Tyrosine Kinase Inhibitors (TKIs) that inhibit BCR-ABL such as imatinib, dasatinib, nilotinib and ponatinib are mainstay therapies in chronic myeloid leukemia (CML) and Philadelphia chromosome+ acute lymphoblastic leukemia (Ph+ ALL). As the use of TKIs becomes more prevalent and the landscape shifts towards chronic therapies, it is imperative that clinicians keep abreast of new adverse drug reactions (ADR).
Renal ADR with BCR-ABL TKIs have traditionally been perceived as uncommon. Frequent ADRs for dasatinib include myelosuppression and fluid retention, while pooled data from clinical trials report an incidence of <1% renal dysfunction and <0.1% proteinuria. [1] Phase I studies of ponatinib found drug-induced proteinuria in 33% of patients, but larger Phase 2 trials failed to identify proteinuria or acute kidney injury (AKI) as an ADR. [2] However, increasing evidence in the literature suggests that AKI and proteinuria are more prevalent with BCR-ABL TKI than previously recognized. [3], [4], [5] Here we present two cases of suspected TKI-induced nephropathy to highlight this underrecognized ADR.
Case 1
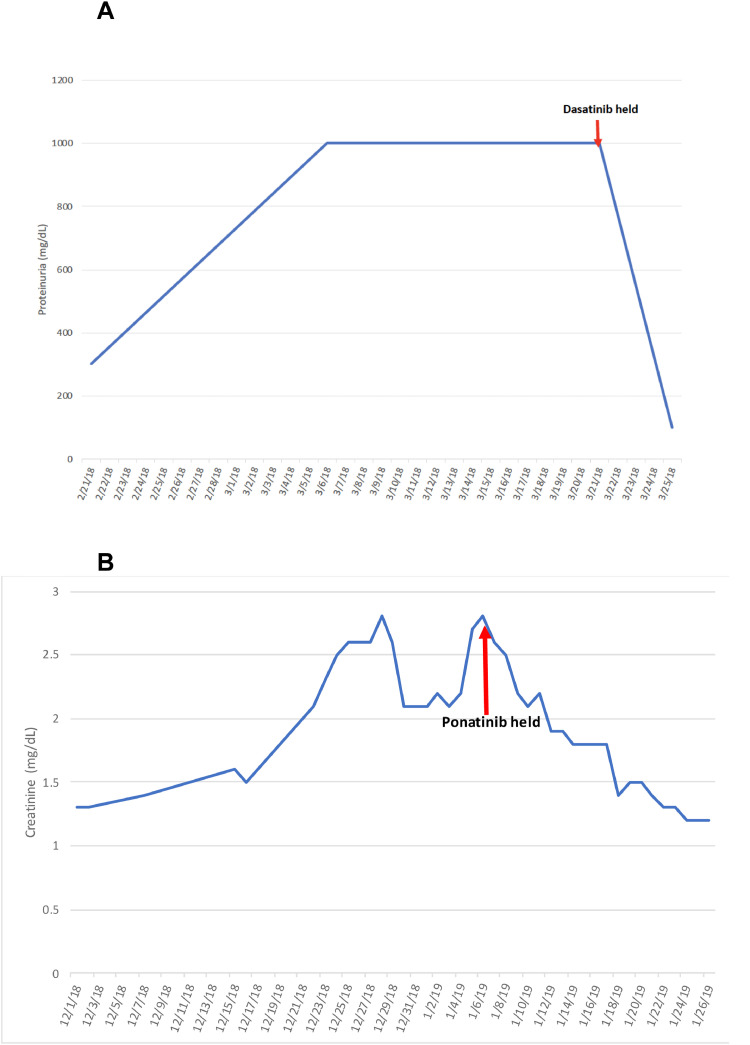
A 53-year-old male with hypertension (HTN) and accelerated-phase CML received therapy with high-dose cytarabine, mitoxantrone and dasatinib (100 mg/day). Several days into treatment, he developed lower extremity and scrotal edema, without shortness of breath or pleural effusions. Urinalysis showed >1.8 g/dL of protein and 24-hour urine revealed 10 g of protein. Hepatic serologies, complement, cryoglobulins, SPEP/UPEP, RPR, were all within normal limits. Dasatinib was discontinued and later replaced with imatinib. Three days later, urine protein decreased to 99 mg/dL (Fig. 1A). He did not experience any renal ADR on imatinib and declined renal biopsy.
Fig. 1.
Lab values from Case 1 and 2 while on BCR-ABL TKI and after discontinuation. (A) Graph depicting protein levels in the urine of case 1 increasing over time while on dasatinib and rapidly resolving after discontinuation. (B) Graph depicting the serum creatinine levels in case 2 increasing over time while on ponatinib and then normalizing after discontinuation.
Case 2
A 57-year-old female with HTN and Ph+ B-ALL was treated with an induction regimen that included imatinib, maintenance therapy with dasatinib, which was subsequently switched to nilotinib due to pleural effusions, and an allogeneic hematopoietic stem cell transplant (HSCT). After relapse, she received ponatinib (45 mg/day) and chemotherapy, but due to cholecystitis, ponatinib was lowered to 30 mg/day. Prior to her second HSCT, ponatinib was increased to 45 mg/day, followed by additional chemotherapy, and five cycles of blinatumomab and ponatinib (30 mg/day).
The patient was in clinical remission on maintenance ponatinib (15 mg/day) for one year and was off immunosuppression when she presented with fever, rash, worsening HTN and AKI in the absence of any viral infection. Renal function rapidly deteriorated, with creatinine rising from 1.5 mg/dL to 2.8 mg/dL with 1 g/dL proteinuria. Due to worsening HTN, elevated LDH (833 U/L), low haptoglobin (<8) and new onset thrombocytopenia, there was concern for drug-related hemolytic uremic syndrome and thrombotic microangiopathy (TMA). Ponatinib was promptly discontinued with quick improvement in proteinuria and creatinine (Fig. 1B). Renal biopsy revealed diffuse sclerotic glomeruli and interstitial inflammation without an overt cause of proteinuria. Skin biopsy revealed perivascular mononuclear cell infiltrate with atypia and interface changes, consistent with inflammatory response. While the patient was on allopurinol which may cause rash, the rash resolved after discontinuation of ponatinib. She was not re-challenged with ponatinib.
Discussion
While multiple factors could have instigated renal injury and nephropathy in these cases, the abrupt onset of renal ADR and rapid improvement upon cessation of the TKI, favor TKI-induced nephropathy. Recent reports in the literature on dasatinib's renal toxicities cite cases of acute tubular necrosis, AKI, proteinuria and rhabdomyolysis. [3], [4],[6], [7], [8] One study found 8.9% of patients receiving dasatinib developed nephrotic-range proteinuria not attributable to any other cause [9], while other reports describe nephrotic syndrome with endothelial injury and TMA, resolving upon dasatinib discontinuation. [4] While our patient was concurrently receiving mitoxantrone which has been shown to cause proteinuria in other cancers [10], our patient's proteinuria resolved despite continuation of mitoxantrone.
Fewer cases detailing overt drug induced proteinuria exist for ponatinib, however, over one-quarter of patients treated with ponatinib in the phase II PACE study developed HTN which may be an early manifestation of renal damage or may act as a pre-disposing factor for proteinuria. [11] Glomerular proteinuria leads to a large amount of plasma proteins in the proximal renal tubules, which compounded with the known tubular toxicity caused directly by BCR-ABL TKI [12], can lead to devasting nephrotoxicity. Results of the renal and skin biopsies also suggest a high level of inflammation, which could have triggered endothelial damage leading to a cascade precipitating TMA.
Both of our patients had pre-existing HTN making it unclear whether HTN acted as a catalyst for renal ADR, or if ADR were an independent effect of the TKI. Our patient in the second case was on three anti-hypertensives with worsening HTN, signaling refractory HTN possibly precipitated by damage to the renal tubule itself as noted above. Regardless, blood pressure control is integral in patients on BCR-ABL TKI, possibly with ACE-I or ARB to mitigate proteinuria. Other ADR and pre-disposing risk factors are summarized in Table 1.
Table. 1.
Pre-disposing Risk Factors and Renal Complications in Patients on Dasatinib and Ponatinib.
| Drug | Reference | No. of patient | Renal complication | Pre-disposing factors | Case or study background | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dasatinib | Ozkurt [3] | 1 | Acute tubular necrosis | None. Labs concerning for pre-renal azotemia 2/2 gastroenteritis so was ruled out as a possible cause of renal failure. | Acute renal failure and gastroenteritis | |||||
| Wallace [4] | 1 | Nephrotic syndrome | None. Discussion includes possible effect of a soluble paraprotein 2/2 CML; cannot be fully ruled out as the cause. | Proteinuria with a kidney biopsy showing chronic thrombotic microangiopathy. | ||||||
| Uz [6] | 1 | Acute renal failure 2/2 rhabdomyolysis | Was initially non-compliant with imatinib. No other chronic diseases or medications that would predispose to rhabdomyolysis or renal failure. | Rhabdomyolysis occurred shortly after the initiation of dasatinib therapy & CK levels normalized after withdrawal of dasatinib. | ||||||
| Kawaguchi [7] | 1 | Massive proteinuria associated with pleural effusion, ascites and HTN | Started on ganciclovir for CMV right before renal event. | Ph+ALL 8 year old female switched to dasatinib secondary to nausea, vomiting, diarrhea on imatinib. | ||||||
| Ochiai [8] | 1 | Nephrotic syndrome | None | Concluded that the main cause of nephrotic syndrome was dasatinib because only switch of the drug improved the patient's proteinuria. | ||||||
| De Luca ML [14] | 1 | Nephrotic range proteinuria with hypercholesteremia | No comorbidities. She was initiated on dasatinib after initially treatment with hydroxyurea | Resolution of proteinuria upon switching to imatinib | ||||||
| Ponatinib | Amin SO [15] | 1 | Renovascular hypertension due to bilateral renal artery stenosis | Smoking history (12 pack year history) and hypertriglyceridemia. | Developed HTN 3 years after starting dasatinib. Responded to revascularization. | |||||
| Hiremath S [16] | 1 | Elevated Cr 2/2 renovascular HTN 2/2 bilateral renal artery stenosis | Responded to revascularization – renal function stabilized | |||||||
2/2 = secondary to; CK = creatinine kinase; CMV = cytomegalovirus; Ph+ ALL= Philadelphia-positive acute lymphoblastic leukemia.
Dasatinib and ponatinib are designed to target BCR-ABL, but they also inhibit Src (Src-i) kinases, which inhibits VEGF (VEGF-i) downstream. [4] Src kinases are ubiquitously found in podocytes and are involved in cytoskeletal organization, thus Src-i has the ability to damage podocytes, disrupt the filtration barrier, and cause proteinuria. Ponatinib is also a direct VEGF-i, which can lead to podocyte damage and proteinuria through decreased nitrous oxide release and endothelial dysfunction, and the development of HTN through pressure natriuresis, and decreased angiogenesis in the lymphatic system creating volume overload. [12]
Notably, in a longitudinal study on patients with chronic phase CML receiving TKI with median follow-up of 52 months, patients on dasatinib who had normal renal function experienced statistically significant reductions in glomerular filtration rate (GFR) from baseline, which stabilized after one year. Patients with baseline CKD (6%) had decrease in GFR, although not statistically significant. [13] This can likely be attributed to the logarithmic relationship between creatinine (Cr) and GFR, where initial increases in Cr cause greater decreases in GFR than at higher Cr levels. Additionally, 4% of patients on dasatinib developed CKD. Unfortunately, proteinuria was not assessed in this study to assess for early signs of glomerular damage.
Questions remain regarding the relationship of renal ADRs and pre-disposing factors, the impact that dose and duration of TKI have on frequency of ADRs, and the permanence of sequelae. Further research is necessary to elucidate the exact mechanism of renal injury to identify which TKI are more likely to contribute to this injury based on their on-and off-target pharmacodynamic properties.
Acknowledgments
Author contributions
M.S. and Z.M. collected data. J.H.P., E.M.S., S.F.C. and J.T. treated the patients and supervised data collection. M.S. and J.T. wrote the manuscript.
Consent for publication
Consent for publication was obtained from the patients.
Research support
J.T. is supported by the Conquer Cancer Foundation of the American Society of Clinical Oncology, the American Association for Cancer Research, the American Society of Hematology (ASH), the Robert Wood Johnson Foundation, and the NIH/NCI (1K08CA230319-01). S.F.C. is supported by the American Society of Hematology, the Mark Foundation for Cancer Research, the Truth 365 and the Rally Foundation for Childhood Cancer Research, the Conquer Cancer Foundation, and a Career Development Program Fellow Award from the Leukemia & Lymphoma Society (545317).
References
- 1.Highlights of Prescribing Information Dasatinib [Internet]. Maryland: food and drug administration; 2017[Reference ID: 4350141:[Available from:https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021986s7s8lbl.pdf.
- 2.LaCivita C., Dempsey M.Risk assessment and risk mitigation review of ponatinib. 2012. [Internet]. Department of Health and Human Services. Public Health Service, Food and Drug Administration, Center for Drug Evaluation and Research Office of Surveillance and Epidemiology, Office of Medication Error Prevention and Risk Management; 2012[Reference ID: 3222501:[Available from: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=2ahUKEwiAiuiYxcnkAhUyuVkKHbklBXgQFjAAegQIABAC&url=https%3A%2F%2Fwww.accessdata.fda.gov%2Fdrugsatfda_docs%2Fnda%2F2012%2F203469Orig1s000RiskR.pdf&usg=AOvVaw108_-L5E2ZxgRZzdaUhyDl.
- 3.Ozkurt S., Temiz G., Acikalin M.F., Soydan M. Acute renal failure under dasatinib therapy. Ren Fail. 2010;32(1):147–149. doi: 10.3109/08860220903391226. [DOI] [PubMed] [Google Scholar]
- 4.Wallace E., Lyndon W., Chumley P., Jaimes E.A., Fatima H. Dasatinib-induced nephrotic-range proteinuria. Am. J. Kidney Dis. 2013;61(6):1026–1031. doi: 10.1053/j.ajkd.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 5.Aguilera D.G., Tsimberidou A.M. Dasatinib in chronic myeloid leukemia: a review. Ther. Clin. Risk Manag. 2009;5(2):281–289. doi: 10.2147/tcrm.s3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uz B., Dolasik I. An unexpected and devastating adverse event of dasatinib: rhabdomyolysis. Leuk. Res. Rep. 2016;5:1–2. doi: 10.1016/j.lrr.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawaguchi H., Tamura Y., Suzuki S., Asano-Murakoshi T., Nonoyama S. Cytomegalovirus infection- and dasatinib-induced proteinuria in Ph+ALL. Pediatr. Int. 2017;59(6):740–741. doi: 10.1111/ped.13251. [DOI] [PubMed] [Google Scholar]
- 8.Ochiai S., Sato Y., Minakawa A., Fukuda A., Fujimoto S. Dasatinib-induced nephrotic syndrome in a patient with chronic myelogenous leukemia: a case report. BMC Nephrol. 2019;20(1):87. doi: 10.1186/s12882-019-1273-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alahmari A., Lipton J.H., Kim D. Dasatinib induced reversible nephrotic range proteinuria occurs more frequently compared to other tyrosine kinase inhibitors in the treatment of chronic myeloid leukemia. Blood. 2017;130(Suppl 1):2880. [Google Scholar]
- 10.Mitoxantrone for injection concentrate [Internet]. Maryland: food and drug administration; 2008[Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/019297s030s031lbl.pdf.
- 11.Cortes J.E., Kim D.W., Pinilla-Ibarz J., le Coutre P., Paquette R., Chuah C. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N. Engl. J. Med. 2013;369(19):1783–1796. doi: 10.1056/NEJMoa1306494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jhaveri K.D., Sakhiya V., Wanchoo R., Ross D., Fishbane S. Renal effects of novel anticancer targeted therapies: a review of the Food and Drug Administration Adverse Event Reporting System. Kidney Int. 2016;90(3):706–707. doi: 10.1016/j.kint.2016.06.027. [DOI] [PubMed] [Google Scholar]
- 13.Yilmaz M., Lahoti A., O'Brien S., Nogueras-González G.M., Burger J., Ferrajoli A. Estimated glomerular filtration rate changes in patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. Cancer. 2015;121(21):3894–3904. doi: 10.1002/cncr.29587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Luca M., Carmosino I., Stefanizzi C., Campanelli M., De Angelis F., Cesini L., et al. Nephrotic Proteinuria Developed under Dasatinib Treatment in a Patient with Chronic Myeloid Leukemia: A Case Report and Review of the Literature. Annals of Hematology & Oncology. 2016;3 (8Th):1106.
- 15.Amin S.O., Ruzicka M., Burns K.D., Bence-Bruckler I.A., Ryan S.E., Hadziomerovic A. Renovascular hypertension from the BCR-ABL tyrosine kinase inhibitor ponatinib. J. Clin. Hypertens. (Greenwich) 2020;22(4):678–682. doi: 10.1111/jch.13843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiremath S., Amin S.O., Ruzicka M., Burns K. Abstract P431: renal artery stenosis caused by a Bcr-Abl tyrosine kinase inhibitor: blood pressure response to revascularization. Hypertension. 2017;70(suppl_1) AP431-AP. [Google Scholar]