Abstract
ABL-class fusions other than BCR-ABL1 characterize around 2–3% of precursor B-cell acute lymphoblastic leukemia. Case series indicated that patients suffering from these subtypes have a dismal outcome and may benefit from the introduction of tyrosine kinase inhibitors. We analyzed clinical characteristics and outcome of 46 ABL-class fusion positive cases other than BCR-ABL1 treated according to AIEOP-BFM (Associazione Italiana di Ematologia-Oncologia Pediatrica-Berlin-Frankfurt-Münster) ALL 2000 and 2009 protocols; 13 of them received a tyrosine kinase inhibitor (TKI) during different phases of treatment. ABL-class fusion positive cases had a poor early treatment response: minimal residual disease levels of ≥5×10−4 were observed in 71.4% of patients after induction treatment and in 51.2% after consolidation phase. For the entire cohort of 46 cases, the 5-year probability of event-free survival was 49.1+8.9% and that of overall survival 69.6+7.8%; the cumulative incidence of relapse was 25.6+8.2% and treatment-related mortality (TRM) 20.8+6.8%. One out of 13 cases with TKI added to chemotherapy relapsed while eight of 33 cases without TKI treatment suffered from relapse, including six in 17 patients who had not received hematopoietic stem cell transplantation. Stem cell transplantation seems to be effective in preventing relapses (only three relapses in 25 patients), but was associated with a very high TRM (6 patients). These data indicate a major need for an early identification of ABL-class fusion positive acute lymphoblastic leukemia cases and to establish a properly designed, controlled study aimed at investigating the use of TKI, the appropriate chemotherapy backbone and the role of hematopoietic stem cell transplantation. (Registered at: clinicaltrials.gov identifier: NTC00430118, NCT00613457, NCT01117441).
Introduction
Continuous optimization of risk-adapted multi-agent treatment has led to excellent curative rates in the majority of children and adolescents suffering from acute lymphoblastic leukemia (ALL).1–9 However, the progress in ALL subtype classification according to the nature of specific sentinel genetic aberrations identified molecular ALL subgroups like low-hypodiploid, KMT2A-rearranged or BCR-ABL1 positive precursor-B-ALL (B-ALL) with distinct biological and clinical characteristics associated with poor outcome. Intensive chemotherapy, including allogeneic hematopoietic stem cell transplantation (HSCT) for some of these patients, is associated with severe toxicity and long-term sequelae.
In this context, one of the first ALL genetic aberrations identified was the gene fusion BCR-ABL1 resulting from the chromosomal translocation t(9;22) (generating the so-called Philadelphia chromosome), translated into the BCR-ABL1 fusion protein, a constitutively active tyrosine kinase, which can be inhibited by tyrosine kinase inhibitors (TKI). This is an excellent example for a successful molecular treatment target: the addition of the first-generation TKI imatinib to intensive chemotherapy back-bone has led, in fact, to a significant improvement of outcome in children with Philadelphia chromosome positive ALL (Ph+ ALL) with cure rates of 60–70%.10–18 The Children’s Oncology Group (COG) studies showed a clear advantage in Ph+ ALL from continuous protracted exposure to TKI combined with chemotherapy, challenging the indications to transplant for all patients with Ph+ ALL.16–18 COG results were confirmed by the European intergroup study group for treatment of Ph+ ALL (EsPhALL) in the EsPhALL2004 and the subsequent EsPhALL2010 studies, showing that intensive chemotherapy combined with imatinib given continuously from induction phase allows a remarkable reduction in the rate of HSCT, without affecting outcome.13–15 However, these trials also demonstrated that the combination of chemotherapy and imatinib is associated with a high rate of treatment-related toxicity and mortality.
In the last decade, different tyrosine kinase gene fusions other than BCR-ABL1 have been identified which are sensitive to TKI similar as BCR-ABL1. These so called ABL-class fusions typically comprise rearrangements of the ABL1, ABL2, PDGFRB and CSF1R genes, each of which can have different fusion partner genes. ABL-class fusion positive B-ALL subtypes other than BCR-ABL1 have been identified showing a gene expression profile largely similar to that of Ph+ ALL. Therefore, they are included in the BCR-ABL1-/Ph-like-ALL group recognized as a provisional entity in the 2016 World Health Organization classification of myeloid neoplasms and acute leukemia although they only make up a minor proportion of patients in this new category. Whereas BCR-ABL1-/Ph-like-ALL accounts for 15–20% of all pediatric B-ALL, the frequency of ABL-class fusion positive B-ALL is estimated to be about 2-3% which is similar to the frequency of BCR-ABL1 pos. ALL.19–21 BCR-ABL1-/Ph-like ALL is associated with other high-risk clinical features, such as older age, elevated white blood cell (WBC) count at diagnosis, high rates of end-induction minimal residual disease (MRD), as well as increased risk of induction failure and of leukemia relapse.22–28
Data on the ABL-class fusion positive ALL other than Ph+ ALL are rare and are limited to anecdotal case reports. In this study, we retrospectively analyzed clinical characteristics and outcome of ABL-class fusion positive cases treated based on contemporary MRD-based protocols of the Associazione Italiana di Ematologia-Oncologia Pediatrica-Berlin-Frankfurt-Münster (AIEOP-BFM ALL study group. The aim was to provide a comprehensive picture of current outcome of these cases without the addition of a TKI to chemotherapy, and to get first data on those cases in which a TKI was added to chemotherapy. It should serve as a basis on which to decide whether the addition of a TKI to chemotherapy may be beneficial, when taking into account the risk of a relevant increase of toxicity and treatment-related mortality (TRM).
Methods
Patients and diagnostics
This retrospective survey of ABL-class fusion positive B-ALL other than Ph+ ALL was performed in patients aged 1–17 years, treated from October 2000 to August 2018 according to the AIEOP-BFM ALL 2000 and 2009 protocols in Centers in Austria, Australia, Czech Republic, Germany, Israel, Italy, and Switzerland.
Routine diagnostics was performed according to national standards based on protocol requirements.8,9,29–31 Diagnosis of ALL was made when 25% or more lymphoblastic cells were present cytomorphologically in the bone marrow. Flow-cytometry immunophenotyping was performed based on the AIEOP-BFM consensus guidelines.29 Complete remission (CR) was defined as the absence of physical signs of leukemia or detectable leukemia cells on blood smears, a bone marrow with active hematopoiesis and <5% blasts, and morphologically normal cerebrospinal fluid. Presence of ETV6-RUNX1, BCR-ABL1 and KMT2A-AFF1 fusion transcripts was screened as previously described.8,9
ABL-class fusions screening, not required by protocols, was performed in a minority of patients according to the policy of individual centers or due to poor response to treatment. Methods used included fluorescence in situ hybridization (FISH, e.g. using probes by Cytocell®, Cambridge, UK), multiplex or singleplex reverse-transcription polymerase chain reaction (PCR),32 array comparative genomic hybridization (CGH) (Agilent Technologies, Waldbronn, Germany) with subsequent confirmation by panel-based RNA-sequencing (e.g. TruSight RNA Pan-Cancer Panel; Illumina, San Diego, CA, USA), whole transcriptome or direct panel-based RNA-sequencing.
In both protocols, patient stratification was mainly based on quantitative assessment of minimal residual disease (MRD) using clone-specific immunoglobulin- and T-cell receptor-gene rearrangements by PCR (PCR-MRD) after induction (treatment day 33) and consolidation (day 78) therapy. In AIEOP-BFM ALL 2009, MRD was additionally measured by flow cytometry on treatment day 15 (FCM-MRD).33 The logistics of the AIEOP-BFM ALL studies, cell sample isolation, and MRD marker identification, as well as MRD-based risk stratification of the AIEOP-BFM ALL 2000 study, have been previously reported.8,9 In the AIEOP-BFM ALL 2009 trial, patients were additionally allocated to the high-risk (HR) group, when PCR-MRD was ≥5×10−4 on day 33 and still measurable on day 78 and/or if FCM-MRD at day 15 was ≥10%.
Details on chemotherapy regimens and randomized treatment interventions, as well as HSCT indication, were reported else-where.8,9,34 The study AIEOP-BFM ALL 2000 is registered at www.clinicaltrials.gov by BFM as NCT00430118 and by AIEOP as NCT00613457; the study AIEOP-BFM ALL 2009 is registered as NCT01117441. The analyses were approved by local Institutional Review Boards and informed consent was obtained from the patients and/or guardians in accordance with the Declaration of Helsinki.
Statistical analysis
Event-free survival (EFS) was calculated from diagnosis to first failure, which was defined as death during induction therapy, resistance, relapse, death in CR, or development of a second malignant neoplasm (SMN). Rates were calculated according to Kaplan-Meier and compared by log-rank test.35,36 Kaplan-Meier plots that compared HSCT with chemotherapy were adjusted to account for the waiting time to transplantation (with a landmark at median time to HSCT). Cumulative incidence of relapse and TRM functions were constructed by the method of Kalbfleisch and Prentice and compared with Gray test.37,38 Proportional differences between patient groups were analyzed by χ2 or Fisher's exact tests.
Results
We identified 46 ABL-class fusion positive cases diagnosed between October 2000 and August 2018 treated according to AIEOP-BFM ALL 2000 and 2009 protocols.
ABL1 fusions were identified in 15 cases, ABL2 fusions in five cases, CSF1R fusions in three cases, and PDGFRB rearrangements in 23 cases (Online Supplementary Table S1).
Overall, 33 patients received chemotherapy without the addition of any TKI (no-TKI group); a TKI was added on an individual basis and not according to protocol during treatment in 13 cases (TKI group; imatinib in 8 and dasatinib in 5 cases) diagnosed between February 2011 and April 2018. In eight of these 13 cases, TKI was introduced at the end of induction or during consolidation therapy, in four during post-consolidation HR-blocks and in one case after HSCT (for details see Online Supplementary Figure S1).
Altogether, 36 of 46 (78.3%) patients were treated in the HR group [(no-TKI 24 of 33 (72.7%)], TKI 12 of 13 (92.3%)), and HSCT in first CR was performed in 25 of 46 (54.3%) patients: in 16 of 33 (48.5%) of no-TKI and in 9 of 13 (69.3%) of TKI-treated patients (Table 1). Compared to the entire group of B-ALL patients of the AIEOP-BFM ALL 2000 study, ABL-class fusion positive cases were older and had higher white blood cell counts at diagnosis (WBC), with a statistically significant difference for NCI-HR features (P<0.0001 each) (Table 1). ABL-class fusion positive cases had a significantly worse response to treatment compared to the entire B-ALL 2000 cohort: prednisone poor response (PPR) was observed in 50% versus 5.6% of patients with data available, high MRD level (≥5×10−4) after induction treatment (EoI) in 71.4% versus 19.2% and after consolidation (EoC) in 51.2% versus 5.1% of cases with data available. There are, however, differences by type of ABL-class fusion: the majority of PDGFRB-fusion positive cases with data available showed a PPR (17 of 23, 73.9%), high EoI-MRD (20 of 21, 95.2% with data available) as well as high EoC-MRD (15 of 20, 75% with data available). In contrast, in ABL1-class positive cases, PPR (2 of 14, 14.3%), high EoI-MRD (6 of 15, 40.0%), and high EoC-MRD (2 of 14, 14.3%) were observed much less frequently (Table 1). Of note, we observed a favorable MRD response (MRD negative or low-positive) at EoC in 5 of 6 ABL-class positive cases with MRD data available in whom a TKI was added to chemotherapy before EoC (three had an ABL1-fusion and two had a PDGFRB-fusion).
Table 1.
Patients' and clinical characteristics and response to treatment according to ABL-class fusion and tyrosine kinase inhibitor (TKI) treatment in comparison with the entire AIEOP-BFM 2000 B-ALL cohort.
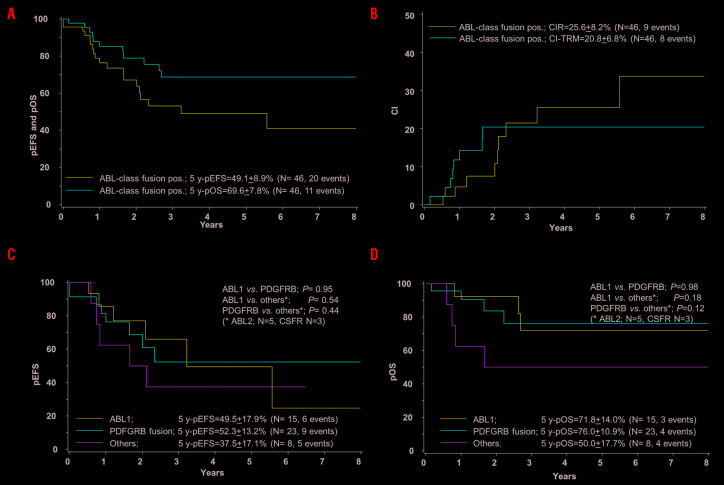
For the entire cohort of 46 cases, 5-year EFS was 49.1+8.9% and 5-year OS 69.6+7.8%; CIR was 25.6+8.2% (9 events) and TRM 20.8+6.8% (8 events) (Figure 1A and B). Six of the nine patients with leukemia relapse in first CR were successfully treated subsequently and are in long-term CR. One case was resistant to treatment (resistance defined as blast persistence after three HR blocks) and two presented with SMN after HSCT (one thyroid cancer and one lymphoma), both patients with SMN were treated successfully. Details of the events are shown in Table 2.
Figure 1.
Treatment outcome of patients with pediatric ABL-class fusion positive acute lymphoblastic leukemia (ALL). Kaplan-Meier estimates for the whole cohort of 46 cases. (A) Event-free survival (pEFS) and overall survival (pOS) at 5 years (y). (B) Cumulative incidence of relapses (CIR) and of treatment-related mortality (CI-TRM) at 5 years. According to ABL-class fusion subtype, ABL1, PDGFRB, others (ABL2 n=5, CSFR n=2): (C) EFS. (D) OS.
Table 2.
Distribution of events according to tyrosine kinase inhibitor (TKI) treatment.
No significant differences in 5-year EFS and 5-year OS were observed comparing the ABL-class subgroups ABL1-fusions, PDGFRB-fusions and other fusions (5 ABL2 and 3 CSFR1 fusions) (Figure 1C and D). Interestingly, the ABL1-fusion positive group, which showed a better treatment sensitivity as compared to the PDGFRB-fusion positive group, had a higher frequency of relapses (5 of 15 patients vs. 3 of 23); of note HSCT was performed only in 2 of 15 ABL1-fusion positive cases versus 17 of 23 PDGFRB-fusion positive cases (Online Supplementary Figure S2A–C).
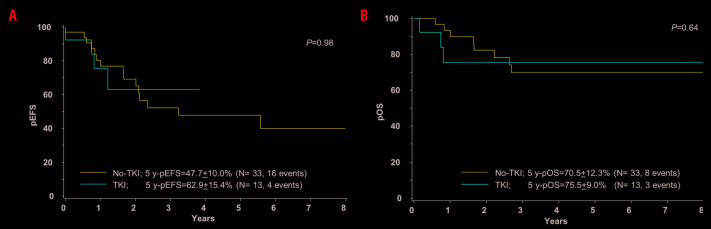
Patients treated with TKI had worse features (12 of 13 were HR vs. 24 of 33 in no-TKI treated patients) and the majority of them underwent HSCT (9 of 13 vs. 16 of 33); their 5-year EFS and 5-year-OS did not differ significantly compared with the no-TKI group: EFS no-TKI 47.7+10.0%, TKI 62.9+5.4%, P=0.98; OS no-TKI 70.9+9.0%, TKI 75.5+12.3%, P=0.64 (Figure 2A and B). Whereas TRM was similar in both groups, only one out of 13 cases of the TKI patients relapsed versus 8 of 33 of no-TKI cases (Online Supplementary Figure S3A and B). Of note, there were four late occurring events in the no-TKI group: two SMN after HSCT and two late relapses in ZMIZ1-ABL1 positive patients not transplanted.
Figure 2.
Treatment outcome of patients with pediatric ABL-class fusion positive acute lymphoblastic leukemia (ALL) according to treatment without or with tyrosine kinase inhibitor (TKI). Kaplan-Meier estimates comparing patients treated without TKI (no-TKI) and with TKI (TKI) are shown. (A) Event-free survival (EFS) at 5 years (y). (B) Overall survival (OS) at 5 years.
No significant difference in outcome between patients treated with or without HSCT were seen: 5-year EFS was 47.9+11.4% versus 55.0+15.5%, P=0.35; 5-year OS 66.7+10.5% versus 84.0+10.6%, P=0.22) (Online Supplementary Figure S4A and B). However, analyzing the events in more detail, it is remarkable that the majority of events in HSCT-treated patients (n=25) were non-relapse events (TRM=6, SMN=2, relapses=3) whereas in patients not transplanted (n= 21) relapses predominated (TRM=1, relapses=6) (Table 2). In those patients who were not treated with TKI, the CIR and TRM rate in transplanted versus not transplanted cases were 13.2+9.3% versus 43.8+17.8%, P=0.06 (CIR) and 32.3+12.4% versus 0.0%, P=0.034 (TRM) (Online Supplementary Figure S4C and D).
Analysis by MRD showed a 5-year EFS of 40.4±11.4% in EoI-MRD≥5×10−4 versus 76.2±14.8% in EoI MRD<5×10−4, P=0.11) (Online Supplementary Figure S5A); CIR was similar in both MRD groups (27.3+11.2% vs. 23.8+15.9%, P=0.74), while TRM in patients with EoI-MRD≥5×10−4 was 25.4+9.4% versus 0.0% in EoI MRD<5×10−4 (P=0.1) (Online Supplementary Figure S5B). By EoC-MRD, 5-year EFS in cases with an MRD≥5×10−4 was 46.2+12.1% versus 56.7+15.4% in those with MRD<5×10−4, P=0.31. However, it should be considered that 19 of 21 patients with EoC-MRD≥5×10−4 received HSCT versus only three of 20 patients with MRD<5×10−4 (Online Supplementary Figure S6).
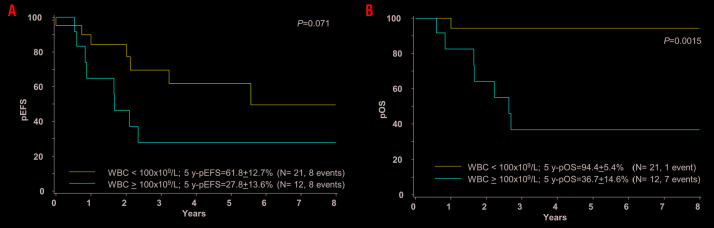
We also analyzed the outcome of patients with a WBC higher or less than 100×109/L: 5-year EFS was 36.8+12.7% versus 59.9+11.6%, respectively, P=0.21, while 5-year OS was significantly lower in patients with a WBC >100×109/L (48.8+12.9% vs. 87.4+6.8%, P=0.036). This difference was more pronounced in the no-TKI group with a 5-year EFS 27.8+13.6% versus 61.8+12.7%, P=0.07 and an OS of 36.7+14.6% versus 94.4+5.4%, respectively, P=0.0015) (Figure 3A and B).
Figure 3.
Treatment outcome of patients with pediatric ABL-class fusion positive acute lymphoblastic leukemia (ALL) according to white blood cell count at diagnosis (WBC). Kaplan-Meier estimates comparing patients with WBC <100×109/L and patients with WBC equal or >100×109/L are shown. (A) Event-free survival (pEFS) at 5 years. (B) Overall survival (pOS) at 5 years.
Discussion
The improvement in genetic diagnostics has allowed the identification of rare ALL subgroups with specific clinical characteristics and target lesions. In this context, ABL-class fusion positive B-ALL other than Ph+ ALL constitutes a challenging entity, which is estimated to represent about 2–3% of childhood ALL. The frequency is reported in the context of the BCR-ABL1-/Ph-like ALL, which is higher in adolescents and associated with higher risk features and a worse outcome as compared to the non-BCR-ABL1-/Ph-like ALL patients.20,21,23,26 First protocols based on “precision medicine” have been designed to identify and treat ALL patients with drugs specific for targetable lesions given in addition to standard chemotherapy (e.g. St Jude Total XVII (NCT03117751) and AALL1131(NCT02883049)). Within the BCR-ABL1-/Ph-like ALL group, it is suggested that patients with ABL-class fusion positive ALL might benefit from the addition of TKI to chemotherapy. However, besides the above mentioned on-going trials, no con-trolled studies have been conducted in this field so far, and the published data on the role of TKI for this subgroup is restricted to case reports.21,39,40
We report here the largest series of ABL-class fusion positive cases, treated according to two consecutive AIEOP-BFM ALL protocols with a stratification mostly based on MRD response. Screening for ABL-class rearrangements was not required by the protocols and was often done retrospectively in the frame of research projects, thus, no conclusions on incidence can be drawn from this retrospective study. Likewise, due to the selection in screening policy, the outcome data need to be interpreted with great caution. Nevertheless, the cohort described here provides interesting information and clearly shows the urgent need for prospective co-operative clinical studies.
When compared with other B-ALL patients recruited in the AIEOP-BFM ALL protocols, the ABL-class fusion positive cases identified in this study included higher proportions of patients aged ten years or older and presenting with hyperleukocytosis (WBC ≥100×109/L). Interestingly, the distribution by age and WBC counts is similar to that of patients with Ph+ ALL included in the EsPhALL studies.15 Poor MRD response at the end of consolidation phase IB (≥5×10−4) was detected in a high proportion of patients with available data (21 of 41, 51%); similar to that of Ph+ ALL patients treated without TKI in the AIEOP-BFM ALL 2000 study, where 22 of 54 (41%) patients had high MRD (≥5×10−4) after consolidation phase.8 Interestingly, in patients who had already been treated with a TKI during consolidation, the majority (6 of 8) had either a low positive or negative EoC-MRD, suggesting a beneficial role of the addition of TKI; however, the low number of TKI-treated patients with EoC-MRD data available does not allow any definitive conclusion to be drawn.
The EFS of the entire cohort was poor, particularly for patients not receiving TKI: <50% at 5 years, very similar to the outcome of Ph+ ALL patients treated in AIEOP-BFM ALL 2000 without TKI.8 This poor outcome was observed even though most patients were treated according to the high-risk schedule and more than half of them underwent HSCT in first CR, a strategy similar to that applied for the cohort of Ph+ ALL patients of the AIEOP-BFM ALL 2000 study.8 Interestingly, the outcome was dismal in patients with WBC ≥100x109/L, as reported also for Ph+ ALL patients treated in the EsPhALL studies.14,15
The impact of HSCT in the cohort reported here is not clear since transplanted patients had an outcome similar to that of patients who were not transplanted. However, patients who received HSCT had worse features, thus, per protocol, an indication for HSCT (and considering the fact that HSCT was associated with a very low rate of relapses), one may infer that HSCT might be effective in disease control. HSCT was, however, also associated with high TRM: six out of 25 patients died of TRM.
Only a minority of our patients (13 of 46) received a TKI (either imatinib or dasatinib). It was not given by protocol; it was used in different schedules, basically decided by treating physicians, and generally due to poor response to chemotherapy. Obviously, the identification of the target lesion had already been achieved during treatment. Of note, most of these patients (9 of 13) also received HSCT, two of them not based on an indication provided by the protocol but on the knowledge of an ABL-class fusion. Although the rate of relapses was low in this small group, their overall outcome was similar to those patients not treated with TKI. Due to the low number of TKI-treated patients, the potentially confounding influence of HSCT and the very short follow up, no conclusions on the benefit of the use of TKI can be drawn from this study. However, given also the biological and clinical similarities with the Ph+ ALL, it is plausible that the early and protracted administration of TKI on top of chemotherapy might improve treatment response and outcome while reducing the need for HSCT in CR1, as shown for Ph+ ALL.14–18
This study, despite being the largest in this field, is limited by its retrospective nature. The complex interaction of confounding factors, such as case selection bias, stratification criteria, chemotherapy intensity, HSCT, and different timing/modalities of delivering targeted therapies, do not allow the benefit of a precision medicine approach to be appropriately assessed. There is, therefore, an urgent need for a prospective controlled clinical trial.
To this purpose, it will be crucial to include the early identification of ABL-class fusion ALL cases in the initial diagnostic work-up of patients and to treat them in a properly designed study to investigate the role of TKI and to identify the appropriate chemotherapy backbone. Given the rarity of this clinical entity, this goal can only be pursued by an international collaborative network, like in pediatric Ph+ ALL.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/105/7/1887
Funding
The project was supported by Grants of Deutsche Krebshilfe, the Madeleine-Schickedanz-Stiftung für Leukämieforschung, the Deutsche Forschungsgemeinschaft (DFG, BE 6555/1-1 and BE 6555/2-1 (GC and AKB)), the Stiftung MHHplus (DSt), the Italian Association for Cancer Research (AIRC) (IG grants to GiC and AB), the Czech Health Research Council - NV15-30626A (MZ) and the Cancer Australia PdCCRs APP1128727 (for RS and DLW). SI and SE were supported by TRAN-SCALL2 from the Israeli Health Ministry and by the Israeli cancer association.
References
- 1.Hunger SP, Mullighan CG. Redefining ALL classification: toward detecting high-risk ALL and implementing precision medicine. Blood. 2015;125(26):3977–3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conter V, Aricò M, Valsecchi MG, et al. Long-term results of the Italian Association of Pediatric Hematology and Oncology (AIEOP) Acute Lymphoblastic Leukemia Studies, 1982–1995. Leukemia. 2000; 14(12):2196–2204. [DOI] [PubMed] [Google Scholar]
- 3.Schrappe M, Reiter A, Zimmermann M, et al. Long-term results of four consecutive trials in childhood ALL performed by the ALL-BFM study group from 1981 to 1995. Leukemia. 2000;14(12):2205–2222. [DOI] [PubMed] [Google Scholar]
- 4.Möricke A, Reiter A, Zimmermann M, et al. Risk-adjusted therapy of acute lymphoblastic leukemia can decrease treatment burden and improve survival: treatment results of 2169 unselected pediatric and adolescent patients enrolled in the trial ALL-BFM 95. Blood. 2008;111(9):4477–4489. [DOI] [PubMed] [Google Scholar]
- 5.Pui CH, Evans WE. Treatment of acute lymphoblastic leukaemia. N Engl J Med. 2006;354(2):166–178. [DOI] [PubMed] [Google Scholar]
- 6.Moghrabi A, Levy DE, Asselin B, et al. Results of the Dana-Farber Cancer Institute ALL Consortium Protocol 95-01 for children with acute lymphoblastic leukemia. Blood. 2007;109(3):896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carroll WL, Bhojwani D, Min DJ, et al. Pediatric acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2003:102–113. [DOI] [PubMed] [Google Scholar]
- 8.Conter V, Bartram CR, Valsecchi MG, et al. Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood. 2010;115(16):3206–3214. [DOI] [PubMed] [Google Scholar]
- 9.Schrappe M, Valsecchi MG, Bartram CR, et al. Late MRD response determines relapse risk overall and in subsets of childhood T-cell ALL: results of the AIEOP-BFM-ALL 2000 study. Blood. 2011;118(8):2077-2084. [DOI] [PubMed] [Google Scholar]
- 10.Bernt KM, Hunger SP. Current concepts in pediatric Philadelphia chromosome-positive acute lymphoblastic leukemia. Front Oncol. 2014;4:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fielding AK. Treatment of Philadelphia chromosome-positive acute lymphoblastic leukemia in adults: a broader range of options, improved outcomes, and more therapeutic dilemmas. Am Soc Clin Oncol Educ Book. 2015:e352–359. [DOI] [PubMed] [Google Scholar]
- 12.Aricò M, Schrappe M, Hunger SP, et al. Clinical outcome of children with newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia treated between 1995 and 2005. J Clin Oncol. 2010;28(31):4755–4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biondi A, Schrappe M, De Lorenzo P, et al. Imatinib after induction for treatment of children and adolescents with Philadelphia-chromosome positive acute lymphoblastic leukaemia (EsPhALL): a randomised, open-label, intergroup study. Lancet Oncol. 2012; 13(9):936–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biondi A, Cario G, De Lorenzo P, et al. Long-term follow up of pediatric Philadelphia positive acute lymphoblastic leukemia treated with the EsPhALL2004 study: high white blood cell count at diagnosis is the strongest prognostic factor. Haematologica. 2019;104(1):13–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biondi A, Gandemer V, De Lorenzo P, et al. Imatinib treatment of paediatric Philadelphia chromosome-positive acute lymphoblastic leukaemia (EsPhALL2010): a prospective, intergroup, open-label, single-arm clinical trial. Lancet Haematol. 2018; 5(12):641–652. [DOI] [PubMed] [Google Scholar]
- 16.Schultz KR, Bowman WP, Aledo A, et al. Improved early event-free survival with imatinib in Philadelphia chromosome-positive acute lymphoblastic leukemia: a children’s oncology group study. J Clin Oncol. 2009;27(31):5175–5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schultz KR, Carroll A, Heerema NA, et al. Long-term follow-up of imatinib in pediatric Philadelphia chromosome-positive acute lymphoblastic leukemia: Children’s Oncology Group Study AALL0031. Leukemia. 2014;28(7):1467–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slayton WB, Schultz KR, Kairalla JA, et al. Dasatinib plus intensive chemotherapy in children, adolescents, and young adults with philadelphia chromosome-positive acute lymphoblastic leukemia: results of Children’s Oncology Group Trial AALL0622. J Clin Oncol. 2018;36(22):2306–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts KG, Yang Y, Turner DP, et al. Oncogenic role and therapeutic targeting of ABL-class and JAK-STAT activating kinase alterations in Ph-like ALL. Blood Adv. 2017;1(20):1657–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Den Boer ML, van Slegtenhorst M, De Menezes RX, et al. A subtype of childhood acute lymphoblastic leukaemia with poor treatment outcome: a genome-wide classification study. Lancet Oncol. 2009;10(2):125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts KG, Li Y, Payne-Turner D, et al. Targetable kinase-activating lesions in Ph- like acute lymphoblastic leukemia. N Engl J Med. 2014;371(11):1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. [DOI] [PubMed] [Google Scholar]
- 23.Boer JM, Koenders JE, van der Holt B, et al. Expression profiling of adult acute lymphoblastic leukemia identifies a BCR-ABL1-like subgroup characterized by high non-response and relapse rates. Haematologica. 2015;100(7):261–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harvey RC, Mullighan CG, Wang X, et al. Identification of novel cluster groups in pediatric high-risk B-precursor acute lymphoblastic leukemia with gene expression profiling: correlation with genome-wide DNA copy number alterations, clinical characteristics, and outcome. Blood. 2010; 116(23):4874–4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Veer A, Waanders E, Pieters R, et al. Independent prognostic value of BCR-ABL1-like signature and IKZF1 deletion, but not high CRLF2 expression, in children with B-cell precursor ALL. Blood. 2013;122(15):2622–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts KG, Pei D, Campana D, et al. Outcomes of children with BCR-ABL1–like acute lymphoblastic leukemia treated with risk-directed therapy based on the levels of minimal residual disease. J Clin Oncol. 2014;32(27):3012–3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boer JM, Steeghs EM, Marchante JR, et al. Tyrosine kinase fusion genes in pediatric BCRABL1-like acute lymphoblastic leukemia. Oncotarget. 2017;8(3):4618–4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reshmi SC, Harvey RC, Roberts KG, et al. Targetable kinase gene fusions in high-risk B-ALL: a study from the Children’s Oncology Group. Blood. 2017; 129(25):3352–3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dworzak MN, Buldini B, Gaipa G, et al. AIEOP-BFM consensus guidelines 2016 for flow cytometric immunophenotyping of Pediatric acute lymphoblastic leukemia. Cytometry B Clin Cytom. 2018;94(1):82–93. [DOI] [PubMed] [Google Scholar]
- 30.van der Velden VH, Cazzaniga G, Schrauder A, et al. European Study Group on MRD detection in ALL (ESG-MRD-ALL). Analysis of minimal residual disease by Ig/TCR gene rearrangements: guidelines for interpretation of real-time quantitative PCR data. Leukemia. 2007;21(4):604–611. [DOI] [PubMed] [Google Scholar]
- 31.van der Does-van den Berg A, Bartram CR, Basso G, et al. Minimal requirements for the diagnosis, classification, and evaluation of the treatment of childhood acute lymphoblastic leukemia (ALL) in the “BFM Family” Cooperative Group. Med Pediatr Oncol. 1992;20(6):497–505. [DOI] [PubMed] [Google Scholar]
- 32.Reshmi SC, Harvey RC, Roberts KG, et al. Targetable kinase gene fusions in high-risk B-ALL: a study from the Children's Oncology Group. Blood. 2017; 129(25):3352–3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basso G, Veltroni M, Valsecchi MG, et al. Risk of relapse of childhood acute lymphoblastic leukemia is predicted by flow cytometric measurement of residual disease on day 15 bone marrow. J Clin Oncol. 2009;27(31):5168–5174. [DOI] [PubMed] [Google Scholar]
- 34.Möricke A, Zimmermann M, Valsecchi MG, et al. Dexamethasone vs prednisone in induction treatment of pediatric ALL: results of the randomized trial AIEOP-BFM ALL 2000. Blood. 2016;127(17):2101–2112. [DOI] [PubMed] [Google Scholar]
- 35.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- 36.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50(3):163–170. [PubMed] [Google Scholar]
- 37.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data (ed 1). New York: John Wiley and sons; 1980:163–188. [Google Scholar]
- 38.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16(3):1141–1154. [Google Scholar]
- 39.Weston BW, Hayden MA, Roberts KG, et al. Tyrosine kinase inhibitor therapy induces remission in a patient with refractory EBF1PDGFRB-positive acute lymphoblastic leukemia. J Clin Oncol. 2013;31(25):413–416. [DOI] [PubMed] [Google Scholar]
- 40.Lengline E, Beldjord K, Dombret H, Soulier J, Boissel N, Clappier E. Successful tyrosine kinase inhibitor therapy in a refractory B-cell precursor acute lymphoblastic leukemia with EBF1-PDGFRB fusion. Haematologica. 2013;98(11):146–148. [DOI] [PMC free article] [PubMed] [Google Scholar]