None of the interactions reached significance (ps > .070).
Fig. 4.
Mean amplitudes of the cue-locked posterior positivity. Error bars indicate standard errors. The amplitude of the initial peak was greater in the external-reward condition than in the other two conditions across all age groups. Further, adults showed more marked posterior positivity on the extended slow-wave for proactive-possible blocks relative to proactive-impossible blocks in all affective conditions, whereas younger children only showed this salient difference in the positive-stimulus condition in which older children showed a similar trend.
3.2.2. Extended posterior slow wave
The effects of age group, F (2, 71) = 6.755, p = .002, ηp2 = .160, and block type, F (1, 71) = 15.419, p < .001, ηp2 = .178, were qualified by two way interactions of Block type × Age group, F (2, 71) = 4.713, p = 0.012, ηp2 = .117, Block type × Affective condition, F (2, 70) = 4.295, p = 0.017, ηp2 = .109, as well as a three-way interaction between age group, block type and affective condition, F (4, 142) = 3.188, p = .015, ηp2 = .082 (Fig. 4). Adults showed greater amplitude in proactive-possible than proactive-impossible blocks in all three affective conditions (ps < .001), suggesting that adults consistently engaged more attentional resources when the cue was informative for the upcoming target. In the positive-stimulus condition, greater amplitude for proactive-possible than proactive-impossible blocks was significant in younger children, F (1, 19) = 5.079, p = .036, ηp2 = .211, and fell short of significance in older children, F (1, 23) = 4.192, p = 0.052, ηp2 = .154. It seems that younger children engaged greater proactive cue preparation when exposed to positive face stimuli. In the other two affective conditions, amplitude did not vary between block types in children (all ps > .066). The other effects were not significant (all ps > .398).
4. Discussion
The present study investigated the effects of external rewards and positive stimuli on children and adults’ cognitive control engagement in a cued task-switching paradigm. The results showed a clear developmental trajectory of proactive control engagement in childhood. In line with previous research (e.g. Chevalier and Blaye, 2009; Chevalier et al., 2015; Lucenet and Blaye, 2014), 5- to 6-year-olds were already able to use proactive control, as shown by higher accuracy, faster RTs and more pronounced posterior positivity in proactive-possible blocks. In older groups, accuracy was high in all blocks, suggesting efficient engagement of either control mode. However, the mean differences of reaction times between proactive-possible blocks and proactive-impossible blocks increased with age, hence confirming that proactive control is engaged more consistently and efficiently with age (e.g. Waxer and Morton, 2011). Surprisingly, we found that both external rewards and positive stimuli promoted cognitive control engagement, but in different ways. Behaviourally, only reward motivation showed an effect on cognitive control. It facilitated both children and adult’s cognitive control in a speed-accuracy trade-off way, as shown by more accurate but slower responses in the external-reward condition than the control and positive-stimulus conditions. However, positive stimuli and external rewards both affected cue-locked posterior positivity ERPs. The expectation of receiving rewards elicited a more pronounced initial posterior positive peak in both proactive-possible and proactive-impossible blocks, whereas positive stimuli were associated with a more marked posterior positivity on the extended slow wave only in proactive-possible blocks in younger children.
The contrasted effects of positive stimuli and external rewards on the posterior positivity ERPs raise the intriguing possibility that they promote different types of proactive control in childhood. Specifically, the more pronounced cue-locked initial peak in the external-reward condition than the other two conditions suggests greater cognitive control engagement in the former. Surprisingly, this effect was observed regardless of whether the cue was informative or not, suggesting rewards did not promote proactive cue preparation but generally facilitated attention-concentration. Both children and adults may have better anchored their attention on the task to maximise the chance of obtaining rewards (Goschke and Bolte, 2014; Braver et al., 2014). In other words, rewards may support task readiness through enhanced mobilisation of attention ahead of the upcoming target. Although we did not predict this finding, it is consistent with a prior study with adults in which the prospect of receiving monetary rewards for correct responses resulted in systematic use of advance cues to prepare for the upcoming task even when the cue was no longer valid (Hefer and Dreisbach, 2017). According to the expectancy-value theory of motivation (Feather, 1982), rewards (i.e. virtual candies in our case) enhance extrinsic motivation which serves as a standard incentive for participants to adopt a surface approach to learning (Biggs and Tang, 2011), that is, engaging more cognitive effort across the board rather than balance benefits and costs to fine-tune effort engagement.
In contrast to reward, the presentation of positive stimuli was associated with a more pronounced extended slow-wave only when the cue was informative, which seems to suggest a greater proactive cue preparation in younger children in this condition. This was again an unexpected result, given prior evidence showed that positive stimuli are associated with more reactive control engagement in adults (e.g. Fröber and Dreisbach, 2014, 2016; Walsh et al., 2019). Nevertheless, our result is consistent with previous evidence that using happy faces as stimuli leads pre-schoolers to better monitor changes in task rules (3-year-olds in Qu and Zelazo, 2007) and task conflicts (5-year-olds in Li et al., 2019). It may be because, under positive emotion, children are able to apply effective strategies to proactively monitor the task cue to anticipate the upcoming task rules (Qu and Zelazo, 2007; Zelazo et al., 2010). The more pronounced extended slow wave in proactive-possible blocks seems to indicate a proactive cue processing. Younger children only proactively engaged cognitive effort when it was efficient to do so, flexibly balancing the cost and benefit of proactive engagement and economically saving attentional resources for other opportunities when proactive cue preparation was not adaptive. This is consistent with the finding in a recent study with adults (Chaillou et al., 2018) that greater attentional preparation was only engaged when a cue signalled the onset of a target probe (i.e. when proactive engagement is efficient and meaningful) under exposure to positive stimuli. Therefore, instead of reducing proactive control (as we originally hypothesized), the presentation of positive stimuli may have encouraged a proactive task-selection strategy in younger children, by which they could more economically and flexibly engage proactive cue preparation depending on the task demands.
Although proactive cue preparation with positive stimuli was significant in younger children and adults only, older children showed a trend (p = 0.052) in the same direction, suggesting this age difference in the effect of positive stimuli needs to be interpreted with caution. The happy faces used in the current task may have elicited a less pronounced positive mood in older children than in younger children, as different age groups may have different level of sensitivity to emotional stimuli. We considered this as one of the limitations of the current study, which will be further discussed below.
On the behavioural level, there was a speed-accuracy trade-off in the external-reward condition across all age groups, which is consistent with a previous study in which encouraging 5- to 6-year-olds to monitor their performance resulted in more pronounced ERP markers of proactive control but slower responses (Hadley et al., 2020). As reward was only related to response accuracy in the present study, it may have encouraged children to prioritise response accuracy over response time. Besides, as greater cognitive control often translates into higher accuracy but slower responses in children (Chevalier et al., 2019; Wiebe et al., 2012), it is likely that slower responses may reflect better performance of children in the present study too. Furthermore, although the presentation of happy faces supported proactive cue preparation as evidenced by ERPs in younger children, it did not translate into any behavioural improvement. This pattern raises the possibility that encouraging proactive control in children may not always translate into behavioural benefits. Children may be still inexperienced and not proficient at using proactive control, and thus only showed limited behavioural benefit. They may attempt proactive cue preparation but fail to effectively use the cue information to instruct their behavioural responses.
In line with previous studies, our behavioural data also suggest that proactive control is engaged more consistently and efficiently with age (e.g. Chevalier et al., 2014, 2015; Waxer and Morton, 2011). The high accuracy of proactive-possible blocks in younger children suggests that 5- to 6-year-olds are already able to engage proactive control. As proactive cue processing is the most effective strategy in the current task, younger children showed higher accuracy when proactive cue processing was allowed than when it was impossible. This accuracy difference between proactive-possible and proactive-impossible blocks was not observed in older children and adults, probably because their accuracy was close to ceiling in all blocks. Meanwhile, not only did all three age groups show faster RTs in proactive-possible blocks than in proactive-impossible blocks, but the RTs difference between these two blocks also increased with age. Although both children and adults can engage proactive control, their efficiency of proactive engagement increases with age.
Overall the present findings raise the intriguing possibility that there may be different ways to engage proactive control within the same task (Sidlauskaite et al., 2020) and these different proactive control strategies might be differentially influenced by external rewards and positive stimuli.
The present study has several limitations. First, because of its relatively small sample size, especially for ERP analyses in children, the results must be interpreted with caution. Although the observed effects in the current study are medium (all ηp2 > .056, which is approximately equal to effect size (f) > .25), considering the debate of replicability in psychological field, we think that accepting a medium effect size without caution may be over optimistic. Therefore, the current findings call for independent replication in future studies. In particular, because of the post-hoc nature of our interpretation of the dissociable effects of positive stimuli and external rewards on cognitive control, the distinction between proactive mobilisation of attention (i.e. readiness) and proactive cue preparation needs to be confirmed in future work purposely designed to directly test it empirically. Second, there is an unbalanced distribution between female and male participants in the adult group. Although we did not observe any gender effects in our preliminary analyses, future studies including balanced female and male samples are needed, as previous research suggests females may be more sensitive to social-emotional signals than males (e.g. Baron-Cohen and Wheelwright, 2004; McDerMott, and Egwuatu, 2019). Third, due to the low reliability of the self-report measurement of emotional arousal in children, especially in younger children, we could not assess to what extent positive stimuli and reward incentives actually enhanced positive mood and motivation in participants. Assessing arousal in future studies would not only ensure manipulations are successful but also probe when a specific arousal threshold is needed to incentivize children to engage proactive strategies. Although happy faces have been commonly used in previous studies for eliciting hedonic feelings in children (e.g. Li et al., 2019; Qu and Zelazo, 2007), it is also possible that happy face expressions might be perceived as social rewards by participants (e.g. Stavropoulos and Carver, 2014; Kohls et al., 2009). However, considering positive face expressions may only be rewarding when participants believe it is contingent to their performance (Krach et al., 2010; Matyjek et al., 2020), the happy faces used as task stimuli not as feedback was less likely perceived as social rewards by participants in the current task. However, future work is needed to understand the exact mechanisms through which positive stimuli influence cognitive control. Fourth, due to the poor signal-to-noise ratio, we could not probe switch and non-switch trials separately with the EEG data. However, we conducted additional analyses to check the effect of trial type on accuracy and RTs (as included in the Supplementary Material), and observed no interaction between cue presentation timing and trial type, hence replicating prior findings (Chevalier et al., 2015). That said, it may be insightful to check the effects of cue presentation timing and trial type on ERPs in future research, especially in light of the present effect of positive stimuli on ERPs but not on behavioural data, suggesting that positive stimuli may have an effect on cognitive control engagement that cannot yet be detected behaviourally.
In sum, our findings suggest that both positive stimuli and external rewards influence cognitive control engagement in childhood, but in different ways. External rewards seem to activate a proactive attention-mobilisation strategy, which helps to swiftly anchor children’s attention on the task. Whereas, the presenting of positive stimuli may be able to elicit a proactive task-selection strategy, by which children only engage their attentional resources as a function of task demands.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported in part by the grant from the Economic and Social Research Council (ES/N018877/1) to Nicolas Chevalier. During the course of this work, Bonnie Auyeung was supported by the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No.813546, and the UK Economic and Social Research Council (ES/N018877/1).
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2020.100806.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Ashby F.G., Isen A.M. A neuropsychological theory of positive affect and its influence on cognition. Psychol. Rev. 1999;106(3):529–550. doi: 10.1037/0033-295X.106.3.529. [DOI] [PubMed] [Google Scholar]
- Atkinson A.L., Waterman A.H., Allen R.J. Can children prioritize more valuable information in working memory? An exploration into the effects of motivation and memory load. Dev. Psychol. 2019;55(5):967–980. doi: 10.1037/dev0000692. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S., Wheelwright S. The empathy quotient: an investigation of adults with asperger syndrome or high functioning autism, and normal sex differences. J. Autism Dev. Disord. 2004;34:163–175. doi: 10.1023/B:JADD.0000022607.19833.00. [DOI] [PubMed] [Google Scholar]
- Biggs J.B., Tang C. McGraw-hill education (UK); 2011. Teaching for Quality Learning at University: What the Student Does. [Google Scholar]
- Bolte A., Goschke T. Thinking and emotion: affective modulation of cognitive processing modes. In: Glatzeder B., editor. Towards a Theory of Thinking. Springer; Berlin, Heidelberg: 2010. pp. 261–277. [DOI] [Google Scholar]
- Botvinick M., Braver T. Motivation and cognitive control: from behavior to neural mechanism. Annu. Rev. Psychol. 2015;66:83–113. doi: 10.1146/annurev-psych-010814-015044. [DOI] [PubMed] [Google Scholar]
- Braver T.S. The variable nature of cognitive control: a dual mechanisms framework. Trends Cogn. Sci. 2012;16(2):106–113. doi: 10.1016/j.tics.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver T.S., Gray J.R., Burgess G.C. Explaining the many varieties of working memory variation: dual mechanisms of cognitive control. In: Conway A., editor. Variation in Working Memory. Oxford University Press; 2007. pp. 75–106. [Google Scholar]
- Braver T.S., Krug M.K., Chiew K.S., Kool W., Westbrook J.A., Clement N.J. Mechanisms of motivation–cognition interaction: challenges and opportunities. Cogn. Affect. Behav. Neurosci. 2014;14(2):443–472. doi: 10.3758/s13415-014-0300-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaillou A.C., Giersch A., Hoonakker M., Capa R.L., Doignon-Camus N., Pham B.T., Bonnefond A. Evidence of impaired proactive control under positive affect. Neuropsychologia. 2018;114:110–117. doi: 10.1016/j.neuropsychologia.2018.04.021. [DOI] [PubMed] [Google Scholar]
- Chatham C.H., Frank M.J., Munakata Y. Pupillometric and behavioral markers of a developmental shift in the temporal dynamics of cognitive control. Proc. Natl. Acad. Sci. 2009;106(14):5529–5533. doi: 10.1073/pnas.0810002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier N. The development of executive function: toward more optimal coordination of control with age. Child Dev. Perspect. 2015;9(4):239–244. doi: 10.1111/cdep.12138. [DOI] [Google Scholar]
- Chevalier N., Blaye A. Setting goals to switch between tasks: effect of cue transparency on children’s cognitive flexibility. Dev. Psychol. 2009;45(3):782–797. doi: 10.1037/a0015409. [DOI] [PubMed] [Google Scholar]
- Chevalier N., James T.D., Wiebe S.A., Nelson J.M., Espy K.A. Contribution of reactive and proactive control to children’s working memory performance: insight from item recall durations in response sequence planning. Dev. Psychol. 2014;50(7):1999–2008. doi: 10.1037/a0036644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier N., Martis S.B., Curran T., Munakata Y. Metacognitive processes in executive control development: the case of reactive and proactive control. J. Cogn. Neurosci. 2015;27(6):1125–1136. doi: 10.1162/jocn_a_00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier N., Jackson J., Roux A.R., Moriguchi Y., Auyeung B. Differentiation in prefrontal cortex recruitment during childhood: evidence from cognitive control demands and social contexts. Dev. Cogn. Neurosci. 2019;36 doi: 10.1016/j.dcn.2019.100629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiew K.S., Braver T.S. Temporal dynamics of motivation-cognitive control interactions revealed by high-resolution pupillometry. Front. Psychol. 2013;4:1–15. doi: 10.3389/fpsyg.2013.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiew K.S., Braver T.S. Dissociable influences of reward motivation and positive emotion on cognitive control. Cogn. Affect. Behav. Neurosci. 2014;14(2):509–529. doi: 10.3758/s13415-014-0280-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBoer T., Scott L.S., Nelson C.A. Event-related potentials in developmental populations. In: Handy T.C., editor. Event-related Potentials, a Methods Handbook. The MIT Press; Cambridge, MA: 2005. pp. 263–298. [Google Scholar]
- Delorme A., Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Dien J. The ERP PCA Toolkit: an open source program for advanced statistical analysis of event-related potential data. J. Neurosci. Methods. 2010;187(1):138–145. doi: 10.1016/j.jneumeth.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Dien J. Evaluating two‐step PCA of ERP data with geomin, infomax, oblimin, promax, and varimax rotations. Psychophysiology. 2010;47(1):170–183. doi: 10.1111/j.1469-8986.2009.00885.x. [DOI] [PubMed] [Google Scholar]
- Dien J. Applying principal components analysis to event-related potentials: a tutorial. Dev. Neuropsychol. 2012;37(6):497–517. doi: 10.1080/87565641.2012.697503. [DOI] [PubMed] [Google Scholar]
- Dien J., Frishkoff G.A. Principal components analysis of event-related potential datasets. In: Handy H.C., editor. Event-Related Potentials: A Methods Handbook. MIT Press; Cambridege, MA: 2005. pp. 189–208. [DOI] [Google Scholar]
- Dreisbach G. How positive affect modulates cognitive control: the costs and benefits of reduced maintenance capability. Brain Cogn. 2006;60(1):11–19. doi: 10.1016/j.bandc.2005.08.003. 10.1016.bandc.2005.08.003 [DOI] [PubMed] [Google Scholar]
- Dreisbach G., Fröber K. On how to be flexible (or not): modulation of the stability-flexibility balance. Curr. Dir. Psychol. Sci. 2019;28(1):3–9. doi: 10.1177/0963721418800030. [DOI] [Google Scholar]
- Elke S., Wiebe S.A. Proactive control in early and middle childhood: an ERP study. Dev. Cogn. Neurosci. 2017;26:28–38. doi: 10.1016/j.dcn.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feather N.T. Lawrence Erlbaum Assoc Incorporated; 1982. Expectations and Actions: Expectancy-value Models in Psychology. [Google Scholar]
- Fredrickson B.L. Positive emotions broaden and build. Adv. Exp. Soc. Psychol. 2013;47:1–53. doi: 10.1016/B978-0-12-407236-7.00001-2. [DOI] [Google Scholar]
- Fröber K., Dreisbach G. The differential influences of positive affect, random reward, and performance-contingent reward on cognitive control. Cogn. Affect. Behav. Neurosci. 2014;14(2):530–547. doi: 10.3758/s13415-014-0259-x. [DOI] [PubMed] [Google Scholar]
- Fröber K., Dreisbach G. How performance (non-) contingent reward modulates cognitive control. Acta Psychol. 2016;168:65–77. doi: 10.1016/j.actpsy.2016.04.008. [DOI] [PubMed] [Google Scholar]
- Goschke T., Bolte A. Emotional modulation of control dilemmas: the role of positive affect, reward, and dopamine in cognitive stability and flexibility. Neuropsychologia. 2014;62:403–423. doi: 10.1016/j.neuropsychologia.2014.07.015. [DOI] [PubMed] [Google Scholar]
- Hadley L.V., Acluche F., Chevalier N. Encouraging performance monitoring promotes proactive control in children. Dev. Sci. 2020 doi: 10.1111/desc.12861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefer C., Dreisbach G. How performance-contingent reward prospect modulates cognitive control: increased cue maintenance at the cost of decreased flexibility. J. Exp. Psychol. Learn. Mem. Cogn. 2017;43(10):1643–1658. doi: 10.1037/xlm0000397. [DOI] [PubMed] [Google Scholar]
- Jamadar S., Michie P., Karayanidis F. Compensatory mechanisms underlie intact task-switching performance in schizophrenia. Neuropsychologia. 2010;48(5):1305–1323. doi: 10.1016/j.neuropsychologia.2009.12.034. [DOI] [PubMed] [Google Scholar]
- Kohls G., Peltzer J., Herpertz‐Dahlmann B., Konrad K. Differential effects of social and non‐social reward on response inhibition in children and adolescents. Dev. Sci. 2009;12(4):614–625. doi: 10.1111/j.1467-7687.2009.00816.x. [DOI] [PubMed] [Google Scholar]
- Krach S., Paulus F.M., Bodden M., Kircher T. The rewarding nature of social interactions. Front. Behav. Neurosci. 2010;4:22. doi: 10.3389/fnbeh.2010.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer H.J., Lagattuta K.H., Sayfan L. Why is happy–sad more difficult? Focal emotional information impairs inhibitory control in children and adults. Emotion. 2015;15(1):61–72. doi: 10.1037/emo0000023. [DOI] [PubMed] [Google Scholar]
- Li D., Liu T., Shi J. Conflict adaptation in 5-year-old preschool children: evidence from emotional contexts. Front. Hum. Neurosci. 2019;13(Article 14):1–11. doi: 10.3389/fnhum.2019.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucenet J., Blaye A. Age-related changes in the temporal dynamics of executive control: a study in 5-and 6-year-old children. Front. Psychol. 2014;5(article 831):1–11. doi: 10.3389/fpsyg.2014.00831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzi A., Nessler D., Czernochowski D., Friedman D. The development of anticipatory cognitive control processes in task‐switching: an ERP study in children, adolescents, and young adults. Psychophysiology. 2011;48(9):1258–1275. doi: 10.1111/j.1469-8986.2011.01192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyjek M., Meliss S., Dziobek I., Murayama K. 2020. A Multidimensional View on Social and Non-social Rewards. March 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott J.M., Egwuatu A.C. More than a face: neural markers of motivated attention toward social and non-social reward-related images in children. Biol. Psychol. 2019;140:1–8. doi: 10.1016/j.biopsycho.2018.08.012. [DOI] [PubMed] [Google Scholar]
- Meiran N. Reconfiguration of processing mode prior to task performance. J. Exp. Psychol. Learn. Mem. Cogn. 1996;22(6):1423–1442. doi: 10.1037/0278-7393.22.6.1423. [DOI] [Google Scholar]
- Meuwissen A.S., Anderson J.E., Zelazo P.D. The creation and validation of the developmental emotional faces stimulus set. Behav. Res. Methods. 2017;49(3):960–966. doi: 10.3758/s13428-016-0756-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mognon A., Jovicich J., Bruzzone L., Buiatti M. Adjust: an automatic eeg artifact detector based on the joint use of spatial and temporal features. Psychophysiology. 2011;48:229–240. doi: 10.1111/j.1469-8986.2010.01061.x. [DOI] [PubMed] [Google Scholar]
- Pessoa L. How do emotion and motivation direct executive control? Trends Cogn. Sci. 2009;13(4):160–166. doi: 10.1016/j.tics.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu L., Finestone D.L., Qin L.J., Reena L.Z. Focused but fixed: the impact of expectation of external rewards on inhibitory control and flexibility in preschoolers. Emotion. 2013;13(3):562–572. doi: 10.1037/a0027263. [DOI] [PubMed] [Google Scholar]
- Qu L., Zelazo P.D. The facilitative effect of positive stimuli on 3-year-olds’ flexible rule use. Cogn. Dev. 2007;22(4):456–473. doi: 10.1016/j.cogdev.2007.08.010. [DOI] [Google Scholar]
- Schultz W. Activity of dopamine neurons in the behaving primate. Semin. Neurosci. 1992;4(2):129–138. doi: 10.1016/1044-5765(92)90011-P. [DOI] [Google Scholar]
- Sidlauskaite J., Dhar M., Sonuga-Barke E., Wiersema J.R. Altered proactive control in adults with ADHD: evidence from event-related potentials during cued task switching. Neuropsychologia. 2020;138 doi: 10.1016/j.neuropsychologia.2019.107330. [DOI] [PubMed] [Google Scholar]
- Somerville L.H., Casey B.J. Developmental neurobiology of cognitive control and motivational systems. Curr. Opin. Neurobiol. 2010;20(2):236–241. doi: 10.1016/j.conb.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavropoulos K.K., Carver L.J. Reward sensitivity to faces versus objects in children: an ERP study. Soc. Cogn. Affect. Neurosci. 2014;9(10):1569–1575. doi: 10.1093/scan/nst149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang N.M., Pollak S.D. Developmental continuity in reward-related enhancement of cognitive control. Dev. Cogn. Neurosci. 2014;10:34–43. doi: 10.1016/j.dcn.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N., Hare T.A., Casey B.J. Behavioral assessment of emotion discrimination, emotion regulation, and cognitive control in childhood, adolescence, and adulthood. Front. Psychol. 2011;2:1–9. doi: 10.3389/fpsyg.2011.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troller‐Renfree S.V., Buzzell G.A., Fox N.A. Changes in working memory influence the transition from reactive to proactive cognitive control during childhood. Dev. Sci. 2020 doi: 10.1111/desc.12959. [DOI] [PubMed] [Google Scholar]
- Walsh A.T., Carmel D., Grimshaw G.M. Reward elicits cognitive control over emotional distraction: evidence from pupillometry. Cogn. Affect. Behav. Neurosci. 2019;19(3):537–554. doi: 10.3758/s13415-018-00669-w. [DOI] [PubMed] [Google Scholar]
- Waxer M., Morton J.B. The development of future-oriented control: an electrophysiological investigation. NeuroImage. 2011;56(3):1648–1654. doi: 10.1016/j.neuroimage.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Wiebe S.A., Sheffield T.D., Andrews Espy K. Separating the fish from the sharks: a longitudinal study of preschool response inhibition. Child Dev. 2012;83(4):1245–1261. doi: 10.1111/j.1467-8624.2012.01765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S.S., Jacques S., Zelazo P.D. A preliminary investigation of the effects of emotional stimuli on 4-year-old children? S abstraction and cognitive flexibility on the flexible item selection task (FIST) Univ. Toronto J. Undergraduate Life Sci. 2008;2(1):34–42. [Google Scholar]
- Zelazo P.D., Carlson S.M. Hot and cool executive function in childhood and adolescence: development and plasticity. Child Dev. Perspect. 2012;6(4):354–360. doi: 10.1111/j.1750-8606.2012.00246.x. [DOI] [Google Scholar]
- Zelazo P.D., Qu L., Kesek A.C. Hot executive function: emotion and the development of cognitive control. In: Calkins S.D., Bell M.A., editors. Human Brain Development. Child Development at the Intersection of Emotion and Cognition. American Psychological Association; Washington, DC, US: 2010. pp. 97–111. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.