Abstract
Heart function relies on the interplay of several specialized cell types and a precisely regulated network of chemical and mechanical stimuli. Over the last few decades, this complexity has often been undervalued and progress in translational cardiovascular research has been significantly hindered by the lack of appropriate research models. The data collected are often oversimplified and these make the translation of results from the laboratory to clinical trials challenging and occasionally misleading. Living myocardial slices are ultrathin (100–400μm) sections of living cardiac tissue that maintain the native multicellularity, architecture, and structure of the heart and can provide information at a cellular/subcellular level. They overcome most of the limitations that affect other in vitro models and they can be prepared from human specimens, proving a clinically relevant multicellular human model for translational cardiovascular research. The publication of a reproducible protocol, and the rapid progress in methodological and technological discoveries which prevent significant structural and functional changes associated with chronic in vitro culture, has overcome the last barrier for the in vitro use of this human multicellular preparations. This technology can bridge the gap between in vitro and in vivo human studies and has the potential to revolutionize translational research approaches.
Keywords: Living myocardial slices, Translational research
Introduction
Multicellular life relies on cellular specialization and complex interplays of various cell types. The orchestrated function of different cell populations in the heart and the elaborate network of inter- and intra-cellular circuits of communications are imperative to maintain a healthy heart. Disruption of these connections and networks gives rise to pathological conditions.
Heart function is precisely regulated by systemic and local stimuli of chemical and mechanical nature, which are capable of regulating both cardiomyocyte and non-myocyte populations. These factors are essential during tissue development and for the maintenance of the adult cardiac phenotype.
Over the last few decades, this complexity has often been undervalued and progress in translational cardiovascular research has been significantly hindered by the lack of appropriate research models. The vast majority of human specimens provided by clinicians to research laboratories are currently used for isolated cell preparations. Following the enzymatic digestion, the complex 3D architecture, the extracellular matrix and the connections between cells are lost. A large number of cells are also killed or damaged with major implications in cell function and structure. The lack of multicellularity makes this in vitro model unsuitable for investigation of cardiac conduction or pharmacological responses.1 Hence, the data collected from isolated cell preparations are often oversimplified and these make the translation of results from laboratory to clinical trials challenging and sometimes misleading. Additionally, adult cardiac tissue, similarly to isolated cells, undergoes a rapid remodelling when removed from the body, which has limited the use of in vitro studies to short (acute) time points. Stem cell-derived cardiomyocytes are a valuable alternative; however, they lack representative adult features and this has hindered their applicability in drug discovery and regenerative medicine. Multicellular models, prepared with animals or human samples, provide a more complex and physiologically relevant research platform. The importance of stromal cells, in primary cardiac fibroblasts and endothelial cells, has long been reported and it is now well recognized.2,3 On the contrary to isolated cell studies, experiments which require the interaction of various cell types such as cardiac conduction measurements, can be investigated and physiologically relevant data acquired. These preparations however suffer for the limited vascularization and/or diffusion of nutrients and oxygen which results in ischaemic damage and altered quality and reliability of results.
Living myocardial slices overcome most of these limitations and therefore are considered the research model that can bridge the gap between in vitro and in vivo translational studies.
Myocardial slices, a multicellular platform to study cardiac biology
Living myocardial slices are ultrathin (100–400 μm) sections of living cardiac tissue, prepared using a high-precision vibratome that maintains the native multicellularity, architecture, and structure of the heart. Their thinness allows the diffusion of oxygen and nutrients into their innermost cells, preserving viability in the absence of coronary perfusion in vitro and preventing ischaemic damage. Myocardial slices can be produced from both small and large animal models but most importantly from human specimens, thus providing a clinically relevant multicellular human model for translational cardiovascular research. They are considered a medium-throughput methodology as several myocardial slices can be produced from the same specimen.
For several years, the lack of a standardized protocol has hampered the diffusion of this technology and limited its utilization to few, specialized laboratories.
The recent description of an optimized and reproducible protocol, published in Nature Protocols,4 results in a preparation with >95% viable cardiomyocytes, robust contractile function, and physiological Ca2+ handling and conduction velocity recordings. Several structural parameters can also be investigated such as cell morphology, gap-junctions, and collagen distribution. In terms of integrated myocardial function, living myocardial slices can serve as a uniquely controlled electromechanical system, in which the bidirectional cross-talk between mechanical activity and electrical excitation can be quantitatively monitored and/or evaluated.
The possibility of preparing living myocardial slices following in vivo experiments [such as echocardiography or magnetic resonance imaging (MRI)] allows investigators to perform additional experiments with the same heart, and acquire informative data on multiple structural, functional, and molecular parameters at the cellular/subcellular level. In the case of human myocardial slices, the data acquired can then be correlated to the parameters obtained from hospitalized patients to extrapolate novel integrated data.
The most recent development of novel technologies that prevent significant structural and functional changes associated with chronic in vitro culture, have overcome the last barrier for the in vitro use of human multicellular preparations.
It was demonstrated that biomimetic electromechanical stimulation with specific physiological preload is required for the maintenance of adult cardiac tissue in vitro.5 The authors also show that biomimetic stimulation affects structure, function, and many gene set expression circuits, which regulate many aspects of the cardiac phenotype, including sarcomeres, Ca2+ handling, and t-tubules.
In parallel, Fisher et al. developed a computerized system where isotonic electromechanical stimulation was able to maintain myocardial slices in vitro for weeks.6 The constant monitoring of the contractile capacity also provides crucial information related to tissue function in real-time.
Biomimetic culture systems are now offering the opportunity to study long-term interventions, pharmacological screening, and genetic manipulation in therapeutically relevant tissue (Figure 1).
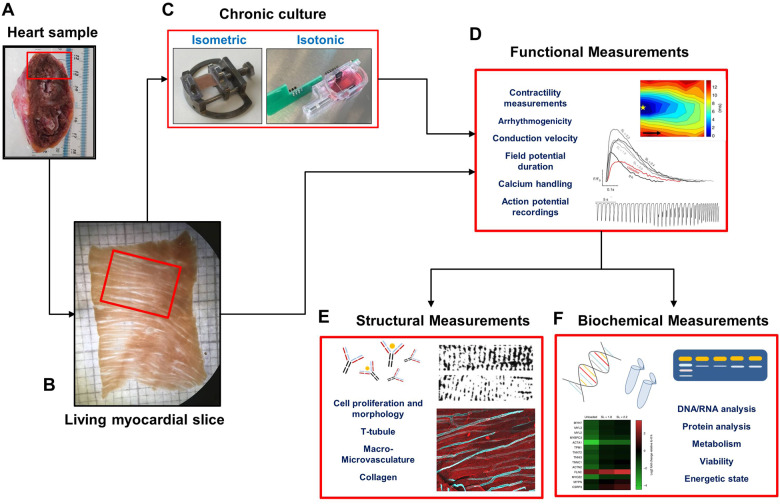
Figure 1.
Flowchart of living myocardial slice preparation, chronic culture, and ex vivo experimental measurements. Living myocardial slices are prepared with both animal and human cardiac specimens. They can be used acutely or following in vitro chronic culture to investigate several functional, structural, or biochemical parameters. Images in this figure have been previously published.3,5
Limitations and new challenges
Acknowledging the manual nature of the tissue block preparation, the main limitation of myocardial slice quality is the method used during the initial dissection. This is primarily influenced by the user’s experience, hospital set up (e.g. distance from clinics to research labs), and manual skill. A considerable time has to be dedicated to exercising the method before high-quality tissue blocks can be consistently prepared. Initial coaching with an experienced researcher in myocardial slice preparation is also recommended.
In the case of human specimen utilization, a limiting factor is often attributed to the time required for the samples to reach the laboratory. Tissue damage is often associated with prolonged hypothermic preservation. Evidence from Fischer et al.6 suggests that the preparation of myocardial slice can be delayed of up to 30 h. Although the authors showed no impairment of maximum twitch force, these observations should be further validated with a more precise characterization of both functional and structural parameters such as calcium handling or conduction velocity.
Interstitial fibrosis, often present in specimens originating from end-stage heart failure patients, is the most frequent complication occurring during the slicing process. The fibrotic regions often impede a smooth cutting, which results in uneven myocardial slices. Researcher’s experience and alteration to the vibratome’s parameters can often overcome this matter. The equipment’s availability and its cost might also be a limiting factor for a broad diffusion of this technology.
Human myocardial slices have mostly been produced from explanted heart specimens (during cardiac transplantation). The main reason is the considerable quantity of tissue available for the operator (1–3 cm3) which also simplifies the preparation of the slicing block. The current stagnation in heart transplant operations performed worldwide1 and therefore the rare and irregular supply of tissue to research laboratories, is driving the researchers to new sources of cardiac tissue. A refinement and adaptation of the tissue block preparation will allow smaller pieces of the myocardium to be used. A septal myectomy or the implantation of a left ventricular assist device can, in fact, generate sufficient tissue for the generation of myocardial slices.
This strategy will broaden the availability of samples and therefore encourage the utilization of this technology.
A new and exciting challenge would be to extend myocardial slice generation to cardiac biopsies. Considering the very small dimensions of these specimens, this approach could be technically challenging; however, it would significantly expand the clinical implications of this methodology to diagnostic use. As an example, contractility parameters can be precisely quantified with isometric force transducer setups and several parameters can be analysed including tissue relaxation. The latter is reported to be altered in several cardiomyopathies and it could hypothetically be used as a diagnostic tool to identify subtle, unrecognized cardiomyopathies (Figure 2).
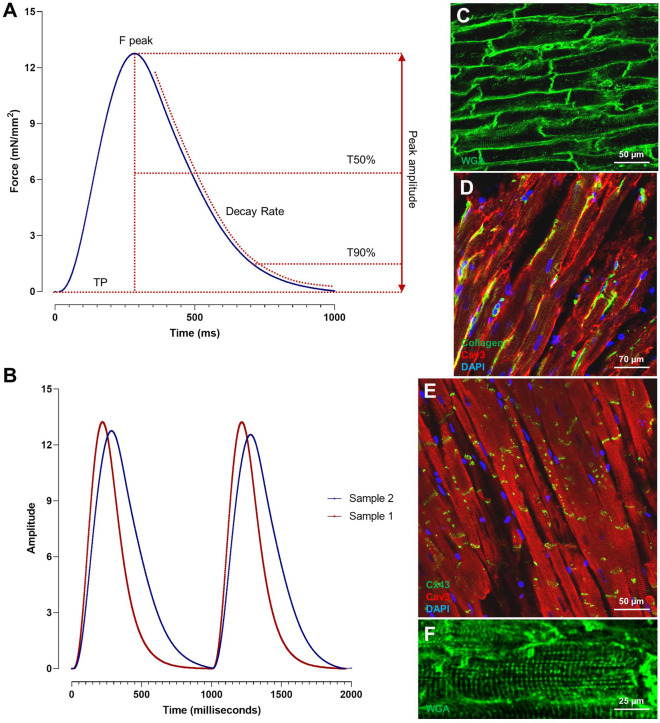
Figure 2.
Contractility recordings and representative confocal images of living myocardial slice. (A) Schematic illustration of contractility parameters (TP = Time to Peak, T50% = Time to 50% relaxation, T90% = Time to 90% relaxation, Decay Rate). (B) Representative traces of the contractile capacity of two myocardial slices displaying a faster (red) and slower (blue) contraction and relaxation kinetics. (C–F) Wheat germ agglutinin (WGA) staining allows the visualization of cell membrane and it is used to study cardiomyocytes dimensions and T-tubule organization. Immunohistochemical staining for Collagen, Connexin43, and Caveolin 3 were used to visualize collagen organization, gap junction distribution, and cardiomyocytes morphology.3,5
During chronic culture, although the application of biomimetic electromechanical stimulation supports the maintenance of the mature adult cardiac phenotype, signs of dedifferentiation are still occurring. The recently described systems allow isometric and isotonic contractions which are simplified representations of the elaborate mechanical load experienced by the myocardium in vivo. Novel technologies that can control both the preload and afterload, and therefore capable of simulating pressure–volume loops, are likely to further delay the dedifferentiation process. Another aspect that can be improved is the generation of media formulations enriched with humoral stimuli and metabolic substrates more appropriate to support the nutritive and humoral needs of the myocardium during in vitro culture. Finally, the non-physiological pacing has been reported to induce alterations to cell function.7 Transient periods of faster and slower stimulation rate could therefore further preserve tissue function and better resemble the physiological in vivo conditions.
Conclusions
The rapid progress in myocardial slice technology and the development of assays to facilitate the acquisition of data from a whole organism to a subcellular level, combined with the recent development of novel technologies that prevent significant structural and functional changes associated with chronic in vitro culture, have enormous potential to revolutionize translational research approaches.
It is realistic to consider the possibility of specimens obtained from patients undergoing a septal myectomy or the implantation of a ventricular assist device, to be sent to the laboratory where several myocardial slices can be produced and functional and structural analysis performed within a few hours. Moreover, pharmacological treatments or genetic manipulation tests can be conducted in the following days/weeks to determine the most suitable treatment for the patient. This could lead to a new era of precision medicine and the development of novel clinical protocols for heart failure treatment.
Funding
Part of the research was supported by grants from ERC Longheart and ERANET CVD Expert and the Deutsche Forschungsgemeinschaft (KFO311/2 and INST 95/1564-1).
Conflict of interest: Dr F.P. declared no conflicts. Prof. T.T. is a founder and shareholder of Cardior Pharmaceuticals GmbH, a clinical-stage spin-off company focused on the development and clinical validation of RNAs therapeutics for patients with cardiovascular disorders.
The opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal or of the European Society of Cardiology.
References
- 1. Fuchs M, Schibilsky D, Zeh W, Berchtold-Herz M, Beyersdorf F, Siepe M.. Does the heart transplant have a future? Eur J Cardio-Thoracic Surg 2019;55:i38–i48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kohl P. Heterogeneous cell coupling in the heart: an electrophysiological role for fibroblasts. Circ Res 2003;93:381–383. [DOI] [PubMed] [Google Scholar]
- 3. Perbellini F, Watson SA, Bardi I, Terracciano CM.. Heterocellularity and cellular cross-talk in the cardiovascular system. Front Cardiovasc Med 2018;5:143.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Watson SA, Scigliano M, Bardi I, Ascione R, Terracciano CM, Perbellini F.. Preparation of viable adult ventricular myocardial slices from large and small mammals. Nat Protoc 2017;12:2623–2639. [DOI] [PubMed] [Google Scholar]
- 5. Watson SA, Duff J, Bardi I, Zabielska M, Atanur SS, Jabbour RJ, Simon A, Tomas A, Smolenski RT, Harding SE, Perbellini F, Terracciano CM.. Biomimetic electromechanical stimulation to maintain adult myocardial slices in vitro. Nat Commun 2019;10:2168.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fischer C, Milting H, Fein E, Reiser E, Lu K, Seidel T, Schinner C, Schwarzmayr T, Schramm R, Tomasi R, Husse B, Cao-Ehlker X, Pohl U, Dendorfer A.. Long-term functional and structural preservation of precision-cut human myocardium under continuous electromechanical stimulation in vitro. Nat Commun 2019;10:117.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vassalle M, Lin C-I.. Calcium overload and cardiac function. J Biomed Sci 2004;11:542–565. [DOI] [PubMed] [Google Scholar]