Highlights
-
•
We describe a rare case with a clitoral metastasis from uterine cervical cancer.
-
•
The patient was treated by concurrent chemoradiotherapy and clitoridectomy.
-
•
Clitoral metastasis should be considered in patients with clitorodynia.
Keywords: Cervical cancer, Metastasis, Clitoris, Clitoridectomy
Abstract
A 81-year-old woman vaginal bleeding and vulvar pain. Pretreatment work-up revealed a 4.5 cm cervical cancer extended to the lower-third of the vagina and an isolated clitoral metastasis. The patient was treated with a multimodal treatment consisting with radiotherapy followed by clitoridectomy. She recovered uneventfully following the multimodal treatment, and is currently free of disease. Clitoral metastasis is extremely rare, however, this condition should be considered in cervical cancer patients during the pretreatment work-up or follow-up period, especially when patients complain of clitoral pain or enlargement.
1. Introduction
The clitoris can be involved in both primary and secondary malignant neoplasms (Czernobilsky et al., 1995, DuPont et al., 2009). In contrast to the primary carcinoma of clitoris which is classified as vulvar carcinoma, the secondary carcinoma of the clitoris is an extremely rare condition. Previous reports have suggested that it can most commonly originate from urinary system cancer (bladder, kidney), followed by endometrium and gastrointestinal system (Jiang et al.,2015). In uterine cervical cancer, only 5 reported cases are available (Table 1) (Jiang et al., 2015, Marek and Hayden, 1950, Bonneau et al., 2011, Papoutsis and Haefner, 2015, Lu et al., 2017).
Table 1.
Summary of reported cases with clitoral metastasis from uterine cervical cancer.
| Author (Year) | Age | FIGO Stage* | Histology | Clitoralpain/enlargement | Size of clitoris | Treatment | Pathological findings from clitoral tumor | Outcome |
|---|---|---|---|---|---|---|---|---|
| Marek CB (1950) | 45 | IIIB<** | SCC | Enlargement | 3 × 1 cm | EBRT + ICBT | SCC LVSI (+) |
NA |
| Bonneau C (2011) | 76 | IVB | SCC | Pain Enlargement | 3 × 1.5 × 1.5 cm | Clitoridectomy | SCC LVSI (+) |
NA |
| Jiang S (2013) | 54 | IIIB | SCC | Pain Enlargement | 3 × 4 cm | Radiotherapy followed by Vulvectomy | SCC | NA |
| Papoutsis D (2015) | 68 | IVB | A | Pain | 1.7 × 1 cm | Clitoridectomy followed by CCRT | A | DOD 7 months after diagnosis |
| Lu YY (2017) | 54 | IVB | SCC | NA | 2 cm | NA | NA | NA |
| Present case | 81 | IIIA | A | Pain Enlargement | 3 × 4.5 × 5 cm | Radiotherapy followed by clitoridectomy | A LVSI (+) |
NED 3 months |
FIGO, The International Federation of Gynecology and Obstetrics; DFI, disease free interval; SCC, squamous cell carcinoma; A, adenocarcinoma; LVSI, lymphovascular space involvement; CCRT, concurrent chemoradiotherapy; S, surgery; NA, not available; NED, no evidence of disease; DOD, died of disease.
*Clitoral lesion was excluded.
** IIIB or IVA.
***Hysterectomy plus pelvic lymphadenectomy
In this report, we describe our experience with a clitoral metastasis from uterine cervical cancer, which was treated by multimodal treatments including concurrent chemoradiotherapy and surgery. Moreover, based on a literature review, we provide information about the clitoral metastasis in cervical cancer patients.
2. Case report
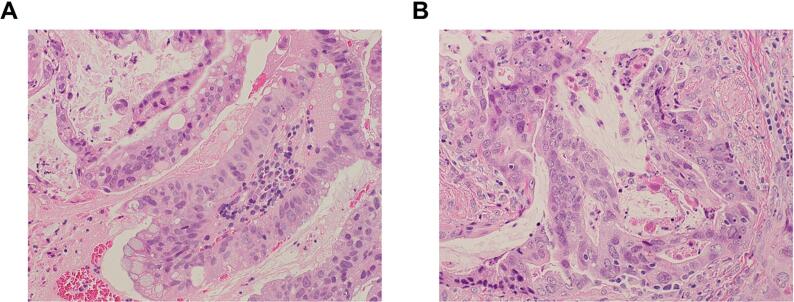
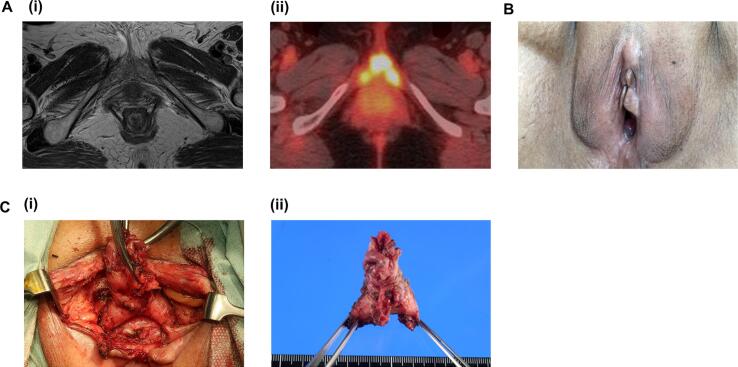
A 81-year-old woman presented with vaginal bleeding and vulvar pain. Her past surgical and medical history was unremarkable except for a hypertension. On evaluation, she was found to have a 4.5 cm friable cervical mass, which was extended to the lower-third of the vagina. The inguinal lymph nodes were not palpable. Bilateral parametrial involvement was also noted. Biopsies from the cervical and vaginal lesions demonstrated an adenocarcinoma (Fig. 1A). A pretreatment workup including a pelvic magnetic resonance imaging (MRI) and a computed tomography (CT) scan of the abdomen and pelvis revealed a cervical tumor and swellings of bilateral obturator nodes, but no abnormal findings in her vulva (Fig. 2A). A fluorine-18-fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) demonstrated a significant FDG-uptake in her uterine cervix and clitoris (Fig. 2A). No other FDG uptake was observed except for bilateral obturator nodes. A detailed pelvic examination under anesthesia demonstrated a firm enlarged clitoris measuring 2 × 2 cm (Fig. 2B). The surface of the clitoris was normal. After the thorough examination, biopsy samples were obtained from the enlarged clitoris. Pathological examination demonstrated an adenocarcinoma (Fig. 1B). The diagnosis of FIGO stage IVB cervical cancer was confirmed.
Fig. 1.
A, Hematoxylin and eosin (H&E) stained sections of the tumor; photomicrographs show atypical cribriform glands with frequent mitotic figures. A, primary tumor (biopsy sample) indicating cervical adenocarcinoma [x20]. B, biopsy sample of clitoral tumors suggesting metastatic adenocarcinoma [x20].
Fig. 2.
The images of the clitoris. A, (i) T2-weighted MRI image; MRI failed to detect a clitoral metastasis. (ii), FDG-PET/CT image; a significant FDG-uptake was observed in her clitoris. B, a representative photo of the enlarged clitoris. C, (i) intraoperative view; (ii), resected clitoris.
Treatment options were discussed with the patient, and multimodal treatment including radiotherapy and surgery has been initiated. One week after the completion of radiotherapy (pelvic external beam radiotherapy followed by intracavitary brachytherapy), clitoridectomy was performed (Fig. 2C). The removed clitoral specimen measured 3 × 4.5 × 5 cm (Fig. 2C), and a metastatic adenocarcinoma with lymphovascular space involvement was demonstrated. Surgical margin was free of disease. She recovered uneventfully following surgery, and is currently free of disease.
3. Discussion
We presented our experience with a clitoral metastasis from uterine cervical cancer. The patient was treated by multimodal treatments including radiotherapy and surgery.
Cervical cancer primarily spreads by direct invasion or through the local lymphatics (Lifshitz and Buchsbaum,1977). Clitoral metastasis is an extremely rare condition, with 5 reported cases available (Table 1). Risk factors for a clitoral metastasis remain unknown, however, all reported cases had locally-advanced (IIIA or greater) or metastatic (IVB) cervical cancer.
As shown in Table 1, 5 out of 6 reported cases with clitoral metastasis from cervical cancer presented with clitoral pain and/or enlargement. Clitoral enlargement can be the sign of an endocrine or intersex disorder, infection, or an underlying neoplastic disease. Benign neoplasms involving the clitoris include clitoral cysts (epidelmoid, mesonephric, or sebaceous) or clitoral tumors (angiokeratoma, hemangioma, leiomyoma, fibroma, or hemangiopericytoma) (Schmidt et al.,1999). Malignant neoplasms include both primary and secondary tumors (Czernobilsky et al., 1995, DuPont et al., 2009). Among the reported cases (Table 1), clitoral pain did not receive sufficient attention at initial presentation, and were misdiagnosed as urinary tract infection or vulvovaginitis in 4 cases (Jiang et al., 2015, Bonneau et al., 2011, Papoutsis and Haefner, 2015, and current case). In cases reported by Jiang and Bonneau et al., the diagnosis of clitoral metastasis was made after the initiation of cervical cancer treatment (Jiang et al., 2015, Bonneau et al., 2011). In such cases, initial staging (FIGO staging) might have been inaccurate.
As described in our case, MRI and a CT scan failed to detect a clitoral metastasis. Although FDG-PET/CT succeeded in the detection of clitoral metastasis, a routine performance of such expensive imaging study is not realistic. Thus, we have to recognize the significance of clitoral enlargement or pain that deserves full attention and thorough physical examination. We also need to recognize that, due to the hypersensitive nature of this organ, the examination of the clitoris should be carefully performed. In cases with significant clitoral pain, examination under anesthesia is recommended.
Due to the rarity of this condition, the standard treatment for clitoral metastasis from uterine cervical cancer has not been established. Although the resection of the clitoris adversely affects a women’s psychosexual function, 4 out of 6 cases received either clitoridectomy or vulvectomy (Table 1). In the present case, as less radical approach may increase the risk of recurrence, we performed clitoridectomy. The radiotherapy focusing on the clitoral area might be an alternative treatment, however, its therapeutic efficacy as well as its impact on women’s psychosexual function have never been evaluated.
In conclusion, we experienced a rare case of isolated clitoral metastasis from cervical cancer. Although clitoral metastasis is rare, this condition should be considered in cervical cancer patients during the pretreatment work-up or follow-up period, especially when patients complain of clitoral pain or enlargement. Given the rarity of this condition, we believe it is important to report individual cases so that optimal treatment can be established.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
4. Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author Contributions
Y.H. assisted surgery and acquired the clinical data; S.M. performed surgery and wrote the manuscript; Y.Y. contributed data analysis; S.M. performed radiation therapy; F.O. acquired the clinical data; T.U. acquired the clinical data and edited the manuscript.
Contributor Information
Yukiko Hino, Email: hinoyukiko@naramed-u.ac.jp.
Yuki Yamada, Email: yuki0528@naramed-u.ac.jp.
Sachiko Miura, Email: sachimiu@naramed-u.ac.jp.
Fumi Okada, Email: fumi-okabe@naramed-u.ac.jp.
Tomoko Uchiyama, Email: uchiyama0403@naramed-u.ac.jp.
Seiji Mabuchi, Email: smabuchi@naramed-u.ac.jp.
References
- Bonneau C., Rodrigues A., Poncelet C., Cornelis F., Chanelles O., Carbillon L., Bricou A. Clitoral metastasis from cervical carcinoma. J Obstet Gynaecol. 2011;31:359–360. doi: 10.3109/01443615.2011.556269. [DOI] [PubMed] [Google Scholar]
- Czernobilsky B., Gat A., Evron R., Dgani R., Ben-Hur H., Lifschitz-Mercer B. Carcinoma of the clitoris: a Histologic study with cytokeratin profile. Int J Gynecol Pathol. 1995;14:274–278. doi: 10.1097/00004347-199507000-00014. [DOI] [PubMed] [Google Scholar]
- DuPont N.C., Mabuchi S., Ries S., Berman M.L. Sclerosing ductal carcinoma of the clitoris with microcystic adnexal carcinoma-like features. J Cutan Pathol. 2009;36:359–361. doi: 10.1111/j.1600-0560.2008.01044.x. [DOI] [PubMed] [Google Scholar]
- Jiang S., Sheng X.G., Song Q.Q., Lu C.H., Pan C.X., Li Q.S. Metastasis of a cervical carcinoma to the clitoris. Jiang J Obstet Gynaecol. 2015;35:213–214. doi: 10.3109/01443615.2014.937335. [DOI] [PubMed] [Google Scholar]
- Lifshitz S.G., Buchsbaum H.J. Spread of cervical carcinoma. Obstet Gynecol Annu. 1977;6:341–354. [PubMed] [Google Scholar]
- Lu Y.Y., Lu C.H., Wang H.Y. Clitoral Metastasis From Advanced Cervical Carcinoma on 18F-FDG-PET/CT. Clin Nucl Med. 2017;42:54–55. doi: 10.1097/RLU.0000000000001410. [DOI] [PubMed] [Google Scholar]
- Marek C.B., Hayden C.R. Metastatic carcinoma of the clitoris. Am J Obstet Gynecol. 1950;60:443–444. doi: 10.1016/0002-9378(50)90491-1. [DOI] [PubMed] [Google Scholar]
- Papoutsis D., Haefner H.K. Metastatic adenocarcinoma to the clitoris from the cervix. Am J Obstet Gynecol. 2015;213(738):E1. doi: 10.1016/j.ajog.2015.05.029. [DOI] [PubMed] [Google Scholar]
- Schmidt A., Lang U., Kiess W. Epidermal cyst of the clitoris: a rare cause of clitorimegaly. Eur J Obstet Gynecol Reprod Biol. 1999;87:163–165. doi: 10.1016/S0301-2115(99)00096-2. [DOI] [PubMed] [Google Scholar]