Abstract
Background
Hypoxia-inducible factor-1α (HIF-1α) is a transcription factor which maintains cellular homeostasis in response to hypoxia. It can trigger apoptosis while stimulating angiogenesis process and decrease neurological deficit after an ischemic stroke. Up until now, this protein complex has not been widely investigated especially in stroke patient.
Objective
Here, we examined the potential of HIF-1α as a marker for neuroplasticity process after ischemic stroke.
Methods
Serum HIF-1α were measured in acute ischemic stroke patients. National Institute of Health Stroke Scale (NIHSS) were assessed on the admission and discharge day (between days 7 and 14). Ischemic stroke divided into 2 groups: large vessel disease (LVD, n = 31) and small vessel disease (SVD, n = 27). Statistical significances were calculated with Spearman rank test.
Results
A total of 58 patients, 31 with large artery atherosclerosis LVD and 27 with small vessel disease (SVD) were included in this study. HIF-1α level in LVD group was 0.5225 ± 0.2459 ng/mL and in SVD group was 0.3815 ± 0.121 ng/mL. HIF-1α was higher (p = 0.004) in LVD group than in SVD group. The initial NIHSS score in LVD group was 15.46 ± 2.61 and discharge NIHSS score was 13.31 ± 3.449. Initial NIHSS score in SVD group was 6.07 ± 1.82 and the discharge NIHSS was 5.703 ± 1.7055. In both SVD and LVD group, HIF-1α were significantly correlated with initial NIHSS (both p < 0.001) and discharge NIHSS (p < 0.0383 r = 0.94, p < 0.001, r = 0.93, respectively).
Conclusions
HIF-1α has a strong correlation with NIHSS and it may be used as predictor in acute ischemic stroke outcome.
Keywords: Health sciences, Clinical genetics, Neurology, Nervous system, Clinical research, Acute ischemic stroke, HIF 1-α, NIHSS, Outcome, Small vessel disease, Large vessel disease
Health sciences; Clinical genetics; Neurology; Nervous system; Clinical research; Acute ischemic stroke, HIF 1-α, NIHSS, Outcome, Small vessel disease, Large vessel disease.
1. Introduction
Stroke is one of the leading causes of mortality, morbidity and severe disability in the world. Ischemic stroke is accounted for eighty percent of all strokes, via narrowing or blockage of arteries, leading to severe blood flow reduction. Prompt management is an important measure to minimize complications [1, 2].
A disproportion in the supply and oxygen demand in cerebral tissue promotes a sequence of biochemical and molecular events that leads to neuron cell death. Following a hypoxia condition, brain responses in many ways. One of the most intriguing ways is by producing preconditioning hypoxia protein which includes transcription factors Hypoxia-inducible factor-1α (HIF-1α) [3, 4, 5]. Studies of HIF-1α in hypoxia was just started recently, although the factor itself has been found for more than 2 decades [4, 6, 7, 8].
Until now, research around HIF-1α has some opposing results. In one hand, it acts as oxygen stability transcription regulator and a main stimulant of adaptive response via upregulaion of several target genes. These genes are important in erythropoiesis, angiogenesis, glucose transport, and metabolism [7, 9, 10, 11]. In the other hand, some studies show the role HIF-1α in the apoptosis cascade by stimulating pro-apoptotic molecules, namely Nix, IL-20 and BNIP30 which result in mitochondrial dysfunction and lead to neuronal cell death [7, 11, 12]. Therefore, a deeper understanding regarding the role of HIF-1α, specifically in ischemic stroke, is required to improve recovery and brain repair process after stroke.
Moreover, to date, all HIF-1α studies related to ischemic stroke by far were done only in animal model. Our study was the first study to determine the association between HIF-1α, the severity of stroke ischemia, and its outcome in patients.
2. Methods
This was a cross-sectional study on acute ischemic stroke patients.
2.1. Clinical and imaging evaluation
Subjects were clinically diagnosed by two senior consultants, and then divided into two groups: large vessel disease (LVD) and small vessel disease (SVD). National Institute of Health Stroke Scale (NIHSS) was assessed in the admission and before discharge. The other inclusion criterion was onset less than 24 h. Exclusion criteria were patients with chronic renal failure, acute myocardial infarction, severe respiratory failure, severe anemia, dementia, acute limb ischemia and history of brain injury. Stroke imaging confirmation was non-contrast brain computerized tomography (CT) scan.
2.2. HIF-1α measurement
Blood sample was collected less than 24 h from onset of stroke. The serum was separated by allowing blood to clot for 30 min and then centrifuging 2500 RPM for 15 min. It was stored in -20 °C – -80 °C if not directly processed. Enzyme linked immuno-sorbent assay was done according to manufacturer protocol (cat no E-EL-H1277, Elabscience Biotechnology Inc.). The result was rounded to the closest 1 decimal value.
2.3. Statistics
Statistical calculations were done using IBM SPSS 22.0 (IBM Corp, Armonk, NY). Spearman rank-test was utilized to examine relationship. P value below 0.05 is considered significant. R coefficient was calculated to establish the correlation.
Ethical approval
This study is approved by Dr. Hasan Sadikin General Hospital Bandung Human Research Ethic Committee. This study had complied with all relevant ethical regulations (including The Declaration of Helsinki). Written consent was obtained from all patients.
3. Results
A total of 58 subjects were enrolled in this study, i.e. 31 subjects with LVD and 27 subjects with SVD). The mean age was 59.52 ± 11.52 years. Laboratory data of risk factor including blood glucose, uric acid, renal and lipid profile showed in Table 1.
Table 1.
Demographic and laboratory data.
| No |
Variable |
Mean |
SD |
Min |
Max |
|---|---|---|---|---|---|
| Demographic | |||||
| 1 | Age (year) | 59.53 | 11.52 | 31 | 91 |
|
Risk factor | |||||
| 2 | Cholesterol (mg/dL) | 200.65 | 45.07 | 83 | 299 |
| 3 | Triglyceride (mg/dL) | 123.15 | 60.13 | 49 | 366 |
| 4 | HDL (mg/dL) | 46.63 | 16.99 | 17 | 99 |
| 5 | LDL (mg/dL) | 141.09 | 38.36 | 60 | 224 |
| 6 | Random Blood Glucose (mg/dL) | 141.17 | 60.02 | 77 | 363 |
| 7 | Urea (mg/dL) | 37.52 | 31.16 | 13 | 267 |
| 8 | Creatine (mg/dL) | 0.93 | 0.32 | 0.38 | 2.4 |
| 9 | Uric acid (mg/dL) | 6.23 | 2.13 | 2.1 | 12.4 |
| 10 | HbA1c (%) | 7.61 | 2.58 | 4.5 | 13.3 |
Control group was enrolled with matched age, and sex. However, the HIF-1α level in this healthy population, was fall below the detection limit of the ELISA kit.
HIF-1α level in LVD group was 0.5225 ± 0.2459 ng/mL (mean ± SD) and SVD group was 0.3815 ± 0.121 ng/mL. The level of HIF-1α was significantly higher (p = 0.004) in the LVD group than in the SVD group (Table 2 and Figure 1).
Table 2.
HIF-1α level in LVD and SVD groups.
| HIF-1α | Mean | SD | Min | Max | p |
|---|---|---|---|---|---|
| Large vessel disease | 0.5225 | 0.2459 | 0.1 | 1.4 | 0.004 |
| Small vessel disease | 0.3815 | 0.121 | 0.1 | 0.6 |
Fig. 1.
HIF-1α level in SVD and LVD groups.
Clinical features in LVD or SVD patients were in accordance with the size of ischemic lesions identified from our non-contrast brain CT results (supplementary data). Wide ischemic changes were found in the LVD group.
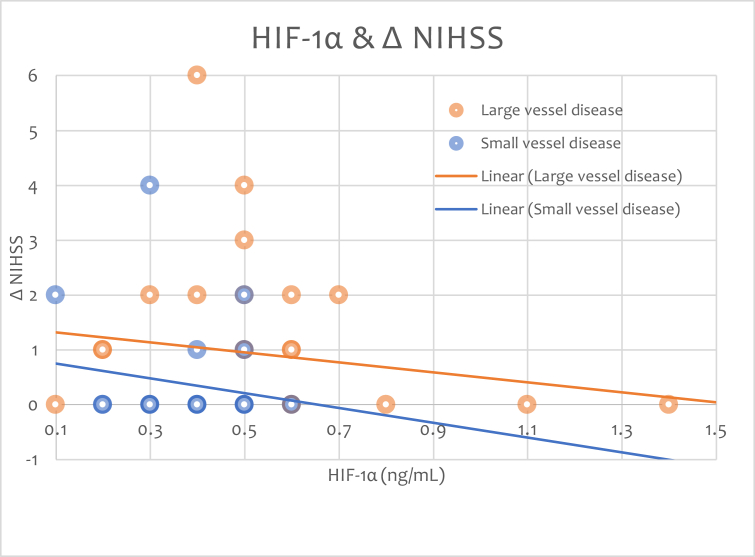
The initial NIHSS score in LVD group was 15.46 ± 2.61 (mean ± SD) and discharge NIHSS score was 13.31 ± 3.449. Initial NIHSS score in SVD group was 6.07 ± 1.82 and the discharge NIHSS was 5.703 ± 1.7055 (Table 3). The higher the HIF-1 α the less NIHSS changes is showed in Figure 2.
Tabel 3.
NIHSS during admission and discharge time points.
| NIHSS |
Mean |
SD |
Min |
Max |
|---|---|---|---|---|
| Large vessel disease | ||||
| On admission | 16.06 | 2.67 | 11 | 21 |
| On discharge | 15.13 | 3.15 | 6 | 20 |
| Small vessel disease | ||||
| On admission | 6.07 | 1.82 | 4 | 9 |
| On discharge | 5.73 | 1.71 | 4 | 9 |
Fig. 2.
HIF-1α level in SVD and LVD groups and NIHSS changes.
Table 4 showed the correlation between HIF-1α level and NIHSS in both groups. Here, we identified a strong correlation between the two variables in both groups.
Table 4.
Correlation of HIF-1α level and NIHSS.
| HIF-1α | NIHSS on admission (mean ± SD) |
NIHSS on discharge (mean ± SD) |
p | r |
|---|---|---|---|---|
| Large Vessel Disease | 16.06 ± 2.67 | 15.13 ± 3.16 | <0.001∗ | 0.9299 |
| Small Vessel Disease | 6.07 ± 1.82 | 5.7 ± 1.7 | <0.001∗ | 0.9394 |
statistically significant.
In the LVD group, the level of HIF-1α was significantly correlated with initial NIHSS (p < 0.001) and discharge NIHSS (p < 0.001). In the SVD group we identified a similar result, the level of HIF-1α significantly correlated with initial NIHSS (p < 0.001) and discharge NIHSS (p = 0.0383).
4. Discussion
Our study includes 58 acute ischemic stroke subjects that grouped into LVD and SVD. Our study is the first time in human. We identified association between HIF-1α level and the subtypes of ischemic stroke.
Due to stroke, HIF-1α starts to accumulate as a response to inadequate oxygen level. Early hypoxia studies of HIF-1α regulation were done in rodents [13]. HIF-1α mRNA expression after hypoxia could be detected within first thirty to sixty minutes [14] and may be increased to 17 folds. HIF-1α induction and transcription activation of target gene is occurred in penumbra. This will lead to increase blood flow, oxygenation, and nutrients transport to the penumbra area [15]. The regulation of HIF-1α, in penumbra, is rising until 7.5 h after onset of ischemia and plateaued for 24 h [13]. The HIF-1α accumulation, in rodents, may persist up to ten days [9].
The level of HIF-1α (Table 2 and Figure 1) was significantly higher in the LVD group than in the SVD group (p = 0.004). This result was in line with physiology and previous studies conducted in animal model of cerebral ischemia [3].
We then analyzed the brain CT. From non-contrast brain CT scans, we identified a relation between HIF-1a concentration to the size of ischemic lesions (supplementary data). Wide ischemic changes were found in the LVD group. This finding showed the extent of neuronal brain damage after ischemic stroke.
This result was in accordance to previous studies which suggest that a wide brain damage is associated with an increase of HIF-1α production. This increase is initiated in the preconditioning-hypoxia sequence induced by neuronal damage [7, 8, 9, 10, 11, 12].
HIF-1α level, as the master regulator of the cellular response to hypoxia, is tightly controlled through synthesis and degradation. HIF-1α protein accumulation during hypoxia is a result from inhibition of its oxygen-dependent degradation by von Hippel Lindau protein (pVHL) pathway. However, recent data described a distinctive new pVHL or oxygen-independent mechanisms for HIF-1α degradation. Despite the global inhibition of protein translation during hypoxia, a constitutive translation of HIF-1α is still occcured [16].
Other study mentioned that normal population also have some levels of. He et al, in 2016 garnered a normal healthy population age over 18, with no major disease, including heart, liver, lung, kidney and other vital organ disease and not in menstrual period for female. In that study, HIF-1α activity median level was 274.92 pg/mL, with a higher concentration on non small cell lung carcinoma patient 297.7 pg/mL [17].
For control, we had already examined HIF-1α in healthy, age, sex matched subject, however HIF-1α were too low to be detected by our ELISA system. Although, given the median HIF-1α concentration that showed by He et al, a common ELISA assay like ours may be able to detect the concentration of normal population.
In a study by Cai et al, they compared HIF-1β and PGC-1β of breast cancer and a healthy patient, however in the body text, the study are comparing HIF-1α and PGC -1α of breast cancer and a benign breast tumor. This was a decent publication, nonetheless confusing, since they were different protein and different type of subject sample, and of course refer to different entity [18].
A wide brain damage also correlates with worsening of neurological deficit in acute ischemic stroke patients [4]. In this study we found that in LVD group initial NIHSS score was 15.46 ± 2.61 and discharge score was 13.31 ± 3.449. In SVD group initial NIHSS score was 6.07 ± 1.82 and the discharge score was 5.703 ± 1.7055 (Table 3).
We then established an association between HIF-1α and NIHSS (Table 4). There were strong correlations between HIF-1α and on admission and discharge NIHSS in both groups. In previous rodent studies, HIF-1α was activated in a biphasic manner lasting up to ten days after a hypoxic stimulation [3]. It stimulates pro-angiogenic factors [4], promototes neuronal viability [7], and ameliorates brain injury [11]. We examined the HIF-1α level only at admission, but as it significantly correlated with the NIHSS, we may suggest that HIF-1α level would decrease on discharge day and is associated with the improvement of clinical outcome, as occurs in mice model. Future study examining serial HIF-1α level needs to be performed to prove this hypothesis.
Our finding is in accordance to Xiu et al. They reported that HIF-1α increased as early as 1 h after acute ischemis stroke and may be used as marker of severity of neuronal damage and recovery in brain ischemic [19].
Lee at al mentioned the feasibility of HIF-1α as therapeutic target in chronic disease conditions to effectively manage or delay the progression of disease (heart, lung, liver and kidney disease). Clinical trials on targeting HIF-1α are being done [20].
Drug or molecule which activates HIF-1α in vitro and in vivo was proved to own a neuroprotective effect in mice cerebral ischemia model [3, 9, 10]. Therefore in the long run, development of molecule regulator for HIF-1α stability and signaling in human may serve as a marker and a therapeutic option to enhance recovery and repair after ischemic stroke.
In the future, analysis of CT perfusion and Magnetic Resonance Imaging Diffusion Weighted Imaging to differentiate infarct volume from infarct core with will be a pivotal aspect, which in the meantime is beyond the scope in our research.
From this study we conclude that HIF-1α may be used to predict the clinical outcome in acute ischemic stroke patients. A further study to establish its precise predictive value is warranted.
5. Conclusion
HIF-1α level was associated with severity of ischemic lesion, large vessel disease had a higher level of HIF-1α compared with small vessel disease. HIF-1α had a strong correlation with NIHSS, both on admission and patients' discharge. From our findings, we can conclude that HIF-1α may serve as a predictor for clinical outcome in acute ischemic stroke patients, the higher HIF-1α the worse the outcome be.
Availability of data and material
The full data showed in supplementary material.
Declarations
Author contribution statement
L. Amalia: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
H. Sadeli, A. Rizal and R. Panigoro: Analyzed and interpreted the data.
I. Parwati: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by the Universitas Padjadjaran.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Data of eligible patients
References
- 1.Petito C.K., editor. The Neuropathology of Focal Brain Ischemia. ISN Neuropath Press; Basel: 2005. pp. 215–221. [Google Scholar]
- 2.Takasawa M., Beech J.S., Fryer T.D., Hong Y.T., Hughes J.L., Igase K. Imaging of brain hypoxia in permanent and temporary middle cerebral artery occlusion in the rat using F-18-fluoromisonidazole and positron emission tomography: a pilot study. J. Cereb. Blood Flow Metab. 2007;27:679–689. doi: 10.1038/sj.jcbfm.9600405. [DOI] [PubMed] [Google Scholar]
- 3.Baranova O., Miranda L.F., Pichiule P., Dragatsis I., Johnson R.S., Chavez J.C. Neuron-specific inactivation of the hypoxia inducible factor 1 alpha increases brain injury in a mouse model of transient focal cerebral ischemia. J. Neurosci. 2007;27:6320–6332. doi: 10.1523/JNEUROSCI.0449-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y., Dong H., Chen M., Liu J., Yang L., Chen S. Preconditioning with repeated hyperbaric oxygen induces myocardial and cerebral protection in patients undergoing coronary artery bypass graft surgery: a prospective, randomized, controlled clinical trial. J. Cardiothorac. Vasc. Anesth. 2011;25:908–916. doi: 10.1053/j.jvca.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 5.Biswas S., Charlesworth P.J., Turner G.D. CD31 angiogenesis and combined expression of HIF-1αlpha and HIF-2alpha are prognostic in primary clear-cell renal cell carcinoma (CC-RCC), but HIF alpha transcriptional products are not: implications for antiangiogenic trials and HIF alpha biomarker studies in primary CC-RCC. Carcinogenesis. 2012;33(9):1717–1725. doi: 10.1093/carcin/bgs222. [DOI] [PubMed] [Google Scholar]
- 6.Kulik T., Kusano Y., Aronhime S., Sandler A.L., Winn H.R. Regulation of cerebral vasculature in normal and ischemic brain. Neuropharmacology. 2008;55:281–288. doi: 10.1016/j.neuropharm.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vangeison G., Carr D., Federoff H.J., Rempe D.A. The good, the bad, and the cell type-specific roles of hypoxia inducible factor-1 alpha in neurons and astrocytes. J. Neurosci. 2008;28:1988–1993. doi: 10.1523/JNEUROSCI.5323-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta S.L., Manhas N., Raghubir R. Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res. Rev. 2007;54:34–66. doi: 10.1016/j.brainresrev.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Bruick R.K. Oxygen sensing in the hypoxic response pathway: regulation of the hypoxia-inducible transcription factor. Genes Dev. 2003;17:2614–2623. doi: 10.1101/gad.1145503. [DOI] [PubMed] [Google Scholar]
- 10.Giusti S., Plazas S.F.D. Neuroprotection by hypoxic preconditioning involves upregulation of hypoxia-inducible factor-1 in a prenatal model of acute hypoxia. J. Neurosci. Res. 2011;22766:468–478. doi: 10.1002/jnr.22766. [DOI] [PubMed] [Google Scholar]
- 11.Shi Q., Zhang P., Zhang J., Chen X., Lu H., Tian Y., Parker T.L., Liu Y. Adenovirus-mediated brain-derived neurotrophic factor expression regulated by hypoxia response element protects brain from injury of transient middle cerebral artery occlusion in mice. Neurosci. Lett. 2009;465:220–225. doi: 10.1016/j.neulet.2009.08.049. [DOI] [PubMed] [Google Scholar]
- 12.Stolze I.P., Tian Y.M., Appelhoff R.J., Turley H., Wykoff C.C., Gleadle J.M., Ratcliffe P.J. Genetic analysis of the role of the asparaginyl hydroxylase factor inhibiting hypoxia-inducible factor (FIH) in regulating hypoxia-inducible factor (HIF) transcriptional target genes. J. Biol. Chem. 2004;279:42719–42725. doi: 10.1074/jbc.M406713200. [DOI] [PubMed] [Google Scholar]
- 13.Bergeron M., Yu A.Y., Solway K.E., Semenza G.L. Sharp FR Induction of hypoxia-inducible factor-1 (HIF-1) and its target genes following focal ischaemia in rat brain. Eur. J. Neurosci. 1999 doi: 10.1046/j.1460-9568.1999.00845.x. [DOI] [PubMed] [Google Scholar]
- 14.Wiener C.M., Booth G., Semenza G.L. In vivo expression of mRNAs encoding hypoxia-inducible factor 1. Biochem. Biophys. Res. Commun. 1996 doi: 10.1006/bbrc.1996.1199. [DOI] [PubMed] [Google Scholar]
- 15.Shi H. Hypoxia inducible factor 1 as a therapeutic target in ischemic stroke. Curr. Med. Chem. 2009 doi: 10.2174/092986709789760779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yee Koh M., Spivak-Kroizman T.R., Powis G. HIF-1 regulation: not so easy come, easy go. Trends Biochem. Sci. 2008 Nov;33(11):526–534. doi: 10.1016/j.tibs.2008.08.002. Epub 2008 Sep 21. [DOI] [PubMed] [Google Scholar]
- 17.He J., Hu Y., Hu M. The relationship between the preoperative plasma level of HIF-1α and clinic pathological features, prognosis in non-small cell lung cancer. Sci. Rep. 2016;6:20586. doi: 10.1038/srep20586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai1 F., Xu C., Pan Xin P., Cai L., Lin Y., Chen S. Prognostic value of plasma levels of HIF-1 a and PGC-1 a in breast cancer. Oncotarget. 2016;7:77793–77806. doi: 10.18632/oncotarget.12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie L., Chen H., Lu K., Huang J., Duan H., Zhao Y. Clinical significance of changes in serum neuroglobin and HIF-1α concentrations during the early-phase of acute ischemic stroke. J. Neurol. Sci. 2017 doi: 10.1016/j.jns.2017.01.039. [DOI] [PubMed] [Google Scholar]
- 20.Lee J.W., Ko Junsuk, Ju C., Eltzschig H.K. Hypoxia signaling in human disease and therapeutic targets. Exp. Mol. Med. 2019;51:68. doi: 10.1038/s12276-019-0235-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data of eligible patients
Data Availability Statement
The full data showed in supplementary material.