Plasmacytomas are local tumor deposits of multiple myeloma (MM) that can cause focal symptoms including nerve compression, pain, and fracture. Although systemic therapy is the primary treatment for MM, focal radiation therapy (RT) can provide effective palliation of symptomatic plasmacytomas.1–5 However, RT can also cause irreversible bone marrow fibrosis and myelosuppression, particularly at doses greater than 30 Gy.6 Adequate bone marrow reserve is essential for receipt of systemic therapy and stem cell transplantation. Focal RT must be used in a way that is mindful of the overall dose to the bone marrow.
The optimal RT dose and fractionation for MM is not clearly established, as existing data were collected from small numbers of patients and the ranges of doses used were limited. Hematologic tumors are radiosensitive and lower doses are expected to produce effective palliation. Several studies have shown palliation from RT doses as low as 8–15 Gy.2 However, for patients with spinal myeloma causing cord compression, hypofractionated treatments have been found to be inferior for maintaining or restoring ambulation.7 Existing data are conflicting regarding the optimal radiation dose but suggest that dose escalation is warranted in some situations. Over the last decade, our practice has been to reduce the dose for palliation of MM. The radiation fields that we use in our practice have evolved to become more aligned with “involved site radiation therapy” as defined by the International Lymphoma Radiation Oncology Group (ILROG).5 We undertook this study to understand palliative radiation treatment patterns in a large cohort of patients with MM and to investigate the relationship between radiation dose and local disease control.
We identified all patients aged ≥18 years with a diagnosis of MM who were treated with RT from 1999 through 2017. Charts were reviewed for age, site of disease, radiation dose, and number of radiation fractions. The biologically effective dose (BED) was calculated assuming an α/β of 10 for tumors.8 A treatment site was defined as a site encompassed by a single radiation field. Reirradiation was defined as treatment at a later date with an overlap of the prescription dose of the new treatment with the prescription dose of the prior treatment. Among patients who received spinal RT, we identified those treatments for which magnetic resonance imaging (or positron emission tomography/computed tomography) of the target lesion had been performed no more than 60 days before RT and at any point after RT. Maximum standard uptake values, Bilsky score,9 and paraspinal extent were measured. Local failure was defined as any evidence of radiographic progression within the radiation field on images obtained after treatment. All statistical analyses were done with SPSS (version 22.0, Armonk, NY, USA). Disease-related outcomes were evaluated using the Kaplan-Meier method. Cox proportional hazards testing was used for univariate analyses while multivariable Cox proportional hazards regression models were generated to analyze the effects of multiple variables of interest on disease-related outcomes. All tests were two-sided, with an α of 0.05 as the threshold for statistical significance.
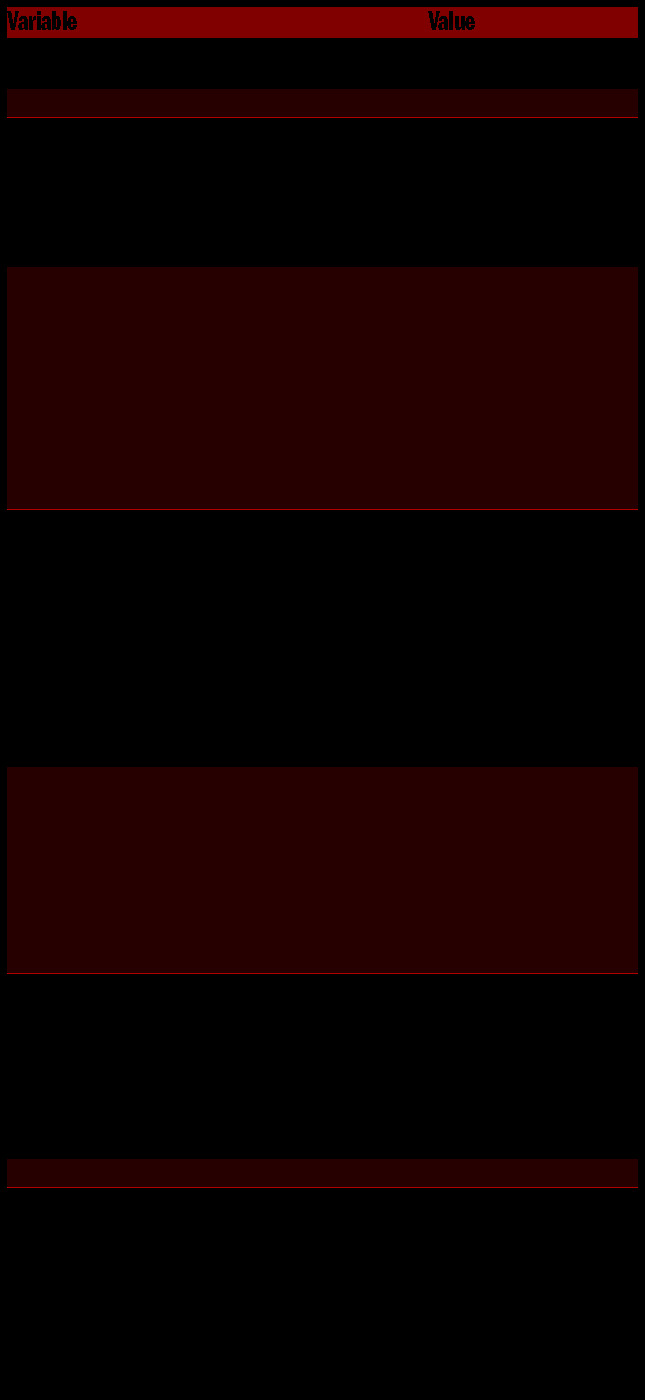
Patient and treatment characteristics are outlined in Table 1. A total of 772 patients given treatment to 1,513 sites were identified. The median follow-up time was 65.6 months after completion of RT. The most common ly treated site was the spine (39.4%, n=596); 55.9% of patients (n=431) were given treatment to a single site, 22.9% (175) were given treatment to two sites and 21.4% (n=166) were given treatment to three or more sites. The maximum number of treated sites in a single patient was 18. The most common dose and fractionation schemes were 2 Gy × 10 (n=413, 27.3%; BED=24 Gy10), 2 Gy × 12 (n=209, 13.8%; BED=28.8 Gy10), 2.5 Gy × 8 (n=135, 8.9%; BED=25 Gy10), and 2.5 Gy × 10 (n=277, 18.3%;, BED=31.25 Gy10). Only 46 sites (3%) were treated with 3 Gy × 10 (BED=39 Gy10). The median BED was 28.8 Gy10 (range, 7.5–60.0 Gy10). Most patients received a regimen involving five or more fractions (n=1,475, 98.5%).
Table 1.
Patient and treatment characteristics.
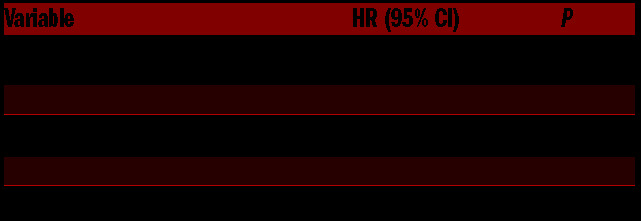
Of the 1,513 sites treated, 39 underwent reirradiation (2.6%). The most common reasons for reirradiation were persistent pain (n=35, 90%) and radiographic progression (n=31, 80%). Analyses to identify dosimetric predictors of reirradiation [RT dose, number of fractions, dose per fraction, and BED (≤ or > 28 Gy10, the population median)] revealed a small but statistically-significant increase in reirradiation rates for BED ≤28 Gy10 (3.25% vs. 1.83%; P=0.02); hazard ratio 6.16 (Table 2). The median overall survival time after completion of the first course of RT was 25.6 months and 1-, 3-, and 5-year rates were 66.5%, 42.0%, and 33.5%, respectively.
Table 2.
Predictors of retreatment.
Since the spine is an important location in which local progression might lead to grave functional disability, we assessed radiographic local control for 82 treated spinal lesions for which magnetic resonance imaging or positron emission tomography/computed tomography scans of the target lesion were available both before and after RT. Most patients (n=56, 68%) had evidence of epidural extension (Bilsky score ≥1) and most had paraspinal extension (n=40, 60%). For the 82 spinal lesions treated, the most common RT regimens were 2 Gy × 10 (n=22, 26.8%; BED=24 Gy10) and 2 Gy × 12 (n=19, 23.2%; BED=28.8 Gy10). Of note, only four of the 82 sites were treated with a total cumulative dose of 30 Gy or higher. Local radiographic failure, defined as failure occurring at least partially within the radiation field, was observed for ten treated sites (12.2%). Local control rates were 86.3% at 2 years and 75.0% at 5 years. Univariate analysis to identify tumor and treatment characteristics able to predict local failure indicated significant associations between local failure and both tumor Bilsky score and paraspinal thickness (Table 3). Sites with a Bilsky score of 3 had a substantially higher local failure rate than sites with a Bilsky score of 0–2 (hazard ratio=7.14; P=0.01). Axial paraspinal extension when treated as a continuous variable was also associated with an increased risk of failure (hazard ratio=1.98; P=0.02), with a notable increase in risk for paraspinal extension greater than 5 mm.
Table 3.
Predictors of local treatment failure in the spine.

The influence of radiation dose on bone marrow fibrosis was reported on by Casanassima et al., who used magnetic resonance imaging to examine changes in adipocytes in treated marrow as a surrogate of marrow recovery after irradiation.6 They found that doses below 30 Gy given in 2-Gy fractions (BED 36 Gy10) were associated with a high probability of marrow recovery, where-as recovery after doses of >36 Gy (BED ≥43.2 Gy10) was rare. Our retrospective analysis shows a low rate of reirradiation in MM patients despite radiation dose reduction. Overall, reirradation was rare at 2.6%, with the majority of patients receiving doses of 20–25 Gy. Long-term survival following RT and multiple courses of RT were common, highlighting the importance of dose reduction to reduce long-term toxicity. Spinal tumors with cord compression or paraspinal extension were associated with an increased risk of local relapse and may warrant dose escalation. Our results are of particular relevance for clinical practice since appropriate radiation dose reduction is expected to spare marrow toxicity in this population of patients.
The ILROG recently published guidelines on the use of RT in MM and recommends doses of 8 Gy in one fraction, 20 Gy in five fractions, or 20–30 Gy in 10 to 20 fractions, for palliation of painful osseous plasmacytomas.5 A dose of 30 Gy in 10–15 fractions is recommended for bulky masses or spinal cord compression when durable control is desired. Our findings are largely consistent with this recommendation; however, we would emphasize that 30 Gy in ten fractions, a dose commonly used in palliative radiation, is unnecessary for uncomplicated painful osseous plasmacytomas not causing local compression. Our study and others have shown a total dose of 20 Gy given in 2-2.5 Gy daily fractions provides durable pain relief, can minimize marrow fibrosis (particularly for the large subset of patients requiring treatment to multiple bony sites), and is low enough that effective salvage reirradiation can be delivered safely without concern for significant reirradiation-related toxicity in most parts of the body.10
Our study has numerous limitations. It is a retrospective analysis spanning a long period of time with a heterogenous patient population. As systemic therapies have improved, local control and reirradiation rates following radiation could have been affected. However the impact of systemic therapy is difficult to tease out in such a large cohort of patients, particularly as radiation is often used as a supportive measure after patients have received multiple lines of systemic therapy. Our data show a low rate of reirradiation even in patients treated with low-dose radiation, and as systemic therapies continue to improve, we expect that reduction of radiation dose will continue to be effective and minimization of chronic toxicities will become even more critical.
In conclusion, we report here a large number of patients treated with radiation at a single institution for plasmacytoma over a period of nearly two decades. We found that for the majority of patients, reirradation was rare, the rate being 2.6%. Dose escalation to a BED of at least 28 Gy is recommended when durable local control is desired. We hope that these findings are useful for practitioners caring for patients with MM and symptomatic plasmacytoma.
Acknowledgments
We thank Christine Wogan, MS, ELS, of MD Anderson’s Division of Radiation Oncology, for her assistance editing this manuscript
Footnotes
Funding: supported in part by Cancer Center Support (Core) Grant P30 CA016672 from the National Cancer Institute, National Institutes of Health, to The University of Texas MD Anderson Cancer Center.
Information on authorship, contributions, and financial & other dis-closures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Catell D, Kogen Z, Donahue B, Steinfeld A. Multiple myeloma of an extremity: must the entire bone be treated? Int J Radiat Oncol Biol Phys. 1998;40(1):117-119. [DOI] [PubMed] [Google Scholar]
- 2.Leigh BR, Kurtts TA, Mack CF, Matzner MB, Shimm DS. Radiation therapy for the palliation of multiple myeloma. Int J Radiat Oncol Biol Phys. 1993;25(5):801-804. [DOI] [PubMed] [Google Scholar]
- 3.Mill WB, Griffith R. The role of radiation therapy in the management of plasma cell tumors. Cancer. 1980;45(4):647-652. [DOI] [PubMed] [Google Scholar]
- 4.Rao G, Ha CS, Chakrabarti I, Feiz-Erfan I, Mendel E, Rhines LD. Multiple myeloma of the cervical spine: treatment strategies for pain and spinal instability. J Neurosurg Spine. 2006;5(2):140-145. [DOI] [PubMed] [Google Scholar]
- 5.Tsang RW, Campbell BA, Goda JS, et al. Radiation therapy for solitary plasmacytoma and multiple myeloma: guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys. 2018;101(4):794-808. [DOI] [PubMed] [Google Scholar]
- 6.Casamassima F, Ruggiero C, Caramella D, Tinacci E, Villari N, Ruggiero M. Hematopoietic bone marrow recovery after radiation therapy: MRI evaluation. Blood. 1989;73(6):1677-1681. [PubMed] [Google Scholar]
- 7.Rades D, Hoskin PJ, Stalpers LJA, et al. Short-course radiotherapy is not optimal for spinal cord compression due to myeloma. Int J Radiat Oncol. 2006;64(5):1452-1457. [DOI] [PubMed] [Google Scholar]
- 8.Hall EJ, Giaccia AJ. Radiobiology for the Radiologist. Eighth edition. Wolters Kluwer; 2018. [Google Scholar]
- 9.Bilsky MH, Laufer I, Fourney DR, et al. Reliability analysis of the epidural spinal cord compression scale. J Neurosurg Spine. 2010;13(3):324-328. [DOI] [PubMed] [Google Scholar]
- 10.Wang S, Salgado LR, Adler A, et al. Dose selection for multiple myeloma in modern era. Pract Radiat Oncol. 2019;9(4):e400-e406. [DOI] [PubMed] [Google Scholar]


