Highlights
-
•
Patients with depression show reduced variability in pro-inflammatory immune measures.
-
•
Patients with depression show increases in pro-inflammatory immune markers mean levels, and reductions in anti-inflammatory IL-4.
Keywords: Depression, Inflammation, Meta-analysis, Heterogeneity, Cytokine, CRP
Abstract
Importance
The magnitude and variability of cytokine alterations in depression are not clear.
Objective
To perform an up to date meta-analysis of mean differences of immune markers in depression, and to quantify and test for evidence of heterogeneity in immune markers in depression by conducting a meta-analysis of variability to ascertain whether only a sub-group of patients with depression show evidence of inflammation.
Data Sources
Studies that reported immune marker levels in peripheral blood in patients with depression and matched healthy controls in the MEDLINE database from inception to August 29th 2018 were examined.
Study Selection
Case-control studies that reported immune marker levels in peripheral blood in patients with depression and healthy controls were selected.
Data Extraction and Synthesis
Means and variances (SDs) were extracted for each measure to calculate effect sizes, which were combined using multivariate meta-analysis.
Main Outcomes and Measures
Hedges g was used to quantify mean differences. Relative variability of immune marker measurements in patients compared with control groups as indexed by the coefficient of variation ratio (CVR).
Results
A total of 107 studies that reported measurements from 5,166 patients with depression and 5,083 controls were included in the analyses. Levels of CRP (g = 0.71; 95%CI: 0.50–0.92; p < 0.0001); IL-3 (g = 0.60; 95%CI: 0.31–0.89; p < 0.0001); IL-6 (g = 0.61; 95%CI: 0.39–0.82; p < 0.0001); IL-12 (g = 1.18; 95%CI: 0.74–1.62; p < 0.0001); IL-18 (g = 1.97; 95%CI: 1.00–2.95; p < 0.0001); sIL-2R (g = 0.71; 95%CI: 0.44–0.98; p < 0.0001); and TNFα (g = 0.54; 95%CI: 0.32–0.76; p < 0.0001) were significantly higher in patients with depression. These findings were robust to a range of potential confounds and moderators. Mean-scaled variability, measured as CVR, was significantly lower in patients with depression for CRP (CVR = 0.85; 95%CI: 0.75–0.98; p = 0.02); IL-12 (CVR = 0.61; 95%CI: 0.46–0.80; p < 0.01); and sIL-2R (CVR = 0.85; 95%CI: 0.73–0.99; p = 0.04), while it was unchanged for IL-3, IL-6, IL-18, and TNF α.
Conclusions and Relevance
Depression is confirmed as a pro-inflammatory state. Some of the inflammatory markers elevated in depression, including CRP and IL-12, show reduced variability in patients with depression, therefore supporting greater homogeneity in terms of an inflammatory phenotype in depression. Some inflammatory marker elevations in depression do not appear due to an inflamed sub-group, but rather to a right shift of the immune marker distribution.
1. Introduction
Depression is a common mental illness and is one of the leading causes of disability worldwide, affecting around 10–20% of the general population in their lifetime (Lim et al., 2018). A better understanding of the pathophysiology of depression is required to identify novel therapeutic targets to improve treatment (Maes et al., 2012). Converging lines of evidence suggest immune dysregulation plays a role in the pathogenesis of depression: early-life infection and autoimmune diseases are associated with a higher risk of depression in adulthood (Benros et al., 2013). Direct evidence of inflammation in depression comes from meta-analyses of cross-sectional studies of inflammatory markers in depression, which have shown increased concentrations of circulating C-reactive protein (CRP), interleukin 6 (IL-6), interleukin-12 (IL-12), tumor necrosis factor-α (TNFα), and reductions in interleukin-4 (IL-4) in acute depression (Howren et al., 2009, Dowlati et al., 2010, Haapakoski et al., 2015, Goldsmith et al., 2016, Köhler et al., 2017). Further evidence for a role of inflammation in psychiatric disorders comes from treatment studies: meta-analyses of clinical trials indicate that anti-inflammatory drugs may have antidepressant effects (Köhler et al., 2014, Kappelmann et al., 2016).
However, it has been proposed that inflammation is a factor only for some patients with depression (Khandaker et al., 2017). Supporting this, some studies have found that higher cytokine levels are only seen in a proportion of patients with depression (Benedetti et al., 2002, Lanquillon et al., 2000, Carvalho et al., 2013); in particular, treatment resistant patients show greater elevations in CRP than treatment responsive patients (Chamberlain et al., 2019). Moreover, it has been shown that inflammatory levels tend to normalise in most patients following recovery, while raised inflammatory markers do not normalise in treatment resistant patients (Maes et al., 1997, O’Brien et al., 2007). Further support to the importance of immune factors in treatment response in depression is that a number of trials have shown a lack of efficacy of anti-inflammatories in depression, and have suggested this variability may be due to heterogeneity in the inflammatory alterations amongst patients with depression (Kappelmann et al., 2018, Raison et al., 2013). Finally, there are ongoing clinical trials in people with depression and an inflamed phenotype at baseline, testing if specific inflammatory cytokines such as IL-6 contribute to the pathogenesis of this type of “inflamed depression”, and if their clinical phenotype differs from people with “non-inflamed” depression (Khandaker et al., 2018). Therefore, individual variability in the peripheral immune marker phenotype might be both contributing to shaping the clinical phenotype, and also to affect outcomes, such as treatment response. Thus, determining if there is evidence for heterogeneity in inflammatory markers in depression is important to determine if clinical trials need to target specific patients, or if inflammation is a general component of the pathophysiology of depression.
Heterogeneity can be systematically compared relative to controls in a meta-analysis of variability (Brugger and Howes, 2017, Pillinger et al., 2018:). To our knowledge no previous meta-analysis has investigated variability in inflammatory cytokines in depression.
Another important issue is that smoking and high BMI, which are common in major depression (Dierker et al., 2002, Stunkard et al., 2003, Anda et al., 1990), can significantly affect peripheral inflammatory marker levels (Nieman et al., 1999, Brooks et al., 2010, Trayhurn and Wood, 2004, Yanbaeva et al., 2007). However, few of the available meta-analyses of immune markers in depression have systematically considered the effect of clinical confounds such as smoking or high BMI on immune alterations (Supplementary Table 1). Thus, it remains unclear to what degree the association between depression and peripheral inflammatory markers is secondary to smoking or high BMI.
We therefore set out to:
Main objective: quantify and test for evidence of heterogeneity in immune markers in depression by conducting a meta-analysis of variability (Brugger and Howes, 2017, Pillinger et al., 2018:);
Secondary objective: perform an up-to-date meta-analysis of mean levels of cytokines in depression, taking into account smoking, high BMI and other potential clinical and demographic confounds.
2. Methods
2.1. Search strategy and study selection
The Pubmed, EMBASE, and PsycINFO databases were independently searched for studies investigating CRP, cytokines, TNFα, transforming growth factor (TGF) and interferon levels in patients with depression and healthy controls. The search was complemented by hand-searching of meta-analyses and review articles.
2.2. Data extraction and processing
We extracted means and variance measures (SDs) of immune parameters for the patient and control groups. In addition, we recorded details of the following potential moderating factors: age, gender, ethnicity, BMI and smoking status.
2.3. Statistical analysis
Original study data was reported as raw or log-transformed in different original studies; data was converted to raw as needed using the formula in (Higgins et al., 2008). As many studies reported on several parameters, multivariate meta-analysis was used, enabling simultaneous estimation of summary effect sizes across all immune parameters, and reducing risk of false positives due to multiple comparisons (Bender et al., 2008). For all meta-analyses, an omnibus test evaluated significance of model coefficients across immune parameters. Where the omnibus test was significant, we moved on to multivariate meta-analysis in order to test the effect separately for each parameter.
A meta-analysis of between group differences in immune parameters was performed, indexed using Hedges g. A random effects model was used owing to expectation of inconsistency across studies.
To measure variability, the natural log of the ratio of estimates of the population standard deviations for each group was calculated to give the log variability ratio (VR), as previously described (Brugger and Howes, 2017, Pillinger et al., 2018:). In biological systems, variance often scales with mean (Eisler et al., 2008). Thus, between group differences in relative variability may, at least partially, be a function of between-group differences in mean. Therefore, a meta-analysis of relative variability of patient compared with control immune parameters scaled to group means was performed: the log coefficient of variation ratio (CVR) (the natural logarithm of the ratio of estimates of population coefficients of variation).
2.4. Moderator and sensitivity analyses
We investigated the potential effects of clinical variables (including medication status, duration of illness and whether patients were experiencing a current depressive episode at the time of blood sampling) on the meta-analytic results where this information was available. Due to data availability, the untreated category includes both antidepressant-naïve and antidepressant-free patients, with no minimum washout period. To determine if findings were influenced by potential confounds, we performed sensitivity analyses to determine if findings remained in studies that matched patients and controls for age, BMI, smoking levels. We also performed sensitivity analyses based on those reporting measures from serum or plasma, on fresh or frozen samples, on ELISA/multiplex-based assays, and excluding poor quality studies.
Publication bias was assessed for mean differences in all parameters by visual inspection of funnel plots of standard errors against immune residuals. Inconsistency between studies was assessed using the I2 statistic (Higgins et al., 2003).
Further details of the search, study selection, data processing and statistical analyses are provided in Supplementary Methods.
3. Results
3.1. Study selection
We retrieved 9,897 citations, and 9,526 were excluded after title/abstract review. Following manuscript review, 269 studies were excluded based on failure to meet inclusion criteria. The final data set included 107 studies (Lanquillon et al., 2000, Maes et al., 1997, O’Brien et al., 2007, Alcocer-Gómez et al., 2014, Alesci et al., 2005, Ali et al., 2018, Bai et al., 2014, Basterzi et al., 2005, Berk et al., 1997, Boettger et al., 2010, Brambilla and Maggioni, 1998, Camardese et al., 2011, Cassano et al., 2017, Chamberlain et al., 2018, Charlton et al., 2018, Chavda et al., 2011, Chen et al., 2017, Crnković et al., 2012, Dannehl et al., 2014, Davami et al., 2016, Dhabhar et al., 2009, Dinan et al., 2009, Diniz et al., 2010, Diniz et al., 2010, Dome et al., 2009, Dunjic-Kostic et al., 2013, Elderkin-Thompson et al., 2012, Eller et al., 2008, Elomaa et al., 2012, Euteneuer et al., 2011, Euteneuer et al., 2012, Fan et al., 2017, Fornaro et al., 2011, Fornaro et al., 2013, Frodl et al., 2012, Frommberger et al., 1997, Gazal et al., 2015, Goyal et al., 2017, Grosse et al., 2016, Häfner et al., 2008, Hernández et al., 2008, Ho et al., 2017, Hocaoglu et al., 2012, Huang and Lin, 2007, Hosseini et al., 2007, Hughes et al., 2012, Hung et al., 2007, Kageyama et al., 2018, Kagaya et al., 2001, Jozuka et al., 2003, Karlović et al., 2012, Kahl et al., 2015, Kéri et al., 2014, Leo et al., 2006, Lehto et al., 2010, Lehto et al., 2010, Lee and Kim, 2006, Lee et al., 2009, Kubera et al., 2000, Kokai et al., 2002, Kling et al., 2007, Kim et al., 2002, Maes et al., 1990, Miller et al., 2005, Mikova et al., 2001, Merendino et al., 2002, Marques-Deak et al., 2007, Manoharan et al., 2016, O'donovan A, Rush G, Hoatam G, Hughes BM, McCrohan A, Kelleher C, , 2013, O'brien SM, Scott LV, Dinan TG. , 2006, Nunes et al., 2011, Narita et al., 2006, Munjiza et al., 2018, Motivala et al., 2005, Mota et al., 2013, Piletz et al., 2009, Pike and Irwin, 2006, Pavón et al., 2006, Owen et al., 2001, Ogłodek, 2018, Tuglu et al., 2003, Thomas et al., 2005, Sutcigil et al., 2008, Sowa-Kućma et al., 2018, Sluzewska et al., 1996, Simon et al., 2008, Seidel et al., 1995, Schlatter et al., 2004, Savitz et al., 2015, Rybka et al., 2013, Rudzki et al., 2017, Rudolf et al., 2014, Rizavi et al., 2016, Rief et al., 2001, Rawdin et al., 2013, Sugimoto et al., 2018, Shen et al., 2010, Schmidt et al., 2014, Zoga et al., 2014, Zincir et al., 2016, Zou et al., 2018, Yoshimura et al., 2010, Yoshimura et al., 2009, Yang et al., 2007, Xia et al., 2018, Wiener et al., 2017, Vaccarino et al., 2008, Wang et al., 2018, Rapaport and Irwin, 1996), covering data on 306 immune measures (Supplementary Fig. 1 and Supplementary Table 2). The number of measures exceeds the number of studies because in some studies subjects had more than one measure, but we adjusted for multiple measures in the analysis (as discussed in the statistical methods). The total sample consisted of 10,249 people (5,166 patients, 5,083 controls), allowing meta-analysis of: IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12, IL-13, IL-18, sIL-1RA, sIL-2R, sIL-6R, TNF α, IFNγ, TGF β, and CRP. The average patient age was 42.53 years, SD 10.94; the median percentage of male patients was 35%, inter-quartile range 22%.
3.2. Proportion of skewed data
Prior to log-transformation, there was strong evidence of skew in 122 out of 306 (39.9%) raw-scaled immune measures. This proportion reduced to 117 out of 306 (38.2%) with log-transformation. For all immune parameters, there was no significant difference in the proportion of immune measures with skew in patients compared with controls, either in raw-scaled (OR = 0.72, p = 0.08) or log-transformed data sets (OR = 1.00, p = 1.00).
3.3. Mean differences
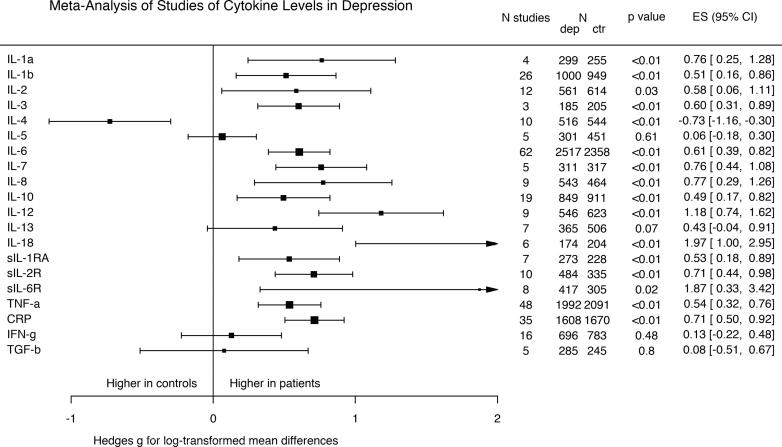
We found a significant overall effect of group on mean concentration across all immune parameters (omnibus χ2 = 217.2, p < 0.0001). Fig. 1 shows that significant elevations in the following parameters were observed in depression: IL-1 α; IL-1 β; IL-2; IL-3; IL-6; IL-7; IL-8; IL-10; IL-12; IL-18; IL-1Ra; IL-2R; IL-6R; TNF α; and CRP. A significant reduction in IL-4 was observed in depression. There were no significant differences between groups for: IL-5; IL-13; IFNγ; and TGF β.
Fig. 1.
Forest plot showing effect sizes for mean differences in immune parameters in depression compared with healthy controls. There were significantly higher levels in patients with depression compared with controls for IL-1 α; IL-1 β; IL-2; IL-3; IL-6; IL-7; IL-8; IL-10; IL-12; IL-18; IL-1Ra; IL-2R; IL-6R; TNF α; and CRP. There was a significant reduction in IL-4 in patients compared with controls. There was no significant difference in TGF β; IFNγ; IL-13; IL-5 in patients compared with controls.
3.3.1. Sensitivity analyses of the influence of psychiatric, clinical and lifestyle predictors on mean differences
Supplementary Table 3 shows the results of sensitivity analyses of psychiatric clinical predictors on mean differences in immune parameters in depression compared with healthy controls. Most parameters showed concordance with the main analysis in sensitivity analyses, with the exception of IL-5, which showed a significant elevation in untreated patients, and of IL-13, which showed a significant elevation in the subset of patients experiencing an active depressive episode. Duration of illness was a significant moderator in analyses of IL-5, IL-7, IL-8 and IFNγ. Full details can be found in Supplementary results and in Supplementary Figs 2 and 3.
Supplementary Table 4 shows the results of sensitivity analyses of lifestyle and medical clinical predictors for mean differences in immune parameters in depression compared with healthy controls. Parameters which did not show concordance with the main analysis were IL-2 and IL-8, which were reduced, and TGF β, which was increased in studies matched for BMI. IFNγ showed an increase in patients of studies matched for smoking. Results for age where all concordant with the main analysis. Full details can be found in Supplementary results and in Supplementary Figs. 4–6.
3.3.2. Sensitivity analyses of the influence of skew, publication bias, sample type and study inconsistency
Supplementary Fig. 7 shows results after removing studies with evidence of persistent severe skew despite log transformation. Results not concordant with the main analysis were seen for TGF β, which showed an increase in patients, and for IL-8, which showed a decrease in patients, while IL-1 β’s increase was no longer significant.
A meta-regression taking into-account sample type (both plasma or serum, and fresh or frozen sample) created a data set of 84 studies (4,419 patients, 4,251 controls); 63 studies (75%) utilised measures from serum, while 73 measures (87%) were taken from previously frozen samples. Results were concordant with the main analysis for IL-1 β, IL-2, IL-4, IL-5, IL-6, IL-12, IL-13, sIL-1RA, sIL-2R, TNF α, CRP, TGF β and IFNγ. For IL-7, IL-8, IL-10 and sIL-6R results were no longer significant in this sensitivity analysis. Full details can be found in Supplementary results.
The funnel plot for publication bias demonstrated symmetry (Supplementary Fig. 8), with one outlier (Camardese et al., 2011). Re-analysis with the outlier excluded (Supplementary Fig. 9) showed that results for IL-6R were no longer significant. Higgins’ I2 inconsistency values (Supplementary Table 5) demonstrated a medium-large degree of inconsistency for all parameters.
3.4. Variability meta-analysis
Given that most cytokines are increased in depression, and variance often scales with mean (Eisler et al., 2008), differences in relative variability may, at least partially, be a function of between-group differences in mean. Therefore, variability ratio results are presented in Supplementary results and Supplementary Fig. 10. Here we present mean scaled coefficient of variation ratios (CVR). We found a significant overall effect of group on log variability ratio across all immune parameters (omnibus χ2 = 72.1, p < 0.0001). Fig. 2 shows that there was significantly lower CVR in patients using for IL-12; IL-13; sIL-2R; CRP; and IFNγ. There was no significant difference found in CVR of IL-1 α; IL-1 β; IL-3; IL-4; IL-5; IL-6; IL-7; IL-8; IL-10; IL-18; IL-1RA; IL-6R; and TNF. Analysis of IL-2 and of TGF β showed both to be more variable in patients according to CVR analysis.
Fig. 2.
Forest plot showing effect sizes for mean-scaled variability of immune parameters in depression compared with healthy controls. The coefficient of variation ratio (CVR) was significant decreased for IL-12, IL-13, IL-2R, CRP, and IFNγ, indicating lower variability of these immune parameters in patients compared with controls, and significantly increased for IL-2 and TGF β, indicating increased variability of these immune parameters in patients compared with controls.
3.4.1. Study quality
Newcastle Ottawa Scale quality scores ranged from 3 to 8 (Supplementary Table 6). Of the 107 studies, 19 were rated as ‘poor-quality’. Following the exclusion of these poor-quality studies, meta-analyses could not be carried out for IL-1 α and IL-3, while results of the primary meta-analyses for IL-2, IL-7, IL-8, IL-13, IL-1RA and IL-6R became non-significant, with implications for reliability of these outcomes (Supplementary Fig. 11).
Excluding poor quality studies, results of the CVR meta-analyses for IL-2R and CRP became non-significant, while IL-8 showed greater variability in controls, and IL-18 and IL-1RA showed greater variability in patients (Supplementary Fig. 12).
Table 1 summarizes the main findings of this meta-analysis, while Supplementary Table 7 summarizes all findings.
Table 1.
Summary of Significant Findings.
The following table summarises the findings of variability and mean differences meta-analyses of inflammatory markers in depression concordant between the main and sensitivity analyses.
| marker | Meta-analysis of mean differences in immune parameters in depression compared with healthy controls | Meta-analysis of variability: CVR |
|---|---|---|
| CRP | ↑ in patients g = 0.71; 95%CI: 0.50–0.92 |
↓ variability in patients CVR = 0.85; 95%CI: 0.75–0.98 |
| IL-3 | ↑ in patients g = 0.60; 95%CI: 0.31–0.89 |
↔ CVR = 0.76; 95%CI: 0.56–1.04 |
| IL-6 | ↑ in patients g = 0.61; 95%CI: 0.39–0.82 |
↔ CVR = 0.92; 95%CI: 0.81–1.05 |
| IL-12 | ↑ in patients g = 1.18; 95%CI: 0.74–1.62 |
↓ variability in patients CVR = 0.61; 95%CI: 0.46–0.80 |
| IL-18 | ↑ in patients g = 1.97; 95%CI: 1.00–2.95 |
↔ CVR = 0.86; 95%CI: 0.67–1.09 |
| sIL-1RA | ↑ in patients g = 0.53; 95%CI: 0.18–0.89 |
↔ CVR = 1.00; 95%CI: 0.84–1.20 |
| sIL-2R | ↑ in patients g = 0.71; 95%CI: 0.44–0.98 |
↓ variability in patients CVR = 0.85; 95%CI: 0.73–0.99 |
| TNF α | ↑ in patients g = 0.54; 95%CI: 0.32–0.76 |
↔ CVR = 0.96; 95%CI: 0.84–1.10 |
4. Discussion
Our meta-analysis finds evidence that mean-scaled variability, measured as CVR, is reduced in patients with depression for CRP, IL-12 and sIL-2R, while it is unchanged for IL-3, IL-6, IL-18 and TNF α. In the same sample, we also find that blood levels of CRP, IL-3, IL-6, IL-12, IL-18, sIL-2R and TNF α are significantly elevated in patients with depression with medium-large effect sizes (range 0.54–1.97), and that these findings are robust to a range of potential confounds and moderators. See Table 1 for a summary of our findings.
Our study is, to our knowledge, the first meta-analysis of variability of immune parameters in individuals with depression compared to matched controls. Mean differences in inflammatory markers in depression have been meta-analysed before (Howren et al., 2009, Dowlati et al., 2010, Haapakoski et al., 2015, Goldsmith et al., 2016, Köhler et al., 2017). However, as shown in Supplementary Table 1, this study is by far the largest meta-analysis of immune markers in depression, including a sample 1.48 times larger than the largest previous one. In addition to this, this is one of the first studies to systematically consider the effect on immune markers of excluding patients not experiencing an active depressive episode (previously only considered in a much smaller study by Goldsmith et al), duration of illness (previously only considered descriptively), study quality (previously only considered in a smaller study by Haapakoski et al), and smoking (previously only considered by Kohler et al). Furthermore, our findings of increased mean levels of CRP, IL-6, IL-12 and TNF α in depression replicate previous meta-analytical findings; the same can be said of no changes in levels of TGF β (Supplementary Table 1). Reductions in IL-4, found in our study with an effect size of −0.73 and resistant to most sensitivity analyses, were not significant in Köhler et al. (2017) nor in Dowlati et al. (2010), however both these studies were based on considerably smaller samples, which could explain the difference. More controversial is the result for IFNγ, which we find not significantly altered in our main analysis and increased in patients when excluding studies not matched for smoking levels between cases and controls. Given that previous, smaller meta-analyses were also non-concordant with regards to IFNγ (Dowlati et al., 2010, Goldsmith et al., 2016, Köhler et al., 2017), we believe that more research is needed to establish the relationship between IFNγ levels and depression.
4.1. Interpretations and implications
4.1.1. Meta-analysis of heterogeneity
In a previous study we have shown that patients with depression show a proportion of high CRP levels at different cut-offs (CRP > 1 mg/L, >3mg/L and > 10 mg/L) that is similar to matched controls (Osimo et al., 2019); this supported the hypothesis that the shape of the CRP distribution curve is similar in patients and controls. In this study we find that mean-scaled variability of CRP and of a number of other immune markers is either reduced or unchanged in patients with depression as compared to healthy controls. A reduced variability implies a narrower distribution in patients than in controls, and possibly even a greater homogeneity in the inflammatory phenotype in depression. Therefore, the findings to date, at least for markers that show elevations of the mean and reductions in heterogeneity such as CRP, support a narrower distribution that is shifted to the right in depression. This is important as in the past there have been suggestions that inflammation in depression could be due to a sub-group of “inflamed and depressed” subjects, who might potentially be part of a separate sub-group of the depressed population (Miller and Cole, 2012). Our findings, instead, point in the direction of a continuous distribution of inflammatory markers in the depressed population, which is more homogenous than the healthy population.
The reduction in variability in CRP is worthy of a special mention here, as CRP is the main inflammatory marker routinely measured in clinical practice (Yeh, 2004), and it is commonly used to stratify patients based on peripheral inflammatory levels in immunopsychiatric studies. Activation of the inflammatory system is thought to underlie antidepressant resistance (Chamberlain et al., 2018, Benedetti et al., 2002, Lanquillon et al., 2000, Carvalho et al., 2013), highlighting a potential involvement in treatment response (Carvalho et al., 2013, Maes et al., 1997, O’Brien et al., 2007, Yoshimura et al., 2009). Therefore, whether targeting inflammatory cytokines could provide therapeutic benefit for patients with depression is a key question that is being investigated in ongoing trials (e.g. NCT02473289; ISRCTN16942542). Our findings will be relevant for future studies assessing inflammation in depression, especially those recruiting patients based on their baseline inflammatory status.
4.1.2. Meta-analysis of mean differences
We found increases in the average levels of type I and other pro-inflammatory cytokines such as IL-3, IL-6, IL-12, IL-18 and TNF α; we also found reductions in IL-4, one of the main anti-inflammatory and immune-modulatory cytokines; finally, we found mean increases in CRP, which is one of the best characterised inflammatory markers in medical (Danesh et al., 2000, Visser et al., 1999) and psychiatric conditions (Fernandes et al., 2016, von Känel et al., 2007, Fernandes et al., 2016). Taken together, these results confirm that acute depression is associated with a pro-inflammatory state.
CRP is one of the best studied inflammatory markers in the field of medicine. Higher levels of CRP have been consistently found in cross-sectional studies and in population-based longitudinal studies of depression, often preceding the onset of illness (Gimeno et al., 2009, Khandaker et al., 2014, Wium-Andersen et al., 2013, Zalli et al., 2016), suggesting that inflammation could be a cause rather than simply a consequence of the illness; supporting this hypothesis, recently Mendelian randomization analyses of the UK Biobank sample found that IL-6 and CRP are likely to be causally linked with depression (Khandaker et al., 2019). Furthermore, elevated peripheral CRP levels have been found to correlate with its level in the central nervous system, with a strong correlation between plasma and CSF CRP (r = 0.855, p < 0.001) (Felger et al., 2018).
TNF α is one of the major pro-inflammatory cytokines; it is produced by dendritic cells and macrophages and is a major activator of downstream inflammatory cascades with multiple effectors (Abbas et al., 2014). During acute infection dendritic cells and macrophages also produce IL-6 and IL-12; both are type I cytokine family members, secreted in response to an acute inflammatory stimulus (Abbas et al., 2014). IL-12 plays a central role in responses to active infection promoting Th1 responses and, hence, cell-mediated immunity (Stern et al., 1996). TNF α, IL-6 and IL-12 increases in current depressive episodes underline the systemic nature of the inflammatory status, showing some similarity to the immune reaction to an active infection.
For markers found to be overall not different between patients and controls, but with variable results in sensitivity analyses (IL-5, IFNγ and TGF β), our results encourage further research, aiming to disentangle their potential role in mediating effects of treatment (IL-5), smoking (IFNγ) or BMI differences (TGF β).
Finally, IL-2 and IL-8 were found to be increased in patients in our main analysis, but produced discordant results in sensitivity analyses due to the effect of BMI-matching; future studies should carefully match participants for BMI as this appears to be a particularly relevant factor affecting immune status.
4.2. Strengths and limitations
The main strength of this work is the use of the largest sample of studies of inflammatory markers in depression to date; the same large sample was used to study heterogeneity and mean differences in patients as compared to controls. Even if we could not make inferences on the shape of the distribution, such as modality, as this would require individual subject data, we were able to obtain the first measure to date of the variability of inflammatory markers in depression.
A further strength of this paper is the employment of a systematic approach to the analysis of potential confounds. Given the large number of studies that focussed on inflammatory markers in depression, we were able to investigate the effect of potential psychiatric (e.g. treatment status, current depressive episode at time of sampling and duration of illness) and lifestyle confounds (e.g. age, BMI and smoking status), as well as statistical and sampling confounds (e.g. data skew and study quality). Sensitivity analyses focussing on studies with strict environmental and physiological matching provided us with greater confidence that depression is associated with the elevation of some immune parameters. Use of a multivariate meta-analytic approach to reduce the influence of multiplicity is a further strength.
Among our limitations, we included cross-sectional studies which used different tools to diagnose depression, even if only studies using ICD or DSM diagnostic criteria were included. Inconsistency between studies was moderate to high. This could reflect methodological factors, e.g. differences in assay sensitivity. However, the random-effects model used is robust to inconsistency, and would not explain our variability findings, because these reflect within-study variation (with methodologic factors common to patient and control groups in any given study). Due to data unavailability, some sensitivity analyses might be subject to type II error, i.e. false negatives; for example, BMI-matched sensitivity analyses often included samples much smaller than that of the main analysis. Furthermore, sensitivity analyses of antidepressant naïve and treatment resistant patients were not possible owing to insufficient studies.
Although all studies included in analyses used well validated quantification techniques, insufficient assay sensitivity may have limited the ability to detect subtle differences in immune parameters between patients and controls, particularly for titres beneath the limit of assay detection. Unfortunately, very few studies (2 out of 106) reported the number of samples below the limit of assay detection, so this factor could not be taken into account. Positive data skew can inflate standard deviation due to outliers within the ‘tail’ of the data (Fayers, 2011). However, we demonstrated no significant difference in the proportion of skewed data sets between patients and controls, suggesting that influence of skew was equal. Thus, excessive skew in healthy controls compared with patients was not likely contributing to results.
We excluded papers that only included patients and controls presenting the same co-morbidity or physiological state in addition to depression (such as studies in autoimmune disorders or pregnancy) to reduce the risk of bias. Most included studies excluded participants with co-morbid medical conditions, and the presence of co-morbidity in participants was assessed as one of the items of our quality assessment of papers. It was not possible to exclude all co-morbidity due to original data quality, but we are confident this issue is not going to significantly affect results as a) we used random effect models to account for additional variation; b) co-morbidity is likely to be equally distributed between cases and controls; and c) our large sample (the largest to date) allows for more individual variation without affecting results.
A very limited number of studies on CRP excluded participants presenting with an acute infection (CRP > 10 mg/L); we decided to include these studies because we previously found that the odds ratio of inflammation in patients vs controls is very similar if considering all patients (OR = 1.46) or excluding patients and controls with CRP > 10 mg/L (OR = 1.44) (Osimo et al., 2019), thus suggesting that an equal proportion of patients and controls present with acute inflammation.
4.3. Conclusions and future directions
In this study we found a reduction in mean-scaled variability in CRP, IL-12 and sIL-2R. We found increases in the mean levels of CRP, IL-3, IL-6, IL-12, IL-18, sIL-2R and TNF α in patients with depression. These results survived sensitivity analyses for psychiatric and lifestyle predictors, influence of skew, influence of poor-quality studies and publication bias.
Our results confirm that acute depression is a pro-inflammatory state, and lend support to the hypothesis that inflammatory marker elevations in depression are not due to an inflamed sub-group, but rather to a right shift of the immune marker distribution. However, future research should specifically address the inflammatory sub-group hypothesis of depression, which can only be directly tested in an individual-patient meta-analysis.
Conflict of interest disclosures
Professor Howes has received investigator-initiated research funding from and/or participated in advisory/speaker meetings organized by Angelini, Autifony, Heptares, Janssen, Lundbeck, Lyden-Delta, Otsuka, Servier, Sunovion, Rand, and Roche. Prof Pariante received research funding from Johnson & Johnson, the UK Medical Research Council and the Wellcome Trust; he is also part of consortia that also include Johnson & Johnson, GSK and Lundbeck. Dr Osimo, Dr Pillinger, Ms Mateos Rodriguez and Dr Khandaker report no conflicts of interest.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments and funding
All authors have approved the final manuscript.
This study was funded by grants MC-A656-5QD30 from the Medical Research Council-UK, and the NIHR Biomedical Research Centre South London and Maudsley Foundation NHS Trust to Professor Howes.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbi.2020.02.010.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Lim G.Y., Tam W.W., Lu Y., Ho C.S., Zhang M.W., Ho R.C. Prevalence of Depression in the Community from 30 Countries between 1994 and 2014. Scientific Rep. 2018;8(1):2861. doi: 10.1038/s41598-018-21243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M., Fišar Z., Medina M., Scapagnini G., Nowak G., Berk M. New drug targets in depression: inflammatory, cell-mediated immune, oxidative and nitrosative stress, mitochondrial, antioxidant, and neuroprogressive pathways. And new drug candidates—Nrf2 activators and GSK-3 inhibitors. Inflammopharmacol. 2012;20(3):127–150. doi: 10.1007/s10787-011-0111-7. [DOI] [PubMed] [Google Scholar]
- Benros M.E., Waltoft B.L., Nordentoft M., Østergaard S.D., Eaton W.W., Krogh J. Autoimmune diseases and severe infections as risk factors for mood disorders: a nationwide study. JAMAPsychiatry. 2013;70(8):812–820. doi: 10.1001/jamapsychiatry.2013.1111. [DOI] [PubMed] [Google Scholar]
- Howren M.B., Lamkin D.M., Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosomatic Medicine. 2009;71(2):171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Dowlati Y., Herrmann N., Swardfager W., Liu H., Sham L., Reim E.K. A meta-analysis of cytokines in major depression. Biol. Psychiatry. 2010;67(5):446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Haapakoski R., Mathieu J., Ebmeier K.P., Alenius H., Kivimäki M. Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain, Behavior, Immun. 2015;49:206–215. doi: 10.1016/j.bbi.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith D., Rapaport M., Miller B. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol. Psychiatry. 2016;21(12):1696–1709. doi: 10.1038/mp.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler C., Freitas T., Maes Md., De Andrade N., Liu C., Fernandes B. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr. Scand. 2017;135(5):373–387. doi: 10.1111/acps.12698. [DOI] [PubMed] [Google Scholar]
- Köhler O., Benros M.E., Nordentoft M., Farkouh M.E., Iyengar R.L., Mors O. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMAPsychiatry. 2014;71(12):1381–1391. doi: 10.1001/jamapsychiatry.2014.1611. [DOI] [PubMed] [Google Scholar]
- Kappelmann N., Lewis G., Dantzer R., Jones P.B., Khandaker G.M. Antidepressant activity of anti-cytokine treatment: a systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Mol. Psychiatry. 2016 doi: 10.1038/mp.2016.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker G.M., Dantzer R., Jones P.B. Immunopsychiatry: important facts. Psychol. Medicine. 2017;47(13):2229–2237. doi: 10.1017/S0033291717000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F., Lucca A., Brambilla F., Colombo C., Smeraldi E. Interleukine-6 serum levels correlate with response to antidepressant sleep deprivation and sleep phase advance. Prog. Neuro-Psychopharmacol. Biol. psychiatry. 2002;26(6):1167–1170. doi: 10.1016/s0278-5846(02)00255-5. [DOI] [PubMed] [Google Scholar]
- Lanquillon S., Krieg J.-C., Bening-Abu-Shach U., Vedder H. Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology. 2000;22(4):370–379. doi: 10.1016/S0893-133X(99)00134-7. [DOI] [PubMed] [Google Scholar]
- Carvalho L., Torre J., Papadopoulos A., Poon L., Juruena M., Markopoulou K. Lack of clinical therapeutic benefit of antidepressants is associated overall activation of the inflammatory system. J. Affect. Disord. 2013;148(1):136–140. doi: 10.1016/j.jad.2012.10.036. [DOI] [PubMed] [Google Scholar]
- Chamberlain S.R., Cavanagh J., de Boer P., Mondelli V., Jones D.N., Drevets W.C. Treatment-resistant depression and peripheral C-reactive protein. Br. J. Psychiatry. 2019;214(1):11–19. doi: 10.1192/bjp.2018.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M., Bosmans E., De Jongh R., Kenis G., Vandoolaeghe E., Neels H. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine. 1997;9(11):853–858. doi: 10.1006/cyto.1997.0238. [DOI] [PubMed] [Google Scholar]
- O’Brien S.M., Scully P., Fitzgerald P., Scott L.V., Dinan T.G. Plasma cytokine profiles in depressed patients who fail to respond to selective serotonin reuptake inhibitor therapy. J. Psychiatric Res. 2007;41(3–4):326–331. doi: 10.1016/j.jpsychires.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Kappelmann N., Lewis G., Dantzer R., Jones P.B., Khandaker G.M. Antidepressant activity of anti-cytokine treatment: a systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Mol. Psychiatry. 2018 doi: 10.1038/mp.2016.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison C.L., Rutherford R.E., Woolwine B.J., Shuo C., Schettler P., Drake D.F. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMAPsychiatry. 2013;70(1):31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker G.M., Oltean B.P., Kaser M., Dibben C.R., Ramana R., Jadon D.R. Protocol for the insight study: a randomised controlled trial of single-dose tocilizumab in patients with depression and low-grade inflammation. BMJOpen. 2018;8(9) doi: 10.1136/bmjopen-2018-025333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugger S.P., Howes O.D. Heterogeneity and homogeneity of regional brain structure in schizophrenia: a meta-analysis. JAMAPsychiatry. 2017;74(11):1104–1111. doi: 10.1001/jamapsychiatry.2017.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillinger T., Osimo E.F., Brugger S., Mondelli V., McCutcheon R.A., Howes O.D. A meta-analysis of immune parameters, variability, and assessment of modal distribution in psychosis and test of the immune subgroup hypothesis. Schizophr. Bull. 2018:sby160. doi: 10.1093/schbul/sby160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierker L.C., Avenevoli S., Stolar M., Merikangas K. Smoking and depression: an examination of mechanisms of comorbidity. Am. J. Psychiatry. 2002;159(6):947–953. doi: 10.1176/appi.ajp.159.6.947. [DOI] [PubMed] [Google Scholar]
- Stunkard A.J., Faith M.S., Allison K.C. Depression and obesity. Biological Psychiatry. 2003;54(3):330–337. doi: 10.1016/s0006-3223(03)00608-5. [DOI] [PubMed] [Google Scholar]
- Anda R.F., Williamson D.F., Escobedo L.G., Mast E.E., Giovino G.A., Remington P.L. Depression and the dynamics of smoking: a national perspective. JAMA. 1990;264(12):1541–1545. [PubMed] [Google Scholar]
- Nieman D.C., Henson D.A., Nehlsen-Cannarella S.L., Ekkens M., Utter A.C., Butterworth D.E. Influence of obesity on immune function. J. Am. Diet. Assoc. 1999;99(3):294–299. doi: 10.1016/S0002-8223(99)00077-2. [DOI] [PubMed] [Google Scholar]
- Brooks G.C., Blaha M.J., Blumenthal R.S. Relation of C-reactive protein to abdominal adiposity. The American journal of cardiology. 2010;106(1):56–61. doi: 10.1016/j.amjcard.2010.02.017. [DOI] [PubMed] [Google Scholar]
- Trayhurn P., Wood I.S. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br. J. Nutr. 2004;92(3):347–355. doi: 10.1079/bjn20041213. [DOI] [PubMed] [Google Scholar]
- Yanbaeva D.G., Dentener M.A., Creutzberg E.C., Wesseling G., Wouters E.F. Systemic effects of smoking. Chest. 2007;131(5):1557–1566. doi: 10.1378/chest.06-2179. [DOI] [PubMed] [Google Scholar]
- Higgins J.P., White I.R., Anzures-Cabrera J. Meta-analysis of skewed data: combining results reported on log-transformed or raw scales. Stat. Med. 2008;27(29):6072–6092. doi: 10.1002/sim.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender R., Bunce C., Clarke M., Gates S., Lange S., Pace N.L. Attention should be given to multiplicity issues in systematic reviews. J. Clin. Epidemiol. 2008;61(9):857–865. doi: 10.1016/j.jclinepi.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Eisler Z., Bartos I., Kertész J. Fluctuation scaling in complex systems: Taylor's law and beyond. Adv. Phys. 2008;57(1):89–142. [Google Scholar]
- Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. Br. Med. J. 2003;327(7414)::557. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcocer-Gómez E., de Miguel M., Casas-Barquero N., Núñez-Vasco J., Sánchez-Alcazar J.A., Fernández-Rodríguez A. NLRP3 inflammasome is activated in mononuclear blood cells from patients with major depressive disorder. Brain Behav. Immun. 2014;36:111–117. doi: 10.1016/j.bbi.2013.10.017. [DOI] [PubMed] [Google Scholar]
- Alesci S., Martinez P.E., Kelkar S., Ilias I., Ronsaville D.S., Listwak S.J. Major depression is associated with significant diurnal elevations in plasma interleukin-6 levels, a shift of its circadian rhythm, and loss of physiological complexity in its secretion: clinical implications. The Journal of Clinical Endocrinology & Metabolism. 2005;90(5):2522–2530. doi: 10.1210/jc.2004-1667. [DOI] [PubMed] [Google Scholar]
- Ali N.S., Hashem A.H.H., Hassan A.M., Saleh A.A., El-Baz H.N. Serum interleukin-6 is related to lower cognitive functioning in elderly patients with major depression. Aging & mental health. 2018;22(5):655–661. doi: 10.1080/13607863.2017.1293005. [DOI] [PubMed] [Google Scholar]
- Bai Y.-M., Chiou W.-F., Su T.-P., Li C.-T., Chen M.-H. Pro-inflammatory cytokine associated with somatic and pain symptoms in depression. J. Affect. Disord. 2014;155:28–34. doi: 10.1016/j.jad.2013.10.019. [DOI] [PubMed] [Google Scholar]
- Basterzi A.D., Aydemir Ç., Kisa C., Aksaray S., Tuzer V., Yazici K. IL-6 levels decrease with SSRI treatment in patients with major depression. Human Psychopharmacology: Clinical and Experimental. 2005;20(7):473–476. doi: 10.1002/hup.717. [DOI] [PubMed] [Google Scholar]
- Berk M., Wadee A., Kuschke R., O'Neill-Kerr A. Acute phase proteins in major depression. J. Psychosom. Res. 1997;43(5):529–534. doi: 10.1016/s0022-3999(97)00139-6. [DOI] [PubMed] [Google Scholar]
- Boettger S., Müller H.-J., Oswald K., Puta C., Donath L., Gabriel H.H. Inflammatory changes upon a single maximal exercise test in depressed patients and healthy controls. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2010;34(3):475–478. doi: 10.1016/j.pnpbp.2010.01.016. [DOI] [PubMed] [Google Scholar]
- Brambilla F., Maggioni M. Blood levels of cytokines in elderly patients with major depressive disorder. Acta Psychiatr. Scand. 1998;97(4):309–313. doi: 10.1111/j.1600-0447.1998.tb10005.x. [DOI] [PubMed] [Google Scholar]
- Camardese G., Pizi G., Marino M., Bartoccioni E., Grillo R., Mattioli B. Alterazioni delle risposte immuno-infiammatorie nei pazienti affetti da disturbo depressivo maggiore. Giorn Ital Psicopat. 2011;17:396–403. [Google Scholar]
- Cassano P., Bui E., Rogers A.H., Walton Z.E., Ross R., Zeng M. Inflammatory cytokines in major depressive disorder: A case–control study. Aust. N. Z. J. Psychiatry. 2017;51(1):23–31. doi: 10.1177/0004867416652736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain S.R., Cavanagh J., de Boer P., Mondelli V., Jones D.N., Drevets W.C. Treatment-resistant depression and peripheral C-reactive protein. The British Journal of Psychiatry. 2018;1–9 doi: 10.1192/bjp.2018.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton R.A., Lamar M., Zhang A., Ren X., Ajilore O., Pandey G.N. Associations between pro-inflammatory cytokines, learning, and memory in late-life depression and healthy aging. International journal of geriatric psychiatry. 2018;33(1):104–112. doi: 10.1002/gps.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavda N., Kantharia N.D., J. Effects of fluoxetine and escitalopram on C-reactive protein in patients of depression. Journal of pharmacology & pharmacotherapeutics. 2011;2(1)::11. doi: 10.4103/0976-500X.77091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Ouyang J., Liu S., Zhang S., Chen P., Jiang T. The Role of Cytokines in the Peripheral Blood of Major Depressive Patients. Clinical laboratory. 2017;63(7):1207–1212. doi: 10.7754/Clin.Lab.2017.170117. [DOI] [PubMed] [Google Scholar]
- Crnković D., Buljan D., Karlović D., Krmek M. Connection between inflammatory markers, antidepressants and depression. Acta clinica Croatica. 2012;51(1):25–32. [PubMed] [Google Scholar]
- Dannehl K., Rief W., Schwarz M.J., Hennings A., Riemer S., Selberdinger V. The predictive value of somatic and cognitive depressive symptoms for cytokine changes in patients with major depression. Neuropsych Dis Treat. 2014;10:1191. doi: 10.2147/NDT.S61640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davami M.H., Baharlou R., Vasmehjani A.A., Ghanizadeh A., Keshtkar M., Dezhkam I. Elevated IL-17 and TGF-β serum levels: a positive correlation between T-helper 17 cell-related pro-inflammatory responses with major depressive disorder. Basic and clinical neuroscience. 2016;7(2):137. doi: 10.15412/J.BCN.03070207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar F.S., Burke H.M., Epel E.S., Mellon S.H., Rosser R., Reus V.I. Low serum IL-10 concentrations and loss of regulatory association between IL-6 and IL-10 in adults with major depression. J. Psychiatr. Res. 2009;43(11):962–969. doi: 10.1016/j.jpsychires.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Dinan T., Siggins L., Scully P., O’Brien S., Ross P., Stanton C. Investigating the inflammatory phenotype of major depression: focus on cytokines and polyunsaturated fatty acids. J. Psychiatr. Res. 2009;43(4):471–476. doi: 10.1016/j.jpsychires.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Diniz B.S., Teixeira A.L., Talib L., Gattaz W.F., Forlenza O.V. Interleukin-1β serum levels is increased in antidepressant-free elderly depressed patients. The American Journal of Geriatric Psychiatry. 2010;18(2):172–176. doi: 10.1097/JGP.0b013e3181c2947f. [DOI] [PubMed] [Google Scholar]
- Diniz B.S., Teixeira A.L., Talib L.L., Mendonça V.A., Gattaz W.F., Forlenza O.V. Increased soluble TNF receptor 2 in antidepressant-free patients with late-life depression. J. Psychiatr. Res. 2010;44(14):917–920. doi: 10.1016/j.jpsychires.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Dome P., Teleki Z., Rihmer Z., Peter L., Dobos J., Kenessey I. Circulating endothelial progenitor cells and depression: a possible novel link between heart and soul. Mol. Psychiatry. 2009;14(5):523. doi: 10.1038/sj.mp.4002138. [DOI] [PubMed] [Google Scholar]
- Dunjic-Kostic B., Ivkovic M., Radonjic N.V., Petronijevic N.D., Pantovic M., Damjanovic A. Melancholic and atypical major depression—Connection between cytokines, psychopathology and treatment. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2013;43:1–6. doi: 10.1016/j.pnpbp.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Elderkin-Thompson V., Irwin M.R., Hellemann G., Kumar A. Interleukin-6 and memory functions of encoding and recall in healthy and depressed elderly adults. The American Journal of Geriatric Psychiatry. 2012;20(9):753–763. doi: 10.1097/JGP.0b013e31825d08d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eller T., Vasar V., Shlik J., Maron E. Pro-inflammatory cytokines and treatment response to escitaloprsam in major depressive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2008;32(2):445–450. doi: 10.1016/j.pnpbp.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Elomaa A.-P., Niskanen L., Herzig K.-H., Viinamäki H., Hintikka J., Koivumaa-Honkanen H. Elevated levels of serum IL-5 are associated with an increased likelihood of major depressive disorder. BMC psychiatry. 2012;12(1):2. doi: 10.1186/1471-244X-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euteneuer F., Schwarz M.J., Hennings A., Riemer S., Stapf T., Selberdinger V. Depression, cytokines and experimental pain: evidence for sex-related association patterns. J. Affect. Disord. 2011;131(1–3):143–149. doi: 10.1016/j.jad.2010.11.017. [DOI] [PubMed] [Google Scholar]
- Euteneuer F., Schwarz M.J., Dannehl K., Hartung A., Westermann S., Rief W. Increased soluble interleukin-2 receptor levels are related to somatic but not to cognitive-affective features in major depression. Brain Behav. Immun. 2012;26(8):1244–1248. doi: 10.1016/j.bbi.2012.06.007. [DOI] [PubMed] [Google Scholar]
- Fan N., Luo Y., Ou Y., He H. Altered serum levels of TNF-α, IL-6, and IL-18 in depressive disorder patients. Human Psychopharmacology: Clinical and Experimental. 2017;32(4) doi: 10.1002/hup.2588. [DOI] [PubMed] [Google Scholar]
- Fornaro M., Martino M., Battaglia F., Colicchio S., Perugi G. Increase in IL-6 levels among major depressive disorder patients after a 6-week treatment with duloxetine 60 mg/day: a preliminary observation. Neuropsych Dis Treat. 2011;7:51. doi: 10.2147/NDT.S16382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornaro M., Rocchi G., Escelsior A., Contini P., Martino M. Might different cytokine trends in depressed patients receiving duloxetine indicate differential biological backgrounds. J. Affect. Disord. 2013;145(3):300–307. doi: 10.1016/j.jad.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Frodl T., Carballedo A., Hughes M., Saleh K., Fagan A., Skokauskas N. Reduced expression of glucocorticoid-inducible genes GILZ and SGK-1: high IL-6 levels are associated with reduced hippocampal volumes in major depressive disorder. Transl. Psychiatry. 2012;2(3) doi: 10.1038/tp.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frommberger U.H., Bauer J., Haselbauer P., Fräulin A., Riemann D., Berger M. Interleukin-6-(IL-6) plasma levels in depression and schizophrenia: comparison between the acute state and after remission. Eur. Arch. Psychiatry Clin. Neurosci. 1997;247(4):228–233. doi: 10.1007/BF02900219. [DOI] [PubMed] [Google Scholar]
- Gazal M., Jansen K., Souza L.D., Oses J.P., Magalhães P.V., Pinheiro R. Association of interleukin-10 levels with age of onset and duration of illness in patients with major depressive disorder. Brazilian Journal of Psychiatry. 2015;37(4):296–302. doi: 10.1590/1516-4446-2014-1452. [DOI] [PubMed] [Google Scholar]
- Goyal S., Srivastava K., Kodange C., Bhat P. Immunological changes in depression. Industrial Psychiatry Journal. 2017;26(2):201–206. doi: 10.4103/ipj.ipj_22_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse L., Ambrée O., Jörgens S., Jawahar M.C., Singhal G., Stacey D. Cytokine levels in major depression are related to childhood trauma but not to recent stressors. Psychoneuroendocrinology. 2016;73:24–31. doi: 10.1016/j.psyneuen.2016.07.205. [DOI] [PubMed] [Google Scholar]
- Häfner S., Baghai T.C., Eser D., Schüle C., Rupprecht R., Bondy B. C-reactive protein is associated with polymorphisms of the angiotensin-converting enzyme gene in major depressed patients. J. Psychiatr. Res. 2008;2(42):163–165. doi: 10.1016/j.jpsychires.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Hernández M.E., Mendieta D., Martínez-Fong D., Loría F., Moreno J., Estrada I. Variations in circulating cytokine levels during 52 week course of treatment with SSRI for major depressive disorder. Eur. Neuropsychopharmacol. 2008;18(12):917–924. doi: 10.1016/j.euroneuro.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Ho P.-S., Yen C.-H., Chen C.-Y., Huang S.-Y., Liang C.-S. Changes in cytokine and chemokine expression distinguish dysthymic disorder from major depression and healthy controls. Psychiatry Res. 2017;248:20–27. doi: 10.1016/j.psychres.2016.12.014. [DOI] [PubMed] [Google Scholar]
- Hocaoglu C., Kural B., Aliyazıcıoglu R., Deger O., Cengiz S. IL-1β, IL-6, IL-8, IL-10, IFN-γ, TNF-α and its relationship with lipid parameters in patients with major depression. Metab. Brain Dis. 2012;27(4):425–430. doi: 10.1007/s11011-012-9323-9. [DOI] [PubMed] [Google Scholar]
- Huang T.-L., Lin F.-C. High-sensitivity C-reactive protein levels in patients with major depressive disorder and bipolar mania. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2007;31(2):370–372. doi: 10.1016/j.pnpbp.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Hosseini R.F., Jabbari Azad F., Talaee A., Miri S., Mokhber N., Farid Hosseini F. Assessment of the immune system activity in Iranian patients with Major Depression Disorder (MDD) Iranian Journal of Immunology. 2007;4(1):38–43. [PubMed] [Google Scholar]
- Hughes M.M., Carballedo A., McLoughlin D.M., Amico F., Harkin A., Frodl T. Tryptophan depletion in depressed patients occurs independent of kynurenine pathway activation. Brain Behav. Immun. 2012;26(6):979–987. doi: 10.1016/j.bbi.2012.05.010. [DOI] [PubMed] [Google Scholar]
- Hung Y.J., Hsieh C.H., Chen Y.J., Pei D., Kuo S.W., Shen D.C. Insulin sensitivity, proinflammatory markers and adiponectin in young males with different subtypes of depressive disorder. Clin. Endocrinol. 2007;67(5):784–789. doi: 10.1111/j.1365-2265.2007.02963.x. [DOI] [PubMed] [Google Scholar]
- Kageyama Y., Kasahara T., Kato M., Sakai S., Deguchi Y., Tani M. The relationship between circulating mitochondrial DNA and inflammatory cytokines in patients with major depression. J. Affect. Disord. 2018;233:15–20. doi: 10.1016/j.jad.2017.06.001. [DOI] [PubMed] [Google Scholar]
- Kagaya A., Kugaya A., Takebayashi M., Fukue-Saeki M., Saeki T., Yamawaki S. Plasma concentrations of interleukin-1β, interleukin-6, soluble interleukin-2 receptor and tumor necrosis factor α of depressed patients in Japan. Neuropsychobiology. 2001;43(2):59–62. doi: 10.1159/000054867. [DOI] [PubMed] [Google Scholar]
- Jozuka H., Jozuka E., Takeuchi S., Nishikaze O. Comparison of immunological and endocrinological markers associated with major depression. J. Int. Med. Res. 2003;31(1):36–41. doi: 10.1177/147323000303100106. [DOI] [PubMed] [Google Scholar]
- Karlović D., Serretti A., Vrkić N., Martinac M., Marčinko D. Serum concentrations of CRP, IL-6, TNF-α and cortisol in major depressive disorder with melancholic or atypical features. Psychiatry Res. 2012;198(1):74–80. doi: 10.1016/j.psychres.2011.12.007. [DOI] [PubMed] [Google Scholar]
- Kahl K.G., Schweiger U., Pars K., Kunikowska A., Deuschle M., Gutberlet M. Adrenal gland volume, intra-abdominal and pericardial adipose tissue in major depressive disorder. Psychoneuroendocrinology. 2015;58:1–8. doi: 10.1016/j.psyneuen.2015.04.008. [DOI] [PubMed] [Google Scholar]
- Kéri S., Szabó C., Kelemen O. Blood biomarkers of depression track clinical changes during cognitive-behavioral therapy. J. Affect. Disord. 2014;164:118–122. doi: 10.1016/j.jad.2014.04.030. [DOI] [PubMed] [Google Scholar]
- Leo R., Di G.L., Tesauro M., Razzini C., Forleo G.B., Chiricolo G. Association between enhanced soluble CD40 ligand and proinflammatory and prothrombotic states in major depressive disorder: pilot observations on the effects of selective serotonin reuptake inhibitor therapy. The Journal of clinical psychiatry. 2006;67(11):1760–1766. doi: 10.4088/jcp.v67n1114. [DOI] [PubMed] [Google Scholar]
- Lehto S.M., Huotari A., Niskanen L., Herzig K.-H., Tolmunen T., Viinamäki H. Serum IL-7 and G-CSF in major depressive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2010;34(6):846–851. doi: 10.1016/j.pnpbp.2010.03.033. [DOI] [PubMed] [Google Scholar]
- Lehto S.M., Niskanen L., Herzig K.-H., Tolmunen T., Huotari A., Viinamäki H. Serum chemokine levels in major depressive disorder. Psychoneuroendocrinology. 2010;35(2):226–232. doi: 10.1016/j.psyneuen.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Lee K.-M., Kim Y.-K. The role of IL-12 and TGF-β1 in the pathophysiology of major depressive disorder. Int. Immunopharmacol. 2006;6(8):1298–1304. doi: 10.1016/j.intimp.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Lee K.S., Chung J.H., Lee K.H., Shin M.-J., Oh B.H., Lee S.H. Simultaneous measurement of 23 plasma cytokines in late-life depression. Neurological sciences. 2009;30(5):435–438. doi: 10.1007/s10072-009-0091-1. [DOI] [PubMed] [Google Scholar]
- Kubera M., Kenis G., Bosmans E., Zieba A., Dudek D., Nowak G. Plasma levels of interleukin-6, interleukin-10, and interleukin-1 receptor antagonist in depression: comparison between the acute state and after remission. Pol. J. Pharmacol. 2000;52(3):237–241. [PubMed] [Google Scholar]
- Kokai M., Kashiwamura S-i, Okamura H., Ohara K., Morita Y. Plasma interleukin-18 levels in patients with psychiatric disorders. J. Immunother. 2002;25:S68–S71. doi: 10.1097/00002371-200203001-00011. [DOI] [PubMed] [Google Scholar]
- Kling M.A., Alesci S., Csako G., Costello R., Luckenbaugh D.A., Bonne O. Sustained low-grade pro-inflammatory state in unmedicated, remitted women with major depressive disorder as evidenced by elevated serum levels of the acute phase proteins C-reactive protein and serum amyloid A. Biol. Psychiatry. 2007;62(4):309–313. doi: 10.1016/j.biopsych.2006.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Suh I., Kim H., Han C., Lim C., Choi S. The plasma levels of interleukin-12 in schizophrenia, major depression, and bipolar mania: effects of psychotropic drugs. Mol. Psychiatry. 2002;7(10):1107. doi: 10.1038/sj.mp.4001084. [DOI] [PubMed] [Google Scholar]
- Maes M., Bosmans E., Suy E., Vandervorst C., De Jonckheere C., Raus J. Immune disturbances during major depression: upregulated expression of interleukin-2 receptors. Neuropsychobiology. 1990;24(3):115–120. doi: 10.1159/000119472. [DOI] [PubMed] [Google Scholar]
- Miller G.E., Rohleder N., Stetler C., Kirschbaum C. Clinical depression and regulation of the inflammatory response during acute stress. Psychosom. Med. 2005;67(5):679–687. doi: 10.1097/01.psy.0000174172.82428.ce. [DOI] [PubMed] [Google Scholar]
- Mikova O., Yakimova R., Bosmans E., Kenis G., Maes M. Increased serum tumor necrosis factor alpha concentrations in major depression and multiple sclerosis. Eur. Neuropsychopharmacol. 2001;11(3):203–208. doi: 10.1016/s0924-977x(01)00081-5. [DOI] [PubMed] [Google Scholar]
- Merendino R.A., Di Rosa A.E., Di Pasquale G., Minciullo P.L., Mangraviti C., Costantino A. Interleukin-18 and CD30 serum levels in patients with moderate-severe depression. Mediators Inflamm. 2002;11(4):265–267. doi: 10.1080/096293502900000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques-Deak A., Neto F.L., Dominguez W., Solis A., Kurcgant D., Sato F. Cytokine profiles in women with different subtypes of major depressive disorder. J. Psychiatr. Res. 2007;41(1–2):152–159. doi: 10.1016/j.jpsychires.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Manoharan A., Rajkumar R.P., Shewade D.G., Sundaram R., Muthuramalingam A., Paul A. Evaluation of interleukin-6 and serotonin as biomarkers to predict response to fluoxetine. Human Psychopharmacology: Clinical and Experimental. 2016;31(3):178–184. doi: 10.1002/hup.2525. [DOI] [PubMed] [Google Scholar]
- O'donovan A, Rush G, Hoatam G, Hughes BM, McCrohan A, Kelleher C, Suicidal ideation is associated with elevated inflammation in patients with major depressive disorder. Depression and anxiety. 2013;30(4):307–314. doi: 10.1002/da.22087. [DOI] [PubMed] [Google Scholar]
- O'brien SM, Scott LV, Dinan TG. Antidepressant therapy and C-reactive protein levels. The British Journal of Psychiatry. 2006;188(5):449–452. doi: 10.1192/bjp.bp.105.011015. [DOI] [PubMed] [Google Scholar]
- Nunes S.O.V., Vargas H.O., Brum J., Prado E., Vargas M.M., Castro M.R.Pd. A comparison of inflammatory markers in depressed and nondepressed smokers. Nicotine Tob. Res. 2011;14(5):540–546. doi: 10.1093/ntr/ntr247. [DOI] [PubMed] [Google Scholar]
- Narita K., Murata T., Takahashi T., Kosaka H., Omata N., Wada Y. Plasma levels of adiponectin and tumor necrosis factor-alpha in patients with remitted major depression receiving long-term maintenance antidepressant therapy. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2006;30(6):1159–1162. doi: 10.1016/j.pnpbp.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Munjiza A., Kostic M., Pesic D., Gajic M., Markovic I., Tosevski D.L. Higher concentration of interleukin 6-A possible link between major depressive disorder and childhood abuse. Psychiatry Res. 2018;264:26–30. doi: 10.1016/j.psychres.2018.03.072. [DOI] [PubMed] [Google Scholar]
- Motivala S.J., Sarfatti A., Olmos L., Irwin M.R. Inflammatory markers and sleep disturbance in major depression. Psychosom. Med. 2005;67(2):187–194. doi: 10.1097/01.psy.0000149259.72488.09. [DOI] [PubMed] [Google Scholar]
- Mota R., Gazal M., Acosta B.A., de Leon P.B., Jansen K., Pinheiro R.T. Interleukin-1β is associated with depressive episode in major depression but not in bipolar disorder. J. Psychiatr. Res. 2013;47(12):2011–2014. doi: 10.1016/j.jpsychires.2013.08.020. [DOI] [PubMed] [Google Scholar]
- Piletz J.E., Halaris A., Iqbal O., Hoppensteadt D., Fareed J., Zhu H. Pro-inflammatory biomakers in depression: treatment with venlafaxine. The World Journal of Biological Psychiatry. 2009;10(4):313–323. doi: 10.3109/15622970802573246. [DOI] [PubMed] [Google Scholar]
- Pike J.L., Irwin M.R. Dissociation of inflammatory markers and natural killer cell activity in major depressive disorder. Brain Behav. Immun. 2006;20(2):169–174. doi: 10.1016/j.bbi.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Pavón L., Sandoval-López G., Hernández M.E., Loría F., Estrada I., Pérez M. Th2 cytokine response in major depressive disorder patients before treatment. J. Neuroimmunol. 2006;172(1–2):156–165. doi: 10.1016/j.jneuroim.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Owen B., Eccleston D., Ferrier I., Young H. Raised levels of plasma interleukin-1β in major and postviral depression. Acta Psychiatr. Scand. 2001;103(3):226–228. doi: 10.1034/j.1600-0447.2001.00162.x. [DOI] [PubMed] [Google Scholar]
- Ogłodek E.A. Changes in the concentrations of inflammatory and oxidative status biomediators (MIP-1 α, PMN elastase, MDA, and IL-12) in depressed patients with and without posttraumatic stress disorder. Pharmacol. Rep. 2018;70(1):110–118. doi: 10.1016/j.pharep.2017.08.008. [DOI] [PubMed] [Google Scholar]
- Tuglu C., Kara S.H., Caliyurt O., Vardar E., Abay E. Increased serum tumor necrosis factor-alpha levels and treatment response in major depressive disorder. Psychopharmacology. 2003;170(4):429–433. doi: 10.1007/s00213-003-1566-z. [DOI] [PubMed] [Google Scholar]
- Thomas A.J., Davis S., Morris C., Jackson E., Harrison R., O’Brien J.T. Increase in interleukin-1β in late-life depression. Am. J. Psychiatry. 2005;162(1):175–177. doi: 10.1176/appi.ajp.162.1.175. [DOI] [PubMed] [Google Scholar]
- Sutcigil L., Oktenli C., Musabak U., Bozkurt A., Cansever A., Uzun O. Pro-and anti-inflammatory cytokine balance in major depression: effect of sertraline therapy. Clinical and Developmental Immunology. 2008;2007 doi: 10.1155/2007/76396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowa-Kućma M., Styczeń K., Siwek M., Misztak P., Nowak R.J., Dudek D. Are there differences in lipid peroxidation and immune biomarkers between major depression and bipolar disorder: Effects of melancholia, atypical depression, severity of illness, episode number, suicidal ideation and prior suicide attempts. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2018;81:372–383. doi: 10.1016/j.pnpbp.2017.08.024. [DOI] [PubMed] [Google Scholar]
- Sluzewska A., Rybakowski J., Bosmans E., Sobieska M., Berghmans R., Maes M. Indicators of immune activation in major depression. Psychiatry Res. 1996;64(3):161–167. doi: 10.1016/s0165-1781(96)02783-7. [DOI] [PubMed] [Google Scholar]
- Simon N., McNamara K., Chow C., Maser R., Papakostas G., Pollack M. A detailed examination of cytokine abnormalities in major depressive disorder. Eur. Neuropsychopharmacol. 2008;18(3):230–233. doi: 10.1016/j.euroneuro.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel A., Arolt V., Hunstiger M., Rink L., Behnisch A., Kirchner H. Cytokine production and serum proteins in depression. Scand. J. Immunol. 1995;41(6):534–538. doi: 10.1111/j.1365-3083.1995.tb03604.x. [DOI] [PubMed] [Google Scholar]
- Schlatter J., Ortuno F., Cervera-Enguix S. Monocytic parameters in patients with dysthymia versus major depression. J. Affect. Disord. 2004;78(3):243–247. doi: 10.1016/S0165-0327(02)00316-6. [DOI] [PubMed] [Google Scholar]
- Savitz J., Drevets W.C., Wurfel B.E., Ford B.N., Bellgowan P.S., Victor T.A. Reduction of kynurenic acid to quinolinic acid ratio in both the depressed and remitted phases of major depressive disorder. Brain Behav. Immun. 2015;46:55–59. doi: 10.1016/j.bbi.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybka J., Kędziora-Kornatowska K., Banaś-Leżańska P., Majsterek I., Carvalho L.A., Cattaneo A. Interplay between the pro-oxidant and antioxidant systems and proinflammatory cytokine levels, in relation to iron metabolism and the erythron in depression. Free Radical Biol. Med. 2013;63:187–194. doi: 10.1016/j.freeradbiomed.2013.05.019. [DOI] [PubMed] [Google Scholar]
- Rudzki L., Pawlak D., Pawlak K., Waszkiewicz N., Małus A., Konarzewska B. Immune suppression of IgG response against dairy proteins in major depression. BMC psychiatry. 2017;17(1):268. doi: 10.1186/s12888-017-1431-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolf S., Greggersen W., Kahl K.G., Hüppe M., Schweiger U. Elevated IL-6 levels in patients with atypical depression but not in patients with typical depression. Psychiatry Res. 2014;217(1–2):34–38. doi: 10.1016/j.psychres.2014.02.016. [DOI] [PubMed] [Google Scholar]
- Rizavi H.S., Ren X., Zhang H., Bhaumik R., Pandey G.N. Abnormal gene expression of proinflammatory cytokines and their membrane-bound receptors in the lymphocytes of depressed patients. Psychiatry Res. 2016;240:314–320. doi: 10.1016/j.psychres.2016.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rief W., Pilger F., Ihle D., Bosmans E., Egyed B., Maes M. Immunological differences between patients with major depression and somatization syndrome. Psychiatry Res. 2001;105(3):165–174. doi: 10.1016/s0165-1781(01)00338-9. [DOI] [PubMed] [Google Scholar]
- Rawdin B., Mellon S., Dhabhar F., Epel E., Puterman E., Su Y. Dysregulated relationship of inflammation and oxidative stress in major depression. Brain Behav. Immun. 2013;31:143–152. doi: 10.1016/j.bbi.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto K., Kakeda S., Watanabe K., Katsuki A., Ueda I., Igata N. Relationship between white matter integrity and serum inflammatory cytokine levels in drug-naive patients with major depressive disorder: diffusion tensor imaging study using tract-based spatial statistics. Transl. Psychiatry. 2018;8(1):141. doi: 10.1038/s41398-018-0174-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Lu P., Wei L., Hu X., Chen W. Fluoxetine treatment for major depression decreases the plasma levels of cytokines. Afr. J. Biotechnol. 2010;9(43):7346–7351. [Google Scholar]
- Schmidt F.M., Lichtblau N., Minkwitz J., Chittka T., Thormann J., Kirkby K.C. Cytokine levels in depressed and non-depressed subjects, and masking effects of obesity. J. Psychiatr. Res. 2014;55:29–34. doi: 10.1016/j.jpsychires.2014.04.021. [DOI] [PubMed] [Google Scholar]
- Zoga M., Oulis P., Chatzipanagiotou S., Masdrakis V.G., Pliatsika P., Boufidou F. Indoleamine 2, 3-dioxygenase and immune changes under antidepressive treatment in major depression in females. in vivo. 2014;28(4):633–638. [PubMed] [Google Scholar]
- Zincir S., Öztürk P., Bilgen A.E., Izci F., Yükselir C. Levels of serum immunomodulators and alterations with electroconvulsive therapy in treatment-resistant major depression. Neuropsych Dis Treat. 2016;12:1389. doi: 10.2147/NDT.S106652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W., Feng R., Yang Y. Changes in the serum levels of inflammatory cytokines in antidepressant drug-naïve patients with major depression. PLoS ONE. 2018;13(6) doi: 10.1371/journal.pone.0197267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura R., Umene-Nakano W., Hoshuyama T., Ikenouchi-Sugita A., Hori H., Katsuki A. Plasma levels of brain-derived neurotrophic factor and interleukin-6 in patients with dysthymic disorder: comparison with age-and sex-matched major depressed patients and healthy controls. Human Psychopharmacology: Clinical and Experimental. 2010;25(7–8):566–569. doi: 10.1002/hup.1155. [DOI] [PubMed] [Google Scholar]
- Yoshimura R., Hori H., Ikenouchi-Sugita A., Umene-Nakano W., Ueda N., Nakamura J. Higher plasma interleukin-6 (IL-6) level is associated with SSRI-or SNRI-refractory depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2009;33(4):722–726. doi: 10.1016/j.pnpbp.2009.03.020. [DOI] [PubMed] [Google Scholar]
- Yang K., Xie G., Zhang Z., Wang C., Li W., Zhou W. Levels of serum interleukin (IL)-6, IL-1β, tumour necrosis factor-α and leptin and their correlation in depression. Aust. N. Z. J. Psychiatry. 2007;41(3):266–273. doi: 10.1080/00048670601057759. [DOI] [PubMed] [Google Scholar]
- Xia Q.-R., Liang J., Cao Y., Shan F., Liu Y., Xu Y.-Y. Increased plasma nesfatin-1 levels may be associated with corticosterone, IL-6, and CRP levels in patients with major depressive disorder. Clin. Chim. Acta. 2018;480:107–111. doi: 10.1016/j.cca.2018.02.004. [DOI] [PubMed] [Google Scholar]
- Wiener C.D., Moreira F.P., Cardoso T.A., Mondin T.C., da Silva Magalhães P.V., Kapczinski F. Inflammatory cytokines and functional impairment in drug-free subjects with mood disorder. J. Neuroimmunol. 2017;307:33–36. doi: 10.1016/j.jneuroim.2017.03.003. [DOI] [PubMed] [Google Scholar]
- Vaccarino V., Brennan M.-L., Miller A.H., Bremner J.D., Ritchie J.C., Lindau F. Association of major depressive disorder with serum myeloperoxidase and other markers of inflammation: a twin study. Biol. Psychiatry. 2008;64(6):476–483. doi: 10.1016/j.biopsych.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Zhong H., Lu M., Song G., Zhang X., Lin M. Higher Serum C Reactive Protein Determined C Reactive Protein Single-Nucleotide Polymorphisms Are Involved in Inherited Depression. Psychiatry investigation. 2018;15(8):824. doi: 10.30773/pi.2018.04.03.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport M.H., Irwin M. Serum soluble interleukin-2 receptors and natural killer cell function in major depression. Research Communications in Biological Psychology and Psychiatry. 1996;21(1/2):73–76. [Google Scholar]
- Osimo E.F., Baxter L.J., Lewis G., Jones P.B., Khandaker G.M. Prevalence of Low-grade Inflammation in Depression: a systematic review and meta-analysis of CRP levels. Psychol. Med. 2019 doi: 10.1017/S0033291719001454. E-pub ahead of print:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G.E., Cole S.W. Clustering of depression and inflammation in adolescents previously exposed to childhood adversity. Biol. Psychiatry. 2012;72(1):34–40. doi: 10.1016/j.biopsych.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh E.T. CRP as a mediator of disease. Circulation. 2004;109(21_suppl_1) doi: 10.1161/01.CIR.0000129507.12719.80. II-11-II-4. [DOI] [PubMed] [Google Scholar]
- Danesh J., Whincup P., Walker M., Lennon L., Thomson A., Appleby P. Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. BMJ. 2000;321(7255):199–204. doi: 10.1136/bmj.321.7255.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M., Bouter L.M., McQuillan G.M., Wener M.H., Harris T.B. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282(22):2131–2135. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- Fernandes B., Steiner J., Bernstein H., Dodd S., Pasco J., Dean O. C-reactive protein is increased in schizophrenia but is not altered by antipsychotics: meta-analysis and implications. Mol. Psychiatry. 2016;21(4):554. doi: 10.1038/mp.2015.87. [DOI] [PubMed] [Google Scholar]
- von Känel R., Hepp U., Kraemer B., Traber R., Keel M., Mica L. Evidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorder. J. Psychiatr. Res. 2007;41(9):744–752. doi: 10.1016/j.jpsychires.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Fernandes B.S., Steiner J., Molendijk M.L., Dodd S., Nardin P., Gonçalves C.-A. C-reactive protein concentrations across the mood spectrum in bipolar disorder: a systematic review and meta-analysis. The Lancet Psychiatry. 2016;3(12):1147–1156. doi: 10.1016/S2215-0366(16)30370-4. [DOI] [PubMed] [Google Scholar]
- Gimeno D., Kivimäki M., Brunner E.J., Elovainio M., De Vogli R., Steptoe A. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychol. Med. 2009;39(3):413–423. doi: 10.1017/S0033291708003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker G.M., Pearson R.M., Zammit S., Lewis G., Jones P.B. Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. JAMA psychiatry. 2014;71(10):1121–1128. doi: 10.1001/jamapsychiatry.2014.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wium-Andersen M.K., Ørsted D.D., Nielsen S.F., Nordestgaard B.G. Elevated C-reactive protein levels, psychological distress, and depression in 73 131 individuals. JAMA psychiatry. 2013;70(2):176–184. doi: 10.1001/2013.jamapsychiatry.102. [DOI] [PubMed] [Google Scholar]
- Zalli A., Jovanova O., Hoogendijk W., Tiemeier H., Carvalho L. Low-grade inflammation predicts persistence of depressive symptoms. Psychopharmacology. 2016;233(9):1669–1678. doi: 10.1007/s00213-015-3919-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker G.M., Zuber V., Rees J.M.B., Carvalho L., Mason A.M., Foley C.N. Shared mechanism between depression and coronary heart disease: findings from Mendelian randomization analysis of a large UK population-based cohort. Mol. Psychiatry. 2019 [Google Scholar]
- Felger J.C., Haroon E., Patel T.A., Goldsmith D.R., Wommack E.C., Woolwine B.J. What does plasma CRP tell us about peripheral and central inflammation in depression? Mol. Psychiatry. 2018 doi: 10.1038/s41380-018-0096-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbas A.K., Lichtman A.H., Pillai S. Cellular and molecular immunology E-book. Elsevier Health Sciences. 2014 [Google Scholar]
- Stern A.S., Magram J., Presky D.H. Interleukin-12 an integral cytokine in the immune response. Life Sci. 1996;58(8):639–654. doi: 10.1016/s0024-3205(96)80003-8. [DOI] [PubMed] [Google Scholar]
- Fayers P. Alphas, betas and skewy distributions: two ways of getting the wrong answer. Adv Health Sci Educ. 2011;16(3):291–296. doi: 10.1007/s10459-011-9283-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.