Abstract
Patient: Male, 74-year-old
Final Diagnosis: Ventricular fibrillation
Symptoms: Altered mental status • slurred speech • somnolence
Medication:—
Clinical Procedure: Left ventricular assist device placement
Specialty: Cardiology • Critical Care Medicine • General and Internal Medicine
Objective:
Unusual clinical course
Background:
Congestive heart failure (CHF) affects over 23 million individuals worldwide and over 5.8 million individuals in the United States. Left ventricular assist device (LVAD) implantation is used as both a bridging and destination therapy for patients with advanced CHF. LVADs are reported to cause ventricular arrhythmias. Ventricular tachycardia and ventricular fibrillation (VF) are common fatal arrhythmias in patients with severe CHF if left untreated. We report a case in which a patient with an LVAD without an implantable cardioverter device (ICD) developed VF with non-classical symptoms with an unknown duration prior to defibrillation.
Case Report:
A 74-year old man was brought to the hospital via Emergency Medical Services (EMS) with a 1-day history of altered mental status, somnolence, and slurred speech. His past medical history was significant for CHF with LVAD Heart Mate II. An initial electrocardiogram (ECG) done by EMS was abnormal but was presumed to be an artifact secondary to LVAD. A 12-lead ECG done in the Emergency Center revealed VF. He required electrical defibrillation. Due to ongoing multiple organ failure, he was admitted to the Intensive Care Unit (ICU) for further care.
Conclusions:
In the management of VF, the time to defibrillation is of paramount importance. LVAD patients could be in VF and present with non-specific symptoms. EMS personnel should be aware of this, as it can appear to be an artifact on ECG.
MeSH Keywords: Arrhythmias, Cardiac; Heart-Assist Devices; Ventricular Fibrillation; Heart Failure
Background
Congestive heart failure (CHF) affects over 23 million individuals worldwide and over 5.8 million individuals in the United States. The lifetime risk of developing CHF in the United States is 1 in 5 [1]. CHF is the inability of the heart to pump blood effectively at a level commensurate with the requirements of metabolizing tissues or with an elevated diastolic filling pressure. This leads to a backflow of blood in the circulatory system and volume overload (i.e., pulmonary and liver congestion, pedal edema, and/or inadequate forward flow for function).
The management of CHF includes lifestyle modifications, pharmacologic agents, device therapies such as implantable cardioverter-defibrillator (ICD), and cardiac resynchronization therapy (CRT). In some cases, failure to improve with these modalities necessitates short-term mechanical circulatory support with the use of an intra-aortic balloon pump (IABP) or even extra-corporeal membrane oxygenation (ECMO). Despite these efforts, many patients continue to have advanced heart failure with worsening left ventricle ejection fraction (LVEF) refractory to medical therapy. The definitive treatment is a heart transplant, but this is contingent on donor availability; because of the inadequate supply of available organs, the use of mechanical circulatory support has gained widespread use. Circulatory support with the use of mechanical heart pumps is used to maintain heart function and perfusion, especially in patients refractory to medical therapy. Several pump types exist – biventricular, right ventricular, or left ventricular – and the latter is most frequently used. A left ventricular assist device (LVAD) is used as both a bridging and destination therapy for patients with advanced CHF.
The use of LVAD results in improved survival rate and quality of life in patients with severe CHF [2]. LVAD is reported to cause de novo ventricular arrhythmias (VA). The incidence of VA in LVAD patients is 22–59% [3]. Ventricular tachycardia and ventricular fibrillation are common fatal arrhythmias in patients with severe CHF, if left untreated [4]. The most important determinant of survival in VF is the time interval to defibrillation.
We report a case in which a patient with an LVAD without an ICD developed ventricular fibrillation with non-classical symptoms with an unknown duration prior to being defibrillated.
Case Report
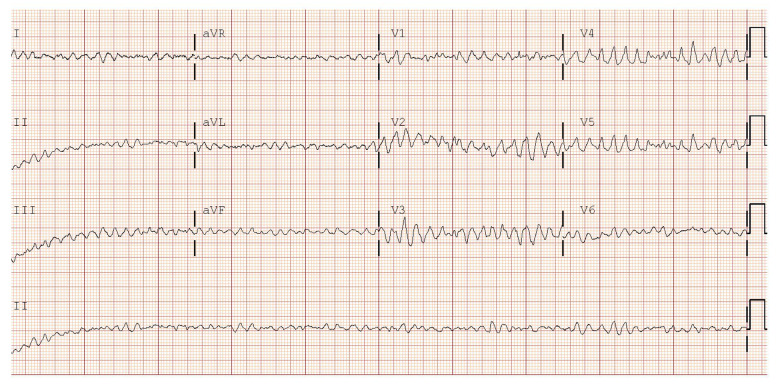
A 74-year old man was brought to the hospital via Emergency Medical Services (EMS). The medical history was obtained from his wife. He presented with a 1-day history of altered mental status, somnolence, and slurred speech. He was slightly lethargic and confused. Four days prior to this presentation, he had flu-like symptoms and cough, which led to a decrease in oral intake and no bowel movements for 3 days. His past medical history was significant for CHF with LVAD (HeartMate II) (set at 6000 revolutions per minute with a flow of 1.0–1.5 L/minute), coronary arterial disease (CAD), myocardial infarction (MI), multiple coronary artery bypass grafting (CABG), peripheral arterial disease (PAD), ischemic cardiomyopathy, mitral regurgitation, hypertension, diabetes, hyperlipidemia, hypothyroidism, and recurrent GI bleeds (Dieulafoy lesion, hemorrhoid, and arteriovenous malformation). He was systemically anticoagulated with warfarin. Prior to admission, a preliminary electrocardiogram (ECG) was obtained via EMS. They noted an abnormal ECG, but presumed it was an artifact secondary to LVAD. Physical examination revealed a pump sound on auscultation consistent with LVAD, no pulse (due to LVAD); respiratory rate of 24; blood pressure of 71/60 mmHg; mean arterial pressure of 64 mmHg, and oxygen saturation of 86% via nasal cannula. A 12-lead ECG done in the Emergency Center revealed VF (Figure 1).
Figure 1.
Electrocardiogram showing ventricular fibrillation.
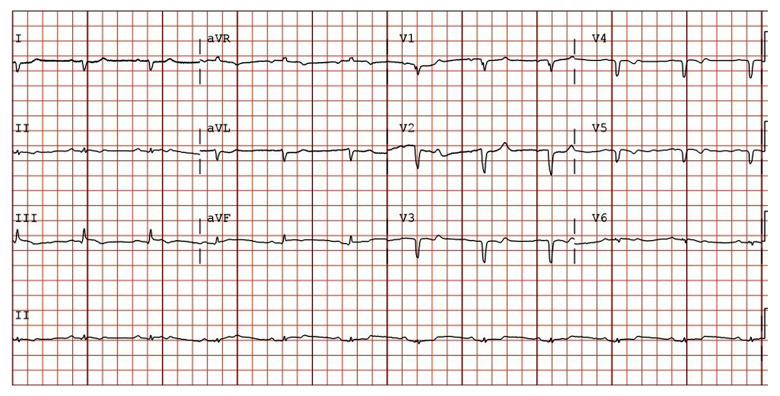
At the Emergency Center, he was shocked with 200 joules, which converted him back to sinus rhythm with a heart rate of 67 beats per minute (Figure 2). Following cardioversion, he was given IV amiodarone and 4 liters of normal saline. On LVAD interrogation, the pump speed was at 8800 revolutions per minute with a flow of 4.8 to 7.7 liter per minute (showing the LVAD was functioning well).
Figure 2.
Electrocardiogram done after cardioversion, showing sinus rhythm.
The hemodynamic compromise due to prolonged VF resulted in hypoperfusion of several vital organs. Most serum electrolyte levels and coagulation profile were abnormal. He had markedly elevated liver function test results, consistent with hypoperfusion of the liver and worsening kidney function (Table 1). He was given fresh frozen plasma (FFP) and vitamin K due to supratherapeutic INR. Due to the ongoing multiple organ failure, he was admitted to the Intensive Care Unit (ICU) for further care.
Table 1.
Metabolic panel, liver function test, and coagulation studies laboratory values obtained.
| Metabolic panel | Patient values | Normal range values |
|---|---|---|
| Sodium | 121 mEq/L (*L) | 135–145 mEq/L |
| Potassium | 4.3 mEq/L | 3.5–5.0 mEq/L |
| Chloride | 84 mEq/L (*L) | 95–105 mEq/L |
| Magnesium | 2.4 mEq/L (*H) | 1.5–2.0 mEq/L |
| Calcium | 7.1 mg/dL (*L) | 8.4–10.2 mg/dL |
| Glucose | 111 mg/dL | 80–140 mg/dL |
| Bicarbonate | 16.4 mEq/L (*L) | 22–28 mEq/L |
| BUN | 120 mg/dL (*H) | 7–18 mg/dL |
| Creatinine | 5.49 mg/dL (*H) | 0.6–1.2 mg/dL |
| Liver function test | Patient values | Normal range values |
|---|---|---|
| AST | 3917 U/L (*H) | 8–40 U/L |
| ALT | 3767 U/L (*H) | 8–40 U/L |
| ALK phosphatase | 248 U/L (*H) | 30–100 U/L |
| Total bilirubin | 7.0 mg/dL (*H) | 0.1–1.0 mg/dL |
| Albumin | 3.7 g/dL | 3.5–5.5 g/dL |
| Total protein | 6.2 g/dL | 6.0–7.8 g/dL |
| Coagulation studies | Patient values | Normal range values |
|---|---|---|
| PT | 120 secs (*H) | 11.0–12.5 secs |
| PTT | 57.3 secs (*L) | 60–70 secs |
| INR | 16 (*H) | 0.8–1.1 |
BUN – blood urea nitrogen; ALT – alanine transaminase; AST – aspartate transaminase; ALK Phosphatase – alkaline phosphatase; PT – prothrombin time; PTT – partial thromboplastin time; INR – international normalized ratio; (*L) – low values; (*H) – high values.
During his hospitalization, a multidisciplinary approach was used; he had a total of 2 units of FFP, and continuous renal replacement therapy (CRRT) was commenced due to worsening renal function. He was subsequently placed on heparin when his INR was less than 2. Two weeks into the hospital admission, he developed atrial fibrillation with rapid ventricular response, which was successfully cardioverted to normal sinus rhythm. Renal function improved on CRRT, but due to his comorbidities, worsening organ dysfunction, and poor prognosis, palliative care was initiated. The family decided to proceed with comfort care and discontinue CRRT and LVAD. Following LVAD deactivation, the patient’s mean arterial pressure dropped to the mid-30s. He died 40 minutes after withdrawal of the LVAD.
Discussion
Ventricular assist devices are mechanical pumps that supplement the function of the damaged ventricle to re-establish normal hemodynamics and end-organ perfusion. Devices can be placed via a median sternotomy into the left, right, or both ventricles of the heart; with the left ventricular devices being the most common. LVADs are used in patients with advanced heart failure as a bridging therapy to cardiac transplantation or as a destination therapy. LVAD consists of an inflow cannula connected to the apex of the left ventricle, an out-flow cannula connected to the ascending aorta, and a pumping chamber that can be placed extracorporeally or intracorporeally. Currently, there are 3 generations of ventricular assist devices: the first-generation devices with pulsatile flow pumps such as Heartmate XVE (Thoratec), the second-generation devices with axial continuous flow pumps such as Heartmate II (Thoratec), and the third-generation devices with centrifugal continuous flow pumps such as Heartmate III (Abbott) and the HVAD (Heart Ware) [5].
The most common complications of LVAD are infections, bleeding, thrombosis, hemolysis, aortic valvular dysfunction, right heart failure, and ventricular arrhythmias (VA) [5–8]. Approximately 20% of patients will develop ventricular arrhythmias (VAs) after LVAD placement, with ventricular fibrillation being the most common [3]. The risk of VAs after LVAD placement is associated with the underlying cardiac pathology, apical scarring around the inflow cannula, arrhythmias from suction events (LV underfilling or high pump speed), and alterations in ion channel gene expression [1,3–8].
Ventricular fibrillation is a life-threatening cardiac arrhythmia characterized by disorganized, high-frequency ventricular contractions that result in diminished cardiac output and hemo-dynamic collapse. Little is known of the pathophysiology of VF secondary to LVAD. Known risk factors of VF include cardiomyopathies, electrolyte abnormalities, acidosis, hypoxemia, and ischemia [5,8,9]. This patient presented with electrolyte and metabolic derangements of unknown duration. We believe that the patient’s hemodynamic instability and laboratory abnormalities prior to defibrillation led to multiple organ failure (heart, kidney, and liver).
Worsening organ dysfunction led to a decision by the patient’s family to switch to comfort-based care. The family was advised that without the use of LVAD and the support of CRRT, there was little chance of survival. Following much explanation at the request of the family, the patient discontinued dialysis, which led to a rise in creatinine and urea nitrogen. He later was disconnected from mechanical circulatory support. Approximately 40 minutes later, he died.
In this case, the patient had CHF refractory to milrinone (New York Heart Association [NYHA] class IV milrinone dependent) which led to the LVAD placement (HeartMate2) on continuous flow set at 6000 revolutions per minute with a flow of 1.0–1.5 L/minute 7 years prior to this event, as a destination therapy. Our patient was not placed on an ICD due to several contraindications (NYHA class IV and failed medical therapy), and he was a poor surgical candidate for heart transplant, which led to the placement of an LVAD without ICD. LVAD was used as a destination therapy due to the patient’s history of diabetes and significant atherosclerotic peripheral vascular disease, status after multiple reconstructions. His LVAD was interrogated and adjusted throughout the years with no significant arrhythmias until the most recent event, which led to his hospitalization. The actual onset of VF is uncertain since the patient did not have an ICD in place. It is also unclear if the patient had a de novo VF due to the LVAD or due to secondary causes (e.g., CHF). To date, there have been several case reports revealing the tolerability of sustained VF with LVADs [9–11]. However, there are limited data on the onset of ventricular fibrillation 7 years after LVAD placement in a patient with NYHA class IV. Several studies have shown that patients on LVAD who survived for more than 2–4 years had NYHA class I and II [12]. In our case, our patient exhibited novel findings. He had diminished cardiac functional capacity (NYHA class IV) at baseline and survived up to 7 years. In addition, unlike cases described in previous reports [9–11], our case exhibited a unique VF with no ICD in situ to help determine the duration and frequency of the VF. With the increasing numbers of LVAD implanted, health care professionals, especially first responders, should be familiar with patients on LVAD, as they may encounter atypical presentation such as a conscious patient in VF without a pulse who needs defibrillation. Such patients should be closely monitored for VF when presenting to the emergency room.
Conclusions
Left ventricular assist devices (LVAD) provide continuous perfusion, and because many patients are pulseless at baseline, it can be difficult to identify VF when it does occur. Therefore, patients with LVADs should be closely monitored for arrhythmias when presenting with any type of illness. The American Heart Association recommends that if one suspects a cardiac arrest in an LVAD patient, bystander cardiopulmonary resuscitation should still be initiated.
In the management of VF, the time to defibrillation is of paramount importance. Because the LVAD patient could be in VF and present with non-specific symptoms, emergency services personnel should be aware of this, as it can appear to be an artifact on ECG.
Acknowledgments
We thank Dr David Parish M.D., M.P.H., F.A.C.P., Professor, Internal Medicine, Mercer University School of Medicine.
Footnotes
Department and Institution where work was done
Advanced Heart Failure Center, Cardiovascular Intensive Care Unit, Medical Center, Navicent Health Macon, GA.; Department of Internal Medicine, Mercer University School of Medicine Macon, GA.
Conflict of interest
None.
References:
- 1.McLarty A. Mechanical circulatory support and the role of LVADs in heart failure therapy. Clin Med Insights Cardiol. 2015;9(2):1–5. doi: 10.4137/CMC.s19694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roehm B, Vest AR, Weiner DE. Left ventricular assist devices, kidney disease, and dialysis. Am J Kidney Dis. 2018;71(2):257–66. doi: 10.1053/j.ajkd.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed A, Amin M, Boilson BA, et al. Ventricular arrhythmias in patients with left ventricular assist device (LVAD) Curr Treat Options Cardio Med. 2019;21(11):75. doi: 10.1007/s11936-019-0783-7. [DOI] [PubMed] [Google Scholar]
- 4.Santangeli P, Rame JE, Birati EY, Marchlinski FE. Management of ventricular arrhythmias in patients with advanced heart failure. J Am Coll Cardiol. 2017;69(14):1842–60. doi: 10.1016/j.jacc.2017.01.047. [DOI] [PubMed] [Google Scholar]
- 5.Prinzing A, Herold U, Berkefeld A, et al. Left ventricular assist devices-current state and perspectives. J Thorac Dis. 2016;8(8):e660–66. doi: 10.21037/jtd.2016.07.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel H, Madanieh R, Kosmas CE, et al. Complications of continuous-flow mechanical circulatory support devices. Clin Med Insights Cardiol. 2015;9(2):15–21. doi: 10.4137/CMC.S19708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jezovnik MK, Gergoric ID, Poredos P. Medical complications in patients with LVAD devices. European Society of Cardiology. 2017;14(37) [Google Scholar]
- 8.Kilic A, Acker MA, Atluri P. Dealing with surgical left ventricular assist device complications. J Thorac Dis. 2015;7(12):2158–64. doi: 10.3978/j.issn.2072-1439.2015.10.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bujo C, Amiya E, Hatano M, et al. Clinical impact of newly developed atrial fibrillation complicated with longstanding ventricular fibrillation during left ventricular assist device support: A case report. BMC Cardiovasc Disord. 2019;19(1):151. doi: 10.1186/s12872-019-1132-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gul EE, Melhem M, Haseeb S, et al. Ineffective ICD shocks for ventricular fibrillation in a patient with a left ventricular assist device: Continuous flow during the electrical storm. J Atr Fibrillation. 2018;11(1):1883. doi: 10.4022/jafib.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butterfield M, Derr C, Keffeler J, Jelic T. Organized cardiac activity in an awake LVAD patient during ventricular fibrillation. Am J Emerg Med. 2017 Jul;35(7):1041.e1–3. doi: 10.1016/j.ajem.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Gosev I, Kiernan MS, Eckman P, et al. Long-term survival in patients receiving a continuous-flow left ventricular assist device. Ann Thorac Surg. 2018;105(3):696–701. doi: 10.1016/j.athoracsur.2017.08.057. [DOI] [PubMed] [Google Scholar]