Abstract
Purpose
To histologically evaluate the use of bovine derived deproteinized xenograft (DBBM), leukocyte and platelet rich fibrin (L-PRF) and the combination of both in Guided Bone Regeneration (GBR) performed in non-critical size defects in rabbit.
Methods
A prospective experimental study was performed. Four bone defects in the tibiae of 12 rabbits were made and each of them was filled with DBBM, L-PRF, a combination of DBBM + L-PRF or was left to heal as control site. All defects were covered with a collagen membrane. Rabbits were randomly distributed in three groups and euthanatized at 3, 6 or 9 weeks. Samples were obtained and histologically analyzed to determine vital bone, connective tissue and remaining graft particles percentage. Analysis of variance, Kruskal Wallis and non-paired t-test where used to evaluate the significance of the results.
Results
At 3 weeks of healing, DBBM showed significantly more vital bone percentage than L-PRF (p = 0,05) and DBBM + L-PRF showed significantly less connective tissue than control (p < 0,05). All other groups showed no statistical difference between them. At 6 weeks of healing, DBBM showed significantly more vital bone percentage than L-PRF (p < 0,05), DBBM + L-PRF (p < 0,05) and control (p < 0,05) and there wasn't any other significant difference regarding to connective tissue or remaining particle percentage between groups. At t 9 weeks healing period, there weren't any significant differences between groups.
Conclusions
DBBM seems to enhance vital bone formation at early healing stages. The use of L-PRF alone or combined with DBBM, didn't show any histological improvement regarding to vital bone formation. The use of DBBM, alone or in conjunction with L-PRF showed a trend to reduce connective tissue percentage. The use of L-PRF combined with DBBM didn't affect the remaining particle percentage.
Keywords: Guided bone regeneration, GBR, Xenograft, DBBM, Platelet rich fibrin, L-PRF
1. Introduction
Using bone regeneration procedures to regain bone width, height or both, lost due to post-extraction alterations, trauma, endodontic lesions and other factors, is a commonly used procedure, to ensure successful implant supported restorations.1
Different kinds of bone graft materials, in form of blocks or particles and also combined with autologous products, have been widely used to improve bone quantity and quality to allow an adequate three-dimensional implant position and a long-term peri-implant tissue stability.2
For this reason, it is of paramount importance to know and understand the biological characteristics of each particular biomaterial before using it for a determined regenerative procedure.
Bovine derived deproteinized xenografts (DBBM), with a slow resorption rate and osteoconductive properties, have shown successful results in guided bone regeneration (GBR) procedures, despite being completely anorganic.3, 4, 5, 6
New generation platelet derivates, which claim to improve bone regeneration, are being used as an attempt give more biological properties and better results in these procedures.7,8
Leukocyte and platelet rich fibrin (L-PRF), is an autologous platelet derivate, which liberates different grow factors, like platelet derived grow factor (PDGF), vascular endothelial grow factor (VEGF), transforming growth factor beta (TGF-β) and others. Growth factors are slowly liberated from the L-PRF matrix, and this liberation can last for up to seven days, which may improve tissue regeneration when used in GBR.9, 10, 11, 12, 13
In vitro and in vivo studies have shown an improvement in tissue regeneration when different grow factor are used alone, or in conjunction with other biomaterials.14,15
The present study was carried out to histologically evaluate and compare the use of DBBM, L-PRF or the combination of both in GBR procedures performed in non-critical size, four-wall defects in rabbit tibia.
2. Materials and methods
The following study was approved by the Bioethics and Biosecurity Committee and the Institutional Board for the Care and Use of Laboratory Animals of the Mayor University in Santiago, Chile (CBB Nº11/2017).
Twelve 6-month-old, healthy female New Zealand rabbits, with an average weight of 2 kg were selected for the study. Sample size was determined using a similar study as reference 3 and using a statistical software (EPIDAT 4.2, Sergas) to search for 20% differences between groups with a power of 0.8 and a confidence level of 95%.
A biochemical profile test (Vetscan Comprehensive Diagnostic Profile, Abaxis ®) was performed to each specimen prior to surgery, to assure the incorporation of healthy specimens and to exclude specimens with a systemic disease or other condition that may alter the study results.16, 17, 18
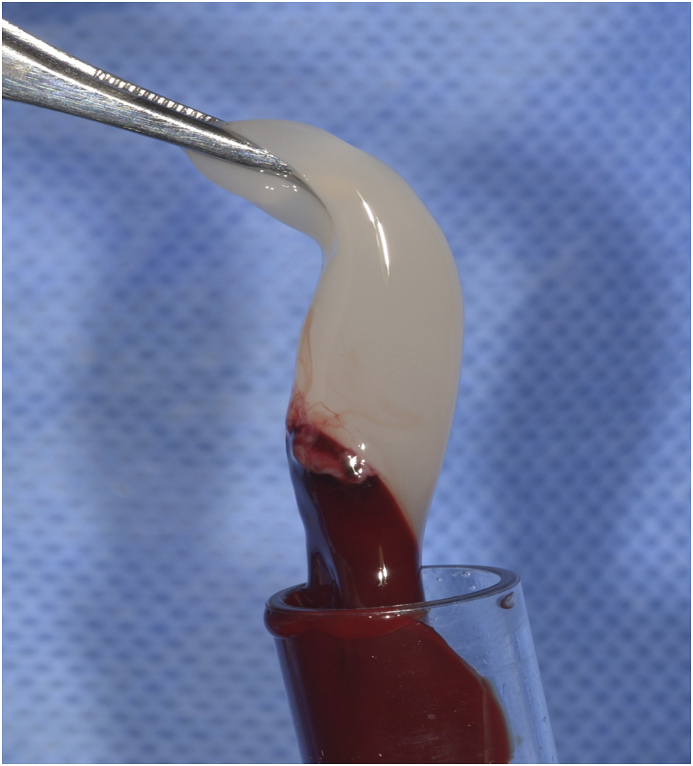
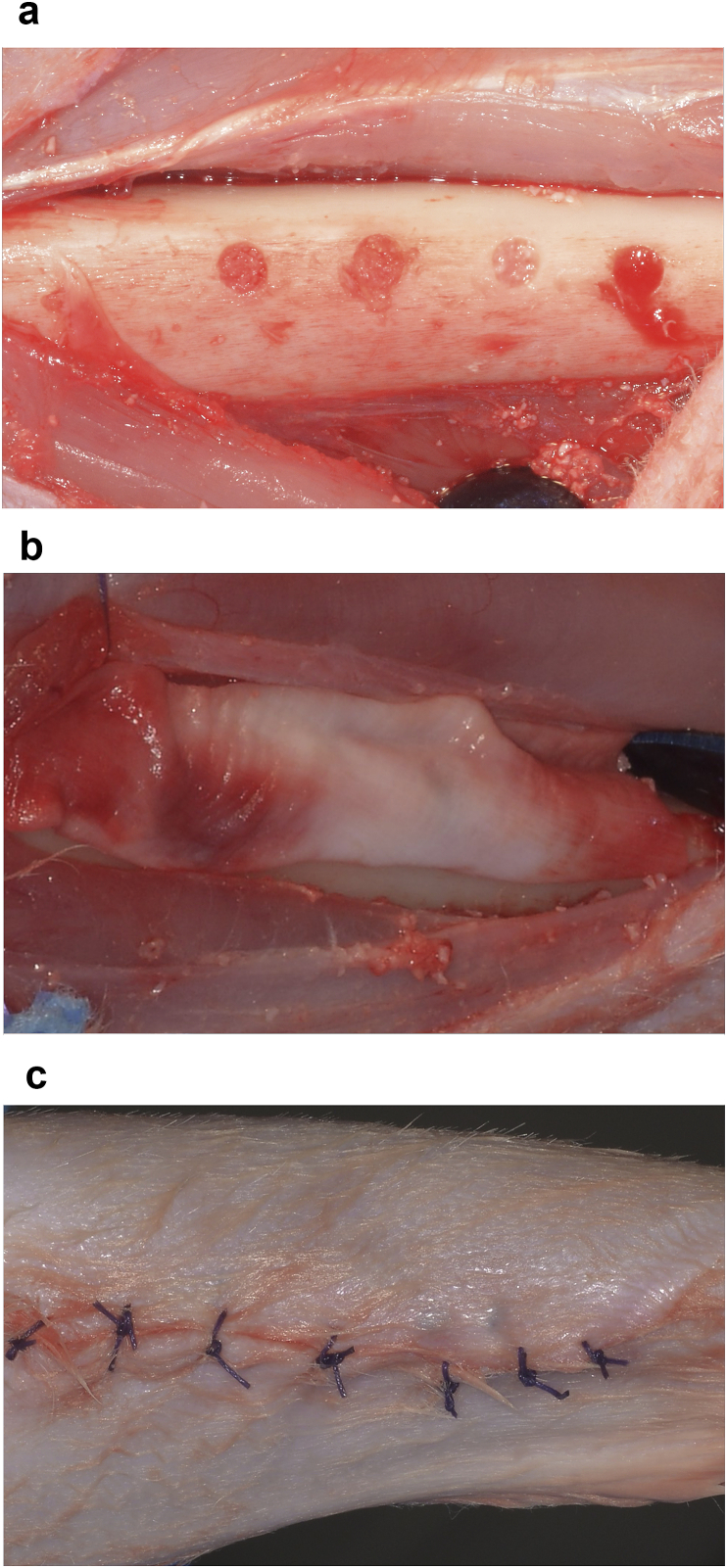
Rabbits were kept in independent 50 cm high, 50 cm wide and 60 long cages, and acclimated two weeks before the study in a ventilated room with a temperature ranging from 18 to 22 °C, 40–70% humidity and 14–16 h of ambient light, and they were fed twice a day with balanced food pellets. Before surgery, food was restricted the night before and water 6 h prior to anesthesia induction. A cannula vas placed in the rabbit's marginal ear vein for drug administration and blood for L-PRF obtention. The tibial zone was shaved and cleaned with 2% chlorhexidine, and cefazoline (30 mg/kg) was administrated as prophylactic antibiotic and then repeated every 30 min. Prior to anesthesia induction, fentanyl (1–2 μg/Kg) and dexmedetomidine (1 μg/Kg) were administrated. For anesthesia induction, propofol (1 mg/kg) and midazolam (0.2 mg/kg) were used. 1% Isoflurane and 100% oxygen were administrated via laryngeal mask for anesthesia maintenance. After general anesthesia was achieved, 8 ml venous blood was extracted and centrifugated at 3000 rpm and 400 g for 10 min to obtain L-PRF (Fig. 1).19 The L-PRF clot was then placed in a metallic PRF box. (PRF box, Dowell). At the tibial surgical site, a full thickness flap was elevated and four 2 mm wide and 6 mm deep defects were made with a surgical drill, with a 5 mm separation between them. The first defect was filled with DBBM (Bio-Oss, Geistlich Pharma AG), the second one with a plug of L-PRF, the third one with a mixture of DBBM and 1–2 mm chopped L-PRF pieces in a 50:50 vol proportion, and the last one was left without any biomaterial as control site. The four defects were covered with a xenogeneic native collagen membrane (Bio-Gide, Geistlich Pharma AG). The muscular and subcutaneous layers were sutured with 6–0 polyglactin and skin was sutured with 4–0 polyglactin (Vicryl, Ethicon) (Fig. 2). Once the procedure was finished, the rabbits were transferred to their cage and carprofen (5 mg/kg) was administrated every 12 h for three days as analgesic. Surgical sites were constantly inspected and maintained clean and dry until complete healing.
Fig. 1.
L-PRF clot.
Fig. 2.
2A. The four 2 × 6 mm bone defects filled with DBBM, L-PRF, L-PRF + DBBM and the control site.
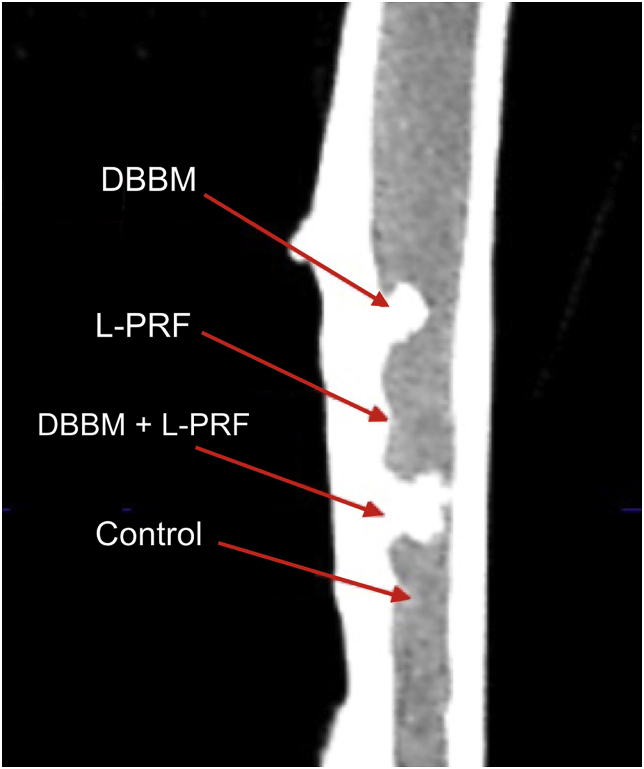
Rabbits were randomly distributed in three groups and euthanatized at 3, 6 or 9 weeks to evaluate different stages of bone healing. Euthanasia was performed by a lethal dose of intravenous propofol (100 mg/kg), previous dexmedetomidine (1 μg/Kg) administration. The tibial bone was extracted and placed in 10% formalin. A cone beam CT was performed to the extracted tibia, to evaluate DBBM graft particle migration between defects which may alter the histological results (Fig. 3). After that, bone samples of each defects were obtained using a 2 mm trephine bur (Osung, Korea) and immediately placed in 10% formalin. Samples were decalcified with a 10% EDTA solution and processed in a histologic tissue processor (STP 120–2, Microm) and embedded in paraffin in an embedding workstation (Histostar, Thermo Fisher Scientific). Histological cuts series were made using a microtome (HR 315, Microm) and Van Gieson's stain was apply to them. Micro-pictures of the samples were taken with a high-resolution camera (ICC50HD, Leica) mounted in an optical microscope (DM500, Leica) at 4x magnification (Fig. 4). A blind and properly calibrated examiner analyzed the pictures using an image processing and analysis software (ImageJ, NIH) to determine the vital bone, connective tissue and remaining graft particles percentage of each total defect sample.5,20
Fig. 3.
A cone beam computed tomography was performed to the extracted tibiae, to evaluate DBBM graft particle migration between defects.
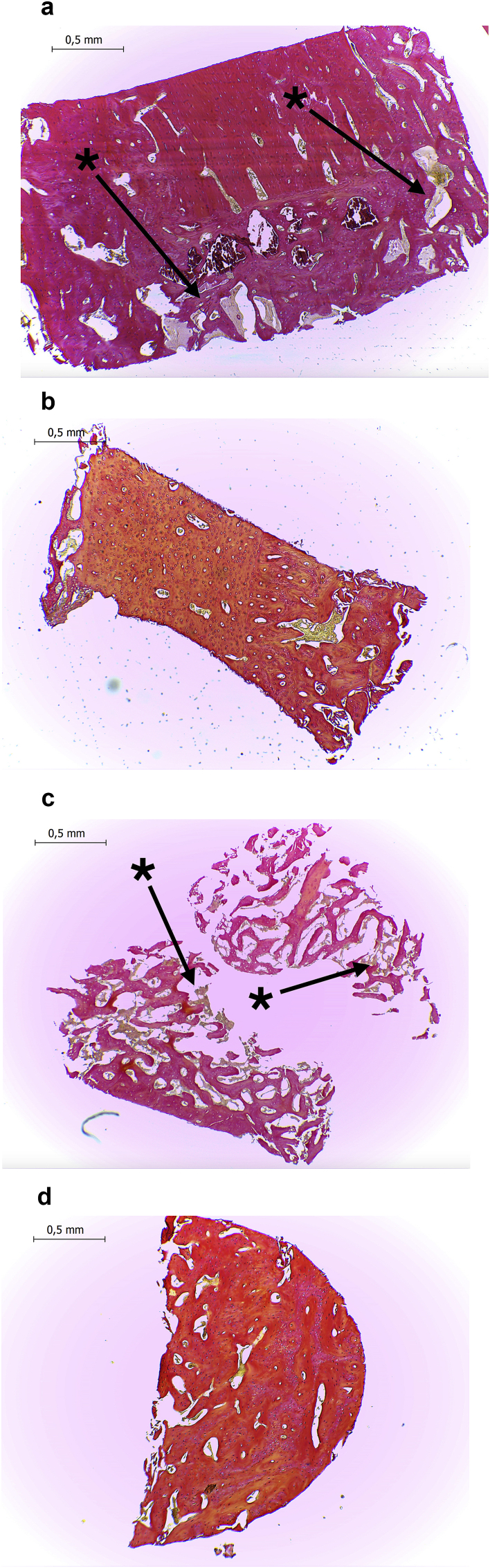
Fig. 4.
Histological samples at 6×weeks of healing and 4× magnification.
Data was arranged according to weeks of healing and vital bone, connective tissue and remaining graft particles percentages were compared between grafting procedures at different healing times. A statistical software (SPSS, IBM) was used for this purpose. For vital bone and connective tissue percentages, variance analysis (ANOVA) and HSD Tukey tests were applied if all samples showed a normal distribution, and Kruskal Wallis and Mann-Whitney U tests were used if at least one group showed an abnormal data distribution. To compare the remaining particle percentage between the DBBM and DBBM + L-PRF groups, non-paired t-test was performed.
3. Results
A total of five samples were excluded, due to graft particle migration between defects one from the L-PRF group at 3 weeks of healing, or sample retrieval inaccuracy one from the L-PRF group at 3 weeks of healing, one from the DBBM + L-PRF group at 6 weeks of healing and three from the 9 week healing period: one from the DBBM, L-PRF and DBBM + L-PRF each.
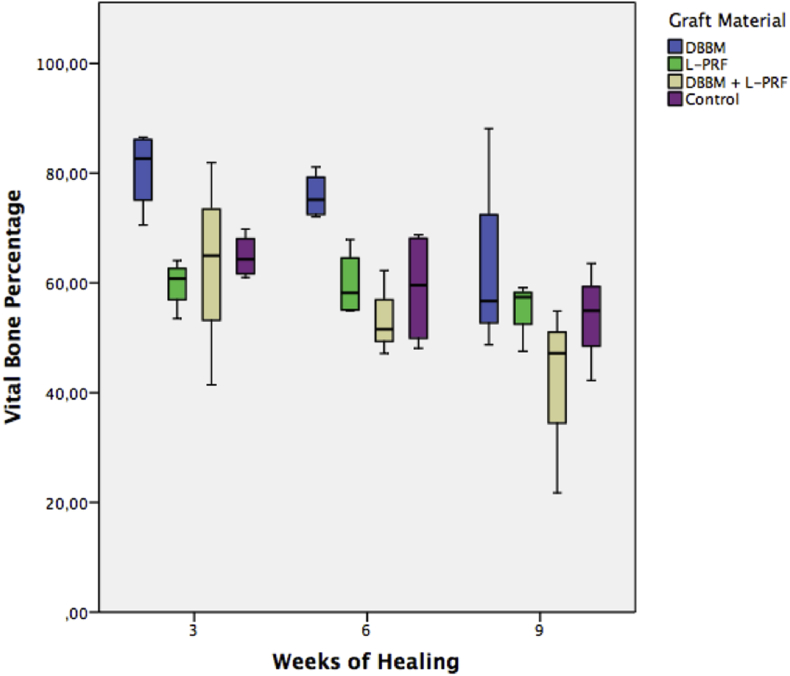
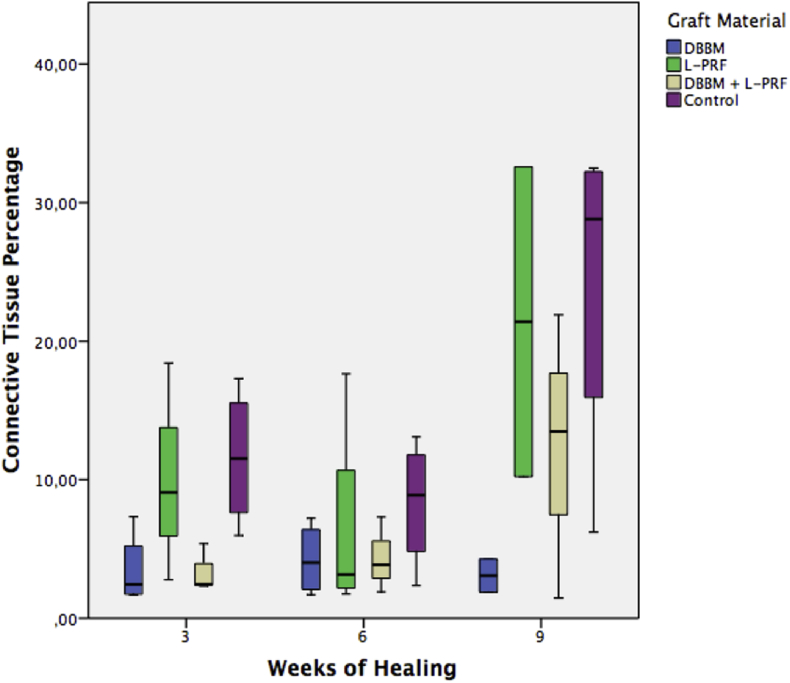
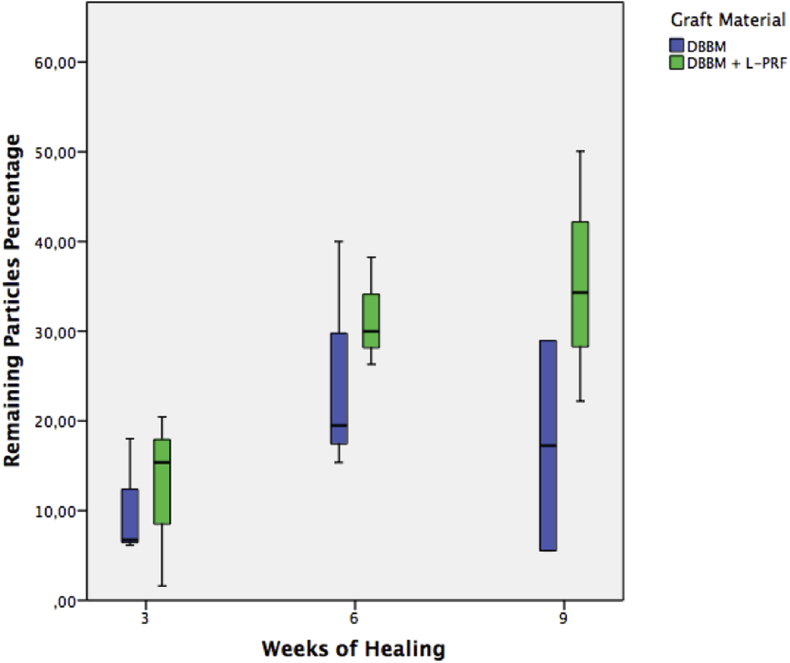
Vital bone, connective tissue and remaining particle percentages are shown in Table 1, Table 2, Table 3 and represented in Graphic 1, Graphic 2, Graphic 3 respectively.
Table 1.
Vital bone percentage at 3, 6 and 9 weeks of healing.
| 3 Weeks | DBBM |
n = 4 mean % = 80,61 (95% CI: 68,86 – 92,35) * |
| L-PRF |
n = 3 mean % = 58,35 (95% CI: 47,84 – 68,86) * |
|
| DBBM + L-PRF |
n = 4 mean % = 63,10 (95% CI: 36,66 – 89,54) |
|
| Control |
n = 4 mean % = 64,86 (95% CI: 58,52 – 71,20) |
|
| ANOVA (p= 0,05); HSD Tukey (p=0,05)* | ||
| 6 Weeks | DBBM |
n = 4 mean % = 75,87 (95% CI: 69,17 – 82,57)*† |
| L-PRF |
n = 4 mean % = 59,80 (95% CI: 50,07 – 69,53)* |
|
| DBBM + L-PRF |
n = 3 mean % = 53,66 (95% CI: 34,33 – 72,98)† |
|
| Control |
n = 4 mean % = 59,00 (95% CI: 42,09 – 75,91)ƒ |
|
| Kruskal Wallis (p = 0,028); Mann-Withney U (p <0,05) *†ƒ | ||
| 9 Weeks | DBBM |
n = 3 mean % = 64,52 (95% CI: 12,76 – 116,27) |
| L-PRF |
n = 3 mean % = 54,69 (95% CI: 39,21 – 70,16) |
|
| DBBM + L-PRF |
n = 3 mean % = 41,27 (95% CI: -1,78 – 84,33) |
|
| Control |
n = 4 mean % = 53,91 (95% CI: 69,17 – 82,57) |
|
| ANOVA (p = 0,311) |
DBBM: bovine derived deproteinized xenograft. L-PRF: leukocyte and platelet rich fibrin. CI: Confidence interval. Symbol*, †and ƒ: significative difference between those groups.
Table 2.
Connective Tissue Percentage at 3, 6 and 9 weeks of healing.
| 3 Weeks | DBBM |
n = 4 mean % = 3,47 (95% CI: -0,74 – 7,69) |
| L-PRF |
n = 3 mean % = 10,09 (95% CI: 9,45 – 29,64) |
|
| DBBM + L-PRF |
n = 4 mean % = 3,16 (95% CI: 0,78 – 5,53)* |
|
| Control |
n = 4 mean % = 11,58 (95% CI: 3,66 – 19,50)* |
|
| Kruskal Wallis (p = 0,049); Mann-Withney U (p < 0,05)* | ||
| 6 Weeks | DBBM |
n = 4 mean % = 4,24 (95% CI: 0,10 – 8,37) |
| L-PRF |
n = 4 mean % = 6,43 (95% CI: -5,54 – 18,39) |
|
| DBBM + L-PRF |
n = 3 mean % = 4,36 (95% CI: -2,43 – 11,15) |
|
| Control |
n = 4 mean % = 8,31 (95% CI: 0,95 – 15,66) |
|
| Kruskal Wallis (p = 0,587) | ||
| 9 Weeks | DBBM |
n = 3 mean % = 4,04 (95% CI: -1,06 – 9,13) |
| L-PRF |
n = 3 mean % = 17,65 (95% CI: -14,48 – 49,77) |
|
| DBBM + L-PRF |
n = 3 mean % = 12,28 (95% CI: -1,78 – 84,33) |
|
| Control |
n = 4 mean % = 24,08 (95% CI: 4,50 – 43,67) |
|
| Kruskal Wallis (p = 0,135) |
DBBM: bovine derived deproteinized xenograft. L-PRF: leukocyte and platelet rich fibrin. CI: Confidence interval. Symbol *: significative difference between those groups.
Table 3.
| (1) |
| (3) |
| (5) |
| 3 Weeks | DBBM |
n = 4 mean % = 10,97 (95% CI: 2,02 – 19,92) |
| DBBM + L-PRF |
n = 4 mean % = 10,12 (95% CI: -4,60 – 24,84) |
|
| Non-paired t test (p = 0,71) | ||
| 6 Weeks | DBBM |
n = 4 mean % = 20,63 (95% CI: -1,36 – 42,62) |
| DBBM + L-PRF |
n = 3 mean % = 31,51 (95% CI: 16,32 – 46,70) |
|
| Non-paired t test (p = 0,344) | ||
| 9 Weeks | DBBM |
n = 3 mean % = 25,32 (95% CI: -20,00 – 70,65) |
| DBBM + L-PRF |
n = 3 mean % = 35,52 (95% CI: 0,83 – 70,21) |
|
| Non-paired t test (p = 0,618) |
DBBM: bovine derived deproteinized xenograft. L-PRF: leukocyte and platelet rich fibrin. CI: Confidence interval.
Graphic 1.
Vital bone percentage at 3, 6 and 9 weeks of healing DBBM: bovine derived deproteinized xenograft. L-PRF: leukocyte and platelet rich fibrin.
Graphic 2.
Connective tissue percentage at 3, 6 and 9 weeks of healing, DBBM: bovine derived deproteinized xenograft. L-PRF: leukocyte and platelet rich fibrin.
Graphic 3.
Remaining particle percentage at 3, 6 and 9 weeks of healing, DBBM: bovine derived deproteinized xenograft. L-PRF: leukocyte and platelet rich fibrin.
At 3 weeks of healing, DBBM showed significantly more vital bone percentage than L-PRF and DBBM + L-PRF showed significantly less connective tissue than control. All other groups showed no statistical difference between them. At 6 weeks of healing, DBBM showed significantly more vital bone percentage than L-PRF, DBBM + L-PRF and control but there wasn't any other significant difference regarding to connective tissue or remaining particle percentage between groups. At the 9-week healing period, there weren't any significant differences between groups.
4. Discussion
In the last years, several efforts have been made to understand the role of different bioactive agents to enhance bone regeneration. Many of them showing a wide range of results and evidence level. Special attention must be paid on controversies between researchers, particularly on the use of platelet concentrates, centrifugation protocols, handling and surgical application. The L-PRF protocol used in the present study was applied following the recommendations of Miron et al.21
New Zealand Rabbits were used as experimental model following all the considerations cited by Stavropoulos et al.17 In addition, the possibility of collecting an amount of blood similar to humans, the chance to perform multiple bone defects in its tibiae, its bone turnover rate, and the ease of handling, made them an appropriate model.15
Miron et al. demonstrated, in a systemic review, the beneficial effect of L-PRF to enhance soft tissue healing, both in medicine and dentistry, but there was no standardization regarding the evaluation of its results.22
A metanalysis performed by Castro et al.12 demonstrated that the use of L-PRF enhances wound healing and periodontal parameters on angular defects and furcations. By the other hand, they were unable to demonstrate benefits when using L-PRF for periodontal plastic surgery. Another metanalysis performed by the same group concluded that L-PRF might have a positive effect on bone regeneration and osseointegration, but that a meta‐analysis couldn't be performed due to the heterogeneity of the data.13
In the present study, at the 3 weeks healing interval, the DBBM group showed significant more vital bone formation than L-PRF. At 6 weeks of healing, the DBBM group had significantly more vital bone formation than the three other groups, which showed no significant differences between them in this parameter. After 9 weeks, no significant differences were observed between any groups regarding this variable, showing no added benefit of L-PRF alone or combined with DBBM in early vital bone formation.
These results are in concordance with those presented by Karayürek et al.7 where they observed that the combination of L-PRF with a Xenograft showed no significant benefit in new bone formation. This may be explained because the growth factors release occurs only during the first 7 days and subsequently the effect of L-PRF may not last enough to promote significant benefits in new bone formation.23 In addition to this, a high percentage of new bone formation was observed in all groups at during the first weeks. This observation may be explained by high rate of bone turnover presented by the rabbit that allows it to reach bone maturation after 8 weeks.24
A recent review concluded that there is insufficient evidence to show that autologous platelet concentrates provide an additional benefit in the regeneration of hard tissues, beyond their benefit in soft tissues and post-surgical discomfort reduction in oral surgery.25
Regarding connective tissue percentage, there was only a significant difference between the DBBM + L-PRF and the control group, showing the latter more connective tissue at 3 weeks. There were no significant differences between groups with regards to this parameter at other time intervals, but nevertheless, there was a trend of less connective tissue percentage in both groups that used DBBM, combined or not with L-PRF. There were also no significant differences regarding remaining particles percentage between DBBM and DBBM + L-PRF groups, at any time interval.
There was a concordance, as published by Nappe et al.,5 that the use of DBBM, combined or not with L-PRF, may reduce the connective tissue percentage of the samples by its remaining particles. This may affect the quality and consistency of the newly formed bone.
The heterogeneity of the data published until now, shows the need for standardize the model to obtain more accurate results to know if there is a clinical benefit of using L-PRF alone or combined with DBBM to enhance bone formation in GBR procedures.
5. Conclusion
Within the limitations of this study, it can be concluded that DBBM seems to enhance vital bone formation at early healing stages. The use of L-PRF alone or combined with DBBM, didn't show any histological improvement regarding to vital bone formation. The use of DBBM, alone or in conjunction with L-PRF showed a trend to reduce connective tissue percentage. The use of L-PRF combined with DBBM didn't affect the remaining particle percentage.
2B. All defects were covered with a native collagen membrane.
2C. Sutured surgical site.
4A. DBBM treated defect.
4B. L-PRF treated defect.
4C. L-PRF + DBBM treated defect.
4D. Control defect.
Van Gieson stain; * Asterisks show DBBM particles.
Acknowledgements
The present study was financed by a FDP Found granted by the Mayor University.
Contributor Information
Andres Rezuc, Email: andres.rezuc@gmail.com.
Christian Saavedra, Email: csaavedrawatson@gmail.com.
Rodrigo Maass, Email: rl.maass@gmail.com.
Christian Nappe, Email: cnappe@uc.cl.
References
- 1.Haugen H.J., Lyngstadaas S.P., Rossi F. Bone grafts: which is the ideal biomaterial? J Clin Periodontol. 2019;46(Suppl. 21):92–102. doi: 10.1111/jcpe.13058. [DOI] [PubMed] [Google Scholar]
- 2.Jepsen S., Schwarz F., Cordaro L. Regeneration of alveolar ridge defects. Consensus report of group 4 of the 15th European Workshop on Periodontology on Bone Regeneration. J Clin Periodontol. 2019;46(Suppl 21):277–286. doi: 10.1111/jcpe.13121. [DOI] [PubMed] [Google Scholar]
- 3.Yoon J.S., Lee S.H., Yoon H.J. The influence of platelet-rich fibrin on angiogenesis in guided bone regeneration using xenogeneic bone substitutes: a study of rabbit cranial defects. J Cranio-Maxillo-Fac Surg. 2014;42(7):1071–1077. doi: 10.1016/j.jcms.2014.01.034. [DOI] [PubMed] [Google Scholar]
- 4.Araujo M.G., da Silva J.C.C., de Mendonca A.F., Lindhe J. Ridge alterations following grafting of fresh extraction sockets in man. A randomized clinical trial. Clin Oral Implants Res. 2015;26:407–412. doi: 10.1111/clr.12366. 6. [DOI] [PubMed] [Google Scholar]
- 5.Nappe C.E., Rezuc A.B., Montecinos A., Donoso F.A., Vergara A.J., Martinez B. Histological comparison of an allograft, a xenograft and alloplastic graft as bone substitute materials. J Osseointegr. 2016;8(2):20–26. [Google Scholar]
- 6.Llanos A.H., Sapata V.M., Jung R.E. Comparison between two bone substitutes for alveolar ridge preservation after tooth extraction: cone-beam computed tomography results of a non-inferiority randomized controlled trial. J Clin Periodontol. 2019;46 doi: 10.1111/jcpe.13079. 373– 38. [DOI] [PubMed] [Google Scholar]
- 7.Karayürek F., Kadiroğlu E.T., Nergis Y. Combining platelet rich fibrin with different bone graft materials: an experimental study on the histopathological and immunohistochemical aspects of bone healing. J Cranio-Maxillo-Fac Surg. 2019;47(5):815–825. doi: 10.1016/j.jcms.2019.01.023. [DOI] [PubMed] [Google Scholar]
- 8.Dragonas P., Katsaros T., Avila-Ortiz G., Chambrone L., Schiavo J.H., Palaiologou A. Effects of leukocyte–platelet-rich fibrin (L-PRF) in different intraoral bone grafting procedures: a systematic review. Int J Oral Maxillofac Surg. 2019;48(2):250–262. doi: 10.1016/j.ijom.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Miron R.J., Dham A., Dham U., Zhang Y., Pikos M.A., Sculean A. The effect of age, gender, and time between blood draw and start of centrifugation on the size outcomes of platelet-rich fibrin (PRF) membranes. Clin Oral Invest. 2019;23(5):2179–2185. doi: 10.1007/s00784-018-2673-x. [DOI] [PubMed] [Google Scholar]
- 10.Cortellini S., Castro A.B., Temmerman A. Leucocyte-and platelet-rich fibrin block for bone augmentation procedure: a proof-of-concept study. J Clin Periodontol. 2018;45(5):624–634. doi: 10.1111/jcpe.12877. [DOI] [PubMed] [Google Scholar]
- 11.Miron R.J., Zucchelli G., Pikos M.A. Use of platelet-rich fibrin in regenerative dentistry: a systematic review. Clin Oral Invest. 2017;21(6):1913–1927. doi: 10.1007/s00784-017-2133-z. [DOI] [PubMed] [Google Scholar]
- 12.Castro A.B., Meschi N., Temmerman A. Regenerative potential of leucocyte- and platelet-rich fibrin. Part A: intra-bony defects, furcation defects and periodontal plastic surgery. A systematic review and meta-analysis. J Clin Periodontol. 2017;44:67–82. doi: 10.1111/jcpe.12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castro A.B., Meschi N., Temmerman A. Regenerative potential of leucocyte- and platelet-rich fibrin. Part B: sinus floor elevation, alveolar ridge preservation, and implant therapy. A systematic review. J Clin Periodontol. 2017;44:225–234. doi: 10.1111/jcpe.12658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miron R.J., Fujioka-Kobayashi M., Bishara M., Zhang Y., Hernandez M., Choukroun J. Platelet-rich fibrin and soft tissue wound healing: a systematic review. Tissue Eng B Rev. 2017;23(1):83–99. doi: 10.1089/ten.TEB.2016.0233. [DOI] [PubMed] [Google Scholar]
- 15.Smith P.C., Martinez C., Caceres M., Martinez J. Research on growth factors in periodontology. Periodontol. 2000;67(1):234–250. doi: 10.1111/prd.12068. 2015. [DOI] [PubMed] [Google Scholar]
- 16.Donos N., Dereka X., Mardas N. Experimental models for guided bone regeneration in healthy and medically compromised conditions. Periodontol. 2000;68(1):99–121. doi: 10.1111/prd.12077. 2015. [DOI] [PubMed] [Google Scholar]
- 17.Stavropoulos A., Sculean A., Bosshardt D.D., Buser D., Klinge B. Pre-clinical in vivo models for the screening of bone biomaterials for oral/craniofacial indications: focus on small-animal models. Periodontol. 2000;68(1):55–65. doi: 10.1111/prd.12065. 2015. [DOI] [PubMed] [Google Scholar]
- 18.Kantarci A., Hasturk H., Van Dyke T.E. Animal models for periodontal regeneration and peri-implant responses. Periodontol. 2000;68(1):66–82. doi: 10.1111/prd.12052. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dohan D.M., Choukroun J., Diss A. Platelet-rich fibrin (PRF): a second generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):e37–44. doi: 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Schindelin J., Arganda-Carreras I., Frise E. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;28(7):676–682. doi: 10.1038/nmeth.2019. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miron R., Choukroun J., Ghanaati S. Controversies related to scientific report describing g-forces from studies on platelet-rich fibrin: necessity for standardization of relative centrifugal force value. Int J Growth Factors Stem Cells Dent. 2012;1(3):80–89. [Google Scholar]
- 22.Miron R., Fujioka M., Bishara M. Platelet Rich Fibrin and soft tissue wound healing. A systemic Review. Tissue Eng Part B Rev. 2017;23(1):83–99. doi: 10.1089/ten.TEB.2016.0233. [DOI] [PubMed] [Google Scholar]
- 23.Ehrenfest D.M.D., de Peppo G.M., Doglioli P., Sammartino G. Slow release of growth factors and thrombospondin-1 in Choukroun's platelet-rich fibrin (PRF): a gold standard to achieve for all surgical platelet concentrates technologies. Growth Factors. 2009;27(1):63–69. doi: 10.1080/08977190802636713. [DOI] [PubMed] [Google Scholar]
- 24.Durmuslar M.C., Balli U., Öngöz Dede F. Evaluation of the effects of platelet-rich fibrin on bone regeneration in diabetic rabbits. J Cranio-Maxillo-Fac Surg. 2016;44(2):126–133. doi: 10.1016/j.jcms.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Donos N., Dereka X., Calciolari E. The use of bioactive factors to enhance bone regeneration: a narrative review. J Clin Periodontol. 2019;46(21):124–161. doi: 10.1111/jcpe.13048. [DOI] [PubMed] [Google Scholar]