Abstract
Background
Research on diagnosing recurrent non‐small cell lung cancer (NSCLC) and applying target gene treatment using exosomes in a less invasive way is very important. Recently, however, it has been argued that exosomes do not contain double‐stranded DNA (dsDNA) or histones. In this study, we describe the expression of extracellular vesicle (EV) markers in specimens from squamous cell carcinoma (SCC) of the lung and analyze their relationship with the prognosis of patients.
Methods
Clinical and pathological data were obtained from 96 patients who had undergone surgery for SCC of the lung. Tissue microarray blocks were made using representative paraffin blocks of samples from patients with SCC of the lung. Two pathologists graded the intensity of CD63, CD9, LC3A/B, P62, and ANXA1 expression as high or low expression. In addition, the authors designated the combined expression of these five independent markers as “positive EV expression” in this article.
Results
SCCs with low CD63 and SCCs with low EV expression showed unfavorable disease‐free survival (DFS) (P‐value = 0.037 and 0.006, respectively) in the survival analysis. The Kaplan‐Meier survival curve confirmed that the low EV expression showed a statistically significant relationship with unfavorable DFS (P‐value = 0.004). There were no statistically significant differences in DFS and disease‐specific survival in each low and high expression group for CD9, LC3A/B, ANXA1, and P62 in the Cox regression analysis.
Conclusions
As EV expression was related to the prognosis of lung SCC patients, a broader approach using different extracellular vesicles rather than a conventional exosome‐dependent one is needed.
Keywords: Amphisome, exosome, extracellular vesicles, lung, squamous cell carcinoma
Introduction
Research on how extracellular vesicles (EVs) shed from cancer cells affect tumorigenesis has been performed.1, 2 In particular, strategies to diagnose recurrent non‐small cell lung cancer (NSCLC) and to apply target gene treatment using EVs are less invasive than percutaneous core needle biopsies. EVs are very small structures of less than 1000 nm in size that are composed of a lipid bilayer and internal cargo and lipids. EVs are secreted from any kind of cell, 3 and they contain several kinds of genetic material, including microRNAs, other noncoding RNAs, and DNA that might be useful for either diagnosing or treating cancers. EVs are generally categorized by size: exosomes, between 30 and 100 nm; microvesicles, between 100 and 1000 nm; and apoptotic bodies, over 1000 nm.4, 5 Jeppesen and colleagues established new nomenclature for extracellular vesicles and proposed markers of intracellular precursors of EVs: CD63 and CD9 for multivesicular endosomes (MVEs), P62, and LC3A/B for amphisomes, and ANXA1 for a novel marker for microvesicles.6, 7 To investigate the prognostic role of EVs in pulmonary SCC patients, each EV marker was analyzed on a pulmonary SCC specimen using immunohistochemical staining. We designated the combined expression of these five immunohistochemical markers as “positive EV expression” in this article. This study aims to confirm the previous in vitro results of Jeppesen et al. 6 and to suggest a broad approach using different extracellular vesicles for patients with SCC of the lung.
Methods
Patients and clinicopathological data
A total of 96 patients who had undergone surgery for SCC of the lung between January 2002 and December 2009 at the Gyeongsang National University Hospital, Jinju, South Korea, were enrolled for the study. Representative hematoxylin and eosin (H&E)‐stained slides from the 96 consecutive patients were re‐examined by two pathologists. Electronic medical records were reviewed, and the clinical and pathological data, including age, sex, smoking history, surgery history, pathologic differentiation, TNM stage, T stage, N stage, disease‐free survival (DFS), and disease‐specific survival (DSS), were obtained. The stages of lung cancer were determined according to the eighth edition guidelines of the American Joint Committee on Cancer (AJCC). This study was approved by the institutional review board of the Gyeongsang National University Hospital (GNUH‐2020‐04‐005).
Tissue microarray construction and immunohistochemistry
Representative H&E‐stained glass slides containing intratumoral lesions were examined. A core (3 mm in size) was collected from the invasive tumor front of each representative paraffin block and transplanted into the recipient tumor microarray (TMA) blocks. Immunohistochemical staining was carried out using an automated immunostainer (Benchmark Ultra, Ventana Medical Systems Inc., Tucson, AZ, USA) with monoclonal antibodies: anti‐CD9 antibody at a dilution of 1:50 (ab2215, Abcam, Cambridge, MA, USA, anti‐CD63 antibody at a dilution of 1:500 (ab134045, Abcam, Cambridge, MA, USA), anti‐LC3A/B antibody at a dilution of 1:100 (ab128025, Abcam, Cambridge, MA, USA), anti‐P62 antibody at a dilution of 1:2000 (ab56416, Abcam, Cambridge, MA, USA), and anti‐ANXA1 antibody at a dilution of 1:100 (ab33061, Abcam, Cambridge, MA, USA).
EV expression
The pattern of immunohistochemical staining was evaluated: nuclear expression for CD63, the membranous expression for CD9, the nuclear and cytoplasmic expression for P62 and LC3AB, and cytoplasmic expression for ANXA1 in the tumor cells. The expression patterns of these markers were compared with each clinicopathological characteristic, including age, sex, smoking and surgical history, pathological differentiation, T stage, and N stage (Table 1). The intensity of stained tumor cells was graded as either high or low. When setting the EV value, the immunohistochemical staining value of each EV marker was characterized as low with one point and as high with two points, and the sum of the scores for the five values was classified as low with six points and as high with more than seven points. Tumor‐infiltrating immune cells were used as the internal control for each marker. Tumor cells that stained stronger than tumor‐infiltrating immune cells were classified as having high expression, and other tumor cells were classified as having low expression. If tumor cells showed a heterogeneous pattern of expression in the same TMA core, the representative value was decided according to the majority of the tumor cells. All the samples were individually reviewed by two pathologists to confirm the reproducibility.
Table 1.
Relationship between each EV markers and clinicopathological characteristics
| CD9 | CD63 | LC3A/B | ANXA1 | P62 | EV | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Low | High | P‐value | Low | High | P‐value | Low | High | P‐value | Low | High | P‐value | Low | High | P‐value | Low | High | P‐value |
| Age (years) | 0.231 | 0.517 | 0.031 | 0.155 | 0.566 | 0.160 | ||||||||||||
| <65 | 30 | 4 | 31 | 2 | 30 | 4 | 31 | 3 | 15 | 18 | 27 | 6 | ||||||
| ≥65 | 47 | 13 | 54 | 6 | 41 | 19 | 48 | 12 | 31 | 29 | 41 | 19 | ||||||
| Gender | 0.556 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | ||||||||||||
| Male | 74 | 16 | 82 | 8 | 69 | 22 | 76 | 15 | 45 | 45 | 66 | 24 | ||||||
| Female | 3 | 1 | 3 | 0 | 2 | 1 | 3 | 0 | 1 | 2 | 2 | 1 | ||||||
| Smoking | 0.546 | 0.675 | 0.413 | 0.753 | 0.593 | 0.809 | ||||||||||||
| Non‐smoker | 21 | 3 | 23 | 1 | 20 | 4 | 21 | 3 | 13 | 11 | 18 | 6 | ||||||
| Smoker | 56 | 14 | 62 | 7 | 51 | 19 | 58 | 12 | 33 | 36 | 50 | 19 | ||||||
| Surgery | 1.000 | 0.343 | 0.551 | 0.016 | 0.450 | 0.387 | ||||||||||||
| Lobectomy | 63 | 14 | 68 | 8 | 57 | 20 | 68 | 9 | 39 | 37 | 57 | 19 | ||||||
| More invasive* | 14 | 3 | 17 | 0 | 14 | 3 | 11 | 6 | 7 | 10 | 11 | 6 | ||||||
| Pathologic differentiation | 0.754 | 0.388 | 0.828 | 0.018 | 0.301 | 0.168 | ||||||||||||
| W/D, M/D | 58 | 14 | 66 | 5 | 54 | 18 | 57 | 15 | 33 | 38 | 49 | 22 | ||||||
| P/D | 19 | 3 | 19 | 3 | 17 | 5 | 22 | 0 | 13 | 9 | 19 | 3 | ||||||
| T stage | 0.048 | 1.000 | 0.008 | 0.519 | 0.218 | 0.229 | ||||||||||||
| T1 | 18 | 8 | 23 | 2 | 14 | 11 | 20 | 5 | 15 | 10 | 16 | 9 | ||||||
| T2,3,4 | 59 | 9 | 62 | 6 | 57 | 12 | 59 | 10 | 31 | 37 | 52 | 16 | ||||||
| N stage | 0.140 | 0.470 | 0.405 | 0.591 | 0.162 | 0.980 | ||||||||||||
| N0 | 44 | 13 | 50 | 6 | 44 | 12 | 48 | 8 | 31 | 25 | 41 | 15 | ||||||
| N1,2,3 | 33 | 4 | 35 | 2 | 27 | 11 | 31 | 7 | 15 | 22 | 27 | 10 | ||||||
Bilobectomy, sleeve lobectomy or pneumonectomy; EV, representative value of panel (value = CD9 + CD63 + LC3A/B + ANXA1 + P62); M/D, moderately‐differentiated; P, P‐value; P/D, poorly‐differentiated; W/D, well‐differentiated.
The number of valid specimen is variable from 93 to 94 due to loss of core.
Statistical analysis
The relationships between EV marker expression and pathological or clinical data were evaluated by Pearson's chi‐square test and Fisher's exact test. DFS and DSS were evaluated by the Kaplan‐Meier method and a Cox proportional hazard regression model among each group. P‐values less than 0.05 were considered statistically significant. All statistical analyses were performed using IBM SPSS ver. 24.0 (IBM Corp., Armonk, NY, USA).
Results
Clinicopathological data of the patients
A total of 96 patients with lung SCC were enrolled in this study. The clinical and pathological parameters of the patients were analyzed for their relationship with each EV marker, and the results are summarized in Table 1. The mean age of the patients was 65.68 years. Among them, 92 (95.8%) were males, and 72 (75.0%) had a smoking history. The pathological differentiation of tumors was as follows: 15 (15.6%) specimens were well‐differentiated, 58 (60.4%) were moderately‐differentiated, and 23 (24.0%) were poorly‐differentiated. Of all the patients, 79 patients (82.3%) underwent lobectomy, while the remaining 17 patients (17.7%) underwent more invasive procedures, including bilobectomy, sleeve lobectomy, and pneumonectomy. In terms of TNM stage, 36 (37.6%) samples were stage I, 45 (46.9%) were stage II, 14 (14.6%) were stage III, and one (1.0%) was stage IV. The distribution of pathological T stages was as follows: T1mi, 0 (0%); T1a, 0 (0%); T1b, 12 (12.5%); T1c, 15 (15.6%); T2a, 33 (34.4%); T2b, 12 (12.5%); T3, 17 (17.7%); and T4, 7 (7.3%). The distribution of N stages was as follows: N0, 58 (60.4%); N1, 35 (36.5%); N2, three (3.1%); and N3, 0 (0%). The distribution of M stages was as follows: M0, 95 (99.0%); M1a, one (1.0%); M1b, 0 (0%); and M1c, 0 (0%).
Relationship between expression of each EV marker and clinicopathological data.
The relationships between the expression of each EV marker and patient characteristics are shown in Table 1. CD9 and LC3A/B expression was significantly related to pathological T stage (P‐value = 0.048 and 0.008, respectively). In addition, LC3A/B expression was also significantly related to the patient's age (P‐value = 0.031). Meanwhile, ANXA1 expression, a novel microvesicle marker, showed a statistically significant association with the operating method and pathological differentiation of SCC (P‐value = 0.016 and 0.018, respectively). EV expression, which was a combination of the representative values of the immunohistochemical staining panel (value = CD9 + CD63 + LC3A/B + ANXA1 + P62), did not show any statistical relevance to any of the variables.
Staining patterns of each EV marker and their statistical significance
Samples with high CD9 expression (Fig 1a) showed weaker but diffuse membranous staining patterns than those with low CD9 expression (Fig 1f). Although the intensity of high CD9 expression was very weak, it was stronger than that of immune cells. Samples with high CD63 expression (Fig 1b) demonstrated stronger patchy positive nuclear intensity than those with low CD63 expression (Fig 1g). Both the samples with high LC3 expression (Fig 1c) and the samples with high P62 expression (Fig 1d) showed strong and diffuse nuclear and cytoplasmic expression. Samples with low P62 expression (Fig. 1i) showed very weakly positive cytoplasmic staining patterns, and it was much weaker than that in the immune cells. High ANXA1 samples (Fig 1e) showed strong and diffuse cytoplasmic expression patterns compared with ANXA1‐negative samples (Fig 1j). Table 2. demonstrates the P‐values of chi‐square tests between each EV marker on each TMA core. Among the five markers described above, CD9 and CD63 showed statistical significance (P‐value = 0.027). In addition, ANXA1, a novel microvesicle marker and CD9 (P‐value = 0.001) and CD63 (P‐value = 0.001) showed statistical significance.
Figure 1.
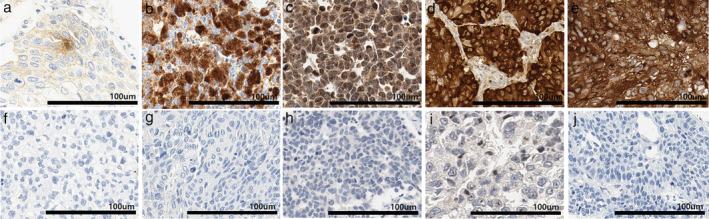
Expression of each extracellular vesicle marker in SSCs. (a) High membranous CD9 expression, (b) high nuclear expression for CD63, (c) high nuclear and cytoplasmic expression for LC3A/B and (d) P62, and (e) high cytoplasmic expression for ANXA1 was seen in tumor cells. In contrast, SCC tumor cells with (f) low CD9 expression, (g) low 63 expression, (h) low LC3A/B expression, (i) low P62 expression, and (j) low ANXA1 expression showed lower staining intensity than intratumoral immune cells.
Table 2.
Statistical significance in each EV marker
| P‐value | CD9 | CD63 | LC3A/B | ANXA1 | P62 | EV |
|---|---|---|---|---|---|---|
| CD9 | 0.027 | 0.072 | 0.001 | 0.286 | <0.001 | |
| CD63 | 0.027 | 0.948 | 0.001 | 0.124 | <0.001 | |
| LC3A/B | 0.072 | 0.948 | 0.588 | 0.152 | 0.008 | |
| ANXA1 | 0.001 | 0.001 | 0.588 | 0.998 | <0.001 | |
| P62 | 0.286 | 0.124 | 0.152 | 0.998 | 0.018 | |
| EV | <0.001 | <0.001 | 0.008 | <0.001 | 0.018 |
Note: P‐values less than 0.05 were considered as significant and checked in bold. EV, representative value of panel (value = CD9 + CD63 + LC3A/B + ANXA1 + P62).
EV expression and survival analysis
The mean follow‐up time of the patients in this study was 113 months. The median DSS time was 39 months. Of all the patients, 54.2% (n = 52) had disease recurrence, and 46.9% (n = 45) died due to lung SCC. The univariate Cox proportional hazards regression analysis of DFS and DSS showed that patients with SCC with low CD63 expression and patients with SCC low EV expression had unfavorable DFS rates (P‐value = 0.037 and 0.006, respectively). To confirm CD63 and EV expression as independent prognostic factors, multivariate analysis was performed. However, SCC samples with low CD63 and SCC samples with low EV expression failed to show a statistically significant relationship with DFS in the multivariate analysis (Table 3). Meanwhile, the Kaplan‐Meier survival curve confirmed that low EV expression had a statistically significant relationship with unfavorable DFS (P‐value = 0.004) (Fig 2a) and a tendency to have a relationship with DSS (P‐value = 0.072) (Fig 2b). There were no significant differences in DFS or DSS between the low and high expression groups for CD9, LC3A/B, ANXA1, and P62.
Table 3.
Cox proportional hazards regression model of DFS and DSS for SCC patients (n = 96)
| Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| DFS | DSS | DFS | DSS | |||||
| Variables | HR (95% CI) | P‐value | HR (95% CI) | P‐value | HR (95% CI) | P‐value | HR (95% CI) | P‐value |
| Age (years) (<65 vs. ≥65) | 1.308 (0.811–2.110) | 0.270 | 1.230 (0.738–2.051) | 0.427 | ||||
| Gender (male vs. female) | 0.519 (0.238–1.132) | 0.099 | 0.316 (0.115–0.871) | 0.026 | 0.355 (0.048–2.630) | 0.311 | ||
| Smoking (non‐smoker vs. smoker) | 0.844 (0.521–1.368) | 0.492 | 0.983 (0.581–1.664) | 0.950 | ||||
| Surgery (lobectomy vs. more invasive*) | 1.594 (0.854–2.973) | 0.143 | 1.494 (0.759–2.944) | 0.246 | ||||
| Pathologic differentiation (W/D, M/D vs. P/D) | 2.142 (1.201–3.821) | 0.010 | 2.089 (1.130–3.861) | 0.019 | 2.031 (1.088–3.794) | 0.026 | 2.088 (1.117–3.902) | 0.021 |
| TNM stage (I, II vs. III, IV) | 2.171 (1.270–3.711) | 0.005 | 1.863 (1.026–3.385) | 0.041 | 1.981 (1.008–3.892) | 0.047 | 1.784 (0.872–3.650) | 0.113 |
| CD9 (low vs. high) | 0.867 (0.481–1.560) | 0.633 | 0.778 (0.404–1.496) | 0.451 | ||||
| CD63 (low vs. high) | 0.515 (0.276–0.960) | 0.037 | 0.606 (0.315–1.165) | 0.133 | 0.981 (0.332–2.900) | 0.972 | ||
| LC3A/B (low vs. high) | 0.594 (0.313–1.130) | 0.113 | 0.734 (0.382–1.411) | 0.353 | ||||
| ANXA1 (low vs. high) | 0.725 (0.398–1.323) | 0.295 | 0.821 (0.427–1.578) | 0.555 | ||||
| P62 (low vs. high) | 0.995 (0.624–1.585) | 0.983 | 1.278 (0.773–2.113) | 0.338 | ||||
| EV (low vs. high) | 0.464 (0.268–0.801) | 0.006 | 0.597 (0.337–1.059) | 0.078 | 0.934 (0.459–1.901) | 0.851 | ||
Bilobectomy, sleeve lobectomy or pneumonectomy; CI, confidence interval; DFS, disease‐free survival; DSS, disease‐specific survival; EV, representative value of panel (value = CD9 + CD63 + LC3A/B + ANXA1 + P62); HR, hazard ratio; M/D, moderately‐differentiated; P/D, poorly‐differentiated; W/D, well‐differentiated. Note: P‐values less than 0.05 were considered as significant and checked in bold.
Figure 2.
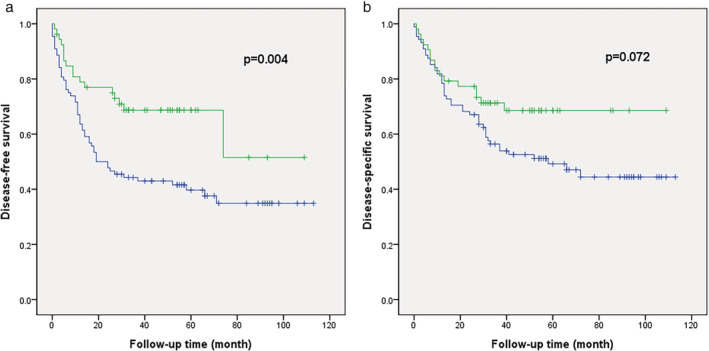
Survival analysis using the Kaplan‐Meier method based on extracellular vesicle (EV) marker expression in samples of SCC of the lung. The low EV marker expression group showed significantly lower disease‐free survival than the high EV marker expression group.  low,
low,  high,
high,  low‐censored,
low‐censored,  high‐censored (a) and a tendency for decreased disease‐specific survival (b),
high‐censored (a) and a tendency for decreased disease‐specific survival (b),  low,
low,  high,
high,  low‐censored,
low‐censored,  high‐censored.
high‐censored.
Discussion
For decades, exosomes have been known as key molecules for cell‐to‐cell communication to transport microRNA, mRNA, dsDNA, protein, and lipids to affect recipient cells.8, 9, 10, 11, 12, 13, 14, 15, 16 Recently, however, Jeppesen et al. challenged how exosomes are classified and reclassified them based on their size and markers; classical exosomes (40–150 nm) and arrestin‐domain‐containing protein 1‐mediated microvesicles (ARMMs) (~40–100 nm) are small EVs, classical microvesicles (~150–1000 nm) and apoptotic bodies (1–5 μm) are large EVs, and nonvesicular fractions (NFs) are nonextracellular vesicles. 6 They suggested that extracellular vesicles have a different composition of RNA, DNA, and protein according to their size. 6 Intracellularly, autophagosomes usually fuse with a lysosome to degrade internal cargo, creating autophagolysosomes. However, sometimes, MVEs may fuse with autophagosomes to make amphisomes. While CD63 and CD9 are well‐known exosomal markers (they both are specific for isolated exosomes and multivesicular endosomes within the cell), P62 and LC3 are autophagosomal markers. Naturally, amphisomes may show colocalized expression of CD63, CD9, P62, or LC3A/B, and amphisomes eventually fuse with the cell plasma membrane for exocytosis of NFs, which contain nonvesicular extracellular matter of histones and dsDNA. Exosomes have been known to have abundant RNA cargos, including miRNAs, which are sorted and packaged into the exosome with the help of Y‐box protein 1.17, 18 When extracellular RNA was extracted and RNA sequencing was employed, however, most microRNAs were more associated with the extracellular NV fraction than with parental cells or small EVs. 6 Additionally, many microRNAs showed different distributions between small EVs and extracellular NV fractions. 6 Since microRNA is an important molecule that works mostly as a tumor suppressor gene in tumor cells, and for the reasons mentioned above, we believe that treatments for lung SCC should not be limited to exosomes alone.
The EV markers we used in this study are markers for the precursor materials of EVs within the cells: CD63 and CD9 for MVEs, LC3A/B, and P62 for amphisomes, and ANXA1 for microvesicles. Hypothetically, if there are many precursor substances that are positive for EV markers intracellularly, there would be many EVs released out of the cell, and tumor exosomes released from tumor cells in such a method would affect tumor processes such as tumor cell metastasis. In this study, classical exosome markers (CD9 and CD63) and microvesicle marker (ANXA1) showed statistical significance in lung SCC cells in vivo. While Dennis et al. tested EV markers in cell line experiments, we assessed EV markers in tissue specimens of lung SCC. To the best of our knowledge, the EV expression, a combination of the representative values of the immunohistochemical staining panel (value = CD9 + CD63 + LC3A/B + ANXA1 + P62) have never been tested in vivo. In the survival analysis, SCC samples with low CD63 and EV expression showed poor DFS. Meanwhile, the Kaplan‐Meier survival curve confirmed that samples with low EV expression showed a statistically significant relationship with unfavorable DFS (p‐value = 0.004). It is possible that the lower the amount of precursors of EVs is, the lower the amount of secreted exosomes or NFs. Being trapped in tumor cells, MVEs or amphisomes may be degraded with their cargos, most importantly, microRNAs. 19 Eventually, this will break the balance in favor of metastasis for the tumor cells. A limitation of this study is that we did not detect any other factors that might have influenced the exocytosis of MVEs, for example, secretory Rabs, which lead to accelerated exosome secretion.19, 20 However, given our previous data, we assumed that multiple factors must associate with EVs, which might affect the prognosis of patients with SCC of the lung. In addition, there were no significant differences in DFS or DSS between the low and high expression groups for CD9, LC3A/B, ANXA1, and P62. We believe that EV expression, which considered the effects of each EV marker, better reflected patient survival than each separate EV marker. Considering all of the different extracellular vesicles rather than just exosomes10, 12, 13 may be necessary.
In conclusion, the combined expression of CD9, CD63, LC3A/B, ANXA1, and P62 as a marker for extracellular vesicles is effective in predicting the prognosis of patients with SCC of the lung. A broader approach using different extracellular vesicles rather than conventional exosome‐dependent methods is required when diagnosing and treating lung cancer patients. The prognostic role of EV expression and its association with DFS in SCC of the lung were elucidated in this study.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Acknowledgments
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Cui S, Cheng Z, Qin W, Jiang L. Exosomes as a liquid biopsy for lung cancer. Lung Cancer 2018; 116: 46–54. [DOI] [PubMed] [Google Scholar]
- 2. Masaoutis C, Mihailidou C, Tsourouflis G, Theocharis S. Exosomes in lung cancer diagnosis and treatment. From the translating research into future clinical practice. Biochimie 2018; 151: 27–36. [DOI] [PubMed] [Google Scholar]
- 3. Szatanek R, Baj‐Krzyworzeka M, Zimoch J, Lekka M, Siedlar M, Baran J. The methods of choice for extracellular vesicles (EVs) characterization. Int J Mol Sci 2017; 18 (6): 1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gaceb A, Martinez MC, Andriantsitohaina R. Extracellular vesicles: New players in cardiovascular diseases. Int J Biochem Cell Biol 2014; 50: 24–8. [DOI] [PubMed] [Google Scholar]
- 5. Théry C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol 2006; 30 (1): 3.22. 1–3.22. 29. [DOI] [PubMed] [Google Scholar]
- 6. Jeppesen DK, Fenix AM, Franklin JL et al Reassessment of exosome composition. Cell 2019; 177 (2): 428–45. e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kowal J, Arras G, Colombo M et al Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A 2016; 113 (8): E968–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L. Exosomes/microvesicles as a mechanism of cell‐to‐cell communication. Kidney Int 2010; 78 (9): 838–48. [DOI] [PubMed] [Google Scholar]
- 9. Lotvall J, Valadi H. Cell to cell signalling via exosomes through esRNA. Cell Adh Migr 2007; 1 (3): 156–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bang C, Thum T. Exosomes: New players in cell–cell communication. Int J Biochem Cell Biol 2012; 44 (11): 2060–4. [DOI] [PubMed] [Google Scholar]
- 11. Zhang C, Ji Q, Yang Y, Li Q, Wang Z. Exosome: Function and role in cancer metastasis and drug resistance. Technol Cancer Res Treat 2018; 17: 1533033818763450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gurunathan S, Kang M, Jeyaraj M, Qasim M, Kim J. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cell 2019; 8 (4): 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science 2020; 367 (6478): eaau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kahlert C, Melo SA, Protopopov A et al Identification of double‐stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer. J Biol Chem 2014; 289 (7): 3869–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang S, Che SP, Kurywchak P et al Detection of mutant KRAS and TP53 DNA in circulating exosomes from healthy individuals and patients with pancreatic cancer. Cancer Biol Ther 2017; 18 (3): 158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thakur BK, Zhang H, Becker A et al Double‐stranded DNA in exosomes: A novel biomarker in cancer detection. Cell Res 2014; 24 (6): 766–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McAndrews KM, Kalluri R. Mechanisms associated with biogenesis of exosomes in cancer. Mol Cancer 2019; 18 (1): 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shurtleff MJ, Temoche‐Diaz MM, Karfilis KV, Ri S, Schekman R. Y‐box protein 1 is required to sort microRNAs into exosomes in cells and in a cell‐free reaction. elife 2016; 5: e19276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. An HJ, Song DH, Koh HM et al RAB27A is an independent prognostic factor in clear cell renal cell carcinoma. Biomark Med 2019; 13 (4): 239–47. [DOI] [PubMed] [Google Scholar]
- 20. Koh HM, Song DH. Prognostic role of Rab27A and Rab27B expression in patients with non‐small cell lung carcinoma. Thoracic Cancer 2019; 10 (2): 143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


