Abstract
Liver metastases in cervical cancer is rare and can be difficult-to-treat. The current guidelines established by the Gynecologic Oncology Group recommend platinum-based systemic chemotherapy in combination with an anti-angiogenic agent such as bevacizumab, however, overall survival remains poor following diagnosis and options for patients who fail chemotherapy are limited. Yttrium-90 (Y90) radioembolization (RE) has shown great promise in the treatment of chemo-refractory colorectal liver metastases. We describe a 30-year-old female with a history of stage IB endocervical adenocarcinoma who later developed metastases to the liver, that were unresponsive to multiple chemotherapeutics and chemoembolization, and was successfully treated with Y90 RE with concurrent systemic Pembrolizumab. The Y90 RE treatment resulted in positive clinical and imaging responses with improvement in her quality of life, all of which continue to persist at the time of writing this manuscript about 8-months into her RE treatment.
Keywords: Endocervical carcinoma, Liver metastasis, Yttrium 90 microspheres, Radioembolization, Pembrolizumab
Introduction
Cervical cancer is the fourth most common malignancy in women with approximately 570,000 new cases reported worldwide in 2018 [1]. Liver metastasis in cervical cancer are uncommon, occurring in only ~5% of patients and portend a poor prognosis [2]. Current guidelines established by the Gynecologic Oncology Group recommend platinum-based chemotherapy in combination with an antiangiogenic agent however, this is not curative and median survival after diagnosis remains 8-13 months [3]. Due to the infrequency of this diagnosis there is little guidance on the role of liver directed treatment in the management of chemorefractory liver metastasis from cervical cancer. Yttrium-90 (Y90) radioembolization (RE) is a well-studied, safe, and effective option used primarily for treatment of chemorefractory, unresectable colorectal liver metastases [4], [5], [6]. Similar efficacy has been observed in primary hepatocellular carcinoma (HCC) [7], liver metastases in chemorefractory breast [8], renal cell carcinoma [9,10], and pancreatic adenocarcinoma [11]. Here we report the unique case of a patient with chemorefractory liver metastases from endocervical adenocarcinoma successfully treated with targeted Y90 RE while concurrently receiving systemic Pembrolizumab (Keytruda, Merck & Co, Kenilworth, NJ, USA).
Case presentation
A 30-year-old Caucasian female initially presented with a 4-month history of progressively worsening back pain. There was no fever, fatigue, weight loss, or constipation. Her past medical history was significant for Stage IB endocervical adenocarcinoma diagnosed 3 years prior. Her cervical cancer had been initially treated with radical hysterectomy, upper vaginectomy, bilateral salpingectomies, and peritoneal lymph node dissection. All nodes were negative and there was no lymphovascular invasion at the original diagnosis. She denied any history of alcohol, tobacco, drug abuse, or exposures to hazardous materials.
On initial physical examination, vital signs were (Heart rate: 88/min, Temperature: 97.7F, Respirations: 18/min, Blood pressure: 120/78 mm Hg, Oxygen saturation: 100% on room air) with no evidence of bony abnormalities, restricted range of motion or pain, abdominal pain, hepatomegaly, or splenomegaly. Laboratory studies were unremarkable other than elevation of creatinine and mild anemia. Given the patient's history of cancer, a computed tomography (CT) abdomen was obtained which demonstrated a 4-cm left-sided retroperitoneal adenopathy involving the left ureter and common iliac artery with left hydronephrosis as well.
Based on the patient's presentation and CT abdomen, a left ureteral stent was placed to relieve the hydronephrosis. Staging positron emission tomography (PET) CT scan 1-month later confirmed the fluorodeoxyglucose (FDG) avid mass encasing the inferior aorta, left common iliac artery/vein, and left ureter. There were no additional lesions/masses detected in the chest, abdomen, or pelvis. Biopsy of the retroperitoneal mass demonstrated metastatic endocervical adenocarcinoma. Hematology-oncology recommended 6 cycles of Cisplatin (Platinol, Bristol-Myers Squibb Company, Princeton, NJ, USA). A 3-month CT after completion of Cisplatin therapy showed a decrease in size of the retroperitoneal mass. However, a subsequent 3-month follow-up PET CT showed a new hypermetabolic 3.5-cm right lobe liver lesion. Treatment with 6 cycles of Carboplatin (Paraplatin, Bristol-Myers Squibb Company, Princeton, NJ, USA), Paclitaxel (Taxol, Bristol-Myers Squibb Company, Princeton, NJ, USA) and Bevacizumab (Avastin, Genentech USA, San Francisco, CA, USA) was initiated. After 2 cycles, when the liver lesion did not respond, a decision was made to treat the patient with transarterial chemoembolization (TACE) using Doxorubicin (Adriamycin, Pfizer, New York, NY, USA) mixed with lipiodol (Guerbet LLC, Bloomington, IN, USA) and continue with the remaining 4 cycles of systemic chemotherapy. TACE was performed to the right lobe via a replaced right hepatic artery achieving good lipiodol deposition (Fig. 1). Post-TACE, a 6-month follow-up PET (Fig. 2) and a concurrent magnetic resonance imaging (MRI) scan, (Fig. 3) demonstrated progressive enlargement of the right inferior lobe lesion. Additionally, there was a new lesion in the segment 7 of the liver, measuring approximately 1.5 cm. This was considered a treatment failure and she was then started on Pembrolizumab.
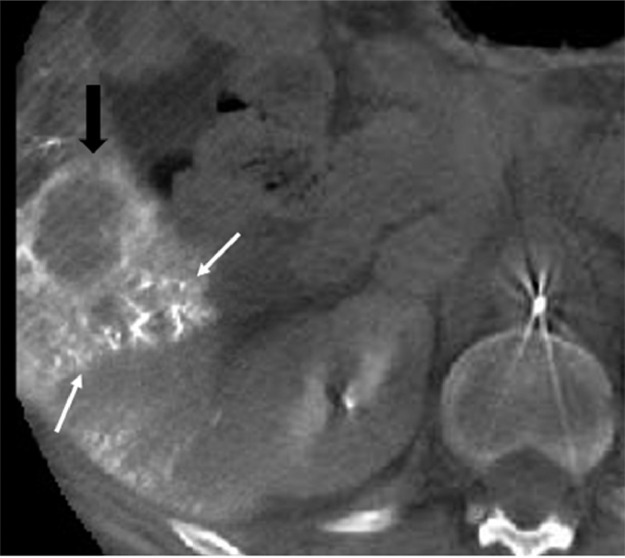
Fig. 1.
8 months after the initiating chemotherapy (cisplatin, followed by carboplatin + Avastin), a TACE was performed to treat the new right lobe liver lesion, that was not responding to systemic therapy. The axial cone beam CT obtained in the IR suite, at the end of the TACE procedure to confirm drug localization, demonstrates Lipiodol accumulation (white arrows) around the segment 5 tumor (black arrows), suggesting a technically successful procedure.
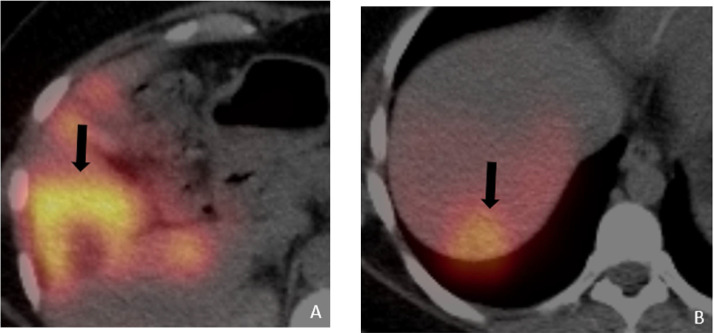
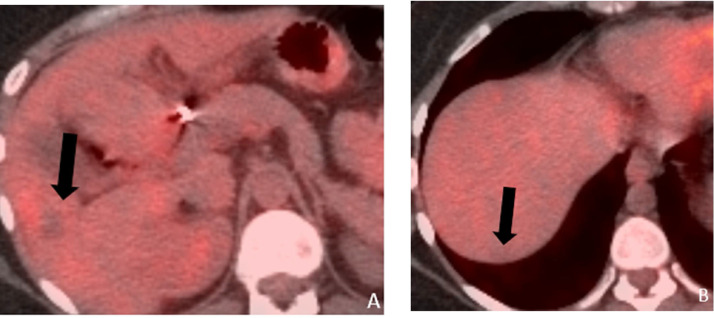
Figure 2.
Axial fused PET/CT (a and b) obtained 6 months following TACE to the right lobe and completion of 6 cycles of carboplatin + Avastin, showing a larger FDG avid lesion in segment V and a new lesion in segment 7 (black arrows), suggesting poor response to the TACE and systemic chemotherapy.
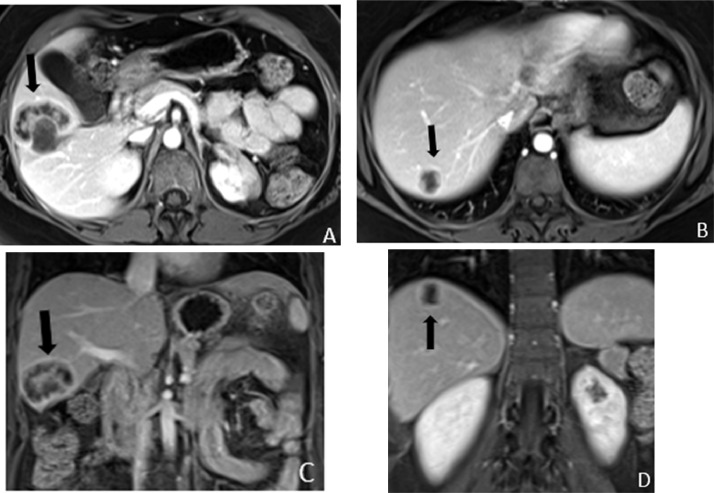
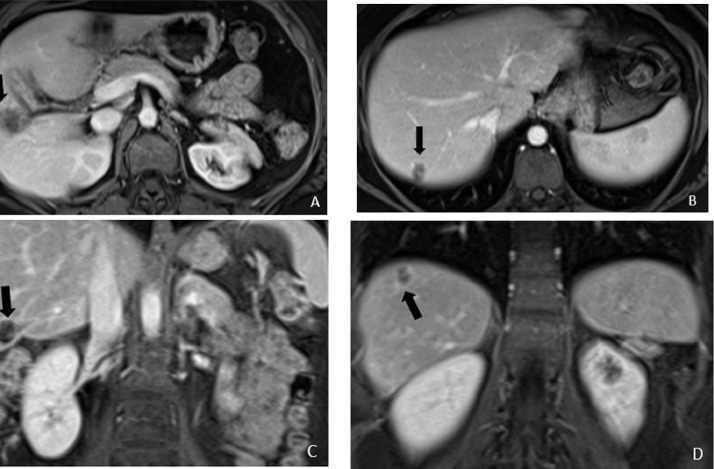
Fig. 3.
Axial (a and b) and coronal (c and d) contrast enhanced MRI of the abdomen obtained concurrently with the PET/CT, to better characterize and evaluate for additional smaller liver lesions, showing heterogeneously enhancing masses in the segment 5 and 7 of the liver (black arrows).
Based on the complexity of the case, the patient was discussed at multidisciplinary tumor board. Given the extent of her disease, she was not considered a candidate for hepatic resection. However, based on her overall good performance status, lack of comorbidities, poor response to chemotherapy, and TACE, it was felt she would be better served with selective internal radiation treatment using Y90. The fact that cervical cancer is sensitive to radiation [12] and as the patient was already treated with radio sensitizing platinum-based drugs, made us speculate radiation with Y90 would be effective to treat the liver lesions.
As a standard part of Y90 RE, an initial mapping angiogram was performed prior to the Y90 RE to map out the tumor supply and estimate a lung shunt fraction. The angiogram was performed via a right common femoral artery access. Selective angiogram of the superior mesenteric artery demonstrated the known replaced right hepatic artery and the hypervascular metastatic lesions within segment 5 and 7 (Fig. 4). The replaced right hepatic artery was then catheterized with a combination of a 5 French cobra catheter and a high-flow microcatheter. The microcatheter was positioned appropriately (Fig. 4) within the right hepatic artery, and approximately 3.9 mCi of technetium 99 (99Tc) macroaggregated albumin was injected. Based on the planar images (Fig. 5) obtained following 99Tc macroaggregated albumin injection, a lung shunt fraction (LSF) of 2.0% was calculated. As per the Y90 manufacturer's recommendation this level of shunting did not necessitate dose reduction. To determine the optimal Y90 microsphere activity, liver 3D volumetrics was performed based off the staging liver MRI. The total liver volume measured 1300 mL. The right lobe measured 955 mL and the left lobe measured 336 mL with a total tumor volume of 100 mL (10.5% of the right lobe treatment area). The Tumor: Normal liver (T: N) ratio was estimated to be 3:1. To deliver a tumor dose of 110 Gray (Gy), an activity 0.9 GBq of Y90 was prescribed based off the partition dosimetry model. The estimated dose to the liver was 38 Gy.
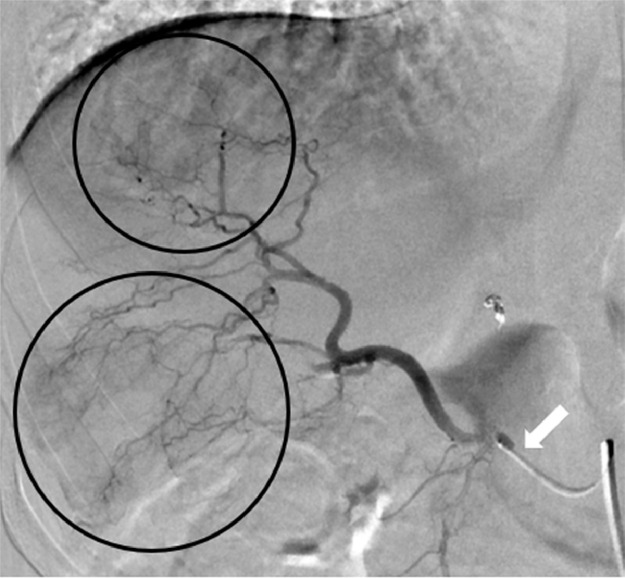
Fig. 4.
Mapping Y90 digital subtraction angiogram of the replaced right hepatic artery performed 2 weeks after the MRI and PET/CT, showing tumor blush in the segment 5 and 7 (black circles). The microcatheter is in the replaced right hepatic artery (white arrows).
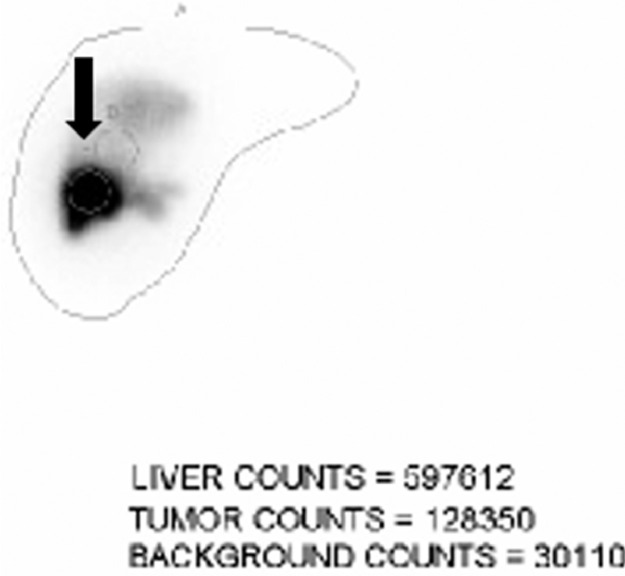
Fig. 5.
Planar image following Tc 99 MAA injection into the right hepatic artery, obtained as a part of the mapping angiogram, shows localization of the isotope to the segment 5 of the liver (black arrow).
The RE treatment was performed 1 week following the mapping angiogram using SIR-spheres (Sirtex Medical Inc., Sydney, Australia), again via the right common femoral artery. The prescribed activity was successfully administered after catheterization of the superior mesenteric artery and subsequently the replaced right hepatic artery with a high-flow microcatheter placed in the same position within the right hepatic artery as in the mapping angiogram. Postprocedure Bremsstrahlung planar and single photon emission computed tomography demonstrated appropriate distribution of Y90 spheres in the right hepatic lobe without evidence of non-target embolization.
The patient tolerated Y90 RE very well and she reported only mild self-limiting right upper quadrant abdominal pain the day after treatment. Follow-up MRI 3 months post Y90 RE, demonstrated a significant decrease in size and enhancement of the treated right hepatic lobe lesions, consistent with good overall response to the RE (Fig. 6). At 8-month follow-up, the patient continued to do well with an improved quality of life. Except for a mild rash attributed to her immunotherapy, she had no other complaints. The most recent PET demonstrated continued decrease in size of the hepatic lesions with no FDG activity, consistent with a sustained response (Fig. 7).
Fig. 6.
Axial (a and b) and coronal (c and d) contrast enhanced MRI of the abdomen obtained 3 months after the Y90 treatment showing much smaller and nonenhancing lesions in segment 5 and 7 (black arrows).
Fig. 7.
PET/CT 8 months following the Y-90 RE showing continued shrinkage of the lesions without FDG activity.
Discussion
Cervical cancer is a common malignancy in middle aged women with an estimated incidence of 8.9 per 100,000 in the United States [13,14]. Globally, there were over 300,000 deaths reported from cervical cancer in 2018 [1,15]. Squamous cell carcinoma is the most observed histological type accounting for ~70% of cases while adenocarcinoma accounts for only 20%. Liver metastasis are rare in cervical cancer and may be synchronous or metachronous. In a large retrospective review of cervical cancer patients with liver metastasis, a majority developed metachronous lesions with a median appearance of 39 months [2] after the initial diagnosis, and were often observed in patients with advanced locoregional disease.
The identification of liver metastasis has important prognostic implications. In a study of 99 patients with newly diagnosed cervical cancer, Yin et al. observed the presence of liver metastases was the most significant determinate of overall survival [16]. Overall survival in patients with liver metastasis form cervical cancer was 20% at 12 months and 8% at 24 months with a median overall survival 6.8 months.
Guidelines for the management of metastatic cervical cancer have been primarily set forth by the Gynecology Oncology Group. In patients with recurrent, persistent disease, a combination of Cisplatin and Paclitaxel is recommended [17,18]. If liver metastases are persistent or progress, the addition of Bevacizumab is also recommended, as in our patient. In a phase III randomized control trial, chemotherapy plus bevacizumab improved overall survival to 16.8 months compared to 13.3 months with chemotherapy alone [19], [20], [21]. However, the patients receiving Bevacizumab had an increased incidence of genitourinary fistula formation. For patients who progress on chemotherapy plus bevacizumab, the FDA recently approved Pembrolizumab (Keytruda) in 2019 which has shown promise in this subset of patients [22,23]. Hepatic resection is also an option for liver metastases from cervical cancer, and has demonstrated a median overall survival 18 months postresection [24,25]. However, liver resection is a major surgery and many patients may not be able to tolerate it or may not be considered a candidate if there is significant disease burden outside of the liver. In the case of our patient, the presence of bulky lymph node disease was indicative of advanced disease, and this made surgical resection a less attractive option
Microwave ablation is a minimally invasive technique that has been increasingly used in treatment of primary and secondary liver tumors in patients who are not surgical candidates [26], [27], [28]. The local control rates following microwave ablation are the highest for liver lesions less than 3 cm [29]. As the largest lesion in our patient was 3.5 cm with an additional lesion in an area which was not readily or safely accessible for microwave ablation, we chose not to use this technique.
Y90 RE is a form of brachytherapy capitalizing on targeted delivery of βparticle-emitting microspheres. The 2 commercial manufacturers of Y-90 microspheres are TheraSpheres (BTG International, ON, Canada) and SIR-spheres (Sirtex Medical Inc., Sydney, Australia). β-rays have a mean penetration range of 2.5 mm in tissue, which limits collateral damage to the liver parenchyma [7,30,31]. In the liver, 75% of the blood supply to the hepatic parenchyma is from the portal venous system whereas 80% of the tumor blood supply derives from the hepatic artery [32,33]. This allows for transarterial delivery of the microspheres preferentially to the tumors. Y90 dosimetry can be performed using empiric, body surface area (BSA) or the partition models. The details pertaining to each model have been previously described [30,34,35]. The Y90 RE treatment is typically performed in 2 stages; an initial mapping angiogram which is performed to map out the vascular supply within the intended area of treatment, coil vessels that may be at risk for nontarget embolization and also estimate the LSF to determine if the prescribed activity needs to be reduced or maintained. Once the vasculature is mapped and the LSF is determined, the patient is scheduled for a treatment with the Y90 microspheres, most often after 7-10 days of the mapping angiogram.
For patients who are not good surgical candidates, palliative liver-directed therapy with transarterial Y90 microspheres has shown promise in treatment of liver metastasis from several cancers including colorectal, appendiceal, breast, renal cell, and pancreatic [36,37]. Based on the historical evidence and the radio-sensitivity of cervical cancers, we hypothesized Y90 RE may slow the progression of our patient's multifocal liver metastases, which if not controlled would have ultimately resulted in liver failure and death.
While there are no reported cases of using immunotherapy in combination with Y90 in cervical cancer, there have been reports of the clinical safety and efficacy of combined therapy in HCC and metastatic renal cell carcinoma [38], [39], [40]. These studies indicate concurrent locoregional therapy and immunotherapy may behave synergistically through immunomodulation and enhanced antigen presentation. A study of the immune profile of tumor infiltrating lymphocytes in Y90 treated HCC demonstrated higher infiltration of granzyme B and tumor necrosis factor-alpha (TNF-α) expressing lymphocytes consistent with local immune activation [41,42]. Other studies have observed significant increases in inflammatory cytokines IL-1, IL-6, IL-8, and TNF-α following Y-90 RE [43,44]. There have been published papers of radiation treatment in the form of stereotactic body radiation therapy inducing and promoting immune responses that mediate antitumorigenic effects [45,46]. Similarly, Y90 may be capable of inducing local and systemic changes in the immune system enhancing innate tumor targeting.
To the best of our knowledge, this is the first report of Y90 RE in combination with immunotherapy to successfully treat hepatic metastatic disease from cervical cancer following failure of chemotherapy and TACE. Most studies using Y90 RE in the salvage setting report partial response of liver lesions. The patient in this case report had a complete response of both liver lesions, presumably from a combination of Y90 RE and immunotherapy. The relative contribution of the 2 individual treatments or any possible synergy between the treatments is unknown, however we speculate that initiating Y-90 RE earlier in the treatment course may have contributed to the excellent control of what was otherwise a rapidly progressive metastatic disease. A larger case series, randomized control trial and/or substantial follow up are needed to define the role of Y90 RE with or without immunotherapy in chemo refractory hepatic metastasis form cervical cancer and provide insight into the possible abscopal/immunomodulatory effects of Y90 RE.
Author contributions
MN wrote the paper and participated in the editing process. AB, GBB participated in the editing process. RD planned andperformed the Y90radioembolization, wrote the paper and participatedin the editing process.
Informed consent
Informed consent was obtained from the patient.
Footnotes
Competing Interest: The authors declare that they have no competing interests. No third party was involved in neither the influence nor outcome of this article.
Funding support: None.
References
- 1.Arbyn M., Weiderpass E., Bruni L., de Sanjosé S., Saraiya M., Ferlay J. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Heal. 2020;8:e191–e203. doi: 10.1016/S2214-109X(19)30482-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim G.E., Lee S.W., Suh C.O., Park T.K., Kim J.W., Park J.T. Hepatic metastases from carcinoma of the uterine cervix. Gynecol Oncol. 1998;70:56–60. doi: 10.1006/gyno.1998.5037. [DOI] [PubMed] [Google Scholar]
- 3.Meir H., Kenter G., Burggraaf J., Kroep J., Welters M., Melief C. The need for improvement of the treatment of advanced and metastatic cervical cancer, the rationale for combined chemo-immunotherapy. Anticancer Agents Med Chem. 2014;14:190–203. doi: 10.2174/18715206113136660372. [DOI] [PubMed] [Google Scholar]
- 4.Sreenivasan N., Kalyanpur A., Bhat A., Sridhar P., Singh J. CT diagnosis of cecal diverticulitis. Indian J Radiol Imaging. 2006;16:451. doi: 10.4103/0971-3026.32244. [DOI] [Google Scholar]
- 5.Saxena A., Bester L., Shan L., Perera M., Gibbs P., Meteling B. A systematic review on the safety and efficacy of yttrium-90 radioembolization for unresectable, chemorefractory colorectal cancer liver metastases. J Cancer Res Clin Oncol. 2014;140:537–547. doi: 10.1007/s00432-013-1564-4. [DOI] [PubMed] [Google Scholar]
- 6.Bhat A., Layfield L.J., Tewari S.O., Gaballah A.H., Davis R., Wu Z. Solitary fibrous tumor of the ischioanal fossa—a multidisciplinary approach to management with radiologic-pathologic correlation. Radiol Case Rep. 2018;13:468–474. doi: 10.1016/j.radcr.2018.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhangoo M.S., Karnani D.R., Hein P.N., Giap H., Knowles H., Issa C. Radioembolization with Yttrium-90 microspheres for patients with unresectable hepatocellular carcinoma. J Gastrointest Oncol. 2015;6:469–478. doi: 10.3978/j.issn.2078-6891.2015.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pieper C.C., Meyer C., Wilhelm K.E., Block W., Nadal J., Ahmadzadehfar H. Yttrium-90 radioembolization of advanced, unresectable breast cancer liver metastases—a single-center experience. J Vasc Interv Radiol. 2016;27:1305–1315. doi: 10.1016/j.jvir.2016.05.028. [DOI] [PubMed] [Google Scholar]
- 9.Schuchardt P., Yasin J., Davis R.M., Thimmappa N., Bhat A.P. Pelvic trauma. Contemp Diagn Radiol. 2019;42:1–6. doi: 10.1097/01.CDR.0000582600.38333.f4. [DOI] [Google Scholar]
- 10.Abdelmaksoud M.H.K., Louie J.D., Hwang G.L., Kothary N., Minor D.R., Sze D.Y. Yttrium-90 radioembolization of renal cell carcinoma metastatic to the liver. J Vasc Interv Radiol. 2012;23:323–330.e1. doi: 10.1016/j.jvir.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Kim A.Y., Frantz S., Brower J., Akhter N. Radioembolization with Yttrium-90 microspheres for the treatment of liver metastases of pancreatic adenocarcinoma: a multicenter analysis. J Vasc Interv Radiol. 2019;30:298–304.e2. doi: 10.1016/j.jvir.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 12.Qin C., Chen X., Bai Q., Davis M.R., Fang Y. Factors associated with radiosensitivity of cervical cancer. Anticancer Res. 2014;34:4649–4656. [PubMed] [Google Scholar]
- 13.Ghouri M.A., Gupta N., Bhat A.P., Thimmappa N.D., Saboo S.S., Khandelwal A. CT and MR imaging of the upper extremity vasculature: pearls, pitfalls, and challenges. Cardiovasc Diagn Ther. 2019;9:S152–S173. doi: 10.21037/cdt.2018.09.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watson M., Saraiya M., Benard V., Coughlin S.S., Flowers L., Cokkinides V. Burden of cervical cancer in the United States, 1998-2003. Cancer. 2008;113:2855–2864. doi: 10.1002/cncr.23756. [DOI] [PubMed] [Google Scholar]
- 15.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 16.Yin Z., Tang H., Li L., Ni J., Yuan S., Lou H. Impact of sites versus number of metastases on survival of patients with organ metastasis from newly diagnosed cervical cancer. Cancer Manag Res. 2019;11:7759–7766. doi: 10.2147/CMAR.S203037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thimmappa N., Bhat A.P., Bishop K., Nagpal P., Prince M.R., Saboo S.S. Preoperative cross-sectional mapping for deep inferior epigastric and profunda artery perforator flaps. Cardiovasc Diagn Ther. 2019;9:S131–S142. doi: 10.21037/cdt.2018.10.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monk B.J., Sill M.W., McMeekin D.S., Cohn D.E., Ramondetta L.M., Boardman C.H. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: a gynecologic oncology group study. J Clin Oncol. 2009;27:4649–4655. doi: 10.1200/JCO.2009.21.8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhat A.P., Pimpalwar A., Dyke P.C. Ultrasonography and X-Ray guided drain placement to evacuate a pneumopericardium/pneumomediastinum in a 1-day-old infant. Indian J Radiol Imaging. 2019;29:94–97. doi: 10.4103/ijri.IJRI_447_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tewari K.S., Sill M.W., Penson R.T., Huang H., Ramondetta L.M., Landrum L.M. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240) Lancet. 2017;390:1654–1663. doi: 10.1016/S0140-6736(17)31607-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Senne J., Davis R., Yasin J., Brimmo O., Evenski A., Bhat A. Computed tomography guided radio-frequency ablation of osteoid osteomas in atypical locations. Indian J Radiol Imaging. 2019;29:253. doi: 10.4103/ijri.ijri_259_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yasin J., Thimmappa N., Kaifi J.T., Avella D.M., Davis R., Tewari S.O. CT-guided cryoablation for post-thoracotomy pain syndrome: a retrospective analysis. Diagn Interv Radiol. 2020;26:53–57. doi: 10.5152/dir.2019.19179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chung H.C., Ros W., Delord J.P., Perets R., Italiano A., Shapira-Frommer R. Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: Results from the phase II KEYNOTE-158 study. J Clin Oncol. 2019;37:1470–1478. doi: 10.1200/JCO.18.01265. [DOI] [PubMed] [Google Scholar]
- 24.Bhat A., Davis R., Bryan W. A rare case of bleeding duodenal varices from superior mesenteric vein obstruction -treated with transhepatic recanalization and stent placement. Indian J Radiol Imaging. 2019;29:313. doi: 10.4103/ijri.ijri_21_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bacalbasa N., Balescu I., Dima S., Popescu I. Hepatic resection for liver metastases from cervical cancer is safe and may have survival benefit. Anticancer Res. 2016;36:3023–3027. [PubMed] [Google Scholar]
- 26.Vogl T.J., Nour-Eldin N.E.A., Hammerstingl R.M., Panahi B., Naguib N.N.N. Microwave ablation (MWA): basics, technique and results in primary and metastatic liver neoplasms - review article. RoFo Fortschritte Auf Dem Gebiet Der Rontgenstrahlen Und Der Bildgeb Verfahren. 2017;189:1055–1066. doi: 10.1055/s-0043-117410. [DOI] [PubMed] [Google Scholar]
- 27.Meloni M.F., Chiang J., Laeseke P.F., Dietrich C.F., Sannino A., Solbiati M. Microwave ablation in primary and secondary liver tumours: technical and clinical approaches. Int J Hyperth. 2017;33:15–24. doi: 10.1080/02656736.2016.1209694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kabeel K., Marjara J., Bhat R., Gaballah A.H., Abdelaziz A., Bhat A.P. Spontaneous hemorrhage of an adrenal myelolipoma treated with transarterial embolization: A case report. Radiol Case Rep. 2020;15:961–965. doi: 10.1016/j.radcr.2020.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tinguely P., Dal G., Bottai M., Nilsson H., Freedman J., Engstrand J. Microwave ablation versus resection for colorectal cancer liver metastases – A propensity score analysis from a population-based nationwide registry. Eur J Surg Oncol. 2020;46:476–485. doi: 10.1016/j.ejso.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Toskich B.B., Liu D.M. Y90 radioembolization dosimetry: concepts for the interventional radiologist. Tech Vasc Interv Radiol. 2019;22:100–111. doi: 10.1053/j.tvir.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 31.Verma M., Yarlagadda B., Hendrani A., Bhat A.P., Kumar S. Simplified rapid protocol for assessing the thoracic aortic dimensions and pathology with noncontrast MR angiography. Int J Angiol. 2019;28:130–136. doi: 10.1055/s-0039-1688473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalva S.P., Thabet A., Wicky S. Recent advances in transarterial therapy of primary and secondary liver malignancies. RadioGraphics. 2008;28:101–117. doi: 10.1148/rg.281075115. [DOI] [PubMed] [Google Scholar]
- 33.Schuchardt P.A., Yasin J.T., Davis R.M., Tewari S.O., Bhat A.P. The role of an IVC filter retrieval clinic-a single center retrospective analysis. Indian J Radiol Imaging. 2019;29:391–396. doi: 10.4103/ijri.IJRI_258_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhat P., Sridhar P., Sreenivasan N., Kalyanpur A. Ct diagnosis of epiploic appendagitis-a case report. Indian J Radiol Imaging. 2006;16:447. doi: 10.4103/0971-3026.32242. [DOI] [Google Scholar]
- 35.Tafti B.A., Padia S.A. Dosimetry of Y-90 microspheres utilizing Tc-99m SPECT and Y-90 PET. Semin Nucl Med. 2019;49:211–217. doi: 10.1053/j.semnuclmed.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 36.Khajornjiraphan N., Thu N.A., Chow P.K.H. Yttrium-90 microspheres: a review of its emerging clinical indications. Liver Cancer. 2015;4:6–15. doi: 10.1159/000343876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhat A.P., Schuchardt P.A., Bhat R., Davis R.M., Singh S. Metastatic appendiceal cancer treated with Yttrium 90 radioembolization and systemic chemotherapy: a case report. World J Radiol. 2019;11:116–125. doi: 10.4329/wjr.v11.i9.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sangro B., Gomez-Martin C., De La Mata M., Iñarrairaegui M., Garralda E., Barrera P. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59:81–88. doi: 10.1016/j.jhep.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 39.Zhan C., Ruohoniemi D., Shanbhogue K.P., Wei J., Welling T.H., Gu P. Safety of combined Yttrium-90 radioembolization and immune checkpoint inhibitor immunotherapy for hepatocellular carcinoma. J Vasc Interv Radiol. 2020;31:25–34. doi: 10.1016/j.jvir.2019.05.023. [DOI] [PubMed] [Google Scholar]
- 40.Adashek J.J., Salgia M., Dizman N., Kessler J., Pal S.K. Concomitant radioembolization and immune checkpoint inhibition in metastatic renal cell carcinoma. Case Rep Oncol. 2018;11:276–280. doi: 10.1159/000489995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel P.J., Hieb R.A., Bhat A.P. Percutaneous revascularization of chronic total occlusions. Tech Vasc Interv Radiol. 2010;13:23–36. doi: 10.1053/j.tvir.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 42.Chew V., Lee Y.H., Pan L., Nasir N.J.M., Lim C.J., Chua C. Immune activation underlies a sustained clinical response to Yttrium-90 radioembolisation in hepatocellular carcinoma. Gut. 2019;68:335–346. doi: 10.1136/gutjnl-2017-315485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fernandez-Ros N., Iñarrairaegui M., Paramo J.A., Berasain C., Avila M.A., Chopitea A. Radioembolization of hepatocellular carcinoma activates liver regeneration, induces inflammation and endothelial stress and activates coagulation. Liver Int. 2015;35:1590–1596. doi: 10.1111/liv.12592. [DOI] [PubMed] [Google Scholar]
- 44.Seidensticker M., Powerski M., Seidensticker R., Damm R., Mohnike K., Garlipp B. Cytokines and 90Y-Radioembolization: Relation to Liver Function and Overall Survival. Cardiovasc Intervent Radiol. 2017;40:1185–1195. doi: 10.1007/s00270-017-1622-4. [DOI] [PubMed] [Google Scholar]
- 45.Brown J.M., Koong A.C. High-dose single-fraction radiotherapy: exploiting a new biology? Int J Radiat Oncol Biol Phys. 2008;71:324–325. doi: 10.1016/j.ijrobp.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 46.Carvalho H.de A., Villar R.C. Radiotherapy and immune response: the systemic effects of a local treatment. Clinics. 2018;73 (suppl 1):e557s. doi: 10.6061/clinics/2018/e557s. [DOI] [PMC free article] [PubMed] [Google Scholar]