Abstract
Aims
Hypertension is a well-established heart failure (HF) risk factor, especially in the context of adverse left ventricular (LV) remodelling. We aimed to use myocardial flow reserve (MFR) and global longitudinal strain (GLS), markers of subclinical microvascular and myocardial dysfunction, to refine hypertensive HF risk assessment.
Methods and results
Consecutive patients undergoing symptom-prompted stress cardiac positron emission tomography (PET)-computed tomography and transthoracic echocardiogram within 90 days without reduced left ventricular ejection fraction (<40%) or flow-limiting coronary artery disease (summed stress score ≥ 3) were included. Global MFR was quantified by PET, and echocardiograms were retrospectively analysed for cardiac structure and function. Patients were followed over a median 8.75 (Q1–3 4.56–10.04) years for HF hospitalization and a composite of death, HF hospitalization, MI, or stroke. Of 194 patients, 155 had adaptive LV remodelling while 39 had maladaptive remodelling, which was associated with lower MFR and impaired GLS. Across the remodelling spectrum, diastolic parameters, GLS, and N-terminal pro-B-type natriuretic peptide were independently associated with MFR. Maladaptive LV remodelling was associated with increased adjusted incidence of HF hospitalization and death. Importantly, the combination of abnormal MFR and GLS was associated with a higher rate of HF hospitalization compared to normal MFR and GLS [adjusted hazard ratio (HR) 3.21, 95% confidence interval (CI) 1.09–9.45, P = 0.034), including in the adaptive remodelling subset (adjusted HR 3.93, 95% CI 1.14–13.56, P = 0.030).
Conclusion
We have demonstrated important associations between coronary microvascular dysfunction and myocardial mechanics that refine disease characterization and HF risk assessment of patients with hypertension based on subclinical target organ injury.
Keywords: Coronary microvascular dysfunction, Hypertension, Heart failure
See page 2376 for the editorial comment on this article (doi: 10.1093/eurheartj/ehaa437)
Introduction
Hypertension is a common cardiovascular (CV) risk factor.1 Beyond associations with stroke and atherosclerosis, hypertension is the most common risk factor for heart failure (HF).1 , 2 Importantly, once hypertensive HF develops, survival is 24–31% at 5 years,2 highlighting the need for early identification and treatment of patients at risk. The development of left ventricular (LV) hypertrophy and maladaptive LV remodelling portends worse outcomes,1 , 3 including HF.4 However, LV remodelling is a late and heterogeneous finding3 , 5 in the natural history of hypertension that is inadequate for actionable clinical risk assessment.
The specific mechanisms by which LV hypertrophy and remodelling predispose to HF remain elusive. Myocardial structural changes have been associated with coronary microvascular dysfunction (CMD), ischaemia, and fibrosis.6 , 7 In addition, CMD has been linked to LV remodelling and systolic mechanical dysfunction in aortic stenosis8 and chronic kidney disease.9 However, there is limited data documenting the interactions between abnormalities in microvascular perfusion, structure, and function, and HF in hypertension. In this study, we performed a retrospective analysis of clinical multi-modality imaging to describe subclinical phenotypes in hypertensive heart disease and their association with adverse clinical outcomes.
Methods
Study population
Consecutive patients who underwent symptom-prompted clinical stress cardiac positron emission tomography (PET)-computed tomography (CT) and transthoracic echocardiography within 90 days of PET at Brigham and Women’s Hospital between 9 January 2006 and 31 October 2017 were included. Patient history, medication use, and ECG were obtained at the time of PET imaging. Patients who lacked natriuretic peptide measurement within 90 days of PET-CT, had perfusion abnormalities by PET suggestive of flow-limiting coronary artery disease (CAD) (summed stress score ≥ 3),10 or had a left ventricular ejection fraction (LVEF) < 40%10–12 were excluded (Figure 1). Patients with a clinical history of CAD, defined as prior myocardial infarction (MI), percutaneous coronary intervention (PCI), or coronary artery bypass grafting (CABG), were included if PET perfusion did not suggest flow-limiting disease. The study was approved by the Partners Healthcare Institutional Review Board and conducted in accordance with institutional guidelines.
Figure 1.
Patient flow diagram.
Circulating biomarkers
As specified, all patients had either B-type natriuretic peptide (BNP) (prior to 2010) or N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels and troponin levels measured within 90 days of PET. Details are available in the Supplementary material online. Analyses described herein are restricted to the subset with NT-proBNP (n = 119 of 194), the current standard of care.
Quantification of myocardial vascular function
Patients were imaged with 82Rubidium or 13N-ammonia PET-CT as described previously.8 , 10 Myocardial scar and ischaemia were evaluated by semiquantitative 17-segment visual assessment with a standard five-point scoring system. Absolute myocardial blood flow (MBF, mL/min/g) was quantified at rest and vasodilator-induced hyperaemia using a validated two-compartment kinetic model.13 Rest MBF was standardized for cardiac work by dividing by the resting rate pressure product/10 000. Corrected global myocardial flow reserve (MFR), a validated measure that integrates epicardial and microvascular function, was calculated as the ratio of stress MBF to corrected rest MBF. We used normal myocardial perfusion (summed stress score < 3) as a surrogate for the absence of focal obstructive CAD based on extensive, consistent evidence that a normal PET myocardial perfusion study has high sensitivity and negative predictive value for excluding focal obstructive CAD.14 , 15 On this basis, a reduced MFR was interpreted as diagnostic of CMD. This method is highly reproducible; in our laboratory, the intra-class correlation coefficient for MFR among four readers is 0.94 [95% confidence interval (CI) 0.88–0.98].16 Coronary microvascular dysfunction was defined as an MFR < 1.8.16 All reported MBF and MFR values reflect global measures.
Assessment of cardiac structure and function
Conventional 2D echocardiography was used to calculate relative wall thickness (RWT) and indexed LV mass for categorization of LV geometry17 into normal geometry, concentric remodelling, concentric hypertrophy, or eccentric hypertrophy. Normal geometry and concentric remodelling were classified as ‘adaptive remodelling’, whereas concentric and eccentric hypertrophy were classified as ‘maladaptive remodelling’. Details of diastolic function assessment are provided in the Supplementary material online.
Speckle tracking 2D echocardiography
Global longitudinal strain (GLS) was measured by speckle tracking 2D echocardiography (TomTec Imaging Systems, Germany). Further details of speckle tracking methodology are provided in the Supplementary material online. More negative values of GLS represent greater contractile function, with less negative values being more abnormal. A cut-off of GLS > −18% was used to define abnormal LV systolic mechanics.18
Clinical outcomes
Patients were followed for a median of 8.75 (Q1–Q3 4.56–10.04) years with follow-up censored on 30 June 2019. The primary outcome was the first occurrence of HF hospitalization. The main secondary outcome was the first occurrence of a composite of all-cause death, HF hospitalization, non-fatal MI, or stroke. Clinical endpoints were adjudicated by a blinded committee of cardiologists using the medical record, Partners Healthcare Research Patient Data Registry, and National Death Index. Heart failure hospitalization was defined by a primary discharge diagnosis of HF, clinical signs and/or symptoms, and escalation of HF therapy.19 Myocardial infarction was defined using the Fourth Universal Definition of MI20 and a hospital discharge diagnosis of MI. Hospitalization within 30 days of PET was excluded as an event.
Statistical analysis
As appropriate, continuous variables are reported as mean ± standard deviation, median [interquartile range], or log-transformed; baseline comparisons between adaptive vs. maladaptive remodelling was performed by Student’s t-test, Wilcoxon rank sum, or χ2.
Univariate and multivariable-adjusted relationships of MFR with indices of diastolic function, systolic function, and wall stress were assessed using linear regression models. Inclusion of covariates was based on clinical significance and univariable screening. Fully adjusted models included age, sex, body mass index (BMI), history of diabetes, history of CAD (MI, PCI, CABG), and rest LVEF by gated PET. Akaike information criterion was used to avoid over-fitting.
The relationship between adverse LV remodelling and clinical outcomes was assessed using Poisson regression. Cox proportional hazards (PH) models were used to examine the association of the combination of normal or abnormal MFR and GLS with HF hospitalization and with composite major adverse cardiac events (MACE) and all-cause death. The Cox PH assumptions test based on graphical methods and Schoenfeld residuals were used to verify PH assumptions. Two-sided P-values < 0.05 were considered statistically significant. Statistical analyses were performed using STATA version 15 (StataCorp, College Station, TX, USA).
Results
Baseline characteristics
Baseline characteristics of the overall cohort and stratified by LV remodelling are presented in Table 1. The study cohort had a mean age of 68 years, a mean BMI of 32 kg/m2, was 56.2% female, and included 59 patients (30%) with prior CAD. There were 155 patients with adaptive remodelling (n = 47 with normal geometry, n = 108 with concentric remodelling), and 39 with maladaptive remodelling (n = 29 with concentric hypertrophy, n = 10 with eccentric hypertrophy). Patients with maladaptive remodelling were more likely to report chest pain as an indication for PET. Although patients with maladaptive remodelling more frequently reported calcium channel blocker use, the total number of anti-hypertensive medications was not different between groups. Other CV history, medications, and systemic haemodynamics were similar (Table 1).
Table 1.
Baseline clinical, haemodynamic, and imaging characteristics
| Total cohort (n = 194) | LV remodelling |
P-value | ||
|---|---|---|---|---|
| Adaptive (n = 155) | Maladaptive (n = 39) | |||
| Demographics | ||||
| Age (years) | 68.52 (11.6) | 68.88 (11.6) | 67.10 (11.7) | 0.39 |
| Male | 85 (43.8) | 72 (46.5) | 13 (33.3) | 0.15 |
| Body mass index (kg/m2) | 32.13 (8.2) | 32.23 (8) | 31.75 (9.2) | 0.74 |
| Medical history | ||||
| CAD | 59 (30.4) | 51 (32.9) | 8 (20.5) | 0.17 |
| Prior MI | 27 (13.9) | 24 (15.5) | 3 (7.7) | 0.30 |
| Prior PCI | 40 (20.6) | 34 (21.9) | 6 (15.4) | 0.51 |
| Prior CABG | 19 (9.8) | 17 (11) | 2 (5.1) | 0.37 |
| Diabetes mellitus | 85 (43.8) | 64 (41.3) | 21 (53.8) | 0.21 |
| Dyslipidaemia | 143 (73.7) | 115 (74.2) | 28 (71.8) | 0.84 |
| Symptoms at PET | ||||
| Chest pain | 95 (49) | 82 (52.9) | 26 (66.7) | 0.03 |
| Dyspnoea | 97 (50) | 76 (49) | 21 (53.8) | 0.72 |
| Palpitations | 12 (6.2) | 7 (4.5) | 5 (12.8) | 0.07 |
| Medications | ||||
| Diuretics | 101 (52.1) | 83 (53.5) | 18 (46.2) | 0.47 |
| Beta blocker | 152 (78.4) | 120 (77.4) | 32 (82.1) | 0.67 |
| Calcium channel blocker | 73 (37.6) | 50 (32.2) | 23 (59) | 0.003 |
| ACEi/ARB | 116 (59.8) | 97 (62.6) | 19 (48.7) | 0.14 |
| Nitrates | 34 (17.5) | 25 (16.1) | 9 (23.1) | 0.35 |
| Aspirin | 143 (73.7) | 115 (74.2) | 28 (71.8) | 0.84 |
| Lipid lowering | 135 (70.7) | 108 (71.1) | 27 (69.2) | 0.85 |
| Number of antihypertensives, n (%) | ||||
| 0 | 2 (1) | 2 (1.3) | 0 | 0.59 |
| 1 | 33 (17) | 26 (16.8) | 7 (17.9) | |
| 2 | 67 (34.5) | 57 (36.8) | 10 (25.6) | |
| 3 | 62 (32) | 48 (31) | 14 (35.9) | |
| >3 | 30 (15.4) | 22 (14.2) | 8 (20.5) | |
| Resting haemodynamics | ||||
| Heart rate (b.p.m.) | 69.7 (13.2) | 69.1 (12.9) | 71.8 (14.6) | 0.26 |
| Systolic BP (mmHg) | 149.9 (25.8) | 147.9 (25.1) | 158.1 (27.1) | 0.025 |
| Diastolic BP (mmHg) | 72.9 (13.0) | 72.6 (12.2) | 74.4 (16.0) | 0.44 |
| Mean arterial pressure (mmHg) | 98.6 (15.4) | 97.7 (14.7) | 102.3 (17.6) | 0.092 |
| Rate pressure product | 11 979.2 (4208.5) | 11 967.4 (433.4) | 12 025.8 (3824.1) | 0.94 |
| Stress haemodynamics | ||||
| Heart rate (b.p.m.) | 85.4 (19.8) | 86.3 (20.3) | 81.6 (17.8) | 0.19 |
| Systolic BP (mmHg) | 139.5 (28.2) | 137.8 (27.4) | 146.5 (30.6) | 0.084 |
| Diastolic BP (mmHg) | 65.8 (13.0) | 65.4 (11.6) | 67.4 (17.4) | 0.39 |
| Mean arterial pressure (mmHg) | 90.4 (16.3) | 89.5 (15.0) | 93.8 (20.4) | 0.15 |
| PET quantitative myocardial blood flow (MBF) | ||||
| Rest MBF (mL/min/g) | 1.09 (0.45) | 1.11 (0.44) | 1.02 (0.46) | 0.29 |
| Corrected rest MBF (mL/min/g) | 1.09 (0.47) | 1.13 (0.47) | 0.93 (0.42) | 0.017 |
| Stress MBF (mL/min/g) | 1.91 (0.81) | 2.01 (0.81) | 1.50 (0.69) | <0.001 |
| Uncorrected MFR | 1.83 (0.66) | 1.90 (0.67) | 1.54 (0.55) | 0.002 |
| Corrected MFR | 1.86 (0.69) | 1.90 (0.70) | 1.70 (0.60) | 0.10 |
| Echocardiography structure and function | ||||
| Time between PET and echocardiogram (days) | 1.3 [0.4–4.1] | 1.2 [0.4–5.0] | 1.3 [0.4–3.4] | 0.71 |
| LVEF (%) | 58.0 (9.7) | 58.9 (9.5) | 53.0 (9.9) | 0.038 |
| LVEDVi (mL/m2) | 44.4 (6.9) | 43.1 (6.1) | 49.8 (7.1) | <0.001 |
| LVESVI (mL/m2) | 30.4 (6.2) | 29.2 (5.5) | 36.2 (6.6) | <0.001 |
| LVSd (cm) | 1.10 (0.23) | 1.05 (0.20) | 1.30 (0.26) | <0.001 |
| PWd (cm) | 1.07 (0.22) | 1.03 (0.17) | 1.28 (0.28) | <0.001 |
| RWT | 0.50 (0.15) | 0.49 (0.12) | 0.54 (0.22) | 0.065 |
| LV mass index, (g/m2) | 86.1 (29.3) | 75.3 (17.8) | 129.1 (26.7) | <0.001 |
| Septal E/e′ | 14.50 (6.39) | 13.74 (5.95) | 17.40 (7.24) | 0.002 |
| Lateral E/e′ | 11.89 (6.81) | 11.20 (6.63) | 14.48 (6.93) | 0.010 |
| Average E/e′ | 12.74 (5.88) | 12.01 (5.50) | 15.62 (6.52) | 0.001 |
| Left atrial volume (mL/m2) | 70.17 (26.56) | 67.96 (24.02) | 93.81 (30.20) | <0.001 |
| Average GLS (%) | −16.42 (4.64) | −16.92 (4.68) | −14.46 (3.93) | 0.003 |
| Laboratory values | ||||
| Time between PET and natriuretic peptide measurement (days) | 1.8 [0.6–7.6] | 1.2 [0.4–5.0] | 1.3 [0.4–3.4] | 0.71 |
| Natriuretic peptides | ||||
| NT-proBNP (pg/mL) (n = 119) | 626.5 [146–1427] | 506 [132–1159] | 2707 [904–10 222] | <0.001 |
| BNP (pg/mL) (n = 83) | 147 [41–284] | 112 [37–250] | 231.5 [132–413] | 0.019 |
Values are mean (standard deviation) or median [interquartile range]. CAD included prior myocardial infarction (MI), percutaneous coronary intervention (PCI), or coronary artery bypass grafting (CABG).
ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; GLS, global longitudinal strain; LVEDVi, LV end-diastolic volume indexed to body surface area (BSA); LVEF, LV ejection fraction (Simpson’s biplane); LVESVi, LV end-systolic volume indexed to BSA; LVSd, LV septal thickness in end-diastole; MFR, myocardial flow reserve; PWd, Posterior wall thickness in end-diastole; RWT, relative wall thickness.
Association between left ventricular remodelling, myocardial flow reserve, and left ventricular mechanics
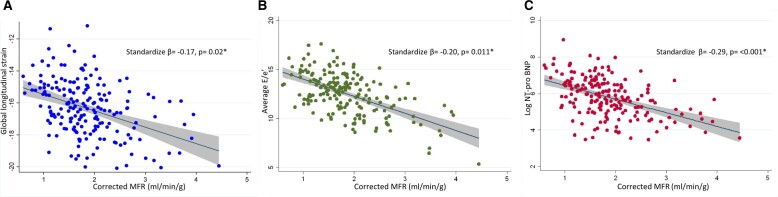
As expected, patients with maladaptive remodelling had greater LV septal wall thickness, posterior wall thickness, and indexed LV mass (Table 1). In addition, patients with maladaptive remodelling had worse diastolic dysfunction, including higher average E/e′, larger left atrial volumes, and higher natriuretic peptide levels, suggestive of elevated filling pressures (Table 1). Maladaptive remodelling was also associated with worse GLS (−14.46 ± 3.93 vs. −16.92 ± 4.68%, P = 0.003), lower peak hyperaemic MBF (1.50 ± 0.69 vs. 2.01 ± 0.81 mL/min/g, P < 0.001), and lower MFR (1.54 (± 0.55) vs. 1.90 (± 0.67), P = 0.002) (Table 1). Average E/e′, GLS, and log NT-proBNP, a proxy for wall stress,21 were significantly associated with global MFR in multivariable-adjusted models, such that a reduced MFR correlated with worse myocardial mechanics and more elevated wall stress (Table 2, Figure 2).
Table 2.
Association between myocardial flow reserve, diastolic dysfunction, left ventricular systolic mechanics, and wall stress
| Association with corrected global MFR |
||||
|---|---|---|---|---|
| Unadjusted |
Adjusted* |
|||
| Standardized β coefficient | P-value | Standardized β coefficient | P-value | |
| Diastolic indices | ||||
| Lateral E/e′ | −0.20 | 0.008 | −0.18 | 0.019 |
| Septal E/e′ | −0.17 | 0.027 | −0.17 | 0.024 |
| Average E/e′ | −0.21 | 0.007 | −0.20 | 0.011 |
| Systolic indices | ||||
| GLS | −0.16 | 0.021 | −0.17 | 0.020 |
| LVEF | −0.10 | 0.188 | −0.10 | 0.503 |
| Wall stress | ||||
| Log NT-proBNP | −0.32 | <0.001 | −0.31 | <0.001 |
| Log BNP | −0.14 | 0.222 | −0.18 | 0.114 |
Beta coefficients and P-values are from linear regression models. Adjusted for age, sex, body mass index, diabetes, coronary artery disease (MI, PCI, or CABG), and left ventricular ejection fraction (LVEF).
GLS, global longitudinal strain; MFR, myocardial flow reserve.
Figure 2.
Myocardial flow reserve is associated with global longitudinal strain (A), E/e′ (B), and log NT-proBNP (C) independent of clinical risk factors and systolic function. *Adjusted for age, sex, body mass index, history of diabetes, history of coronary artery disease (myocardial infarction, percutaneous coronary intervention, or coronary artery bypass grafting), and left ventricular ejection fraction. GLS, global longitudinal strain; MFR, myocardial flow reserve.
Association between left ventricular structural and functional abnormalities, coronary microvascular dysfunction, and adverse cardiovascular outcomes
There were 53 HF hospitalizations. The secondary outcome of composite MACE (n = 106) included 48 deaths, 53 hospitalizations for HF, 2 for non-fatal MI, and 3 for stroke. Compared to adaptive remodelling, maladaptive LV remodelling was associated with increased adjusted annualized incidence rates of HF hospitalization (6.77 vs. 3.41 events/year, P < 0.001) and of composite MACE (13.95 vs. 7.45 events/year, P < 0.001) and all-cause death (8.40 vs. 5.34 events/year, P = 0.019).
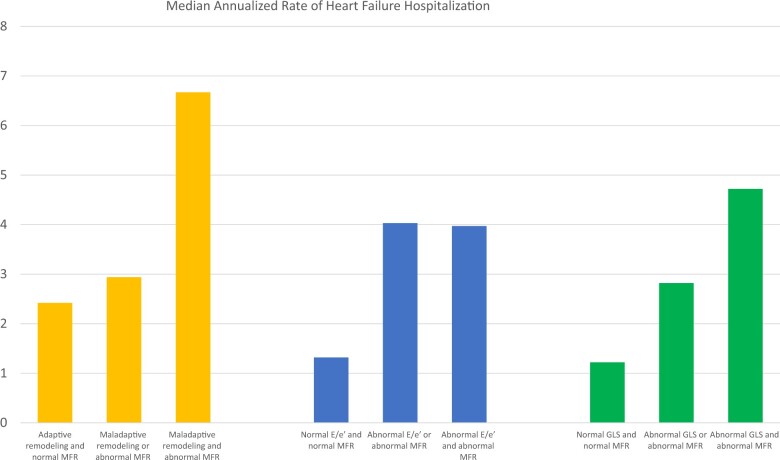
We then turned our attention to the potential role of CMD in the pathway of adverse LV remodelling and HF events. The presence of CMD concurrent with abnormalities in cardiac structure, diastolic function, or subclinical systolic mechanics was associated with significantly higher adjusted annualized rate of HF hospitalization (all P < 0.001) (Figure 3, Supplementary material online, Table S1) and lower survival free of HF hospitalization, independent of remodelling severity and clinical risk factors [adjusted hazard ratio (HR) 3.21, 95% CI 1.09–9.45, P = 0.034] (Figure 4). There was no significant association between abnormal MFR and GLS and risk of composite MACE or all-cause death (Supplementary material online, Table S2).
Figure 3.
Adjusted* median annualized rates of heart failure hospitalization by left ventricular structure or function and myocardial flow reserve. *Adjusted for age, sex, history of diabetes, and history of coronary artery disease (myocardial infarction, percutaneous coronary intervention, or coronary artery bypass grafting). Myocardial flow reserve: normal myocardial flow reserve ≥ 1.8, abnormal myocardial flow reserve < 1.8. Diastolic function: normal E/e′ ≤ 15, abnormal E/e′ > 15. Global longitudinal strain: normal GLS ≤ −18%, abnormal GLS > −18%.
Figure 4.
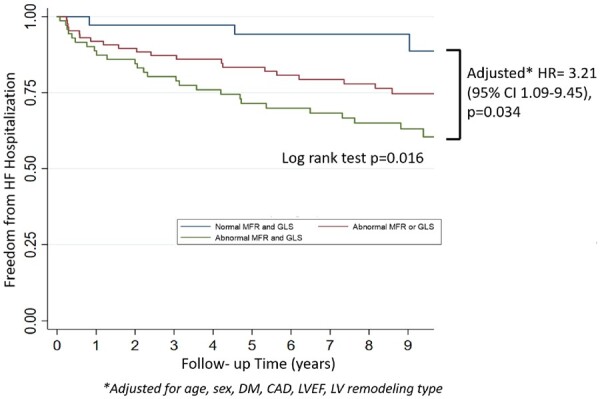
Freedom from heart failure hospitalization over a median follow-up time of 8.75 years. *Adjusted for age, sex, history of diabetes, and history of coronary artery disease (myocardial infarction, percutaneous coronary intervention, or coronary artery bypass grafting), left ventricular ejection fraction, and left ventricular remodelling type. The Kaplan–Meier survival curves are shown for heart failure hospitalization with patients categorized by normal vs. abnormal myocardial flow reserve (MFR ≥ 1.8 vs. <1.8) and normal vs. abnormal global longitudinal strain (GLS ≤ −18% vs. >−18%). Hazard ratios and P-values are displayed from Cox proportional hazard models comparing those with both abnormal vs. both normal myocardial flow reserve and global longitudinal strain.
Finally, in an analysis stratified by adaptive vs maladaptive remodelling, the association between CMD, abnormal systolic mechanics, and HF hospitalization was present only in patients with adaptive LV remodelling (adjusted HR 3.93, 95% CI 1.14–13.56, P = 0.030) and not in those who already displayed maladaptive remodelling (Take home figure), supporting a prognostic role of subclinical markers of target organ dysfunction before overt adverse remodelling has developed.
Take home figure.
The Kaplan–Meier survival curves heart failure HF hospitalization based on adaptive vs. maladaptive remodelling. *Adjusted for age, sex, history of diabetes, and history of coronary artery disease (myocardial infarction, percutaneous coronary intervention, or coronary artery bypass grafting), and left ventricular ejection fraction. The presence of subclinical injury is associated with a higher risk of heart failure hospitalization in the context of adaptive remodelling (left panel) but not once maladaptive remodelling has developed (right panel).
Discussion
We have demonstrated that, in patients with hypertension and without reduced LVEF or flow-limiting epicardial CAD, CMD and subclinical myocardial mechanical dysfunction are predictive of HF hospitalization independent of overt adverse LV remodelling. In patients with apparently adaptive remodelling, reduced MFR and GLS predicted risk of HF hospitalization and thus should be considered more sensitive metrics of hypertensive cardiac target organ damage.
These results build on prior findings supporting the significance of CMD and its relationship to cardiac structure, function, and outcomes. Coronary microvascular dysfunction may be the aetiology for angina in patients with hypertension and LVH without epicardial obstruction22 and serves as a powerful prognostic indicator for CV events in patients with diabetes,23 obesity,19 aortic stenosis,8 and chronic kidney disease.9 Additionally, CMD was independently associated both with markers of diastolic dysfunction and adverse CV events, particularly HF hospitalization.10 Further, CMD11 assessed by transthoracic Doppler echocardiography, another modality for measuring MFR,24 was highly prevalent in a large prospective cohort with HF with preserved EF and associated with right ventricular function.
Notably, in hypertension, both haemodynamic and non-haemodynamic effects may contribute to LV remodelling and HF hospitalization risk, precipitated by changes in microvascular function. The haemodynamic load of hypertension may lead to downstream impairment of coronary microcirculation and myocardial perfusion.25 , 26 In aortic stenosis, similar relationships between alterations in myocardial perfusion and systolic mechanical function have been identified8 and may reflect a common pathway of injury and myocardial adaptation to elevated afterload that precede clinical events. Non-haemodynamic factors characterized by the sympathetic nervous system, renin–angiotensin–aldosterone system, and oxidative stress have also been hypothesized to contribute to the interplay of CMD and cardiac remodelling.27–29 Thus, CMD may play an integral role across haemodynamic and non-haemodynamic causes of LV remodelling in hypertension.
Exactly how CMD and LV remodelling lead to increased risk of HF hospitalization cannot be determined from this study. Prior studies have proposed a cycle of repetitive myocardial injury via endothelial dysfunction, chronic subendocardial ischaemia, capillary rarefaction, and fibrosis leading to subclinical alterations in myocardial mechanical function5 , 10 , 30 as a mechanistic explanation for the relationship between myocardial perfusion, structure, function, and clinical HF. In the current study, the prognostic value of abnormal MFR and GLS was substantially less in patients with maladaptive remodelling (Take home figure), potentially because the impact of CMD is eclipsed by established fibrosis at later stages of disease.
While identifying subclinical hypertensive target organ damage is important for the recognition of risk, the ultimate goal is to inform treatment. Intensive blood pressure lowering has been convincingly demonstrated to reduce CV events,31 including through the prevention of HF.32 However, residual risk remains,31 , 33 potentially mediated by non-haemodynamic mechanisms of microvascular dysfunction. Several therapies, including both classical antihypertensives and non-antihypertensives such as statins and ranolazine, have been tested for the treatment of CMD.34 Whether treatment focused on the coronary microvasculature can be translated into the prevention of HF development and hospitalization warrants further investigation.
Strengths and limitations
Our study has several strengths, including state-of-the-art imaging analysis and follow-up for adjudicated clinical events; however, the findings should be taken in the context of several limitations. First, this is a retrospective, single-centre analysis with PET scans and echocardiograms obtained for clinical symptoms introduces potential selection bias. However, patients may better reflect a clinical population, supporting generalizability. Although the routine assessment of MFR and GLS is not advisable in all patients with hypertension, the findings provide a rationale for future studies. Second, data on hypertension duration and control at the time of PET are unavailable. However, there were no significant differences in the number of antihypertensives by remodelling profile. Similarly, longitudinal clinical details of medical therapy and adjunctive vascular function metrics that may inform HF hospitalization risk were not ascertained. However, if a finding of abnormal MFR did influence subsequent treatment intensity, this would be expected to bias the results toward the null, with more aggressive treatment in those with more abnormal MFR potentially mitigating the progression to HF hospitalization. Third, coronary angiography was not available in all patients. However, we excluded patients with perfusion defects indicating focal flow-limiting coronary stenosis, a highly sensitive method for diagnosis of obstructive CAD. Finally, while outcomes were assessed longitudinally, the cross-sectional imaging data do not allow for assessment of the evolution of LV remodelling. Prospective studies are needed to examine the impact of CMD on myocardial structure and function with serial imaging and whether using these subclinical markers to select high-risk patients for intensive and/or novel therapies can prevent HF.
In conclusion, we have shown important associations between CMD and myocardial mechanics that refine both disease characterization and risk assessment of patients with hypertension on the basis of subclinical target organ injury. Given the high and growing prevalence of hypertension and hypertension-mediated HF, efforts to improve risk stratification and identify mechanisms of risk have the potential for substantial impact on resource allocation, therapeutic intervention, and patient outcomes.
Funding
National Institutes of Health (5T32HL094301 to W.Z., A.C., S.D., and BW), (T32HL007604 to J.M.B.), (K23HL135438 to V.R.T.), and (R01HL132021 to M.D.C.).
Conflict of interest: W.Z. is a full-time U.S. federal government employee. S.D. reports personal fees from GE Healthcare and grants and fees from Pfizer and Proclara. R.B. reports grants from Amgen and Astellas. M.F.D.C. reports grants from Gilead Sciences and Spectrum Dynamics and consulting honoraria from Bayer and Janssen. P.O. reports support from Medtrace. The other authors declared that they have no relevant relationships to disclose.
Supplementary Material
Contributor Information
Wunan Zhou, Cardiology Branch, Division of Intramural Research, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD 20814, USA; Cardiovascular Imaging Program, Division of Nuclear Medicine and Molecular Imaging, Department of Radiology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA.
Jenifer M Brown, Division of Cardiovascular Medicine, Department of Medicine, Heart and Vascular Center, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA.
Navkaranbir S Bajaj, Division of Cardiovascular Disease, Department of Medicine, University of Alabama at Birmingham, Birmingham, AL 35294, USA.
Alvin Chandra, Division of Cardiology, Department of Internal Medicine, University of Texas Southwestern Medical Center, Dallas, TX 75390, USA.
Sanjay Divakaran, Division of Cardiovascular Medicine, Department of Medicine, Heart and Vascular Center, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA.
Brittany Weber, Cardiovascular Imaging Program, Division of Nuclear Medicine and Molecular Imaging, Department of Radiology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA.
Courtney F Bibbo, Cardiovascular Imaging Program, Division of Nuclear Medicine and Molecular Imaging, Department of Radiology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA.
Jon Hainer, Cardiovascular Imaging Program, Division of Nuclear Medicine and Molecular Imaging, Department of Radiology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA.
Viviany R Taqueti, Cardiovascular Imaging Program, Division of Nuclear Medicine and Molecular Imaging, Department of Radiology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA; Division of Cardiovascular Medicine, Department of Medicine, Heart and Vascular Center, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA.
Sharmila Dorbala, Cardiovascular Imaging Program, Division of Nuclear Medicine and Molecular Imaging, Department of Radiology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA; Division of Cardiovascular Medicine, Department of Medicine, Heart and Vascular Center, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA.
Ron Blankstein, Cardiovascular Imaging Program, Division of Nuclear Medicine and Molecular Imaging, Department of Radiology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA; Division of Cardiovascular Medicine, Department of Medicine, Heart and Vascular Center, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA.
Dale Adler, Division of Cardiovascular Medicine, Department of Medicine, Heart and Vascular Center, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA.
Patrick O’Gara, Division of Cardiovascular Medicine, Department of Medicine, Heart and Vascular Center, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA.
Marcelo F Di Carli, Cardiovascular Imaging Program, Division of Nuclear Medicine and Molecular Imaging, Department of Radiology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA; Division of Cardiovascular Medicine, Department of Medicine, Heart and Vascular Center, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA.
References
- 1. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I, De Backer G, Heagerty AM, Agewall S, Bochud M, Borghi C, Boutouyrie P, Brguljan J, Bueno H, Caiani EG, Carlberg B, Chapman N, Cífková R, Cleland JGF, Collet J-P, Coman IM, de Leeuw PW, Delgado V, Dendale P, Diener H-C, Dorobantu M, Fagard R, Farsang C, Ferrini M, Graham IM, Grassi G, Haller H, Hobbs FDR, Jelakovic B, Jennings C, Katus HA, Kroon AA, Leclercq C, Lovic D, Lurbe E, Manolis AJ, McDonagh TA, Messerli F, Muiesan ML, Nixdorff U, Olsen MH, Parati G, Perk J, Piepoli MF, Polonia J, Ponikowski P, Richter DJ, Rimoldi SF, Roffi M, Sattar N, Seferovic PM, Simpson IA, Sousa-Uva M, Stanton AV, van de Borne P, Vardas P, Volpe M, Wassmann S, Windecker S, Zamorano JL, Windecker S, Aboyans V, Agewall S, Barbato E, Bueno H, Coca A, Collet J-P, Coman IM, Dean V, Delgado V, Fitzsimons D, Gaemperli O, Hindricks G, Iung B, Jüni P, Katus HA, Knuuti J, Lancellotti P, Leclercq C, McDonagh TA, Piepoli MF, Ponikowski P, Richter DJ, Roffi M, Shlyakhto E, Simpson IA, Sousa-Uva M, Zamorano JL, Tsioufis C, Lurbe E, Kreutz R, Bochud M, Rosei EA, Jelakovic B, Azizi M, Januszewics A, Kahan T, Polonia J, van de Borne P, Williams B, Borghi C, Mancia G, Parati G, Clement DL, Coca A, Manolis A, Lovic D, Benkhedda S, Zelveian P, Siostrzonek P, Najafov R, Pavlova O, De Pauw M, Dizdarevic-Hudic L, Raev D, Karpettas N, Linhart A, Olsen MH, Shaker AF, Viigimaa M, Metsärinne K, Vavlukis M, Halimi J-M, Pagava Z, Schunkert H, Thomopoulos C, Páll D, Andersen K, Shechter M, Mercuro G, Bajraktari G, Romanova T, Trušinskis K, Saade GA, Sakalyte G, Noppe S, DeMarco DC, Caraus A, Wittekoek J, Aksnes TA, Jankowski P, Polonia J, Vinereanu D, Baranova EI, Foscoli M, Dikic AD, Filipova S, Fras Z, Bertomeu-Martínez V, Carlberg B, Burkard T, Sdiri W, Aydogdu S, Sirenko Y, Brady A, Weber T, Lazareva I, Backer TD, Sokolovic S, Jelakovic B, Widimsky J, Viigimaa M, Pörsti I, Denolle T, Krämer BK, Stergiou GS, Parati G, Trušinskis K, Miglinas M, Gerdts E, Tykarski A, de Carvalho Rodrigues M, Dorobantu M, Chazova I, Lovic D, Filipova S, Brguljan J, Segura J, Gottsäter A, Pechère-Bertschi A, Erdine S, Sirenko Y, Brady A; ESC Scientific Document Group. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 2018;39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 2. Levy D, Larson MG, Vasan RS, Kannel WB, Ho K. The progression from hypertension to congestive heart failure. J Am Med Assoc 1996;275:1557–1562. [PubMed] [Google Scholar]
- 3. Shah AM, Cikes M, Prasad N, Li G, Getchevski S, Claggett B, Rizkala A, Lukashevich I, O’Meara E, Ryan JJ, Shah SJ, Mullens W, Zile MR, Lam CSP, McMurray JJV, Solomon SD. Echocardiographic features of patients with heart failure and preserved left ventricular ejection fraction. J Am Coll Cardiol 2019;74:2858–2873. [DOI] [PubMed] [Google Scholar]
- 4. Peters MN, Seliger SL, Christenson RH, Hong-Zohlman SN, Daniels LB, Lima JAC, de Lemos JA, Neeland IJ, de Filippi CR. ‘Malignant’ left ventricular hypertrophy identifies subjects at high risk for progression to asymptomatic left ventricular dysfunction, heart failure, and death: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Heart Assoc 2018;7. pii: e006619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Drazner MH. The progression of hypertensive heart disease. Circulation 2011;123:327–334. [DOI] [PubMed] [Google Scholar]
- 6. Raman SV. The hypertensive heart. J Am Coll Cardiol 2010;55:91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med 2007;356:830–840. [DOI] [PubMed] [Google Scholar]
- 8. Zhou W, Bajaj N, Gupta A, Sun YP, Divakaran S, Bibbo C, Hainer J, Taqueti V, Dorbala S, Blankstein R, Shah P, Kaneko T, Adler D, O’Gara P, Carli MD. Coronary microvascular dysfunction, left ventricular remodeling, and clinical outcomes in aortic stenosis. J Nucl Cardiol 2019;doi: 10.1007/s12350-019-01706-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bajaj NS, Singh A, Zhou W, Gupta A, Fujikura K, Byrne C, Harms HJ, Osborne MT, Bravo P, Andrikopolou E, Divakaran S, Bibbo CF, Hainer J, Skali H, Taqueti V, Steigner M, Dorbala S, Charytan DM, Prabhu SD, Blankstein R, Deo RC, Solomon SD, Di Carli MF. Coronary microvascular dysfunction, left ventricular remodeling and clinical outcomes in patients with chronic kidney impairment. Circulation 2020;141:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Taqueti VR, Solomon SD, Shah AM, Desai AS, Groarke JD, Osborne MT, Hainer J, Bibbo CF, Dorbala S, Blankstein R, Carli MD. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur Heart J 2018;39:840–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shah SJ, Lam CSP, Svedlund S, Saraste A, Hage C, Tan RS, Beussink-Nelson L, Faxén UL, Fermer ML, Broberg MA, Gan LM, Lund LH. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur Heart J 2018;39:3439–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola V-P, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; Authors/Task Force Members; Document Reviewers. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur J Heart Fail 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 13. Fakhri G, El Kardan A, Sitek A, Dorbala S, Abi-Hatem N, Lahoud Y, Fischman A, Coughlan M, Yasuda T, Carli MD. Reproducibility and accuracy of quantitative myocardial blood flow assessment with 82Rb PET: comparison with 13N-ammonia PET. J Nucl Med 2009;50:1062–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Parker MW, Iskandar A, Limone B, Perugini A, Kim H, Jones C, Calamari B, Coleman CI, Heller GV. Diagnostic accuracy of cardiac positron emission tomography versus single photon emission computed tomography for coronary artery disease: a bivariate meta-analysis. Circ Cardiovasc Imaging 2012;5:700–707. [DOI] [PubMed] [Google Scholar]
- 15. Takx RAP, Blomberg BA, Aidi H, El Habets J, Jong PA, De Nagel E, Hoffmann U, Leiner T. Diagnostic accuracy of stress myocardial perfusion imaging compared to invasive coronary angiography with fractional flow reserve meta-analysis. Circ Cardiovasc Imaging 2014;8:7. [DOI] [PubMed] [Google Scholar]
- 16. Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Carli G, Di Blankstein R, Dorbala S, Sitek A, Pencina MJ, Carli MD. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation 2011;124:2215–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt J-U. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 18. Marwick TH, Leano RL, Brown J, Sun JP, Hoffmann R, Lysyansky P, Becker M, Thomas JD. Myocardial strain measurement with 2-dimensional speckle-tracking echocardiography. Definition of normal range. JACC Cardiovasc Imaging 2009;2:80–84. [DOI] [PubMed] [Google Scholar]
- 19. Bajaj NS, Osborne MT, Gupta A, Tavakkoli A, Bravo PE, Vita T, Bibbo CF, Hainer J, Dorbala S, Blankstein R, Bhatt DL, Di Carli MF, Taqueti VR. Coronary microvascular dysfunction and cardiovascular risk in obese patients. J Am Coll Cardiol 2018;72:707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD. Fourth Universal Definition of Myocardial Infarction (2018). J Am Coll Cardiol 2018;72:2231–2264. [DOI] [PubMed] [Google Scholar]
- 21. Krittayaphong R, Boonyasirinant T, Saiviroonporn P, Thanapiboonpol P, Nakyen S, Udompunturak S. Correlation between NT-Pro BNP levels and left ventricular wall stress, sphericity index and extent of myocardial damage: a magnetic resonance imaging study. J Card Fail 2008;14:687–694. [DOI] [PubMed] [Google Scholar]
- 22. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ, Neumann F-J, Sechtem U, Banning AP, Bonaros N, Bueno H, Bugiardini R, Chieffo A, Crea F, Czerny M, Delgado V, Dendale P, Flachskampf FA, Gohlke H, Grove EL, James S, Katritsis D, Landmesser U, Lettino M, Matter CM, Nathoe H, Niessner A, Patrono C, Petronio AS, Pettersen SE, Piccolo R, Piepoli MF, Popescu BA, Räber L, Richter DJ, Roffi M, Roithinger FX, Shlyakhto E, Sibbing D, Silber S, Simpson IA, Sousa-Uva M, Vardas P, Witkowski A, Zamorano JL, Achenbach S, Agewall S, Barbato E, Bax JJ, Capodanno D, Cuisset T, Deaton C, Dickstein K, Edvardsen T, Escaned J, Funck-Brentano C, Gersh BJ, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Prescott E, Saraste A, Storey RF, Svitil P, Valgimigli M, Windecker S, Aboyans V, Baigent C, Collet J-P, Dean V, Delgado V, Fitzsimons D, Gale CP, Grobbee D, Halvorsen S, Hindricks G, Iung B, Jüni P, Katus HA, Landmesser U, Leclercq C, Lettino M, Lewis BS, Merkely B, Mueller C, Petersen S, Petronio AS, Richter DJ, Roffi M, Shlyakhto E, Simpson IA, Sousa-Uva M, Touyz RM, Benkhedda S, Metzler B, Sujayeva V, Cosyns B, Kusljugic Z, Velchev V, Panayi G, Kala P, Haahr-Pedersen SA, Kabil H, Ainla T, Kaukonen T, Cayla G, Pagava Z, Woehrle J, Kanakakis J, Tóth K, Gudnason T, Peace A, Aronson D, Riccio C, Elezi S, Mirrakhimov E, Hansone S, Sarkis A, Babarskiene R, Beissel J, Maempel AJC, Revenco V, de Grooth GJ, Pejkov H, Juliebø V, Lipiec P, Santos J, Chioncel O, Duplyakov D, Bertelli L, Dikic AD, Studenčan M, Bunc M, Alfonso F, Bäck M, Zellweger M, Addad F, Yildirir A, Sirenko Y, Clapp B; ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020;41:407–477. [DOI] [PubMed] [Google Scholar]
- 23. Murthy VL, Naya M, Foster CR, Gaber M, Hainer J, Klein J, Dorbala S, Blankstein R, Carli MF. Di Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation 2012;126:1858–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C, Ferreira JR, Gersh BJ, Gitt AK, Hulot J-S, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabaté M, Senior R, Taggart DP, van der Wall EE, Vrints CJM, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Knuuti J, Valgimigli M, Bueno H, Claeys MJ, Donner-Banzhoff N, Erol C, Frank H, Funck-Brentano C, Gaemperli O, Gonzalez-Juanatey JR, Hamilos M, Hasdai D, Husted S, James SK, Kervinen K, Kolh P, Kristensen SD, Lancellotti P, Maggioni AP, Piepoli MF, Pries AR, Romeo F, Rydén L, Simoons ML, Sirnes PA, Steg PG, Timmis A, Wijns W, Windecker S, Yildirir A, Zamorano JL; Document Reviewers. 2013 ESC guidelines on the management of stable coronary artery disease. Eur Heart J 2013;34:2949–3003. [DOI] [PubMed] [Google Scholar]
- 25. Brush JE, Cannon RO, Schenke WH, Bonow RO, Leon MB, Maron BJ, Epstein SE. Angina due to coronary microvascular disease in hypertensive patients without left ventricular hypertrophy. N Engl J Med 1988;319:1302–1307. [DOI] [PubMed] [Google Scholar]
- 26. Levy BI, Ambrosio G, Pries AR, Struijker-Boudier H. Microcirculation in hypertension: a new target for treatment? Circulation 2001;104:735–740. [DOI] [PubMed] [Google Scholar]
- 27. Scholten BJ, Von Hansen CS, Hasbak P, Kjaer A, Rossing P, Hansen TW. Cardiac autonomic function is associated with the coronary microcirculatory function in patients with type 2 diabetes. Diabetes 2016;65:3129–3138. [DOI] [PubMed] [Google Scholar]
- 28. Vaccarino V, Khan D, Votaw J, Faber T, Veledar E, Jones DP, Goldberg J, Raggi P, Quyyumi AA, Bremner JD. Inflammation is related to coronary flow reserve detected by positron emission tomography in asymptomatic male twins. J Am Coll Cardiol 2011;57:1271–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Garg R, Rao AD, Baimas-George M, Hurwitz S, Foster C, Shah RV, Jerosch-Herold M, Kwong RY, Di Carli MF, Adler GK. Mineralocorticoid receptor blockade improves coronary microvascular function in individuals with type 2 diabetes. Diabetes 2015;64:236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Olivotto I, Cecchi F, Gistri R, Lorenzoni R, Chiriatti G, Girolami F, Torricelli F, Camici PG. Relevance of coronary microvascular flow impairment to long-term remodeling and systolic dysfunction in hypertrophic cardiomyopathy. J Am Coll Cardiol 2006;47:1043–1048. [DOI] [PubMed] [Google Scholar]
- 31. Wright JT SPRINT Research GroupWilliamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Arnold JMO, Yusuf S, Young J, Mathew J, Johnstone D, Avezum A, Lonn E, Pogue J, Bosch J. Prevention of heart failure in patients in the Heart Outcomes Prevention Evaluation (HOPE) study. Circulation 2003;107:1284–1290. [DOI] [PubMed] [Google Scholar]
- 33. Nadir MA, Rekhraj S, Wei L, Lim TK, Davidson J, MacDonald TM, Lang CC, Dow E, Struthers AD. Improving the primary prevention of cardiovascular events by using biomarkers to identify individuals with silent heart disease. J Am Coll Cardiol 2012;60:960–968. [DOI] [PubMed] [Google Scholar]
- 34. Marinescu MA, Löffler AI, Ouellette M, Smith L, Kramer CM, Bourque JM. Coronary microvascular dysfunction, microvascular angina, and treatment strategies. JACC Cardiovasc Imaging 2015;8:210–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.