Abstract
Objective: This study aims to evaluate the efficacy and safety of stereotactic body radiation therapy (SBRT) using Cyber Knife (CK) in the treatment of patients with recurrent pancreatic cancer after surgery, and analyze its survival-related factors. Methods: The primary endpoint was freedom from local progression (FFLP) and local control (LC) rate after CK. The secondary endpoints were overall survival (OS), progression-free survival (PFS), symptom relief and toxicities. Receiver operating characteristic (ROC) curves were used to determine the optimal cut-off values of inflammatory composite indicators NLR, PLR, SII and PNI. The prognostic factors that affected these patients were analyzed by univariate and multivariate analysis, respectively. Results: A total of 27 patients were enrolled. Median local recurrence disease free interval(DFI)was 11.3 (1.3-30.6) months, LC was 81.5% and 37.0% at 6 and 12 months, respectively. Median PFS was 7.1 (1.3-27.1) months. Median OS was 11.3 (1.3-30.6) months. Symptom alleviation was observed in 16 of 17 patients (94.1%) within 2 weeks after CK. Subsequent chemotherapy, CA199≥50% decrease after CK were independent prognostic factors for OS (all P <0.05). Conclusion: SBRT is a safe and effective treatment approach for recurrent pancreatic adenocarcinoma. Encouraging local control rate, low toxicity, and effective symptom relief suggests the vital role of CK in the treatment of these patients. This clinical application needs to be further studied in the combination of CK and multimodal therapy.
Introduction
Pancreatic cancer is a common malignant tumor of the digestive system. However, its prognosis is extremely poor. Even with radical surgical resection, approximately 80% of patients will relapse within two years after surgery [[1], [2], [3], [4], [5]]. Furthermore, although the postoperative recurrence pattern of pancreatic cancer is well-known [6], its optimal treatment strategy remains unclear [7,8]. Due to the disorder of the anatomical structure of the pancreas and its surroundings in the first operation, in most cases, local recurrent disease cannot be re-operated [[9], [10], [11]]. In addition, patients with recurrence after surgery often have obvious local symptoms, including pain, biliary obstruction, etc. [12] Local progression is an important factor that affects quality of life, and is also correlated to short PFS [13]. Hence, it is particularly important to relieve the symptoms and improve quality of life through local radiotherapy.
Due to the special anatomic location of the pancreas, conventional radiotherapy has low fractionated doses, long treatment cycles and large adverse reactions, which may adversely affect other comprehensive treatments, such as chemotherapy. In addition, pancreatic cancer has low response to conventional radiotherapy and poor local control. Stereotactic body radiation therapy (SBRT) is a precise radiotherapy method. Its advantages are precise positioning, precise planning and precise irradiation. At the same time, during high-dose irradiation, the doses to the surrounding organs are reduced, thereby reducing the incidence of serious adverse reactions. The present study aims to evaluate the efficacy, safety and survival of patients with recurrence of pancreatic cancer after CK.
Materials and methods
Study design and clinical data
The prospectively collected database of patients with recurrent pancreatic cancer of the investigators was retrospectively queried. Patients were treated with SBRT using CK between January 2009 and June 2019. Inclusion criteria: (1) any age; (2) imaging and clinical evidence supporting the local tumor recurrence; (3) complete clinical case data and follow-up data; (4) signed informed consent. Exclusion criteria: (1) previous radiation therapy; (2) contraindications to radiotherapy; (3) uncontrollable comorbidities. The study protocol was in accordance with the ethical guidelines of the Declaration of Helsinki, and was approved by independent ethics committees in Jinling Hospital. In addition, all included patients provided a written informed consent.
The following were collected and recorded from the study subjects: the gender, age and pain level (based on the Numerical Rating Scale [NRS]), Eastern Cooperation Oncology Group (ECOG) score, tumor staging, systemic immune-inflammation index (SII), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), prognostic nutrition index (Prognostic nutritional index [PNI]) before treatment, median biological effective dose (BED10), serum CA-199 levels before and after treatment, and the presence or absence of follow-up treatment.
Treatment plan implementation
Preparation before treatment: Three 6.0 × 0.8 mm gold markers were implanted within or around the tumor using a CT-guided 19G needle. CT scan was performed to determine whether the markers were in the proper positions at 24 h after implantation. The CT scan was repeated at seven days after implantation. At this time, the local hemorrhage and edema subsided around the gold seed fiducial, and became relatively stable and immobile.
Tumor localization and target area delineation: The patient took the supine position, and was fixed with a vacuum pad. Then, CT enhanced scanning (layer thickness: 1 mm) was performed for positioning. The patients were fasted for more than 4 h before positioning. The oral contrast agents were taken before the CT scan to better display the gastrointestinal tract. The scanning range was 15 cm above and below the pancreatic lesion. The target area was delineated on the soft tissue window of the CT. The gross tumor volume (GTV) was the tumor volume observed through the imaging. The GTV was placed at 2–3 mm outward on all sides, in order to form a planning target volume (PTV). If the tumor was close to the organs at risk (OAR, the gap between the tumor and OAR was <3 mm), the GTV was not placed in this direction. Normal tissues, including the esophagus, stomach, duodenum, small intestine, spinal cord, liver and kidneys, are sketched.
Treatment schedule: Before treatment, a respiratory monitoring device was used to continuously detect the position of the infrared generator placed on the patient's chest, in order to create a dynamic respiratory rhythm. Then, the X-ray digital imaging of the kV level was collected at different time points of the respiratory rhythm, in order to obtain the dynamics model between the gold seed fiducial (tumor) position and respiratory rhythm. Afterwards, the respiratory model was used to guide the accelerator in tracking the lesions within the pancreas, and provide dynamic radiation. The prescription dose provided to the lesions was 25–50 Gy (median dose: 40 Gy) once per day, at a fraction number of 4–7.
Follow-up and adverse reactions
These patients were observed at one month after completion of the treatment, every three months for the first year, and every six months thereafter, until December 2019. These patients were monitored for imaging data, related hematological indicators, adverse events, and compliance during follow-up. The OS, PFS and FFLP were recorded. The overall survival time was the time from the start of treatment to death or follow-up. The progression-free survival time was the time from the start of treatment to the progression of any lesion or onset of new metastasis. FFLP was the time from the start of treatment to the progression of the local lesion. Acute and long-term toxicities were defined as adverse events that occurred at <3 months and >3 months after SBRT, respectively. Toxicity was scored according to the Common Terminology Criteria for Adverse Events version 4.0.
End point
The primary end-point was the FFLP and LC rate after CK, which was determined using the Response Evaluation Criteria in Solid Tumors version 1.1.16. The LC rate for recurrent disease was assessed at six months after CK, in order to avoid the uncertainty associated with early transient radiographic changes within the high-dose region. The secondary end points were OS, PFS, symptom alleviation and toxicity.
Statistical analysis
The SPSS 22.0 software was used for the statistical analysis of the research data. ROC curves were used to determine the best cut-off values for NLR, PLR, SII and PNI, and the area under the curve (AUC) was recorded. The OS and PFS rate estimates were calculated using the Kaplan–Meier method, and compared using the stratified log-rank test. The multivariate analysis of survival was carried out using the Cox's regression model. P < 0.05 was considered statistically significant.
Results
Clinical characteristics
According to the inclusion criteria, the present study included a total of 27 patients: 18 male patients (66.7%), nine female patients (33.3%). Their median age was 58 years old (43–81 years old). The median time from operation to recurrence was 10.0 (0–34.2) months, and the median time from relapse to CK was 1.6 months (0.4–11.9). The median time from surgery to CK was 11.5 (1.7–37.2) months. A total of 18 (66.7%) patients underwent follow-up chemotherapy after the CK. Table 1, Table 2 presents the summary of the clinical and treatment characteristics of all patients.
Table 1.
Summary of patient characteristics.
| Name | Sex | Age (years) | Surgical staging | Type of surgery | Surgical pathology | Interval between surgery and recurrence (months) | Interval between surgery and SBRT (months) | ECOG | Symptoms | Synchronous metastases |
|---|---|---|---|---|---|---|---|---|---|---|
| LZJ | Male | 46 | T3N0M0 | R0 | Sarcomatoid carcinoma | 1.3 | 1.7 | 2 | Abdominal pain | Liver |
| WBH | Male | 71 | T3N1M0 | R0 | Adenocarcinoma | 10.5 | 13.6 | 1 | No | |
| LB | Male | 56 | T3N1M0 | R1 | Mucinous adenocarcinoma | 13.1 | 16.2 | 1 | No | |
| SXG | Male | 50 | T3N2M0 | R0 | Adenocarcinoma | 10.6 | 11.5 | 3 | Abdominal pain | |
| HXQ | Male | 47 | T4N0M0 | R2 | Adenocarcinoma | 0 | 4.5 | 1 | Abdominal pain | |
| WMH | Female | 61 | T4N0M0 | R0 | Adenocarcinoma | 11.2 | 12.3 | 2 | Lumbago | |
| ZXS | Male | 72 | T2N0M0 | R0 | Adenocarcinoma | 34.2 | 37.2 | 2 | Abdominal pain | |
| LQB | Male | 68 | T2N1M0 | R0 | Adenocarcinoma | 6.4 | 11.5 | 1 | No | |
| ZQ | Female | 70 | T3N0M0 | R0 | Adenocarcinoma | 10.5 | 12.3 | 1 | No | |
| WDC | Male | 69 | T1N0M0 | R0 | Adenocarcinoma | 11.4 | 23.3 | 1 | Lumbago | |
| ZH | Female | 48 | T3N1M0 | R0 | Adenocarcinoma | 14.3 | 19.9 | 3 | Lumbago | |
| YCH | Male | 80 | T3N1M0 | R0 | Adenocarcinoma with focal squamous cell carcinoma | 4 | 5.4 | 2 | No | |
| WZJ | Male | 69 | T3N0M0 | R0 | Adenocarcinoma | 19 | 22 | 1 | Abdominal pain | |
| LYM | Female | 53 | T3N1M0 | R0 | Adenocarcinoma | 5.5 | 6.9 | 3 | No | Lymphonodus of neck and armpit |
| ZL | Male | 43 | T3N0M0 | R1 | Adenocarcinoma | 5.3 | 5.8 | 1 | No | |
| WWJ | Male | 79 | T2N2M0 | R0 | Adenocarcinoma | 10.9 | 11.5 | 2 | No | |
| GLY | Female | 81 | T1N0M0 | R0 | Adenocarcinoma | 10 | 10.9 | 2 | Abdominal pain | |
| SST | Male | 51 | T3N0M0 | R0 | Adenocarcinoma | 4.7 | 5.8 | 1 | Abdominal pain and anorexia | |
| LW | Male | 50 | T4N1M0 | R0 | Adenocarcinoma | 6 | 6.4 | 1 | Abdominal pain | |
| XGD | Male | 53 | T3N0M0 | R0 | Adenocarcinoma | 15.4 | 17.6 | 1 | Lumbago | |
| ZJB | Male | 53 | T4N1M0 | R2 | Adenocarcinoma | 0 | 2.3 | 1 | Abdominal pain | |
| CQH | Female | 60 | T4N1M0 | R0 | Adenocarcinoma | 3.7 | 8.6 | 2 | No | |
| GHL | Female | 74 | T3N1M0 | R0 | Adenocarcinoma | 9 | 10.6 | 2 | Abdominal pain | |
| XZ | Male | 73 | T3N2M0 | R0 | Adenocarcinoma | 7.7 | 8.3 | 2 | Abdominal pain | |
| WHM | Female | 56 | T2N1M0 | R0 | Adenocarcinoma | 10 | 12 | 1 | Abdominal pain | |
| LAG | Male | 56 | T3N1M0 | R0 | Adenocarcinoma | 12.2 | 13 | 1 | Lumbago | |
| WYZ | Female | 62 | T2N0M0 | R0 | Adenocarcinoma | 7 | 7.6 | 1 | No |
Abbreviations: SBRT, stereotactic body radiation therapy.
Note: The R0, R1 and R2 were defined as negative margins, microscopically positive margins, and macroscopically positive margins after surgery, respectively.
Table 2.
Summary of treatment characteristics.
| Name | Prescription dose (Gy)/fractionation | BED10 (Gy) | Isodose (%) | History of CT | Follow-up of CT | Location of PD | FFLP (months) | PFS (months) | OS (months) | Status (cause) |
|---|---|---|---|---|---|---|---|---|---|---|
| LZJ | 50/5 | 100 | 71 | No CT | Gemcitabine-based | Liver and adrenal gland metastasis | 11.9 | 2.6 | 13.3 | Death (tumor) |
| WBH | 44/4 | 92.4 | 68 | No gemcitabine-based | Gemcitabine-based | Liver metastasis | 12.3 | 6.6 | 12.3 | Death (tumor) |
| LB | 40/5 | 72 | 70 | Gemcitabine-based | No CT | No | 3.6 | 3.6 | 3.6 | Death (gastrointestinal hemorrhage) |
| SXG | 40/4 | 80 | 77 | No CT | No CT | Abdominal wall metastasis | 2.9 | 1.3 | 2.9 | Death (tumor) |
| HXQ | 50/5 | 100 | 75 | Gemcitabine-based | 5-Fu-based | Liver metastasis | 26.3 | 22.6 | 26.3 | Death (tumor) |
| WMH | 42/7 | 67.2 | 76 | Gemcitabine-based | No CT | Liver and lung metastasis | 6.8 | 3.3 | 6.8 | Death (tumor) |
| ZXS | 40/5 | 72 | 75 | Gemcitabine-based | 5-Fu-based | Liver metastasis | 21.8 | 16.7 | 21.8 | Death (tumor) |
| LQB | 40/5 | 72 | 75 | Gemcitabine-based | No CT | No | 3.6 | 3.6 | 3.6 | Death (heart accident) |
| ZQ | 45/5 | 85.5 | 71 | No CT | Gemcitabine-based | Liver metastasis | 13.5 | 10.3 | 13.5 | Death (tumor) |
| WDC | 42/6 | 71.4 | 75 | Gemcitabine-based | 5-Fu-based | Liver and lung metastasis | 30.6 | 27.1 | 30.6 | Death (tumor) |
| ZH | 50/5 | 100 | 75 | Gemcitabine-based | No CT | Treatment site and liver metastasis | 1.3 | 1.3 | 1.3 | Death (tumor) |
| YCH | 40/5 | 72 | 70 | No gemcitabine-based | No CT | Liver metastasis | 8.9 | 7.1 | 8.9 | Death (tumor) |
| WZJ | 42/7 | 67.2 | 81 | Gemcitabine-based | 5-Fu-based | Treatment site | 22.3 | 22.3 | 26.5 | Death (tumor) |
| LYM | 40/5 | 72 | 70 | Gemcitabine-based | 5-Fu-based | Lymphonodus of neck and armpit | 11.3 | 7.6 | 11.3 | Death (tumor) |
| ZL | 40/5 | 72 | 67 | Gemcitabine-based | 5-Fu-based | Liver and retroperitoneal metastasis | 17.7 | 14.2 | 17.7 | Death (tumor) |
| WWJ | 40/5 | 72 | 80 | No CT | 5-Fu-based | Lung metastasis | 15.2 | 9.3 | 15.2 | Death (tumor) |
| GLY | 35/5 | 59.5 | 75 | No CT | No CT | Liver metastasis | 6.9 | 4.3 | 6.9 | Death (tumor) |
| SST | 35/5 | 59.5 | 77 | Gemcitabine-based | No CT | Peritoneum metastasis | 5.3 | 4.1 | 5.3 | Death (tumor) |
| LW | 35/5 | 59.5 | 77 | Gemcitabine-based | 5-Fu-based | Liver metastasis | 6.4 | 2.8 | 6.4 | Death (tumor) |
| XGD | 35/5 | 59.5 | 81 | Gemcitabine-based | Gemcitabine-based | Liver metastasis | 17.1 | 13.6 | 17.1 | Death (tumor) |
| ZJB | 35/5 | 59.5 | 79 | No CT | Gemcitabine-based | Liver and lung metastasis | 15.9 | 14.2 | 15.9 | Death (tumor) |
| CQH | 35/5 | 59.5 | 80 | No CT | Oxaliplatin-based | No | 11.9 | 11.9 | 11.9 | Alive |
| GHL | 35/5 | 59.5 | 77 | No gemcitabine-based | Oxaliplatin-based | No | 11.3 | 11.3 | 11.3 | Alive |
| XZ | 35/5 | 59.5 | 76 | No CT | No CT | Peritoneum metastasis | 7.5 | 5.9 | 7.5 | Death (tumor) |
| WHM | 25/5 | 37.5 | 75 | Gemcitabine-based | 5-Fu-based | No | 9.1 | 9.1 | 9.1 | Alive |
| LAG | 35/5 | 59.5 | 75 | Gemcitabine-based | Irinotecan-based | No | 6.1 | 6.1 | 6.1 | Alive |
| WYZ | 30/5 | 48 | 83 | Gemcitabine-based | Oxaliplatin-based | No | 6.1 | 6.1 | 6.1 | Alive |
Abbreviations: BED, biologically equivalent dose; CT, chemotherapy; PD, progressive disease; FFLP, freedom from local progression; PFS, progression free survival; OS, overall survival time.
Dosimetric index
The isodose level of the prescription dose in the treatment plan was 82.33–94.66%, with a median value of 91%. The irradiation fields involved 150–200 non-coplanar fields. The treatment plan revealed that the median CI, nCI and HI of the pancreatic lesions in all patients was 1.13, 1.52 and 1.33 respectively (Table 3).
Table 3.
The dosimetry index of the patients during Cyber-Knife radiosurgery treatment.
| Item | CI | n CI | HI | Coverage (%) | PTV (cm3) | Prescription dose (Gy) | BED10 (Gy) | Isodose (%) |
|---|---|---|---|---|---|---|---|---|
| Range | 1.01–1.56 | 1.15–2.28 | 1.2–1.52 | 82.33–94.65 | 18.38–170.38 | 25–50 | 37.5–100 | 67–83 |
| Mean | 1.16 | 1.52 | 1.33 | 89 | 87.13 | 39 | 70 | 75 |
| Median | 1.13 | 1.52 | 1.33 | 91 | 44.14 | 40 | 71.4 | 75 |
Abbreviations: Coverage: the coverage is volume of the tumor receiving greater than or equal to the prescription dose divided by the total volume of the tumor times 100; CI, conformity index;
n CI, new conformity index; HI, homogeneity index.
Determination of critical values of inflammatory composite indicators
The ROC curve for each inflammatory composite index was drawn with the survival time as the end-point (Fig. 1). The results revealed that the AUC for SII, NLR, PLR and PNI was 0.6636, 0.7727, 0.5091 and 0.6455, respectively. The best cut-off values for each indicator in the present study were as follows: SII was 821.9, NLR was 1.26, PLR was 105.2, and PNI was 49.88. These were used to analyze the relationship between inflammatory composite indicators and OS, and PFS.
Fig. 1.
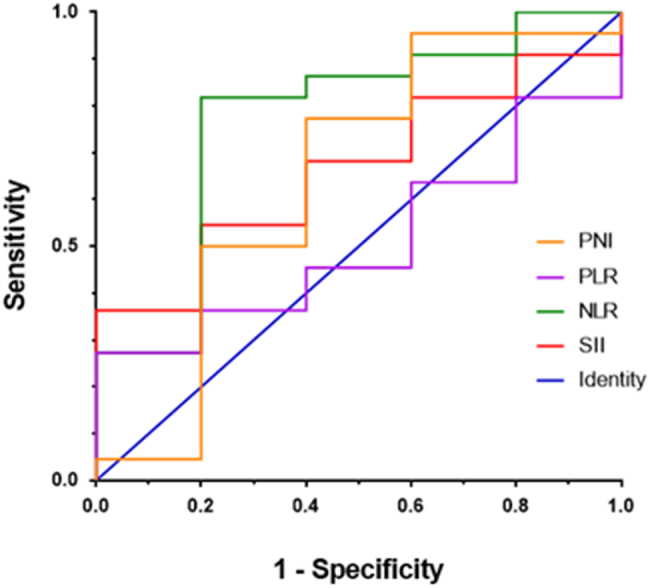
Receiver operation characteristic curve of the patients according to the neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), systemic immune-inflammation index (SII) and prognostic nutrition index (PNI).
LC rate, OS and prognostic factors
Among the 27 patients, one (3.7%) patient achieved complete response, 18 (66.7%) patients exhibited partial response, six (22.2%) patients presented with a stable disease, and two (7.4%) patients developed progressive disease. The median local recurrence disease free interval (DFI) was 11.3 (1.3–30.6) months. The 6-month and 12-month LC rate was 81.5% and 37.0%, respectively. At six months after the CK, 16 (94.1%) of 17 patients with pain were relieved to varying degrees.
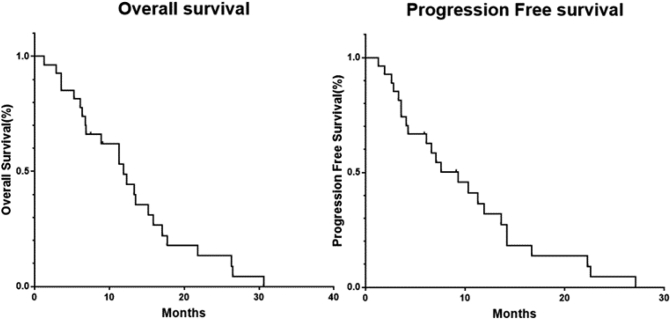
The median OS of the 27 patients was 11.3 months (1.3–30.6 months), and the median PFS was 7.1 months (1.3–27.1 months). The 6-month and 12-month survival rate was 81.8% and 40.7%, respectively, and the 6-month and 12-month progression-free survival rate was 63.0% and 26.0%, respectively (Fig. 2).
Fig. 2.
Kaplan–Meier analysis of overall survival and progression free survival for patients.
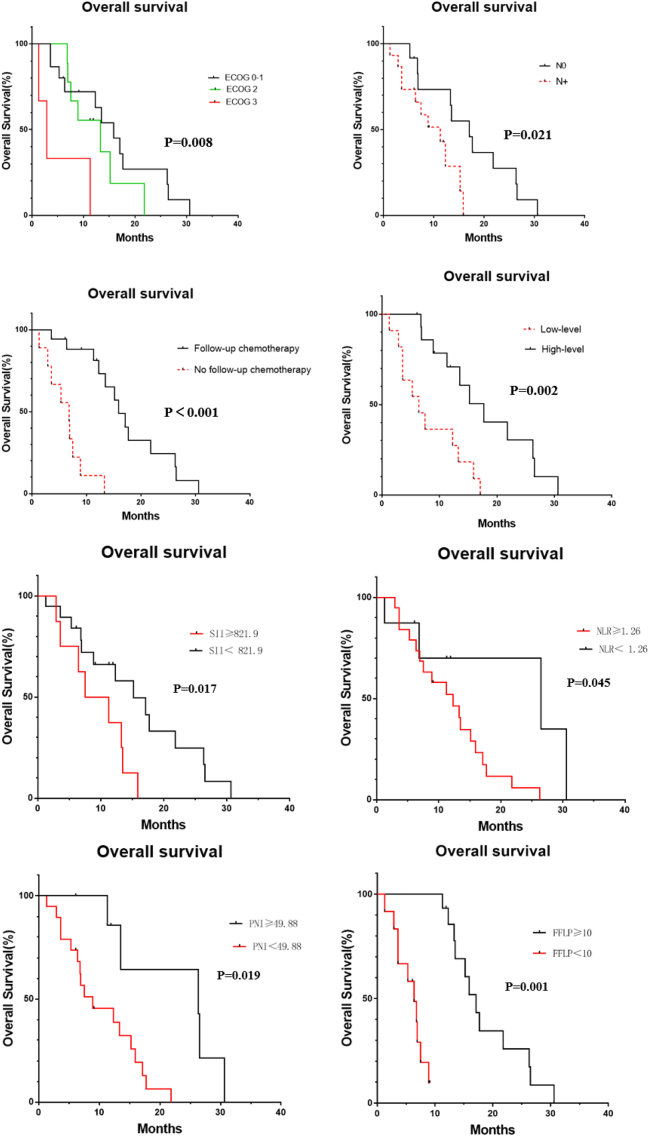
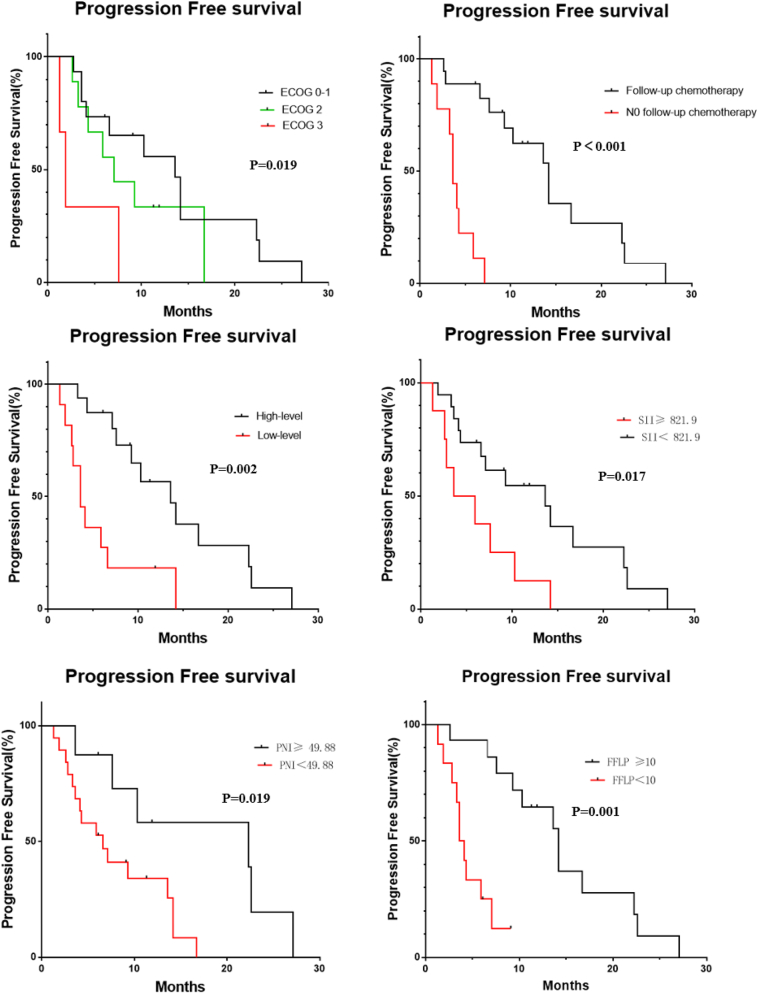
The univariate analysis results revealed the following: the ECOG score, postoperative lymph node staging, follow-up treatment, CA199 decline, SII, NLR, PNI and FFLP were factors correlated to OS (all, P < 0.05; Table 4, Fig. 3). Patients with a lower ECOG score, no lymph node metastasis after surgery, continued treatment after radiotherapy, and a decrease in CA199 of ≥50% after treatment had a longer OS. Patients with SII <821.9 had a better OS, when compared to patients with SII ≥821.9 (P = 0.017). The OS was shorter in the high NLR group than in the low NLR group. Patients with PNI ≥49.88 were significantly better, when compared to patients with PNI <49.88 (P = 0.019). The ECOG score, follow-up treatment, CA199 decline after treatment, SII, PNI and FFLP were the related factors that affected PFS, (all, P < 0.05; Table 4, Fig. 4).
Table 4.
The univariate analysis of OS and PFS.
| Variable | N | OS Median (months) |
P | PFS Median (months) |
P |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 18 | 12.8 | 0.490 | 6.9 | 0.993 |
| Female | 9 | 9.1 | 7.6 | ||
| Age | |||||
| ≤60 | 14 | 10.2 | 0.413 | 6.9 | 0.570 |
| >60 | 13 | 11.3 | 7.1 | ||
| ECOG | |||||
| 0–1 | 15 | 12.3 | 0.008 | 9.1 | 0.019 |
| 2 | 9 | 11.3 | 7.1 | ||
| 3 | 3 | 2.9 | 1.9 | ||
| N stage | |||||
| N0 | 12 | 15.3 | 0.021 | 11.9 | 0.131 |
| N+ | 15 | 8.9 | 6.6 | ||
| Follow-up chemotherapy | |||||
| No | 9 | 5.3 | <0.001 | 3.6 | <0.001 |
| Yes | 18 | 13.4 | 10.8 | ||
| Degree of decline of CA199 | |||||
| <50% | 11 | 6.4 | 0.002 | 3.6 | 0.002 |
| ≥50% | 16 | 11.6 | 9.8 | ||
| BED10 | |||||
| ≥60 | 16 | 12.8 | 0.834 | 7.4 | 0.637 |
| <60 | 11 | 7.5 | 6.1 | ||
| NRS | |||||
| ≤3 | 10 | 11.6 | 0.319 | 7.35 | 0.480 |
| ≥4 | 17 | 9.1 | 6.1 | ||
| SII | |||||
| ≥821.9 | 8 | 9.4 | 0.017 | 4.8 | 0.017 |
| <821.9 | 19 | 11.3 | 9.1 | ||
| NLR | |||||
| ≥1.26 | 19 | 11.3 | 0.045 | 7.1 | 0.045 |
| <1.26 | 8 | 9.05 | 8.7 | ||
| PLR | |||||
| ≥105.2 | 21 | 8.9 | 0.052 | 6.1 | 0.107 |
| <105.2 | 6 | 20.8 | 15.8 | ||
| PNI | |||||
| ≥49.88 | 8 | 12.7 | 0.019 | 11.1 | 0.019 |
| <49.88 | 19 | 7.5 | 6.1 | ||
| FFLP | |||||
| ≥10 | 14 | 15.6 | 0.001 | 12.8 | 0.001 |
| <10 | 13 | 6.1 | 4.1 |
Fig. 3.
Overall survival curves of 27 patients according ECOG score, postoperative lymph node staging, follow-up treatment, CA199 decline, SII, NLR, PNI, and FFLP.
Fig. 4.
Progression free survival curves of 27 patients according ECOG score, follow-up treatment, CA199 decline, SII, PNI, and FFLP.
The multivariate analysis results revealed that subsequent chemotherapy and the CA199 ≥ 50% decrease after CK are independent prognostic factors for OS, while subsequent chemotherapy was an independent prognostic factor for PFS (all, P < 0.05; Table 5).
Table 5.
The multivariate analysis of OS and PFS.
| Variable | OS |
|||||
|---|---|---|---|---|---|---|
| B | SE | Wald | P | RR | 95% CI | |
| Follow-up chemotherapy (no vs yes) | 3.732 | 1.128 | 10.954 | 0.001 | 41.751 | 4.581–227.862 |
| Degree of decline of CA199 (<50% vs ≥50%) | 1.462 | 0.564 | 6.716 | 0.001 | 4.316 | 1.428–13.045 |
| Variable | PFS |
|||||
|---|---|---|---|---|---|---|
| B | SE | Wald | P | RR | 95% CI | |
| Follow-up chemotherapy (no vs yes) | 2.036 | 0.729 | 7.811 | 0.005 | 7.662 | 1.837–31.955 |
Side effects and patterns of failure
All patients completed the CK without treatment breaks or dose reductions. As indicated in Table 6, 21 patients (77.8%) experienced CTCAE version 4.0 grade 1–2 acute toxicities that manifested as diarrhea, abdominal distention, nausea and vomiting, thrombocytopenia, weakness and anorexia. One patient (3.7%) who received gemcitabine-based chemotherapy experienced grade 3 acute toxicity due to thrombocytopenia. These toxicities were generally transient, and were resolved with conservative management. One patient (3.7%) suffered from late grade 3 duodenal ulcer, which needed endoscopic treatment. No treatment-related deaths were observed.
Table 6.
Side effects in 27 patents with pancreatic carcinoma in the treatment of Cyber-Knife.
| Any grade, n (%) | Grade 3, n (%) | Grade 4, n (%) | Grade 5, n (%) | |
|---|---|---|---|---|
| Acute toxicities | ||||
| Diarrhea | 2 (7.4) | 0 | 0 | 0 |
| Abdominal pain | 0 | 0 | 0 | 0 |
| Abdominal distention | 1 (4.2) | 0 | 0 | 0 |
| Agranulocytosis | 0 | 0 | 0 | 0 |
| Nausea and vomiting | 10 (37.0) | 0 | 0 | 0 |
| Thrombocytopenia | 1 (4.2) | 1 (4.2) | 0 | 0 |
| Weak | 3 (11.1) | 0 | 0 | 0 |
| Anorexia | 5 (18.5) | 0 | 0 | 0 |
| Late toxicities | ||||
| Intestinal obstruction | 0 | 0 | 0 | 0 |
| Intestinal perforation | 0 | 0 | 0 | 0 |
| Gastric perforation | 0 | 0 | 0 | 0 |
| Stomach ulcer | 0 | 0 | 0 | 0 |
| Duodenal ulcer | 1 (4.2) | 1 (4.2) | 0 | 0 |
Two patients (7.4%) exhibited relapse within the PTV. Furthermore, out-of-field progression was detected in 19 patients (66.7%) within a median of 7.1 months after the CK (range: 1.3–27.1 months), while progression was not observed after the CK in seven patients (25.9%).
Discussion
The treatment of patients with recurrent pancreatic cancer following surgical resection was mainly based on the comprehensive treatment, which included reoperation, chemotherapy and radiotherapy. Nevertheless, the treatment effect on these patients was not ideal, and most of these patients were not suitable for reoperation due to simultaneous distant metastases, vascular involvement, or poor physical condition [1,14]. The present results suggest that the CK is a safe and effective treatment approach for recurrent pancreatic carcinoma, and the related prognostic factors were analyzed for the first time.
Due to the high radiation accuracy of SBRT, tumors can be given higher radiation doses, while minimizing the radiation doses to the surrounding endangered organs. Compared with conventional radiation therapy, this can significantly shorten the treatment time without affecting the patient's overall treatment plan. The patient's local symptoms were effectively alleviated, and their quality of life was significantly improved, providing more time and opportunities for subsequent comprehensive treatment. The role of SBRT for advanced pancreatic cancer has been confirmed in many studies, but its application in patients with recurrent pancreatic carcinoma has been less studied. Five retrospective case series reported on SBRT for the treatment of isolated local recurrence (Table 7). In three studies, all patients received prior radiation in association with initial surgical resection. Rwigema et al. [15] reported on 71 patients with advanced adenocarcinoma of the pancreas, who were treated with SBRT, and 11 (16%) of these patients had local recurrence following surgical resection. These patients had a median survival of 13 months, and no grade 3 or above toxicity was observed. Wild et al. [16] included 15 patients with isolated local recurrence after surgical resection and three patients with local progression after definitive chemoradiotherapy for locally advanced pancreatic cancer. All patients received GEM-based maintenance chemotherapy before SBRT. The median OS was nine months, and no acute toxicity of grade 3 or above was observed, while one patient developed grade 3 advanced toxicity (intestinal obstruction). Among the seven patients who presented with abdominal/back pain prior to SBRT, an effective symptom palliation was achieved in four patients. Dagoglu et al. [17] further described 15 patients with isolated local recurrence treated by SBRT. All of these patients received some form of chemotherapy prior to or after SBRT. The median OS was 13 months, and no acute or chronic toxicity above level 3 was found. Furthermore, all four patients who presented with pain symptoms experienced the alleviation of these symptoms as a result of the SBRT. Zeng et al. [18] reported 24 patients with SBRT after the recurrence of pancreatic cancer after surgery, with a median OS of 12 months and one year, and an LC rate of 83.8%. Nine patients developed grade 1–2 acute toxicity, and one patient developed grade 3 toxicity due to thrombocytopenia. However, no late toxicity was found. Comito et al. [19] reported 31 patients with isolated local recurrence after R0, and the median follow-up was 12 months. FFLP was 91% and 82% in one year and two years, respectively. The median OS was 18 months. No acute or chronic toxicity of grade 3 or above was observed, and the treatment was tolerable for all patients. In the present study, the median FFLP was 11.3 months, and the median OS and PFS was 11.3 and 7.1 months, respectively. The 6-month and 12-month LC rates were 81.5% and 37.0%, respectively. The 6-month and 12-month survival rate was 81.8% and 40.7%, respectively, and the 6-month and 12-month progression-free survival rate was 63.0% and 26.0%, respectively. Among the 27 patients, two patients had relapses within PTV, while the remaining patients had distant metastases. Therefore, SBRT should be combined with systemic therapy to reduce the rate of distant metastases.
Table 7.
Study outcomes of stereotactic body radiation therapy for the treatment of locally recurrent pancreatic cancer.
| Reference | Relevant patients (total) | Median radiation dose (Gy) | Acute toxicity ≥Grade 3 |
Late toxicity ≥Grade 3 |
LC (1 year) | Median survival (months) |
|---|---|---|---|---|---|---|
| Rwigema et al. [16] | 11 (71) | 24 (18–25) | 3 | 0 | 19% | 13 |
| Wild et al. [17] | 15 (18) | 25 (20–27) | 0 | 1 | 62% | 9 (8–12) |
| Dagoglu et al. [18] | 15 (30) | 25 (24–36) | 0 | 0 | 72% | 16 (3−32) |
| Zeng et al. [19] | 19 (24) | 45 (42–50) | 0 | 0 | 42% | 12 (4–23) |
| Comito et al. [20] | 31 (31) | 45 (45–45) | 0 | 0 | 91% | 18 (11.1–24.9) |
| Total | 91 (174) | NA | 3 of 135 (2.2%) | 1 of 63 (1.6%) | NA | NA |
Abbreviations: NA, not available.
Inflammatory response plays a vital role in all stages of tumorigenesis, development and treatment. The level of neutrophils, lymphocytes and platelets in peripheral blood are significantly correlated to tumor progression [[20], [21], [22], [23]]. The combinations of different indicators, such as NLR, PLR and SII, have been shown to correlate with the prognosis of various malignancies. SII can more objectively reflect the balance between inflammatory and immune responses in patients [[24], [25], [26]]. The higher SII in tumor patients is associated with the high level of circulating tumor cells (CTCs) in the body, which in turn is associated with tumor invasion and metastasis, ultimately leading to poor prognosis. In addition, the prognostic nutrition index (PNI), which is a combination of serum albumin levels and lymphocyte counts, has also been considered to be correlated to the prognosis in a variety of tumors [[27], [28], [29]]. In the present study, the survival factors of recurrent pancreatic carcinoma after SBRT treatment were analyzed, and the relationship between inflammatory composite indicators and the prognosis of patients was initially explored. The univariate analysis results revealed that patients had a low ECOG score, good physical fitness (P = 0.008), no lymph node metastasis after the operation (P = 0.021), continued treatment after radiotherapy (P < 0.001), and a decrease in CA199 by ≥50% after treatment (P = 0.002), while patients with PPLP ≥10 months (P = 0.001) had a longer OS. Furthermore, patients with SII <821.9 had a better OS than patients with SII ≥821.9 (P = 0.017). The OS of patients with high NLR was shorter, when compared to patients with low NLR (P = 0.045). The OS of patients with PNI ≥49.88 was significantly better than patients with PNI <49.88 (P = 0.019). The ECOG score, radiotherapy follow-up treatment, CA199 degree after treatment, SII, PNI and FFLP were the related factors that affects a patient's PFS. The follow-up treatment of radiotherapy (P = 0.001) and the decrease in CA199 ≥ 50% after treatment (P = 0.001) were independent prognostic factors of OS. The follow-up treatment of radiotherapy (P = 0.005) was an independent prognostic factor of PFS. In the present study, two patients had a R1 resection and two patients had a R2 resection. Furthermore, two patients had distant metastasis at the time the recurrence was found, and nine patients did not follow-up treatment after SBRT. Patients with longer FFLP had a better OS. Therefore, more studies are needed to explore the combination of SBRT and other treatment models, providing a higher LC rate and better OS. Cytokines secreted by neutrophils can change the tumor microenvironment through endogenous and exogenous pathways, in order to increase invasiveness and evade immune surveillance [30]. Furthermore, this can also lead to T lymphocyte activation disorders [26]. Lymphocytes mediate the body's immune response to tumor cells. By inducing cytotoxic cell death, and inhibiting the proliferation and migration of tumor cells, these play an important role in tumor defense and immune surveillance. When the number of CD4+ T helper lymphocytes is reduced, the patient's anti-cancer ability is weakened, and the prognosis becomes poor [31]. Therefore, the immunotherapy of pancreatic cancer needs to select specific antigens for labeling, according to the heterogeneity. Furthermore, subsequent studies need to explore the changes in the immune inflammatory complex indexes of patients after SBRT treatment, in order to identify more accurate immunotherapy markers, and further explore the best mode of SBRT combined immunotherapy.
The whole management of patients with recurring pancreatic cancer after surgery remains as a challenging subject, because the disease typically causes symptoms that negatively impact the patient's quality of life, such as abdominal and back pain. These symptoms are not typically transient, and cannot be resolved with conservative management. However, in the present study, the majority of patients achieved complete symptoms relief, typically within two weeks of treatment. These present findings concur with published studies, suggesting that SBRT can reduce pain and improve the quality of life of patients with localized advanced pancreatic cancer. In the present study, most of the patients experienced CTCAE grade 1–2 acute toxic events, and most of these symptoms were transient and resolved with conservative management. One patient developed grade 3 thrombocytopenia, and the blood returned to normal after symptomatic treatment. One patient developed grade 1 radioactive enteritis, which was cured after conservative medical treatment. One patient developed grade 3 radioactive enteritis, which required combined medical and surgical treatment. The patient's overall tolerance was better.
These present results confirm the efficacy and safety of SBRT using the CK for patients with recurrent pancreatic cancer after surgery. To the best of our knowledge, this study is the first to analyze the related prognostic factors, and explore the relationship between the inflammatory complex index and the prognosis of patients. However, the present study is limited by its retrospective nature, and the patient characteristics and treatment plan are not unified. However, in light of the paucity of literature on the outcomes of CK for these patients, these present results further recommend the clinical use of CK. At the time of precision therapy, more prospective studies are needed to combine SBRT with gene-guided multi-mode (chemotherapy, targeted therapy, immunotherapy and anti-vascular therapy) therapy, in order to achieve comprehensive treatment, provide a cure or control to the greatest extent, prolong patient survival, and improve the patient's quality of life.
CRediT author statement
Jing Li: Conceptualization, Methodology, Software, Writing - original draft preparation. Zhen Wang: Visualization, Investigation, Writing - review & editing, Formal analysis. Ao-mei Li: Project administration. Han Zhou: Data curation, Investigation, Writing - review & editing. Xi-xu Zhu: Funding acquisition, Supervision, Validation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the Science and Technology Plan of Jiangsu Province, Social Development-General Project of China (No BE2015688). No benefits in any form have been or will be received from a commercial party directly or indirectly related to the subject of this article.
References
- 1.Kleeff J., Reiser C., Hinz U. Surgery for recurrent pancreatic ductal adenocarcinoma. Ann. Surg. 2007;245(4):566–572. doi: 10.1097/01.sla.0000245845.06772.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vincent A., Herman J., Schulick R. Pancreatic cancer. Lancet. 2011;378(9791):607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamada S., Fujii T., Kanda M. Value of peritoneal cytology in potentially resectable pancreatic cancer. Br. J. Surg. 2013;100(13):1791–1796. doi: 10.1002/bjs.9307. [DOI] [PubMed] [Google Scholar]
- 4.Hartwig W., Werner J., Jäger D. Improvement of surgical results for pancreatic cancer. Lancet Oncol. 2013;14(11):e476–e485. doi: 10.1016/S1470-2045(13)70172-4. [DOI] [PubMed] [Google Scholar]
- 5.Moningi S., Dholakia A.S., Raman S.P. The role of stereotactic body radiation therapy for pancreatic cancer: a single-institution experience. Ann. Surg. Oncol. 2015;22(7):2352–2358. doi: 10.1245/s10434-014-4274-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oettle H., Post S., Neuhaus P. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297(3):267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 7.Gangl O., Fröschl U., Dutta-Függer B. Elective pancreatic reresection – report of a series and review of the literature. Eur Surg. 2010;34(1):91–95. 42. [Google Scholar]
- 8.Kyriazanos I.D., Tsoukalos G.G., Papageorgiou G. Local recurrence of pancreatic cancer after primary surgical intervention: how to deal with this devastating scenario? Surg. Oncol. 2011;20(4):e133–e142. doi: 10.1016/j.suronc.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y., Frampton A.E., Kyriakides C. Loco-recurrence after resection for ductal adenocarcinoma of the pancreas: predictors and implications for adjuvant chemoradiotherapy. J. Cancer Res. Clin. Oncol. 2012;138(6):1063–1071. doi: 10.1007/s00432-012-1165-7. [DOI] [PubMed] [Google Scholar]
- 10.Habermehl D., Brecht I.C., Bergmann F. Chemoradiation in patients with isolated recurrent pancreatic cancer - therapeutical efficacy and probability of re-resection. Radiat. Oncol. 2013;8(1):27. doi: 10.1186/1748-717X-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakamura A., Itasaka S., Takaori K. Radiotherapy for patients with isolated local recurrence of primary resected pancreatic cancer. Strahlenther. Onkol. 2014;190:485–490. doi: 10.1007/s00066-014-0610-8. [DOI] [PubMed] [Google Scholar]
- 12.Ogawa K., Shibuya H., Uchida N. Postoperative external beam radiotherapy for resected pancreatic adenocarcinoma: impact of chemotherapy on local control and survival. Anticancer Res. 2010;30:2959–2967. [PubMed] [Google Scholar]
- 13.Rudra S., Narang A.K., Pawlik T.M. Evaluation of predictive variables in locally advanced pancreatic adenocarcinoma patients receiving definitive chemoradiation. Pract. Radiat. Oncol. 2012;2:77–85. doi: 10.1016/j.prro.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zacharias T., Oussoultzoglou E., Jaeck D., Pessaux P., Bachellier P. Surgery for recurrence of periampullary malignancies. J. Gastrointest. Surg. 2009;13(4):760–767. doi: 10.1007/s11605-008-0769-3. [DOI] [PubMed] [Google Scholar]
- 15.Rwigema J.C.M., Parikh S.D., Heron D.E. Stereotactic body radiotherapy in the treatment of advanced adenocarcinoma of the pancreas. Am. J. Clin. Oncol. 2011;34:63–69. doi: 10.1097/COC.0b013e3181d270b4. [DOI] [PubMed] [Google Scholar]
- 16.Wild A.T., Hiniker S.M., Chang D.T. Re-irradiation with stereotactic body radiation therapy as a novel treatment option for isolated local recurrence of pancreatic cancer after multimodality therapy: experience from two institutions. J. Gastrointest. Oncol. 2013;4:343–351. doi: 10.3978/j.issn.2078-6891.2013.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dagoglu N., Callery M., Moser J. Stereotactic body radiotherapy (SBRT) reirradiation for recurrent pancreas cancer. J. Cancer. 2016;7:283–288. doi: 10.7150/jca.13295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng X., Wang H., Meng M. Stereotactic body radiation therapy for patients with recurrent pancreatic adenocarcinoma at the abdominal lymph npdes or postoperative stump including pancreatic stump and other stump. OncoTargets Ther. 2016;9:3985–3992. doi: 10.2147/OTT.S102784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Comito T., Cozzi L., Zerbi A. Clinical results of stereotactic body radiotherapy (SBRT) in the treatment of isolated local recurrence of pancreatic cancer after R0 surgery: a retrospective study. Eur. J. Surg. Oncol. (EJSO) 2017;43(4):735–742. doi: 10.1016/j.ejso.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 20.Elinav Eran, Nowarski Roni, Christoph A. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer. 2013;13(11):759–771. doi: 10.1038/nrc3611. [DOI] [PubMed] [Google Scholar]
- 21.Chai E.Z., Siveen K., Shanmugam M. Analysis of the intricate relationship between chronic inflammation and cancer. Biochem. J. 2015;468(1):1–15. doi: 10.1042/BJ20141337. [DOI] [PubMed] [Google Scholar]
- 22.Coolslartigue J., Spicer J., Mcdonald B. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J. Clin. Investig. 2013;123(8):3446–3458. doi: 10.1172/JCI67484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Myriam Labelle. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011 doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Passardi A., Scarpi E., Cavanna L. Inflammatory indexes as predictors of prognosis and bevacizumab efficacy in patients with metastatic colorectal cancer. Oncotarget. 2016;7(22) doi: 10.18632/oncotarget.8901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kyoung-Min C., Hyunkyung P., Do-Youn O. Neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and their dynamic changes during chemotherapy is useful to predict a more accurate prognosis of advanced biliary tract cancer. Oncotarget. 2017;8(2) doi: 10.18632/oncotarget.13731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geng Y., Shao Y., Zhu D. Systemic immune-inflammation index predicts prognosis of patients with esophageal squamous cell carcinoma: a propensity score-matched analysis. Sci. Rep. 2016;6(1):39482. doi: 10.1038/srep39482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naito Tateaki, Tanaka Fumihiro, Ono Akira. Prognostic impact of circulating tumor cells in patients with small cell lung cancer. J. Thorac. Oncol. 2012;7(3):512–519. doi: 10.1097/JTO.0b013e31823f125d. [DOI] [PubMed] [Google Scholar]
- 28.Ys Tong, Tan H., Xl Zhou. Systemic immune-inflammation index predicting chemoradiation resistance and poor outcome in patients with stage III non-small cell lung cancer. J. Transl. Med. 2017;15(1):221–231. doi: 10.1186/s12967-017-1326-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun K., Chen S., Xu J. The prognostic significance of the prognostic nutritional index in cancer: a systematic review and meta-analysis. J. Cancer Res. Clin. Oncol. 2014;140(9):1537–1549. doi: 10.1007/s00432-014-1714-3. [DOI] [PubMed] [Google Scholar]
- 30.Wang K., Diao F., Ye Z. Prognostic value of systemic immune-inflammation index in patients with gastric cancer. Chin. J. Cancer. 2017;38(09):34–40. doi: 10.1186/s40880-017-0243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raghavan S., Quiding-JaRbrink M. Regulatory T cells in gastrointestinal tumors. Expert Rev. Gastroenterol. Hepatol. 2011;5(4):489–501. doi: 10.1586/egh.11.44. [DOI] [PubMed] [Google Scholar]