Abstract
Exosomes are small membranous vesicles implicated in intercellular signalling. Through their uncanny ability to carry and deliver donor cellular cargo (biomolecules) to target cells, they exert a profound effect on the regular functioning of healthy cells and play a significant role in pathogenesis and progression of several diseases, including cancer. The composition and number of endogenously circulating exosomes frequently vary, which is often reflective of the pathophysiological status of the cell. Applicability of exosomes derived from normal cells as a drug carrier with or without modifying their intraluminal and surface components are generally tested. Conversely, exosomes also are reported to contribute to resistance towards several anti-cancer therapies. Therefore, it is necessary to carefully evaluate the role of exosomes in cancer progression and resistance and the potential use of exosomes as delivery vehicle of cancer therapeutics. In this review, we summarize the recent advancements in the exploitation of exosomes as a drug delivery vehicle. We also discuss the role of exosomes in conferring resistance to anti-cancer therapeutics. It is to be noted that while the present article provides information about the characteristics, composition and outcome of exosome-mediated therapeutics in cancer, the role of exosome in resistance and exosome-based drug delivery is also applicable to other human diseases.
Keywords: Extracellular vesicles, nanocarriers, tumor-derived exosome, tangential flow filtration, tumor-microenvironment
Introduction
Efficacy of anticancer drugs is limited by poor drug solubility, short half-life, and inefficient drug delivery to the tumor cells resulting in drug resistance, significant local and systemic toxicity [1]. To override these clinical challenges, testing of various nanoformulations (liposome, biodegradable polymers, microspheres, hydrogels, etc.) has been undertaken. While each of the formulations has shown promise in preclinical studies, their clinical translation has been hampered by off-target effects and toxicity albeit lower than that observed with chemotherapeutic drugs. Therefore, new and improved nanocarriers having desirable properties such as biocompatibility, ability to carry hydro- and hydrophilic drugs, have longer half-life in vivo are warranted. Ultimately, these nanocarriers need to efficiently target and deliver anticancer drugs to the tumor cells [2]. It is in this set up that researchers were attracted to test extracellular vesicles (EVs) including exosomes as natural drug delivery vehicle for cancer therapy.
EVs are secreted by almost all cell types and play significant roles in intercellular communication [3, 4]. EVs are classified into three types; exosomes, microvesicles (MVs), and apoptotic bodies based primarily on their size and pathway of generation. Among them, exosomes have garnered greater attention and are the most widely studied in cancer biology and therapy. Exosomes are 30–150 nm sized vesicles generated by the endocytic process and secreted by the fusion of multi-vesicular bodies (MVBs) with the plasma membrane [5]. Exosomes are enriched with distinct membrane proteins called tetraspanin proteins such as CD9, CD37, CD63, CD81 and CD82. Additionally, proteins such as the tumor susceptibility gene (TSG)-101 and Alix of exosome sorting complex required for transport (ESCRT) are also routinely used as markers to characterize exosomes [6].
The ever-increasing body of literature suggests that exosomes are involved in the regulation of normal functioning of cells. Additionally, they have also been reported to be involved in the pathology of several human diseases that include cardiovascular, neurodegenerative and autoimmune diseases and cancer [7–10]. The exosome composition (lipids, nucleic acids, metabolites, proteins, and peptides) reflects its cellular origin. However, the composition in diseased cells under pathological conditions is dysregulated compared to normal healthy cells [11]. Efforts to characterize the exosomal composition necessitated the emergence of databases like ExoCarta, Vesiclepedia, and EV miRNA which document and present the relevant information from various publications [12–14]. A growing number of reports have demonstrated exosomes also contribute to resistance against anticancer drugs via autocrine and paracrine mode of communication. This exosome mediated resistance mechanism eventually results in therapy failure and disease recurrence [15].
In this short review, we will first discuss the accumulating knowledge on the strategies applied to use exosomes as drug delivery systems and in targeted delivery of therapeutics; and in the later part about their role in carting factors that contribute to resistance to a variety of anti-cancer therapies.
1. Exosomes in cancer drug delivery
Exosomes are suitable as drug delivery vehicles due to their small size, biocompatibility, and reduced toxicity in comparison with synthetic nanoformulations such as liposomes, dendrimers, and polymers [2]. Additionally, delivery of anticancer drugs contained in exosomes demonstrated improved pharmacokinetic and pharmacodynamic properties and enhanced anticancer activity in vivo compared to free drug [16]. Similarly, loading of therapeutic nucleic acids such as small interfering (si) RNA into exosomes protects the RNA from nucleases and increases cellular uptake and therapeutic effect compared to free RNAs that are rapidly degraded by nucleases [17, 18]. Above all, exosomes have the ability to cross the blood brain barrier and penetrate deep tissues with improved efficacy compared to that of synthetic nanocarriers [19]. Thus, exosomes are demonstrably an effective and necessary tool for carrying and delivering the anti-cancer therapeutics. In the sections outlined below, we discuss the current status and research strides made in using exosomes as delivery vehicles for cancer treatment.
1.1. The cellular source of exosomes. Does it matter?
Exosomes can serve as a natural and potential carrier for cancer drug delivery. However, exosomes obtained from different biological sources and cell types behave differently in vivo thus impacting the anticipated antitumor activity. So, it is imperative to find an appropriate biological or cellular source for exosome isolation with desirable characteristics for use as drug carrier. For instance, exosomes isolated from macrophages had better doxorubicin loading capacity than did exosomes isolated from pancreatic cancer cells or pancreatic stellate cells [20]. Similarly, autologous exosomes (same cell type) were reported to function with higher potential in drug loading capacity and delivering of gemcitabine to pancreatic cells than did heterologous (different cell type) exosomes [21]. Additionally, a study by Emam et al., demonstrated that the autologous exosomes of C26 colorectal cancer cells were taken up more efficiently than the allogeneic exosomes from B16-BL6 melanoma cells in a murine model [22]. Thus, it is evident that exosomes from the same cell type are taken up effectively than the exosomes from another cell type from the same species. However, this is not always a beneficial criterion for selecting the cells source for exosome isolation. Sometimes, exosomes isolated from donor cell that is different from the recipient cell function better as drug delivery vehicle. Indeed, exosomes isolated from certain cell types show organotropism towards certain cancers wherein the exosomes get accumulated selectively in the major cell type of an organ rather than in adjacent cell types. For instance, overexpression of integrin beta 4 (ITG-B4) on the surface of human MDA-MB-231 breast cancer cell line, and its subsequent interaction with surfactant protein C (SPC+) on the surface of human A549 lung cancer cells are reasoned for the observed lung-tropism of exosomes from breast cancer cells. This finding could be explored for developing a drug delivery vehicle for lung cancer [23].
Studies report tumor-derived exosomes (TDEs) as drug carrier for cancer therapy. The rationale is that TDEs express different kinds of ligands and receptors on their surface that can be utilized to target the actively growing in situ tumor. By this approach, surface modification of exosomes for tumor-targeted drug delivery which is the norm for synthetic drug carriers, is avoided. As an example, TDEs loaded with the heat shock protein (HSP)-90 inhibitor geldanamycin exhibited improved antitumor activity compared to administration of free drug [24]. Similarly, induction of robust cytotoxic T-cell response against lung cancer was observed with tumor-associated exosomes compared to tumor cell lysate suggesting exosomes as better cancer vaccine candidate [25]. Contrary to these reports, studies demonstrate exosomes are notorious in their function and could operate as both pro-tumorigenic and anti-tumorigenic based on the source from which exosomes are derived. For example, exosome from menstrual mesenchymal stem cells (MenSc) exhibited antiangiogenic properties in vitro and in vivo while exosomes from bone marrow derived stem cells promoted tumor growth in vivo [26]. Adding to the literature, studies from our own laboratory have observed exosomes from human lung tumor cells are pro-tumorigenic (unpublished) and could be deleterious in their use as drug carriers. Therefore, we have resorted to using exosomes from normal lung fibroblast cells as drug carriers for cancer treatment [27].
Taken together, it is evident that the source of exosomes to be isolated and used for anticancer drug delivery needs to be prudently chosen that offer benefit and not harm.
1.2. Methods to obtain a high yield of exosomes
Although, exosomes possess favourable characteristics for drug delivery, producing them in adequate quantity for pre-clinical and clinical trial applications remains a challenge. Conventional methods applied for isolating exosomes include differential ultracentrifugation, ultrafiltration, precipitation, immunoaffinity capture beads-based methods. Application of these methods while improving the quality of exosomes is limited by the low yield and occasionally containing contaminants including microvesicles in the preparation [28, 29]. Recently, acoustic and fluidic device-based purification methods were described which again focuses primarily in improving the quality [30]. Application of tangential flow filtration (TFF) has also been shown to improve the quality and more importantly useful for concentrating the exosome number. Since cell culture supernatant is often the source of exosomes, multiple methods were described to increase the secretion of exosomes from cell culture into the medium thereby the above mentioned purification methods can be exploited efficiently to achieve adequate quantity of exosomes. For example, application of chemical, electrical stimulation, hypoxia and silencing of specific endo-lysosomal traffic proteins have shown to enhance exosome production and secretion from cells (Figure 1). Large-scale exosome production using laboratory and industrial grade bioreactors are also being evaluated for clinical testing.
Figure 1.
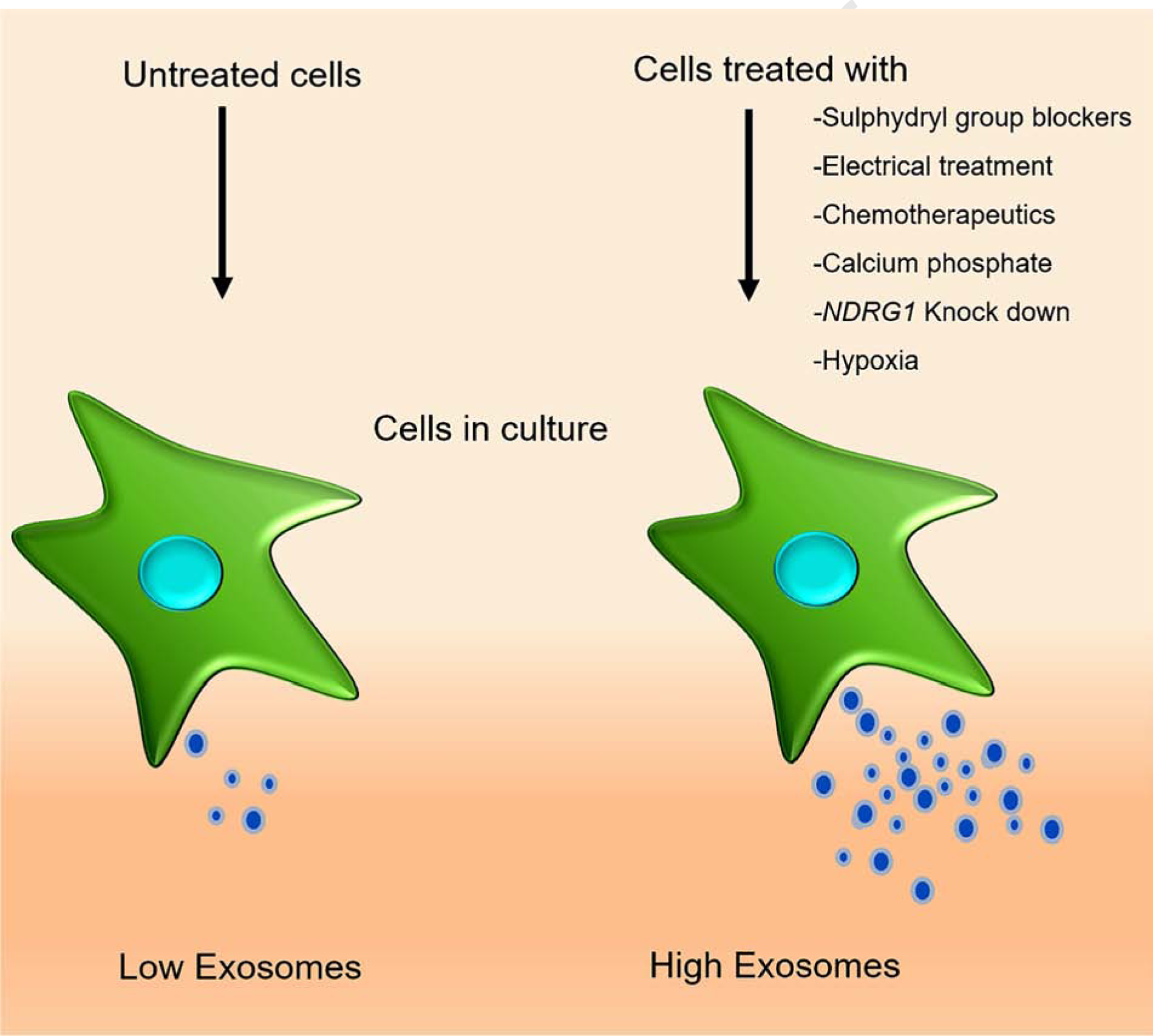
Strategies to induce exosome induction from cells. Exosome production can be increased by subjecting the cells to electrical signal; knockdown of endo-lysosomal gene (NDRG1); treating cells with calcium phosphate; membrane protein sulfhydryl group blockers; chemotherapy agents; and hypoxia.
In case of chemical induction of exosome production, paraformaldehyde and dithiothreitol (DTT)-mediated blocking of sulfhydryl group on the receptor proteins increased exosome blebbing and quantity [31]. Exosomes prepared this way showed improved release of Dox to the recipient cells leading to reduced tumor growth in mice compared to mice receiving liposome preparations. Similarly, calcium phosphate (CaP) treatment of THP-1 monocytes like cells or RAW264.7 macrophage cells increased the production of exosomes [32]. Along these lines, inhibition of endo-lysosomal trafficking by silencing the N-Myc downstream regulated-1 (NDRG1) gene increased exosome secretion [33]. On the other hand, exosomes secreted from stress induced cells were able to facilitate antitumor function. As such, hepatocellular carcinoma (HCC) cells that are resistant to chemotherapeutic drugs secreted exosomes with increased levels of Hsp-60, −70 and −90 proteins. These exosomes eventually bolstered the function of natural killer (NK) cells thereby boosting immune surveillance [34]. Similarly, hypoxia-induced stress is known to activate exosome production and secretion by multiple cell lines in vitro that can be further explored for their applicability as drug delivery vehicles. However, it is to be noted that hypoxia induced exosomes carry proteins such as protein-lysine-6-oxidase (LOX), thrombospondin-1 (TSP-1) and vascular derived endothelial growth factor (VEGF) that are known to play a role in metastasis, invasion, angiogenesis, and tumor progression. Hence, phenotypic outcome of these hypoxia associated exosomes on the recipient cells needs to be determined before being used as a drug carrier [35–38]. Another novel approach used electrical signal to induce exosomes in cell culture. Minimal electric treatment (0.34 mA/cm2) to murine melanoma and fibroblast cells ended up in yielding more exosomes. This noticeable increase in the production of exosomes operate through the activation of Rho GTPase signalling [39]. These methods while useful in enhancing the exosome production, continue to be inadequate for industrial scale production. To fill this gap, microcarriers and hollow-fiber bioreactors with increased surface area are being developed and tested for producing exosome in large-scale quantities [40, 41]. However, these bioreactor-based methods have intrinsic steps that influence and modify the phenotype of the cells over time thereby reducing the quality of the exosomes. For example, interleukin (IL)-2 was reduced in the exosomes from T cells when subjected to agitation at 180 rpm [42]. Thus, it is important to carefully select and optimize the method of large-scale exosome production that ensures the quality of exosomes is maintained without influencing the exosome source adversely.
Ensuring the quality and quantity of exosomes isolated from bodily fluids such as blood and urine is another challenging task. Enrichment of exosomes with specific receptor protein is explored besides using conventional methods to address this requirement. For example, purification of transferrin receptor (TfR+) containing exosomes from blood using the superparamagnetic nanoparticle cluster method with transferrin (Tf) surface conjugated nanoparticles was shown to result in exosomes with increased biocompatibility and deliverability [43]. Recently, an exosome total isolation chip (ExoTic) was launched that works primarily by filtration to isolate exosome from bodily fluids and resulted in a 1000-fold increased yield [44]. Altogether, these methods can be used to produce sufficient quantities of exosomes for their subsequent use in the drug delivery applications.
1.3. Methods of exosome preservation with the retention of quality and function
To successfully utilize exosomes as a delivery vehicle it is important to optimize the storage methods for the isolated exosomes. It is necessary because the native exosomes lose quality and functionality during standard storage condition such as freezing at −80°C and repeated freeze thaw cycles rupture or aggregate the exosomes [45]. Multiple storage conditions were tested to find a suitable method. For instance, Frank et al., studied the enzyme activity of beta-glucuronidase loaded onto exosomes isolated from mesenchymal stem cells, endothelial cells and cancer cells. They stored the exosomes at −80°C, +4°C, room temperature and lyophilized; of which lyophilization worked most efficiently [46]. Similarly, a recent study demonstrated that exosomes modified with a peptide rich in arginine for enhanced penetration of the cell retained their quality during lyophilization [47]. Furthermore, Charoenviriyakul et al., demonstrated that the lyophilization of B16BL6 melanoma cell-derived exosomes with the cryoprotectant, trehalose, reduces their aggregation during the lyophilization step [48]. However, Akers et al., argued that lyophilization reduced the EV number and their RNA content when compared to exosome storage at room temperature [45]. Thus, prior consideration of the appropriate method for long-term storage of exosomes specific to a cellular source would increase the beneficial applications.
1.4. Exosome loading with desired content
Once the exosomes are isolated from the cellular or biological source of choice, they are loaded with the therapeutic drug of interest, thereby making them more effective for drug delivery. Different types of synthetic and natural biomolecules, including nucleic acids, lipids, proteins and small chemical drugs are loaded into the exosomes. There are two kinds of strategies followed in the exosomal loading of any content that includes the endogenous and exogenous loading. In the endogenous loading method, overexpression of nucleic acid and/or protein in the source (donor) cells results in generation of exosomes pre-loaded or enriched with the component of interest (Figure 2). In the exogenous loading method, exosomes are directly loaded with nucleic acids and small molecular therapeutics using various methods including chemical transfection, electroporation, sonication and co-incubation.
Figure 2.
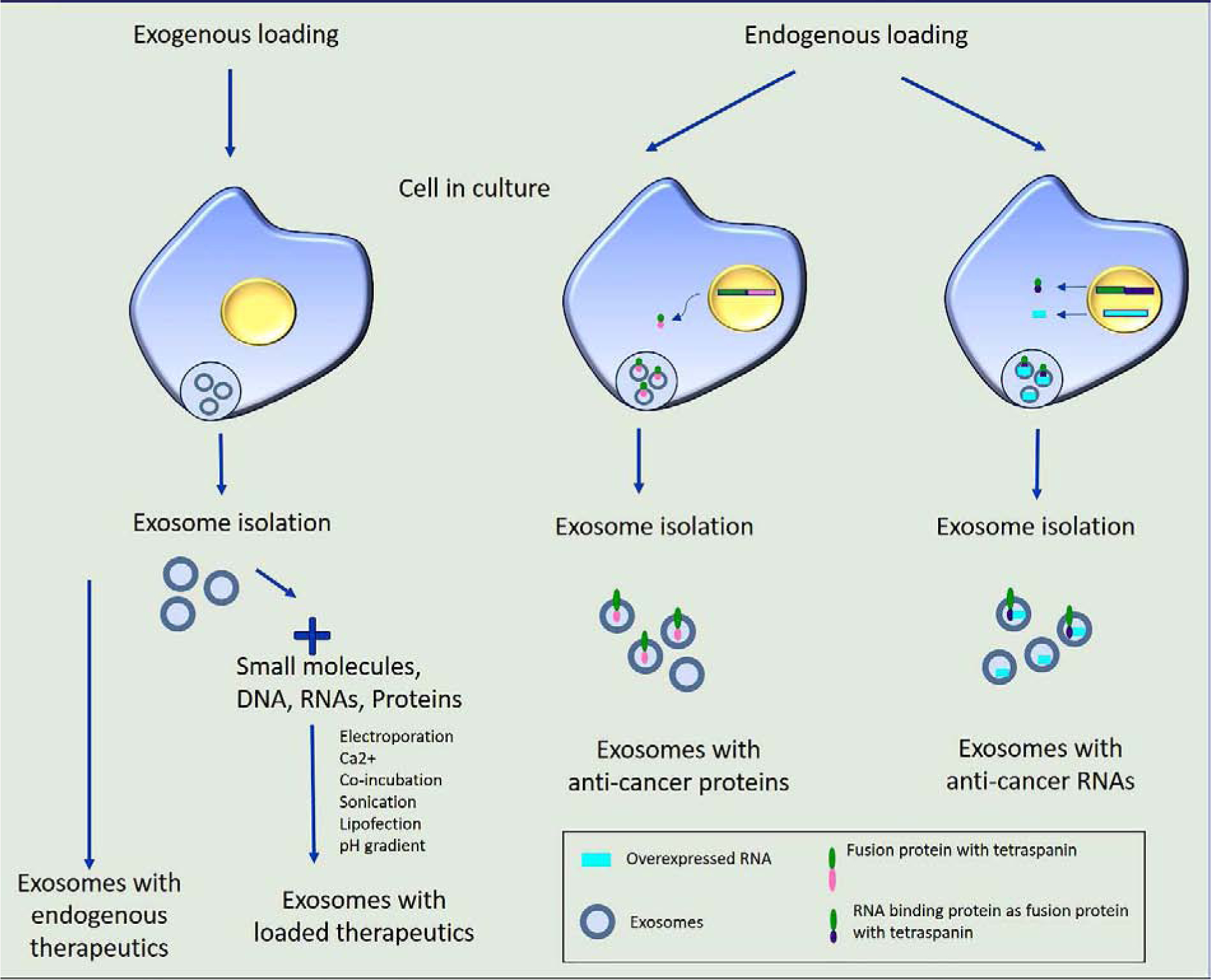
Method of loading therapeutics in exosome. Exosomes isolated from certain cell types show antitumor effects with its endogenously loaded composition. Exosomes can also be equipped exogenously with therapeutic proteins, nucleic acids, and small molecules. Endogenous loading of exosomes is achieved using a plasmid vector based expression of protein as a fusion protein with the tetraspanin proteins.
Each of the loading methods have pros and cons of their own. The required method is often decided based on the therapeutic component that is to be loaded into these exosomes. For instance, loading the mRNA transcripts into exosomes often results in a reduced yield than when loading them with short RNAs such as siRNAs and miRNAs. In this scenario, loading the exosomes with mRNA is preferably done through an endogenous method. A recent study utilized a nanoporation method where-in the source cells were transfected with plasmid DNA to express the desired biomolecule and transiently stimulated with an electric signal. This method yielded a 50-fold increase in exosome production and 1000-fold increase in the exosomes enriched in mRNAs and peptides compared with other standard methods [49].
Efforts to further enhance the endogenous loading of exosomes with desired RNA were also attempted. A recent study described an enhanced RNA packaging into exosomes by overexpressing RNA binding protein, HuR, as a fusion protein with tetraspanins such as CD9 in the host cells used for exosome isolation. Co-overexpression of HuR-CD9 fusion protein with either miR-155 inhibitor RNA or guide RNA (gRNA) for CRISPR/Cas9 resulted in the specific enrichment of these RNAs in the isolated exosomes. Such exosomal systems were demonstrated to be efficient for in vitro and in vivo gene therapy applications [50]. The endogenous loading of exosomes with protein of interest is often carried out as a fusion protein along with the surface marker proteins of exosomes. A recent study tested four exosomal membrane localization proteins such as CD63, Palm, PB and CAAX and determined the loading efficiency of fusion proteins. The authors individually expressed these proteins as fusion protein with mCherry reporter protein for tracking of exosome loading. The results showed that CD63 followed by Palm had the highest sorting and packaging of cargo (mCherry) protein in the exosome. Fusion with PB and CAAX led to the expression of the reporter protein mCherrry on the plasma membrane rather than in the exosomes [51]. This study revealed a strategy for enhancing endogenous packaging of desired therapeutic proteins into exosomes for achieving maximum treatment outcomes.
To simplify the exosomal loading with desired therapeutic components, researchers developed and successfully demonstrated methods that enabled direct loading of exosomes with therapeutic compounds. For instance, exosomes transfected with miRNA mimics or inhibitors using the modified calcium chloride-based method respectively resulted in an efficient overexpression or depletion of the target miRNAs in the recipient cells [52]. As an alternative, sonication was also shown to be effective in loading the short nucleic acid sequences into exosomes. For instance, the loading of siRNA against HER2 in exosomes resulted in a significant reduction in HER2 mRNAs in the recipient cells making it a viable option for breast cancer therapy [53]. On the other hand, treatment of SKOV3 ovarian cancer cells with exosomes loaded via electroporation with plasmid DNA expressing sgRNA for poly (ADP-ribose) polymerase-1 (PARP-1) suppressed cell growth and sensitized the cells to cisplatin [54]. Another study reported pH gradient based direct loading of RNAs, especially small RNAs into exosomes [55]. Above all, a simple co-incubation of hydrophobic synthetic siRNAs with exosomes yielded successful encapsulation within exosomes [56]. Thus, exogenous loading of exosomes with different biomolecules offers a rapid production of therapeutic exosomes. However, direct loading of exosomes with siRNA and DNA were also shown to result in inefficient loading and delivery of the contents that became in-effective in the recipient cells [57]. Altogether, direct loading of exosomes with therapeutic content requires further optimization and scale-up methods to make it a viable approach for cancer therapy.
1.5. Surface engineering of exosomes
Exosomes while proven to be useful as anti-cancer drug carrier in vitro, it use for delivering anticancer drugs in vivo could be limited due to non-specific toxicity and off-target effects similar to that observed with conventional chemodrugs. This necessitates equipping the drug-loaded exosome’s surface with tumor-targeted ligands (eg: peptide, antibody, aptamer) for achieving tumor-specific drug delivery and reduced non-specific toxicity. The ligands used are often targeted against receptors that are overexpressed in cancer cells. Such ligand/antibody displaying exosomes were reported to function effectively in suppressing tumor growth since they are taken up by recipient tumor cells through a receptor mediated endocytosis process that avoids endosome encapsulation and trapping [54]. Several strategies for surface modification of the exosome with ligands have been tested. For instance, exosomes obtained from the noncancerous HEK293T cells were anchored with lipophilic hyaluronic acid (lipHA-hEVs) as a ligand to target CD44 overexpressing MCF7/ADR breast cancer cells to deliver doxorubicin. These lipHA-hEVs were shown to enrich doxorubicin specifically in breast cancer cells thereby reducing the tumor mass by 89% and increasing the animal survival by 50% [58]. Similarly, lipophilic linker mediated surface tagging of exosomes with monoclonal antibodies (mAb) is another common strategy widely endorsed. For example, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine lipophilic linker conjugated with polyethylene glycol (PEG) and SSTR2 mAb was used to modify the exosome surface to deliver FK228 histone deacetylase inhibitor to pancreatic neuroendocrine xenograft model in mouse where the SSTR2 is overexpressed [59]. Another example is the exosomal surface modification with antibodies capable of releasing checkpoint inhibition for improved cancer therapy. Overexpression of CD47 is observed in many cancer cells that enables them to escape from being engulfed by macrophages. Exosomes derived from M1 macrophages anchored on their membrane with dibenzocyclooctyne-modified antibodies against CD47 or SIRPα via a pH responsive benzoic imine bond resulted in the enhanced targeting of tumor cells by macrophages. Notably, this modification stimulated a conversion of M2 pro-tumoral macrophage phenotype to M1 anti-tumoral phenotype characteristics [60, 61].
Receptor protein engineering is another strategy for preparing exosomes for targeted delivery. Recently, a novel method was described in HEK293 cells that enabled partitioning of reporter proteins into the inner or outer surface of exosome by utilizing specific locations on the tetraspanin proteins for fusion protein engineering. Such methods immensely allow engineering of exosomes to suit better for the targeted delivery of favourable therapeutic content [62]. On these lines, chimeric antigen receptor (CAR)-T therapy is becoming an appealing and promising approach for cancer treatment. Exosomes obtained from CAR-T cells contain high number of CAR on the surface, which elicits enhanced tumor cell cytotoxicity. These CAR exosomes lack programmed cell death protein 1 (PD-1) expression on their membrane and hence bypass the immunosuppressive mechanisms to which CAR-T cells are subjected. Moreover, CAR exosomes do not show the acute toxicities observed with CAR-T cell therapy. These characteristics make the exosomes derived from CAR-T cells an ideal candidate for anti-cancer therapy [63, 64]. Likewise, utilizing the sulfhydryl groups on the exosome membrane proteins for the targeted delivery has also been described in the literature. Engineering the surface of exosomes with functionalized DNA linked to quantum dots (EXO-DNA-QDs) enhances the specific uptake of these exosomes by tumor cells up to 85%. Thus, this method can be another effective strategy for improved tumor targeted delivery [65].
All these methods significantly improve the tumor-targeted drug delivery of the native exosomes and helps reduce the systemic toxicity otherwise observed with free anticancer drugs.
1.6. Exosome hybrids in drug delivery
The composition of native exosomes varies widely and as stated earlier their yield is also usually low thus restricting their potential for translational adoption. To address this, exosome-mimetic nanosystems (EMNs) were produced by sequential extrusion of the cells through multiple filters with downgraded pore size [66]. Such EMNs result in 100 times more yield in nanovesicles and with defined composition. Alternatively, incorporating the liposome technology for generating these EMNs was shown to result in 1000 times more yield in nanovesicles. Liposomes based on various lipids such as DOTAP, POPC, DPPC and POPG are commonly fused with exosomes to produce these EMNs hybrid particles with desired size range of 100 nm. These EMNs can further be improved for an exosome like properties such as tagging them with integrin α6β4 to enable organotropism [67]. Fusion of membrane proteins or exosomes obtained from red blood cells (RBCs), and leukocytes were also reported to improve these liposomes based EMNs in exerting antitumor activity. Moreover, these EMNs were effective in delivering the benefits of natural exosomes such as improved tumor enrichment, stability, controlled production, and drug loading capacity [68–70].
On the other hand, combining exosomes with nanoparticles has been reported to reduce the immunogenicity associated toxicity of the nanoparticles. For example, Wang et al., [71] used a “cocktail therapy”, that combined exosomes from natural killer cells with their biomimetic core shell nanoparticles. This cocktail therapy efficiently delivered miRNAs to neuroblastoma cells in an in vivo model resulting in increased tumor prevention. In another study, exosomes were loaded with gold nanoparticles pre-conjugated with doxorubicin via a pH sensitive hydrazine linker to achieve tumor-specific and controlled release of drug to lung cancer cells [29]. This approach specifically is useful when chemotherapeutics are planned for cancer therapy.
1.7. Exosomes of non-human origin for cancer therapy
It’s intriguing to note that exosomes obtained from non-human origin such as from bacteria and plants showed anti-tumor functionality. They were suggested to be useful in cancer therapy mainly due to their reduced toxicity and comparative ease in large scale production. Moreover, these exosomes retained the functionality comparable to the exosomes prepared from animal and human origin. EVs or outer membrane vesicles in the size range of 40 nm obtained from gram-negative and gram-positive bacteria accumulated in the tumor environment and stimulated the production of anti-tumor cytokines, CXCL10 and interferon-γ [72]. Likewise, EVs from a gram-positive bacterium, Lactobacillus rhamnosus induced apoptosis in hepatic cancer cells through the regulation of anti-apoptotic Bcl-2 and pro-apoptotic Bax proteins [73]. On the other hand, plant derived EVs have been well documented and their drug delivery applications reported as well. Particularly, intranasal delivery of grapefruit derived EVs loaded with miR-17 and decorated with folic acid were selectively taken up by tumor cells and inhibited brain tumor in mice [71]. Similarly, citrus-derived exosomes induced TRAIL-mediated apoptosis and reduced growth of chronic myeloid leukaemia (CML) in NOD/SCID mice [74]. Thus, such extended sources of exosomes are a valuable alternative that can be availed as a carrier for encapsulating the anti-cancer therapeutics for an effective cancer therapy in humans.
1.8. Exosome mediated delivery vehicles in pre-clinical and clinical trials
Since exosomes have tremendous potential to serve as drug delivery vehicles in vitro and in vivo, their translation into clinical application have been initiated and several are in progress. Currently, a phase I clinical trial in progress utilizes a plant derived exosome to deliver curcumin for colon cancer treatment (www.clinicaltrials.gov; NCT01294072). Similarly, dexosomes are small vesicles secreted by the dendritic cells with an enriched membrane content of major histocompatibility complex (MHC) class I and II molecules along with tetraspanins and CD86 proteins. These dexosomes are implicated in stimulating the immune response. Results from preclinical studies and phase I clinical trial demonstrated the dexosomes as efficient activator of the innate and adaptive immune response towards antitumor surveillance [75]. In fact, a phase II clinical trial with tumor antigen loaded dexosomes for cancer vaccination is currently in progress (NCT01159288). Similarly, a phase II clinical trial using IFN-γ on dexosomes showed that they could elicit anti-tumor function through natural killer (NK) cells [76]. The results from these clinical trials highlight the significant strides made with exosome as drug delivery vehicles and provide enormous promise for additional clinical trials in the future that utilize exosomes for therapeutic applications.
2. Contribution of exosomes in anticancer drug resistance
Common cancer treatment options include monotherapy or a combination of the modalities, such as chemotherapy, immunotherapy, cellular therapy, radiotherapy, hormonal therapy, and surgery. However, development of resistance to these treatments is common and the disease on relapse often exhibit an aggressive behaviour. While several factors contributing to drug resistance has been studied in the past, the role of exosomes in drug resistance is relatively new. For example, enhanced metastasis post-surgery has been documented, and exosomes are thought to be a contributing factor [77, 78]. Similarly, exosomes produced by drug resistant tumor cells can reshape and reconfigure the infiltrating immune cells (T-cell, macrophage, dendritic cells) present within the tumor microenvironment and perturb their function resulting in their inability to induce host immune response [79, 80]. Furthermore, exosomes undeniably mediate drug resistance as evidenced by a study that showed exosomes from drug-resistant human microvascular endothelial cells (HMVEC) promoted epithelial-mesenchymal transition (EMT) and growth of nasopharyngeal carcinoma cells [81]. However, the tumor growth promoting effects of exosomes from the drug resistant HMVEC was abrogated when treated with the exosome inhibitor, GW4869. In the section below, we discuss exosome-mediated resistance mechanisms to currently used anti-cancer therapies.
2.1. Exosomes in chemotherapy resistance
Chemotherapy is widely used in cancer treatment. There have been a variety of classes of chemotherapies described in the literature based on their mode of action such as alkylating agents, inhibitors of mitosis, and corticosteroids to name a few [82]. However, occurrence of resistance (innate and acquired) to chemotherapy is common causing disease recurrence. While several mechanisms of drug resistance have been reported in the past, more recently resistance contributed by TDEs has emerged. Table 1 lists exosome-mediated drug resistance towards commonly used anti-cancer drugs. Factors involved in drug resistant mechanisms for a select list of drugs are discussed below.
Table 1.
List of anticancer drugs with reported resistance mechanisms mediated by exosomes.
| Drug | Resistance factor | Expression change in resistant cells | Target | Cancer | Reference | |
|---|---|---|---|---|---|---|
| 1. | Oxaliplatin | hsa_circ_0005963 | Up | miR-122/PKM2 | Colorectal cancer | [118] |
| 2. | Palbociclib | Thymidine Kinase 1 (TK1), CDK9 | Up | - | Breast cancer | [119] |
| 3. | 5-fluorouracil (5-FU) | p-STAT3 and GSTP1 | Up | - | Colorectal Cancer | [120] |
| 4. | Temozolomide (TMZ) | lncRNA SBF2-AS1 | Up | miR-151a-3p/XRCC4 | Glioblasto ma | [121] |
| miR-1238 | Up | CAV1/EGFR | Glioblasto ma | [122] | ||
| 5. | Docetaxel | miR-9–5p, miR-195–5p, miR-203a-3p | Up | ONECUT2 | Breast cancer | [123] |
| 6. | Doxorubicin | miR-501–5p | Up | BLID | Gastric cancer | [79] |
| 7. | Melphalan or Bortezomib | Acid sphingomyelina se (ASM) | Up | Concerts sphingomyel in into ceramide | Multiple myeloma | [124] |
| 8. | Vincristine | Chloride intracellular channel 1 (CLIC1) | Up | - | Gastric cancer | [125] |
Cisplatin (cis-diamminedichloridoplatinum; CDDP) is an alkylating agent that is widely used against multiple human solid tumors [82]. In ovarian cancer cells, exosome mediated transfer of cytosolic gelsolin (pGSN) protein caused CDDP-sensitive cells to become resistant [15]. Similarly, treatment of lung cancer cells with miR-425–3p packaged exosomes isolated from CDDP-treated cells conferred resistance by stimulating the autophagic process via AKT1 [80]. In MDA-MB-231 triple negative breast cancer cells, addition of miR (miR-370–3p, miR-423–5p, and miR-373) enriched exosome conferred CDDP resistance [81].
Oxaliplatin is another platinum-based alkylating agent and anti-cancer drug used in the treatment of cancers. Poor response to the oxaliplatin treatment is increasingly attributed to the long-noncoding RNA (lncRNA) cargo in the exosomes from drug resistant cells. In colorectal cancer (CRC) cells, transfer of exosomes containing colorectal cancer-associated lncRNA (CCAL) from cancer associated fibroblasts (CAF) to CRC cells resulted in suppression of apoptosis, activation of beta-catenin and drug resistance. Functional studies showed CCAL interacted with the mRNA binding human antigen R (HuR) and increased β-catenin mRNA and protein levels contributing to chemoresistance [83]. Similarly, miR-46146 was observed to be enriched in exosomes obtained from CRC cells and was shown to influence PDCD10 to exert chemoresistance in the recipient cells [84]. Resistance to combinatorial therapies have also been reported in CRC. Oxaliplatin combined with the anti-metabolite 5-fluorouracil (5-FU) was reported to be challenged by the generation of a resistant mechanism through transcription factor activating enhancer binding protein 2e (TFAP2E) mediated upregulation of miRNAs miR106a5p and miR421 in MGC803/5FU CRC cells [81, 85].
Sorafenib, a multi-kinase inhibitor that inhibits tumor growth by blocking the Raf kinase, vascular endothelial growth factor (VEGF), and platelet-derived growth factor (PDGF) is widely used in the treatment of liver, kidney, and thyroid cancers. Resistance to sorafenib was reported to be mediated by exosomes in human renal cell carcinoma (RCC) via miR-31–5p and MLH1 mediated mechanisms both in vitro and in vivo. Furthermore, RCC patients with progressive disease receiving sorafenib treatment showed increased amounts of miR-31–5p in the exosomes [86]. Similarly, resistance towards sorafenib due to reduced miR-744 content in exosomes was demonstrated in hepatocellular carcinoma (HCC) [87]. In the same study, the authors showed that addition of miR-744 enriched exosomes to HCC reverted to chemosensitization and inhibited tumor cell proliferation. Exosome-mediated resistance mechanisms to multiple anti-cancer drugs including docetaxel, temozolomide, bortezomib and doxorubicin were also reported [Table 1].
Another mechanism by which exosomes can contribute to drug resistance is by carrying the drug efflux components that attribute for multi-drug resistance (MDR). Frequently, more than 50% of cancer patients with MDR are linked with elevated levels of adenosine triphosphate (ATP)-binding cassette transporter (ABC) family of proteins such as, P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP). The ABC transporters are reported to impart resistance to over 20 different lead anti-cancer drugs [88, 89]. Conceivably, transfer of constituents of these multi-drug efflux pump can be a viable mechanism for cancer cells to combat anti-cancer therapy. Notably, exosomes either directly carry these ABC transporter proteins or carry miRNA/mRNA components from drug resistant cells that can stimulate expression of the ABC transporter proteins in drug-sensitive cancer cell population and making them become resistant. Intriguingly, if the ABC transporters are placed inverted in the exosome membrane, they can remove anti-tumor drugs from the intercellular space as well [90–92]. Conversely, direct transfer of chemotherapeutic drugs from resistant to susceptible tumor cells via exosomes is another substantial mode of development of chemoresistance. Such exosome mediated resistance was conferred for cisplatin, pixantrone and doxorubicin drugs in melanoma, breast and ovarian cancers [93–96]. Thus, multiple-modes of resistance mechanisms is operational to escape chemotherapy.
2.2. Exosomes contribute to immunotherapy resistance
Until recently, cancer treatment options were limited to conventional chemotherapy, radiotherapy, and surgery. However, the recent application of immunotherapy has seen great success due to efficacious and durable response by patients. Immunotherapy strategies include the usage of monoclonal antibodies, vaccines, immune checkpoint inhibitors, lymphocyte-activating cytokines, CAR-T cells, bispecific antibodies, and oncolytic viruses [97–99]. Immune checkpoint proteins restrict both the immune responses to pathogens and self-antigens generated by the carcinogenesis process. Cancer cells are known to overexpress checkpoint proteins such as programmed death ligand (PDL)-1 that interacts with programmed cell death (PD)-1 receptor to inactivate T cells. Application of antibodies to regulate the immune checkpoint has improved response to therapy by 20–30%. Below are some of the recent immunotherapy approaches and the reported resistance mechanisms mediated by exosomes.
Exosomes are reported to disseminate resistance either by carrying receptor proteins that compete with the targeted therapy antibodies or deliver a cargo of noncoding RNAs and proteins to the sensitive cells. For instance, increasing evidence supports that the tumor secreted exosomes contain increased levels of PDL-1 that function as decoy for the PDL-1 targeted therapy [100]. A similar phenomenon was observed with anti-CD20 targeted therapy. Exosomes secreted by B-cell lymphoma cells have been shown to carry CD-20 to block antibodies targeted against CD-20. Furthermore, these exosomes complexed with anti-CD20 antibodies attracted complement proteins, thereby evading the antibody mediated lysis of cancer cells [101]. Contribution of noncoding RNAs and protein cargo present in the exosomes have also been shown to contribute to resistance against trastuzumab-targeted therapy. Trastuzumab is a monoclonal antibody against human epidermal growth factor receptor (HER)-2 that is used as a targeted therapy for breast, metastatic gastric, and other HER2 overexpressing cancers [102]. In breast cancer, overexpression of lncRNA-small nucleolar RNA host gene 14 (SNHG14) was associated with resistance to trastuzumab targeted therapy [103]. Similarly, overexpression of lncRNAs such as the actin filament associated protein 1 antisense RNA 1 (AFAP1-AS1) and AGAP2 antisense RNA 1 (AGAP2-AS1) in trastuzumab resistant breast cancer cells and their exosomes contributed to therapy resistance [104, 105]. Exosome mediated resistant mechanisms were reported not only for HER2-targeted therapy but also for other cancer targeted therapies. For example, Cetuximab is a human and mouse chimeric monoclonal antibody against epidermal growth factor receptor (EGFR). This antibody is widely used for treatment of colon cancer patients carrying the wild-type KRAS gene. In RKO colon cancer cells, exosome conferred resistance to cetuximab by regulating the phosphatase and tensin homolog (PTEN)/Akt pathway [106]. Similarly, exosome mediated resistance to the anti-apoptotic survivin protein was observed in glioma patients receiving anti-survivin vaccine (SurVaxM) [107]. The survivin containing exosomes were higher in the glioma patients than in healthy control individuals. Overall, the resistance mechanisms contributed by the exosomes significantly reduce the effectiveness of immunotherapy in cancer patients.
2.3. Radiotherapy/proton therapy resistance mediated by exosomes
Radiation therapy or radiotherapy uses ionizing radiation to kill cancer cells. Although, radiotherapy alone is effective in killing cancer cells, it is often combined with other cancer treatments such as chemotherapy, hormonal therapy and surgery to contain cancer more efficiently [108]. Nevertheless, exosome mediated resistance to radiotherapy results in the recurrence of metastatic cancers. For instance, exposing BHY and FaDu head and neck cancer cells to gamma radiation stimulated increased secretion of exosomes. Co-culturing of these exosomes with BHY cells conferred radioresistance and promoted proliferation of recipient cells as early as 6 h post exosome treatment. Mechanistically, these exosomes exerted their resistance function through enhanced DNA double strand break repair [109]. Similarly, latent membrane protein-1 (LMP1) containing exosomes from CNE1 nasopharyngeal carcinoma cells were observed to induce radioresistance by activating the p38 MAPK pathway in recipient cells [110]. Also, exosomes from hypoxic glioblastoma cells were found to contain miR-301a which targeted the tumor-suppressor TCEAL7 gene and conferred radioresistance to corresponding normoxic recipient cells [111]. Likewise, Ni et al., have extensively discussed the aspects of exosome mediated radiation-resistance to multiple cancer treatments [112]. Conversely, exosomes also carry tumor suppressor miRNAs that suppressed the radioresistance of nasopharyngeal carcinoma [113].
Proton beam therapy (PBT) is a type of radiotherapy with improved therapeutic effects and reduced side effects compared with conventional X-ray based radiotherapy. Notably, PBT has been increasingly employed as a treatment option for multiple cancer types [114]. However, exosome mediated PBT resistance mechanisms have been reported as well. Proton irradiated HeLa cervical carcinoma cells were shown to secrete exosomes with higher content of survivin [115] thereby favouring tumor growth and treatment resistance. Thus, it is evident that exosomes play an important role in conferring resistance to radiation and proton therapy and strategies overriding radiotherapy resistance is warranted.
2.4. Involvement of exosomes in the resistance to anti-cancer hormonal therapy
Pathogenesis of certain malignancies such as breast and prostate cancer are influenced by hormones. Hence, first and second-generation drugs targeting hormone specific pathways are recommended for treating these malignancies. However, exosome mediated resistance mechanisms have been reported that decrease the efficiency of hormonal therapies. Notably, MCF-7 breast cancer cells resistant to the anti-estrogen, tamoxifen, secrete exosomes that confer resistance to tamoxifen treatment upon its delivery to sensitive cells [116]. Moreover, transfer of mitochondrial DNA by exosomes to oxidative phosphorylation-deficient breast cancer cells enabled the cells to bypass hormonal therapy-induced dormancy and develop resistance to hormonal therapy [117].
Conclusion and perspectives
Within the past decade, the field of exosome research has disclosed enormous information on these nanosized natural particles that can be harnessed in a broad range of basic and translational applications. This was achieved due to keen interest in the field combined with parallel discovery and evolution of supportive isolation and purification technologies; direct/indirect exosome drug loading/manipulating methods; in vivo imaging and high-throughput methods for exosomal RNA/protein profiling. However, limitations exist in the use of exosomes as drug delivery vehicle. Major issues related to their use as drug carriers include isolation methods, purity, homogeneity, immunogenicity, and scalability. For example, exosomes have been shown to exhibit low immunogenicity compared synthetic nanocarriers. However, whether exosomes derived from different cell types exhibit varying immunogenicity is not known. Thus, comparative understanding on the immunogenicity of exosomes isolated from different cellular and biological sources will allow researchers to choose appropriate exosome source for use as drug delivery vehicle and advancing to clinical translation. Similarly, information about why exosomes from certain cell types demonstrate high intrinsic drug loading capacity and efficient drug delivery to tumor sites than others can potentially improve exosome’s application for clinical use.
In the context of drug resistance, improved understanding of the exosome biology has allowed in demonstrating their role in resistance and therapy failure. However, majority of the studies have focused on resistance towards a single agent. For clinical relevance where combinatorial treatments are applied, it is important to investigate the contribution of exosome-mediated resistance towards combination therapies. Therefore, designing studies to closely monitor the emergence of exosome mediated resistance to numerous current and novel anti-cancer therapies, will enable researchers to quickly re-engineer these therapies to reduce or avoid emergence of exosome mediated therapy resistance. These necessary steps will stimulate the application of exosomes as a drug carrier and for the targeted delivery of anti-cancer therapeutics.
Highlights.
Exosomes are potential, natural drug delivery vehicles.
Engineering of exosome cargo and surface receptors can make exosomes suitable for targeted drug delivery.
Exosomes mediate resistance to multiple anti-cancer therapies.
Acknowledgments
This study was supported by grants received from the National Institutes of Health (NIH), (R01 CA167516 and R01CA233201); from the National Institute of General Medical Sciences (P20 GM103639) of the National Institutes of Health; a Merit Grant (101BX003420A1) from the Department of Veterans Affairs; a Pilot Grant, Seed Grant, and Student Trainee Grant funded by the National Cancer Institute Cancer Center Support Grant P30CA225520 awarded to the University of Oklahoma Stephenson Cancer Center; from the Department of Defense (DOD) through the Lung Cancer Research Program (LCRP) under award no.W81XWH-18-1-0637 & W81XWH-19-1-0647; a grant (HR18-088) from the Oklahoma Center for Advanced Science and Technology (OCAST); funds received from the Presbyterian Health Foundation Seed Grant (AM), Presbyterian Health Foundation Bridge Grant, and the Jim and Christy Everest Endowed Chair in Cancer Developmental Therapeutics.
The authors thank Ms. Kathy Kyler at the Office of the Vice President for Research, OUHSC, for editorial assistance. Rajagopal Ramesh is an Oklahoma TSET Research Scholar and holds the Jim and Christy Everest Endowed Chair in Cancer Developmental Therapeutics.
The content is solely the responsibility of the authors. The opinions, interpretations, conclusions and recommendations are those of the author and not necessarily endorsed by or representative of the official views by the NIH, DOD, or Department of Veterans Affairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors report no conflicts of interest in this work.
References
- [1].Livshits Z, Rao RB, Smith SW, An approach to chemotherapy-associated toxicity, Emerg Med Clin North Am, 32 (2014) 167–203. [DOI] [PubMed] [Google Scholar]
- [2].Hossen S, Hossain MK, Basher MK, Mia MNH, Rahman MT, Uddin MJ, Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: A review, J Adv Res, 15 (2019) 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Adem B, Vieira PF, Melo SA, Decoding the Biology of Exosomes in Metastasis, Trends Cancer, 6 (2020) 20–30. [DOI] [PubMed] [Google Scholar]
- [4].Dini L, Tacconi S, Carata E, Tata AM, Vergallo C, Panzarini E, Microvesicles and exosomes in metabolic diseases and inflammation, Cytokine Growth Factor Rev, (2020). [DOI] [PubMed] [Google Scholar]
- [5].Gurunathan S, Kang MH, Jeyaraj M, Qasim M, Kim JH, Review of the Isolation, Characterization, Biological Function, and Multifarious Therapeutic Approaches of Exosomes, Cells, 8 (2019) 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yoshioka Y, Konishi Y, Kosaka N, Katsuda T, Kato T, Ochiya T, Comparative marker analysis of extracellular vesicles in different human cancer types, J Extracell Vesicles, 2 (2013) 20424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bei Y, Chen T, Banciu DD, Cretoiu D, Xiao J, Circulating Exosomes in Cardiovascular Diseases, Adv Exp Med Biol, 998 (2017) 255–269. [DOI] [PubMed] [Google Scholar]
- [8].Howitt J, Hill AF, Exosomes in the Pathology of Neurodegenerative Diseases, J Biol Chem, 291 (2016) 26589–26597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Baharlooi H, Azimi M, Salehi Z, Izad M, Mesenchymal Stem Cell-Derived Exosomes: A Promising Therapeutic Ace Card to Address Autoimmune Diseases, Int J Stem Cells, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Steinbichler TB, Dudas J, Riechelmann H, Skvortsova II, The role of exosomes in cancer metastasis, Semin Cancer Biol, 44 (2017) 170–181. [DOI] [PubMed] [Google Scholar]
- [11].Doyle LM, Wang MZ, Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis, Cells, 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Keerthikumar S, Chisanga D, Ariyaratne D, Al Saffar H, Anand S, Zhao K, Samuel M, Pathan M, Jois M, Chilamkurti N, Gangoda L, Mathivanan S, ExoCarta: A Web-Based Compendium of Exosomal Cargo, J Mol Biol, 428 (2016) 688–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pathan M, Fonseka P, Chitti SV, Kang T, Sanwlani R, Van Deun J, Hendrix A, Mathivanan S, Vesiclepedia 2019: a compendium of RNA, proteins, lipids and metabolites in extracellular vesicles, Nucleic Acids Res, 47 (2019) D516–D519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Liu T, Zhang Q, Zhang J, Li C, Miao YR, Lei Q, Li Q, Guo AY, EVmiRNA: a database of miRNA profiling in extracellular vesicles, Nucleic Acids Res, 47 (2019) D89–D93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Asare-Werehene M, Nakka K, Reunov A, Chiu CT, Lee WT, Abedini MR, Wang PW, Shieh DB, Dilworth FJ, Carmona E, Le T, Mes-Masson AM, Burger D, Tsang BK, The exosome-mediated autocrine and paracrine actions of plasma gelsolin in ovarian cancer chemoresistance, Oncogene, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Melzer C, Rehn V, Yang Y, Bahre H, von der Ohe J, Hass R, Taxol-Loaded MSC-Derived Exosomes Provide a Therapeutic Vehicle to Target Metastatic Breast Cancer and Other Carcinoma Cells, Cancers (Basel), 11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Musacchio T, Torchilin VP, siRNA delivery: from basics to therapeutic applications, Front Biosci (Landmark Ed), 18 (2013) 58–79. [DOI] [PubMed] [Google Scholar]
- [18].Dowdy SF, Overcoming cellular barriers for RNA therapeutics, Nat Biotechnol, 35 (2017) 222–229. [DOI] [PubMed] [Google Scholar]
- [19].Chen CC, Liu L, Ma F, Wong CW, Guo XE, Chacko JV, Farhoodi HP, Zhang SX, Zimak J, Segaliny A, Riazifar M, Pham V, Digman MA, Pone EJ, Zhao W, Elucidation of Exosome Migration across the Blood-Brain Barrier Model In Vitro, Cell Mol Bioeng, 9 (2016) 509–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kanchanapally R, Deshmukh SK, Chavva SR, Tyagi N, Srivastava SK, Patel GK, Singh AP, Singh S, Drug-loaded exosomal preparations from different cell types exhibit distinctive loading capability, yield, and antitumor efficacies: a comparative analysis, Int J Nanomedicine, 14 (2019) 531–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Li YJ, Wu JY, Wang JM, Hu XB, Cai JX, Xiang DX, Gemcitabine loaded autologous exosomes for effective and safe chemotherapy of pancreatic cancer, Acta Biomater, 101 (2020) 519–530. [DOI] [PubMed] [Google Scholar]
- [22].Emam SE, Abu Lila AS, Elsadek NE, Ando H, Shimizu T, Okuhira K, Ishima Y, Mahdy MA, Ghazy FS, Ishida T, Cancer cell-type tropism is one of crucial determinants for the efficient systemic delivery of cancer cell-derived exosomes to tumor tissues, Eur J Pharm Biopharm, 145 (2019) 27–34. [DOI] [PubMed] [Google Scholar]
- [23].Nie H, Xie X, Zhang D, Zhou Y, Li B, Li F, Li F, Cheng Y, Mei H, Meng H, Jia L, Use of lung-specific exosomes for miRNA-126 delivery in non-small cell lung cancer, Nanoscale, 12 (2020) 877–887. [DOI] [PubMed] [Google Scholar]
- [24].Dhayapulay A, Kanapathipillai M, Exosomes Based Geldanamycin Delivery to Cancer Cells with Increased Therapeutic Efficacy, J Biomed Nanotechnol, 15 (2019) 2202–2208. [DOI] [PubMed] [Google Scholar]
- [25].Wang C, Huang X, Wu Y, Wang J, Li F, Guo G, Tumor Cell-associated Exosomes Robustly Elicit Anti-tumor Immune Responses through Modulating Dendritic Cell Vaccines in Lung Tumor, Int J Biol Sci, 16 (2020) 633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rosenberger L, Ezquer M, Lillo-Vera F, Pedraza PL, Ortuzar MI, Gonzalez PL, Figueroa-Valdes AI, Cuenca J, Ezquer F, Khoury M, Alcayaga-Miranda F, Stem cell exosomes inhibit angiogenesis and tumor growth of oral squamous cell carcinoma, Sci Rep, 9 (2019) 663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Srivastava A, Amreddy N, Babu A, Panneerselvam J, Mehta M, Muralidharan R, Chen A, Zhao YD, Razaq M, Riedinger N, Kim H, Liu S, Wu S, Abdel-Mageed AB, Munshi A, Ramesh R, Nanosomes carrying doxorubicin exhibit potent anticancer activity against human lung cancer cells, Sci Rep, 6 (2016) 38541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Patel GK, Khan MA, Zubair H, Srivastava SK, Khushman M, Singh S, Singh AP, Comparative analysis of exosome isolation methods using culture supernatant for optimum yield, purity and downstream applications, Sci Rep, 9 (2019) 5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Srivastava A, Amreddy N, Pareek V, Chinnappan M, Ahmed R, Mehta M, Razaq M, Munshi A, Ramesh R, Progress in extracellular vesicle biology and their application in cancer medicine, Wiley Interdiscip Rev Nanomed Nanobiotechnol, (2020) e1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Li P, Kaslan M, Lee SH, Yao J, Gao Z, Progress in Exosome Isolation Techniques, Theranostics, 7 (2017) 789–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ingato D, Edson JA, Zakharian M, Kwon YJ, Cancer Cell-Derived, Drug-Loaded Nanovesicles Induced by Sulfhydryl-Blocking for Effective and Safe Cancer Therapy, ACS Nano, 12 (2018) 9568–9577. [DOI] [PubMed] [Google Scholar]
- [32].Shyong YJ, Chang KC, Lin FH, Calcium phosphate particles stimulate exosome secretion from phagocytes for the enhancement of drug delivery, Colloids Surf B Biointerfaces, 171 (2018) 391–397. [DOI] [PubMed] [Google Scholar]
- [33].Ortega FG, Roefs MT, de Miguel Perez D, Kooijmans SA, de Jong OG, Sluijter JP, Schiffelers RM, Vader P, Interfering with endolysosomal trafficking enhances release of bioactive exosomes, Nanomedicine, 20 (2019) 102014. [DOI] [PubMed] [Google Scholar]
- [34].Lv LH, Wan YL, Lin Y, Zhang W, Yang M, Li GL, Lin HM, Shang CZ, Chen YJ, Min J, Anticancer drugs cause release of exosomes with heat shock proteins from human hepatocellular carcinoma cells that elicit effective natural killer cell antitumor responses in vitro, J Biol Chem, 287 (2012) 15874–15885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kore RA, Edmondson JL, Jenkins SV, Jamshidi-Parsian A, Dings RPM, Reyna NS, Griffin RJ, Hypoxia-derived exosomes induce putative altered pathways in biosynthesis and ion regulatory channels in glioblastoma cells, Biochem Biophys Rep, 14 (2018) 104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hsu YL, Hung JY, Chang WA, Lin YS, Pan YC, Tsai PH, Wu CY, Kuo PL, Hypoxic lung cancer-secreted exosomal miR-23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO-1, Oncogene, 36 (2017) 4929–4942. [DOI] [PubMed] [Google Scholar]
- [37].Panigrahi GK, Praharaj PP, Peak TC, Long J, Singh R, Rhim JS, Abd Elmageed ZY, Deep G, Hypoxia-induced exosome secretion promotes survival of African-American and Caucasian prostate cancer cells, Sci Rep, 8 (2018) 3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Park JE, Dutta B, Tse SW, Gupta N, Tan CF, Low JK, Yeoh KW, Kon OL, Tam JP, Sze SK, Hypoxia-induced tumor exosomes promote M2-like macrophage polarization of infiltrating myeloid cells and microRNA-mediated metabolic shift, Oncogene, 38 (2019) 5158–5173. [DOI] [PubMed] [Google Scholar]
- [39].Fukuta T, Nishikawa A, Kogure K, Low level electricity increases the secretion of extracellular vesicles from cultured cells, Biochem Biophys Rep, 21 (2020) 100713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gerlach JC, Lin YC, Brayfield CA, Minteer DM, Li H, Rubin JP, Marra KG, Adipogenesis of human adipose-derived stem cells within three-dimensional hollow fiber-based bioreactors, Tissue Eng Part C Methods, 18 (2012) 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Mendt M, Kamerkar S, Sugimoto H, McAndrews KM, Wu CC, Gagea M, Yang S, Blanko EVR, Peng Q, Ma X, Marszalek JR, Maitra A, Yee C, Rezvani K, Shpall E, LeBleu VS, Kalluri R, Generation and testing of clinical-grade exosomes for pancreatic cancer, JCI Insight, 3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Carswell KS, Papoutsakis ET, Culture of human T cells in stirred bioreactors for cellular immunotherapy applications: shear, proliferation, and the IL-2 receptor, Biotechnol Bioeng, 68 (2000) 328–338. [DOI] [PubMed] [Google Scholar]
- [43].Yang L, Han D, Zhan Q, Li X, Shan P, Hu Y, Ding H, Wang Y, Zhang L, Zhang Y, Xue S, Zhao J, Hou X, Wang Y, Li P, Yuan X, Qi H, Blood TfR+ exosomes separated by a pH-responsive method deliver chemotherapeutics for tumor therapy, Theranostics, 9 (2019) 7680–7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Liu F, Vermesh O, Mani V, Ge TJ, Madsen SJ, Sabour A, Hsu EC, Gowrishankar G, Kanada M, Jokerst JV, Sierra RG, Chang E, Lau K, Sridhar K, Bermudez A, Pitteri SJ, Stoyanova T, Sinclair R, Nair VS, Gambhir SS, Demirci U, The Exosome Total Isolation Chip, ACS Nano, 11 (2017) 10712–10723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Akers JC, Ramakrishnan V, Yang I, Hua W, Mao Y, Carter BS, Chen CC, Optimizing preservation of extracellular vesicular miRNAs derived from clinical cerebrospinal fluid, Cancer Biomark, 17 (2016) 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Frank J, Richter M, de Rossi C, Lehr CM, Fuhrmann K, Fuhrmann G, Extracellular vesicles protect glucuronidase model enzymes during freeze-drying, Sci Rep, 8 (2018) 12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Noguchi K, Hirano M, Hashimoto T, Yuba E, Takatani-Nakase T, Nakase I, Effects of Lyophilization of Arginine-rich Cell-penetrating Peptide-modified Extracellular Vesicles on Intracellular Delivery, Anticancer Res, 39 (2019) 6701–6709. [DOI] [PubMed] [Google Scholar]
- [48].Charoenviriyakul C, Takahashi Y, Nishikawa M, Takakura Y, Preservation of exosomes at room temperature using lyophilization, Int J Pharm, 553 (2018) 1–7. [DOI] [PubMed] [Google Scholar]
- [49].Yang Z, Shi J, Xie J, Wang Y, Sun J, Liu T, Zhao Y, Zhao X, Wang X, Ma Y, Malkoc V, Chiang C, Deng W, Chen Y, Fu Y, Kwak KJ, Fan Y, Kang C, Yin C, Rhee J, Bertani P, Otero J, Lu W, Yun K, Lee AS, Jiang W, Teng L, Kim BYS, Lee LJ, Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation, Nat Biomed Eng, 4 (2020) 69–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Li Z, Zhou X, Wei M, Gao X, Zhao L, Shi R, Sun W, Duan Y, Yang G, Yuan L, In Vitro and in Vivo RNA Inhibition by CD9-HuR Functionalized Exosomes Encapsulated with miRNA or CRISPR/dCas9, Nano Lett, 19 (2019) 19–28. [DOI] [PubMed] [Google Scholar]
- [51].Huang L, Wang D, Gu N, Zhang XE, [Construction of engineered exosomes with high loading efficiency of cellular endogenous proteins], Sheng Wu Gong Cheng Xue Bao, 35 (2019) 1537–1545. [DOI] [PubMed] [Google Scholar]
- [52].Zhang D, Lee H, Zhu Z, Minhas JK, Jin Y, Enrichment of selective miRNAs in exosomes and delivery of exosomal miRNAs in vitro and in vivo, Am J Physiol Lung Cell Mol Physiol, 312 (2017) L110–L121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lamichhane TN, Jeyaram A, Patel DB, Parajuli B, Livingston NK, Arumugasaamy N, Schardt JS, Jay SM, Oncogene Knockdown via Active Loading of Small RNAs into Extracellular Vesicles by Sonication, Cell Mol Bioeng, 9 (2016) 315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kim SM, Yang Y, Oh SJ, Hong Y, Seo M, Jang M, Cancer-derived exosomes as a delivery platform of CRISPR/Cas9 confer cancer cell tropism-dependent targeting, J Control Release, 266 (2017) 8–16. [DOI] [PubMed] [Google Scholar]
- [55].Jeyaram A, Lamichhane TN, Wang S, Zou L, Dahal E, Kronstadt SM, Levy D, Parajuli B, Knudsen DR, Chao W, Jay SM, Enhanced Loading of Functional miRNA Cargo via pH Gradient Modification of Extracellular Vesicles, Mol Ther, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Didiot MC, Hall LM, Coles AH, Haraszti RA, Godinho BM, Chase K, Sapp E, Ly S, Alterman JF, Hassler MR, Echeverria D, Raj L, Morrissey DV, DiFiglia M, Aronin N, Khvorova A, Exosome-mediated Delivery of Hydrophobically Modified siRNA for Huntingtin mRNA Silencing, Mol Ther, 24 (2016) 1836–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kooijmans SAA, Stremersch S, Braeckmans K, de Smedt SC, Hendrix A, Wood MJA, Schiffelers RM, Raemdonck K, Vader P, Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles, J Control Release, 172 (2013) 229–238. [DOI] [PubMed] [Google Scholar]
- [58].Liu J, Ye Z, Xiang M, Chang B, Cui J, Ji T, Zhao L, Li Q, Deng Y, Xu L, Wang G, Wang L, Wang Z, Functional extracellular vesicles engineered with lipid-grafted hyaluronic acid effectively reverse cancer drug resistance, Biomaterials, 223 (2019) 119475. [DOI] [PubMed] [Google Scholar]
- [59].Si Y, Kim S, Zhang E, Tang Y, Jaskula-Sztul R, Markert JM, Chen H, Zhou L, Liu XM, Targeted Exosomes for Drug Delivery: Biomanufacturing, Surface Tagging, and Validation, Biotechnol J, 15 (2020) e1900163. [DOI] [PubMed] [Google Scholar]
- [60].Nie W, Wu G, Zhang J, Huang LL, Ding J, Jiang A, Zhang Y, Liu Y, Li J, Pu K, Xie HY, Responsive Exosome Nano-bioconjugates for Synergistic Cancer Therapy, Angew Chem Int Ed Engl, 59 (2020) 2018–2022. [DOI] [PubMed] [Google Scholar]
- [61].Koh E, Lee EJ, Nam GH, Hong Y, Cho E, Yang Y, Kim IS, Exosome-SIRPalpha, a CD47 blockade increases cancer cell phagocytosis, Biomaterials, 121 (2017) 121–129. [DOI] [PubMed] [Google Scholar]
- [62].Stickney Z, Losacco J, McDevitt S, Zhang Z, Lu B, Development of exosome surface display technology in living human cells, Biochem Biophys Res Commun, 472 (2016) 53–59. [DOI] [PubMed] [Google Scholar]
- [63].Fu W, Lei C, Liu S, Cui Y, Wang C, Qian K, Li T, Shen Y, Fan X, Lin F, Ding M, Pan M, Ye X, Yang Y, Hu S, CAR exosomes derived from effector CAR-T cells have potent antitumour effects and low toxicity, Nat Commun, 10 (2019) 4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Tang XJ, Sun XY, Huang KM, Zhang L, Yang ZS, Zou DD, Wang B, Warnock GL, Dai LJ, Luo J, Therapeutic potential of CAR-T cell-derived exosomes: a cell-free modality for targeted cancer therapy, Oncotarget, 6 (2015) 44179–44190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Fan Z, Xiao K, Lin J, Liao Y, Huang X, Functionalized DNA Enables Programming Exosomes/Vesicles for Tumor Imaging and Therapy, Small, 15 (2019) e1903761. [DOI] [PubMed] [Google Scholar]
- [66].Jang SC, Kim OY, Yoon CM, Choi DS, Roh TY, Park J, Nilsson J, Lotvall J, Kim YK, Gho YS, Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors, ACS Nano, 7 (2013) 7698–7710. [DOI] [PubMed] [Google Scholar]
- [67].Vazquez-Rios AJ, Molina-Crespo A, Bouzo BL, Lopez-Lopez R, Moreno-Bueno G, de la Fuente M, Exosome-mimetic nanoplatforms for targeted cancer drug delivery, J Nanobiotechnology, 17 (2019) 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Zhang KL, Wang YJ, Sun J, Zhou J, Xing C, Huang G, Li J, Yang H, Artificial chimeric exosomes for anti-phagocytosis and targeted cancer therapy, Chem Sci, 10 (2019) 1555–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Jhan YY, Prasca-Chamorro D, Palou Zuniga G, Moore DM, Arun Kumar S, Gaharwar AK, Bishop CJ, Engineered extracellular vesicles with synthetic lipids via membrane fusion to establish efficient gene delivery, Int J Pharm, 573 (2020) 118802. [DOI] [PubMed] [Google Scholar]
- [70].Chen Q, Chen Y, Sun Y, He W, Han X, Lu E, Sha X, Leukocyte-mimicking Pluronic-lipid nanovesicle hybrids inhibit the growth and metastasis of breast cancer, Nanoscale, 11 (2019) 5377–5394. [DOI] [PubMed] [Google Scholar]
- [71].Wang G, Hu W, Chen H, Shou X, Ye T, Xu Y, Cocktail Strategy Based on NK Cell-Derived Exosomes and Their Biomimetic Nanoparticles for Dual Tumor Therapy, Cancers (Basel), 11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Kim OY, Park HT, Dinh NTH, Choi SJ, Lee J, Kim JH, Lee SW, Gho YS, Bacterial outer membrane vesicles suppress tumor by interferon-gamma-mediated antitumor response, Nat Commun, 8 (2017) 626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Behzadi E, Mahmoodzadeh Hosseini H, Imani Fooladi AA, The inhibitory impacts of Lactobacillus rhamnosus GG-derived extracellular vesicles on the growth of hepatic cancer cells, Microb Pathog, 110 (2017) 1–6. [DOI] [PubMed] [Google Scholar]
- [74].Raimondo S, Naselli F, Fontana S, Monteleone F, Lo Dico A, Saieva L, Zito G, Flugy A, Manno M, Di Bella MA, De Leo G, Alessandro R, Citrus limon-derived nanovesicles inhibit cancer cell proliferation and suppress CML xenograft growth by inducing TRAIL-mediated cell death, Oncotarget, 6 (2015) 19514–19527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, Valente N, Shreeniwas R, Sutton MA, Delcayre A, Hsu DH, Le Pecq JB, Lyerly HK, A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer, J Transl Med, 3 (2005) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Besse B, Charrier M, Lapierre V, Dansin E, Lantz O, Planchard D, Le Chevalier T, Livartoski A, Barlesi F, Laplanche A, Ploix S, Vimond N, Peguillet I, Thery C, Lacroix L, Zoernig I, Dhodapkar K, Dhodapkar M, Viaud S, Soria JC, Reiners KS, Pogge von Strandmann E, Vely F, Rusakiewicz S, Eggermont A, Pitt JM, Zitvogel L, Chaput N, Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC, Oncoimmunology, 5 (2016) e1071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Fu Q, Zhang Q, Lou Y, Yang J, Nie G, Chen Q, Chen Y, Zhang J, Wang J, Wei T, Qin H, Dang X, Bai X, Liang T, Primary tumor-derived exosomes facilitate metastasis by regulating adhesion of circulating tumor cells via SMAD3 in liver cancer, Oncogene, 37 (2018) 6105–6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Fu Q, Zhang Q, Lou Y, Yang J, Nie G, Chen Q, Chen Y, Zhang J, Wang J, Wei T, Qin H, Dang X, Bai X, Liang T, Correction: Primary tumor-derived exosomes facilitate metastasis by regulating adhesion of circulating tumor cells via SMAD3 in liver cancer, Oncogene, 38 (2019) 5740–5741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Liu X, Lu Y, Xu Y, Hou S, Huang J, Wang B, Zhao J, Xia S, Fan S, Yu X, Du Y, Hou L, Li Z, Ding Z, An S, Huang B, Li L, Tang J, Ju J, Guan H, Song B, Exosomal transfer of miR-501 confers doxorubicin resistance and tumorigenesis via targeting of BLID in gastric cancer, Cancer Lett, 459 (2019) 122–134. [DOI] [PubMed] [Google Scholar]
- [80].Jang JY, Lee JK, Jeon YK, Kim CW, Exosome derived from epigallocatechin gallate treated breast cancer cells suppresses tumor growth by inhibiting tumor-associated macrophage infiltration and M2 polarization, BMC Cancer, 13 (2013) 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Huang L, Hu C, Chao H, Zhang Y, Li Y, Hou J, Xu Z, Lu H, Li H, Chen H, Drug-resistant endothelial cells facilitate progression, EMT and chemoresistance in nasopharyngeal carcinoma via exosomes, Cell Signal, 63 (2019) 109385. [DOI] [PubMed] [Google Scholar]
- [82].Huang CY, Ju DT, Chang CF, Muralidhar Reddy P, Velmurugan BK, A review on the effects of current chemotherapy drugs and natural agents in treating non-small cell lung cancer, Biomedicine (Taipei), 7 (2017) 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Deng X, Ruan H, Zhang X, Xu X, Zhu Y, Peng H, Zhang X, Kong F, Guan M, Long noncoding RNA CCAL transferred from fibroblasts by exosomes promotes chemoresistance of colorectal cancer cells, Int J Cancer, 146 (2020) 1700–1716. [DOI] [PubMed] [Google Scholar]
- [84].Xu Y, Zhu M, Novel exosomal miR-46146 transfer oxaliplatin chemoresistance in colorectal cancer, Clin Transl Oncol, (2019). [DOI] [PubMed] [Google Scholar]
- [85].Jingyue S, Xiao W, Juanmin Z, Wei L, Daoming L, Hong X, TFAP2E methylation promotes 5fluorouracil resistance via exosomal miR106a5p and miR421 in gastric cancer MGC803 cells, Mol Med Rep, 20 (2019) 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].He J, He J, Min L, He Y, Guan H, Wang J, Peng X, Extracellular vesicles transmitted miR-31–5p promotes sorafenib resistance by targeting MLH1 in renal cell carcinoma, Int J Cancer, 146 (2020) 1052–1063. [DOI] [PubMed] [Google Scholar]
- [87].Wang G, Zhao W, Wang H, Qiu G, Jiang Z, Wei G, Li X, Exosomal MiR-744 Inhibits Proliferation and Sorafenib Chemoresistance in Hepatocellular Carcinoma by Targeting PAX2, Med Sci Monit, 25 (2019) 7209–7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Januchowski R, Sterzynska K, Zaorska K, Sosinska P, Klejewski A, Brazert M, Nowicki M, Zabel M, Analysis of MDR genes expression and cross-resistance in eight drug resistant ovarian cancer cell lines, J Ovarian Res, 9 (2016) 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Moitra K, Lou H, Dean M, Multidrug efflux pumps and cancer stem cells: insights into multidrug resistance and therapeutic development, Clin Pharmacol Ther, 89 (2011) 491–502. [DOI] [PubMed] [Google Scholar]
- [90].Sousa D, Lima RT, Vasconcelos MH, Intercellular Transfer of Cancer Drug Resistance Traits by Extracellular Vesicles, Trends Mol Med, 21 (2015) 595–608. [DOI] [PubMed] [Google Scholar]
- [91].Corcoran C, Rani S, O’Brien K, O’Neill A, Prencipe M, Sheikh R, Webb G, McDermott R, Watson W, Crown J, O’Driscoll L, Docetaxel-resistance in prostate cancer: evaluating associated phenotypic changes and potential for resistance transfer via exosomes, PLoS One, 7 (2012) e50999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Bebawy M, Combes V, Lee E, Jaiswal R, Gong J, Bonhoure A, Grau GE, Membrane microparticles mediate transfer of P-glycoprotein to drug sensitive cancer cells, Leukemia, 23 (2009) 1643–1649. [DOI] [PubMed] [Google Scholar]
- [93].Safaei R, Larson BJ, Cheng TC, Gibson MA, Otani S, Naerdemann W, Howell SB, Abnormal lysosomal trafficking and enhanced exosomal export of cisplatin in drug-resistant human ovarian carcinoma cells, Mol Cancer Ther, 4 (2005) 1595–1604. [DOI] [PubMed] [Google Scholar]
- [94].Shedden K, Xie XT, Chandaroy P, Chang YT, Rosania GR, Expulsion of small molecules in vesicles shed by cancer cells: association with gene expression and chemosensitivity profiles, Cancer Res, 63 (2003) 4331–4337. [PubMed] [Google Scholar]
- [95].Federici C, Petrucci F, Caimi S, Cesolini A, Logozzi M, Borghi M, D’Ilio S, Lugini L, Violante N, Azzarito T, Majorani C, Brambilla D, Fais S, Exosome release and low pH belong to a framework of resistance of human melanoma cells to cisplatin, PLoS One, 9 (2014) e88193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Koch R, Aung T, Vogel D, Chapuy B, Wenzel D, Becker S, Sinzig U, Venkataramani V, von Mach T, Jacob R, Truemper L, Wulf GG, Nuclear Trapping through Inhibition of Exosomal Export by Indomethacin Increases Cytostatic Efficacy of Doxorubicin and Pixantrone, Clin Cancer Res, 22 (2016) 395–404. [DOI] [PubMed] [Google Scholar]
- [97].Yang Y, Cancer immunotherapy: harnessing the immune system to battle cancer, J Clin Invest, 125 (2015) 3335–3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Velcheti V, Schalper K, Basic Overview of Current Immunotherapy Approaches in Cancer, Am Soc Clin Oncol Educ Book, 35 (2016) 298–308. [DOI] [PubMed] [Google Scholar]
- [99].Tang B, Guo ZS, Bartlett DL, Yan DZ, Schane CP, Liu J, McFadden G, Thomas DL, Shisler JL, Roy EJ, Synergistic Combination of Oncolytic Virotherapy and Immunotherapy for Glioma, Clin Cancer Res, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Xie F, Xu M, Lu J, Mao L, Wang S, The role of exosomal PD-L1 in tumor progression and immunotherapy, Mol Cancer, 18 (2019) 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Aung T, Chapuy B, Vogel D, Wenzel D, Oppermann M, Lahmann M, Weinhage T, Menck K, Hupfeld T, Koch R, Trumper L, Wulf GG, Exosomal evasion of humoral immunotherapy in aggressive B-cell lymphoma modulated by ATP-binding cassette transporter A3, Proc Natl Acad Sci U S A, 108 (2011) 15336–15341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Sarosiek T, Morawski P, [Trastuzumab and its biosimilars], Pol Merkur Lekarski, 44 (2018) 253–257. [PubMed] [Google Scholar]
- [103].Dong H, Wang W, Chen R, Zhang Y, Zou K, Ye M, He X, Zhang F, Han J, Exosome-mediated transfer of lncRNASNHG14 promotes trastuzumab chemoresistance in breast cancer, Int J Oncol, 53 (2018) 1013–1026. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [104].Han M, Gu Y, Lu P, Li J, Cao H, Li X, Qian X, Yu C, Yang Y, Yang X, Han N, Dou D, Hu J, Dong H, Exosome-mediated lncRNA AFAP1-AS1 promotes trastuzumab resistance through binding with AUF1 and activating ERBB2 translation, Mol Cancer, 19 (2020) 26. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [105].Zheng Z, Chen M, Xing P, Yan X, Xie B, Increased Expression of Exosomal AGAP2-AS1 (AGAP2 Antisense RNA 1) In Breast Cancer Cells Inhibits Trastuzumab-Induced Cell Cytotoxicity, Med Sci Monit, 25 (2019) 2211–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Zhang S, Zhang Y, Qu J, Che X, Fan Y, Hou K, Guo T, Deng G, Song N, Li C, Wan X, Qu X, Liu Y, Exosomes promote cetuximab resistance via the PTEN/Akt pathway in colon cancer cells, Braz J Med Biol Res, 51 (2017) e6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Galbo PM Jr., Ciesielski MJ, Figel S, Maguire O, Qiu J, Wiltsie L, Minderman H, Fenstermaker RA, Circulating CD9+/GFAP+/survivin+ exosomes in malignant glioma patients following survivin vaccination, Oncotarget, 8 (2017) 114722–114735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Hennequin C, Barillot I, Azria D, Belkacemi Y, Bollet M, Chauvet B, Cowen D, Cutuli B, Fourquet A, Hannoun-Levi JM, Leblanc M, Mahe MA, [Radiotherapy of breast cancer], Cancer Radiother, 20 Suppl (2016) S139–146. [DOI] [PubMed] [Google Scholar]
- [109].Mutschelknaus L, Peters C, Winkler K, Yentrapalli R, Heider T, Atkinson MJ, Moertl S, Exosomes Derived from Squamous Head and Neck Cancer Promote Cell Survival after Ionizing Radiation, PLoS One, 11 (2016) e0152213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Zhang Z, Yu X, Zhou Z, Li B, Peng J, Wu X, Luo X, Yang L, LMP1-positive extracellular vesicles promote radioresistance in nasopharyngeal carcinoma cells through P38 MAPK signaling, Cancer Med, 8 (2019) 6082–6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Yue X, Lan F, Xia T, Hypoxic Glioma Cell-Secreted Exosomal miR-301a Activates Wnt/beta-catenin Signaling and Promotes Radiation Resistance by Targeting TCEAL7, Mol Ther, 27 (2019) 1939–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Ni J, Bucci J, Malouf D, Knox M, Graham P, Li Y, Exosomes in Cancer Radioresistance, Front Oncol, 9 (2019) 869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Wan FZ, Chen KH, Sun YC, Chen XC, Liang RB, Chen L, Zhu XD, Exosomes overexpressing miR-34c inhibit malignant behavior and reverse the radioresistance of nasopharyngeal carcinoma, J Transl Med, 18 (2020) 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Hu M, Jiang L, Cui X, Zhang J, Yu J, Proton beam therapy for cancer in the era of precision medicine, J Hematol Oncol, 11 (2018) 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Khan S, Jutzy JM, Aspe JR, McGregor DW, Neidigh JW, Wall NR, Survivin is released from cancer cells via exosomes, Apoptosis, 16 (2011) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Semina SE, Scherbakov AM, Vnukova AA, Bagrov DV, Evtushenko EG, Safronova VM, Golovina DA, Lyubchenko LN, Gudkova MV, Krasil’nikov MA, Exosome-Mediated Transfer of Cancer Cell Resistance to Antiestrogen Drugs, Molecules, 23 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Sansone P, Savini C, Kurelac I, Chang Q, Amato LB, Strillacci A, Stepanova A, Iommarini L, Mastroleo C, Daly L, Galkin A, Thakur BK, Soplop N, Uryu K, Hoshino A, Norton L, Bonafe M, Cricca M, Gasparre G, Lyden D, Bromberg J, Packaging and transfer of mitochondrial DNA via exosomes regulate escape from dormancy in hormonal therapy-resistant breast cancer, Proc Natl Acad Sci U S A, 114 (2017) E9066–E9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Wang X, Zhang H, Yang H, Bai M, Ning T, Deng T, Liu R, Fan Q, Zhu K, Li J, Zhan Y, Ying G, Ba Y, Exosome-delivered circRNA promotes glycolysis to induce chemoresistance through the miR-122-PKM2 axis in colorectal cancer, Mol Oncol, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Del Re M, Bertolini I, Crucitta S, Fontanelli L, Rofi E, De Angelis C, Diodati L, Cavallero D, Gianfilippo G, Salvadori B, Fogli S, Falcone A, Scatena C, Naccarato AG, Roncella M, Ghilli M, Morganti R, Fontana A, Danesi R, Overexpression of TK1 and CDK9 in plasma-derived exosomes is associated with clinical resistance to CDK4/6 inhibitors in metastatic breast cancer patients, Breast Cancer Res Treat, 178 (2019) 57–62. [DOI] [PubMed] [Google Scholar]
- [120].Zhang Q, Liu RX, Chan KW, Hu J, Zhang J, Wei L, Tan H, Yang X, Liu H, Exosomal transfer of p-STAT3 promotes acquired 5-FU resistance in colorectal cancer cells, J Exp Clin Cancer Res, 38 (2019) 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Zhang Z, Yin J, Lu C, Wei Y, Zeng A, You Y, Exosomal transfer of long non-coding RNA SBF2-AS1 enhances chemoresistance to temozolomide in glioblastoma, J Exp Clin Cancer Res, 38 (2019) 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Yin J, Zeng A, Zhang Z, Shi Z, Yan W, You Y, Exosomal transfer of miR-1238 contributes to temozolomide-resistance in glioblastoma, EBioMedicine, 42 (2019) 238–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Shen M, Dong C, Ruan X, Yan W, Cao M, Pizzo D, Wu X, Yang L, Liu L, Ren X, Wang SE, Chemotherapy-Induced Extracellular Vesicle miRNAs Promote Breast Cancer Stemness by Targeting ONECUT2, Cancer Res, 79 (2019) 3608–3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Faict S, Oudaert I, D’Auria L, Dehairs J, Maes K, Vlummens P, De Veirman K, De Bruyne E, Fostier K, Vande Broek I, Schots R, Vanderkerken K, Swinnen JV, Menu E, The Transfer of Sphingomyelinase Contributes to Drug Resistance in Multiple Myeloma, Cancers (Basel), 11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Zhao K, Wang Z, Li X, Liu JL, Tian L, Chen JQ, Exosome-mediated transfer of CLIC1 contributes to the vincristine-resistance in gastric cancer, Mol Cell Biochem, 462 (2019) 97–105. [DOI] [PubMed] [Google Scholar]


