Abstract
The main recurrence pattern for lung cancer patients after radical surgery is distant metastasis. The probability of pancreatic metastasis in patients diagnosed with lung squamous cell carcinoma is 0.02%, with a poor prognosis. Chemotherapy is the preferred treatment for recurrence. Single lesions or oligometastasis can be surgically resected, and local lesions with compression symptoms can be treated with radiotherapy. The FDA and NMPA have approved first‐line indications for immunotherapy for lung squamous cell carcinoma. Here, we report the case of a 57‐year‐old male patient with lung squamous cell carcinoma who developed pancreatic metastasis after radical resection. The disease progressed after first‐line chemotherapy, and the patient was treated with immunotherapy combined with radiotherapy. We subsequently observed the abscopal effect of intensity modulated radiation therapy (IMRT) and pembrolizumab with disappearance of lung metastasis after radiotherapy for pancreatic metastasis. The patient’s tumor symptoms were relieved with prolonged survival.
Keywords: Abscopal effect, immunotherapy, lung cancer, metastasis, pancreatic, radiotherapy
Introduction
The abscopal effect is a phenomenon in which localized radiation treatment is associated with cancer regression at a site distant from that irradiated.1, 2, 3 Intensity modulated radiation therapy (IMRT) delivers precise high‐dose radiation treatments to tumors while minimizing exposure to surrounding normal tissues.4
The abscopal effect after radiation treatment is theorized to result from radiation “priming” of the immune system, enhancing immune recognition through increased antigen presentation resulting in antitumor effects outside irradiated areas. Here, we report a rare case of primary lung cancer regression after IMRT and pembrolizumab with disappearance of lung metastasis after radiotherapy for pancreatic metastasis.
Case report
A 57‐year‐old Asian male with 34 years history of smoking 20 cigarettes a day was diagnosed with right upper lung squamous cell carcinoma (cT1bN0M0, Ia, AJCC V7) by tracheobronchoscopy on 27 May 2016. The positron emission tomography‐computed tomography (PET‐CT) scan demonstrated the lesion size was 2.1 × 1.5 × 1.8 cm with SUVmax 9.1.
Video‐assisted thoracoscopic surgery (VATS) right upper lung resection, bronchoplasty and mediastinal lymph node dissection was performed on 23 June. An excisional biopsy of the mass demonstrated poorly‐differentiated lung squamous cell carcinoma, invading the bronchus, without involving the lung membrane, and lymph nodes with chronic inflammation. The resection margin of the tumor was 0.4 cm. Pathological staging was pT1bN0M0, Ia, AJCC V7. Postoperatively, the multidisciplinary team (MDT) recommended adjuvant radiotherapy (6 MV X‐ray intensity‐modulated radiotherapy, 55 Gy/25 fractions).
The PET‐CT scan demonstrated pancreatic metastasis (rcTxNxM1) on 11 May 2017. Laparoscopic radical pancreatic body tail and splenectomy were performed on 24 May. An excisional biopsy of the mass demonstrated invasion of medium differentiated squamous cell carcinoma, invasion of peripancreatic adipose tissue, intravascular tumor thrombus, and lymph node metastasis (rpTxNxM1). One month post operation, chest and abdomen CT scan demonstrated metastatic nodules in the left lower lobe, with no recurrence in the abdominal cavity.
Four cycles of chemotherapy were administered from 27 June to 8 September. The treatment regimen consisted of gemcitabine (1000 mg/m2, d1, d8) plus cisplatin (65 mg/m2, d1) (GP). According to the CT scan on 19 September, there was stable disease. The maintenance phase of gemcitabine for one cycle started on 24 October.
The expression rates of PD‐L1 (SP‐263) in primary lung cancer and pancreatic metastasis were 60% and 70%, respectively (Fig 1). The whole exome sequencing (WES) analysis of pancreatic metastatic tissue showed PLXND1–ALK fusion which was not included in the Catalogue of Somatic Mutations in Cancer (COSMIC) database. We tested the primary and metastatic tissues using a fluorescent in situ hybridization (FISH) assay which indicated a negative ALK genetic status. ALK inhibitors were therefore not considered for further treatment. The CT demonstrated the left lung lesions were enlarged, and a new neoplasm was found in the pancreatic head on 16 November, at which stage progression‐free survival (PFS) had reached 4.6 months. The patient then received three cycles pembrolizumab (150 mg, 2 mg/kg) from 22 November to 2 January 2018. The pancreatic head received IMRT (60.2 Gy/28 fractions, Fig 2) from 5 December to 16 January 2018, and the patient’s abdominal pain was relieved. The CT scan demonstrated that the left lung nodules had disappeared, and the metastatic head of the pancreas was stable on 22 February (Fig 3). However, a pancreatic fistula subsequently occurred which led to aggravated abdominal pain.
Figure 1.
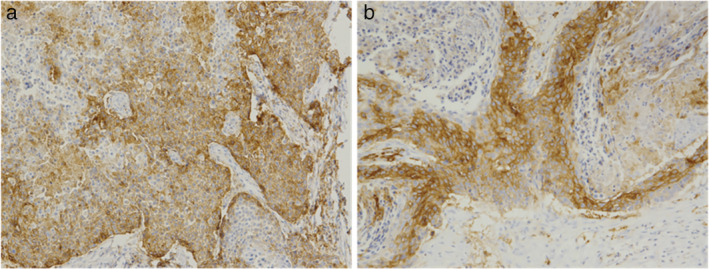
The PD‐L1 expression rates in (a) primary lung lesions were 60% and (b) pancreatic metastasis were 70% after adjuvant radiotherapy.
Figure 2.
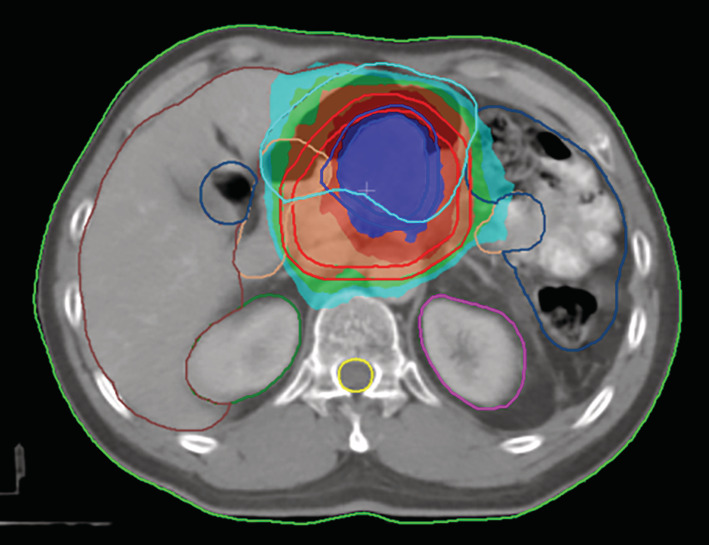
Transverse images of the dose distribution of radiotherapy. The patient received intensity modulated radiation therapy (IMRT); isodose lines of 6020 cGy (dark blue), 5544 cGy (dark red), 5040 cGy (orange), 4536 cGy (green), 4032 cGy (medium green), 3800 cGy (light green) and the planned target volume (shadow area) are shown.
Figure 3.
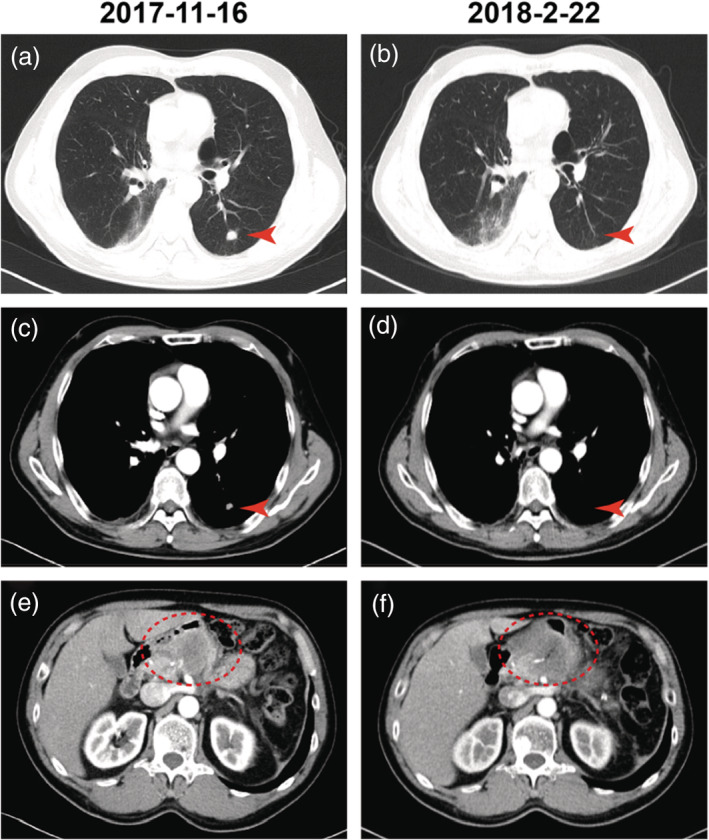
Computed tomography (CT) scans of the patient demonstrate disease regression. (a), (c), (e) CT scans indicate the lesions before and (b), (d) ,(f) 92 days after the induction of pembrolizumab and intensity modulated radiation therapy (IMRT). The red arrow indicates the primary lung lesions, and the red dotted oval circle indicates pancreatic head metastatic lesions.
After symptomatic treatment, the patient died in March 2018. The PFS of immunotherapy combined with radiotherapy was three months, and the overall survival (OS) was 21.1 months.
Discussion
Surgical resection is the preferred treatment for early stage lung cancer. However, the five‐year survival rate of stage I lung cancer is reported to be only 70%. For patients with oligometastasis, palliative resection can be considered. The patient in our study had stage Ia squamous cell carcinoma and underwent surgery.5 The tumor margin was 0.4 cm away from the cut end, and the patient received adjuvant radiotherapy which can prevent local recurrence. In the case reported here, local pancreatic metastasis occurred after disease‐free survival (DFS) for 11 months and was then resected.
Gemcitabine plus cisplatin was administered as the first‐line treatment recommended for advanced squamous cell carcinoma according to ECOG 1594.6 A phase III trial reported that patients with KPS ≥80 benefited from single‐agent gemcitabine maintenance treatment.7 In our study, the CT scan showed stable disease after four cycles of GP and one cycle of gemcitabine maintenance therapy, and the PFS of the patient reached 4.6 months.
The immunotherapy had been approved for the second line of lung cancer at that time. The PD‐L1 expression detection was not required. In this case, the expression of PD‐L1 (SP263) in the primary lesion was 60%, but increased to 70% after radiotherapy. Several studies have reported that radiotherapy can induce the upregulation of PD‐L1 expression in tumors and predict the efficacy of immunotherapy.8, 9
The abscopal effect of radiotherapy combined with immunotherapy in the treatment of advanced lung cancer and Hodgkin's lymphoma have been reported to be highly efficient.5, 10 To our knowledge, here we have reported the first case of the abscopal effect in lung squamous cell carcinoma. After radiotherapy combined with pembrolizumab, the lung lesions disappeared and the patient’s pancreatic metastasis was stable.
Poleszczuk et al. found that the efficacy and safety of the abscopal effect was related to the site of radiotherapy.4 A previous study found that a combination of radiotherapy targeting intra‐abdominal organ metastasis and immune checkpoint inhibitors was more effective in liver than in lung cancer.11 The probability of pancreatic metastasis in lung squamous cell carcinoma has been reported to be about 0.02%,12 and the data available on radiotherapy targeting the pancreas combined with immunotherapy is unclear. In the case reported here, the abdominal pain was relieved after radiotherapy combined with immunotherapy.
In our study, trafficking and distribution between metastatic lesions of activated T lymphocytes was thought to have resulted in the disappearance of lung metastasis, while tumor necrosis could have caused the pancreatic fistula during tumor shrinkage. However, the exact cause of death was not confirmed due to the inability to perform an autopsy. Questions remain about how to avoid and treat this life‐threatening complication, and radiotherapy dose segmentation, irradiation site, timing and so on. The combination of immunotherapy and radiotherapy needs further exploration. Our colleagues should therefore be alerted to this potential complication when treating patients with pancreatic lesions with a combination of immunotherapy and radiotherapy.
Disclosure
The authors report that there are no potential conflicts of interest.
Contributor Information
Weiwei Wang, Email: wangwwben@126.com.
Li Li, Email: lili11011@sina.com.
Shanqing Li, Email: lidanqing@pumch.cn.
References
- 1. Reynders K, Illidge T, Siva S, Chang JY, De Ruysscher D. The abscopal effect of local radiotherapy: Using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev 2015; 41 (6): 503–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abuodeh Y, Venkat P, Kim S. Systematic review of case reports on the abscopal effect. Curr Prob Cancer 2016; 40(1): 25–37. 10.1016/j.currproblcancer.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 3. Garnett CT, Palena C, Chakraborty M, Tsang KY, Schlom J, Hodge JW. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res 2004; 64 (21): 7985–94. [DOI] [PubMed] [Google Scholar]
- 4. Poleszczuk JT, Luddy KA, Prokopiou S e a. Abscopal benefits of localized radiotherapy depend on activated T‐cell trafficking and distribution between metastatic lesions. Cancer Res 2016; 76 (5): 1009–18. [DOI] [PubMed] [Google Scholar]
- 5. Bitran J. The abscopal effect exists in non‐small cell lung cancer: A case report and review of the literature. Cureus 2019; 11 (2): e4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hoang T, Dahlberg SE, Schiller JH, Johnson DH. Does histology predict survival of advanced non‐small cell lung cancer patients treated with platin‐based chemotherapy? An analysis of the eastern cooperative oncology group study E1594. Lung Cancer 2013; 81 (1): 47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brodowicz T, Krzakowski M, Zwitter M et al Cisplatin and gemcitabine first‐line chemotherapy followed by maintenance gemcitabine or best supportive care in advanced non‐small cell lung cancer: A phase III trial. Lung Cancer 2006; 52 (2): 155–63. [DOI] [PubMed] [Google Scholar]
- 8. Dovedi SJ, Adlard AL, Lipowska‐Bhalla G e a. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD‐L1 blockade. Cancer Res 2014; 74 (19): 5458–68. [DOI] [PubMed] [Google Scholar]
- 9. Yoneda K, Kuwata T, Kanayama M e a. Alteration in tumoural PD‐L1 expression and stromal CD8‐positive tumour‐infiltrating lymphocytes after concurrent chemo‐radiotherapy for non‐small cell lung cancer. Br J Cancer 2019; 121 (6): 490–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Michot JM, Mazeron R, Dercle L e a. Abscopal effect in a Hodgkin lymphoma patient treated by an anti‐programmed death 1 antibody. Eur J Cancer 2016; 66: 91–4. [DOI] [PubMed] [Google Scholar]
- 11. Tang C, Welsh JW, de Groot P e a. Ipilimumab with stereotactic ablative radiation therapy: Phase I results and immunologic correlates from peripheral T cells. Clin Cancer Res 2017; 23 (6): 1388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang Y, Chen M, Zhao J, Zhong W, Xu Y, Wang M. [a retrospective study of 42 lung cancer patients with pancreatic metastases]. Zhongguo fei ai za zhi = . Chin J Lung Cancer 2019; 22 (4): 228–32. [DOI] [PMC free article] [PubMed] [Google Scholar]


