Abstract
The use of face masks in public settings has been widely recommended by public health officials during the current COVID-19 pandemic. The masks help mitigate the risk of cross-infection via respiratory droplets; however, there are no specific guidelines on mask materials and designs that are most effective in minimizing droplet dispersal. While there have been prior studies on the performance of medical-grade masks, there are insufficient data on cloth-based coverings, which are being used by a vast majority of the general public. We use qualitative visualizations of emulated coughs and sneezes to examine how material- and design-choices impact the extent to which droplet-laden respiratory jets are blocked. Loosely folded face masks and bandana-style coverings provide minimal stopping-capability for the smallest aerosolized respiratory droplets. Well-fitted homemade masks with multiple layers of quilting fabric, and off-the-shelf cone style masks, proved to be the most effective in reducing droplet dispersal. These masks were able to curtail the speed and range of the respiratory jets significantly, albeit with some leakage through the mask material and from small gaps along the edges. Importantly, uncovered emulated coughs were able to travel notably farther than the currently recommended 6-ft distancing guideline. We outline the procedure for setting up simple visualization experiments using easily available materials, which may help healthcare professionals, medical researchers, and manufacturers in assessing the effectiveness of face masks and other personal protective equipment qualitatively.
Infectious respiratory illnesses can exact a heavy socio-economic toll on the most vulnerable members of our society, as has become evident from the current COVID-19 pandemic.1,2 The disease has overwhelmed healthcare infrastructure worldwide,3 and its high contagion rate and relatively long incubation period4,5 have made it difficult to trace and isolate infected individuals. Current estimates indicate that about 35% of infected individuals do not display overt symptoms6 and may contribute to the significant spread of the disease without their knowledge. In an effort to contain the unabated community spread of the disease, public health officials have recommended the implementation of various preventative measures, including social-distancing and the use of face masks in public settings.7
The rationale behind the recommendation for using masks or other face coverings is to reduce the risk of cross-infection via the transmission of respiratory droplets from infected to healthy individuals.8,9 The pathogen responsible for COVID-19 is found primarily in respiratory droplets that are expelled by infected individuals during coughing, sneezing, or even talking and breathing.10–15 Apart from COVID-19, respiratory droplets are also the primary means of transmission for various other viral and bacterial illnesses, such as the common cold, influenza, tuberculosis, SARS (Severe Acute Respiratory Syndrome), and MERS (Middle East Respiratory Syndrome), to name a few.16–19 These pathogens are enveloped within respiratory droplets, which may land on healthy individuals and result in direct transmission, or on inanimate objects, which can lead to infection when a healthy individual comes in contact with them.10,18,20,21 In another mode of transmission, the droplets or their evaporated contents may remain suspended in the air for long periods of time if they are sufficiently small. This can lead to airborne transmission19,22 when they are breathed in by another person, long after the infected individual may have left the area.
Several studies have investigated respiratory droplets produced by both healthy and infected individuals when performing various activities. The transport characteristics of these droplets can vary significantly depending on their diameter.23–28 The reported droplet diameters vary widely among studies available in the literature and usually lie within the range 1 µm–500 µm,29 with a mean diameter of ∼10 µm.30 The larger droplets (diameter >100 µm) are observed to follow ballistic trajectories under the effects of gravity and aerodynamic drag.20,31 Intermediate-sized droplets20,31,32 may get carried over considerable distances within a multiphase turbulent cloud.33–35 The smallest droplets and particles (diameter < 5 µm–10 µm) may remain suspended in the air indefinitely, until they are carried away by a light breeze or ventilation airflow.20,32
After being expelled into the ambient environment, the respiratory droplets experience varying degrees of evaporation depending on their size, ambient humidity, and temperature. The smallest droplets may undergo complete evaporation, leaving behind a dried-out spherical mass consisting of the particulate contents (e.g., pathogens), which are referred to as “droplet nuclei.”36 These desiccated nuclei, in combination with the smallest droplets, are potent transmission sources on account of two factors: (1) they can remain suspended in the air for hours after the infected individual has left the area, potentially infecting unsuspecting individuals who come into contact with them and (2) they can penetrate deep into the airways of individuals who breathe them in, which increases the likelihood of infection even for low pathogen loads. At present, the role of droplet nuclei in the transmission of COVID-19 is not known with certainty and the matter is the subject of ongoing studies.37–39 In addition to generating microscopic droplets, the action of sneezing can expel sheet-like layers of respiratory fluids,40 which may break apart into smaller droplets through a series of instabilities. The majority of the fluid contained within the sheet falls to the ground quickly within a short distance.
Regardless of their size, all droplets and nuclei expelled by infected individuals are potential carriers of pathogens. Various studies have investigated the effectiveness of medical-grade face masks and other personal protective equipment (PPE) in reducing the possibility of cross-infection via these droplets.13,33,41–47 Notably, such respiratory barriers do not prove to be completely effective against extremely fine aerosolized particles, droplets, and nuclei. The main issue tends to be air leakage, which can result in aerosolized pathogens being dispersed and suspended in the ambient environment for long periods of time after a coughing/sneezing event has occurred. A few studies have considered the filtration efficiency of homemade masks made with different types of fabric;48–51 however, there is no broad consensus regarding their effectiveness in minimizing disease transmission.52,53 Nonetheless, the evidence suggests that masks and other face coverings are effective in stopping larger droplets, which, although fewer in number compared to the smaller droplets and nuclei, constitute a large fraction of the total volume of the ejected respiratory fluid.
While detailed quantitative measurements are necessary for the comprehensive characterization of PPE, qualitative visualizations can be invaluable for rapid iteration in early design stages, as well as for demonstrating the proper use of such equipment. Thus, one of the aims of this Letter is to describe a simple setup for visualization experiments, which can be assembled using easily available materials. Such setups may be helpful to healthcare professionals, medical researchers, and industrial manufacturers, for assessing the effectiveness of face masks and other protective equipment qualitatively. Testing designs quickly and early on can prove to be crucial, especially in the current pandemic scenario where one of the central objectives is to reduce the severity of the anticipated resurgence of infections in the upcoming months.
The visualization setup used in the current study is shown in Fig. 1 and consists of a hollow manikin head which was padded on the inside to approximate the internal shape and volume of the nasal- and buccal-cavities in an adult. In case a more realistic representation is required, such a setup could include 3D-printed or silicone models of the internal airways. The manikin was mounted at a height of ∼5 ft and 8 in. to emulate respiratory jets expelled by an average human male. The circular opening representing the mouth is 0.75 in. in diameter. The pressure impulse that emulates a cough or a sneeze may be delivered via a manual pump, as shown in Fig. 1, or via other sources such as an air compressor or a pressurized air canister. The air capacity of the pump is 500 ml, which is comparable to the lower end of the total volume expelled during a cough.54 We note that the setup here emulates a simplified representation of an actual cough, which is an extremely complex and dynamic problem.55 We use a recreational fog/smoke machine to generate tracer particles for visualizing the expelled respiratory jets, using a liquid mixture of distilled water (4 parts) and glycerin (1 part). Both the pressure- and smoke-sources were connected to the manikin using clear vinyl tubing and NPT fittings wherever necessary.
FIG. 1.
Left—experimental setup for qualitative visualization of emulated coughs and sneezes. Right—a laser sheet illuminates a puff emerging from the mouth.
The resulting “fog” or “smoke” is visible in the right panel of Fig. 1 and is composed of microscopic droplets of the vaporized liquid mixture. These are comparable in size to the smallest droplets expelled in a cough jet (∼1 µm–10 µm). We estimate that the fog droplets are less than 10 µm in diameter, based on Stokes’ law and our observation that they could remain suspended for up to 3 min in completely still air with no perceptible settling. The laser source used to generate the visualization sheet is an off-the-shelf 5 mW green laser pointer with 532 nm wavelength. A plane vertical sheet is created by passing the laser beam through a thin cylindrical rod (diameter 5 mm) made of borosilicate glass.
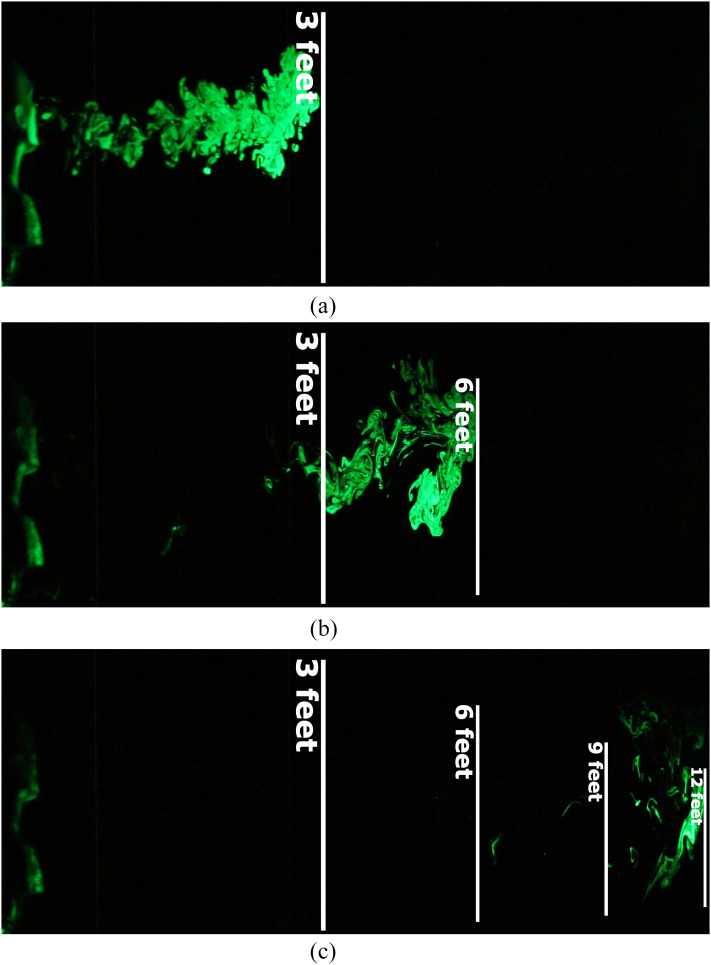
We first present visualization results from an emulation of an uncovered heavy cough. The spatial and temporal evolution of the resulting jet is shown in Fig. 2. The aerosolized microscopic droplets visible in the laser sheet act as tracer particles, revealing a two-dimensional cross section of the conical turbulent jet. These tracers depict the fate of the smallest ejected droplets and any resulting nuclei that may form. We observed high variability in droplet dispersal patterns from one experimental run to another, which was caused by otherwise imperceptible changes in the ambient airflow. This highlights the importance of designing ventilation systems that specifically aim to minimize the possibility of cross-infection in a confined setting.23,56–58
FIG. 2.
An emulated heavy cough jet travels up to 12 ft in ∼50 s, which is twice the CDC’s recommended distancing guideline of 6 ft.7 Images taken at (a) 2.3 s, (b) 11 s, and (c) 53 s after the initiation of the emulated cough.
Despite high variability, we consistently observed jets that traveled farther than the 6-ft minimum distance proposed by the U.S. Centers for Disease Control and Prevention (CDC’s).7 In the images shown in Fig. 2, the ejected tracers were observed to travel up to 12 ft within ∼50 s. Moreover, the tracer droplets remained suspended midair for up to 3 min in the quiescent environment. These observations, in combination with other recent studies,35,59 suggest that current social-distancing guidelines may need to be updated to account for the aerosol-based transmission of pathogens. We note that although the unobstructed turbulent jets were observed to travel up to 12 ft, a large majority of the ejected droplets will fall to the ground by this point. Importantly, both the number and concentration of the droplets will decrease with increasing distance,59 which is the fundamental rationale behind social- distancing.
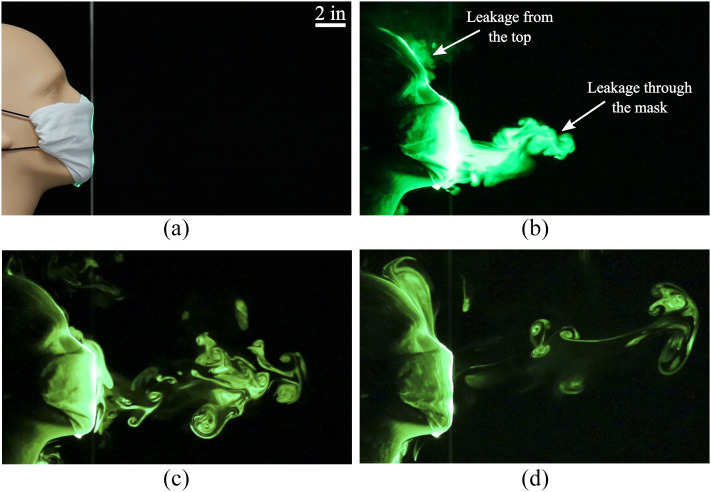
We now discuss dispersal patterns observed when the mouth opening was blocked using three different types of face masks. For these results, we focus on masks that are readily accessible to the general public, which do not draw away from the supply of medical-grade masks and respirators for healthcare workers. Figure 3 shows the impact of using a folded cotton handkerchief mask on the expelled respiratory jet. The folded mask was constructed by following the instructions recommended by the U.S. Surgeon General.60 It is evident that while the forward motion of the jet is impeded significantly, there is notable leakage of tracer droplets through the mask material. We also observe a small amount of tracers escaping from the top edge of the mask, where gaps exist between the nose and the cloth material. These droplets remained suspended in the air until they were dispersed by ambient disturbances. In addition to the folded handkerchief mask discussed here, we tested a single-layer bandana-style covering (not shown) which proved to be substantially less effective in stopping the jet and the tracer droplets.
FIG. 3.
(a) A face mask constructed using a folded handkerchief. Images taken at (b) 0.5 s, (c) 2.27 s, and (d) 5.55 s after the initiation of the emulated cough.
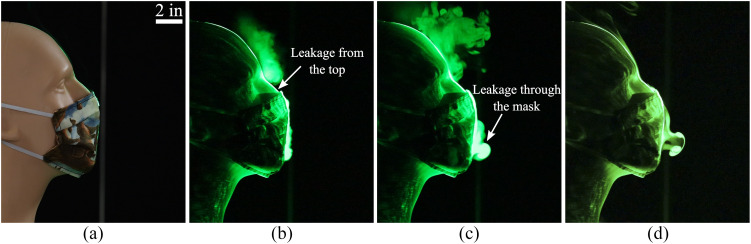
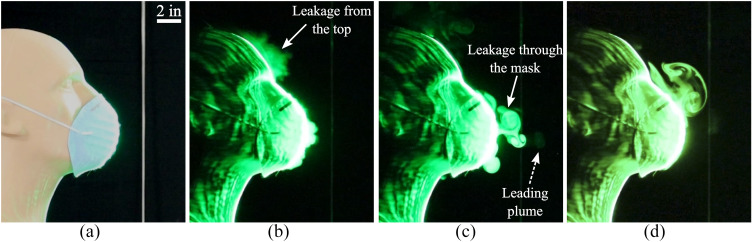
We now examine a homemade mask that was stitched using two-layers of cotton quilting fabric consisting of 70 threads/in. The mask’s impact on droplet dispersal is shown in Fig. 4. We observe that the mask is able to arrest the forward motion of the tracer droplets almost completely. There is minimal forward leakage through the material, and most of the tracer-escape happens from the gap between the nose and the mask along the top edge. The forward distance covered by the leaked jet is less than 3 in. in this case. The final mask design that we tested was a non-sterile cone-style mask that is available in most pharmacies. The corresponding droplet-dispersal visualizations are shown in Fig. 5, which indicate that the flow is impeded significantly compared to Figs. 2 and 3. However, there is noticeable leakage from gaps along the top edge. The forward distance covered by the leaked jet is ∼6 in. from the mouth opening, which is farther than the distance for the stitched mask in Fig. 4.
FIG. 4.
(a) A homemade face mask stitched using two-layers of cotton quilting fabric. Images taken at (b) 0.2 s, (c) 0.47 s, and (d) 1.68 s after the initiation of the emulated cough.
FIG. 5.
(a) An off-the-shelf cone style mask. (b) 0.2 s after initiation of the emulated cough. (c) 0.97 s after initiation of the emulated cough. The leading plume, which has dissipated considerably, is faintly visible. (d) 3.7 s after initiation of the emulated cough.
A summary of the various scenarios examined in this study is provided in Table I, along with details about the mask material and the average distances traveled by the respiratory jets. We observe that a single-layer bandana-style covering can reduce the range of the expelled jet to some extent, compared to an uncovered cough. Importantly, both the material and construction techniques have a notable impact on the masks’ stopping-capability. The stitched mask made of quilting cotton was observed to be the most effective, followed by the commercial mask, the folded handkerchief, and, finally, the bandana. Importantly, our observations suggest that a higher thread count by itself is not sufficient to guarantee better stopping-capability; the bandana covering, which has the highest thread count among all the cloth masks tested, turned out to be the least effective.
TABLE I.
A summary of the different types of masks tested, the materials they are made of, and their effectiveness in impeding droplet-dispersal. The last column indicates the distance traveled by the jet beyond which its forward progression stops. The average distances have been computed over multiple runs, and the symbol “∼” is used to indicate the presence of high variability in the first two scenarios listed.
| Mask type | Material | Threads/in. | Average jet distance |
|---|---|---|---|
| Uncovered | … | … | ∼8 ft |
| Bandana | Elastic T-shirt material | 85 | ∼3 ft 7 in. |
| Folded handkerchief | Cotton | 55 | 1 ft 3 in. |
| Stitched mask | Quilting cotton | 70 | 2.5 in. |
| Commercial maska | Unknown | Randomly assorted fibres | 8 in. |
CVS Cone Face Mask.
We note that it is likely that healthcare professionals trained properly in the use of high-quality fitted masks will not experience leakage to the extent that we have observed in this study. However, leakage remains a likely issue for members of the general public who often rely on loose-fitting homemade masks. Additionally, the masks may get saturated after prolonged use, which might also influence their filtration capability. We reiterate that although the non-medical masks tested in this study experienced varying degrees of flow leakage, they are likely to be effective in stopping larger respiratory droplets.
In addition to providing an initial indication of the effectiveness of protective equipment, the visuals used in this study can help convey to the general public the rationale behind social-distancing guidelines and recommendations for using face masks. Promoting widespread awareness of effective preventative measures is crucial, given the high likelihood of a resurgence of COVID-19 infections in the fall and winter.
DATA AVAILABILITY
The data that support the findings of this study are available within this article.
Note: This paper is part of the Special Topic, Flow and the Virus.
Contributor Information
Siddhartha Verma, Email: .
Manhar Dhanak, Email: .
John Frankenfield, Email: .
REFERENCES
- 1.United Nations, “A UN framework for the immediate socio-economic response to COVID-19,” Technical Report, United Nations, April 2020, available at https://unsdg.un.org/sites/default/files/2020-04/UN-framework-for-the-immediate-socio-economic-response-to-COVID-19.pdf. [Google Scholar]
- 2.Nicola M., Alsafi Z., Sohrabi C., Kerwan A., Al-Jabir A., Iosifidis C., Agha M., and Agha R., “The socio-economic implications of the coronavirus pandemic (COVID-19): A review,” Int. J. Surg. 78, 185–193 (2020). 10.1016/j.ijsu.2020.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Emanuel E. J., Persad G., Upshur R., Thome B., Parker M., Glickman A., Zhang C., Boyle C., Smith M., and Phillips J. P., “Fair allocation of scarce medical resources in the time of covid-19,” N. Engl. J. Med. 382, 2049–2055 (2020). 10.1056/nejmsb2005114 [DOI] [PubMed] [Google Scholar]
- 4.Lauer S. A., Grantz K. H., Bi Q., Jones F. K., Zheng Q., Meredith H. R., Azman A. S., Reich N. G., and Lessler J., “The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: Estimation and application,” Ann. Intern. Med. 172, 577–582 (2020). 10.7326/m20-0504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He X., Lau E. H. Y., Wu P., Deng X., Wang J., Hao X., Lau Y. C., Wong J. Y., Guan Y., Tan X., Mo X., Chen Y., Liao B., Chen W., Hu F., Zhang Q., Zhong M., Wu Y., Zhao L., Zhang F., Cowling B. J., Li F., and Leung G. M., “Temporal dynamics in viral shedding and transmissibility of COVID-19,” Nat. Med. 26, 672–675 (2020). 10.1038/s41591-020-0869-5 [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention, “COVID-19 pandemic planning scenarios,” https://www.cdc.gov/coronavirus/2019-ncov/hcp/planning-scenarios. html, May 2020.
- 7.Centers for Disease Control and Prevention, “Social distancing, quarantine, and isolation,” https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/ social-distancing.html, May 2020.
- 8.MacIntyre C. R., Cauchemez S., Dwyer D. E., Seale H., Cheung P., Browne G., Fasher M., Wood J., Gao Z., Booy R., and Ferguson N., “Face mask use and control of respiratory virus transmission in households,” Emerging Infect. Dis. 15, 233–241 (2009). 10.3201/eid1502.081166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacIntyre C. R. and Chughtai A. A., “A rapid systematic review of the efficacy of face masks and respirators against coronaviruses and other respiratory transmissible viruses for the community, healthcare workers and sick patients,” Int. J. Nurs. Stud. 108, 103629 (2020). 10.1016/j.ijnurstu.2020.103629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morawska L., “Droplet fate in indoor environments, or can we prevent the spread of infection?,” Indoor Air 16, 335–347 (2006). 10.1111/j.1600-0668.2006.00432.x [DOI] [PubMed] [Google Scholar]
- 11.Stelzer-Braid S., Oliver B. G., Blazey A. J., Argent E., Newsome T. P., Rawlinson W. D., and Tovey E. R., “Exhalation of respiratory viruses by breathing, coughing, and talking,” J. Med. Virol. 81, 1674–1679 (2009). 10.1002/jmv.21556 [DOI] [PubMed] [Google Scholar]
- 12.Morawska L., Johnson G. R., Ristovski Z. D., Hargreaves M., Mengersen K., Corbett S., Chao C. Y. H., Li Y., and Katoshevski D., “Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities,” J. Aerosol Sci. 40, 256–269 (2009). 10.1016/j.jaerosci.2008.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen C., Lin C.-H., Jiang Z., and Chen Q., “Simplified models for exhaled airflow from a cough with the mouth covered,” Indoor Air 24, 580–591 (2014). 10.1111/ina.12109 [DOI] [PubMed] [Google Scholar]
- 14.Stadnytskyi V., Bax C. E., Bax A., and Anfinrud P., “The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission,” Proc. Natl. Acad. Sci. U. S. A. 117, 11875–11877 (2020). 10.1073/pnas.2006874117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bahl P., Doolan C., de Silva C., Chughtai A. A., Bourouiba L., and MacIntyre C. R., “Airborne or droplet precautions for health workers treating COVID-19?,” J. Infect. Dis. 2020, 1–8. 10.1093/infdis/jiaa189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jennings L. C. and Dick E. C., “Transmission and control of rhinovirus colds,” European Journal of Epidemiology 3, 327–335 (1987). 10.1007/bf00145641 [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention, “Core curriculum on tuberculosis: What the clinician should know,” Technical Report CS234269, 2013, available at https://www.cdc.gov/tb/education/corecurr/pdf/corecurr_all.pdf.
- 18.Kutter J. S., Spronken M. I., Fraaij P. L., Fouchier R. A., and Herfst S., “Transmission routes of respiratory viruses among humans,” Curr. Opin. Virol. 28, 142–151 (2018). 10.1016/j.coviro.2018.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tellier R., Li Y., Cowling B. J., and Tang J. W., “Recognition of aerosol transmission of infectious agents: A commentary,” BMC Infect. Dis. 19, 1–9 (2019). 10.1186/s12879-019-3707-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tellier R., “Review of aerosol transmission of influenza A virus,” Emerging Infect. Dis. 12, 1657–1662 (2006). 10.3201/eid1211.060426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernstrom A. and Goldblatt M., “Aerobiology and its role in the transmission of infectious diseases,” J. Pathog. 2013, 1–13. 10.1155/2013/493960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang J. W., Noakes C. J., Nielsen P. V., Eames I., Nicolle A., Li Y., and Settles G. S., “Observing and quantifying airflows in the infection control of aerosol- and airborne-transmitted diseases: An overview of approaches,” J. Hosp. Infect. 77, 213–222 (2011). 10.1016/j.jhin.2010.09.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang J. W., Li Y., Eames I., Chan P. K. S., and Ridgway G. L., “Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises,” J. Hosp. Infect. 64, 100–114 (2006). 10.1016/j.jhin.2006.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu S. W., Kato S., and Yang J.-H., “Study on transport characteristics of saliva droplets produced by coughing in a calm indoor environment,” Build. Environ. 41, 1691–1702 (2006). 10.1016/j.buildenv.2005.06.024 [DOI] [Google Scholar]
- 25.Xie X., Li Y., Chwang A. T. Y., Ho P. L., and Seto W. H., “How far droplets can move in indoor environments—Revisiting the Wells evaporation–falling curve,” Indoor Air 17, 211–225 (2007). 10.1111/j.1600-0668.2007.00469.x [DOI] [PubMed] [Google Scholar]
- 26.Liu S. and Novoselac A., “Transport of airborne particles from an unobstructed cough jet,” Aerosol Sci. Technol. 48, 1183–1194 (2014). 10.1080/02786826.2014.968655 [DOI] [Google Scholar]
- 27.Nishimura H., Sakata S., and Kaga A., “A new methodology for studying dynamics of aerosol particles in sneeze and cough using a digital high-vision, high-speed video system and vector analyses,” PLoS One 8, e80244 (2013). 10.1371/journal.pone.0080244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gralton J., Tovey E., McLaws M.-L., and Rawlinson W. D., “The role of particle size in aerosolised pathogen transmission: A review,” J. Infect. 62, 1–13 (2011). 10.1016/j.jinf.2010.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han Z. Y., Weng W. G., and Huang Q. Y., “Characterizations of particle size distribution of the droplets exhaled by sneeze,” J. R. Soc., Interface 10, 20130560 (2013). 10.1098/rsif.2013.0560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chao C. Y., Wan M. P., Morawska L., Johnson G. R., Ristovski Z. D., Hargreaves M., Mengersen K., Corbett S., Li Y., Xie X., and Katoshevski D., “Characterization of expiration air jets and droplet size distributions immediately at the mouth opening,” J. Aerosol Sci. 40, 122–133 (2009). 10.1016/j.jaerosci.2008.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wells W. F., “On air-borne infection: Study II. Droplets and droplet nuclei,” Am. J. Epidemiol. 20, 611–618 (1934). 10.1093/oxfordjournals.aje.a118097 [DOI] [Google Scholar]
- 32.Duguid J. P., “The size and the duration of air-carriage of respiratory droplets and droplet-nuclei,” J. Hyg. 44, 471–479 (1946). 10.1017/S0022172400019288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang J. W., Liebner T. J., Craven B. A., and Settles G. S., “A schlieren optical study of the human cough with and without wearing masks for aerosol infection control,” J. R. Soc., Interface 6, 727–736 (2009). 10.1098/rsif.2009.0295.focus [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bourouiba L., Dehandschoewercker E., and Bush J. W., “Violent expiratory events: On coughing and sneezing,” J. Fluid Mech. 745, 537–563 (2014). 10.1017/jfm.2014.88 [DOI] [Google Scholar]
- 35.Bourouiba L., “Turbulent gas clouds and respiratory pathogen emissions: Potential implications for reducing transmission of COVID-19,” JAMA, J. Am. Med. Assoc. 323, 1837–1838 (2020). 10.1001/jama.2020.4756 [DOI] [PubMed] [Google Scholar]
- 36.Nicas M., Nazaroff W. W., and Hubbard A., “Toward understanding the risk of secondary airborne infection: Emission of respirable pathogens,” J. Occup. Environ. Hyg. 2, 143–154 (2005). 10.1080/15459620590918466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y., Ning Z., Chen Y., Guo M., Liu Y., Gali N. K., Sun L., Duan Y., Cai J., Westerdahl D., Liu X., Xu K., Ho K.-f., Kan H., Fu Q., and Lan K., “Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals,” Nature (published online 2020). 10.1038/s41586-020-2271-3 [DOI] [PubMed] [Google Scholar]
- 38.Ong S. W. X., Tan Y. K., Chia P. Y., Lee T. H., Ng O. T., Wong M. S. Y., and Marimuthu K., “Air, surface environmental, and personal protective equipment contamination by severe Acute respiratory Syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient,” JAMA, J. Am. Med. Assoc. 323, 1610–1612 (2020). 10.1001/jama.2020.3227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cai J., Sun W., Huang J., Gamber M., Wu J., and He G., “Indirect virus transmission in cluster of COVID-19 cases, wenzhou, China, 2020,” Emerging Infect. Dis. 26, 1343–1345 (2020). 10.3201/eid2606.200412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scharfman B. E., Techet A. H., Bush J. W., and Bourouiba L., “Visualization of sneeze ejecta: Steps of fluid fragmentation leading to respiratory droplets,” Exp. Fluids 57, 1–9 (2016). 10.1007/s00348-015-2078-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ha’eri G. B. and Wiley A. M., “The efficacy of standard surgical face masks: An investigation using “tracer particles”,” Clin. Orthop. Relat. Res. 148, 160–162 (1980). 10.1097/00003086-198005000-00024 [DOI] [PubMed] [Google Scholar]
- 42.Johnson D. F., Druce J. D., Birch C., and Grayson M. L., “A quantitative assessment of the efficacy of surgical and N95 masks to filter influenza virus in patients with acute influenza infection,” Clin. Infect. Dis. 49, 275–277 (2009). 10.1086/600041 [DOI] [PubMed] [Google Scholar]
- 43.Lindsley W. G., King W. P., Thewlis R. E., Reynolds J. S., Panday K., Cao G., and Szalajda J. V., “Dispersion and exposure to a cough-generated aerosol in a simulated medical examination room,” J. Occup. Environ. Hyg. 9, 681–690 (2012). 10.1080/15459624.2012.725986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lindsley W. G., Noti J. D., Blachere F. M., Szalajda J. V., and Beezhold D. H., “Efficacy of face shields against cough aerosol droplets from a cough simulator,” J. Occup. Environ. Hyg. 11, 509–518 (2014). 10.1080/15459624.2013.877591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zayas G., Chiang M. C., Wong E., Macdonald F., Lange C. F., Senthilselvan A., and King M., “Effectiveness of cough etiquette maneuvers in disrupting the chain of transmission of infectious respiratory diseases,” BMC Public Health 13, 1–11 (2013). 10.1186/1471-2458-13-811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leung N. H. L., Chu D. K. W., Shiu E. Y. C., Chan K.-H., McDevitt J. J., Hau B. J. P., Yen H.-L., Li Y., Ip D. K. M., Peiris J. S. M., Seto W.-H., Leung G. M., Milton D. K., and Cowling B. J., “Respiratory virus shedding in exhaled breath and efficacy of face masks,” Nat. Med. 26, 676–680 (2020). 10.1038/s41591-020-0843-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou S. S., Lukula S., Chiossone C., Nims R. W., Suchmann D. B., and Ijaz M. K., “Assessment of a respiratory face mask for capturing air pollutants and pathogens including human influenza and rhinoviruses,” J. Thorac. Dis. 10, 2059–2069 (2018). 10.21037/jtd.2018.03.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rengasamy S., Eimer B., and Shaffer R. E., “Simple respiratory protection—Evaluation of the filtration performance of cloth masks and common fabric materials against 20-1000 nm size particles,” Ann. Occup. Hyg. 54, 789–798 (2010). 10.1093/annhyg/meq044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davies A., Thompson K.-A., Giri K., Kafatos G., Walker J., and Bennett A., “Testing the efficacy of homemade masks: Would they protect in an influenza pandemic?,” Disaster Med. Public Health Preparedness 7, 413–418 (2013). 10.1017/dmp.2013.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bae S., Kim M.-C., Kim J. Y., Cha H.-H., Lim J. S., Jung J., Kim M.-J., Oh D. K., Lee M.-K., Choi S.-H., Sung M., Hong S.-B., Chung J.-W., and Kim S.-H., “Effectiveness of surgical and cotton masks in blocking SARS-CoV-2: A controlled comparison in 4 patients,” Ann. Intern. Med. M20, 1342 (2020). 10.7326/m20-1342 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Konda A., Prakash A., Moss G. A., Schmoldt M., Grant G. D., and Guha S., “Aerosol filtration efficiency of common fabrics used in respiratory cloth masks,” ACS Nano 14, 6339–6347 (2020). 10.1021/acsnano.0c03252 [DOI] [PubMed] [Google Scholar]
- 52.Feng S., Shen C., Xia N., Song W., Fan M., and Cowling B. J., “Rational use of face masks in the COVID-19 pandemic,” Lancet Respir. Med. 8, 434–436 (2020). 10.1016/s2213-2600(20)30134-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xiao J., Shiu E. Y. C., Gao H., Wong J. Y., Fong M. W., Ryu S., and Cowling B. J., “Nonpharmaceutical measures for pandemic influenza in nonhealthcare settings-personal protective and environmental measures,” Emerging Infect. Dis. 26, 967–975 (2020). 10.3201/eid2605.190994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gupta J. K., Lin C.-H., and Chen Q., “Flow dynamics and characterization of a cough,” Indoor Air 19, 517–525 (2009). 10.1111/j.1600-0668.2009.00619.x [DOI] [PubMed] [Google Scholar]
- 55.Hsu J. Y., Stone R., Logan-Sinclair R., Worsdell M., Busst C., and Chung K., “Coughing frequency in patients with persistent cough: Assessment using a 24 hour ambulatory recorder,” Eur. Respir. J. 7, 1246–1253 (1994). 10.1183/09031936.94.07071246 [DOI] [PubMed] [Google Scholar]
- 56.Bjorn E. and Nielsen P. V., “Dispersal of exhaled air and personal exposure in displacement ventilated rooms,” Indoor Air 12, 147–164 (2002). 10.1034/j.1600-0668.2002.08126.x [DOI] [PubMed] [Google Scholar]
- 57.Qian H., Li Y., Nielsen P. V., Hyldgaard C. E., Wong T. W., and Chwang A. T. Y., “Dispersion of exhaled droplet nuclei in a two-bed hospital ward with three different ventilation systems,” Indoor Air 16, 111–128 (2006). 10.1111/j.1600-0668.2005.00407.x [DOI] [PubMed] [Google Scholar]
- 58.Li Y., Leung G. M., Tang J. W., Yang X., Chao C. Y., Lin J. Z., Lu J. W., Nielsen P. V., Niu J., Qian H., Sleigh A. C., Su H.-J., Sundell J., Wong T. W., and Yuen P. L., “Role of ventilation in airborne transmission of infectious agents in the built environment–A multidisciplinary systematic review,” Indoor Air 17, 2–18 (2007). 10.1111/j.1600-0668.2006.00445.x [DOI] [PubMed] [Google Scholar]
- 59.Dbouk T. and Drikakis D., “On coughing and airborne droplet transmission to humans,” Phys. Fluids 32, 053310 (2020). 10.1063/5.0011960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Centers for Disease Control and Prevention (CDC), “How to Make Your own Face Covering,” https://www.youtube.com/watch?v=tPx1yqvJgf4, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available within this article.