Abstract
Autoimmune diseases are common diseases of the immune system that are characterized by the loss of self-tolerance and the production of autoantibodies; the breakdown of immune tolerance and the prolonged inflammatory reaction are undisputedly core steps in the initiation and maintenance of autoimmunity. Peroxisome proliferator-activated receptors (PPARs) are ligand-dependent transcription factors that belong to the nuclear hormone receptor family and act as ligand-activated transcription factors. There are three different isotypes of PPARs: PPARα, PPARγ, and PPARβ/δ. PPARγ is an established regulator of glucose homeostasis and lipid metabolism. Recent studies have demonstrated that PPARγ exhibits anti-inflammatory and anti-fibrotic effects in multiple disease models. PPARγ can also modulate the activation and polarization of macrophages, regulate the function of dendritic cells and mediate T cell survival, activation, and differentiation. In this review, we summarize the signaling pathways and biological functions of PPARγ and focus on how PPARγ and its agonists play protective roles in autoimmune diseases, including autoimmune thyroid diseases, multiple sclerosis, rheumatoid arthritis, systemic sclerosis, systemic lupus erythematosus, primary Sjogren syndrome and primary biliary cirrhosis.
Keywords: PPARγ, Nuclear hormone receptors, Autoimmune diseases
1. Introduction
Autoimmune diseases are a wide spectrum of diseases that are characterized by the loss of self-tolerance and the production of autoantibodies [1]. There are both organ-specific autoimmune diseases, such as autoimmune thyroid diseases, multiple sclerosis and rheumatoid arthritis, and systemic autoimmune diseases, such as systemic sclerosis and systemic lupus erythematosus [2]. Although the pathogenic mechanisms underlying autoimmune diseases remain to be elucidated, the breakdown of immune tolerance and the prolonged inflammatory reaction are undisputedly core steps in the initiation and maintenance of autoimmunity [3]. Thus, the molecules that participate in immune feedback may be potential therapeutic targets for the treatment of autoimmune diseases.
Peroxisome proliferator-activated receptors (PPARs) are ligand-activated transcription factors which belong to the nuclear hormone receptor family [4]. There are three different isoforms of PPARs, namely, PPARα, PPARβ/δ and PPARγ, all of which are encoded by different genes [5]. These isoforms heterodimerize with the retinoid X receptor. When activated, this complex can regulate gene expression by binding to specific peroxisome proliferator response elements (PPREs), which are located in the regulatory site of each gene [6]. Although the three different isoforms of PPARs share a high degree of structural similarity, they have different ligands and distinct patterns of distribution [7]. PPARα was the first PPAR subtype to be cloned. The basic function of PPARα is to regulate the oxidation of fatty acids. PPARα is highly expressed in multiple organs and tissues, particularly in the liver, heart, kidneys, brown adipose tissue and skeletal muscles [8]. PPARβ/δ not only takes part in the metabolism of lipids but is also involved in many other physiological processes, such as wound healing, embryonic development and inflammation [9]. PPARβ/δ is ubiquitously expressed but is expressed at higher levels in the digestive tract and heart. In addition, PPARβ/δ is the predominant isotype in the skin [10]. PPARγ is an established regulator of glucose homeostasis and lipid metabolism [11]. PPARγ also plays an anti-inflammatory role [12]. PPARγ has two different protein isoforms, namely, PPARγ1 and PPARγ2 [7]. PPARγ1 is expressed in many different tissues and inflammatory cells, including macrophages, lymphocytes and dendritic cells. PPARγ2 is mainly expressed in adipocytes [13]. PPARγ is extensively expressed in immune cells and inhibits inflammatory processes [14]. PPARγ can inhibit the activation and function of macrophages and dendritic cells [12,15] and mediate the survival, activation and differentiation of T cells [16]. In this review, we will summarize the signaling pathways and biological functions of PPARγ and focus on how PPARγ plays a protective role in autoimmune diseases.
2. Structure, ligands, and signaling pathways of PPARγ
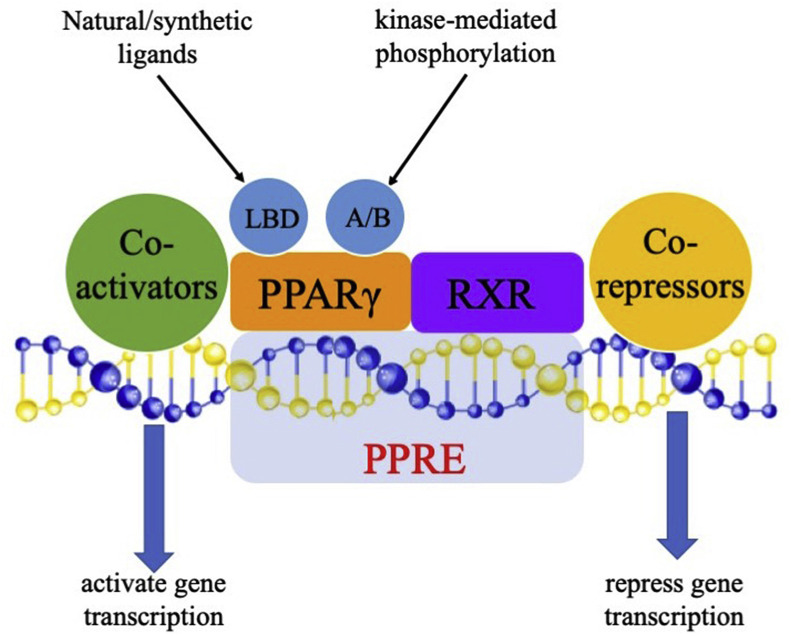
The three-dimensional structure of PPARγ is composed of four domains, including the transactivation and phosphorylation domain (A/B domain), a DNA binding domain (DBD) in the N-terminus, a hinge region, and a ligand-binding domain (LBD) in the C-terminus [17,18]. The A/B domain comprises an activation function 1region that is required for ligand-independent activation. The DBD is conserved across the nuclear receptor superfamily and functions as a sequence-specific binding site for genomic DNA. The hinge region can modulate the DNA-binding ability and is required for receptor dimerization [19]. The LBD comprises 12 α-helices (H1–H12), leads to heterodimerization with retinoid X receptors (RXRs), and contains an activation function 2 region, which is required for ligation, dimerization, recruitment of coactivators and release of corepressors [20].
Many natural and synthetic compounds can act as ligands of PPARγ [21]. The natural ligands of PPARγ, also known as endogenous agonists, can be divided into four subgroups: (A) the eicosanoid prostaglandin-A1 and the cyclopentenone prostaglandin 15-deoxy- Δ12,14-Prostaglandin J2 (15D-PGJ2), (B) unsaturated fatty acids, (C) nitroalkanes, and (D) oxidized phospholipids [7]. However, the natural modulators of PPARγ do not always lead to PPARγ activation and target gene transcription [22]. The synthetic ligands of PPARγ are pharmacological agonists and can be divided into 5 subgroups: (I) the thiazolidinedione (TZD) family, including rosiglitazone, pioglitazone and troglitazone, which were the first ligand family developed to bind and activate PPARγ [23], (II) non-TZD agonists, such as cilitazone, netoglitazone and rivoglitazone [24], (III) selective PPARγ modulators (SPPARγM), which minimize the adverse effects of full PPAR-γ agonists [25], (IV) dual α/γ agonists [26], and (V) pan α/δ/γ agonists [27]. These drugs are mainly used to treat type 2 diabetes mellitus [28] (Table 1 ).
Table 1.
The ligands of PPARγ.
| Ligands of PPARγ | Examples | Effects | References |
|---|---|---|---|
| Natural ligands | the eicosanoid prostaglandin-A1 and the cyclopentenone prostaglandin 15-deoxy-Δ12,14-Prostaglandin J2(15D-PGJ2) | rapid expression and ability to contribute to a natural defense mechanism | [147] |
| unsaturated fatty acids | Upregulate PPARγ expression | [148] | |
| Nitrated fatty acids (NFAs) | anti-inflammatory and anti-fibrotic effects via PPAR-γ activation | [149] | |
| Nitroalkenes | activate PPARγ in monocytes and upregulate FABP4 expression | [150] | |
| oxidized phospholipids | dual effect on bile acid-induced CCL2 expression in pancreatic acini | [151] | |
| Synthetic ligands | the thiazolidinedione (TZD) family | treatment of type 2 diabetes mellitus | [28] |
| the non-TZD agonists | treatment of type 2 diabetes mellitus | [28] | |
| the selective PPARγ modulators (SPPARγM) | mediate Tissue-Dependent PPARγ activation and insulin sensitization | [152] | |
| the dual α/γ agonists | treatment of type 2 diabetes mellitus | [28] | |
| pan α/δ/γ agonists | treatment of type 2 diabetes mellitus | [28] |
The activation of PPARγ is either ligand-dependent due to the conformational change of the LBD or ligand-independent due to the kinase-mediated phosphorylation of the A/B domain [10]. Primed PPARγ can regulate target gene expression both positively and negatively by binding to specific PPREs in the regulatory sites of these genes [29]. The large Y-shaped ligand binding domain allows PPARγ to recognize many different ligands and to flexibly interact with ligands, which makes it possible for PPARγ to respond to various environmental stimuli and to modulate the expression of target genes [30]. Upon binding to the specific ligand, PPARγ forms a heterodimer with RXR and then translocates to the PPREs of the target genes [31]. This complex can also activate or repress gene transcription directly in a ligand-independent manner through recruitment of coactivator proteins, like peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α), or corepressor proteins, like nuclear receptor corepressor 1 (NCoR1) [32] (Fig. 1 ).
Fig. 1.
Signaling pathway of PPARγ. PPARγ can be activated either by its ligands, which bind to the LBD domain, or by the kinase-mediated phosphorylation of its A/B domain. Primed PPARγ can recruit another nuclear receptor, retinoid X receptor (RXR), to form a heterodimer and then bind to the peroxisome proliferator response elements (PPREs) in the promoter regions of the target genes. PPARγ can also recruit coactivator proteins, such as peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α), or corepressor proteins, such as nuclear receptor corepressor 1 (NCoR1), to activate or repress the transcription of direct target genes in the absence of ligands.
3. Biological roles and functions of PPARγ
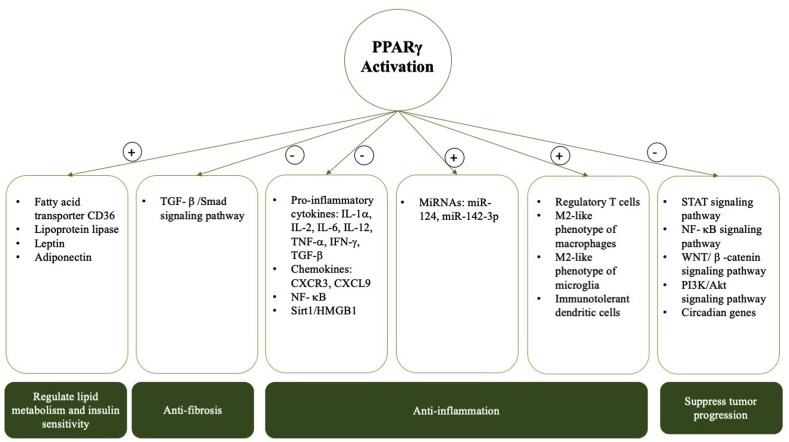
PPARγ exhibits multiple functions in cell biology and participates in pathogenesis of metabolism, inflammation and tumor progression. PPARγ has drawn great medical attention as it is a pivotal transcriptional regulator related to glucose and fatty acid metabolism [33]. PPARγ has become a significant target for the treatment of type 2 diabetes [34]. Both isoforms of PPARγ are essential in the regulation of lipid metabolism and insulin sensitivity regulation [35]. Activated PPARγ regulates the expression of genes involved in the release, transportation, and storage of lipids, such as the fatty acid transporter CD36 and lipoprotein lipase [36,37]. PPARγ also promotes balanced and sufficient production of adipocytokines, such as leptin and adiponectin, which modulate insulin function in peripheral tissues [38]. In recent years, PPARγ has been discovered to contribute to the repression of proinflammatory genes, such as NF-κB [39]. PPARγ aslo exerts anti-inflammatory effects through inhibiting the expression of a multitude of pro-inflammatory cytokines and chemokines including interleukin (IL)-1α, IL-2, IL-6, IL-12, tumor necrosis factor (TNF)-α, interferon (IFN)-γ, transforming growth factor (TGF)-β, chemokine (C-X-C motif) receptor (CXCR)3 and chemokine (C-X-C motif) ligand (CXCL)9 [40,41]. Via regulating TGF-β/Smad signaling pathway, PPARγ exhibits anti-fibrosis property [42]. The activation of PPARγ has also been suggested to regulate microRNA expression to inhibit inflammatory responses. PPARγ could upregulate microRNA (miR)-124 in vitro and in vivo to inhibit the production of pro-inflammatory cytokines [43], and it could enhance the expression of miR-142-3p in vitro and in vivo to inhibit the expression of the pro-inflammatory mediator high mobility group box-1 (HMGB1) which level is increased in multiple autoimmune diseases [44]. In addition, PPARγ can restrain the translocation of HMGB1 through upregulation of protein deacetylase Sirt1 [45]. PPARγ can modulate macrophage and dendritic cell responses and phenotypes, thus ameliorating inflammation [[46], [47], [48], [49]]. Mice bearing macrophage-specific PPARγ ablation develop autoimmune kidney disease and show deficiencies in phagocytosis and clearance of debris from apoptotic cells which leads to the loss of immune-tolerance [50]. Activation of PPARγ can induce the polarization of macrophages towards an immune-modulatory M2-like phenotype and reduce neutrophil migration [51]. PPARγ also alters the T helper (Th)1/Th2 and Th17/regulatory T cells (Treg) ratios. PPARγ can induce the differentiation of Treg cells and suppress the Th17 cells [52]. Mice with T cell-specific PPARγ ablation showed a skewed balance towards Th2 and Treg cells [51]. Microglia plays a critical role in the neuroinflammation and are categorized into classical (M1) and alternative (M2) phenotypes. Pioglitazone can mediate microglia to differentiate into the anti-inflammatory M2 subset which exerts protective effects in neuroinflammation [53]. Due to the anti-inflammatory capacity of PPARγ, Pasquinelli G et al. suggest that agonists of PPARγ may be candidates to prevent or treat the cytokine storm in the COVID-19 disease [54]. Moreover, PPARγ also take part in the regulation of cancer development [55]. PPARγ is downregulated in most, but not all, cancers [56]. Activated PPARγ can suppress tumor progression via the inhibition of some signaling pathways, such as the WNT/β -catenin, PI3K/Akt, signal transducer and activator of transcription (STAT) and nuclear transcription factor-κB (NF-κB) pathways, and the regulation of certain key circadian genes, like brain and muscle aryl-hydrocarbon receptor nuclear translocator-like 1 (Bmal1) [[56], [57], [58]]. However, due to its anti-inflammatory effects, the role of PPARγ in autoimmune diseases has attracted great interest and has been studied by many researchers in recent years (Fig. 2 ).
Fig. 2.
Functional roles of PPARγ. PPARγ is essential in lipid metabolism and control of insulin sensitivity. It is a key transcriptional regulator for fatty acid and glucose metabolism. PPARγ exhibits anti-fibrosis capacity by the inhibition of TGF-β/Smad signaling pathway. Activation of PPARγ can also inhibit inflammatory responses through directly repressing genes expression or by regulating microRNAs. PPARγ also participates in the regulation of cancer development via regulating some signaling pathways and circadian genes. Symbols: + enhance, - suppress. Abbreviations: PPARγ, Peroxisome proliferator-activated receptor γ; HA, hyaluronan; TGF-β, transforming growth factor β; IL, interleukin; TNF- α, tumor necrosis factor α; IFN, interferon; CXCR, chemokine (C-X-C motif) receptor; CXCL, chemokine (C-X-C motif) ligand; Th, T helper cells; Treg, regulatory T cells; miR, microRNA; HMGB1, high mobility group box-1; Sirt1:Sirtuin1; NF-κB: nuclear transcription factor-κB.
4. The roles of PPARγ and PPARγ agonists in autoimmune diseases
4.1. PPARγ and PPARγ agonists in autoimmune thyroid diseases
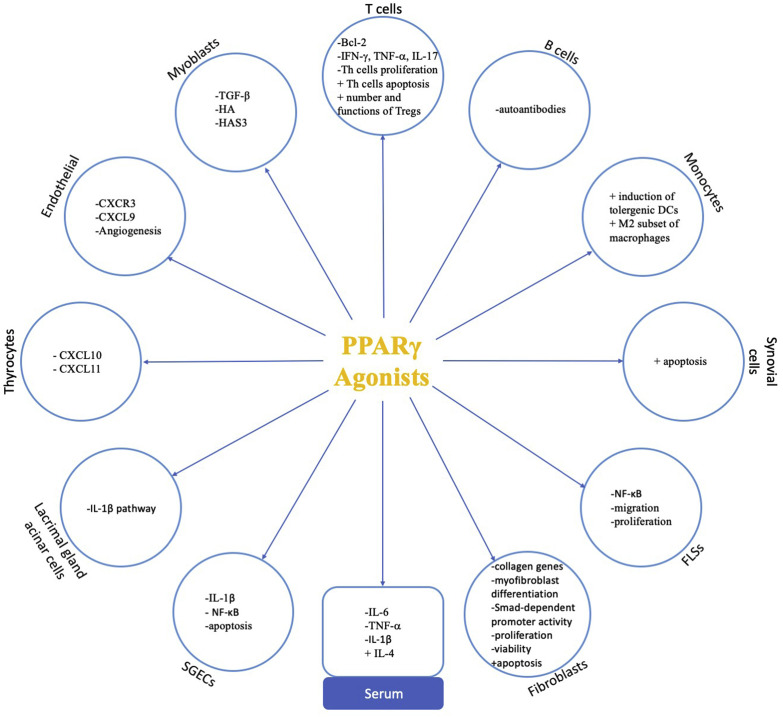
Autoimmune thyroid diseases (AITDs), for example Graves' disease (GD) and Hashimoto's thyroiditis (HT), are a group of thyroid diseases that are characterized by the autoimmune-mediated damage of thyroid tissues [59]. The prevalence rate of AITD is more than 5% in the general population, but elevated levels of IgG anti-thyroid autoantibodies (AAbs) are detected in more than 10% of the general population [60]. The PPARγ expression level increased significantly in adipose or connective tissues from Graves' ophthalmopathy (GO) patients of the active stage compared to normal controls [61]. In vitro experiments demonstrated that PPARγ expression was significantly upregulated in TNF-α-treated GO myoblasts but not in non-GO myoblasts. When treated with pioglitazone, which is a PPARγ agonist, the expression of TNF-α-induced TGF-β, hyaluronan (HA), and HAS3 was substantially diminished in myoblasts isolated from patients with GO, which demonstrated PPARγ agonists to be a promising treatment of GO [62]. Moreover, a recent study demonstrated that caffeine may contribute to the prevention of GO by inhibiting the expression level of PPARγ, C/EBPα, and C/EBPβ [63]. The anti-inflammatory role of PPARγ in thyroid autoimmunity has also been indicated through modulation of proinflammatory cytokines and chemokines. IFNγ-dependent chemokines, such as CXCL9-11, and CXCR3 participate in the development of AITD [64]. These chemokines can induce Th1 lymphocytes to migrate into thyroid tissues to secrete more TNF-α and IFN-γ, which in turn stimulate the production of these chemokines and inhibit the expression of PPAR-γ, thus perpetuating the inflammatory cascade [65]. In vitro studies have demonstrated that PPAR-γ agonists exert an inhibitory effect on the regulation of the chemokines CXCR3 and CXCL9 in the endothelial cells, and CXCL10 and CXCL11 in the thyrocytes [[66], [67], [68]]. The pathogenesis of PPARγ involved in the development of AIDs has been summarized in Fig. 3 . And the protective roles of PPARγ and its agonists in AIDs are summarized in Table 2 .
Fig. 3.
Protective roles of PPARγ involved in autoimmune diseases. Activation of PPARγcan downregulate the expression of CXCL10 and CXCL11 in thyrocytes; decrease the expression of CXCR3 and CXCL9 in endothelial cells and inhibit the angiogenesis process; inhibit TGF-β, HA and HAS3 in myoblasts; promote the proliferation and fucntion of Tregs and suppress the differentiation and function of Th17 cells, induce apoptosis of Th cells via inhibit the expression of Bcl-2; decrease the production of autoantibodies; promote the differentiation of M2 phenotype of macrophages and the tolergenic DCs; induce the apoptosis of synovial cells; repress the NF-κB signaling pathway in FLSs and inhibit the migration and proliferation of FLSs; suppress the TGF-β induced collagen genes expression, the differentiation of myofibroblasts, the Smad-dependent promoter activity in fibroblasts, and inhibit the proliferation and viability of fibroblasts while induce the apoptosis of fibroblasts; suppress the IL-1β and NF-κB signaling pathways in SGECs and inhibit the apoptosis of SGECs; inhibit the IL-1β pathway in lacrimal gland acinar cells; decrease the levels of IL-6, TNF-α and IL-1βwhile increase the level of IL-4 in the serum. Thus, activation of PPARγcan inhibit the inflammatory reactions, modulate the balance between immune cells and protect the target organs in autoimmune diseases. Symbols: + enhance,- suppress. Abbreviations: PPARγ, Peroxisome proliferator-activated receptor γ; HA, hyaluronan; TGF-β, transforming growth factor β; IL, interleukin; TNF-α, tumor necrosis factor α; IFN-γ, interferon γ; CXCR, chemokine receptor; CXCL, chemokine (C-X-C motif) ligand; Th, T helper cells; Treg, regulatory T cells; miR, microRNA; NF-κB: nuclear transcription factor-κB; FLSs, fibroblast-like synoviocytes; SGECs, salivary gland epithelial cells; DCs, dendritic cells.
Table 2.
Studies of the protective roles of PPARγ in autoimmune diseases.
| Autoimmune diseases | Cell types | Animal models | PPARγ agonists | Effects | Ref |
|---|---|---|---|---|---|
| Graves ophthalmopathy (GO) | Myoblasts from extraocular muscles (EOM) | pioglitazone | Diminish the expression of TNF-α-induced TGF-β, hyaluronan (HA), and HAS3 | [62] | |
| Autoimmune thyroid diseases | thyrocytes | Pioglitazone and RGZ | Inhibit the expression and secretion of the chemokines CXCL10 and CXCL11 | [66,67] | |
| Multiple sclerosis | thiazolidinedione pioglitazone, ziglitazone and nonthiazolidinedione PPAR-γ agonist GW347845 | Reduce the T cell proliferation and production of the cytokines TNF- α and IFN- γ induced by phytohemagglutinin | [79] | ||
| Rheumatoid arthritis | Pioglitazone | Alleviate insulin resistance | [91] | ||
| Systemic sclerosis | myofibroblast | Both natural and synthetic agonists | Abrogate the TGF-beta-induced stimulation of collagen synthesis and myofibroblast differentiation. | [102] | |
| mouse model of bleomycin-induced scleroderma | 15-deoxy-Delta (12,14)-prostaglandin J(2) | (1) Reduce dermal sclerosis, hydroxyproline content, and dermal thickness (2) Downregulate expression of transforming growth factor beta and connective tissue growth factor |
[103] | ||
| mouse model of bleomycin-induced scleroderma | Rosiglitazone | Attenuate inflammation, dermal fibrosis, and subcutaneous lipoatrophy | [104] | ||
| mouse model of bleomycin-induced scleroderma | Ajulemic acid | (1) Prevent experimental bleomycin-induced dermal fibrosis and modestly reduce its progression (2) Counteract the progression of pulmonary fibrosis |
[117,118] | ||
| mouse model of bleomycin-induced scleroderma | triterpenoid CDDO | Attenuate TGF-β signaling and dermal fibrosis | [119] | ||
| mouse model of bleomycin-induced scleroderma | Pan PPAR agonist IVA337 | (1) Decrease extracellular matrix deposition and reduce expression of phosphorylated SMAD2/3-intracellular effector of TGF-β1 (2) Downregulate several markers of inflammation |
[120] | ||
| Mice bearing fibroblast-specific deletion of PPARγ | Fibroblast-specific deletion of PPARγ results in enhanced susceptibility to bleomycin-induced skin fibrosis | [105] | |||
| Mice bearing adipocyte-specific deletion of PPARγ nuclear corepressor (NCoR) | Adipocyte-specific deletion of PPARγ nuclear corepressor (NCoR) showed protective effects on experimental skin fibrosis and inflammation. | [106] | |||
| mouse model of bleomycin-induced scleroderma | EHP-101 | Inhibit the expression of genes involved in the inflammation, vasculogenesis and fibrogenesis. | [116] | ||
| SSc fibroblasts | rosiglitazone and pioglitazone | Reduce cell proliferation and cell viability and increase apoptosis | [108] | ||
| ECV304 cells | Lack of PPARγ results in an angiogenic potential | [111] | |||
| Mice with targeted deletion of PPARγ in SMCs | Spontaneously develop PAH | [112] | |||
| Systemic lupus erythematosus | human THP-1 and SLE patient-derived macrophages | MRL-lpr mice deficient in adiponectin | rosiglitazone and pioglitazone | (1) Increased PPARγ expression represses the CD40/CD40L signaling pathway (2) Induce transcriptional repression of various genes involved in T cell responses (3) Reduce the production of autoantibodies |
[124,126,127] |
| DCs derived from SLE monocytes | Rosiglitazone combined with dexamethasone | Induce stable autologous tolerogenic dendritic cells | [128] | ||
| monocyte-derived macrophages from SLE patients | Pioglitazone | Induce the M2 phenotype of monocyte-derived macrophages from SLE patients | [130] | ||
| Sjogren Syndrome | SGEC | Inhibit activation of the NF-κB and IL-1β pathways and apoptosis induced by proinflammatory agents | [134] | ||
| Cultured lacrimal gland acinar cells | Inhibit the IL-1β pathway | [135] | |||
| nonobese diabetic mice with Sjogren's syndrome | Rosiglitazone | Ameliorates histopathologic changes in the salivary glands through the reduction in Th1 cytokines | [136] | ||
| Primary biliary cirrhosis | MRL/lpr mice with a PBC-like cholangitis | prostaglandin D metabolite 15-deoxy-Δ (12,14)-prostaglandin J2 (15d-PGJ2) | Reduce portal inflammation and T cell numbers in portal tracts | [140] |
4.2. PPARγ and PPARγ agonists in multiple sclerosis
Multiple sclerosis (MS) is a progressive neurodegenerative disease that is characterized by demyelination of the central nervous system (CNS), immune responses, chronic inflammation, and destruction of the blood-brain barrier [69]. The pathogenesis of MS is not clear, but it may be caused by genetic and environmental factors [70]. During the demyelinating processes in MS, PPAR-γ is downregulated [71]. The lack of PPAR-γ aggravates the clinical signs in the EAE model [72]. However, PPAR-γ can alleviate inflammation and allow remyelination in an MS oligodendrocyte (OL) model [73]. Moringin has been found to have a protective effect in EAE by increasing the level of PPAR-γ to inhibit inflammatory factors and can prevent neurodegenerative diseases [[74], [75], [76]]. Ursolic acid is also demonstrated to have a dual effect of anti-inflammation and direct remyelination on the treatment of MS through PPARγ/CREB signaling pathway [77]. Some studies have indicated that PPAR-γ agonists can reduce the clinical expression of EAE [78]. Bright et al. demonstrated that more acute EAE could be observed after treatment with PPAR-γ antagonists. PPAR-γ agonists, such as thiazolidinedione pioglitazone, ziglitazone and the nonthiazolidinedione PPAR-γ agonist GW347845, can reduce the T cell proliferation and IFN-γ and TNF-α production induced by phytohemagglutinin. Interestingly, pretreatment of a PPAR-γ agonist could further inhibit T cell proliferation and cytokine secretion. It has also been proven that PPAR-γ agonists reduce the bcl2 expression and induce apoptosis in activated T cells [79]. In addition, several studies have shown that continuous stimulation of PPAR-γ can prevent the decreased expression of the receptor caused by inflammation. These studies laid the foundation for future application of PPAR-γ agonists in the treatment of MS [80]. In both murine CD4+ T cells and human models, PPARγ agonists decrease Th17 differentiation. In the infiltrating CD4+ T cells of the central nervous system, the expression of IL-17 is weakened by the overexpression of PPARγ. The anti-inflammatory effect of PPARγ leads to a decrease in the release of inflammatory cytokines and a decrease in the expansion of brain-derived Th1 and Th17 cells and B lymphocytes [71,81]. Treatment with pioglitazone can significantly decrease the secretion of inflammatory cytokines and enhance the number and functions of regulatory T cells [82].
4.3. PPARγ and PPARγ agonists in rheumatoid arthritis
Rheumatoid arthritis (RA) is a chronic organ-specific autoimmune disease that is characterized by inflammatory cells infiltrating into the synovium of the joint, resulting in bone and articular cartilage damage [83,84]. In the synovium of RA patients, the abnormal migration, proliferation, and activation of fibroblast-like synoviocytes (FLSs) are observed in the pannus of bone and cartilage [85]. The histological and immunological characteristics of adjuvant arthritis (AA) in rats are similar to those of RA in humans. Marder W et al. found that expression of PPAR-γ in the FLSs of RA and AA was significantly decreased compared with that in normal FLSs as shown by in Western blot and immunohistochemistry [86]. Some assays suggested that the downregulated PPARγ expression significantly enhanced the migration and proliferation of FLSs in AA rats and normal rats and that the upregulated PPARγ expression significantly reduced the migration and proliferation of FLSs in AA rats [87]. Moreover, it has been indicated that PPARγ ligands can induce synovial cell apoptosis. NF-κB is a necessary transcription factor for the maintenance of rheumatoid synovitis, and stimulation of FLSs with PPARγ can inhibit the pro-inflammatory activity of NF-κB [88].
The incidence of insulin resistance in patients with rheumatoid arthritis is more than twice as high as that in normal subjects [89], and hyperinsulinemia may aggravate inflammation and is closely related to disease activity [90]. Pioglitazone is a PPARγ agonist. Studies have found that the addition of pioglitazone to RA treatment regimens can alleviate insulin resistance [91]. Pioglitazone has been shown to significantly improve the arthritis index, which is related to the significant decrease in oxidative stress markers and serum cytokines (IL-1β and TNF-α) [92]. In addition, patients with RA also have vascular dysfunction and an increased augmentation index, which is related to coronary artery atherosclerosis [93]. Pioglitazone can improve certain indexes of vascular function in RA patients, including diastolic blood pressure and the augmentation index, which is not mediated by insulin sensitivity [94]. Besides pioglitazone, another natural PPAR-γ agonist, 15d-PGJ2 also modulate bone metabolism through PPAR-γ dependent pathways [95]. In addition, some natural agents can also improve arthritis by targeting PPAR-γ. The results of a randomized clinical trial revealed that ginger supplementation can upregulate the expression of PPAR-γ and ameliorate disease manifestations [96]. And morin, a natural flavonoid, can activate PPAR-γ signaling pathway to attenuate synovial angiogenesis [97].
4.4. PPARγ and PPARγ agonists in systemic sclerosis
Systemic sclerosis (SSc) is still a grave disease which is characterized by microvascular dysfunction, autoimmune reactivity and organ fibrosis [98]. Therapies that have been found to be effective in randomized controlled trials (RCTs) are limited, and the advances in treatment observed in other areas have not yet been observed in this field [99]. Fibrosis in multiple organs is the final common pathway in SSc [100]. The underlying mechanism of the uncontrolled progression of fibrosis in SSc remains unclear. However, the impaired PPAR-γ expression or function in SSc may partly explain the reason [101]. As early as 2004, researchers demonstrated the expression of PPARγ in normal dermal fibroblasts and found that PPARγ ligation could abrogate the TGFβ-induced collagen gene expression, inhibit myofibroblast differentiation, and repress Smad-dependent promoter activity of normal fibroblasts [102]. Later, Kohno S et al. found that naturally occurring PPARγ ligands, such as 15-deoxy-Delta(12,14)-prostaglandin J(2), could reduce dermal sclerosis and decrease the expression levels of connective tissue growth factor and TGFβ in bleomycin-induced scleroderma [103]. Wu M et al. found that the synthetic PPARγ ligand rosiglitazone, which is widely used as an insulin sensitizer, could also attenuate inflammation, dermal fibrosis, and subcutaneous lipoatrophy in an animal model of scleroderma [104]. Mice bearing conditional knockout of PPARγ in fibroblast are more susceptible to develop skin fibrosis induced by bleomycin, as indicated by increased dermal thickness and collagen content, and enhanced inflammation and sensitivity of fibroblasts to TGFβ 1 [105]. And mice bearing conditional knockout of PPARγ nuclear corepressor (NCoR) in adipocyte showed significant protection from inflammation and experimental skin fibrosis [106]. All these studies established the role of PPARγ in regulating TGF-β-dependent fibrogenesis. Moreover, the unrestrained TGFβ activity in turn accounted for the markedly diminished expression and impaired function of PPAR-γ in SSc [107]. In addition to its effects on fibrogenesis, the activation of PPARγ by rosiglitazone and pioglitazone could also significantly reduce cell proliferation and viability and increase apoptosis of fibroblasts in SSc [108]. Furthermore, PPARγ is also important in the regulation of adipogenesis. In SSc patients and mice treated systemically with bleomycin, PPARγ expression in adipocytes was decreased, and the subcutaneous adipose layer was diminished [109].
In addition to fibrosis, pulmonary arterial hypertension (PAH) is another lethal complication in patients with SSc. Defective PPAR-γ expression or function also participate in the pathogenesis of PAH [110]. A landmark study reported that both the gene and protein levels of PPARγ were reduced in lung tissue from patients with severe PAH, and the loss of PPARγ expression in their complex vascular lesions led to angiogenic endothelial cell growth and impaired apoptosis [111]. Mouse experiments also demonstrated the antiproliferative effect of PPARγ in the pathogenesis of PAH. Mice with a targeted deletion of PPARγ in SMCs spontaneously develop PAH [112].
Additional evidence supporting the role of PPARγ in SSc is a genome-wide association study (GWAS) follow-up study, which suggested a possible role for PPARγ in susceptibility to systemic sclerosis [113]. Moreover, a single PPARG intronic SNP (rs10865710) is associated with susceptibility to SSc and PAH [114].
Because of its anti-fibrotic and anti-PAH effects, PPARγ might be a therapeutic target for the control of fibrosis and the pathological vascular remodeling underlying PAH and, therefore, might be a potential drug target for SSc [115]. In recent years, many synthetic agonists of PPARγ have been indicated to be a promising adjuvant in the prevention and treatment of fibrosis in animal experiments, such as cannabinoid derivative EHP-101 [116], synthetic cannabinoid ajulemic acid (AjA) [117,118], 2-cyano-3,12-dioxo-olean-1,9-dien-28-oic acid, synthetic oleanane triterpenoid [119], and IVA337 [120], which is a pan PPAR agonist. However, more work needs to be conducted to advance these drugs into clinical use.
4.5. PPARγ and PPARγ agonists in systemic lupus erythematosus
Systemic lupus erythematosus is a spectrum of autoimmune disease that is characterized by multiple organ dysfunction and abnormalities in several cell types, such as APCs and T and B cells [121]. The production of autoantibodies and pro-inflammatory cytokines plays a crucial role in the pathogenesis of SLE [122]. Although research on the molecular pathogenesis of systemic lupus erythematosus (SLE) has advanced in recent years, treatment of SLE is still a challenge [123]. However, the increased expression of PPARγ may play a protective role in the pathogenesis of SLE [124]. The PPARγ agonists pioglitazone and rosiglitazone are beneficial for the early prevention of systemic lupus erythematosus and the related atherosclerosis in mice [125]. Zhao et al. also found that pioglitazone treatment could transcriptional regulate various molecules involved in several T cell-related signaling pathways in the PBMCs and particularly in the isolated CD4+ T cells from lupus patients. Moreover, pioglitazone could induce the differentiation of T regulatory cells and repress the activation and proliferation of effector T cells in lupus [126]. Furthermore, PPARγ agonist rosiglitazone can reduce the production of autoantibodies, prevent atherosclerosis and renal diseases in mice models of systemic lupus erythematosus, which is based on the induction of adiponectin [127]. In addition, rosiglitazone combined with dexamethasone can induce stable tolerogenic dendritic cells (tolDCs) from monocytes derived from SLE patients [128]. Due to the modulatory role of PPARγ in the differentiation of monocytes and monocyte-derived macrophages, both natural and synthetic agents targeted PPARγ could promote the differentiation of monocytes towards a M2 phenotype and improve the outcome of SLE, which may be an adjuvant to the treatment of this complicated autoimmune disease [129,130].
4.6. PPARγ and PPARγ agonists in Sjogren's syndrome and primary biliary cirrhosis
Sjogren's syndrome (SS) is a classic autoimmune disease that is characterized by the infiltration of lymphocytes and destruction of exocrine glands, leading to the loss of secretory function [131]. Salivary and lacrimal glands are predominantly affected, which leads to the disease hallmarks of severe dryness of the eyes and mouth [132]. Salivary gland epithelial cells (SGECs) derived from SS patients exhibit persistent inflammation. Although the etiology and mechanism of SS remain undefined, a variety of pro-inflammatory cytokines, particularly the persistent activated type I interferon system, are crucial to the pathogenesis of SS [133]. PPARγ and its agonists could modulate the activity of the type I interferon system in SS patients. A previous study found that the transcriptional activity, expression level, and anti-inflammatory function of PPARγ were reduced in ductal epithelial cells from SS patients, and this reduced PPARγ activity promoted the cell-autonomous activation of the IL-1β and NF-κB pathways. Moreover, treatment with PPARγ agonists could repress the activity of NF-κB and prevent proinflammatory agents-induced apoptosis in control SGEC lines and exhibited favorable effects on SS-SGEC lines [134]. PPARγ agonists could also inhibit the IL-1β pathway in lacrimal gland acinar cells [135]. Animal experiments also demonstrated the anti-inflammatory function of PPARγ agonists in nonobese diabetic mouse (NOD mouse) models of SS. Compared with the control mice, mice treated with PPAR-γ agonists show ameliorated histopathological changes in the salivary glands, decreased expression of IL-6 and TNF-α, and increased expression of IL-4 in the serum, which indicated the modulatory role of PPAR-γ in the balance between Th1 and Th2 cells [136]. In addition, Stergios Katsiougiannis et al. found that endoplasmic reticulum stress contributed to the pathogenesis of SS; therefore, PPAR agonists may be a potential treatment for SS by upregulating adiponectin to modulate the energy metabolism of SGECs [137].
Primary biliary cirrhosis (PBC) is a chronic and progressive autoimmune disease that is characterized by destruction of small intrahepatic bile ducts, leading to potential cirrhosis [138]. PBC patients with extrahepatic conditions had a 56.1% probability of developing SS [139]. PPARγ and its agonists also exhibit immunomodulatory roles in PBC. Nozaki Y et al. found that a PPARγ ligand, the prostaglandin D metabolite 15-deoxy-Δ (12,14)-prostaglandin J2 (15d-PGJ2), could attenuate portal inflammation in the lupus-prone mouse model with PBC-like cholangitis [140]. PPARγ ligands have exhibited anti-inflammatory properties in SS and PBC, which may add to the therapeutic options available to patients with SS and/or PBC.
5. Clinical implications of PPARγ agonists in autoimmune diseases
Owing to the protective role of PPARγ in the development of autoimmune diseases, the natural ligands of PPARγ, for example 15d-PGJ2, virgin olive oil or ginger, may be recommended as a daily supplement to the diet of patients with autoimmune diseases [96,129]. Synthetic ligands of PPARγ have already been widely prescribed to treat type 2 diabetes mellitus. The thiazolidinedione (TZD) family, including the above mentioned rosiglitazone and pioglitazone, were the first synthetic ligands discovered to activate PPARγ [141]. TZDs can enhance insulin sensitivity and regulate lipid and glucose metabolism [142]. Due to the anti-inflammatory properties of thiazolidinedione derivatives, studies have been conducted to examine their use in the control of autoimmune diseases. It has been proven that PPARγ agonists, particularly rosiglitazone and pioglitazone, can ameliorate inflammatory responses and improve the symptoms of autoimmune diseases in animal experiments and in vitro assays. The data of some randomized controlled clinical studies reported that RA patients received additional pioglitazone showed significant improvement in disease activity, insulin resistance, vascular function and lower C reactive protein (CRP) level with minimal safety issues [[143], [144], [145]]. These results suggest that PPARγ agonists may be used as an adjuvant to the standard therapy of autoimmune diseases, particular for those combined with diabetes, obesity or glycometabolism disorder. PPARγ agonists may also protect the target organs, like cardiovascular system, joints or kidney, in the systemic autoimmune diseases. However, the side effects of TZDs, such as heart failure, sodium retention, peripheral edema, weight gain and hemodilution, may limit the use of TZDs [146]. One of the future direction of studies of PPARγ agonists may be the development of novel agents that target PPARγ with minimal adverse events. To achieve a better understanding of the clinical implications of PPARγ agonists, additional clinical trials need to be conducted.
6. Conclusion and future directions
In recent years, autoimmune diseases have attracted increasing attention. Autoimmune diseases are characterized by excessive immune responses that cause damage to and dysfunction of certain organs and tissues. Increasing numbers of experiments have shown that PPAR-γ and its agonists play many different protective roles in autoimmune diseases, which provides novel therapeutic option of the autoimmune diseases. Pioglitazone and rosiglitazone are commonly used PPAR-γ agonists with excellent safety, which will provide convenience for future clinical trials. However, the current research cannot clearly explain the mechanisms underlying PPAR-γ protective roles. Further explorations are needed to identify the specific connection between PPAR-γ and autoimmune diseases to provide a more comprehensive understanding of their correlation and a theoretical basis for future clinical application.
Author contributions
Yu Liu collected data and wrote the manuscript, Jiayu Wang made tables and graphs, Shuangyan Luo and Yi Zhan provided technical support and suggestions, Qianjin Lu critically revised the manuscript and provided suggestions.
Fundings
This project is supported by grants from the National Natural Science Foundation for Young Scientists of China (Grant No. 81502732) to YL, the National Natural Science Foundation of China (Grant No. 81974477) to SL, and the National Natural Science Foundation of China (No. 81830097) to QL.
Declaration of competing interest
There is no conflict of interest to declare.
References
- 1.Lu Q. Unmet needs in autoimmunity and potential new tools. Clin. Rev. Allergy Immunol. 2014;47(2):111–118. doi: 10.1007/s12016-014-8414-2. [DOI] [PubMed] [Google Scholar]
- 2.Papp G., Boros P., Nakken B., Szodoray P., Zeher M. Regulatory immune cells and functions in autoimmunity and transplantation immunology. Autoimmun. Rev. 2017;16(5):435–444. doi: 10.1016/j.autrev.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Liu Y., Yin H., Zhao M., Lu Q. TLR2 and TLR4 in autoimmune diseases: a comprehensive review. Clin. Rev. Allergy Immunol. 2014;47(2):136–147. doi: 10.1007/s12016-013-8402-y. [DOI] [PubMed] [Google Scholar]
- 4.V, ella V., Nicolosi M.L., Giuliano S., Bellomo M., Belfiore A., Malaguarnera R. PPAR-gamma agonists as antineoplastic agents in cancers with dysregulated IGF Axis. Front. Endocrinol. 2017;8:31. doi: 10.3389/fendo.2017.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Derosa G., Sahebkar A., Maffioli P. THE role of various peroxisome proliferator-activated receptors and their ligands in clinical practice. J. Cell. Physiol. 2018 Jan;233(1) doi: 10.1002/jcp.25804. [DOI] [PubMed] [Google Scholar]
- 6.Vallee A., Lecarpentier Y. Alzheimer disease: crosstalk between the canonical wnt/beta-catenin pathway and PPARs alpha and gamma. Front. Neurosci. 2016;10:459. doi: 10.3389/fnins.2016.00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corona J.C., Duchen M.R. PPARgamma as a therapeutic target to rescue mitochondrial function in neurological disease. Free Radic. Biol. Med. 2016;100:153–163. doi: 10.1016/j.freeradbiomed.2016.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kersten S., Stienstra R. The role and regulation of the peroxisome proliferator activated receptor alpha in human liver. Biochimie. 2017;136:75–84. doi: 10.1016/j.biochi.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 9.Chinetti-Gbaguidi G., Staels B. PPARbeta in macrophages and atherosclerosis. Biochimie. 2017;136:59–64. doi: 10.1016/j.biochi.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Gupta M., Mahajan V.K., Mehta K.S., Chauhan P.S., Rawat R. Peroxisome proliferator-activated receptors (PPARs) and PPAR agonists: the 'future' in dermatology therapeutics? Arch. Dermatol. Res. 2015;307(9):767–780. doi: 10.1007/s00403-015-1571-1. [DOI] [PubMed] [Google Scholar]
- 11.Kvandova M., Majzunova M., Dovinova I. The role of PPARgamma in cardiovascular diseases. Physiol. Res. 2016;65(Supplementum 3):S343–S363. doi: 10.33549/physiolres.933439. [DOI] [PubMed] [Google Scholar]
- 12.Griffiths H.R., Gao D., Pararasa C. Redox regulation in metabolic programming and inflammation. Redox biology. 2017;12:50–57. doi: 10.1016/j.redox.2017.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamers C., Schubert-Zsilavecz M., Merk D. Therapeutic modulators of peroxisome proliferator-activated receptors (PPAR): a patent review (2008-present) Expert Opin. Ther. Pat. 2012;22(7):803–841. doi: 10.1517/13543776.2012.699042. [DOI] [PubMed] [Google Scholar]
- 14.Scheen A.J., Esser N., Paquot N. Antidiabetic agents: potential anti-inflammatory activity beyond glucose control. Diabetes Metabol. 2015;41(3):183–194. doi: 10.1016/j.diabet.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Kiss M., Czimmerer Z., Nagy L. The role of lipid-activated nuclear receptors in shaping macrophage and dendritic cell function: from physiology to pathology. J. Allergy Clin. Immunol. 2013;132(2):264–286. doi: 10.1016/j.jaci.2013.05.044. [DOI] [PubMed] [Google Scholar]
- 16.Choi J.M., Bothwell A.L. The nuclear receptor PPARs as important regulators of T-cell functions and autoimmune diseases. Mol. Cell. 2012;33(3):217–222. doi: 10.1007/s10059-012-2297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Vallve S., Guasch L., Tomas-Hernandez S., del Bas J.M., Ollendorff V., Arola L., Pujadas G., Mulero M. Peroxisome proliferator-activated receptor gamma (PPARgamma) and ligand choreography: newcomers take the stage. J. Med. Chem. 2015;58(14):5381–5394. doi: 10.1021/jm501155f. [DOI] [PubMed] [Google Scholar]
- 18.Naim M.J., Alam M.J., Ahmad S., Nawaz F., Shrivastava N., Sahu M., Alam O. Therapeutic journey of 2,4-thiazolidinediones as a versatile scaffold: an insight into structure activity relationship. Eur. J. Med. Chem. 2017;129:218–250. doi: 10.1016/j.ejmech.2017.02.031. [DOI] [PubMed] [Google Scholar]
- 19.Zoete V., Grosdidier A., Michielin O. Peroxisome proliferator-activated receptor structures: ligand specificity, molecular switch and interactions with regulators. Biochim. Biophys. Acta. 2007;1771(8):915–925. doi: 10.1016/j.bbalip.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Muralikumar S., Vetrivel U., Narayanasamy A., ND U. Probing the intermolecular interactions of PPARgamma-LBD with polyunsaturated fatty acids and their anti-inflammatory metabolites to infer most potential binding moieties. Lipids Health Dis. 2017;16(1):17. doi: 10.1186/s12944-016-0404-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo J., Qu J., Yang R., Ge M.X., Mei Y., Zhou B.T., Qu Q. Phytochemicals mediate the expression and activity of OCTN2 as activators of the PPARgamma/RXRalpha pathway. Front. Pharmacol. 2016;7:189. doi: 10.3389/fphar.2016.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grygiel-Gorniak B. Peroxisome proliferator-activated receptors and their ligands: nutritional and clinical implications–a review. Nutr. J. 2014;13:17. doi: 10.1186/1475-2891-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiu M., McBeth L., Sindhwani P., Hinds T.D. Deciphering the roles of thiazolidinediones and PPARgamma in bladder cancer. PPAR Res. 2017;2017:4810672. doi: 10.1155/2017/4810672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wuertz B.R., Darrah L., Wudel J., Ondrey F.G. Thiazolidinediones abrogate cervical cancer growth. Exp. Cell Res. 2017;353(2):63–71. doi: 10.1016/j.yexcr.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 25.Tan Y., Muise E.S., Dai H., Raubertas R., Wong K.K., Thompson G.M., Wood H.B., Meinke P.T., Lum P.Y., Thompson J.R., et al. Novel transcriptome profiling analyses demonstrate that selective peroxisome proliferator-activated receptor gamma (PPARgamma) modulators display attenuated and selective gene regulatory activity in comparison with PPARgamma full agonists. Mol. Pharmacol. 2012;82(1):68–79. doi: 10.1124/mol.111.076679. [DOI] [PubMed] [Google Scholar]
- 26.Bopst M., Atzpodien E.A. Non-clinical safety evaluation and risk assessment to human of aleglitazar, a dual PPAR alpha/gamma agonist, and its major human metabolite. Regul. Toxicol. Pharmacol. : RTP (Regul. Toxicol. Pharmacol.) 2017;86:107–116. doi: 10.1016/j.yrtph.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 27.Kummer M.P., Schwarzenberger R., Sayah-Jeanne S., Dubernet M., Walczak R., Hum D.W., Schwartz S., Axt D., Heneka M.T. Pan-PPAR modulation effectively protects APP/PS1 mice from amyloid deposition and cognitive deficits. Mol. Neurobiol. 2015;51(2):661–671. doi: 10.1007/s12035-014-8743-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferroni P., Della-Morte D., Pileggi A., Riondino S., Rundek T., Ricordi C., Guadagni F. Pleiotropic effects of PPARgamma agonist on hemostatic activation in type 2 diabetes mellitus. Curr. Vasc. Pharmacol. 2013;11(3):338–351. doi: 10.2174/1570161111311030008. [DOI] [PubMed] [Google Scholar]
- 29.Chung S., Kim Y.J., Yang S.J., Lee Y., Lee M. Nutrigenomic functions of PPARs in obesogenic environments. PPAR Res. 2016;2016:4794576. doi: 10.1155/2016/4794576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waku T., Shiraki T., Oyama T., Fujimoto Y., Maebara K., Kamiya N., Jingami H., Morikawa K. Structural insight into PPARgamma activation through covalent modification with endogenous fatty acids. J. Mol. Biol. 2009;385(1):188–199. doi: 10.1016/j.jmb.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 31.Wang L., Waltenberger B., Pferschy-Wenzig E.M., Blunder M., Liu X., Malainer C., Blazevic T., Schwaiger S., Rollinger J.M., Heiss E.H., et al. Natural product agonists of peroxisome proliferator-activated receptor gamma (PPARgamma): a review. Biochem. Pharmacol. 2014;92(1):73–89. doi: 10.1016/j.bcp.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sauer S. Ligands for the nuclear peroxisome proliferator-activated receptor gamma. Trends Pharmacol. Sci. 2015;36(10):688–704. doi: 10.1016/j.tips.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 33.Dubois V., Eeckhoute J., Lefebvre P., Staels B. Distinct but complementary contributions of PPAR isotypes to energy homeostasis. J. Clin. Invest. 2017;127(4):1202–1214. doi: 10.1172/JCI88894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jung Y., Cao Y., Paudel S., Yoon G., Cheon S.H., Bae G.U., Jin L.T., Kim Y.K., Kim S.N. Antidiabetic effect of SN158 through PPARalpha/gamma dual activation in ob/ob mice. Chem. Biol. Interact. 2017;268:24–30. doi: 10.1016/j.cbi.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 35.Bugge A., Holst D. PPAR agonists, - could tissue targeting pave the way? Biochimie. 2017;136:100–104. doi: 10.1016/j.biochi.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 36.Henning A.L., McFarlin B.K. Consumption of a high-fat, high-calorie meal is associated with an increase in intracellular co-localization of PPAR-gamma mRNA and protein in monocytes. Methods (San Diego, Calif) 2017;112:182–187. doi: 10.1016/j.ymeth.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 37.Blanchard P.G., Turcotte V., Cote M., Gelinas Y., Nilsson S., Olivecrona G., Deshaies Y., Festuccia W.T. Peroxisome proliferator-activated receptor gamma activation favours selective subcutaneous lipid deposition by coordinately regulating lipoprotein lipase modulators, fatty acid transporters and lipogenic enzymes. Acta Physiol. 2016;217(3):227–239. doi: 10.1111/apha.12665. [DOI] [PubMed] [Google Scholar]
- 38.Holguin F., Rojas M., Hart C.M. The peroxisome proliferator activated receptor gamma (PPARgamma) ligand rosiglitazone modulates bronchoalveolar lavage levels of leptin, adiponectin, and inflammatory cytokines in lean and obese mice. Lung. 2007;185(6):367–372. doi: 10.1007/s00408-007-9035-9. [DOI] [PubMed] [Google Scholar]
- 39.Baker R.G., Hayden M.S., Ghosh S. NF-kappaB, inflammation, and metabolic disease. Cell Metabol. 2011;13(1):11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Y Y., W C., L J., M D., X C., L D., W A. Atorvastatin protects against postoperative neurocognitive disorder via a peroxisome proliferator-activated receptor-gamma signaling pathway in mice. J. Int. Med. Res. 2020;48(5) doi: 10.1177/0300060520924251. 300060520924251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.S R., W D., U L., G B., M C., E A., T G., vR N., H L.C., K L., et al. Pioglitazone-mediated peroxisome proliferator-activated receptor γ activation aggravates murine immune-mediated hepatitis. Int. J. Mol. Sci. 2020;(7):21. doi: 10.3390/ijms21072523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.M M.N., E-K D.H. Saroglitazar attenuates renal fibrosis induced by unilateral ureteral obstruction via inhibiting TGF-β/Smad signaling pathway. Life Sci. 2020;253:117729. doi: 10.1016/j.lfs.2020.117729. [DOI] [PubMed] [Google Scholar]
- 43.Wang D., Shi L., Xin W., Xu J., Xu J., Li Q., Xu Z., Wang J., Wang G., Yao W., et al. Activation of PPARgamma inhibits pro-inflammatory cytokines production by upregulation of miR-124 in vitro and in vivo. Biochem. Biophys. Res. Commun. 2017;486(3):726–731. doi: 10.1016/j.bbrc.2017.03.106. [DOI] [PubMed] [Google Scholar]
- 44.Yuan Z., Luo G., Li X., Chen J., Wu J., Peng Y. PPARgamma inhibits HMGB1 expression through upregulation of miR-142-3p in vitro and in vivo. Cell. Signal. 2016;28(3):158–164. doi: 10.1016/j.cellsig.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 45.H J.S., L W.J., K E.S., H S.A., Y T., P K.S., L D.S., D J.T., S H.G. Ligand-activated peroxisome proliferator-activated receptor-δ and -γ inhibit lipopolysaccharide-primed release of high mobility group box 1 through upregulation of SIRT1. Cell Death Dis. 2014;5:e1432. doi: 10.1038/cddis.2014.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klotz L., Burgdorf S., Dani I., Saijo K., Flossdorf J., Hucke S., Alferink J., Nowak N., Beyer M., Mayer G., et al. The nuclear receptor PPAR gamma selectively inhibits Th17 differentiation in a T cell-intrinsic fashion and suppresses CNS autoimmunity. J. Exp. Med. 2009;206(10):2079–2089. doi: 10.1084/jem.20082771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu Z., Wang G., Zhu Y., Liu R., Song J., Ni Y., Sun H., Yang B., Hou M., Chen L., et al. PPAR-gamma agonist ameliorates liver pathology accompanied by increasing regulatory B and T cells in high-fat-diet mice. Obesity. 2017;25(3):581–590. doi: 10.1002/oby.21769. [DOI] [PubMed] [Google Scholar]
- 48.Nagy L., Szanto A., Szatmari I., Szeles L. Nuclear hormone receptors enable macrophages and dendritic cells to sense their lipid environment and shape their immune response. Physiol. Rev. 2012;92(2):739–789. doi: 10.1152/physrev.00004.2011. [DOI] [PubMed] [Google Scholar]
- 49.Xia H., Chen L., Liu H., Sun Z., Yang W., Yang Y., Cui S., Li S., Wang Y., Song L., et al. Protectin DX increases survival in a mouse model of sepsis by ameliorating inflammation and modulating macrophage phenotype. Sci. Rep. 2017;7(1):99. doi: 10.1038/s41598-017-00103-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.R T., M-G M.P., L M.I., A D., N V., L M.A., F T., R M. Autoimmune kidney disease and impaired engulfment of apoptotic cells in mice with macrophage peroxisome proliferator-activated receptor gamma or retinoid X receptor alpha deficiency. J. Immunol. 2011;186(1):621–631. doi: 10.4049/jimmunol.1002230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.A H.B., N M.H., L A.H., dMM A.G., C T.M., VD T.E., CN J.T. Activation of PPAR-γ induces macrophage polarization and reduces neutrophil migration mediated by heme oxygenase 1. Int. Immunopharm. 2020;84:106565. doi: 10.1016/j.intimp.2020.106565. [DOI] [PubMed] [Google Scholar]
- 52.L W., Z Z., Z K., X Z., L Y., Z Z., Z L., G C., Z Q., H J., et al. Arctigenin suppress Th17 cells and ameliorates experimental autoimmune encephalomyelitis through AMPK and PPAR-γ/ROR-γt signaling. Mol. Neurobiol. 2016;53(8):5356–5366. doi: 10.1007/s12035-015-9462-1. [DOI] [PubMed] [Google Scholar]
- 53.Q X., W W., W H., L D., W R., X J., J H., P F. PPARγ-mediated microglial activation phenotype is involved in depressive-like behaviors and neuroinflammation in stressed C57BL/6J and ob/ob mice. Psychoneuroendocrinology. 2020;117:104674. doi: 10.1016/j.psyneuen.2020.104674. [DOI] [PubMed] [Google Scholar]
- 54.C C., M I., V S., P G. Pharmacological (or synthetic) and nutritional agonists of PPAR-γ as candidates for cytokine storm modulation in COVID-19 disease. Molecules. 2020;25(9) doi: 10.3390/molecules25092076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lecarpentier Y., Claes V., Vallee A., Hebert J.L. Interactions between PPAR gamma and the canonical wnt/beta-catenin pathway in type 2 diabetes and colon cancer. PPAR Res. 2017;2017:5879090. doi: 10.1155/2017/5879090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lecarpentier Y., Claes V., Vallee A., Hebert J.L. Thermodynamics in cancers: opposing interactions between PPAR gamma and the canonical WNT/beta-catenin pathway. Clin. Transl. Med. 2017;6(1):14. doi: 10.1186/s40169-017-0144-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.V A., L Y., V J.N. Hypothesis of opposite interplay between the canonical WNT/beta-catenin pathway and PPAR gamma in primary central nervous system lymphomas. Curr. Issues Mol. Biol. 2019;31:1–20. doi: 10.21775/cimb.031.001. [DOI] [PubMed] [Google Scholar]
- 58.V A., L Y., V J.N. Targeting the canonical WNT/β-Catenin pathway in cancer treatment using non-steroidal anti-inflammatory drugs. Cells. 2019;8(7) doi: 10.3390/cells8070726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kolkhir P., Metz M., Altrichter S., Maurer M. Comorbidity of chronic spontaneous urticaria and autoimmune thyroid diseases: a systematic review. Allergy. 2017 Oct;72(10) doi: 10.1111/all.13182. [DOI] [PubMed] [Google Scholar]
- 60.Wang B., Shao X., Song R., Xu D., Zhang J.A. The emerging role of epigenetics in autoimmune thyroid diseases. Front. Immunol. 2017;8:396. doi: 10.3389/fimmu.2017.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mimura L.Y., Villares S.M., Monteiro M.L., Guazzelli I.C., Bloise W. Peroxisome proliferator-activated receptor-gamma gene expression in orbital adipose/connective tissues is increased during the active stage of Graves' ophthalmopathy. Thyroid : official journal of the American Thyroid Association. 2003;13(9):845–850. doi: 10.1089/105072503322401032. [DOI] [PubMed] [Google Scholar]
- 62.Cheng A.M., Yin H.Y., Chen A., Liu Y.W., Chuang M.C., He H., Tighe S., Sheha H., Liao S.L. Celecoxib and pioglitazone as potential therapeutics for regulating TGF-beta-induced hyaluronan in dysthyroid myopathy. Invest. Ophthalmol. Vis. Sci. 2016;57(4):1951–1959. doi: 10.1167/iovs.15-18018. [DOI] [PubMed] [Google Scholar]
- 63.K J., K J.Y., K J.W., Y J.S. Anti-oxidative and anti-adipogenic effects of caffeine in an in vitro model of Graves' orbitopathy. Endocr. J. 2020;67(4):439–447. doi: 10.1507/endocrj.EJ19-0521. [DOI] [PubMed] [Google Scholar]
- 64.Fallahi P., Ferrari S.M., Elia G., Nasini F., Colaci M., Giuggioli D., Vita R., Benvenga S., Ferri C., Antonelli A. Novel therapies for thyroid autoimmune diseases. Expet Rev. Clin. Pharmacol. 2016;9(6):853–861. doi: 10.1586/17512433.2016.1157468. [DOI] [PubMed] [Google Scholar]
- 65.Werion A., Joris V., Hepp M., Papasokrati L., Marique L., de Ville de Goyet C., Van Regemorter V., Mourad M., Lengele B., Daumerie C., et al. Pioglitazone, a PPARgamma agonist, upregulates the expression of caveolin-1 and catalase, essential for thyroid cell homeostasis: a clue to the pathogenesis of hashimoto's thyroiditis. Thyroid : official journal of the American Thyroid Association. 2016;26(9):1320–1331. doi: 10.1089/thy.2015.0625. [DOI] [PubMed] [Google Scholar]
- 66.F S.M., R F., P S.R., N F., N M., F S.S., F P., A A. Differential modulation of CXCL8 versus CXCL10, by cytokines, PPAR-gamma, or PPAR-alpha agonists, in primary cells from Graves' disease and ophthalmopathy. Autoimmun. Rev. 2019;18(7):673–678. doi: 10.1016/j.autrev.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 67.A A., F S.M., M C., M V., P C., C M., F C., F E., F P. Interferon-α, -β and -γ induce CXCL11 secretion in human thyrocytes: modulation by peroxisome proliferator-activated receptor γ agonists. Immunobiology. 2013;218(5):690–695. doi: 10.1016/j.imbio.2012.08.267. [DOI] [PubMed] [Google Scholar]
- 68.M N., M F., S A., L J.H., S M.N., R R.M., L P., P J., L A.D. Peroxisome proliferator-activated receptor-gamma activators inhibit IFN-gamma-induced expression of the T cell-active CXC chemokines IP-10, Mig, and I-TAC in human endothelial cells. J. Immunol. 2000;164(12):6503–6508. doi: 10.4049/jimmunol.164.12.6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.D T., T L., L C., P E., Z S., G C., B L., L S., A X., R R.M., et al. CD70 defines a subset of proinflammatory and CNS-pathogenic T1/T17 lymphocytes and is overexpressed in multiple sclerosis. Cell. Mol. Immunol. 2019;16(7):652–665. doi: 10.1038/s41423-018-0198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.T K., K W., H A., H K. Association between vitamin D receptor polymorphisms and multiple sclerosis: systematic review and meta-analysis of case-control studies. Cell. Mol. Immunol. 2015;12(2):243–252. doi: 10.1038/cmi.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vallee A., Lecarpentier Y., Guillevin R., Vallee J.N. Demyelination in multiple sclerosis: reprogramming energy metabolism and potential PPARgamma agonist treatment approaches. Int. J. Mol. Sci. 2018;19(4) doi: 10.3390/ijms19041212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Natarajan C., Bright J.J. Peroxisome proliferator-activated receptor-gamma agonists inhibit experimental allergic encephalomyelitis by blocking IL-12 production, IL-12 signaling and Th1 differentiation. Gene Immun. 2002;3(2):59–70. doi: 10.1038/sj.gene.6363832. [DOI] [PubMed] [Google Scholar]
- 73.Huang J.K., Jarjour A.A., Nait Oumesmar B., Kerninon C., Williams A., Krezel W., Kagechika H., Bauer J., Zhao C., Baron-Van Evercooren A., et al. Retinoid X receptor gamma signaling accelerates CNS remyelination. Nat. Neurosci. 2011;14(1):45–53. doi: 10.1038/nn.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Giacoppo S., Galuppo M., Montaut S., Iori R., Rollin P., Bramanti P., Mazzon E. An overview on neuroprotective effects of isothiocyanates for the treatment of neurodegenerative diseases. Fitoterapia. 2015;106:12–21. doi: 10.1016/j.fitote.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 75.Galuppo M., Giacoppo S., De Nicola G.R., Iori R., Navarra M., Lombardo G.E., Bramanti P., Mazzon E. Antiinflammatory activity of glucomoringin isothiocyanate in a mouse model of experimental autoimmune encephalomyelitis. Fitoterapia. 2014;95:160–174. doi: 10.1016/j.fitote.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 76.Giacoppo S., Soundara Rajan T., De Nicola G.R., Iori R., Bramanti P., Mazzon E. Moringin activates Wnt canonical pathway by inhibiting GSK3beta in a mouse model of experimental autoimmune encephalomyelitis. Drug Des. Dev. Ther. 2016;10:3291–3304. doi: 10.2147/DDDT.S110514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Z Y., L X., C B., C M.T., C W.J., R A., Z G.X. A dual effect of ursolic acid to the treatment of multiple sclerosis through both immunomodulation and direct remyelination. Proc. Natl. Acad. Sci. U.S.A. 2020;117(16):9082–9093. doi: 10.1073/pnas.2000208117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Racke M.K., Gocke A.R., Muir M., Diab A., Drew P.D., Lovett-Racke A.E. Nuclear receptors and autoimmune disease: the potential of PPAR agonists to treat multiple sclerosis. J. Nutr. 2006;136(3):700–703. doi: 10.1093/jn/136.3.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.S S., M E., S M., S M., F D.L., H M.T. Anti-inflammatory and antiproliferative actions of PPAR-gamma agonists on T lymphocytes derived from MS patients. J. Leukoc. Biol. 2004;75(3):478–485. doi: 10.1189/jlb.0803402. [DOI] [PubMed] [Google Scholar]
- 80.D P.D., X J., R M.K. PPAR-gamma: therapeutic potential for multiple sclerosis. PPAR Res. 2008;2008:627463. doi: 10.1155/2008/627463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu J., Drew P.D. Peroxisome proliferator-activated receptor-γ agonists suppress the production of IL-12 family cytokines by activated glia. J. Immunol. 2007;178(3):1904–1913. doi: 10.4049/jimmunol.178.3.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.N L., F M.F., C J. Immunologic effects of metformin and pioglitazone treatment on metabolic syndrome and multiple sclerosis. JAMA neurology. 2016;73(5):520–528. doi: 10.1001/jamaneurol.2015.4807. [DOI] [PubMed] [Google Scholar]
- 83.Smolen J.S., Aletaha D., McInnes I.B. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–2038. doi: 10.1016/S0140-6736(16)30173-8. [DOI] [PubMed] [Google Scholar]
- 84.Leiming Zhang M.Z., Minmin Li1, Yuan Du, Sijin Duan, Yanan, Huang Y.L., Jianqiao Zhang, Tian Wang, Fenghua Fu. Ginsenoside Rg1 attenuates adjuvant-induced arthritis in rats via modulation of PPAR-γ/NF-κB signal pathway. Oncotarget. 2017:55384–55393. doi: 10.18632/oncotarget.19526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pap T., van der Laan W.H., Aupperle K.R., Gay R.E., Verheijen J.H., Firestein G.S., Gay S., Neidhart M. Modulation of fibroblast-mediated cartilage degradation by articular chondrocytes in rheumatoid arthritis. Arthritis Rheum. 2000;43(11):2531–2536. doi: 10.1002/1529-0131(200011)43:11<2531::AID-ANR21>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 86.Marder W., Khalatbari S., Myles J.D., Hench R., Lustig S., Yalavarthi S., Parameswaran A., Brook R.D., Kaplan M.J. The peroxisome proliferator activated receptor-gamma pioglitazone improves vascular function and decreases disease activity in patients with rheumatoid arthritis. J Am Heart Assoc. 2013;2(6) doi: 10.1161/JAHA.113.000441. e000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li X.F., Sun Y.Y., Bao J., Chen X., Li Y.H., Yang Y., Zhang L., Huang C., Wu B.M., Meng X.M., et al. Functional role of PPAR-gamma on the proliferation and migration of fibroblast-like synoviocytes in rheumatoid arthritis. Sci. Rep. 2017;7(1):12671. doi: 10.1038/s41598-017-12570-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Amasaki S.Y., Nakashima T., Wakami A.K., Ashita T.M., Ida H., Migita K., Nakata K., Eguchi K. Functional changes in rheumatoid fibroblast-like synovial cells through activation of peroxisome proliferator-activated receptor γ mediated signalling pathway. Clin. Exp. Immunol. 2002;129:379–384. doi: 10.1046/j.1365-2249.2002.01876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chung C.P., Oeser A., Solus J.F., Gebretsadik T., Shintani A., Avalos I., Sokka T., Raggi P., Pincus T., Stein C.M. Inflammation-associated insulin resistance: differential effects in rheumatoid arthritis and systemic lupus erythematosus define potential mechanisms. Arthritis Rheum. 2008;58(7):2105–2112. doi: 10.1002/art.23600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shahin D., Eltoraby E., Mesbah A., Houssen M. Insulin resistance in early untreated rheumatoid arthritis patients. Clin. Biochem. 2010;43(7-8):661–665. doi: 10.1016/j.clinbiochem.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 91.Ormseth M.J., Oeser A.M., Cunningham A., Bian A., Shintani A., Solus J., Tanner S.B., Stein C.M. Peroxisome proliferator-activated receptor γ agonist effect on rheumatoid arthritis: a randomized controlled trial. Arthritis Res. Ther. 2013;15:2–9. doi: 10.1186/ar4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shahin D., Toraby E.E., Abdel-Malek H., Boshra V., Elsamanoudy A.Z., Shaheen D. Effect of peroxisome proliferator-activated receptor gamma agonist (pioglitazone) and methotrexate on disease activity in rheumatoid arthritis (experimental and clinical study) Clin. Med. Insights Arthritis Musculoskelet. Disord. 2011;4:1–10. doi: 10.4137/CMAMD.S5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Avalos I., Chung C.P., Oeser A., Gebretsadik T., Shintani A., Kurnik D., Raggi P., Sokka T., Pincus T., Stein C.M. Increased augmentation index in rheumatoid arthritis and its relationship to coronary artery atherosclerosis. J. Rheumatol. 2007;34(12):2388–2394. [PubMed] [Google Scholar]
- 94.Ormseth M.J., Oeser A.M., Cunningham A., Bian A., Shintani A., Solus J., Tanner S.B., Stein C.M. Reversing vascular dysfunction in rheumatoid arthritis: improved augmentation index but not endothelial function with peroxisome proliferator-activated receptor gamma agonist therapy. Arthritis Rheum. 2014;66(9):2331–2338. doi: 10.1002/art.38686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.X Z., L P., Z J., T M. 15-Deoxy-Δ-prostaglandin J as a potential regulator of bone metabolism via PPARγ-dependent and independent pathways: a review. Drug Des. Dev. Ther. 2019;13:1879–1888. doi: 10.2147/DDDT.S206695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.A N., S F., M M., T H., Y B., A T., JK S. The effect of ginger supplementation on some immunity and inflammation intermediate genes expression in patients with active Rheumatoid Arthritis. Gene. 2019;698:179–185. doi: 10.1016/j.gene.2019.01.048. [DOI] [PubMed] [Google Scholar]
- 97.Y M., Z N., X Y., W Z., D Y. Morin exerts anti-arthritic effects by attenuating synovial angiogenesis via activation of peroxisome proliferator activated receptor-γ. Mol. Nutr. Food Res. 2018;62(21):e1800202. doi: 10.1002/mnfr.201800202. [DOI] [PubMed] [Google Scholar]
- 98.Maria A.T., Maumus M., Le Quellec A., Jorgensen C., Noel D., Guilpain P. Adipose-derived mesenchymal stem cells in autoimmune disorders: state of the art and perspectives for systemic sclerosis. Clin. Rev. Allergy Immunol. 2017;52(2):234–259. doi: 10.1007/s12016-016-8552-9. [DOI] [PubMed] [Google Scholar]
- 99.Aringer M., Erler A. Recent advances in managing systemic sclerosis. F1000Research. 2017;6:88. doi: 10.12688/f1000research.10022.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wei J., Bhattacharyya S., Tourtellotte W.G., Varga J. Fibrosis in systemic sclerosis: emerging concepts and implications for targeted therapy. Autoimmun. Rev. 2011;10(5):267–275. doi: 10.1016/j.autrev.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dantas A.T., Pereira M.C., de Melo Rego M.J., da Rocha L.F., Jr., Pitta Ida R., Marques C.D., Duarte A.L., Pitta M.G. The role of PPAR gamma in systemic sclerosis. PPAR Res. 2015;2015:124624. doi: 10.1155/2015/124624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ghosh A.K., Bhattacharyya S., Lakos G., Chen S.J., Mori Y., Varga J. Disruption of transforming growth factor beta signaling and profibrotic responses in normal skin fibroblasts by peroxisome proliferator-activated receptor gamma. Arthritis Rheum. 2004;50(4):1305–1318. doi: 10.1002/art.20104. [DOI] [PubMed] [Google Scholar]
- 103.Kohno S., Endo H., Hashimoto A., Hayashi I., Murakami Y., Kitasato H., Kojima F., Kawai S., Kondo H. Inhibition of skin sclerosis by 15deoxy delta12,14-prostaglandin J2 and retrovirally transfected prostaglandin D synthase in a mouse model of bleomycin-induced scleroderma. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2006;60(1):18–25. doi: 10.1016/j.biopha.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 104.Wu M., Melichian D.S., Chang E., Warner-Blankenship M., Ghosh A.K., Varga J. Rosiglitazone abrogates bleomycin-induced scleroderma and blocks profibrotic responses through peroxisome proliferator-activated receptor-gamma. Am. J. Pathol. 2009;174(2):519–533. doi: 10.2353/ajpath.2009.080574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kapoor M., McCann M., Liu S., Huh K., Denton C.P., Abraham D.J., Leask A. Loss of peroxisome proliferator-activated receptor gamma in mouse fibroblasts results in increased susceptibility to bleomycin-induced skin fibrosis. Arthritis Rheum. 2009;60(9):2822–2829. doi: 10.1002/art.24761. [DOI] [PubMed] [Google Scholar]
- 106.K B., M R.G., L G., O J., T W., V J. Adipocyte-specific repression of PPAR-gamma by NCoR contributes to scleroderma skin fibrosis. Arthritis Res. Ther. 2018;20(1):145. doi: 10.1186/s13075-018-1630-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wei J., Ghosh A.K., Sargent J.L., Komura K., Wu M., Huang Q.Q., Jain M., Whitfield M.L., Feghali-Bostwick C., Varga J. PPARgamma downregulation by TGFss in fibroblast and impaired expression and function in systemic sclerosis: a novel mechanism for progressive fibrogenesis. PloS One. 2010;5(11):e13778. doi: 10.1371/journal.pone.0013778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Antonelli A., Ferri C., Ferrari S.M., Colaci M., Ruffilli I., Sebastiani M., Fallahi P. Peroxisome proliferator-activated receptor gamma agonists reduce cell proliferation and viability and increase apoptosis in systemic sclerosis fibroblasts. Br. J. Dermatol. 2013;168(1):129–135. doi: 10.1111/j.1365-2133.2012.11199.x. [DOI] [PubMed] [Google Scholar]
- 109.Lee R., Reese C., Carmen-Lopez G., Perry B., Bonner M., Zemskova M., Wilson C.L., Helke K.L., Silver R.M., Hoffman S., et al. Deficient adipogenesis of scleroderma patient and healthy african American monocytes. Front. Pharmacol. 2017;8:174. doi: 10.3389/fphar.2017.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wei J., Bhattacharyya S., Jain M., Varga J. Regulation of matrix remodeling by peroxisome proliferator-activated receptor-gamma: a novel link between metabolism and fibrogenesis. Open Rheumatol. J. 2012;6:103–115. doi: 10.2174/1874312901206010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ameshima S., Golpon H., Cool C.D., Chan D., Vandivier R.W., Gardai S.J., Wick M., Nemenoff R.A., Geraci M.W., Voelkel N.F. Peroxisome proliferator-activated receptor gamma (PPARgamma) expression is decreased in pulmonary hypertension and affects endothelial cell growth. Circ. Res. 2003;92(10):1162–1169. doi: 10.1161/01.RES.0000073585.50092.14. [DOI] [PubMed] [Google Scholar]
- 112.Hansmann G., de Jesus Perez V.A., Alastalo T.P., Alvira C.M., Guignabert C., Bekker J.M., Schellong S., Urashima T., Wang L., Morrell N.W., et al. An antiproliferative BMP-2/PPARγ/apoE axis in human and murine SMCs and its role in pulmonary hypertension. J. Clin. Invest. 2008;118(5):1846–1857. doi: 10.1172/JCI32503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lopez-Isac E., Bossini-Castillo L., Simeon C.P., Egurbide M.V., Alegre-Sancho J.J., Callejas J.L., Roman-Ivorra J.A., Freire M., Beretta L., Santaniello A., et al. A genome-wide association study follow-up suggests a possible role for PPARG in systemic sclerosis susceptibility. Arthritis Res. Ther. 2014;16(1):R6. doi: 10.1186/ar4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Marangoni R.G., Korman B.D., Allanore Y., Dieude P., Armstrong L.L., Rzhetskaya M., Hinchcliff M., Carns M., Podlusky S., Shah S.J., et al. A candidate gene study reveals association between a variant of the Peroxisome Proliferator-Activated Receptor Gamma (PPAR-gamma) gene and systemic sclerosis. Arthritis Res. Ther. 2015;17:128. doi: 10.1186/s13075-015-0641-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wei J., Bhattacharyya S., Varga J. Peroxisome proliferator-activated receptor gamma: innate protection from excessive fibrogenesis and potential therapeutic target in systemic sclerosis. Curr. Opin. Rheumatol. 2010;22(6):671–676. doi: 10.1097/BOR.0b013e32833de1a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.G-M A., G-R M., N C., C D., P B., D J., R A., A G., M E. Cannabinoid derivatives acting as dual PPARγ/CB2 agonists as therapeutic agents for systemic sclerosis. Biochem. Pharmacol. 2019;163:321–334. doi: 10.1016/j.bcp.2019.02.029. [DOI] [PubMed] [Google Scholar]
- 117.Gonzalez E.G., Selvi E., Balistreri E., Akhmetshina A., Palumbo K., Lorenzini S., Lazzerini P.E., Montilli C., Capecchi P.L., Lucattelli M., et al. Synthetic cannabinoid ajulemic acid exerts potent antifibrotic effects in experimental models of systemic sclerosis. Ann. Rheum. Dis. 2012;71(9):1545–1551. doi: 10.1136/annrheumdis-2011-200314. [DOI] [PubMed] [Google Scholar]
- 118.Lucattelli M., Fineschi S., Selvi E., Garcia Gonzalez E., Bartalesi B., De Cunto G., Lorenzini S., Galeazzi M., Lungarella G. Ajulemic acid exerts potent anti-fibrotic effect during the fibrogenic phase of bleomycin lung. Respir. Res. 2016;17(1):49. doi: 10.1186/s12931-016-0373-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wei J., Zhu H., Komura K., Lord G., Tomcik M., Wang W., Doniparthi S., Tamaki Z., Hinchcliff M., Distler J.H., et al. A synthetic PPAR-gamma agonist triterpenoid ameliorates experimental fibrosis: PPAR-gamma-independent suppression of fibrotic responses. Ann. Rheum. Dis. 2014;73(2):446–454. doi: 10.1136/annrheumdis-2012-202716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ruzehaji N., Frantz C., Ponsoye M., Avouac J., Pezet S., Guilbert T., Luccarini J.M., Broqua P., Junien J.L., Allanore Y. Pan PPAR agonist IVA337 is effective in prevention and treatment of experimental skin fibrosis. Ann. Rheum. Dis. 2016;75(12):2175–2183. doi: 10.1136/annrheumdis-2015-208029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Y H., W H., C Y., Z J., Z M., C G., L L., L Q. The therapeutic and pathogenic role of autophagy in autoimmune diseases. Front. Immunol. 2018;9(undefined):1512. doi: 10.3389/fimmu.2018.01512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.L Q., W H., L W., Z M., C V., L L., Z M., C G., Z J., L C.S., et al. A comprehensive review of immune-mediated dermatopathology in systemic lupus erythematosus. J. Autoimmun. 2018;93(undefined):1–15. doi: 10.1016/j.jaut.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 123.Cannon G.W., Erickson A.R., Teng C.C., Huynh T., Austin S., Wade S.W., Stolshek B.S., Collier D.H., Mutebi A., Sauer B.C. Tumour necrosis factor inhibitor exposure and radiographic outcomes in Veterans with rheumatoid arthritis: a longitudinal cohort study. Rheumatol Adv Pract. 2019;3(1) doi: 10.1093/rap/rkz015. rkz015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.O D.S., G L.C., B E., L-S T., P L.A., L I., K S., B D.F., F D., L F.R., et al. PPARγ expression is increased in systemic lupus erythematosus patients and represses CD40/CD40L signaling pathway. Lupus. 2011;20(6):575–587. doi: 10.1177/0961203310392419. [DOI] [PubMed] [Google Scholar]
- 125.Aprahamian T.R., Bonegio R.G., Weitzner Z., Gharakhanian R., Rifkin I.R. Peroxisome proliferator-activated receptor gamma agonists in the prevention and treatment of murine systemic lupus erythematosus. Immunology. 2014;142(3):363–373. doi: 10.1111/imm.12256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhao W., Berthier C.C., Lewis E.E., McCune W.J., Kretzler M., Kaplan M.J. The peroxisome-proliferator activated receptor-gamma agonist pioglitazone modulates aberrant T cell responses in systemic lupus erythematosus. Clin. Immunol. 2013;149(1):119–132. doi: 10.1016/j.clim.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Aprahamian Tamar, Bonegio Ramon G., Richez Christophe, Yasuda Kei, Chiang Lo-Ku, Sato Kaori, Walsh Kenneth, Rifkin2 Ian R. The peroxisome proliferator-activated receptor γ agonist rosiglitazone ameliorates murine lupus by induction of adiponectin. J. Immunol. 2009;182(1):340–346. doi: 10.4049/jimmunol.182.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Obreque J., Vega F., Torres A., Cuitino L., Mackern-Oberti J.P., Viviani P., Kalergis A., Llanos C. Autologous tolerogenic dendritic cells derived from monocytes of systemic lupus erythematosus patients and healthy donors show a stable and immunosuppressive phenotype. Immunology. 2017;152(4):648–659. doi: 10.1111/imm.12806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.A-S M., M-dlP S., S-H M., C A., B B., M F.J.G., A-d-l-L C. Virgin olive oil and its phenol fraction modulate monocyte/macrophage functionality: a potential therapeutic strategy in the treatment of systemic lupus erythematosus. Br. J. Nutr. 2018;120(6):681–692. doi: 10.1017/S0007114518001976. [DOI] [PubMed] [Google Scholar]
- 130.M S., S-J M., S S., M A. Immunomodulation in systemic lupus erythematosus: induction of M2 population in monocyte-derived macrophages by pioglitazone. Lupus. 2017;26(12):1318–1327. doi: 10.1177/0961203317701842. [DOI] [PubMed] [Google Scholar]
- 131.Z Y., Z Y., H J., Y M., Z J., J T. Transitional B cells involved in autoimmunity and their impact on neuroimmunological diseases. J. Transl. Med. 2020;18(1):131. doi: 10.1186/s12967-020-02289-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.B A., M J., W-H M. Environmental factors in the pathogenesis of primary Sjögren’s syndrome. J. Intern. Med. 2020 May;287(5) doi: 10.1111/joim.13032. [DOI] [PubMed] [Google Scholar]
- 133.B P.S. Sjogren-larsson syndrome: mechanisms and management. Appl. Clin. Genet. 2020;13:13–24. doi: 10.2147/TACG.S193969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.V A.G., P A., K E.K., T D., M M.N. Impaired anti-inflammatory activity of PPARγ in the salivary epithelia of Sjögren's syndrome patients imposed by intrinsic NF-κB activation. J. Autoimmun. 2018;86:62–74. doi: 10.1016/j.jaut.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 135.B C., B P.C. Peroxisome proliferator-activated receptor agonists inhibit interleukin-1beta-mediated nitric oxide production in cultured lacrimal gland acinar cells. J. Ocul. Pharmacol. Therapeut. : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2003;19(6):579–587. doi: 10.1089/108076803322660495. [DOI] [PubMed] [Google Scholar]
- 136.L X., X B., W Y., W L. Anti-inflammatory effect of peroxisome proliferator-activated receptor-γ (PPAR-γ) on non-obese diabetic mice with Sjogren's syndrome. Int. J. Clin. Exp. Pathol. 2014;7(8):4886–4894. [PMC free article] [PubMed] [Google Scholar]
- 137.Katsiougiannis S., Tenta R., Skopouli F.N. Autoimmune epithelitis (Sjögren's syndrome); the impact of metabolic status of glandular epithelial cells on auto-immunogenicity. J. Autoimmun. 2019;104:102335. doi: 10.1016/j.jaut.2019.102335. [DOI] [PubMed] [Google Scholar]
- 138.C E.J., A A.H., L K.D. Primary biliary cirrhosis. Lancet (London, England) 2015;386(10003):1565–1575. doi: 10.1016/S0140-6736(15)00154-3. [DOI] [PubMed] [Google Scholar]
- 139.F A., F I., C N., S A., B A., F P., B V., G M.E. Extrahepatic autoimmune conditions associated with primary biliary cirrhosis. Clin. Rev. Allergy Immunol. 2015;48:192–197. doi: 10.1007/s12016-014-8427-x. [DOI] [PubMed] [Google Scholar]
- 140.N Y., H K., S T., N Y. PPARγ ligand attenuates portal inflammation in the MRL-lpr mouse: a new strategy to restrain cholangiopathy in primary biliary cirrhosis. Med. Mol. Morphol. 2013;46(3):153–159. doi: 10.1007/s00795-013-0017-0. [DOI] [PubMed] [Google Scholar]
- 141.Corona J.C., Duchen M.R. PPARγ as a therapeutic target to rescue mitochondrial function in neurological disease. Free Radic. Biol. Med. 2016;100:153–163. doi: 10.1016/j.freeradbiomed.2016.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mirza A.Z., Althagafi, Shamshad H. Role of PPAR receptor in different diseases and their ligands: physiological importance and clinical implications. Eur. J. Med. Chem. 2019;166:502–513. doi: 10.1016/j.ejmech.2019.01.067. [DOI] [PubMed] [Google Scholar]
- 143.S D., T E.E., A-M H., B V., E A.Z., S D. Effect of peroxisome proliferator-activated receptor gamma agonist (pioglitazone) and methotrexate on disease activity in rheumatoid arthritis (experimental and clinical study) Clin. Med. Insights Arthritis Musculoskelet. Disord. 2011;4:1–10. doi: 10.4137/CMAMD.S5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.O M.J., O A.M., C A., B A., S A., S J., T S.B., S C.M. Reversing vascular dysfunction in rheumatoid arthritis: improved augmentation index but not endothelial function with peroxisome proliferator-activated receptor γ agonist therapy. Arthritis & rheumatology (Hoboken, NJ) 2014;66(9):2331–2338. doi: 10.1002/art.38686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.O M.J., O A.M., C A., B A., S A., S J., T S., S C.M. Peroxisome proliferator-activated receptor γ agonist effect on rheumatoid arthritis: a randomized controlled trial. Arthritis Res. Ther. 2013;15(5):R110. doi: 10.1186/ar4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Krishnaswami A., Ravi-Kumar S., Lewis J.M. Thiazolidinediones: a 2010 perspective. Perm. J. 2010;14(3):64–72. doi: 10.7812/tpp/09-052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li J., Guo C., Wu J. 15-Deoxy–(12,14)-Prostaglandin J2 (15d-PGJ2), an endogenous ligand of PPAR-gamma: function and mechanism. PPAR Res. 2019;2019:7242030. doi: 10.1155/2019/7242030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.A H, S M.H., D H., E A., K H.S., A S.M. The effect of ω-fatty acids on mRNA expression level of PPARγ in patients with gastric adenocarcinoma. Exp. Oncol. 2016;38(3):191–194. [PubMed] [Google Scholar]
- 149.N V.R., S P.A., N V.R., S F.B., P K. The role of nitrated fatty acids and peroxisome proliferator-activated receptor gamma in modulating inflammation. Int. Immunopharm. 2014;23(1):283–287. doi: 10.1016/j.intimp.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 150.Lamas Bervejillo M., Bonanata J., Franchini G.R., Richeri A., Marqués J.M., Freeman B.A., Schopfer F.J., Coitiño E.L., Córsico B., Rubbo H., et al. A FABP4-PPARγ signaling axis regulates human monocyte responses to electrophilic fatty acid nitroalkenes. Redox Biol. 2020;29(101376) doi: 10.1016/j.redox.2019.101376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mateu A., De Dios I., Manso M.A., Ramudo L. Oxidized phospholipids exert a dual effect on bile acid-induced CCL2 expression in pancreatic acini. Pancreatology. 2017;17(3):372–380. doi: 10.1016/j.pan.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 152.Plant L.D., Webster N.J., Boyle J.P., Ramsden M., Freir D.B., Peers C., Pearson H.A. Amyloid beta peptide as a physiological modulator of neuronal 'A'-type K+ current. Neurobiol. Aging. 2006;27(11):1673–1683. doi: 10.1016/j.neurobiolaging.2005.09.038. [DOI] [PubMed] [Google Scholar]