This editorial refers to ‘Effects of dapagliflozin in DAPA-HF according to background heart failure therapy’†, by K.F. Docherty et al., on page 2379.
Many drugs that antagonize endogenous neurohormonal systems reduce the risk of death in patients with heart failure and a reduced ejection fraction. Importantly, the magnitude of the survival benefit with each agent has not been influenced by concomitant treatment with other life-prolonging drugs. In the post-infarction patient, angiotensin-converting enzyme (ACE) inhibitors decreased the mortality rate in patients already receiving beta-blockers. Conversely, in patients with established heart failure due to systolic dysfunction, beta-blockers reduced the risk of death in patients already receiving ACE inhibitors. The mortality reduction with mineralocorticoid receptor antagonists in an early trial where few patients were treated with a beta-blocker was similar to that seen in a later trial where most patients were receiving a beta-blocker. In subgroup analyses of clinical trials, the survival benefits of a neurohormonal antagonist have not been meaningfully affected by the intensity of use of other important treatments for heart failure.
The concept that each neurohormonal antagonist exerts favourable effects that are independent of the benefits of other treatments is critically important to physicians. In clinical practice, most patients with heart failure and a reduced ejection fraction are treated with only a conventional inhibitor of the renin–angiotensin system and a beta-blocker, often in doses that are much smaller than the target doses that were effective in large-scale clinical trials.1 Presumably, some practitioners believe that such limited neurohormonal antagonism is sufficient, perhaps based on an assumption that a combination of an ACE inhibitor and a beta-blocker achieves adequate normalization of the neurohormonal environment. However, this belief is not supported by the available evidence. The addition of a mineralocorticoid receptor antagonist and a neprilysin inhibitor to an ACE inhibitor and a beta-blocker leads to a further substantial reduction in the risk of death; modelling estimates suggest that mortality can be reduced by an additional 40–50% with more comprehensive neurohormonal blockade.
Influence of background therapy on the heart failure benefits of SGLT2 inhibitors
The subgroup analyses of the DAPA-HF trial published in this issue of the European Heart Journal 2 strongly reinforce this finding. This trial demonstrated that dapagliflozin—a sodium–glucose co-transporter 2 (SGLT2) inhibitor—reduced the combined risk of cardiovascular death and hospitalization for heart failure by 26% and the risk of cardiovascular death alone by 18%. Both effects were clinically meaningful, and the magnitude of these benefits was not influenced by the use of concomitant treatments for heart failure, regardless of the specific combinations and doses that were prescribed by each physician. Another large-scale trial with empagliflozin in chronic heart failure and a reduced ejection fraction (EMPEROR-Reduced) is nearing completion and is enrolling many patients at higher risk. This trial is likely to confirm and extend the findings of DAPA-HF and, additionally, it is expected that background neurohormonal antagonism will not influence the size of the benefit of empagliflozin.
The finding that the effect of SGLT2 inhibition in the DAPA-HF trial is not influenced by background therapy for heart failure is consistent with the findings of large-scale cardiovascular outcomes trials in type 2 diabetes. In these trials, SGLT2 inhibitors reduced the risk of cardiovascular death and hospitalization for heart failure by ∼30%; yet most patients in these trials were not receiving mineralocorticoid receptor antagonists or neprilysin inhibitors.3 In contrast, in the DAPA-HF trial, the use of broad-based neurohormonal antagonism was strongly encouraged, and yet the magnitude of the benefit with SGLT2 inhibitors was similar to that seen in the trials in type 2 diabetes.
The additive benefit of SGLT2 inhibitors is particularly noteworthy in patients receiving neprilysin inhibitors. Compared with older treatments for heart failure, both SGLT2 inhibitors and sacubitril/valsartan are more expensive, raising the question as to whether physicians can simply prescribe only one of the two newer drugs. The subgroup analyses of DAPA-HF demonstrate that treatment with sacubtril/valsartan does not attenuate the benefit of dapagliflozin, confirming that neprilysin inhibitors and SGLT2 inhibitors provide independently important benefits in reducing the risk of cardiovascular death. Therefore, it is not reasonable to propose that physicians must choose between the two newer approaches. If mortality reduction is important, then a combination of an SGLT2 inhibitor and a neprilysin inhibitor represents the only acceptable standard of care. The addition of both drugs would be expected to yield an incremental ∼35% reduction in the risk of death in patients already receiving an inhibitor of the renin–angiotensin system, a beta-blocker, and a mineralocorticoid receptor antagonist.
Drug–drug interactions can provide insights into mechanism of action
Analyses of drug–drug interactions may provide insights into the mechanism of action of pharmacological interventions. If two agents have distinctly different modes of action, then the magnitude of benefit of one drug should not be influenced by co-administration of the other. The reverse would be true if a meaningful drug–drug interaction were observed. For example, renin inhibitors, ACE inhibitors, and angiotensin receptor blockers act in broadly similar ways. Therefore, the finding that the addition of a renin inhibitor or an angiotensin receptor blocker to an ACE inhibitor yielded few incremental benefits confirmed our understanding of the mechanism of action of these drugs.
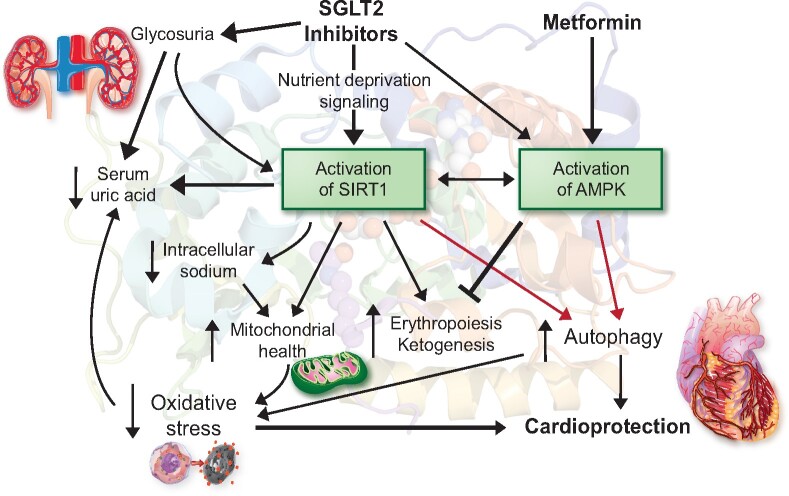
Therefore, the observation in DAPA-HF that the magnitude of the benefit of SGLT2 inhibitors is not influenced by background therapy strongly indicates that the mechanism of the cardioprotective effects of these drugs is not mediated by interference with the sympathetic nervous system, renin–angiotensin system, aldosterone, or neprilysin. In experimental models, SGLT2 inhibitors act to reduce oxidative stress, ameliorate mitochondrial dysfunction, and attenuate proinflammatory pathways; potentially, all of these effects may be mediated by an effect of these drugs to promote autophagy.5 This pattern of biological responses is consistent with an effect of SGLT2 inhibitors to induce a fasting transcriptional paradigm and, thereby, promote the activation of nutrient deprivation sensors—predominantly, sirtuin-1 (SIRT1) and (to a much lesser degree) AMP-activated protein kinase (AMPK) (Figure 1).4 , 5 These enzymes are master regulators of hundreds of genes and proteins that regulate cellular stress, and their up-regulation yields important cardioprotective effects. SIRT1 activation may also underlie the striking effects of SGLT2 inhibitors to reduce the risk of serious adverse renal events.6
Figure 1.
Proposed framework to explain the mechanism of the cardioprotective effect of SGLT2 inhibitors and the potential for an interaction with metformin. SGLT2 inhibitors promote erythropoiesis, presumably through SIRT1-mediated activation of hypoxia-inducible factor-2α, which is suppressed by metformin. Gluconeogenesis—a critical contributor to ketogenesis—is stimulated by SIRT1 and SGLT2 inhibitors, but inhibited by metformin. AMPK, AMP-activated protein kinase; SGLT2, sodium–glucose co-transporter 2; SIRT1, sirtuin-1.
Intriguingly, signalling through SIRT1 may also explain the action of SGLT2 inhibitors to promote ketogenesis and erythrocytosis and to lower uric acid (a biomarker of oxidative stress).4 , 6 , 7 Changes in haemoglobin and serum urate are the most powerful predictors of the reduction in serious heart failure events with SGLT2 inhibitors in large-scale trials.8 , 9 Furthermore, activation of SIRT1 may contribute to the ability of SGLT2 inhibitors to mitigate the increased intracellular sodium concentration seen in diabetic cardiomyocytes.4 , 10 Interestingly, another antihyperglycaemic drug—metformin—is a known agonist of AMPK (Figure 1). Yet, presumably because it does not concomitantly promote SIRT1 signalling to a meaningful degree, metformin does not enhance ketogenesis or erythropoiesis or decrease uric acid or intracellular sodium concentrations.4 , 5 , 11–13
The possibility of a partial mechanistic overlap between SGLT2 inhibitors and metformin (with respect to AMPK activation) is supported by the finding of a drug–drug interaction in large-scale trials in diabetes.14 In the CANVAS trials, canagliflozin reduced the risk of cardiovascular death or hospitalization for heart failure by 36% in patients not receiving metformin, but by only 12% in those receiving metformin (interaction P = 0.03). In the EMPA-REG OUTCOME trial, empagliflozin reduced the risk of cardiovascular death by 54% in patients not receiving metformin and by 29% in those receiving metformin (interaction P = 0.07); no interaction was seen with respect to the effect on hospitalizations for heart failure. In the same trial, metformin modestly attenuated the benefits of empagliflozin on the clinical course of nephropathy (53% risk reduction in metformin users vs. 32% risk reduction in metformin non-users, interaction P = 0.01).
The contribution of AMPK activation with the effects of SGLT2 inhibitors may vary among members of the drug class. Canagliflozin has a more striking effect to activate AMPK than empagliflozin and dapagliflozin,15 thus explaining why the magnitude of the metformin interaction was greater in the CANVAS trials than in the EMPA-REG OUTCOME study. In light of these observations, it is important for the investigators of trials with dapagliflozin to report their findings with respect to the influence of background therapy with metformin. To date, we do not know if metformin attenuated the benefit of dapagliflozin on heart failure events among patients with type 2 diabetes, either in DECLARE-TIMI58 or in the DAPA-HF trials. The experimental data suggest that empagliflozin and dapaglifozin primarily signal through sirtuin-1, rather than AMPK.
Clinical implications
With the completion of two large-scale trials of SGLT2 inhibitors in patients with chronic heart failure and a reduced ejection fraction, we are poised to add yet another drug to our portfolio of cardioprotective agents. These disease-modifying drugs target important, but distinct, pathways that promote cardiomyocyte dysfunction and demise, and it is critical that physicians prescribe all of them in combination to all appropriate patients who do not have demonstrable intolerance. Yet, <1% of patients with chronic heart failure are receiving currently recommended drugs at doses that have been shown to prolong life.1 According to modelling estimates, when compared with no neurohormonal blockade, the use of a broad-based combination of disease-modifying drugs at target doses may reduce the risk of death by as much as 75%. It is time that physicians who treat patients with heart failure took notice.
Conflict of interest: M.P. has consulted for Abbvie, Actavis, Akcea, Amgen, AstraZeneca, Boehringer Ingelheim, Cardiorentis, Daiichi Sankyo, Johnson & Johnson, NovoNordisk, Pfizer, Relypsa, Sanofi, Synthetic Biologics, and Theravance. He is the chair of the Executive Committee for the trial programme that is evaluating empagliflozin in chronic heart failure.
Footnotes
† doi: 10.1093/eurheartj/ehaa183.
The opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal or of the European Society of Cardiology.
References
- 1. Greene SJ, Butler J, Albert NM, DeVore AD, Sharma PP, Duffy CI, Hill CL, McCague K, Mi X, Patterson JH, Spertus JA, Thomas L, Williams FB, Hernandez AF, Fonarow GC. Medical therapy for heart failure with reduced ejection fraction: the CHAMP-HF registry. J Am Coll Cardiol 2018;72:351–366. [DOI] [PubMed] [Google Scholar]
- 2. Docherty KF, Jhund PS, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, DeMets DL, Sabatine MS, Bengtsson O, Sjöstrand M, Langkilde AM, Desai AS, Diez M, Howlett JG, Katova T, Ljungman CEA, O’Meara E, Petrie MC, Schou M, Verma S, Vinh PN, Solomon SD, McMurray JJV on behalf of the DAPA-HF Investigators and Committees. Effects of dapagliflozin in DAPA-HF according to background heart failure therapy. Eur Heart J 2020;41:2379–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fitchett D, Zinman B, Wanner C, Lachin JM, Hantel S, Salsali A, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME trial investigators. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME trial. Eur Heart J 2016;37:1526–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Packer M. Autophagy stimulation and intracellular sodium reduction as mediators of the cardioprotective effect of sodium–glucose cotransporter 2 inhibitors. Eur J Heart Fail 2020;doi: 10.1002/ejhf.1732. [DOI] [PubMed] [Google Scholar]
- 5. Packer M. SGLT2 inhibitors produce cardiorenal benefits by promoting adaptive cellular reprogramming to induce a state of fasting mimicry: a paradigm shift in understanding their mechanism of action. Diabetes Care 2020;43:508–511. [DOI] [PubMed] [Google Scholar]
- 6. Packer M. Interplay of adenosine monophosphate-activated protein kinase/sirtuin-1 activation and sodium influx inhibition mediates the renal benefits of sodium–glucose co-transporter-2 inhibitors in type 2 diabetes: a novel conceptual framework. Diabetes Obes Metab 2020;22:734–742 [DOI] [PubMed] [Google Scholar]
- 7. Wang J, Zhu XX, Liu L, Xue Y, Yang X, Zou HJ. SIRT1 prevents hyperuricemia via the PGC-1α/PPARγ-ABCG2 pathway. Endocrine 2016;53:443–452. [DOI] [PubMed] [Google Scholar]
- 8. Inzucchi SE, Zinman B, Fitchett D, Wanner C, Ferrannini E, Schumacher M, Schmoor C, Ohneberg K, Johansen OE, George JT, Hantel S, Bluhmki E, Lachin JM. How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA-REG OUTCOME trial. Diabetes Care 2018;41:356–363. [DOI] [PubMed] [Google Scholar]
- 9. Li JW, Woodward M, Perkovic V, Figtree GA, Heerspink HJL, Mahaffey KW, de Zeeuw D, Vercruysse F, Shaw W, Matthews DR, Neal B. Mediators of the effects of canagliflozin on heart failure in patients with type 2 diabetes. JACC Heart Fail 2020;8:57–66. [DOI] [PubMed] [Google Scholar]
- 10. Yuan Q, Zhou QY, Liu D, Yu L, Zhan L, Li XJ, Peng HY, Zhang XL, Yuan XC. Advanced glycation end-products impair Na+/K+-ATPase activity in diabetic cardiomyopathy: role of the adenosine monophosphate-activated protein kinase/sirtuin 1 pathway. Clin Exp Pharmacol Physiol 2014;41:127–133. [DOI] [PubMed] [Google Scholar]
- 11. Fulgencio JP, Kohl C, Girard J, Pégorier JP. Effect of metformin on fatty acid and glucose metabolism in freshly isolated hepatocytes and on specific gene expression in cultured hepatocytes. Biochem Pharmacol 2001;62:439–446. [DOI] [PubMed] [Google Scholar]
- 12. Nistala R, Raja A, Pulakat L. mTORC1 inhibitors rapamycin and metformin affect cardiovascular markers differentially in ZDF rats. Can J Physiol Pharmacol 2017;95:281–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ségalen C, Longnus SL, Baetz D, Counillon L, Van Obberghen E. 5-aminoimidazole-4-carboxamide-1-beta-d-ribofuranoside reduces glucose uptake via the inhibition of Na+/H+ exchanger 1 in isolated rat ventricular cardiomyocytes. Endocrinology 2008;149:1490–1498. [DOI] [PubMed] [Google Scholar]
- 14. Packer M. Concerns about the use of metformin as a first-line agent to slow the progression of chronic kidney disease in diabetes. Diabetes Res Clin Pract 2020;160:108024. [DOI] [PubMed] [Google Scholar]
- 15. Hawley SA, Ford RJ, Smith BK, Gowans GJ, Mancini SJ, Pitt RD, Day EA, Salt IP, Steinberg GR, Hardie DG. The Na+/glucose cotransporter inhibitor canagliflozin activates AMPK by inhibiting mitochondrial function and increasing cellular AMP levels. Diabetes 2016;65:2784–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]