Abstract
Shenling Baizhu additive powder (SLBZ-AP), a formulation of a variety of natural medicinal plants, has clinical efficacy in treating cancers in previous studies. We explored the effect of SLBZ-AP in bone metastasis of lung cancer (BMLC) mice, and the possible mechanism involved was further investigated in the present study. Mice model of BMLC was made and treated with SLBZ-AP. Pain behavioral tests were performed to explore the effect on BMLC-induced pain in mice. TUNEL staining was used to investigate apoptosis. The mRNA expression of markers in the PI3K/Akt/mTOR pathway was measured by qPCR, and protein expression was detected by western blotting and immunohistochemistry analysis. SLBZ-AP relieved BMLC-induced pain and prolonged animals' survival, promoted cell apoptosis in the marrow from the tibia of BMLC mice, and inhibited mRNA and protein expression of AKT, mTOR, P70S6, and VEGF, as well as protein expression of p-AKT, p-mTOR, p-P70S6, and VEGF upregulation in the marrow of tibia induced by BMLC, an effect which was similar to rapamycin. Our results suggested that SLBZ-AP may have antinociceptive effect and prolong survival of BMLC mice at least partially by inhibiting cell proliferation and promoting apoptosis through the PI3K/Akt/mTOR signaling pathway. SLBZ-AP may be a potential candidate for BMLC therapy.
1. Introduction
Small- cell lung cancer (SCLC) is one form of lung cancer, which is the leading cause of cancer-related deaths worldwide [1]. Lung cancer tends to develop into bone metastases (BMLC) strongly and about 30–40% of lung cancer patients develop bone metastasis [2–4]. Development of BMLC is associated with poor prognosis and high morbidity and BMLC-induced pain has strong impact on the patients' quality of life [5, 6]. Therefore, prevention of BMLC and relieving the pain to improve lung cancer patients' quality of life are critical. It is necessary to find safe and effective drug therapies for BMLC currently. PI3K/Akt/mTOR signaling pathway plays a pivotal role in a variety of biological activities to regulate cell proliferation, survival, and migration [7, 8]. The PI3K/AKT/mTOR axis commonly contributes to possible mechanisms of oncogenic transformation including stimulation of proliferation, survival, invasion/metastasis, and metabolic reprogramming, as well as suppression of autophagy [9]. PI3K/Akt/mTOR signaling pathway is one of the major signaling cascades which is frequently activated in various human cancers including lung cancer [10, 11]. It has been confirmed by researchers that suppression of PI3K/Akt/mTOR signaling pathway activation may inhibit cells proliferation and metastasis in lung cancer [12–15]. PI3K/Akt/mTOR signaling pathway has been considered a promising therapeutic target.
Shenling Baizhu Powder (SLBZ-AP) is a well-known Chinese medicine formula. The prescription principle of SLBZ-AP is to replenish Qi and invigorate the spleen [16], resolve dampness [17], and relieve diarrhea [18] according to traditional Chinese medicine (TCM) theory [19]. SLBZ-AP is reported to be commonly used to treat patients with shortness of breath, poor appetite, abdominal distension, loose stool, prolapsed anus, lassitude, dysphasia, and spontaneous sweating [20, 21]. It was reported that SLBZ-AP could increase abundance of beneficial gut microbiota and decrease levels of LPS in the portal vein of rats to indicate effects on nonalcoholic fatty liver disease (NAFLD) [22]. SLBZ-AP induced TNF-α, IL-1, and IL-6 significantly decreased in Kupffer cells of nonalcoholic steatohepatitis rats [23]. Studies showed that SLBZ-AP is also effective in curing chronic enteritis [24, 25]. Researchers reported that SLBZ-AP plays an important role in tumor therapy. SLBZ-AP could improve spleen deficiency syndrome after gastric cancer [26]. SLBZ-AP treatment reduced the death rate of mice and decreased the incidence and multiplicity of colonic neoplasms, as well as lowered myeloid-derived suppressor cells infiltration and alleviated TGF-β1 induced epithelial mesenchymal transition (EMT) to exert its effects of anticolitis associated colorectal cancer [19]. SLBZ-AP combined with chemotherapy in postoperative treatment can improve the patient's efficacy after colon cancer surgery while not showing more side effects [27]. SLBZ-AP inhibit tumor growth and increase IL-2, IFN-γ and TNF-α in mice with xenografted hepatocellular cancers [28]. SLBZ-AP combined with gefitinib/erlotinib in the treatment of advanced lung cancer showed more efficacy in clinical practice [29]. According to some researchers, it has been found that SLBZ-AP treatment had a good curative effect on lung cancer and improved quality of life of lung cancer patients [29, 30]. BMLC is associated with poor prognosis and high morbidity and patients' quality of life was strongly affected by BMLC-induced pain. In our previous study, SLBZ-AP tends to show effects on BMLC patients [31, 32].
In the present study, we investigated the effect of SLBZ-AP on BMLC-induced pain and survival in BMLC model mice and further explored the possible signaling pathway involved, as PI3K-Akt-mTOR. The results of the present study provided prospective TCM therapeutic approaches for BMLC and improving BMLC-induced pain.
2. Materials and Methods
2.1. Cell Lines and Culture
Human small-cell lung cancer (SCLC) cell line SBC-5 was obtained from Japanese Collection of Research Bioresources Cell Bank (JCRB0819, Japan) and was maintained in RPMI-1640 medium (Gibco, Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco, Thermo Fisher Scientific, Inc., Waltham, MA, USA). Cells were incubated at 37°C in an atmosphere with 5% CO2.
2.2. Drugs
Shenling Baizhu additive powder (SLBZ-AP) contains twenty-eight species of traditional Chinese medicines (TCM), named according to Chinese Pharmacopoeia [33], as listed in Table 1 (supplied by Pharmacy of Ruikang Hospital Affiliated to Guangxi University of Chinese Medicine, China). All the TCM involved in the formula are crushed into fine powder, sieved, and mixed to obtain SLBZ-AP. According to a previous study [22], SLBZ-AP was dissolved in distilled water to prepare decoction and stored at −4°C. The water decoction was characterized according to the method mentioned in the previous reports [34, 35]. All the plant materials included in Shenling Baizhu used in our study are identified in laboratory department of Pharmacy of Ruikang Hospital Affiliated to Guangxi University of Chinese Medicine according to Chinese Pharmacopoeia. All the voucher specimens of the material have been deposited in Guangxi University of Chinese Medicine.
Table 1.
Twenty-eight species of traditional Chinese medicines involved in Shenling Baizhu additive powder (SLBZ-AP).
| Chinese name | Latin name∗ | Ratio |
|---|---|---|
| Dang shen | Codonopsis Radix | 12 |
| Bai Zhu | Atractylodis Macrocephalae Rhizoma | 12 |
| Fu Ling | Poria | 12 |
| Shan Yao | Dioscoreae Rhizoma | 12 |
| Gan Cao | Glycyrrhizae Radix et Rhizoma | 6 |
| Bai Bian Dou | Semen Lablab Album | 12 |
| Dang Gui | Angelicae sinensis Radix | 15 |
| Chuan Xiong | Chuanxiong Rhizoma | 15 |
| Bai shao | Paeoniae Radix Alba | 15 |
| Sheng Di | Rehmanniae Radix | 15 |
| Lian Zi | Nelumbinis semen | 10 |
| Yi Yi Ren | Coicis semen | 10 |
| Sha Ren | Amomi Fructus | 5 |
| Jie Geng | Platycodonis Radix | 6 |
| Huang Qi | Astragali Radix | 8 |
| Wu Gong | Scolopendra | 2 |
| Fu Zi | Aconiti Lateralis Radix Praeparata | 10 |
| Rou Gui | Cinnamomi cortex | 5 |
| Shan Zhu Yu | Corni Fructus | 10 |
| Tu Si Zi | Cuscutae semen | 10 |
| Du Zhong Ye | Eucommiae Folium | 10 |
| Niu Xi | Achyranthis Bidentatae Radix | 10 |
| Lu Jiao Jiao | Cervi Cornus Colla | 10 |
| Shi Hu | Dendrobii Caulis | 10 |
| Mai Dong | Ophiopogonis Radix | 10 |
| Shi chang Pu | Acori Tatarinowii Rhizoma | 10 |
| Yuan Zhi | Polygalae Radix | 10 |
| Da Huang | Rhei Radix et Rhizoma | 6 |
∗According to Chinese Pharmacopoeia.
2.3. Animals and Experimental Design
The animal studies were conducted and approved by Medical Animal Ethics Committee of Guangxi University of Chinese Medicine. A total of 30 male BALB/c nude mice (6–8 weeks old) were purchased from Shanghai Experimental Animal Center of Chinese Academy of Science (Shanghai, China). All the animals had free access to food and water in a controlled environment with temperature of 25 ± 2°C, 50 ± 10% humidity, and 12 h light/dark cycle. After adaptive feeding for 1 week, the mice were randomly divided into 6 groups with 5 mice per group: (1) sham group which was injected with sterile PBS (pH 7.2) into the right tibia of each mouse; (2) vehicle group, where the mice model of bone metastasis of lung cancer (BMLC) was made according to the previous reports [36]; briefly, 2 × 106 SBC-5 cells/mouse were injected into the right tibia of the animal which then received 0.9% saline i.g. (volume was equivalent to therapeutic drugs); (3) low dose group (L-D), where BMLC model mice were treated with 15 g·kg−1·day−1 for 2 weeks; (4) medium dose group (M-D), where BMLC model mice were treated with 30 g·kg−1·day−1 for 2 weeks; (5) high dose group (H-D), where BMLC model mice were treated with 60 g·kg−1·day−1 for 2 weeks; and (6) rapamycin group, where BMLC model mice were treated with 4 mg·kg−1·day−1 for 2 weeks. The dosage was adjusted according to the mice weight measured daily. The survival of animals was observed daily. The mice were euthanized with 100% carbon dioxide and sacrificed at 12 h after the last treatment and the right tibia specimens were collected and decalcification was performed followed by paraffin embedding and histopathological examination.
2.4. Pain Behavioral Tests
Pain intensity to mechanical stimulus was measured using Von Frey monofilaments. Mice were placed on an elevated wire framework in a transparent box, and then different pressure intensities (equivalent to 1 to 30 g) of von Frey filaments were applied to the plantar surface of light hind. Each filament, from the smallest von Frey filament and increasing gradually, was applied to the plantar surface of the hind paw until the filament bent. The lowest pressure filament in two out of three applications that induced paw licking, paw lifting, and paw withdrawal was used to determine paw withdrawal mechanical threshold (g) (PWMT).
2.5. RNA Extraction and Quantitative Real-Time PCR Analysis (qPCR)
Total RNA was isolated from marrow of tibia specimens from mice using Trizol (TaKaRa, Japan) according to the manufacturer's instructions. Reverse transcription was performed using the reverse transcription kit (Invitrogen, USA). The same amounts of RNA were added to a reverse transcriptase reaction mix with oligo-dT as a primer. Quantitative real-time PCR analyses were performed using the SYBR Green PCR Master Mix kit (Thermo Scientific, USA) according to the manufacturer's instructions. After an initial incubation at 95°C for 2 min, amplification was performed for 40 cycles at 95°C for 15 s, 60°C for 20 s, and 72°C for 20 s. The corresponding specific primers were showed in Table 2.
Table 2.
Primers used in qPCR assay.
| Gene | Primer | Primer sequence (5′-3′) | Product |
|---|---|---|---|
| ACTB | Forward | CCCATCTATGAGGGTTACGC | 150 bp |
| Reverse | TTTAATGTCACGCACGATTTC | ||
|
| |||
| AKT | Forward | CCGAAGGACGGGAGCAG | 151 bp |
| Reverse | CTCTCAGGCTGGCGCTC | ||
|
| |||
| mTOR | Forward | CTTAGAGGACAGCGGGGAAG | 90 bp |
| Reverse | TCCAAGCATCTTGCCCTGAG | ||
|
| |||
| P70S6 | Forward | ACGAAAGCGCAAGAAATCCC | 100 bp |
| Reverse | GGAGGCTCCAGGGCATTAGA | ||
|
| |||
| VEGF | Forward | TTCCGAGACAGGGAAGCTGA | 273 bp |
| Reverse | ATCATTGCCTTTCCATAGCCCC | ||
2.6. Western Blot Analysis
The marrow of tibia specimens were lysed in cold RIPA buffer and centrifuged at 10,000 ×g for 10 min at 4°C. The lysates were then run on 10% SDS-PAGE gel and transferred onto PVDF membranes (Millipore, Shanghai, China).After blocking the membranes in Tris-buffered saline (TBS) buffer with 5% skim milk for 6 h at room temperature, the membranes were incubated with primary antibodies against β-actin (Santa, USA), p-AKT (CST, USA), p-mTOR (CST, USA), p-P70S6k (CST, USA), and VEGF (CST, USA) (1 : 500 to 1 : 1,000) overnight at 4°C.Then, the membranes were washed and incubated with horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. Immunoblots were detected using enhanced chemiluminescence reagents (Invitrogen, USA) and analyzed by Image J software to quantify. The results were normalized to β-actin to correct for loading.
2.7. Histological Analysis
The marrow of tibia specimens was harvested and then stored in 10% formal saline and embedded in paraffin blocks. 3–5 μm sections were prepared from the paraffin blocks for further staining.
Sections of the marrow of tibia specimens were stained with hematoxylin and eosin (HE) in our research to visualize the tissue morphology.
For immunohistochemistry analysis, sections of the marrow of tibia specimens were deparafinized with xylene and then gradient alcohol was used to hydrate. The endogenous peroxidase activity was inhibited using hydrogen peroxide accompanied with a protein blocking agent. Next, the sections were incubated with the appropriate primary antibodies against p-AKT (CST, USA), p-mTOR (CST, USA), p-P70S6k (CST, USA), and VEGF (CST, USA) (1 : 500 to 1 : 1,000) overnight at 4°C and with the secondary antibody for 1hsubsequently. Nucleus was counterstained using hematoxylin. Photomicrograph of all stained sections photomicrograph was taken (Nikon, Japan).
2.8. TUNEL Staining
The marrow of tibia sections was permeabilized with 0.1% Triton ×100 (Beyotime, Shanghai, China). An in situ cell death detection kit (Roche, Mannheim, Germany) was used to perform TUNEL staining according to the manufacturer's instructions. And DAPI was used to counterstain the nucleus. Images were captured using the microscope.
2.9. Statistical Analysis
All data are presented as mean ± SD from the least three independent experiments. All data were analyzed using the GraphPad Prism 8 software (USA). Differences between two groups were analyzed using Student's t-test. Differences were considered statistically significant when p value <0.05. Survival rate was analyzed with Kaplan-Meier survival analysis.
3. Results
3.1. Shenling Baizhu Additive Powder (SLBZ-AP) Relieves Bone Metastasis of Lung Cancer- (BMLC-) Induced Pain and Prolongs Animals' Survival
We investigated the effect of SLBZ-AP on pain and survival in BMLC mice. Paw withdrawal mechanical threshold (g) (PWMT) is used to analyze pain behavior. Compared with the sham group, pain sensitization was significantly decreased in mice of the placebo group (vehicle) (p < 0.01) (Figure 1(a)). 15 (L-D), 30 (M-D), 60 (H-D) g·kg−1·day−1 doses of SLBZ-AP showed relieved effect on BMLC induced pain, especially H-D treatment. It is remarkable that SLBZ-AP showed significant relieved effect on BMLC induced pain compared with rapamycin.
Figure 1.
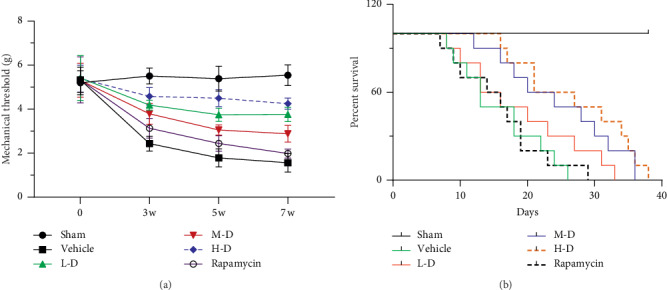
The effect of Shenling Baizhu Additive Powder (SLBZ-AP) on pain and survival of mice with bone metastasis of lung cancer (BMLC). (a) Pain behavior of mice was analyzed using paw withdrawal mechanical threshold (g) (PWMT). (b) The survival of animals was observed daily and percent survival was calculated. Vehicle: BMLC mice treated with placebo (saline). L-D: BMLC mice treated with 15 g·kg−1·day−1 SLBZ-AP for 2 weeks. M-D: BMLC mice treated with 30 g·kg−1·day−1 SLBZ-AP for 2 weeks. H-D: BMLC mice treated with 60 g·kg−1·day−1 SLBZ-AP for 2 weeks. Rapamycin: BMLC mice treated with 4 mg·kg−1·day−1 rapamycin for 2 weeks.
We further explored the effect of SLBZ-AP on survival of mice with BMLC. As shown in Figure 1(b), M-D (30 g·kg−1·day−1) and H-D (60 g·kg−1·day−1) treatment prolonged effect while L-D (15 g·kg−1·day−1) treatment has no change on survival of mice with BMLC. Rapamycin showed almost no effect on survival of mice with BMLC, which was similar to the result of pain behavioral analysis.
3.2. Effect of SLBZ-AP on Alleviating Bone Metastasis of Lung Cancer (BMLC) in Mice
Tumor cells can be identified by deep purple color and irregular nuclear shape in images of H&E staining. It is shown in Figure 2 that more tumor cells were found in the marrow from tibia of BMLC mice compared with the sham group. Treatment with L-D, M-D, or H-D SLBZ-AP decreased tumor cells, and M-D and H-D showed effect more significantly. L-D SLBZ-AP and rapamycin have almost equivalent effects on BMLC.
Figure 2.
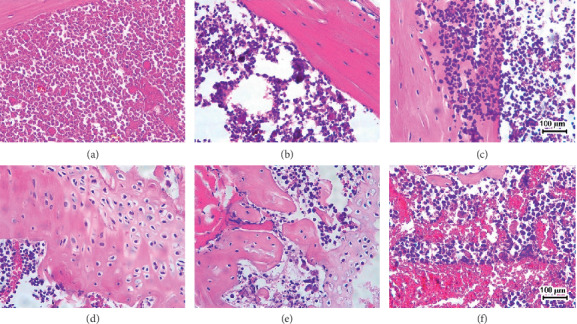
H&E staining of the marrow of tibia in mice with BMLC. Tumor cells in H&E staining images can be identified by deep purple color and irregular nuclear shape. Vehicle: BMLC mice treated with placebo (saline). L-D: BMLC mice treated with 15 g·kg−1·day−1 SLBZ-AP for 2 weeks. M-D: BMLC mice treated with 30 g·kg−1·day−1 SLBZ-AP for 2 weeks. H-D: BMLC mice treated with 60 g·kg−1·day−1 SLBZ-AP for 2 weeks. Rapamycin: BMLC mice treated with 4 mg·kg−1·day−1 rapamycin for 2 weeks. (a) Sham. (b) Vehicle. (c) L-D. (d) M-D. (e) H-D. (f) Rapamycin.
3.3. SLBZ-AP Affects Apoptosis in BMLC Mice
TUNEL staining was performed to evaluate the effect of SLBZ-AP on apoptosis of the marrow from tibia of BMLC mice. Less apoptosis was observed in the sham or vehicle group. L-D, M-D, or H-D SLBZ-AP treatment promoted cells apoptosis in marrow from tibia of BMLC mice significantly (Figure 3). The effect of H-D SLBZ-AP was similar to that of rapamycin on cell apoptosis in the marrow from the tibia of BMLC mice.
Figure 3.
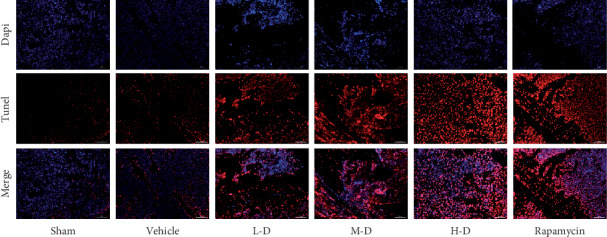
TUNEL staining of the marrow of tibia in mice with BMLC. Dapi was used to stain nucleus. TUNEL staining (red) indicates apoptosis. Vehicle: BMLC mice treated with placebo (saline). L-D: BMLC mice treated with 15 g·kg−1·day−1 SLBZ-AP for 2 weeks. M-D: BMLC mice treated with 30 g·kg−1·day−1 SLBZ-AP for 2 weeks. H-D: BMLC mice treated with 60 g·kg−1·day−1 SLBZ-AP for 2 weeks. Rapamycin: BMLC mice treated with 4 mg ·kg−1·day−1 rapamycin for 2 weeks.
3.4. Change in mRNA Expression of AKT, mTOR, P70S6, and VEGF after SLBZ-AP Treatment
qPCR assay was performed to detect the mRNA expression of AKT, mTOR, P70S6, and VEGF in the marrow of tibia from BMLC mice after SLBZ-AP treatment. BMLC induced the mRNA expression of AKT, mTOR, P70S6, and VEGF upregulation significantly (p < 0.01) (Figure 4). SLBZ-AP treatment downregulated AKT, mTOR, P70S6, or VEGF expression in the marrow of tibia from BMLC mice in a dose-dependent manner (15–60 g·kg−1·day−1) compared with the sham group. Rapamycin inhibited BMLC-induced increased expression of mTOR, P70S6, and VEGF particularly. There is no change of AKT expression in BMLC mice treated with rapamycin compared with the vehicle group (Figure 4(a)).
Figure 4.

The mRNA expression of AKT, mTOR, P70S6, and VEGF in the marrow of tibia of mice with BMLC was measured by qPCR. (a) The AKT expression. (b) The mTOR expression. (c) The P70S6 expression. (d) The VEGF expression. Vehicle: BMLC mice treated with placebo (saline). L-D: BMLC mice treated with 15 g·kg−1·day−1 SLBZ-AP for 2 weeks. M-D: BMLC mice treated with 30 g·kg−1·day−1 SLBZ-AP for 2 weeks. H-D: BMLC mice treated with 60 g·kg−1·day−1 SLBZ-AP for 2 weeks. Rapamycin: BMLC mice treated with 4 mg·kg−1·day−1 rapamycin for 2 weeks.
3.5. Change in Protein Expression of AKT, mTOR, P70S6, and VEGF after SLBZ-AP Treatment Analyzed by Immunohistochemistry
The protein expression of AKT, mTOR, P70S6, and VEGF in the marrow of tibia in mice was analyzed using immunohistochemistry analysis. Compared with the sham group, BMLC induced AKT (Figure 5(a)), mTOR (Figure 5(b)), P70S6 (Figure 5(c)), and VEGF (Figure 5(d)) protein expression significantly. All the animals treated with SLBZ-AP (15–60 g·kg−1·day−1) showed decreasing protein expression of AKT, mTOR, P70S6, and VEGF. It is similar to the results of mRNA expression assay which showed that rapamycin inhibited mTOR, P70S6, and VEGF protein expression significantly and tended to show no effect on AKT compared with the vehicle group.
Figure 5.
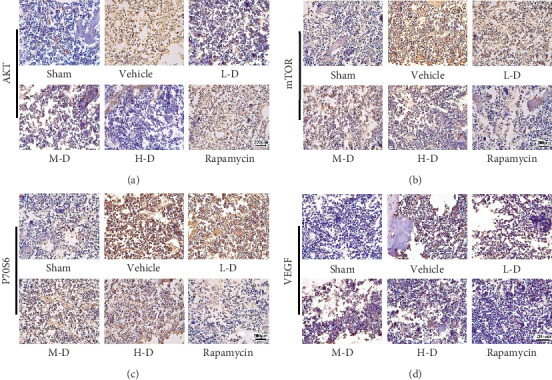
The protein expression of AKT, mTOR, P70S6, and VEGF in the marrow of tibia of mice with BMLC was analyzed by immunohistochemistry. (a) The AKT protein expression. (b) The mTOR protein expression. (c) The P70S6 protein expression. (d) The VEGF protein expression. Vehicle: BMLC mice treated with placebo (saline). L-D: BMLC mice treated with 15 g·kg−1·day−1 SLBZ-AP for 2 weeks. M-D: BMLC mice treated with 30 g·kg−1·day−1 SLBZ-AP for 2 weeks. H-D: BMLC mice treated with 60 g·kg−1·day−1 SLBZ-AP for 2 weeks. Rapamycin: BMLC mice treated with 4 mg·kg−1·day−1 rapamycin for 2 weeks.
3.6. Change in Protein Expression of p-AKT, p-mTOR, p-P70S6, and VEGF after SLBZ-AP Treatment Detected by Western Blotting
We further measured the protein expression of p-AKT, p-mTOR, p-P70S6, and VEGF in the marrow of tibia in mice by western blotting. The protein expression of p-AKT, p-mTOR, p-P70S6, and VEGF was increased significantly in BMLC mice (p < 0.01) (Figure 6), which was downregulated by SLBZ-AP treatment, especially by 30 g·kg−1·day−1 SLBZ-AP (M-D). The effect of 60 g·kg−1·day−1 SLBZ-AP on p-AKT, p-mTOR, p-P70S6, and VEGF tends to be less than that of L-D and M-D SLBZ-AP. Rapamycin and L-D SLBZ-AP treatment indicated equivalent effect on p-AKT, p-mTOR, p-P70S6, and VEGF protein expression.
Figure 6.
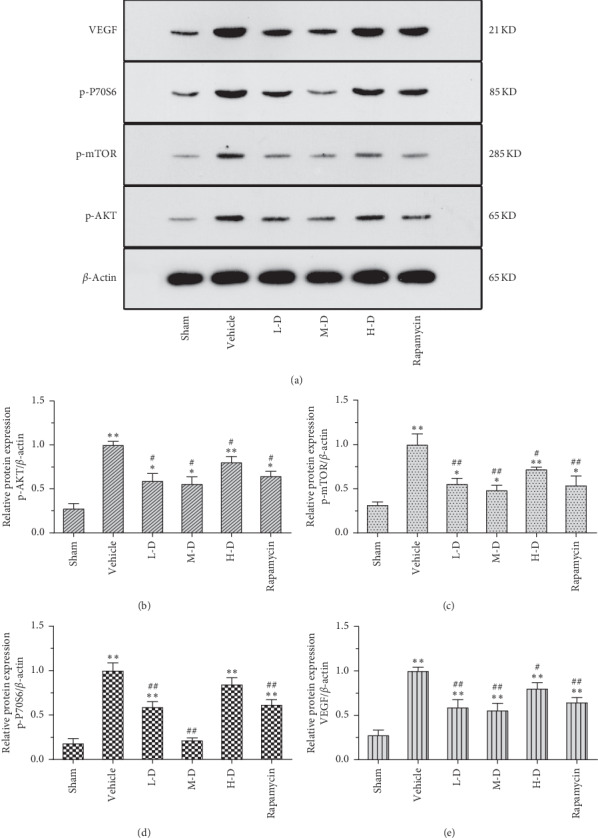
The protein expression of p-AKT, p-mTOR, p-P70S6, and VEGF in the marrow of tibia of mice with BMLC was analyzed by immunohistochemistry. (a) Western blots of p-AKT, p-mTOR, p-P70S6, and VEGF. (b) The p-AKT protein expression. (c) The p-mTOR protein expression. (d) The p-P70S6 protein expression. (e) The VEGF protein expression. Vehicle: BMLC mice treated with placebo (saline). L-D: BMLC mice treated with 15 g·kg−1·day−1 SLBZ-AP for 2 weeks. M-D: BMLC mice treated with 30 g·kg−1·day−1 SLBZ-AP for 2 weeks. H-D: BMLC mice treated with 60 g·kg−1·day−1 SLBZ-AP for 2 weeks. Rapamycin: BMLC mice treated with 4 mg·kg−1·day−1 rapamycin for 2 weeks.
4. Discussion
Lung cancer is the leading cause of cancer-related deaths worldwide [1]. The incidence rate of lung cancer developing into bone metastases (BMLC) is higher, which is related to poor prognosis and high morbidity, and BMLC-induced pain affects the patients' quality of life strongly [5, 6]. Safe and effective drug therapies to prevent BMLC and relieve the pain to improve lung cancer patients' quality of life remain necessary currently. As a canonical and well-known Chinese medicine formula, SLBZ-AP is first described in “The Prescriptions of the Bureau of Taiping People's Welfare Pharmacy” in Song-dynasty [19] and contains twenty-eight species of TCM as Dangshen, Fuling, Baizhu, Gancao, and so on . There are chemical components as saccharides (Dang Shen, Fu Ling, Bai Bian Dou) [37, 38], glycosides (Shan Yao, Jie Geng, Shan Zhu Yu, Bai Shao) [39–42], and flavonoids (Bai Zhu, Gan Cao) [43, 44] mainly in SLBZ-AP. Saccharides have immunomodulation effects [37]. Glycosides as saponins [39] and flavonoids [44] have anti-inflammatory effects. It also has been reported that saponins could inhibit invasion and metastasis in colorectal cancer cell through NF-κB signaling pathway and EMT [45]. Flavonoids also showed anticancer effect [46]. Dang gui could ameliorate skin inflammation [47] and has activities against cancer [48]. The other or the above mentioned drugs may have multiple as well as unclear chemical components which remain to be further explored. As reported in many previous studies, SLBZ-AP plays an important role in tumor therapy including lung cancer. SLBZ-AP combined with Gefitinib/Erlotinib had a good curative effect for advanced non-small-cell lung cancer with spleen deficiency [29]. SLBZ-AP treatment applied in the advanced non-small-cell lung cancer can improve the clinical efficacy, relieve clinical symptoms, and improve the quality of life of patients [30]. SLBZ-AP improve clinical chemotherapy benefit and quality of life of patients with non-small-cell lung cancer by upregulating the expression of VEGF and MMP-9. In addition, SLBZ-AP treatment has higher safety [49]. The effect of SLBZ-AP on BMLC-induced pain and survival of BMLC mice as well as involved signaling pathway was explored in our study.
Mice model of BMLC was made and treatment with SLBZ-AP was performed. Our data showed that SLBZ-AP relieves BMLC-induced pain and prolongs animals' survival. It is remarkable that SLBZ-AP showed significant relieved effect on BMLC-induced pain and survival of BMLC mice compared with rapamycin. H&E staining of the marrow of tibia in animals showed that M-D and H-D of SLBZ-AP has effect on inhibition of cell proliferation more significantly. Results of TUNEL staining indicated that L-D, M-D, or H-D of SLBZ-AP treatment promoted cells apoptosis in marrow from tibia of BMLC mice significantly. Our results suggested that SLBZ-AP have antinociceptive effect and may prolong survival of BMLC mice by inhibiting cell proliferation and promoting apoptosis.
Many researchers confirmed that PI3K/Akt/mTOR signaling pathway is related to a few biological activities to affect cell proliferation, survival, and migration [7, 8], especially to stimulate proliferation, survival, invasion/metastasis, and metabolic reprogramming and suppress autophagy, which is involved in possible mechanisms of oncogenic transformation [9]. PI3K/Akt/mTOR signaling pathway is commonly activated and suppression of its activation may inhibit cells proliferation and metastasis in cancers of the lung [10, 11, 50], colorectal [51, 52], esophagus [53], breast [54–56], liver [57, 58], and kidney [59, 60], which has been considered a promising therapeutic target. Whether the PI3K/Akt/mTOR signaling pathway involved in SLBZ-AP affects BMLC was further explored in our study, results of which indicated that the mRNA and protein expression of AKT, mTOR, P70S6, and VEGF as well as protein expression of p-AKT, p-mTOR, p-P70S6, and VEGF was significantly increased in the marrow of tibia from BMLC mice. Rapamycin, also known as Sirolimus, is a specific mTOR inhibitor. Rapamycin could block targets known to be downstream of mTOR such as inhibition of p70S6K activity to inhibit metastatic tumor growth [61, 62]. The phosphorylation of p70S6K has been used as a hallmark of activation by mTOR and correlated with autophagy inhibition in various situations. Pain is one of the major signs of bone metastasis in lung cancer. Furthermore, bone metastasis in lung cancer is associated with tumor cells survival and proliferation. It has been reported in the previous studies that suppression PI3K/Akt/mTOR signaling pathway activation may inhibit cells proliferation and metastasis in lung cancer. In our study, we preliminarily explored the effect of SLBZ-AP on sensitivity to pain in bone metastasis with small-cell lung cancer model mice and cells proliferation and apoptosis of lung cancer as well as possible markers involved. Moreover, signaling by mTOR has been implicated in the development of chronic pain [63]. p-mTOR in the spinal cord may play an important role in the regulation of antinociception induced by acute immobilization stress and the tolerance development induced by chronic immobilization stress [64]. PI3K/p-Akt upregulation may be correlated with chronic osteoarthritis pain [65]. mTOR and its downstream pathway are activated in the dorsal root ganglion and spinal cord after peripheral inflammation which subsequently contribute to the development of chronic inflammatory pain [66]. According to previous studies, rapamycin treatment at the dosage of 4 mg·kg−1·day−1 was performed in BMLC mice.
Results of our study showed that SLBZ-AP exhibited more antinociceptive and L-D of SLBZ-AP have almost equivalent effects to rapamycin on survival of BMLC mice as well as the enhancement of apoptosis by H-D SLBZ-AP which was similar to that of rapamycin. Similar to rapamycin, SLBZ-AP treatment downregulated AKT, mTOR, P70S6, and VEGF mRNA and protein expression in the marrow of tibia from BMLC mice. Moreover, BMLC-induced protein expression increase of p-AKT, p-mTOR, p-P70S6, and VEGF was downregulated by SLBZ-AP treatment, especially by M-D dosage of SLBZ-AP. However, H-D SLBZ-AP (60 g·kg−1·day−1) tends to show less effect on p-AKT, p-mTOR, p-P70S6, and VEGF protein expression induced by BMLC and the effect of M-D SLBZ-AP is more than that of L-D SLBZ-AP, which may be due to the fact that the expression of p-AKT, p-mTOR, p-P70S6, and VEGF in the marrow from tibia from bone metastasis with small-cell lung cancer (BMLC) mice was measured and cells in the marrow from tibia might include cancer cells and normal cells. The effect of H-D SLBZ-AP remains to be explored further. Rapamycin inhibited BMLC-induced increased expression of mTOR, P70S6, and VEGF particularly, which has equivalent effect on p-AKT, p-mTOR, p-P70S6, and VEGF protein expression to L-D SLBZ-AP although it showed no effect on AKT expression. In our further study, we will perform more behavioral or neurochemical markers of pain and explore the role of chemical components in bone metastasis with small-cell lung cancer.
5. Conclusions
In conclusion, with higher incidence rate, BMLC associates with poor prognosis and high morbidity. Furthermore, the patients' quality of life is affected by BMLC-induced pain strongly. PI3K/Akt/mTOR signaling pathway is involved in possible mechanisms of oncogenic transformation as stimulation of proliferation, survival, invasion/metastasis, metabolic reprogramming, and suppression of autophagy and is commonly activated in lung cancer to correlate with cells proliferation and metastasis. As one of Chinese medicine formulas, SLBZ-AP is reported to have curative effect on lung cancer. We investigated the effect of SLBZ-AP on BMLC-induced pain and survival of BMLC mice as well as the involved signaling pathway in present study. Our results indicated that SLBZ-AP relieves BMLC-induced pain and prolongs animals' survival. Results of TUNEL staining indicated that SLBZ-AP treatment promoted cells apoptosis in marrow from tibia of BMLC mice. SLBZ-AP inhibited mRNA and protein expression of AKT, mTOR, P70S6, and VEGF as well as protein expression of p-AKT, p-mTOR, p-P70S6, and VEGF upregulation in the marrow of tibia induced by BMLC, an effect which was similar to rapamycin. Considering that SLBZ-AP showed more effects on pain and survival than rapamycin, and further studies need to be performed. Our results suggested that SLBZ-AP may have antinociceptive effect and prolong survival of BMLC mice at least partially by inhibiting cell proliferation and promoting apoptosis through PI3K/Akt/mTOR signaling pathway. SLBZ-AP may be a potential candidate for BMLC therapy.
Acknowledgments
The Regional Science Foundation Project of the Nation Science Foundation of China Research Agency (no. 81560779) and the High-level Talent Team Cultivation Project of “Qi Huang” of Guangxi University of Traditional Chinese Medicine (no. 04B1804804) supported the animals' experiments and data analysis.
Abbreviations
- BMLC:
Bone metastasis of lung cancer
- SLBZ-AP:
Shenling Baizhu additive powder
- TCM:
Traditional Chinese medicine
- EMT:
Epithelial mesenchymal transition
- SCLC:
Human small-cell lung cancer
- PI3K:
Phosphatidylinositol 3-kinase
- mTOR:
Mammalian target of rapamycin
- IL-2:
Interleukin 2
- IFN-γ:
Interferon-γ
- TNF-α:
Tumor necrosis factor-α.
Contributor Information
Ziyi Feng, Email: 944068464@qq.com.
GuoJian Chen, Email: 343925232@qq.com.
Zhenwei Cui, Email: 121722500@qq.com.
Data Availability
All data generated or analyzed during this study are included in this published article.
Ethical Approval
All experiments were approved and performed by the Medical Animal Ethics Committee of Guangxi University of Chinese Medicine.
Conflicts of Interest
All the authors declare that they have no conflicts of interest.
Authors' Contributions
ZF, JH, WMC, GJC, and ZWC conceived and designed the study. ZF, JH, WMC, BS, JM, XJF, and STF performed the article search and data extraction. ZF, GJC, PH, LH, and ZWC evaluated the methodological quality of each study. FZ analyzed the data and wrote the paper, which was improved by GJC, and ZWC supervised the research. All authors read and approved the final manuscript. Zhe Feng and Ziyi Feng contributed equally to this work.
References
- 1.Chi Y.-H., Hsiao J.-K., Lin M.-H., Chang C., Lan C.-H., Wu H.-C. Lung cancer-targeting peptides with multi-subtype indication for combinational drug delivery and molecular imaging. Theranostics. 2017;7(6):1612–1632. doi: 10.7150/thno.17573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang L., Gong Z. Clinical characteristics and prognostic factors in bone metastases from lung cancer. Medical Science Monitor. 2017;23:4087–4094. doi: 10.12659/msm.902971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riihimäki M., Hemminki A., Fallah M., et al. Metastatic sites and survival in lung cancer. Lung Cancer. 2014;86(1):78–84. doi: 10.1016/j.lungcan.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 4.De Castro J., García R., Garrido P., et al. Therapeutic potential of denosumab in patients with lung cancer: beyond prevention of skeletal complications. Clinical Lung Cancer. 2015;16(6):431–446. doi: 10.1016/j.cllc.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Dohzono S., Sasaoka R., Takamatsu K., Hoshino M., Nakamura H. Low paravertebral muscle mass in patients with bone metastases from lung cancer is associated with poor prognosis. Supportive Care in Cancer. 2020;28(1):389–394. doi: 10.1007/s00520-019-04843-9. [DOI] [PubMed] [Google Scholar]
- 6.Cho Y. J., Cho Y. M., Kim S. H., Shin K. H., Jung S. T., Kim H. S. Clinical analysis of patients with skeletal metastasis of lung cancer. BMC Cancer. 2019;19(1):p. 303. doi: 10.1186/s12885-019-5534-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin R., Jin Y. Y., Tang Y. L., Yang H. J., Zhou X. Q., Lei Z. GPNMB silencing suppresses the proliferation and metastasis of osteosarcoma cells by blocking the PI3K/Akt/mTOR signaling pathway. Oncology Reports. 2018;39(6):3034–3040. doi: 10.3892/or.2018.6346. [DOI] [PubMed] [Google Scholar]
- 8.Ke M., Mo L., Li W., Zhang X., Li F., Yu H. Ubiquitin ligase SMURF1 functions as a prognostic marker and promotes growth and metastasis of clear cell renal cell carcinoma. FEBS Open Bio. 2017;7(4):577–586. doi: 10.1002/2211-5463.12204. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Aoki M., Fujishita T. Oncogenic roles of the PI3K/AKT/mTOR Axis. Current Topics in Microbiology and Immunology. 2017;407:153–189. doi: 10.1007/82_2017_6. [DOI] [PubMed] [Google Scholar]
- 10.Beck J. T., Ismail A., Tolomeo C. Targeting the phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) pathway: an emerging treatment strategy for squamous cell lung carcinoma. Cancer Treatment Reviews. 2014;40(8):980–989. doi: 10.1016/j.ctrv.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Heavey S., O’Byrne K. J., Gately K. Strategies for co-targeting the PI3K/AKT/mTOR pathway in NSCLC. Cancer Treatment Reviews. 2014;40(3):445–456. doi: 10.1016/j.ctrv.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Zhou X., Zhou R., Li Q., et al. Cardamonin inhibits the proliferation and metastasis of non-small-cell lung cancer cells by suppressing the PI3K/Akt/mTOR pathway. Anti-Cancer Drugs. 2019;30(3):241–250. doi: 10.1097/cad.0000000000000709. [DOI] [PubMed] [Google Scholar]
- 13.Baek S. H., Ko J.-H., Lee J. H., et al. Ginkgolic acid inhibits invasion and migration and TGF-β-induced EMT of lung cancer cells through PI3K/Akt/mTOR inactivation. Journal of Cellular Physiology. 2017;232(2):346–354. doi: 10.1002/jcp.25426. [DOI] [PubMed] [Google Scholar]
- 14.Yang X., Song X., Wang X., Liu X., Peng Z. Downregulation of TM7SF4 inhibits cell proliferation and metastasis of A549 cells through regulating the PI3K/AKT/mTOR signaling pathway. Molecular Medicine Reports. 2017;16(5):6122–6127. doi: 10.3892/mmr.2017.7324. [DOI] [PubMed] [Google Scholar]
- 15.Lv X., Li C. Y., Han P., Xu X. Y. MicroRNA-520a-3p inhibits cell growth and metastasis of non-small cell lung cancer through PI3K/AKT/mTOR signaling pathway. European Review for Medical and Pharmacological Sciences. 2018;22(8):2321–2327. doi: 10.26355/eurrev_201804_14822. [DOI] [PubMed] [Google Scholar]
- 16.Li Z.-H., Wang J., Cai R.-L., Sun J., Ye M.-G. Effect of Shenling Baizhu San on the protein expression of NF-κB p65 and the serum level of related inflammatory cytokines in the colon tissue of rats with ulcerative colitis due to dampness retention and spleen deficiency. Journal of Beijing University of Traditional Chinese Medicine. 2015;38(5):315–317. [Google Scholar]
- 17.Han H., Song G., Hu S. Mechanism of function of Shenlibaizhu powder on diarrhea due to spleen asthenia by Rhubarb. Modern Journal of Integrated Traditional Chinese and Western Medicine. 2008;17(1):15–16. [Google Scholar]
- 18.Ma X., Li Z. Treatment of 35 cases of chronic functional diarrhea with shenqi baizhu powder and zhongzhong decoction. Shanxi Traditional Chinese Medicine. 2011;32(5):542–543. [Google Scholar]
- 19.Lin X., Xu W., Shao M., et al. Shenling Baizhu San supresses colitis associated colorectal cancer through inhibition of epithelial-mesenchymal transition and myeloid-derived suppressor infiltration. BMC Complementary and Alternative Medicine. 2015;15(1):p. 126. doi: 10.1186/s12906-015-0649-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu L., Han L., Wong D. Y. L., et al. Effects of Si-Jun-Zi decoction polysaccharides on cell migration and gene expression in wounded rat intestinal epithelial cells. British Journal of Nutrition. 2005;93(1):21–29. doi: 10.1079/bjn20041295. [DOI] [PubMed] [Google Scholar]
- 21.Ding Y.-J., Wang B.-C., Wen C.-C., et al. Evaluation of the teratogenic effects of three traditional Chinese medicines, Si jun zi tang, liu jun zi tang and shenling baizhu san, during zebrafish pronephros development. Journal of Toxicologic Pathology. 2015;28(3):141–149. doi: 10.1293/tox.2013-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y., Tang K., Deng Y., et al. Effects of shenling baizhu powder herbal formula on intestinal microbiota in high-fat diet-induced NAFLD rats. Biomedicine & Pharmacotherapy. 2018;102:1025–1036. doi: 10.1016/j.biopha.2018.03.158. [DOI] [PubMed] [Google Scholar]
- 23.Gong X.-W., Xu Y.-J., Yang Q.-H., et al. Effect of soothing Gan (liver) and invigorating pi (spleen) recipes on TLR4-p38 MAPK pathway in kupffer cells of non-alcoholic steatohepatitis rats. Chinese Journal of Integrative Medicine. 2019;25(3):216–224. doi: 10.1007/s11655-018-2829-6. [DOI] [PubMed] [Google Scholar]
- 24.Tang B. Clinical observation of Shenling Baizhu San on 60 cases of chronic enteritis. Internal Medicine China. 2011;6(3):225–226. [Google Scholar]
- 25.Li X. Clinical observation of the efficacy of Shenling Baizhu powder on treatment of chronic enteritis. China Modern Medicine. 2012;19(15):90–91. [Google Scholar]
- 26.Li P. Observation on the effect of Shenling Baizhu Decoction on improving spleen deficiency syndrome in patients with gastric cancer with colorectal cancer. Journal of Tianjin University of Traditional Chinese Medicine. 2005;24(3):151–152. [Google Scholar]
- 27.Yu P. Clinical observation of shenling baizhu decoction combined with chemotherapy in postoperative treatment of colon cancer. Modern Diagnosis and Treatment. 2015;23:5310–5311. [Google Scholar]
- 28.Huang Z. Effects of Shenling Baizhu San on serum IL-2, INF-γ , TNF-α in tumor bearing mice. Guangming Journal Chinese Medicine. 2010;25(9):1584–1586. [Google Scholar]
- 29.Zhang X. Clinical study of Shenling Baizhu Granule combined with gefitinib/erlotinib in the treatment of advanced non-small cell lung cancer with spleen deficiency. Journal of New Chinese Medicine. 2014;1:127–129. [Google Scholar]
- 30.Li H. Clinical observation of 50 cases of advanced non-small cell lung cancer with Shenling Baizhu Powder. Jian Kang Qian Yan. 2016;25(11) [Google Scholar]
- 31.Feng Z. Effect of shenling baizhu powder on the expression of COX-2 and VEGF in bone metastasis of lung cancer. Shizhen Guoguo Guoyao. 2013;24(1):105–107. [Google Scholar]
- 32.Feng Z. Clinical study on shenling baizhu powder for treating bone metastasis of lung cancer. Chinese Medicine Herald. 2013;10:1–3. [Google Scholar]
- 33.Commission C. Chinese Pharmacopoeia. Vol. 1. China: Chinese Medical Science and Technology Press; 2015. [Google Scholar]
- 34.Liu C., Zhu Q. Study on characteristic fingerprint of Shenling Baizhu powder by HPLC and determination of five index components. China Pharmaceuticals. 2018;27(466(15)):18–22. [Google Scholar]
- 35.Li J. Study on fingerprint and multi-component quality of Shenling Baizhu powder based on UPLC. China Pharmacist. 2019;22(2):18–22. [Google Scholar]
- 36.Shen W., Pang H., Xin B., Duan L., Liu L., Zhang H. Biological effects of BMP7 on small-cell lung cancer cells and its bone metastasis. International Journal of Oncology. 2018;53(3):1354–1362. doi: 10.3892/ijo.2018.4469. [DOI] [PubMed] [Google Scholar]
- 37.Zou Y. F., Zhang Y.-Y., Fu Y.-P., et al. A polysaccharide isolated from codonopsis pilosula with immunomodulation effects both in vitro and in vivo. Molecules. 2019;24(20) doi: 10.3390/molecules24203632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang W., Chen L., Li P., Zhao J., Duan J. Antidepressant and immunosuppressive activities of two polysaccharides from Poria cocos (Schw.) Wolf. International Journal of Biological Macromolecules. 2018;120:1696–1704. doi: 10.1016/j.ijbiomac.2018.09.171. [DOI] [PubMed] [Google Scholar]
- 39.Guo Y., Xing E., Liang X., Song H., Dong W. Effects of total saponins from Rhizoma Dioscoreae Nipponicae on expression of vascular endothelial growth factor and angiopoietin-2 and Tie-2 receptors in the synovium of rats with rheumatoid arthritis. Journal of the Chinese Medical Association. 2016;79(5):264–271. doi: 10.1016/j.jcma.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 40.Ma X. Q., Li S.-M., Chan C. L., et al. Influence of sulfur fumigation on glycoside profile in Platycodonis Radix (Jiegeng) Chinese Medicine. 2016;11(1):p. 32. doi: 10.1186/s13020-016-0101-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Du K., Li J., Bai Y., An M., Gao X.-M., Chang Y.-X. A green ionic liquid-based vortex-forced MSPD method for the simultaneous determination of 5-HMF and iridoid glycosides from Fructus Corni by ultra-high performance liquid chromatography. Food Chemistry. 2018;244:190–196. doi: 10.1016/j.foodchem.2017.10.057. [DOI] [PubMed] [Google Scholar]
- 42.Zheng M., Liu C., Fan Y., Shi D., Jian W. Total glucosides of paeony (TGP) extracted from Radix Paeoniae Alba exerts neuroprotective effects in MPTP-induced experimental parkinsonism by regulating the cAMP/PKA/CREB signaling pathway. Journal of Ethnopharmacology. 2019;245 doi: 10.1016/j.jep.2019.112182.112182 [DOI] [PubMed] [Google Scholar]
- 43.Xu Y., Cai H., Cao G., et al. Profiling and analysis of multiple constituents in Baizhu Shaoyao San before and after processing by stir-frying using UHPLC/Q-TOF-MS/MS coupled with multivariate statistical analysis. Journal of Chromatography B. 2018;1083:110–123. doi: 10.1016/j.jchromb.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 44.Yang X. L., Liu D., Bian K., Zhang D. D. Study on in vitro anti-inflammatory activity of total flavonoids from Glycyrrhizae Radix et Rhizoma and its ingredients. Zhongguo Zhong Yao Za Zhi. 2013;38(1):99–104. [PubMed] [Google Scholar]
- 45.Xia L., Zhang B., Yan Q., Ruan S. Effects of saponins of patrinia villosa against invasion and metastasis in colorectal cancer cell through NF-κB signaling pathway and EMT. Biochemical and Biophysical Research Communications. 2018;503(3):2152–2159. doi: 10.1016/j.bbrc.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 46.Kopustinskiene D. M., Jakstas V., Savickas A., Bernatoniene J. Flavonoids as anticancer agents. Nutrients. 2020;12(2):p. 457. doi: 10.3390/nu12020457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nguyen L. T. H., Ahn S.-H., Nguyen U. T., Yang I.-J. Dang-Gui-Liu-Huang Tang a traditional herbal formula, ameliorates imiquimod-induced psoriasis-like skin inflammation in mice by inhibiting IL-22 production. Phytomedicine. 2018;47:48–57. doi: 10.1016/j.phymed.2018.04.051. [DOI] [PubMed] [Google Scholar]
- 48.Chunhua M., Hongyan L., Weina Z., Xiaoli H., Yajie Z., Jie R. Dang Gui Bu Xue Tang ameliorates coronary artery ligation-induced myocardial ischemia in rats. Biomedicine & Pharmacotherapy. 2017;88:617–624. doi: 10.1016/j.biopha.2017.01.079. [DOI] [PubMed] [Google Scholar]
- 49.Xingzi H. Effect of shenling baizhu jiawei decoction on expression of serum vascular endothelial growth factor and matrix metalloproteinase-9 in patients with non-small cell lung cancer. China Medical Herald. 2013;10(29):72–75. [Google Scholar]
- 50.Dong M., Ye T., Bi Y., et al. A novel hybrid of 3-benzyl coumarin seco-B-ring derivative and phenylsulfonylfuroxan induces apoptosis and autophagy in non-small-cell lung cancer. Phytomedicine. 2019;52:79–88. doi: 10.1016/j.phymed.2018.09.216. [DOI] [PubMed] [Google Scholar]
- 51.Soleimani A., Rahmani F., Ferns G. A., Ryzhikov M., Avan A., Hassanian S. M. Role of regulatory oncogenic or tumor suppressor miRNAs of PI3K/AKT signaling Axis in the pathogenesis of colorectal cancer. Current Pharmaceutical Design. 2018;24(39):4605–4610. doi: 10.2174/1381612825666190110151957. [DOI] [PubMed] [Google Scholar]
- 52.Kumar S., Agnihotri N. Piperlongumine, a piper alkaloid targets Ras/PI3K/Akt/mTOR signaling axis to inhibit tumor cell growth and proliferation in DMH/DSS induced experimental colon cancer. Biomedicine & Pharmacotherapy. 2019;109:1462–1477. doi: 10.1016/j.biopha.2018.10.182. [DOI] [PubMed] [Google Scholar]
- 53.Javadinia S. A., Shahidsales S., Fanipakdel A., et al. The esophageal cancer and the PI3K/AKT/mTOR signaling regulatory microRNAs: a novel marker for prognosis, and a possible target for immunotherapy. Current Pharmaceutical Design. 2019;24(39):4646–4651. doi: 10.2174/1381612825666190110143258. [DOI] [PubMed] [Google Scholar]
- 54.Li Z. W., Xue M., Zhu B.-X., Yue C.-L., Chen M., Qin H.-H. microRNA-4500 inhibits human glioma cell progression by targeting IGF2BP1. Biochemical and Biophysical Research Communications. 2019;513(4):800–806. doi: 10.1016/j.bbrc.2019.04.058. [DOI] [PubMed] [Google Scholar]
- 55.Guerrero-Zotano A., Mayer I. A., Arteaga C. L. PI3K/AKT/mTOR: role in breast cancer progression, drug resistance, and treatment. Cancer and Metastasis Reviews. 2016;35(4):515–524. doi: 10.1007/s10555-016-9637-x. [DOI] [PubMed] [Google Scholar]
- 56.Sharma V. R., Gupta G. K., Sharma A. K., et al. PI3K/Akt/mTOR intracellular pathway and breast cancer: factors, mechanism and regulation. Current Pharmaceutical Design. 2017;23(11):1633–1638. doi: 10.2174/1381612823666161116125218. [DOI] [PubMed] [Google Scholar]
- 57.Wu T., Dong X., Yu D., Shen Z., Yu J., Yan S. Natural product pectolinarigenin inhibits proliferation, induces apoptosis, and causes G2/M phase arrest of HCC via PI3K/AKT/mTOR/ERK signaling pathway. OncoTargets and Therapy. 2018;11:8633–8642. doi: 10.2147/ott.s186186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pu X. H., Li F., Miao X. L., Ye J. L., Lu L. G. Transforming growth factor beta regulates hepatic progenitor cells migration via PI3K/AKT/mTOR/p70S6K pathway. Zhonghua Gan Zang Bing Za Zhi. 2018;26(9):680–685. doi: 10.3760/cma.j.issn.1007-3418.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 59.Wei C., Yang C., Wang S., et al. Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis. Molecular Cancer. 2019;18(1):p. 64. doi: 10.1186/s12943-019-0976-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhu Y., Rao Q., Zhang X., Zhou X. Galangin induced antitumor effects in human kidney tumor cells mediated via mitochondrial mediated apoptosis, inhibition of cell migration and invasion and targeting PI3K/AKT/mTOR signalling pathway. Journal of Balkan Union of Oncology. 2018;23(3):795–799. [PubMed] [Google Scholar]
- 61.Guba M., von Breitenbuch P., Steinbauer M., et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nature Medicine. 2002;8(2):128–135. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- 62.Auyeung K. K., Ko J. K. Angiogenesis and oxidative stress in metastatic tumor progression: pathogenesis and novel therapeutic approach of colon cancer. Current Pharmaceutical Design. 2017;23(27):3952–3961. doi: 10.2174/1381612823666170228124105. [DOI] [PubMed] [Google Scholar]
- 63.Choi S., Kim K., Cha M., Kim M., Lee B. H. mTOR signaling intervention by Torin1 and XL388 in the insular cortex alleviates neuropathic pain. Neuroscience Letters. 2020;718 doi: 10.1016/j.neulet.2020.134742.134742 [DOI] [PubMed] [Google Scholar]
- 64.Feng J.-H., Lee H.-J., Suh H.-W. The molecular signatures of acute-immobilization-induced antinociception and chronic-immobilization-induced antinociceptive tolerance. Experimental Neurobiology. 2019;28(6):670–678. doi: 10.5607/en.2019.28.6.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Batalle G., Cabarga L., Pol O. The inhibitory effects of slow-releasing hydrogen sulfide donors in the mechanical allodynia, grip strength deficits, and depressive-like behaviors associated with chronic osteoarthritis pain. Antioxidants (Basel) 2019;9(1) doi: 10.3390/antiox9010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liang L., Tao B., Fan L., Yaster M., Zhang Y., Tao Y.-X. mTOR and its downstream pathway are activated in the dorsal root ganglion and spinal cord after peripheral inflammation, but not after nerve injury. Brain Research. 2013;1513:17–25. doi: 10.1016/j.brainres.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


